Abstract
The last 100 years witnessed a rapid and progressive development of the body of knowledge concerning the neural control of the cardiovascular system in health and disease. The understanding of the complexity and the relevance of the neuroregulatory system continues to evolve and as a result raises new questions. The purpose of this review is to articulate results from studies involving experimental models in animals as well as in humans concerning the interaction between the neural mechanisms mediating the hemodynamic responses during exercise. The review describes the arterial baroreflex, the pivotal mechanism controlling mean arterial blood pressure and its fluctuations along with the two main activation mechanisms to exercise: central command (parallel activation of central somatomotor and autonomic descending pathways) and the muscle metaboreflex, the metabolic component of exercise pressor reflex (feedback from ergoreceptors within contracting skeletal muscles). In addition, the role of the cardiopulmonary baroreceptors in modulating the resetting of arterial baroreflex is identified, and the mechanisms in the central nervous system involved with the resetting of baroreflex function during dynamic exercise are also described. Approaching a very relevant clinical condition, the review also presents the concept that the impaired arterial baroreflex function is an integral component of the metaboreflex-mediated exaggerated sympathetic tone in subjects with heart failure. This increased sympathetic activity has a major role in causing the depressed ventricular function observed during submaximal dynamic exercise in these patients. The potential contribution of a metaboreflex arising from respiratory muscles is also considered.
Keywords: autonomic, baroreflex, central command, exercise, metaboreflex
this article is part of a collection on 1st Pan American Congress of Physiological Sciences: Physiology Without Borders. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
A wide range of complex but routine behaviors such as feeding, shivering, locomotion, aggressive/defensive, and sexual behaviors triggers concomitant activation of somatomotor and autonomic outflows (47). Dynamic exercise is a complex physiological challenge involving highly coordinated activity of skeletal muscles, rapid and immediate increases in respiratory function, heart rate (HR), stroke volume and cardiac output associated with changes in regional vascular resistance, and redistribution of systemic blood flow that cause a slight increase in mean blood pressure (108). The increased blood flow to active muscles provides the necessary oxygen and glucose supply for muscle contractions (52). Therefore, during an acute bout of exercise, there is a tight coupling between the somatic motor system, the efferent system that innervates skeletal muscle, and the autonomic nervous system that innervates visceral organs (108).
The coordinated and simultaneous activation of both neural outflows involves coordinated activity of integrated brain circuits and different classes of receptors/afferent projections that detect and codify peripheral changes induced by exercise. The current model of circulatory control during exercise holds that it is governed by two main neural mechanisms: 1) the central command (CC), a feedforward control mechanism that sets the basic pattern of motor activity to skeletal muscles and drives cardiorespiratory activation (22, 33, 62, 70), and 2) the feedback control mechanisms driven by intrinsic/extrinsic receptors from cardiovascular areas and receptors within the active muscles (57, 71, 95, 109).
The increased muscle activity and cardiovascular and respiratory adjustments during dynamic exercise simultaneously activate several receptors (Fig. 1): 1) the reduced venous capacitance (with increased venous return) and increased cardiac filling stimulate cardiopulmonary receptors; 2) increases in cardiac output and changes in vascular resistance (renal and gastrointestinal vasoconstriction along with working muscle vasodilatation) causes an increase in blood pressure that activates arterial baroreceptors; 3) the increased oxygen consumption and carbon dioxide by active muscles combined with respiratory and peripheral capillary blood flow dynamics may change arterial Po2 and Pco2 under certain conditions activating peripheral (and central) chemoreceptors; 4) thermoreceptors are also activated if exercise is performed in a hot or cold environment or during strenuous or prolonged physical activity; 5) muscle contractions and metabolic byproducts of muscular work activate thin muscle fibers, i.e., groups III and IV afferents of the so-called exercise pressor reflex (EPR). Afferent signals from contracting muscles initially relay on the dorsal horn of the spinal cord where a complex network of neurons and interneurons interact via various types of receptors sensitive to vasopressin, excitatory amino acids, serotonin, opioids, neurokinin-1, and nitric oxide among others (37, 56, 74, 105, 116). Along with these previous publications in animal models, studies involving human subjects have also shown that stimulation of opioid receptors can modulate the reflex cardiorespiratory responses to exercise (1, 5). Peripheral stimuli are conducted via appropriate afferents, including skeletal muscle mechano- and metabosensitive fibers, to the nucleus of the solitary tract in the brain stem (NTS, a primary integrative area, Fig. 1), ascending from there to higher integrative areas in the central nervous system. Integration of afferent signals within the brain stem results in efferent parasympathetic and sympathetic outflow from the nucleus ambiguus/dorsomotor nucleus of the vagus and rostroventrolateral medulla, respectively.
Fig. 1.
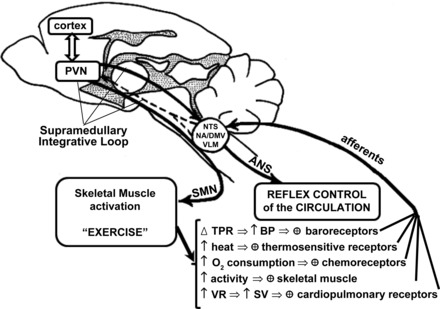
Schematic representation of somatomotor and autonomic outflows controlling muscle activity and the reflex control of the circulation. Different receptors detect changes in muscle contraction/perfusion, cardiac filling, blood pressure (BP), blood gases, and temperature during muscular activity and send this information to brain stem and supramedullary areas involved in reflex control of the circulation. ANS, autonomic nervous system; DMV, dorsomotor nucleus of the vagus; NA, nucleus ambiguous; NTS, nucleus of the solitary tract; PVN, paraventricular nucleus of the hypothalamus; SMN, somatomotor neurons; SV, stroke volume; TPR, total peripheral resistance; VLM, ventrolateral medulla; VR, venous return. Modified from Fig. 3 in Michelini and Morris (68).
CC encompasses higher brain areas involving, at the onset and during dynamic exercise, the simultaneous and parallel activation of two separate networks: one signaling the autonomic neural control of the cardiovascular system and the other signaling the neuromotor control of the active skeletal muscles. Although the CC effect has been widely accepted, the structures responsible for its activity are not clearly defined. Several experimental animal and human studies have suggested the involvement of insular cortex, medial prefrontal area, amygdaloid and hypothalamic nuclei, ventral tegmental field, and periaqueductal gray and subthalamic nucleus in the mesencephalon, besides the mesencephalic locomotor region and the ventromedial medulla (6, 22, 47, 60, 62, 108, 112).
Systematic studies on brain stem and supramedullary pathways activated by baroreceptors afferents during exercise and following exercise training revealed changes on the plasticity/activity of the neuronal circuitry (functional remodeling), showing an important link between reflex control of the circulation and the CC. It has been shown that acute bouts of dynamic exercise repeated over long periods of time (exercise training) 1) increase the sensitivity of aortic nerve afferents and augment afferent NTS signaling (9), 2) increase noradrenergic ascending inputs from the NTS to the preautonomic neurons in the paraventricular nucleus of the hypothalamus (PVN) (40), 3) result in structural remodeling (i.e., increased dendritic branching and neuron surface area) of preautonomic PVN neurons projecting to the dorsal brain stem and augment their excitability (11, 43, 69), and 4) increase the density/intensity of descending PVN oxytocinergic neurons projecting to the NTS and augment the density of oxytocinergic terminals within this area (11, 69). It was also shown that the affinity of NTS vasopressinergic receptors for its endogenous ligand is significantly increased after training (21). In addition, PVN-NTS vasopressinergic projections are strongly activated during an acute bout of exercise (26, 68). These observations demonstrate the existence of a supramedullary integrative loop connecting brain stem and PVN, comprising of Fig. 1: 1) ascending noradrenergic projections from the NTS and caudal ventrolateral medulla, and 2) descending PVN oxytocinergic and vasopressinergic neurons projecting to brainstem areas (69). From these observations, it is likely that both feedforward (CC) and feedback neural controllers of the circulation (driven by baroreceptors, chemoreceptors, cardiopulmonary receptors, and skeletal muscle receptors) interact to regulate cardiovascular and respiratory responses during exercise. Although not clearly demonstrated, other loops between diencephalic and/or mesencephalic areas and higher integrative centers may participate in the integration of CC with brain stem pathways involved in reflex control of the circulation.
The purpose of this review is to integrate studies involving experimental models in animals as well as humans concerning the interaction between CC, arterial baroreflex, and muscle metaboreflex. The mechanisms in the central nervous system involved with the changes in baroreflex function during dynamic exercise and exercise training are initially addressed. The second part of the review presents the concept that the impaired arterial baroreflex function is an integral component of the excessive sympathetic tone in subjects with heart failure. This hyperadrenergic state is driven by muscle metaboreflex and plays a key role in causing depressed ventricular function during submaximal dynamic exercise in these patients. The potential contribution of a metaboreflex arising from respiratory muscles is also considered.
Arterial Baroreflex
In 1863, Marey (59) described the inverse reflex relationship between HR and arterial blood pressure (ABP) at rest in humans. Subsequent animal investigations by Hering (1927) (38), Koch and Miles (1929) (51), and finally Heymans and Neil (1958) (39) described the underlying neural mechanisms of the arterial baroreflex responses to changes in ABP at rest, i.e., increases in ABP accompanied by reflex bradycardia and decreases in ABP accompanied by reflex tachycardia and vasoconstriction (38, 39, 51). Nowadays, it is well known that arterial baroreceptors are essential mechanisms for correcting blood pressure changes and oscillations. Transient increases and decreases in pressure, codified by baroreceptors in frequency of action potentials, are integrated at bulbar and supramedullary levels (Fig. 1), changing sympathetic and parasympathetic outflows that result in appropriate responses of venous capacitance, cardiac output, and total peripheral resistance to promptly correct pressure changes. Although transient pressure increases determine reflex bradycardia and generalized reflex vasodilatation, exercise-induced pressure increases are accompanied by tachycardia, skeletal muscle, and coronary vasodilatation simultaneously with renal and mesenteric vasoconstriction (66). In 1913, Krogh and Lindhard (54) identified in exercising human subjects a parallel increase in HR and ABP at the start of dynamic exercise, thereby raising the question as to why the HR did not decrease during the transition from rest in relation to the exercise-induced increase in ABP.
Addressing the question.
In 1957, Ernsting and Parry (28) developed a neck collar system that could provide pressure and suction around the neck of humans at rest to noninvasively decrease and increase transmural pressure around the carotid sinus. Despite technical limitations of the neck collar to assess carotid baroreflex control of ABP at rest, Bevegaard and Shepherd (1966) (7) clearly demonstrated that neck suction (simulating hypertension) resulted in equal increases in forearm blood flow and increases in radial artery diameter at rest and during mild and moderate leg cycling exercise (7). These findings suggested that the operating point (OP) pressure of the arterial baroreflex during exercise was “reset” to an increased arterial pressure that was related to the intensity of the exercise (44). However, at the beginning of the 1970s, a pharmacological method of sequentially increasing and decreasing ABP in humans was developed at Oxford to examine the arterial baroreflex at rest and during exercise, subsequently identified as the “Oxford Technique” (8, 19, 86, 87). Because the Oxford Technique used vasoactive drugs that controlled the amount of peripheral blood vessel vasomotor tone for a given dose of drug, the ABP-HR reflex OP gain was the only outcome measurement available in assessing the arterial baroreflex's sensitivity at rest and during exercise. The major finding of these studies indicated that as the intensity of dynamic exercise increased from rest, the gain (sensitivity) of the ΔABP-ΔR-R interval reflex at the OP pressure decreased, suggesting that the arterial baroreflex control of ABP was “switching off” in an intensity related manner (8, 19, 86, 87). Once again raising the question as to whether the arterial baroreflex was “switched off” or “reset” during dynamic exercise.
In the 1980s, David Donal's group at the Mayo Clinic performed a series of investigations using treadmill exercising canines with and without surgically isolated carotid sinus in combination with or without vagotomy (20, 64, 109, 110). The major finding of these studies were that 1) the carotid-HR reflex was reset to a higher OP pressure in association with the increasing exercise intensities and 2) vagal afferent neural information from cardiopulmonary baroreceptors was important for establishing the resetting of the OP pressure. However, these findings were not confirmed in human subjects performing isometric exercise in combination with or without dynamic exercise (100). Subsequent work by Sheriff et al. (1990) (102) identified in exercising canines that the arterial baroreflex functioned as a brake on the exercise-induced increase in blood pressure (102). These contrasting findings raised the question as to whether the differences were related to differences in species, exercise mode, experimental designs, or measurement technologies.
A series of technological developments to the neck collar system involving computer-controlled neck pressure and neck suction stimuli and data acquisition and logistic function modeling (27, 85, 90) helped to answer the questions raised by Rowell and O'Leary's (1990) (95) proposed hypothetical model of arterial baroreflex upward and rightward resetting during exercise (see Fig. 2). Initial investigations (75, 90) using our customized neck pressure/neck suction system examined whether the OP pressure of the arterial baroreflex was reset from rest upward and rightward during dynamic exercise of 25, 50, 75, and near 100% exercise intensity (Fig. 3). The major findings of these experiments identified that 1) the arterial baroreflex was progressively reset upward and rightward to function at a higher OP pressure in direct relation to the exercise intensity; 2) the maximum gain (Gmax, i.e., sensitivity) at the centering point (CP) of the carotid-HR and carotid-ABP baroreflex function curves at the 25, 50, 75, and near 100% work intensity was unchanged from rest; 3) the OP pressure of the carotid-HR baroreflex function curve moves to a point of reduced gain, operating range, and response range in direct relation to work intensities above 50%; and 4) the OP pressure and CP pressure of the carotid-ABP reflex pressure curve were reset upward and rightward in direct relation to the increasing intensity of the exercise without a change in Gmax, OP gain, response range, or operating range. These findings confirm those of Melcher and Donald (64) in their exercising dog model and the earlier work of Bevegaard and Shepherd (7) in exercising human subjects that the arterial baroreflex was reset during dynamic exercise. Furthermore, the relocation of the OP pressure to a point of lesser gain on the carotid-HR baroreflex function provides an explanation as to the apparent “switch off” of the HR reflex during exercise identified by the earlier investigations using the Oxford Technique (8, 19, 86, 87). Within a few months of Potts et al.'s (90) investigation, Papelier et al. (84) provided confirmatory data describing carotid arterial baroreflex resetting from rest to 75% maximum oxygen consumption exercise; however, the investigators neglected to model the baroreflex function curves.
Fig. 2.
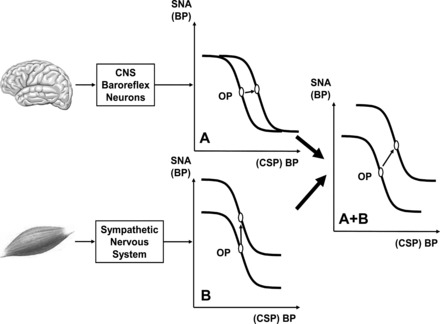
Hypothetical stimulus-response curves for arterial baroreflex, expressed as relationship between sympathetic nervous activity (SNA) and systemic BP or often in experiments as relationship between BP and isolated carotid sinus pressure (CSP). Operating point (OP) is BP “sought” by arterial baroreflex (often point of maximal gain). A: resetting of baroreflex by central command, a stimulus acting on neuron pool receiving baroreceptor afferent. OP is shifted laterally to a higher BP. CNS, central nervous system. B: vertical shift of baroreflex function curve by muscle chemoreflex, a stimulus that increases SNA and raises BP without changing OP; i.e., influence is confined to efferent arm of baroreflex. A + B: hypothetical combined effects of both stimuli on curve during exercise (upward and rightward resetting). Modified from Fig. 4 in Rowell and O'Leary (95).
Fig. 3.
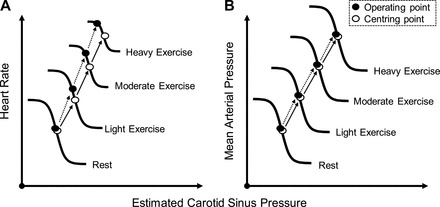
Representative illustration of the intensity-dependent resetting of the carotid baroreflex during dynamic exercise of 7 subjects. The centering point (CP) is the point at which there is an equal depressor and pressor response to a given change in BP; the OP is the prestimulus BP. Both the carotid baroreflex-heart rate (HR; A) and mean arterial pressure (MAP; B) stimulus-response curves progressively reset during exercise in an intensity-dependent manner without significant changes in maximal gain (sensitivity). A consistent observation for the baroreflex control of HR is the relocation of the OP away from the CP and closer to the threshold of the stimulus-response curve along with a reduction in the response range as exercise intensity increases (A). The relocation of the OP for HR control positions the baroreflex in a more optimal position to counter hypertensive stimuli with increasing exercise intensity. In contrast, for the carotid baroreflex-MAP, stimulus-response curve, the OP does not relocate away from the CP and the response range remains the same as at rest with an upward and rightward shift in parallel with an increase in the intensity of exercise (B). Previously published in Fadel and Raven (29).
Central command: arterial baroreflex interaction.
In the hypothetical model of arterial baroreflex resetting during exercise proposed by Rowell & O'Leary (95) (Fig. 2), both feedforward (CC) and feedback neural controllers (EPR) interact to regulate cardiovascular and respiratory responses during exercise. DiCarlo and Bishop (23) identified in exercising rabbits that at the onset of exercise, the OP pressure of the arterial baroreflex is reset to a higher pressure. In human subjects, Strange et al. (106) confirmed the hypothesis of Rowell and O'Leary (95) using an elegantly designed experiment in which they compared voluntary cycling to electrically stimulated cycling (no CC) and electrically stimulated cycling with epidural anesthesia (no CC and no EPR). During the no CC and no EPR cycling, the ABP did not increase during the exercise, confirming Rowell and O'Leary's (95) hypothesis that one or both CC and EPR were required to be activated for the exercise-induced increase in ABP to occur. In another study the selective activation of CC, using suggested increases in effort while human subjects were hypnotized and performing steady-state 100-W exercise or imagined exercise, resulted in increases in ABP, HR, and ratings of perceived exertion (113, 114). Decreases in hypnotic-suggested effort while performing steady-state 100-W exercise reduced ratings of perceived exertion but did not reduce ABP or HR. Other means of selectively increasing CC was to compare dynamic and static handgrip exercise with and without muscle weakness produced by 2% lidocaine (91) or static and dynamic leg exercise with and without neuromuscular blockade using vecuronium, a curare derivative (31), and constructing complete baroreflex stimulus response curves using our customized neck pressure/neck suction system. The baroreflex function curves for HR and ABP generated during control exercise were reset upward and rightward above rest. During the muscle weakness exercise, requiring an increase in CC above control, the exercise baroreflex function curves were further reset upward and rightward above the control exercise trial. Ogoh et al. (82) using the innovative tendon vibration technique to perform agonist and antagonist patellar tendon vibration to decrease and increase CC, respectively, also demonstrated that by selectively decreasing and increasing CC above control CC of 30% maximum voluntary contraction static exercise, the carotid baroreflex function curves were reset downward and leftward and upward and rightward, respectively, compared with the control exercise reflex function curves.
EPR: arterial baroreflex interaction.
In a recent review on EPR, Kaufman (45) showed it has two major roles in evoking cardiovascular responses to exercise: 1) when the blood supply of oxygen to the active skeletal muscles is insufficient to meet the metabolic needs of the contracting muscles, an exaggerated afferent neural signal is transmitted to the central nervous system via c-fiber afferents; and 2) even though the blood supply of oxygen to the active skeletal muscles is sufficient to meet the metabolic needs, there remains an active neural c-fiber afferent signal to the central nervous system associated with the intensity of the exercise. In Secher and Amann's review (97) of investigations of the EPR in humans, the anesthetic reduction of the neural afferent information transmitted to the central nervous system from the active skeletal muscle attenuates the exercise induced increase in ABP and cardiac output. In addition, electrically induced leg exercise in patients with tetraplegia, results in an attenuated ABP response and no ABP response in patients with paraplegia or in healthy subjects with anesthetic-induced paralysis, even though the electrical stimulation enhances lactate release and reduces muscle glycogen. These findings indicated that EPR increases sympathetic activity, cardiac output, and ABP to redistributing blood flow from the splanchnic vasculature, maintaining adequate blood flow to the active skeletal muscles.
In humans, selective activation of the intramuscular mechanoreceptors without stimulating metaboreceptors at rest and during exercise is technically challenging. However, in a series of experiments designed to preferentially accentuate mechanoreceptor activation above the metaboreceptor activation in healthy subjects, we employed leg compression using medical antishock trousers (MAST) with and without epidural anesthesia at rest (115) and with and without leg compression using MAST during steady-state exercise while constructing baroreflex function curves (32), lower body positive pressure (LBPP) at rest and during exercise (111), and 4) LBPP with and without thigh-cuff occlusion at 90 mmHg (30). In a separate investigation, the subjects performed submaximal dynamic exercise with and without epidural anesthesia with construction of the baroreflex function curves (103). In each of the investigations designed to accentuate the activation of the leg muscle's mechanoreceptors using either MAST or LBPP, the mean arterial pressure was increased above control at rest and during exercise, indicating a resetting of the arterial baroreceptor OP pressure. In the study that employed MAST at rest and during exercise, the baroreflex function curve was reset upward and rightward above control (31). In the investigation that used epidural anesthesia to attenuate the amount of afferent neural information arising from the exercised legs, the amount of resetting of the baroreflex function was attenuated compared with that in the control exercise (103). Collectively, these studies identify that activation of the EPR during dynamic and static exercise results in upward and rightward baroreflex resetting. Furthermore, a reduction or absence of EPR activation during leg exercise results in attenuation or absence, respectively, of exercise-induced baroreflex resetting.
Cardiopulmonary receptors: arterial baroreflex interaction.
Changes in central blood volume (CBV) are sensed by cardiopulmonary baroreceptors connected by c-fiber vagal afferents to the NTS (83). Information regarding increases and decreases in CBV results in reflex decreases and increases, respectively, in the sympathetic nerve activity (34, 93). During lower body negative pressure, reductions in CBV of humans increased the Gmax (sensitivity) of the carotid-HR and carotid-ABP baroreflex function curves (85). Invasive investigations in animals using procainamide infused into the pericardial sac of rats accentuated renal sympathetic nerve activity and increased vascular resistance (14, 24, 76). These findings confirm the involvement of cardiac receptors in providing afferent neural information regarding cardiac volume to the NTS and subsequently modulating the outflow of central sympathetic nerve activity.
In resting and exercising humans, increases in CBV can be accomplished by changing the posture from upright to supine (107), by infusion of serum albumin (79, 81) or infusion of a blood volume expander, such as low molecular weight dextran, and by increases in pedal frequency, for example, from 60 to 80 rpm at the same oxygen uptake (81). Decreases in CBV during rest and exercise can be accomplished by changing posture from supine to upright (81) and using lower body negative pressure (78, 79). The primary findings of these investigations identified that CBV modulated the resetting of the OP pressure of arterial baroreflex, i.e., increases in CBV during exercise reduced the OP pressure of the reflex, whereas reductions in CBV increased OP pressure of the reflex.
Mechanisms of Interaction Between CC, EPR, and Arterial Baroreflex During Exercise
The observations that baroreceptors are functionally active and reflex sensitivity is maintained during exercise (23, 53, 57, 95) indicate that other mechanism(s) is (are) activated simultaneously with baroreceptors' afferents by an acute bout of exercise.
Several studies in the last 10–20 years confirmed these observations and the interaction proposed by Rowell and O'Leary (95) (Fig. 2) between CC, EPR, and baroreceptors afferent signaling to reset reflex control of the circulation during exercise. As shown in Fig. 4, during exercise there is a concomitant activation of afferent projections to second-order neurons within the NTS that originate from the arterial baroreceptors, from the skeletal muscle receptors (EPR) and from the PVN (which is part of the CC). During a transient pressure increase (no exercise), the activation of baroreceptors' afferents causes a strong excitation of second-order neurons resulting in increased vagal outflow (via activation of parasympathetic preganglionic neurons located in the dorsal motor nucleus of the vagus and nucleus ambiguus) simultaneously with sympathetic withdrawal (mediated by the activation of GABAergic neurons projecting from caudalventrolateral medulla to premotor sympathetic neurons located in the rostroventrolateral medulla). The simultaneous activation of ascending neural feedback from skeletal muscle receptors (EPR) and descending neural input from the PVN during an exercise-induced pressure increase (Fig. 4) partially inhibits the baroreceptor-mediated activation of NTS second-order neurons, thus reducing both vagal outflow and sympathetic withdrawal to heart and vessels.
Fig. 4.
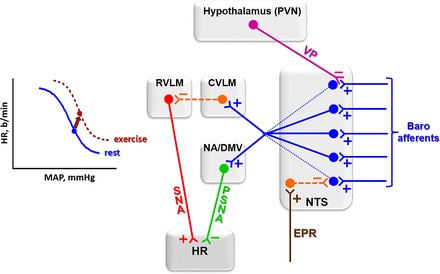
Proposed interaction between arterial baroreceptor afferents, exercise pressor reflex (EPR), and PVN vasopressinergic (VP) pathways that project to second-order neurons within the NTS. During exercise, both EPR (through a GABAergic projection) and VP (through an axo-axonal inhibitory synapse) partially inhibit afferent signaling by baroreceptors, thus limiting the degree of baroinhibition of the heart during pressure increases. These exercise-induced effects instantaneously reset baroreflex function (left). Blue, excitatory glutamatergic pathways; orange, GABAergic inhibitory pathways; pink, descending vasopressinergic projections; green, parasympathetic nervous activity (PSNA); red, SNA to the heart. CVLM, caudal ventrolateral medulla; RVLM, rostral ventrolateral medulla. b/min, Beats/min.
As elegantly demonstrated by Potts and coworkers (89, 90), the neural feedback from muscle receptors activates NTS GABAergic interneurons, which reduce the excitability of barosensitive neurons and limit the degree of baroinhibition of the heart (94).
We also showed that dynamic exercise, via supramedullary integrative loop (Fig. 1), simultaneously activates PVN vasopressinergic neurons projecting to the NTS, which release vasopressin into this area (see Fig. 5, top left) (26, 66). Bailey and coworkers (4) demonstrated that vasopressinergic projections make axo-axonal synapses with baroreceptors afferents projecting to NTS second-order neurons (Fig. 4), thus reducing the glutamate release probability and/or increasing transmission failures. These effects reduce NTS excitatory postsynaptic currents, the degree of baroreceptor afferent signaling, and the bradycardic response for a given pressure increase.
Fig. 5.
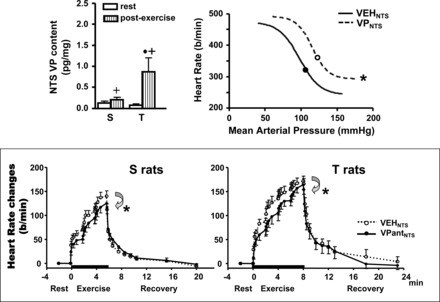
Graphs depicting the effect of exercise on the activation of descending PVN-NTS vasopressinergic projections [top left: modified from Fig. 4 in Michelini (66)] and the functional responses induced by NTS administration of vasopressin on baroreceptor reflex control of HR [top right: redrawn from data published in Michelini and Bonagamba (67)] and by NTS VP antagonist blockade on HR response during graded exercise on treadmill [bottom: reproduced with permission from Michelini and Stern (69)]. S and T, sedentary and trained groups, respectively; Veh, vehicle; VP, vasopressin; VPant, VP antagonist. The parameters for sigmoidal fitting are as follows: VehNTS: inferior plateau = 242 ± 10 beats/min; range = 232 ± 17 beats/min; blood pressure at half of the HR range = 101 ± 3 mmHg; gain = −3.55 ± 0.45 beats·min−1·mmHg−1; VPNTS: inferior plateau = 293 ± 9 beats/min*; range = 192 ± 15 beats/min*; blood pressure at half of the HR range = 116 ± 2 mmHg*; gain = −4.05 ± 0.48 beats·min−1·mmHg−1. *P < 0.05 vs. Veh treatment; +P < 0.05 vs. rest;·P < 0.05 vs. S group.
By reducing the excitability of barosensitive neurons, both EPR and PVN modulation act in concert to displace baroreceptor reflex control of HR toward higher blood pressure and HR levels (Fig. 4, left).
Indeed, the central nervous system differentiates between an exercise-induced pressure increase from an effortless pressure elevation by the simultaneous activation of both the CC (33) and the skeletal muscle receptors (15, 61) together with baroreceptors' activation.
The efficacy of vasopressin to reset HR-pressure relationship toward higher pressure levels was previously confirmed by loading/unloading baroreceptors before and after NTS pretreatment with vasopressin in resting, conscious rats. Similar to exercise in humans (Fig. 3) (92), it displaces rightward and upward the baroreceptor reflex control of HR (Fig. 5, top right) (67). Importantly, the upward and rightward displacement does not change reflex sensitivity, an effect similar to that observed during exercise in humans (92).
It was also shown that vasopressin-induced resetting of the reflex is due to reduced sympathoinhibition of the heart during blood pressure increase, since the upward and rightward displacement of the reflex response is blocked by intravenous propranolol and not changed after atropine blockade (66). Based on published evidence (4, 26, 66–68), we suggested that descending PVN-NTS vasopressinergic projections are an important mechanism to explain the instantaneous resetting of baroreceptor reflex control of HR during dynamic exercise.
Vasopressinergic modulation of HR control also participates in the genesis of exercise tachycardia. The exercise-induced increase in vasopressin content within the NTS of both sedentary and trained groups (Fig. 5, top left) facilitates exercise tachycardia at submaximal intensities during graded exercise, since the blockade of V1-receptors within this area significantly reduces the magnitude of exercise tachycardia in both groups (Fig. 5, bottom) (26, 69) without changing the blood pressure response of rats running on a treadmill (data not shown). Together, these findings demonstrate that descending PVN-NTS vasopressinergic projections are activated during exercise, helping to occlude reflex bradycardia and to facilitate exercise tachycardia during exercise-induced pressure increase, thus resulting in improved cardiac output. Indeed, it was shown that PVN vasopressinergic neurons are activated during the EPR since static muscle contractions elicited by electrical stimulation augmented Fos-like immunoreactivity in those neurons (55). It is important to note that this mechanism does not exclude the participation of complimentary mechanisms such as the EPR and other possible pathways of the CC in the modulation of HR control during exercise.
Impaired Baroreflex Buffering of Muscle Metaboreflex in Heart Failure
The central integration of afferent signals causes reflex adjustments of the autonomic outflow to the circulation characterized by inhibition sympathetic tone at rest and increased sympathetic drive during dynamic exercise that ultimately lead to the hemodynamic responses. In subjects with cardiovascular diseases, there is a disruption in this complex integrative process in the initial stages as well as during progression of the disease. For example, in subjects with heart failure, increased sympathetic activity is a hallmark (10) along with depressed parasympathetic modulation, an emerging target for treatment (98).
Resting sympathetic activity is elevated in subjects with compromised ventricular function and during strenuous exercise can rise to severe levels, causing massive vasoconstriction of inactive vascular beds, and even the normal coronary vasodilation with exercise may be reduced (16, 36). One potential mechanism that may elicit this “sympathetic storm” is overexcitation of the muscle metaboreflex. In heart failure, there is compromised oxygen delivery to the active skeletal muscles as well as altered kinetics of oxygen uptake (88), which may lead to increased metabolite accumulation and excitation of group III/IV skeletal muscle afferents. During mild, dynamic exercise, the muscle metaboreflex is likely not very active since skeletal muscle blood flow must be reduced below a clear threshold before substantial reflex increases in HR, cardiac output, and peripheral vasoconstriction occur (3) (see Fig. 6). As workload increases, this threshold level of skeletal muscle blood flow becomes closer and closer to the normal level of flow such that at moderate workloads, little, if any, threshold exists, and the reflex appears tonically active (1, 3). In heart failure, skeletal muscle blood flow is lower during exercise and the muscle metaboreflex may become engaged at lower workloads and overactivated during moderate to heavier workloads as the skeletal muscle blood flow falls well below the metaboreflex threshold (35, 36). Thus the excessive tachycardia and peripheral vasoconstriction seen in during exercise subjects with heart failure may stem, in part, from overexcitation of the muscle metaboreflex.
Fig. 6.
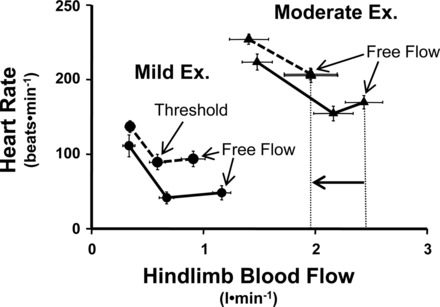
Relationship between hindlimb blood flow and HR during mild and moderate exercise (Ex) before (solid lines) and after (dashed lines) induction of heart failure. Hindlimb blood flow was reduced via transient vascular occlusion of the terminal aorta. During mild exercise, a clear threshold exists for metaboreflex activation both before and after induction of heart failure as hindlimb blood flow must be reduced below threshold from the free flow (no occlusion) levels before tachycardia occurs. However, during moderate exercise in heart failure, the free flow level of hindlimb blood flow (indicated by vertical dashed lines) is now below the threshold seen in control experiments, indicating that the reflex is now tonically active [modified from Hammond et al. (35)].
During submaximal dynamic exercise in normal subjects, the primary mechanism mediating the metaboreflex pressor response is the reflex increase in cardiac output (3). Although some systemic vasoconstriction occurs (3, 72), recent evidence shows that this may be buffered by reflex increases in epinephrine release, which evokes β2-mediated vasodilation (46). The rise in cardiac output occurs via substantial increases in HR and ventricular contractility coupled with enhanced CBV mobilization, which combined maintains or even increases stroke volume (96, 101). Normally, such substantial increases in cardiac work (large increase in cardiac output pumped against markedly elevated afterload) would be expected to elicit metabolic coronary vasodilation; however, coronary vascular conductance is little changed, and the increase in coronary blood flow is primarily due to the increase in arterial pressure forcing more flow through the same caliber vessels (77). During graded exercise in canines, the normal coronary metabolic vasodilation is restrained by concurrent α-mediated coronary vasoconstriction as revealed by larger increases in coronary blood flow seen after α-blockade (25, 42). The metaboreflex-induced rise in sympathetic activity to the heart, which caused such large increases in cardiac work, also prevented coronary vasodilation; after α-receptor blockade, substantial increases in coronary blood flow occurred because of increases in coronary vascular conductance accompanying increases in driving pressure (16). As a consequence of this increase of coronary blood flow, ventricular function also rose, substantially causing even greater increases in cardiac output (16). In subjects with heart failure, the ability of the muscle metaboreflex to raise ventricular function is markedly diminished (18, 35). The reflex increases in cardiac output are much smaller, and despite modest increases in cardiac work (small increases in output pumped against elevated afterload), actual coronary vasoconstriction occurs (2, 17). After α1-adrenergic receptor blockade, this coronary vasoconstriction is reversed to vasodilation, the increases in coronary blood flow are much greater, and there is a partial return of ventricular function (17). Thus the inability to raise cardiac output during metaboreflex activation in subjects with heart failure stems not only from the inherent ventricular dysfunction but also from accentuated coronary vasoconstriction that limits oxygen delivery to the myocardium, thereby limiting increases in cardiac function (17).
With the limited ability to raise cardiac output during metaboreflex activation, the mechanism of the pressor response shifts from a flow-mediated rise in arterial pressure to peripheral vasoconstriction (18, 35). What causes this shift is unclear but may involve impaired arterial baroreflex buffering of the metaboreflex pressor response. Normally, the arterial baroreflex buffers the rise in arterial pressure induced by metaboreflex activation. After sinoarterial baroreceptor denervation, the metaboreflex pressor response increases twofold (49, 102). In this setting, substantial peripheral vasoconstriction now accompanies the rise in cardiac output (50). Thus the arterial baroreflex buffers the muscle metaboreflex mainly by preventing peripheral vasoconstriction. This is consistent with studies in canines as well as in humans showing that unloading of the arterial baroreceptors during exercise raises arterial pressure mainly via peripheral vasoconstriction (13, 80). In heart failure, the strength of the arterial baroreflex is depressed and sinoaortic denervation neither markedly increases the magnitude nor alters mechanisms of the metaboreflex pressor response; a somewhat larger rise in arterial pressure occurs via even more peripheral vasoconstriction (48, 49).
Thus, with metaboreflex activation in heart failure, the limited ability to raise cardiac output is due to both inherent myocardial dysfunction as well as accentuated coronary vasoconstriction. The depressed arterial baroreflex function decreases the ability to buffer metaboreflex-induced peripheral vasoconstriction. To what extent this peripheral vasoconstriction extends to the active skeletal muscle is unknown; however, given the magnitude of the changes in total vascular conductance seen and that most of total vascular conductance is that to the active skeletal muscle, vasoconstriction within the active muscle appears likely (49). This may create a “perfect storm” scenario wherein under perfusion of active muscle in heart failure triggers a large increase in sympathetic activity back to the active muscle. Whether the ischemic muscle itself becomes a target for vasoconstriction is unknown.
Metaboreflex Originating from Respiratory Muscles
The EPR encompasses the reflex adjustments in autonomic function, causing the proportional increase in blood pressure during activation of mechanosensitive type III and metabosensitive type IV muscle afferents during contraction (61). The origin of the metaboreflex is located in the appendicular skeletal muscles, since the reflex has been investigated during dynamic exercise involving limb movements (for example, running in dogs and leg cycling in humans). However, the reflex is triggered by the buildup of metabolites within the muscle that is sensed by type III and IV fibers, which are present in virtually all skeletal muscle groups. Thus, in theory, not only limb muscle groups can trigger the metaboreflex but also trunk and respiratory muscles. Indeed, Dempsey and his coworkers (94) proposed that a metaboreflex arising from respiratory muscles plays a major role in contributing to the reflex increase in sympathetic drive. The experimental basis for the afferent arm of a respiratory metaboreflex considered the observation that diaphragm fatigue in anesthetized rats increases the firing rate of type IV afferents (metabosensitive), whereas the activity of type III mechanosensitive fibers was unchanged (41). In a series of publications involving healthy human volunteers, it has been shown that increasing the work of breathing resulting in respiratory muscle fatigue, leg muscle sympathetic nerve activity increases independent of central respiratory motor drive and following a pattern that suggests progressive accumulation of metabolites (104) along with a reduction in limb vascular conductance and blood flow (99).
Respiratory muscle fatigue is a critical component of the diminished exercise capacity in subjects with heart failure (65). Respiratory fatigue may hamper exercise capacity not only because of the consequent impaired hematosis leading to arterial blood oxygen desaturation and even hypercapnia at latter stages of the disease but also by triggering the respiratory metaboreflex. During respiratory muscle fatigue, blood flow is reduced to resting and exercising limbs in subjects with heart failure, an effect that is diminished after inspiratory muscle training (12). The trigger for the metaboreflex is the accumulation of metabolites within skeletal muscle developed by an oxygen demand/delivery mismatch. With the use of near-infrared spectroscopy, deoxygenation of the serratus anterior muscle during cycling maximal exercise in heart failure without concomitant arterial blood oxygen desaturation has been shown (58). During a protocol with progressive inspiratory pressure, respiratory muscle fatigue was induced in subjects with heart failure at rest, revealing deoxygenation not only in intercostal muscles but also in resting forearm, an effect not seen in healthy controls, suggesting underperfusion of respiratory muscles in those patients (73).
Increased sympathetic drive is a well-known key factor in the pathophysiology of heart failure. Modulation of sympathetic activity is an essential physiological mechanism in the normal cardiovascular system. However, in subjects with heart failure, the progressive increase in adrenergic drive produces a vicious cycle leading to deterioration of clinical status and eventually death. During submaximal dynamic exercise in these subjects, the muscle metaboreflex causes a reflex increase in sympathetic activity that, on its turn, causes accentuated coronary vasoconstriction, an important mechanism limiting the increased in ventricular function by reducing myocardial perfusion (17). Whether the respiratory metaboreflex is responsible for at least part of this increase in sympathetic drive causing coronary vasoconstriction is currently unknown.
Conclusions and Future Directions
There has been a long quest for understanding the organization and functioning of the neural mechanisms controlling the cardiovascular system during exercise. The integration of cardiovascular physiologists and neurophysiologists made it possible to build a description of a complementary and redundant neural circuitry that drives and modulates the autonomic and hemodynamic changes during physical exercise.
Understanding the major components of a single specific reflex circuitry is a challenging task, but even more challenging is to explore and understand the complex interaction between all those input and reflexes causing the normal physiological hemodynamic response to exercise and the possible changes occurring during the natural history of very prevalent cardiometabolic diseases, such as diabetes, hypertension, and heart failure. The more the body of knowledge advances, more questions arise. This review reports the subjects addressed in a symposium presented at the recent “1st Pan American Congress of Physiological Sciences: Physiology Without Borders” meeting, which addressed key issues in the field, presenting complementary studies involving both animal models and studies in human subjects.
A detailed and robust description of the neural control of the circulation during exercise is now available, but not without controversies and open gaps with answered questions. For example, there is not a full description of the consecutive neural stimulation/inhibition cross connecting the activation of the somatomotor neural network causing skeletal muscle contraction and the modulation of the autonomic nervous system. This still incomplete morphofunctional network of the parallel somatomotor-autonomic activation is the basis for the well-established CC as a concept. However, both fast-developing neuroimaging techniques and the use of direct electrical recording/stimulation of specific areas within the brain in awake humans have been providing in recent years and will continue to provide new elements for the mapping of the neural circuitry underlying the CC.
Despite many relevant contributions that have been discussed in this review, future research efforts should involve at the least the following lines of investigation: 1) identification of mediators of the mechanical and metabolic input to the skeletal muscle afferents during contractions, 2) description of other central areas and peripheral components (chemoreflex, for example) playing a physiological relevant role in the overall integration of reflex responses, 3) mapping of the neurochemical mechanisms for baroreflex resetting after exercise training, 4) description of the pathophysiological mechanism causing the increased sympathetic drive and depressed vagal tone in hypertension and heart failure, 5) identification of the clinical impact of these pathophysiological changes concerning vascular dysfunction and end-organ damage. Rapid progress and widespread availability of many different techniques should continue to provide opportunities to advance toward a full description of the neural mechanisms controlling the circulation during exercise.
GRANTS
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo Grant 2011/51410-9 (to L. C. Michelini); Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro Grant E-26/102-752/12 (to A. C. L. Nóbrega); Conselho Nacional de Desenvolvimento Científico e Tecnológico Grants 304060/2011-9 (to L. C. Michelini) and 306953/2013-7 (to A. C. L. Nóbrega); National Heart, Lung, and Blood Institute Grants HL-04457 (to P. B. Raven) and HL-55743 (to D. S. O'Leary); and University of North Texas Health Science Center Institutional Bridge Grants RI6128 and RI6100 (to P. B. Raven).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
L.C.M., D.S.O., P.B.R., and A.C.L.N. conception and design of research; L.C.M., D.S.O., P.B.R., and A.C.L.N. prepared figures; L.C.M., D.S.O., P.B.R., and A.C.L.N. drafted manuscript; L.C.M., D.S.O., P.B.R., and A.C.L.N. edited and revised manuscript; L.C.M., D.S.O., P.B.R., and A.C.L.N. approved final version of manuscript; A.C.L.N. interpreted results of experiments.
ACKNOWLEDGMENTS
We thank Alessandra C. Toledo-Arruda for expert editorial assistance.
REFERENCES
- 1.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol (1985) 109: 966–976, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansorge EJ, Augustyniak RA, Perinot ML, Hammond RL, Kim JK, Sala-Mercado JA, Rodriguez J, Rossi NF, O'Leary DS. Altered muscle metaboreflex control of coronary blood flow and ventricular function in heart failure. Am J Physiol Heart Circ Physiol 288: H1381–H1388, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Augustyniak RA, Collins HL, Ansorge EJ, Rossi NF, O'Leary DS. Severe exercise alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 280: H1645–H1652, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Bailey TW, Jin YH, Doyle MW, Smith SM, Andresen MC. Vasopressin inhibits glutamate release via two distinct modes in the brainstem. J Neurosci 26: 6131–6142, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbosa TC, Fernandes IA, Magalhães-Jr N, Cavalcanti IL, Secher NH, Nóbrega AC, Vianna LC. Oscillatory blood pressure response to the onset of cycling exercise in men: role of group III/IV muscle afferents. Exp Physiol 100: 302–311, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Basnayake SD, Green AL, Paterson DJ. Mapping the central neurocircuitry that integrates the cardiovascular response to exercise in humans. Exp Physiol 97: 29–38, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Bevegard BS, Shepherd JT. Circulatory effects of stimulating the carotid arterial stretch receptors in man at rest and during exercise. J Clin Invest 45: 132–142, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bristow JD, Cunningham DJ, Howson MG, Petersen ES, Pickering TG, Sleight P. Effect of bicycling on the baroreflex regulation of pulse interval. Circ Res 28: 582–592, 1971. [Google Scholar]
- 9.Brum PC, Da Silva GJ, Moreira ED, Ida F, Negrao CE, Krieger EM. Exercise training increases baroreceptor gain sensitivity in normal and hypertensive rats. Hypertension 36: 1018–1022, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Brunner-La Rocca HP, Esler MD, Jennings GL, Kaye DM. Effect of cardiac sympathetic nervous activity on mode of death in congestive heart failure. Eur Heart J 22: 1136–1143, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Cavalleri MT, Burgi K, Cruz JC, Jordao MT, Ceroni A, Michelini LC. Afferent signaling drives oxytocinergic preautonomic neurons and mediates training-induced plasticity. Am J Physiol Regul Integr Comp Physiol 301: R958–R966, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Chiappa GR, Roseguini BT, Vieira PJ, Alves CN, Tavares A, Winkelmann ER, Ferlin EL, Stein R, Ribeiro JP. Inspiratory muscle training improves blood flow to resting and exercising limbs in patients with chronic heart failure. J Am Coll Cardiol 51: 1663–1671, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Collins HL, Augustyniak RA, Ansorge EJ, O'Leary DS. Carotid baroreflex pressor responses at rest and during exercise: cardiac output vs. regional vasoconstriction. Am J Physiol Heart Circ Physiol 280: H642–H648, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Collins HL, DiCarlo SE. Cardiac afferents attenuate the muscle metaboreflex in the rat. J Appl Physiol (1985) 75: 114–120, 1993. [DOI] [PubMed] [Google Scholar]
- 15.Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coutsos M, Sala-Mercado JA, Ichinose M, Li Z, Dawe EJ, O'Leary DS. Muscle metaboreflex-induced coronary vasoconstriction functionally limits increases in ventricular contractility. J Appl Physiol (1985) 109: 271–278, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coutsos M, Sala-Mercado JA, Ichinose M, Li Z, Dawe EJ, O'Leary DS. Muscle metaboreflex-induced coronary vasoconstriction limits ventricular contractility during dynamic exercise in heart failure. Am J Physiol Heart Circ Physiol 304: H1029–H1037, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crisafulli A, Salis E, Tocco F, Melis F, Milia R, Pittau G, Caria MA, Solinas R, Meloni L, Pagliaro P, Concu A. Impaired central hemodynamic response and exaggerated vasoconstriction during muscle metaboreflex activation in heart failure patients. Am J Physiol Heart Circ Physiol 292: H2988–H2996, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Cunningham DJ, Petersen ES, Peto R, Pickering TG, Sleight P. Comparison of the effect of different types of exercise on the baroreflex regulation of heart rate. Acta Physiol Scand 86: 444–455, 1972. [PubMed] [Google Scholar]
- 20.Daskalopoulos DA, Shepherd JT, Walgenbach SC. Cardiopulmonary reflexes and blood pressure in exercising sinoaortic-denervated dogs. J Appl Physiol Respir Environ Exercise Physiol 57: 1417–1421, 1984. [DOI] [PubMed] [Google Scholar]
- 21.De Souza CG, Michelini LC, Fior-Chadi DR. Receptor changes in the nucleus tractus solitarii of the rat after exercise training. Med Sci Sports Exerc 33: 1471–1476, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Degtyarenko AM, Kaufman MP. MLR-induced inhibition of barosensory cells in the NTS. Am J Physiol Heart Circ Physiol 289: H2575–H2584, 2005. [DOI] [PubMed] [Google Scholar]
- 23.DiCarlo SE, Bishop VS. Onset of exercise shifts operating point of arterial baroreflex to higher pressures. Am J Physiol Heart Circ Physiol 262: H303–H307, 1992. [DOI] [PubMed] [Google Scholar]
- 24.DiCarlo SE, Bishop VS. Regional vascular resistance during exercise: role of cardiac afferents and exercise training. Am J Physiol Heart Circ Physiol 258: H842–H847, 1990. [DOI] [PubMed] [Google Scholar]
- 25.Dodd-o JM, Gwirtz PA. Coronary alpha 1-adrenergic constrictor tone varies with intensity of exercise. Med Sci Sports Exerc 28: 62–71, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Dufloth DL, Morris M, Michelini LC. Modulation of exercise tachycardia by vasopressin in the nucleus tractus solitarii. Am J Physiol Regul Integr Comp Physiol 273: R1271–R1282, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Eckberg DL, Cavanaugh MS, Mark AL, Abboud FM. A simplified neck suction device for activation of carotid baroreceptors. J Lab Clin Med 85: 167–173, 1975. [PubMed] [Google Scholar]
- 28.Ernsting J, Parry DJ. Some observations on the effects of stimulating the stretch receptors in the carotid artery of man. J Physiol 137: 45P–46P, 1957. [Google Scholar]
- 29.Fadel PJ, Raven PB. Human investigations into the arterial and cardiopulmonary baroreflexes during exercise. Exp Physiol 97: 39–50, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallagher KM, Fadel PJ, Smith SA, Norton KH, Querry RG, Olivencia-Yurvati A, Raven PB. Increases in intramuscular pressure raise arterial blood pressure during dynamic exercise. J Appl Physiol (1985) 91: 2351–2358, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Gallagher KM, Fadel PJ, Stromstad M, Ide K, Smith SA, Querry RG, Raven PB, Secher NH. Effects of exercise pressor reflex activation on carotid baroreflex function during exercise in humans. J Physiol 533: 871–880, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallagher KM, Fadel PJ, Stromstad M, Ide K, Smith SA, Querry RG, Raven PB, Secher NH. Effects of partial neuromuscular blockade on carotid baroreflex function during exercise in humans. J Physiol 533: 861–870, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol 226: 173–190, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hainsworth R. Reflexes from the heart. Physiol Rev 71: 617–658, 1991. [DOI] [PubMed] [Google Scholar]
- 35.Hammond RL, Augustyniak RA, Rossi NF, Churchill PC, Lapanowski K, O'Leary DS. Heart failure alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 278: H818–H828, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Hammond RL, Augustyniak RA, Rossi NF, Lapanowski K, Dunbar JC, O'Leary DS. Alteration of humoral and peripheral vascular responses during graded exercise in heart failure. J Appl Physiol (1985) 90: 55–61, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Hand GA, Kramer GL, Petty F, Ordway GA, Wilson LB. Excitatory amino acid concentrations in the spinal dorsal horn of cats during muscle contraction. J Appl Physiol (1985) 81: 368–373, 1996. [DOI] [PubMed] [Google Scholar]
- 38.Hering H. Die Karotissinusreflexe auf Herz und Gefässe vom normalphysiologischen, pathologisch-physiologischen und klinischen Standpunkt. Dresden: Steinkopff, 1927. [Google Scholar]
- 39.Heymans C, Neil E. Reflexogenic areas of the cardiovascular system. London: Churchill, 1958. [DOI] [PubMed] [Google Scholar]
- 40.Higa-Taniguchi KT, Silva FC, Silva HM, Michelini LC, Stern JE. Exercise training-induced remodeling of paraventricular nucleus (nor)adrenergic innervation in normotensive and hypertensive rats. Am J Physiol Regul Integr Comp Physiol 292: R1717–R1727, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Hill JM. Discharge of group IV phrenic afferent fibers increases during diaphragmatic fatigue. Brain Res 856: 240–244, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Huang AH, Feigl EO. Adrenergic coronary vasoconstriction helps maintain uniform transmural blood flow distribution during exercise. Circ Res 62: 286–298, 1988. [DOI] [PubMed] [Google Scholar]
- 43.Jackson K, Silva HM, Zhang W, Michelini LC, Stern JE. Exercise training differentially affects intrinsic excitability of autonomic and neuroendocrine neurons in the hypothalamic paraventricular nucleus. J Neurophysiol 94: 3211–3220, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Joyner MJ. Baroreceptor function during exercise: resetting the record. Exp Physiol 91: 27–36, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Kaufman MP. The exercise pressor reflex in animals. Exp Physiol 97: 51–58, 2012. [DOI] [PubMed] [Google Scholar]
- 46.Kaur J, Spranger MD, Hammond RL, Krishnan AC, Alvarez A, Augustyniak RA, O'Leary DS. Muscle metaboreflex activation during dynamic exercise evokes epinephrine release resulting in β2-mediated vasodilation. Am J Physiol Heart Circ Physiol 308: H524–H529, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kerman IA. Organization of brain somatomotor-sympathetic circuits. Exp Brain Res 187: 1–16, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Kim JK, Augustyniak RA, Sala-Mercado JA, Hammond RL, Ansorge EJ, Rossi NF, O'Leary DS. Heart failure alters the strength and mechanisms of arterial baroreflex pressor responses during dynamic exercise. Am J Physiol Heart Circ Physiol 287: H1682–H1688, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Kim JK, Sala-Mercado JA, Hammond RL, Rodriguez J, Scislo TJ, O'Leary DS. Attenuated arterial baroreflex buffering of muscle metaboreflex in heart failure. Am J Physiol Heart Circ Physiol 289: H2416–H2423, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Kim JK, Sala-Mercado JA, Rodriguez J, Scislo TJ, O'Leary DS. Arterial baroreflex alters strength and mechanisms of muscle metaboreflex during dynamic exercise. Am J Physiol Heart Circ Physiol 288: H1374–H1380, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Koch E, Miles H. Chronischer Arterieller Hochtruck Durch Experimentelle Dauerasschaltung der Blutdruckzugler. Krankheitsforschung 7: 241–256, 1929. [Google Scholar]
- 52.Koga S, Rossiter HB, Heinonen I, Musch TI, Poole DC. Dynamic heterogeneity of exercising muscle blood flow and O2 utilization. Med Sci Sports Exerc 46: 860–876, 2014. [DOI] [PubMed] [Google Scholar]
- 53.Krieger EM, Brum PC, Negrao CE. Role of arterial baroreceptor function on cardiovascular adjustments to acute and chronic dynamic exercise. Biol Res 31: 273–279, 1998. [PubMed] [Google Scholar]
- 54.Krogh A, Lindhard J. The regulation of respiration and circulation during the initial stages of muscular work. J Physiol 47: 112–136, 1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li J, Hand GA, Potts JT, Mitchell JH. Identification of hypothalamic vasopressin and oxytocin neurons activated during the exercise pressor reflex in cats. Brain Res 752: 45–51, 1997. [DOI] [PubMed] [Google Scholar]
- 56.Li J, Mitchell JH. Role of NO in modulating neuronal activity in superficial dorsal horn of spinal cord during exercise pressor reflex. Am J Physiol Heart Circ Physiol 283: H1012–H1018, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Ludbrook J, Graham WF. Circulatory responses to onset of exercise: role of arterial and cardiac baroreflexes. Am J Physiol Heart Circ Physiol 248: H457–H467, 1985. [DOI] [PubMed] [Google Scholar]
- 58.Mancini DM, Ferraro N, Nazzaro D, Chance B, Wilson JR. Respiratory muscle deoxygenation during exercise in patients with heart failure demonstrated with near-infrared spectroscopy. J Am Coll Cardiol 18: 492–498, 1991. [DOI] [PubMed] [Google Scholar]
- 59.Marey EJ. Physiologie medicale de la circulation du sang Paris: Delahaye, 1863. [Google Scholar]
- 60.Matsukawa K. Central command: control of cardiac sympathetic and vagal efferent nerve activity and the arterial baroreflex during spontaneous motor behaviour in animals. Exp Physiol 97: 20–28, 2012. [DOI] [PubMed] [Google Scholar]
- 61.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McIlveen SA, Hayes SG, Kaufman MP. Both central command and exercise pressor reflex reset carotid sinus baroreflex. Am J Physiol Heart Circ Physiol 280: H1454–H1463, 2001. [DOI] [PubMed] [Google Scholar]
- 63.Meintjes AF, Nobrega AC, Fuchs IE, Ally A, Wilson LB. Attenuation of the exercise pressor reflex. Effect of opioid agonist on substance P release in L-7 dorsal horn of cats. Circ Res 77: 326–334, 1995. [DOI] [PubMed] [Google Scholar]
- 64.Melcher A, Donald DE. Maintained ability of carotid baroreflex to regulate arterial pressure during exercise. Am J Physiol Heart Circ Physiol 241: H838–H849, 1981. [DOI] [PubMed] [Google Scholar]
- 65.Meyer FJ, Borst MM, Zugck C, Kirschke A, Schellberg D, Kubler W, Haass M. Respiratory muscle dysfunction in congestive heart failure: clinical correlation and prognostic significance. Circulation 103: 2153–2158, 2001. [DOI] [PubMed] [Google Scholar]
- 66.Michelini LC. Differential effects of vasopressinergic and oxytocinergic pre-autonomic neurons on circulatory control: reflex mechanisms and changes during exercise. Clin Exp Pharmacol Physiol 34: 369–376, 2007. [DOI] [PubMed] [Google Scholar]
- 67.Michelini LC, Bonagamba LG. Baroreceptor reflex modulation by vasopressin microinjected into the nucleus tractus solitarii of conscious rats. Hypertension 11: I75–I79, 1988. [DOI] [PubMed] [Google Scholar]
- 68.Michelini LC, Morris M. Endogenous vasopressin modulates the cardiovascular responses to exercise. Ann NY Acad Sci 897: 198–211, 1999. [DOI] [PubMed] [Google Scholar]
- 69.Michelini LC, Stern JE. Exercise-induced neuronal plasticity in central autonomic networks: role in cardiovascular control. Exp Physiol 94: 947–960, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mitchell JH. Cardiovascular control during exercise: central and reflex neural mechanisms. Am J Cardiol 55: 34D–41D, 1985. [DOI] [PubMed] [Google Scholar]
- 71.Mitchell JH. J.B. Wolffe memorial lecture. Neural control of the circulation during exercise. Med Sci Sports Exerc 22: 141–154, 1990. [PubMed] [Google Scholar]
- 72.Mittelstadt SW, Bell LB, O'Hagan KP, Clifford PS. Muscle chemoreflex alters vascular conductance in nonischemic exercising skeletal muscle. J Appl Physiol (1985) 77: 2761–2766, 1994. [DOI] [PubMed] [Google Scholar]
- 73.Moreno AM, Castro RR, Silva BM, Villacorta H, Junior MS, Nobrega AC. Intercostal and forearm muscle deoxygenation during respiratory fatigue in patients with heart failure: potential role of a respiratory muscle metaboreflex. Braz J Med Biol Res. 2014 Aug 29; 0:0 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nobrega AC, Meintjes AF, Ally A, Wilson LB. Modulation of reflex pressor response to contraction and effect on substance P release by spinal 5-HT1A receptors. Am J Physiol Heart Circ Physiol 268: H1577–H1585, 1995. [DOI] [PubMed] [Google Scholar]
- 75.Norton KH, Boushel R, Strange S, Saltin B, Raven PB. Resetting of the carotid arterial baroreflex during dynamic exercise in humans. J Appl Physiol (1985) 87: 332–338, 1999. [DOI] [PubMed] [Google Scholar]
- 76.O'Hagan KP, Bell LB, Mittelstadt SW, Clifford PS. Cardiac receptors modulate the renal sympathetic response to dynamic exercise in rabbits. J Appl Physiol (1985) 76: 507–515, 1994. [DOI] [PubMed] [Google Scholar]
- 77.O'Leary DS, Sala-Mercado JA, Hammond RL, Ansorge EJ, Kim JK, Rodriguez J, Fano D, Ichinose M. Muscle metaboreflex-induced increases in cardiac sympathetic activity vasoconstrict the coronary vasculature. J Appl Physiol (1985) 103: 190–194, 2007. [DOI] [PubMed] [Google Scholar]
- 78.Ogoh S, Brothers RM, Barnes Q, Eubank WL, Hawkins MN, Purkayastha S, O-Yurvati A, Raven PB. Cardiopulmonary baroreflex is reset during dynamic exercise. J Appl Physiol (1985) 100: 51–59, 2006. [DOI] [PubMed] [Google Scholar]
- 79.Ogoh S, Brothers RM, Barnes Q, Eubank WL, Hawkins MN, Purkayastha S, O-Yurvati A, Raven PB. Effects of changes in central blood volume on carotid-vasomotor baroreflex sensitivity at rest and during exercise. J Appl Physiol (1985) 101: 68–75, 2006. [DOI] [PubMed] [Google Scholar]
- 80.Ogoh S, Fadel PJ, Nissen P, Jans O, Selmer C, Secher NH, Raven PB. Baroreflex-mediated changes in cardiac output and vascular conductance in response to alterations in carotid sinus pressure during exercise in humans. J Physiol 550: 317–324, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ogoh S, Fisher JP, Fadel PJ, Raven PB. Increases in central blood volume modulate carotid baroreflex resetting during dynamic exercise in humans. J Physiol 581: 405–418, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ogoh S, Wasmund WL, Keller DM, O-Yurvati A, Gallagher KM, Mitchell JH, Raven PB. Role of central command in carotid baroreflex resetting in humans during static exercise. J Physiol 543: 349–364, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paintal AS. Vagal sensory receptors and their reflex effects. Physiol Rev 53: 159–227, 1973. [DOI] [PubMed] [Google Scholar]
- 84.Papelier Y, Escourrou P, Gauthier JP, Rowell LB. Carotid baroreflex control of blood pressure and heart rate in men during dynamic exercise. J Appl Physiol (1985) 77: 502–506, 1994. [DOI] [PubMed] [Google Scholar]
- 85.Pawelczyk JA, Raven PB. Reductions in central venous pressure improve carotid baroreflex responses in conscious men. Am J Physiol Heart Circ Physiol 257: H1389–H1395, 1989. [DOI] [PubMed] [Google Scholar]
- 86.Pickering TG, Gribbin B, Petersen ES, Cunningham DJ, Sleight P. Effects of autonomic blockade on the baroreflex in man at rest and during exercise. Circ Res 30: 177–185, 1972. [DOI] [PubMed] [Google Scholar]
- 87.Pickering TG, Gribbin B, Sleight P. Comparison of the reflex heart rate response to rising and falling arterial pressure in man. Cardiovasc Res 6: 277–283, 1972. [DOI] [PubMed] [Google Scholar]
- 88.Poole DC, Hirai DM, Copp SW, Musch TI. Muscle oxygen transport and utilization in heart failure: implications for exercise (in)tolerance. Am J Physiol Heart Circ Physiol 302: H1050–H1063, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Potts JT. Inhibitory neurotransmission in the nucleus tractus solitarii: implications for baroreflex resetting during exercise. Exp Physiol 91: 59–72, 2006. [DOI] [PubMed] [Google Scholar]
- 90.Potts JT, Shi XR, Raven PB. Carotid baroreflex responsiveness during dynamic exercise in humans. Am J Physiol Heart Circ Physiol 265: H1928–H1938, 1993. [DOI] [PubMed] [Google Scholar]
- 91.Querry RG, Smith SA, Stromstad M, Ide K, Raven PB, Secher NH. Neural blockade during exercise augments central command's contribution to carotid baroreflex resetting. Am J Physiol Heart Circ Physiol 280: H1635–H1644, 2001. [DOI] [PubMed] [Google Scholar]
- 92.Raven PB, Fadel PJ, Ogoh S. Arterial baroreflex resetting during exercise: a current perspective. Exp Physiol 91: 37–49, 2006. [DOI] [PubMed] [Google Scholar]
- 93.Ray CA, Rea RF, Clary MP, Mark AL. Muscle sympathetic nerve responses to dynamic one-legged exercise: effect of body posture. Am J Physiol Heart Circ Physiol 264: H1–H7, 1993. [DOI] [PubMed] [Google Scholar]
- 94.Rodman JR, Henderson KS, Smith CA, Dempsey JA. Cardiovascular effects of the respiratory muscle metaboreflexes in dogs: rest and exercise. J Appl Physiol (1985) 95: 1159–1169, 2003. [DOI] [PubMed] [Google Scholar]
- 95.Rowell LB, O'Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol (1985) 69: 407–418, 1990. [DOI] [PubMed] [Google Scholar]
- 96.Sala-Mercado JA, Hammond RL, Kim JK, McDonald PJ, Stephenson LW, O'Leary DS. Heart failure attenuates muscle metaboreflex control of ventricular contractility during dynamic exercise. Am J Physiol Heart Circ Physiol 292: H2159–H2166, 2007. [DOI] [PubMed] [Google Scholar]
- 97.Secher NH, Amann M. Human investigations into the exercise pressor reflex. Exp Physiol 97: 59–69, 2012. [DOI] [PubMed] [Google Scholar]
- 98.Serra SM, Costa RV, Teixeira De Castro RR, Xavier SS, Nobrega AC. Cholinergic stimulation improves autonomic and hemodynamic profile during dynamic exercise in patients with heart failure. J Card Fail 15: 124–129, 2009. [DOI] [PubMed] [Google Scholar]
- 99.Sheel AW, Derchak PA, Morgan BJ, Pegelow DF, Jacques AJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex reduction in resting leg blood flow in humans. J Physiol 537: 277–289, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shepherd JT, Blomqvist CG, Lind AR, Mitchell JH, Saltin B. Static (isometric) exercise. Retrospection and introspection. Circ Res 48: I179–I188, 1981. [PubMed] [Google Scholar]
- 101.Sheriff DD, Augustyniak RA, O'Leary DS. Muscle chemoreflex-induced increases in right atrial pressure. Am J Physiol Heart Circ Physiol 275: H767–H775, 1998. [DOI] [PubMed] [Google Scholar]
- 102.Sheriff DD, O'Leary DS, Scher AM, Rowell LB. Baroreflex attenuates pressor response to graded muscle ischemia in exercising dogs. Am J Physiol Heart Circ Physiol 258: H305–H310, 1990. [DOI] [PubMed] [Google Scholar]
- 103.Smith SA, Querry RG, Fadel PJ, Gallagher KM, Stromstad M, Ide K, Raven PB, Secher NH. Partial blockade of skeletal muscle somatosensory afferents attenuates baroreflex resetting during exercise in humans. J Physiol 551: 1013–1021, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.St Croix CM, Morgan BJ, Wetter TJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex sympathetic activation in humans. J Physiol 529: 493–504, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stebbins CL, Ortiz-Acevedo A, Hill JM. Spinal vasopressin modulates the reflex cardiovascular response to static contraction. J Appl Physiol (1985) 72: 731–738, 1992. [DOI] [PubMed] [Google Scholar]
- 106.Strange S, Secher NH, Pawelczyk JA, Karpakka J, Christensen NJ, Mitchell JH, Saltin B. Neural control of cardiovascular responses and of ventilation during dynamic exercise in man. J Physiol 470: 693–704, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Volianitis S, Yoshiga CC, Vogelsang T, Secher NH. Arterial blood pressure and carotid baroreflex function during arm and combined arm and leg exercise in humans. Acta Physiol Scand 181: 289–295, 2004. [DOI] [PubMed] [Google Scholar]
- 108.Waldrop TG, Eldridge F, Iwamoto GA, Mitchell JH. Central neural control of respiration and circulation during exercise. New York: Oxford University Press, 1996. [Google Scholar]
- 109.Walgenbach SC, Donald DE. Cardiopulmonary reflexes and arterial pressure during rest and exercise in dogs. Am J Physiol Heart Circ Physiol 244: H362–H369, 1983. [DOI] [PubMed] [Google Scholar]
- 110.Walgenbach SC, Donald DE. Inhibition by carotid baroreflex of exercise-induced increases in arterial pressure. Circ Res 52: 253–262, 1983. [DOI] [PubMed] [Google Scholar]
- 111.Williamson JW, Crandall CG, Potts JT, Raven PB. Blood pressure responses to dynamic exercise with lower-body positive pressure. Med Sci Sports Exerc 26: 701–708, 1994. [DOI] [PubMed] [Google Scholar]
- 112.Williamson JW, Fadel PJ, Mitchell JH. New insights into central cardiovascular control during exercise in humans: a central command update. Exp Physiol 91: 51–58, 2006. [DOI] [PubMed] [Google Scholar]
- 113.Williamson JW, McColl R, Mathews D. Evidence for central command activation of the human insular cortex during exercise. J Appl Physiol (1985) 94: 1726–1734, 2003. [DOI] [PubMed] [Google Scholar]
- 114.Williamson JW, McColl R, Mathews D, Mitchell JH, Raven PB, Morgan WP. Hypnotic manipulation of effort sense during dynamic exercise: cardiovascular responses and brain activation. J Appl Physiol (1985) 90: 1392–1399, 2001. [DOI] [PubMed] [Google Scholar]
- 115.Williamson JW, Mitchell JH, Olesen HL, Raven PB, Secher NH. Reflex increase in blood pressure induced by leg compression in man. J Physiol 475: 351–357, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wilson LB, Hand GA. Segmental effect of spinal NK-1 receptor blockade on the pressor reflex. Am J Physiol Heart Circ Physiol 275: H789–H796, 1998. [DOI] [PubMed] [Google Scholar]


