Abstract
Intrauterine growth restriction (IUGR) increases the risk of adult type 2 diabetes (T2D) and obesity. Neonatal exendin-4 treatment can prevent diabetes in the IUGR rat, but whether this will be effective in a species where the pancreas is more mature at birth is unknown. Therefore, we evaluated the effects of neonatal exendin-4 administration after experimental restriction of placental and fetal growth on growth and adult metabolic outcomes in sheep. Body composition, glucose tolerance, and insulin secretion and sensitivity were assessed in singleton-born adult sheep from control (CON; n = 6 females and 4 males) and placentally restricted pregnancies (PR; n = 13 females and 7 males) and in sheep from PR pregnancies that were treated with exendin-4 as neonates (daily sc injections of 1 nmol/kg exendin-4; PR + exendin-4; n = 11 females and 7 males). Placental restriction reduced birth weight (by 29%) and impaired glucose tolerance in the adult but did not affect adult adiposity, insulin secretion, or insulin sensitivity. Neonatal exendin-4 suppressed growth during treatment, followed by delayed catchup growth and unchanged adult adiposity. Neonatal exendin-4 partially restored glucose tolerance in PR progeny but did not affect insulin secretion or sensitivity. Although the effects on glucose tolerance are promising, the lack of effects on adult body composition, insulin secretion, and insulin sensitivity suggest that the neonatal period may be too late to fully reprogram the metabolic consequences of IUGR in species that are more mature at birth than rodents.
Keywords: intrauterine growth restriction, exendin-4, glucose tolerance, insulin action, body composition
intrauterine growth restriction (IUGR) in humans impairs adult insulin action in part via impaired insulin sensitivity and secretion and hence, increases the risk of type 2 diabetes (T2D) (23, 37). Poor growth before birth increases the risk of T2D, accounting for ∼18% of the population lifetime risk of this disease (13). Even after correcting for gestational age, low-birth weight individuals remain at elevated risk of impaired glucose tolerance, inadequate insulin secretion, and risk of T2D as adults (21, 24, 31, 34, 37, 38, 56). Prevention of T2D is a clinical challenge. Lifestyle interventions improve short-term glucose control (28, 55) but do not reduce long-term risk of cardiovascular morbidity or mortality (33). These poor outcomes of interventions initiated after disease onset lead us to suggest that the better approach to improve long-term outcomes in those at elevated risk of T2D would be via prevention of disease onset, including interventions that reverse the mechanisms that are initiated during IUGR and underlie the increased risk of T2D.
One intervention that may ameliorate the risk of T2D after IUGR is short-term neonatal exendin-4 administration. Exendin-4 is a long-acting analog of the hormone glucagon-like peptide 1 (GLP-1), which stimulates β-cell proliferation and function in rodents and human islets in vitro, with evidence of increased β-cell mass following short- and medium-term treatment (days to weeks) in rodents (reviewed in Ref. 29). Chronic treatment with exendin-4 and other GLP-1 receptor agonists improves insulin secretion in diabetic rats (61) and in human T2D patients, where current evidence from clinical trials suggests that GLP-1 receptor agonists increase β-cell function and may preserve existing β-cells but probably do not increase β-cell mass (reviewed in Ref. 29). Daily administration of exendin-4 for the first 6 days of neonatal life (1 nmol/kg body wt) successfully prevents development of diabetes in the IUGR rat without altering glucose metabolism in control progeny (52). Furthermore, neonatal exendin-4 treatment of IUGR rats reduced adult fasting plasma insulin concentrations, prevented oxidative stress in the liver, reversed defects in insulin signaling, and normalized hepatic insulin sensitivity (43). A well-defined mechanism by which neonatal exendin-4 normalizes insulin secretion in the IUGR rat is via increased expression of pancreatic and duodenal homeobox 1 (Pdx1), which is a regulator of β-cell function and mass. Neonatal exendin-4 administration reverses IUGR-induced deacetylation of histone tails in the promoter of Pdx1, preventing subsequent progression to DNA methylation of the Pdx1 promoter and associated Pdx1 gene silencing with age and normalizing β-cell mass in IUGR progeny (40). In rodents, β-cells develop late in gestation, with remodeling and functional maturation occurring ∼10–17 days postnatal age, and the rat pancreas is still functionally immature at birth (7, 48). Therefore, both experimental restriction of the prenatal nutrient supply and neonatal exendin-4 treatment occur prior to pancreatic maturation in this species. Whether neonatal exendin-4 will also improve adult metabolic outcomes after IUGR in a species where pancreatic maturation is largely complete before birth is unknown.
Development of the pancreas in humans and sheep occurs mostly before birth, with β-cells appearing within the first quarter of gestation, islet development by midgestation, and substantial remodeling to a functional endocrine pancreas near term (26, 41, 46, 59). In fetal sheep, in vivo glucose-stimulated insulin secretion, particularly early or first-phase insulin secretion, matures from midgestation and increases during the last quarter of gestation (4, 15). IUGR induced by surgical removal of placental attachment sites before pregnancy [placental restriction (PR)] in sheep restricts placental growth and function and hence, fetal growth throughout gestation and has similar consequences for postnatal growth and metabolism as that which occurs in human IUGR (2, 10, 11, 17, 39, 47). The effects of IUGR and PR in sheep include accelerated neonatal growth leading to accumulation of excess visceral fat by the end of the neonatal catchup growth period, impaired insulin action, including insulin resistance in animals measured throughout the first month of life, particularly in muscle, and inadequate insulin secretion from early postnatal life through to adulthood, with impaired glucose tolerance in adult males (9–11, 18, 39), consistent with the consequences of IUGR in humans (21, 23, 24, 31, 34, 37, 38, 56). The similarity in the timing of pancreatic development and the similarities in postnatal consequences of IUGR make sheep a useful species in which to investigate long-term effects of IUGR and perinatal interventions. In our initial studies, where in utero growth was restricted by twinning, progeny undergoing neonatal exendin-4 treatment (1 nmol/kg body wt daily from days 1 to 16 after birth) had impaired glucose tolerance and insulin sensitivity during treatment, which was possibly due to the appetite-suppressing effects of exendin-4 (19). In that study, β-cell mass was increased in IUGR lambs treated with exendin-4 compared with control lambs, although it was not significantly different from untreated IUGR lambs, and in vitro insulin secretion was normalized in IUGR lambs treated with exendin-4 (19). This suggests that β-cell proliferation as well as function may be responsive to short-term treatment with GLP-1 agonists in sheep, as it is in rodents (reviewed in Ref. 29). Given that neonatal exendin-4 also halved visceral fat mass by the end of treatment in twin lambs and increased insulin secretion and proportional β-cell mass compared with unrestricted controls (19), we predicted that adult adiposity would be decreased and metabolic function would be improved after neonatal exendin-4 treatment in the IUGR lamb. We dosed neonatal lambs in the present study at the same dose as that which protects neonatal IUGR rats from subsequent development of diabetes and insulin resistance (43, 52) and as used in our previous studies in the twin IUGR lamb (19). Therefore, the present study evaluated the effects of neonatal exendin-4 treatment on adult metabolic outcomes in the PR sheep, including body composition and insulin-regulated glucose homeostasis.
MATERIALS AND METHODS
Animal Cohort and Treatments
All experimental and surgical procedures were approved by the University of Adelaide Animal Ethics Committee (M-2009-145 and M-2011-055) and SA Pathology/CHN Adelaide Health Sciences (M/135a/09) and conducted in accordance with the Australian Code for the Care and Use of Animals for Scientific Purposes (36).
Placental size and function in primiparous Merino × Border Leicester ewes was restricted by surgical removal of all but four visible endometrial caruncles (placental attachment sites) from each uterine horn (2, 47) at least 10 wk prior to mating. Control (CON) ewes remained unoperated. Ewes underwent timed mating, with pregnancy confirmed via ultrasound at ∼60 days gestational age (term ∼150 days). Housing and nutrition of the ewes and progeny cohort has been described previously (60). Briefly, pregnant CON and PR ewes were housed indoors from 110 days of gestation until offspring were weaned at 13 wk of age. All ewes and offspring had ad libitum access to lucerne chaff and water and were fed 1 kg of Rumevite daily (Ridley AgriProducts, Melbourne, Australia). From weaning until ∼327 days of age, progeny were housed outdoors in seasonal pastures with ad libitum access to oaten hay and water in same-sex groups of similar ages and fed 0.5 kg of Rumevite/sheep daily.
Placentally restricted singleton offspring were randomly allocated within each sex to remain untreated (PR; n = 13 females and 7 males) or to receive neonatal treatment with exendin-4 (PR + EX-4; n = 12 females and 7 males). PR + EX-4 lambs were injected daily with exendin-4 (Bachem, Bubendorf, Switzerland) from day 1 to day 16 postnatal age (1 nmol/kg body wt sc; prepared as a 5-μM exendin-4 solution in 0.5% methanol, 0.9% NaCl). Approximately one-third of the PR lambs (n = 6 females and 1 male) were injected with equivalent volumes of vehicle (0.5% methanol, 0.9% NaCl). Outcomes were not affected by vehicle treatment, so vehicle-treated PR offspring were combined with the untreated PR offspring in all further analyses. Singleton CON (n = 6 females and 4 males) offspring were untreated as neonates.
Size at Birth, Growth, and Adult Body Composition
Gestational age and litter size were recorded at birth. Body weight was measured at birth and at 2-day intervals until 16 days and then weekly until weaning. Body size was measured within 24 h of birth, at 16 days, 3.5 wk, 8 wk, and at weaning, and included measurements of spinal length (crown-rump length), long bone lengths (shoulder height), soft tissue growth (abdominal and thoracic circumferences), and head size (skull width and length) (11). Absolute neonatal growth rates (AGR) and fractional neonatal growth rates (FGR) between birth and 16 days (covering the period of neonatal exendin-4 treatment) and between birth and 30 days (covering the period of catchup growth in PR lambs) were calculated by linear regression (11). From weaning, body weight, crown-rump length, and shoulder height were measured at ∼5 wk intervals.
Body composition was assessed on day 316 ± 1 after an overnight fast using dual X-ray absorptiometry (DEXA) (35). Briefly, general anesthesia was induced with thiopentone sodium (15 mg/kg body wt iv; Troy Laboratories) and maintained by isoflurane inhalation anesthetic (Bomac). Sheep were scanned in a supine position with flexed forelimbs and stretched hindlimbs using a Lunar DPX-IQ dual-energy X-ray bone densitometer (software version 4.7e; GE Medical Systems Lunar). Body composition was assessed for the whole body, the abdominal region (bordered by the diaphragm, left and right side of the abdomen, approximately midway along the femur and the posterior edge of pelvis), and the upper abdominal region (bordered by the diaphragm and posterior edge of the ribcage perpendicular to spine; Fig. 1). These regions were defined on the basis of clear and repeatable morphological features, with abdominal fat regions of particular interest given the emerging evidence that visceral fat is a better predictor of cardiometabolic disease risks in humans (5). Based on postmortem findings, fat in the abdominal fat region consists of omental fat (surrounding the rumen), abdominal fat (associated with intestines), and perirenal and retroperitoneal fat depots, as well as some subcutaneous fat, whereas the upper abdominal region contains predominantly omental fat. After scanning, animals were housed in pens during a 1-wk recovery period and then transferred to indoor housing (12:12-h light-dark cycle) in individual metabolic crates. During this period, sheep were fed 1 kg of Rumevite per day with ad libitum access to lucerne chaff and water, except when fasted for assessment of metabolic outcomes, as described below.
Fig. 1.
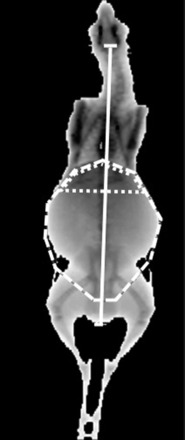
Regions of interest used to determine abdominal fatness and upper abdominal fatness. Abdominal region is bordered by the diaphragm, left and right side of the abdomen, and posterior edge of the pelvis (dashed line). Upper abdominal region is bordered by the diaphragm and the bottom of the ribcage (dotted line). Solid vertical line depicts measurement for crown-rump length.
In Vivo Metabolic Tests
At ∼332 days of age, catheters were inserted into the left and right femoral artery and vein of each sheep under general anesthesia (10) and induced and maintained as described above. Antibiotics (cephazolin, 1 g in 2.5 ml im of sterile water; Hospira Australia, Mulgrave, VIC, Australia) were administered after induction of anesthesia. Analgesia (150 mg im ketoprofen; Troy Laboratories) was administered at extubation and again on the day after surgery. Daily blood samples (3 ml) were then collected, and blood glucose was measured using a HemoCue 201 RT glucometer (HemoCue, Angelholm, Sweden) to determine average nonfasted and fasted blood glucose concentrations. Catheter patency was maintained by daily flushing with heparinized saline (500 U/ml) for the remainder of the experiment, delivering a dose of ∼20 U/kg body wt heparin each day, which is >10-fold lower than heparin doses that alter (reduce) circulating free fatty acids in sheep (49). After 3 days of recovery, three metabolic tests were performed on each animal, with ≥48 h between commencing each test. Sheep were fasted overnight prior to each metabolic test.
Intravenous arginine tolerance test.
At ∼337 days of age, blood samples (2 ml) were collected before and for 3.5 h after administration of an intravenous bolus of l-arginine (100 mg/kg fasting body wt; Sigma-Aldrich) (16). Blood was centrifuged and plasma stored at −20°C for later plasma insulin concentration analysis.
Intravenous glucose tolerance test.
At ∼339 days of age, blood samples (2 ml) were collected before and for 3.5 h after the administration of an intravenous bolus of glucose (0.25 g/kg fasting body wt glucose; Baxter Healthcare) (17, 39). Blood was centrifuged and plasma stored at −20°C for later plasma insulin and glucose concentration analysis.
Hyperinsulinemic euglycemic clamp.
At ∼341 days of age, blood was sampled (0.2 ml every 5 min throughout the clamp) and blood glucose measured using a HemoCue 201 RT glucometer (HemoCue) to obtain a measure of whole body insulin sensitivity. Human insulin was infused for 120 min (2 mU·kg−1·min−1 for 120 min, Actrapid; Novo Nordisk, Bagsvaerd, Denmark), and a glucose infusion was commenced 15 min after the start of the insulin infusion. Glucose was initially infused at 2 mg·kg−1·min−1 iv, with glucose infusion rate adjusted every 5 min to restore and maintain blood glucose at the nonfasted blood glucose concentration (average blood glucose as determined from routine daily measurements of blood glucose) using a previously published algorithm (12, 17). Larger blood samples (2 ml) were collected before (every 5 min for 20 min) and during the clamp (every 15 min, from 60 to 120 min of insulin infusion) and centrifuged to collect plasma for later analysis of insulin. The intra-animal coefficient of variation (CV) for blood glucose during the steady-state period of the clamps averaged 5.7%, and blood glucose during the steady-state period of the clamps averaged 99.2 ± 0.4% of the nonfasted blood glucose concentration (n = 44 clamp studies).
Calculations of in vivo insulin secretion and sensitivity measures.
Indices of glucose tolerance, insulin secretion, and relative insulin secretion [insulin secretion during the intravenous glucose tolerance test (IVGTT) corrected for the glucose stimulus] were calculated as described previously, with first-phase insulin secretion defined to be complete by 20 min after glucose bolus (10, 17, 39). Insulin sensitivity, the metabolic clearance rate of insulin, basal and maximal posthepatic insulin delivery rates, and basal and maximal insulin-stimulated glucose disposition indices (DI; insulin secretion relative to insulin sensitivity, a measure of insulin action) were calculated as described previously (17). Indices for insulin sensitivity (SI), glucose effectiveness (Sg) and insulin-stimulated glucose disposition (DI) were also derived by minimal modeling by simultaneous fitting of glucose and insulin curves from each IVGTT using MINMOD Millenium software (MinMod, Los Angeles, CA) (6), which successfully fitted indices for 44 of 47 IVGTT data sets.
Analysis of Plasma Glucose and Insulin Concentrations
Plasma glucose concentrations were measured by colorimetric enzymatic analysis on a Cobas Integra 400 autoanalyzer (Roche Diagnostics, Mannheim, Germany), using a Roche Glucose HK Gen. 3 kit (Roche Diagnostics). Plasma insulin concentrations were measured in duplicate by a double-antibody, solid-phase radioimmunoassay using a commercially available kit for human insulin (HI-14K; Merck Millipore). Cross-reactivity of this assay is 100% with human insulin and 62% with bovine insulin (manufacturer's information) and would be expected to be similar for ovine and bovine given the 97% homology between bovine and ovine insulin protein sequences. Because of the infusion of human insulin, circulating insulin during the steady state of hyperinsulinemic euglycemic clamps is predominantly human insulin. An ovine plasma QC pool was included in all assays; this changed once during the course of the study. The intra-assay CVs for the insulin assay were 8.9 and 10.9%, and the interassay CVs were 25.1 and 19.4% for these two ovine QC samples, which contained 9.1 and 14.3 mU/l insulin, used in n = 35 and n = 46 assays, respectively. The intra- and interassay CVs for a human insulin QC pool that contained 44.0 mU/l insulin were 7.4 and 13.1%, respectively, and this reflects lower assay variability for measurement of the elevated human insulin concentrations measured during hyperinsulinaemic euglycemic clamps.
Statistical Analyses
Effects of treatment (CON, PR, or PR + EX-4) and sex difference were analyzed using a general linear mixed model and including dam as a random factor (SPSS Statistics, version 20.0; IBM). Animal weight throughout life was analyzed by repeated-measures general linear model for effects of age, treatment, sex difference, and interactions, including dam as a random factor. Where treatment effects or trends were apparent (P < 0.1), we then compared means by the least significant difference method based on a priori questions and as described previously (19) to 1) determine the effects of IUGR (CON cf. PR treatments), 2) determine the effects of exendin-4 in PR progeny (PR cf. PR + EX-4 treatments), and 3) assess whether exendin-4 restored values to those of controls (CON cf. PR + EX-4 treatments). Associations of AGRweight and FGRweight with birth weight were assessed by Pearson's correlation, and slopes and intercepts of these relationships were compared between groups (62). Insulin data were not distributed normally, and therefore, all insulin data and data derived from measurements of insulin were log transformed prior to analysis. Remaining data were assessed for normality, and an appropriate transformation was applied as necessary. Unless otherwise stated, data are expressed as estimated means ± SE. Significance was accepted at P < 0.05.
RESULTS
Size at Birth and Postnatal Growth
Birth characteristics.
Gestational age at birth was unaffected by placental restriction (146.4 ± 0.3 days, P = 0.280). Birth weight differed between treatments, being reduced in PR (23%, 4.7 ± 0.3 kg, P = 0.004) and PR + EX-4 (29%, 4.3 ± 0.3 kg, P < 0.001) compared with CON (6.1 ± 0.4 kg) offspring, did not differ between PR and PR + EX-4 (P > 0.3), and was similar between sexes. Crown-rump length, thoracic circumference, and skull length and width, but not abdominal circumference or shoulder height, were reduced in PR compared with CON offspring at birth (P < 0.05 for all, Table 1). All measurements of body size were reduced in PR + EX-4 compared with CON offspring, but most measurements of birth size were similar in PR + EX-4 and PR offspring, and sex difference did not alter size at birth (Table 1).
Table 1.
Effect of PR and neonatal exendin-4 on body size from birth to adulthood and neonatal growth rates
| CON |
PR |
PR + EX-4 |
Significance |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Female (n = 6) | Male (n = 4) | Female (n = 14) | Male (n = 7) | Female (n = 12) | Male (n = 7) | Treatment | Sex | Interaction | |
| Size at birth, cm | |||||||||
| Abdominal circumference | 38.2 ± 1.9 | 39.7 ± 2.2 | 34.6 ± 1.2 | 36.5 ± 1.7 | 33.9 ± 1.3 | 34.6 ± 1.8 | 0.042† | 0.316 | 0.914 |
| Thoracic circumference | 40.5 ± 1.7 | 40.0 ± 1.9 | 35.6 ± 1.1 | 37.5 ± 1.5 | 35.6 ± 1.1 | 35.2 ± 1.6 | 0.014*† | 0.796 | 0.665 |
| Skull width | 6.6 ± 0.1 | 6.7 ± 0.1 | 6.3 ± 0.1 | 6.5 ± 0.1 | 6.2 ± 0.1 | 6.1 ± 0.1 | <0.001*†‡ | 0.525 | 0.221 |
| Skull length | 13.8 ± 0.3 | 13.3 ± 0.3 | 12.6 ± 0.2 | 13.1 ± 0.3 | 12.5 ± 0.2 | 12.3 ± 0.3 | 0.002*† | 0.647 | 0.219 |
| Size at 16 days, cm | |||||||||
| Abdominal circumference | 54.0 ± 1.7 | 53.8 ± 2 | 49.2 ± 1.1 | 51.3 ± 1.5 | 46.5 ± 1.2 | 46.7 ± 1.5 | <0.001*†‡ | 0.572 | 0.701 |
| Thoracic circumference | 52.8 ± 1.4 | 52.6 ± 1.6 | 48.1 ± 0.9 | 49.8 ± 1.1 | 45.7 ± 0.9 | 45.1 ± 1.2 | <0.001*†‡ | 0.770 | 0.568 |
| Skull width | 7.5 ± 0.1 | 7.3 ± 0.2 | 6.9 ± 0.1 | 7.2 ± 0.1 | 6.8 ± 0.1 | 6.8 ± 0.1 | <0.001*†‡ | 0.593 | 0.218 |
| Skull length | 14.9 ± 0.2 | 14.9 ± 0.3 | 14.3 ± 0.1 | 14.8 ± 0.2 | 14.4 ± 0.2 | 14.1 ± 0.2 | 0.026† | 0.701 | 0.102 |
| Size at 25 days, cm | |||||||||
| Abdominal circumference | 59.8 ± 2 | 58.4 ± 2.3 | 54.5 ± 1.3 | 57.9 ± 1.8 | 52.1 ± 1.3 | 53.0 ± 1.7 | 0.003†‡ | 0.499 | 0.442 |
| Thoracic circumference | 56.0 ± 1.5 | 55.7 ± 1.7 | 52.9 ± 1 | 54.5 ± 1.3 | 50.2 ± 1 | 50 ± 1.3 | <0.001†‡ | 0.709 | 0.679 |
| Skull width | 7.3 ± 0.2 | 7.5 ± 0.2 | 7.1 ± 0.1 | 7.7 ± 0.2 | 7.0 ± 0.1 | 7.1 ± 0.1 | 0.024†‡ | 0.029 | 0.237 |
| Skull length | 15.2 ± 0.7 | 15.3 ± 0.8 | 15.5 ± 0.4 | 16.7 ± 0.6 | 15.1 ± 0.5 | 15.6 ± 0.6 | 0.303 | 0.252 | 0.628 |
| Size at 56 days | |||||||||
| Abdominal circumference | 73.7 ± 2.7 | 77.4 ± 3 | 72.2 ± 1.6 | 76.3 ± 2.3 | 70.9 ± 1.7 | 70.5 ± 2.5 | 0.110 | 0.208 | 0.518 |
| Thoracic circumference | 67.7 ± 2 | 70.0 ± 2.2 | 66.8 ± 1.2 | 69.7 ± 1.6 | 65.5 ± 1.3 | 66.0 ± 1.8 | 0.153 | 0.183 | 0.716 |
| Skull width | 7.8 ± 0.3 | 8.6 ± 0.4 | 8.0 ± 0.2 | 8.1 ± 0.3 | 7.9 ± 0.2 | 7.8 ± 0.3 | 0.502 | 0.327 | 0.305 |
| Skull length | 19.0 ± 0.8 | 19.2 ± 0.9 | 20.2 ± 0.5 | 20.8 ± 0.7 | 19.2 ± 0.5 | 19.9 ± 0.7 | 0.108 | 0.318 | 0.948 |
| Size at 91 days (weaning) | |||||||||
| Abdominal circumference | 86.4 ± 2.2 | 90.8 ± 2.5 | 86.6 ± 1.4 | 91.6 ± 2.0 | 86.5 ± 1.4 | 88.0 ± 2.2 | 0.586 | 0.035 | 0.616 |
| Thoracic circumference | 80.7 ± 2.2 | 79.3 ± 2.4 | 78.7 ± 1.5 | 84.3 ± 2 | 78.7 ± 1.4 | 81.0 ± 2.2 | 0.615 | 0.184 | 0.234 |
| Skull width | 9.1 ± 1.0 | 13.5 ± 1.1 | 9.1 ± 0.6 | 9.9 ± 0.9 | 9.0 ± 0.6 | 9.7 ± 1.0 | 0.089 | 0.010 | 0.104 |
| Skull length | 21.3 ± 0.8 | 21.7 ± 0.9 | 22.1 ± 0.5 | 24.4 ± 0.7 | 22.2 ± 0.5 | 22.9 ± 0.8 | 0.076 | 0.067 | 0.308 |
| Neonatal growth rate | |||||||||
| AGRweight (days 0–16), kg/day | 0.398 ± 0.026 | 0.389 ± 0.031 | 0.349 ± 0.018 | 0.391 ± 0.023 | 0.292 ± 0.018 | 0.269 ± 0.023 | <0.001†‡ | 0.860 | 0.287 |
| FGRweight (days 0–16), days | 0.067 ± 0.010 | 0.062 ± 0.012 | 0.081 ± 0.006 | 0.102 ± 0.009 | 0.065 ± 0.007 | 0.063 ± 0.009 | 0.002*‡ | 0.530 | 0.238 |
| AGRweight (days 16–30) kg/day | 0.245 ± 0.031 | 0.289 ± 0.038 | 0.251 ± 0.02 | 0.322 ± 0.029 | 0.271 ± 0.023 | 0.277 ± 0.029 | 0.793 | 0.096 | 0.461 |
| FGRweight (days 16–30), days | 0.020 ± 0.003 | 0.023 ± 0.003 | 0.025 ± 0.002 | 0.028 ± 0.002 | 0.030 ± 0.002 | 0.033 ± 0.002 | 0.001*†‡ | 0.116 | 0.998 |
Data are estimated means ± SE.
CON, control; PR, placental restriction; EX-4, exendin-4.
Where treatment effects or trends were apparent (P < 0.1), we compared means for each treatment by the least significant difference (LSD) method based on a priori questions to 1) determine the effects of intrauterine growth restriction (IUGR; CON cf. PR treatments), 2) determine effects of exendin-4 in PR progeny (PR cf. PR + EX-4 treatments), and 3) assess whether exendin-4 restored values to those of controls (CON cf. PR + EX-4 treatments). Between-group differences for specific contrasts are shown as follows:
P < 0.05 CON cf. PR;
P < 0.05 CON cf. PR + EX-4;
P < 0.05 PR cf. PR + EX-4.
Postnatal changes in weight.
Body weight (Fig. 2) increased with age (P < 0.001), was similar between treatment groups (P = 0.269), and was greater in males than females (P = 0.033). Effects of age on weight differed between treatment groups (P < 0.001) and between males and females (P = 0.001). Therefore, effects of treatment and sex difference on body weight were analyzed separately at each age.
Fig. 2.
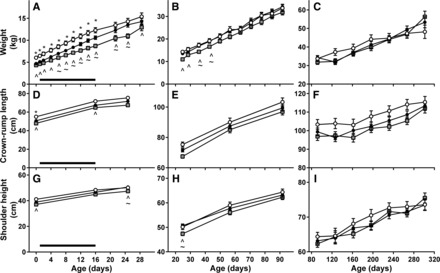
Effect of PR and neonatal exendin-4 on postnatal growth. Body weight (A–C), crown-rump length (D–F), and shoulder height (G–I) of CON (○), PR (●), and PR + EX-4 offspring (gray squares) are shown from birth to day 28 (A, D, and G), from day 28 to weaning on day 91 (B, E, and H), and from weaning to adulthood on day 301 (C, F, and I). Data are estimated means ± SE. Horizontal black bar represents period of exendin-4 treatment. *P < 0.05, CON cf. PR; ^P < 0.05 CON cf. PR+EX-4; ∼P < 0.05 PR cf. PR + EX-4.
Neonatal growth during treatment period.
PR and PR + EX-4 lambs were lighter than CON lambs until day 16, and PR + EX-4 lambs were lighter than PR lambs from days 6 to 16 (P < 0.05 for all; Fig. 2A). AGRweight from birth to day 16 (Table 1) was similar in PR and CON lambs (P = 0.352) but was lower in PR + EX-4 lambs than in PR or CON lambs (P < 0.001 for both). In PR and PR + EX-4 lambs, but not CON lambs, AGRweight in the first 16 days correlated positively with birth weight (Fig. 3A). The slope of the correlation between AGRweight and birth weight was similar in PR and PR + EX-4 lambs (P > 0.1), but the intercept was higher in PR lambs than in PR + EX-4 lambs (P = 0.001), reflecting a downward shift in growth rate during exendin-4 treatment regardless of birth weight. FGRweight was higher in PR than CON lambs (P = 0.007) and was reduced during exendin-4 treatment (PR vs. PR + EX-4, P = 0.001; Table 1) to values similar to those of CON lambs (Table 1). FGRweight in the first 16 days correlated negatively with birth weight in all groups (Fig. 3B). The intercept for the correlation between FGRweight and birth weight was lower in PR + EX-4 than in CON or PR lambs (P < 0.001 for both) and similar in CON and PR lambs (P = 1.0), whereas the slope of this relationship was similar in all groups (P > 0.4), also indicating that reduction in FGRweight in response to exendin-4 was unaffected by birth weight. On day 16 of age, abdominal and thoracic circumferences and skull width were reduced in PR compared with CON lambs (P < 0.05 for all; Table 1) and were further reduced in PR + EX-4 lambs (PR vs. PR + EX-4, P < 0.05 for all; Table 1); crown-rump length and skull length of PR + EX-4 lambs were lower than those of CON lambs (Table 1). Shoulder height was unaffected by treatment (Fig. 2). Neonatal growth rates and all measurements of body size on day 16 were similar between sexes.
Fig. 3.
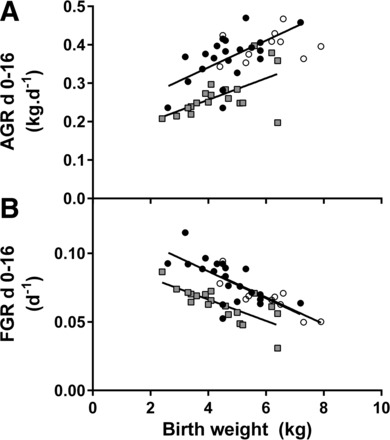
Relationships between birth weight and absolute (AGR; A) and fractional growth rates (FGR; B) during the neonatal treatment period between birth and day 16. A: birth weight correlated positively with AGRweight in PR (r = 0.594, P = 0.002, n = 21) and PR + EX-4 (r = 0.594, P = 0.004, n = 19) but not CON (r = 0.259, P = 0.235, n = 10) lambs. B: birth weight correlated negatively with FGRweight in all treatment groups (CON: r = −0.864, P = 0.001, n = 10; PR: r = −0.672, P < 0.001, n = 21; PR + EX-4: r = −0.752, P < 0.001, n = 18). ○, CON; ●, PR; gray squares, PR + EX-4. Regression lines are shown only for significant relationships.
Neonatal growth following treatment period.
Between days 16 and 30, AGRweight was similar in each treatment, whereas FGRweight differed with treatment (P = 0.001; Table 1). FGRweight (Table 1) was higher in PR and PR + EX-4 offspring than in CON offspring (P < 0.05 for both) and was higher in PR + EX-4 offspring than in PR offspring (P = 0.023). PR + EX-4 offspring remained lighter than CON offspring during this period and were also lighter than PR offspring until day 25 (Fig. 2A). On day 25, all measurements of size were similar in CON and PR offspring, whereas shoulder height, abdominal and thoracic circumferences, and skull width were reduced in PR + EX-4 offspring compared with PR or CON offspring (Table 1 and Fig. 2, D and G).
Growth to weaning.
Body weights of PR and CON offspring did not differ from 28 days until weaning. PR + EX-4 offspring remained lighter than CON offspring and were lighter than PR offspring between days 35 and 42, and from day 51, body weight was similar between treatment groups (Fig. 2B). Males became heavier than females on day 84 and remained heavier until weaning (P < 0.05 at each age). On day 56 and at weaning (day 91), all body size measurements were similar in treatment groups. Shoulder height was greater in males than in females on day 56 (males: 59.3 ± 1.0 cm; females: 55.9 ± 0.8 cm) and at weaning (males: 65.1 ± 0.8 cm; females: 61.5 ± 0.8 cm), whereas skull width was greater in males than in females at weaning, but not on day 56 (Table 1).
Growth to adulthood.
From weaning (day 91) to adulthood, body weight (Fig. 2C) remained similar in all treatment groups (P > 0.055 at each age), and males remained heavier than females (P < 0.017 at each age). Crown-rump length and shoulder height were similar in all treatment groups from weaning to adulthood (Fig. 2, F and I).
Adult Body Composition
Total body.
At DEXA scan (316 ± 1 day of age), body weight was similar in each treatment group, and males were heavier than females (Table 2). Total body fat (absolute and relative to body weight), lean mass (absolute and relative to body weight), and the ratio of fat mass to lean mass were unaffected by treatment (P > 0.1 for each). Absolute total body lean mass was greater in males than females (Table 2). Absolute and relative fat mass, relative lean mass, and the ratio of fat mass to lean mass were similar in males and females (Table 2).
Table 2.
Effect of PR and neonatal exendin-4 on adult body composition
| CON |
PR |
PR+EX-4 |
Significance |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Female (n = 6) | Male (n = 4) | Female (n = 14) | Male (n = 7) | Female (n = 12) | Male (n = 7) | Treatment | Sex | Interaction | |
| Body weight, kg | 42.0 ± 3.1 | 47.5 ± 3.7 | 41.0 ± 2 | 53.3 ± 2.8 | 39.6 ± 2.2 | 50.3 ± 2.8 | 0.600 | <0.001 | 0.517 |
| Total body | |||||||||
| Fat mass, kg | 3.98 ± 1.30 | 2.34 ± 1.58 | 4.87 ± 0.85 | 4.22 ± 1.20 | 4.18 ± 0.92 | 3.9 ± 1.2 | 0.751 | 0.932 | 0.948 |
| Fat mass, %body wt | 8.82 ± 2.53 | 3.36 ± 3.03 | 10.66 ± 1.65 | 7.86 ± 2.29 | 10.52 ± 1.77 | 7.46 ± 2.29 | 0.145 | 0.114 | 0.135 |
| Lean mass, kg | 35.8 ± 2.1 | 44.2 ± 2.5 | 33.4 ± 1.4 | 44.4 ± 1.9 | 32.6 ± 1.5 | 42.8 ± 1.9 | 0.514 | <0.001 | 0.806 |
| Lean mass, %body wt | 87.9 ± 2.4 | 91.7 ± 2.9 | 85.6 ± 1.6 | 88.7 ± 2.2 | 86.2 ± 1.7 | 88.9 ± 2.2 | 0.405 | 0.116 | 0.999 |
| Fat mass/lean mass | 0.106 ± 0.033 | 0.053 ± 0.040 | 0.138 ± 0.021 | 0.091 ± 0.030 | 0.127 ± 0.023 | 0.086 ± 0.03 | 0.333 | 0.058 | 0.970 |
| Total abdominal region | |||||||||
| Fat mass, kg | 2.12 ± 0.74 | 1.11 ± 0.90 | 2.60 ± 0.48 | 2.09 ± 0.68 | 2.18 ± 0.52 | 1.94 ± 0.68 | 0.776 | 0.771 | 0.991 |
| Fat mass, %body wt | 4.69 ± 1.39 | 2.32 ± 1.68 | 5.90 ± 0.91 | 3.85 ± 1.27 | 5.47 ± 0.98 | 3.71 ± 1.27 | 0.374 | 0.029 | 0.997 |
| Fat mass, %total body fat mass | 50.7 ± 1.8 | 47.5 ± 2.1 | 51.2 ± 1.2 | 47.2 ± 1.5 | 50.3 ± 1.2 | 48.9 ± 1.6 | 0.934 | 0.031 | 0.644 |
| Lean mass, kg | 18.3 ± 1.0 | 21.6 ± 1.2 | 16.8 ± 0.7 | 21.5 ± 0.9 | 16.2 ± 0.7 | 20.7 ± 0.9 | 0.342 | <0.001 | 0.800 |
| Lean mass, % body wt | 44.5 ± 1.5 | 45.1 ± 1.8 | 43.0 ± 1.0 | 43.0 ± 1.4 | 42.7 ± 1.1 | 43.2 ± 1.4 | 0.414 | 0.777 | 0.971 |
| Fat mass/lean mass | 0.109 ± 0.038 | 0.051 ± 0.046 | 0.144 ± 0.025 | 0.093 ± 0.035 | 0.137 ± 0.027 | 0.089 ± 0.035 | 0.362 | 0.051 | 0.997 |
| Upper abdominal region | |||||||||
| Fat mass, kg | 0.472 ± 0.171 | 0.238 ± 0.207 | 0.550 ± 0.112 | 0.494 ± 0.157 | 0.505 ± 0.120 | 0.480 ± 0.157 | 0.488 | 0.665 | 0.962 |
| Fat mass, %body wt | 1.05 ± 0.32 | 0.50 ± 0.39 | 1.26 ± 0.21 | 0.90 ± 0.29 | 1.27 ± 0.23 | 0.91 ± 0.29 | 0.179 | 0.031 | 0.991 |
| Fat mass, %total body fat mass | 10.9 ± 0.8 | 10.1 ± 0.9 | 11.4 ± 0.5 | 10.9 ± 0.7 | 11.9 ± 0.5 | 11.6 ± 0.7 | 0.293 | 0.359 | 0.942 |
| Fat mass, %total abdominal fat mass | 21.2 ± 1.2 | 21.5 ± 1.5 | 22.4 ± 0.8 | 22.9 ± 1.1 | 23.4 ± 0.9 | 23.5 ± 1.1 | 0.223 | 0.732 | 0.982 |
| Fat mass/lean mass | 0.107 ± 0.038 | 0.051 ± 0.045 | 0.142 ± 0.024 | 0.092 ± 0.034 | 0.134 ± 0.026 | 0.088 ± 0.034 | 0.346 | 0.050 | 0.994 |
Data are estimated means ± SE.
Abdominal region.
Treatment did not alter fat or lean masses in the abdominal region (Table 2). Total abdominal fat mass relative to body weight and relative to total body fat mass was greater in females than males (Table 2). Absolute abdominal fat mass and the ratio of abdominal fat mass to lean mass did not differ between sexes (Table 2). Absolute abdominal lean mass, but not abdominal lean mass relative to body weight, was greater in males than in females (Table 2).
Upper abdominal region.
In the upper abdominal region, fat mass (absolute and relative to body weight, total body fat, and abdominal fat mass) was similar in each treatment (Table 2). Upper abdominal fat relative to body weight was greater in females than in males (Table 2). Upper abdominal absolute fat mass and fat mass relative to total body fat mass and total abdominal fat mass were similar in males and females (Table 2).
Insulin Secretion, Sensitivity, and Action in Adult Progeny
Fasting glucose metabolism.
Fasting plasma glucose concentrations tended to be higher in PR + EX-4 sheep than in CON (P = 0.054) or PR (P = 0.088) offspring but were similar in CON and PR sheep (P = 0.585; Table 3 and Fig. 4). Fasting plasma insulin concentrations and insulin/glucose ratios did not differ between treatment groups (Table 3). Fasting plasma glucose and insulin concentration and the insulin/glucose ratio were similar in males and females (Table 3).
Table 3.
Effect of PR and neonatal exendin-4 on adult glucose tolerance, glucose- and arginine-stimulated insulin secretion, and insulin action
| CON |
PR |
PR + EX-4 |
Significance |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Female (n = 6) | Male (n = 4) | Female (n = 14) | Male (n = 7) | Female (n = 12) | Male (n = 7) | Treatment | Sex | Interaction | |
| Fasting | |||||||||
| Plasma glucose, mmol/l | 3.47 ± 0.22 | 3.52 ± 0.26 | 3.57 ± 0.15 | 3.65 ± 0.20 | 3.80 ± 0.16 | 4.03 ± 0.20 | 0.098 | 0.465 | 0.893 |
| Plasma insulin, mU/l | 5.52 ± 1.38 | 6.23 ± 1.61 | 5.67 ± 0.95 | 9.47 ± 1.20 | 7.27 ± 0.99 | 8.72 ± 1.21 | 0.398 | 0.306 | 0.323 |
| Plasma insulin/glucose, mU/mmol | 1.59 ± 0.34 | 1.66 ± 0.40 | 1.59 ± 0.23 | 2.67 ± 0.30 | 1.87 ± 0.24 | 2.09 ± 0.30 | 0.481 | 0.356 | 0.216 |
| AUCglucose (IVGTT, mmol·min·l−1) | |||||||||
| Total | 279 ± 38 | 215 ± 44 | 339 ± 26 | 312 ± 33 | 275 ± 27 | 303 ± 34 | 0.097* | 0.457 | 0.423 |
| 1st Phase | 104 ± 9 | 98 ± 11 | 122 ± 6 | 114 ± 8 | 117 ± 7 | 117 ± 8 | 0.125 | 0.545 | 0.895 |
| 2nd Phase | 175 ± 31 | 117 ± 36 | 218 ± 21 | 196 ± 27 | 158 ± 22 | 186 ± 27 | 0.097* | 0.446 | 0.323 |
| AUCinsulin (IVGTT, mU·min·l−1) | |||||||||
| Total | 755 ± 205 | 1,272 ± 251 | 979 ± 145 | 972 ± 190 | 758 ± 152 | 1,201 ± 190 | 0.914 | 0.068 | 0.291 |
| 1st Phase | 185 ± 75 | 437 ± 92 | 243 ± 53 | 434 ± 70 | 204 ± 56 | 369 ± 70 | 0.873 | 0.001 | 0.571 |
| 2nd Phase | 569 ± 166 | 835 ± 203 | 737 ± 117 | 538 ± 153 | 554 ± 123 | 833 ± 153 | 0.678 | 0.422 | 0.292 |
| AUCinsulin/AUCglucose (IVGTT, mU/mmol) | |||||||||
| Total | 2.62 ± 0.75 | 5.78 ± 0.92 | 2.89 ± 0.53 | 3.77 ± 0.69 | 2.76 ± 0.55 | 3.98 ± 0.69 | 0.601 | 0.016 | 0.192 |
| 1st Phase | 1.74 ± 1.14 | 4.33 ± 1.31 | 2.04 ± 0.78 | 4.65 ± 0.96 | 1.98 ± 0.80 | 3.26 ± 0.99 | 0.962 | 0.001 | 0.636 |
| 2nd Phase | 3.16 ± 0.83 | 7.06 ± 1.01 | 3.36 ± 0.59 | 3.09 ± 0.77 | 3.59 ± 0.61 | 4.34 ± 0.77 | 0.239 | 0.169 | 0.121 |
| AUCinsulin (arginine test, mU·min·l−1) | |||||||||
| Total | 239 ± 186 | 108 ± 189 | 168 ± 125 | 329 ± 133 | 397 ± 109 | 285 ± 143 | 0.545 | 0.824 | 0.522 |
| 1st Phase | 143 ± 49 | 76 ± 49 | 117 ± 33 | 193 ± 29 | 134 ± 26 | 156 ± 37 | 0.540† | 0.718 | 0.228 |
| 2nd Phase | 97 ± 158 | 33 ± 161 | 52 ± 106 | 125 ± 116 | 268 ± 94 | 129 ± 122 | 0.487 | 0.675 | 0.641 |
| Insulin sensitivity, mg·l·mU−1·kg−1·min−1 | 0.035 ± 0.007 | 0.019 ± 0.008 | 0.035 ± 0.005 | 0.026 ± 0.007 | 0.036 ± 0.005 | 0.025 ± 0.006 | 0.807 | 0.041 | 0.806 |
| Basal DI, mg·ml·kg−2·min−2 | 4.15 ± 1.77 | 1.02 ± 2.16 | 5.00 ± 1.37 | 3.88 ± 1.77 | 5.09 ± 1.37 | 3.12 ± 1.63 | 0.278 | 0.227 | 0.263 |
| Maximal DI, mg·ml·kg−2·min−2 | 15.15 ± 7.62 | 6.63 ± 8.57 | 18.88 ± 5.70 | 12.70 ± 5.87 | 19.04 ± 5.15 | 20.85 ± 6.48 | 0.520 | 0.412 | 0.799 |
| Indices derived from minimal modeling | |||||||||
| SI (mU·l−1·min−1) | 30.1 ± 6.7 | 9.7 ± 8.2 | 18.4 ± 4.9 | 11.1 ± 6.7 | 17.3 ± 4.9 | 9.6 ± 6.7 | 0.865 | 0.009 | 0.720 |
| Sg, min | 0.011 ± 0.002 | 0.013 ± 0.002 | 0.011 ± 0.002 | 0.013 ± 0.002 | 0.012 ± 0.002 | 0.010 ± 0.002 | 0.837 | 0.533 | 0.641 |
| DI | 1,842 ± 530 | 1,507 ± 650 | 2,029 ± 392 | 1,627 ± 531 | 1,443 ± 392 | 1,645 ± 531 | 0.706 | 0.787 | 0.922 |
Data are estimated means ± SE.
AUC, area under the curve; IVGTT, intravenous glucose tolerance test; DI, disposition index; SI, insulin sensitivity; Sg, glucose effectiveness.
Where treatment effects or trends were apparent (P < 0.1) we compared means for each treatment by the LSD method based on a priori questions to 1) determine the effects of IUGR (CON cf. PR treatments), 2) determine the effects of exendin-4 in PR progeny (PR cf. PR + EX-4 groups), and 3) assess whether exendin-4 restored values to those of controls (CON cf. PR + EX-4 treatments). Between-group differences for specific contrasts are shown as follows:
P < 0.05 CON cf. PR;
P < 0.05 male CON cf. male PR;
Fig. 4.
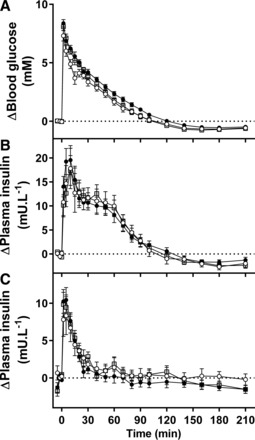
Blood glucose (A) and plasma insulin (B) during an intravenous glucose tolerance test and plasma insulin (C) during an arginine test in CON (○), PR (●), and PR + EX-4 (gray square) offspring. Data are estimated means ± SE and are shown as change from fasting (pretest) concentrations.
Glucose tolerance.
PR sheep had poorer glucose tolerance [higher area under the curve for plasma glucose (AUCglucose) during the IVGTT; Fig. 4] than CON sheep (P = 0.035), and glucose tolerance was similar in PR + EX-4 compared with CON or PR sheep (P > 0.2 for each; Table 3 and Fig. 4). Treatment did not alter first-phase AUCglucose. PR sheep had higher second-phase AUCglucose than CON sheep (P = 0.041), and second-phase AUCglucose was similar in PR + EX-4 compared with CON or PR sheep (P > 0.1 for each; Table 3). Overall, first-phase and second-phase AUCglucose were similar in males and females (Table 3).
Glucose-stimulated insulin secretion.
Overall, first- and second-phase area under the curve of plasma insulin (AUCinsulin) during the IVGTT (Fig. 4) were similar in all treatment groups (Table 3). Male sheep tended to have higher overall AUCinsulin than female sheep, and first-phase AUCinsulin was higher in male than female sheep (Table 3). In contrast, second-phase AUCinsulin did not differ between males and females (Table 3). Overall, first- and second-phase AUCinsulin/AUCglucose did not differ between treatment groups (Table 3). Overall and first-phase but not second-phase AUCinsulin/AUCglucose were higher in males in than females (Table 3).
Arginine-stimulated insulin secretion.
Fasting plasma insulin concentration was similar in all treatment groups and in males and females (Table 3). During the intravenous arginine test (Fig. 4), overall AUCinsulin was similar in all treatment groups and in males and females (Table 3). First-phase AUCinsulin was similar in all treatment groups and between sexes overall but was higher in male PR sheep than in male CON sheep (Table 3). Second-phase insulin secretion was unaffected by treatment or sex difference (Table 3).
Insulin sensitivity and DI.
SI measured by hyperinsulinemic euglycemic clamp and basal and maximal DI calculated from IVGTT and hyperinsulinemic euglycemic clamp did not differ between treatment groups (Table 3). SI was higher in female than in male sheep, whereas basal and maximal disposition index did not differ between males and females (Table 3).
Indices from minimal modelling of IVGTT data.
SI, Sg, and DI derived from minimal modeling did not differ between groups (Table 3). SI was greater in females than in males, and Sg and DI did not differ between sexes (Table 3). SI derived from minimal modeling did not correlate with direct measurements of insulin sensitivity by hyperinsulinemic euglycemic clamp in this cohort (r = 0.170, P = 0.288, n = 41). Similarly, DI obtained by minimal modeling did not correlate with basal or maximal disposition indices calculated from insulin secretion data or direct measurements of insulin sensitivity (P > 0.8 for each).
DISCUSSION
Contrary to our hypothesis, neonatal exendin-4 did not decrease adult adiposity despite suppressing neonatal growth during treatment in the PR lamb. Growth accelerated following the cessation of exendin-4 treatment, resulting in delayed catchup growth in this group compared with PR animals. Nevertheless, both PR and PR + EX-4 sheep caught up to CON progeny in weight by ∼2 mo of age. As adults, glucose tolerance of exendin-4-treated PR offspring was intermediate between control and PR levels, suggesting some benefits of neonatal exendin-4 treatment for adult glucose metabolism after IUGR in this species. These effects of exendin-4 in the PR sheep are thus relatively subtle, and probably of limited clinical significance, at least in healthy young adults not subject to additional challenges such as obesity. This is in contrast to the marked benefits of neonatal exendin-4 on adult metabolism and complete protection against diabetes observed in the PR rat (52).
Species differences in response to neonatal exendin-4 might reflect differences in timing of pancreas development (4, 15, 26, 41, 46, 48, 59) and hence, timing of the IUGR insult and exendin-4 intervention relative to pancreas development. Although the adult sheep is a ruminant, we do not consider that this explains the smaller effects of neonatal exendin-4 on adult glucose tolerance in this species. As neonates, lambs are not functionally ruminants and derive sugars from intestinal absorption after suckling in addition to hepatic gluconeogenesis when fasting (25, 51). Their source of glucose changes as ruminant digestion develops, and in later postnatal life, circulating glucose is derived largely from hepatic gluconeogenesis (30). Although they are not exposed to large fluxes of circulating glucose from the diet, adolescent and adult (ruminant) sheep exhibit similar glucose profiles and robust biphasic insulin secretion responses following a bolus of intravenous glucose (16, 17, 39), as occurs during IVGTT in humans (e.g., see Ref. 27). Although developmental and tissue-specific expression of the GLP-1 receptor has not been characterized in the sheep, we previously observed a profound reduction in growth during treatment and normalized in vitro insulin secretion in IUGR lambs during exendin-4 treatment (19). These responses suggest that the receptor is present in gastrointestinal and/or brain sites and pancreas in early life in sheep, consistent with expression patterns in humans and rodents, where the GLP-1 receptor is expressed in gastrointestinal tract, brain, heart, kidney, lung, and pancreatic islets, particularly β-cells (22, 42). Overall, our results suggest that neonatal exendin-4 is unlikely to substantially ameliorate the adverse long-term metabolic effects of IUGR in species where the majority of the pancreatic development occurs before birth, and earlier intervention windows should be evaluated to develop interventions that are likely to be effective in humans.
In the present cohort, PR offspring were smaller at birth than control offspring, with a 29% reduction in birth weight in PR and PR + EX-4 offspring compared with control offspring. This is at the larger end of size reductions observed in our published PR cohorts (11, 18, 39, 60), with less variation in birth size within groups, reflecting the restriction of the present cohort to singletons only and hence, removal of natural IUGR due to twinning (58). PR progeny caught up in weight to that of CON lambs by 21 days of age, consistent with our previous observations (11) and similar to the early life catchup growth observed after human IUGR (1). Neonatal fractional growth rates in CON and PR lambs correlated negatively with size at birth, probably reflecting increased appetite, as suggested by increased feeding activity in PR neonates (8, 11) and preferential allocation of nutrient intake toward growth. Adult size was not different in PR and control progeny, either in the present cohort or in our previous study (39). Consistent with increased fat mass in humans of low birth weight (38), in the present study, we observed an association between lower birth weight and increased adult absolute and relative fat masses measured by DEXA. Intriguingly, this relationship was only present in control progeny and was not present in PR progeny, and PR also did not increase adult fatness overall. This suggests that exposure to a restricted intrauterine environment does not necessarily increase adult adiposity in the sheep, although visceral adiposity is increased in PR compared with control sheep late in the catchup growth period at ∼43 days of age (11).
Ovine fetal growth becomes progressively more limited by placental capacity to deliver nutrients and is more closely correlated with placental size as gestation progresses, and therefore, PR is particularly restrictive in later gestation (2, 3). Interestingly, restricted fetal nutrient supply in humans due to severe maternal undernutrition as reported from the Dutch Winter Hunger famine increased BMI in 19-yr-old men and in 50-yr-old adult women only when exposure occurred during early gestation and not if exposed during midgestation or late gestation (44, 45). The lack of effect of PR on adult adiposity in the present study is consistent with this suggestion that early rather than late gestation growth restriction programs later obesity. Also in the present cohort, glucose tolerance was poorer in PR than in control offspring, although the underlying determinants, insulin secretion, insulin sensitivity, and glucose effectiveness, did not differ between CON and PR adults, suggesting that changes in glucose tolerance reflect multiple small changes in each determinant rather than a single factor. Consistent with the impaired glucose tolerance we observed in sheep born from restricted pregnancies in the present cohort, we also observed progressive impairment of glucose tolerance with decreasing birth weight in males in a previous adult cohort that included singleton and twin CON and PR progeny, although glucose tolerance was not impaired by PR in that study (39). In that cohort, low-birth weight females were protected from impairment of adult glucose tolerance due to increasing insulin sensitivity with decreasing birth weight (39). At younger postnatal ages, insulin sensitivity is reduced in PR and low-birth weight lambs, concurrent with reduced expression of insulin signaling components in skeletal muscle (9, 10). Therefore, impaired adult glucose tolerance in the present cohort might reflect subtle differences in muscle insulin signaling or insulin secretion. In the present cohort, PR did not alter glucose effectiveness in adulthood, which has been reported in conjunction with impaired insulin secretion and sensitivity in adult progeny of obese pregnancies in sheep (32). In contrast, glucose effectiveness determined by minimal modeling of IVGTT responses in young adult men increased as birth weight decreased, suggesting that IUGR may improve glucose effectiveness (14). Although both minimal modeling indices and hyperinsulinemic euglycemic clamp measurements of insulin sensitivity identified higher insulin sensitivity in females than in males, insulin sensitivity derived by minimal modeling did not correlate with direct measurements of insulin in this cohort. Similarly, insulin-stimulated glucose disposition derived from minimal modeling did not correlate with disposition indices calculated from independent measurements of insulin sensitivity and secretion, and therefore, this approach may be of limited accuracy in assessing determinants of glucose tolerance in the sheep.
Consistent with our previous studies in the spontaneously IUGR twin lamb (19), exendin-4 profoundly suppressed neonatal growth during treatment. Intriguingly, this suppression of neonatal growth completely blocks the accelerated neonatal growth characteristic of PR progeny in sheep (11) and after IUGR in humans (1) but is not observed during neonatal exendin-4 treatment in control or PR rats (43). Exendin-4 injections given once daily cause transient reductions in food consumption in db/db mice and in Zucker fatty rats, two rodent models of insulin resistance (20, 54). When exendin-4 injections were given twice daily, a sustained reduction in food intake and weight gain was observed in Zucker fatty rats (54). Therefore, the lack of appetite suppression in response to once-daily exendin-4 injections in neonatal control and PR rodents (43) might reflect frequency of administration in this study. Exendin-4 and other GLP-1 receptor agonists reduce food intake via delaying gastric emptying, which increases postmeal gastric distension via peripheral vagal nerve activation and also acts centrally in the brain to induce satiety (reviewed in Ref. 57). Therefore, a lack of suppression of food intake and growth by exendin-4 in neonatal rodents might also reflect immaturity of one or more of these pathways. Direct measurements of food intake during exendin-4 treatment and of gastrointenstinal and brain GLP-1 receptor expression in neonatal rodents and sheep might be useful in determining mechanisms of effect in each species. The markedly reduced growth in PR offspring during neonatal exendin-4 treatment from 1 to 16 days after birth was followed by accelerated growth in the week following treatment in the present study, suggesting that exendin-4 delayed but did not prevent catchup growth. In the twin IUGR lamb, neonatal exendin-4 treatment at the same dose and duration halved visceral fat mass at the end of treatment in conjunction with preventing accelerated neonatal growth (19). PR + EX-4 lambs achieved similar body weights and abdominal circumferences as CON and PR lambs by 56 days of age, consistent with increased soft tissue deposition during catchup growth, even if it was delayed for the first 16 days of life by exendin-4 treatment. These results and those of other studies suggest that catchup growth is associated with increased deposition of adipose tissue, whether it occurs following restriction of growth before birth or in growing animals postnatally (11, 38, 53). Given the efficacy of the same dose of neonatal exendin-4 in suppressing neonatal growth in these PR lambs and previously in reducing size and visceral fatness in twin IUGR lambs and juvenile and adult control and PR rats (19, 43, 52), we were somewhat surprised to see a lack of effect of neonatal exendin-4 on adult body size or body composition in the present study. Thus the sheep appears to retain a capacity for complete catchup growth of body size and soft tissue deposition after growth restriction in early postnatal as well as intrauterine life. It is possible that a longer duration of suppressed neonatal growth, perhaps spanning the entirety of the first month of postnatal life when neonatal catchup growth in weight occurs after PR (11), would permanently reduce adult size or adiposity in the sheep.
Adult glucose tolerance in the PR sheep was partially normalized by neonatal exendin-4 treatment such that glucose tolerance did not differ between exendin-4 and either control or PR groups, with relatively small differences between all groups. Consistent with the lack of persistent effect of neonatal exendin-4 on adult body size and fatness in PR sheep, however, neonatal exendin-4 did not alter the determinants of insulin action and secretion and sensitivity or glucose effectiveness in the present study, suggesting that small changes in multiple determinants account for the effects on glucose tolerance. Neither glucose- nor arginine-stimulated insulin secretion were altered by neonatal exendin-4 treatment in these PR sheep. This might partially reflect a lack of impairment in insulin secretion in untreated PR sheep in the present cohort. We also observed that insulin sensitivity in adult PR sheep was unaffected by prior neonatal exendin-4 treatment. This confirms that reduced insulin sensitivity during exendin-4 treatment in the twin IUGR lamb (19) is transient and probably reflects acute effects of suppressed appetite and nutrient intake during this period. The unchanged insulin secretion and sensitivity after neonatal exendin-4 treatment in PR sheep in the present study is in marked contrast to its effects in IUGR rats. Neonatal exendin-4 treatment of IUGR rats completely normalized glucose tolerance and β-cell mass throughout postnatal life and increased whole body insulin sensitivity by 75% in young adults compared with untreated IUGR rats (43, 52). Consequently, neonatal exendin-4 completely prevents the later development of T2D induced by intrauterine growth restriction in the Sprague-Dawley rat (50, 52). We hypothesize that the poorer efficacy of neonatal exendin-4 in improving subsequent insulin secretion after IUGR in sheep, compared with rats, reflects the need to administer exendin-4 at critical windows prior to pancreas maturation. The rat pancreas is immature at birth, with maturational events, including a wave of β-cell apoptosis and development of the adult glucose-responsive phenotype occurring in the second and third weeks of life (7, 48). Therefore, neonatal exendin-4 treatment occurs during a much earlier phase of pancreas development in the rat than in the sheep, where β-cells develop much earlier in gestation than in rodents, and the pancreas exhibits a glucose-responsive phenotype from late gestation (4, 15, 46). Together, these results suggest that exendin-4 may need to be given prior to birth to improve later insulin secretion after IUGR in species in which the pancreas is more mature at birth.
Perspectives and Significance
This is the first study to show a partial restoration of adult glucose tolerance in IUGR progeny by neonatal exendin-4 treatment in a species where the pancreas is substantially mature at birth, and therefore, maturational effects occur during IUGR and prior to exendin-4 treatment. The relatively small effects of neonatal exendin-4 on adult glucose tolerance may be of limited clinical significance. Consistent with this, the lack of effects of neonatal exendin-4 on adult body composition, insulin secretion, and insulin sensitivity in the present study suggest that the neonatal period may be too late to fully reprogram the metabolic consequences of IUGR in species that are more mature at birth than rodents, such as the sheep or human.
GRANTS
This work was supported by project grants (ID nos. 627123 and 1011767) from the National Health and Medical Research Council of Australia. H. Liu was supported by a University of Adelaide Faculty of Health Sciences Postgraduate Scholarship. D. S. Hunter and A. L. Woolridge were supported by Australian Postgraduate Awards and A. L. Woolridge was the recipient of a Healthy Development Adelaide scholarship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.L., C.G.S., M.J.D.B., A.M.P., G.K.H., H.H., D.S.H., A.L.W., K.L.K., and K.L.G. performed experiments; H.L., C.G.S., L.C.G., and K.L.G. analyzed data; H.L., R.A.S., J.A.O., and K.L.G. interpreted results of experiments; H.L. and K.L.G. prepared figures; H.L. and K.L.G. drafted manuscript; H.L., C.G.S., M.J.D.B., A.L.W., K.L.K., L.C.G., R.A.S., J.A.O., and K.L.G. edited and revised manuscript; H.L., C.G.S., M.J.D.B., A.M.P., G.K.H., H.H., D.S.H., A.L.W., K.L.K., L.C.G., R.A.S., J.A.O., and K.L.G. approved final version of manuscript; M.J.D.B., R.A.S., J.A.O., and K.L.G. conception and design of research.
ACKNOWLEDGMENTS
We thank the staff of Laboratory Animal Service for their excellence in animal care.
Present address of M. J. De Blasio: Baker IDI Heart and Diabetes Institute, Melbourne, Victoria, Australia.
REFERENCES
- 1.Albertsson-Wikland K, Boguszewski M, Karlberg J. Children born small-for-gestational age: postnatal growth and hormonal status. Horm Res 49, Suppl 2: 7–13, 1998. [PubMed] [Google Scholar]
- 2.Alexander G. Studies on the placenta of the sheep (Ovis aries L.). Effect of surgical reduction in the number of caruncles. J Reprod Fertil 7: 307–322, 1964. [DOI] [PubMed] [Google Scholar]
- 3.Alexander G. Studies on the placenta of the sheep (Ovis aries L.). Placental size. J Reprod Fertil 7: 289–305, 1964. [DOI] [PubMed] [Google Scholar]
- 4.Bassett JM. Glucagon, insulin and glucose homeostasis in the fetal lamb. Ann Rech Vet 8: 362–373, 1977. [PubMed] [Google Scholar]
- 5.Bastien M, Poirier P, Lemieux I, Després JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis 56: 369–381, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther 5: 1003–1015, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho CP, Martins JC, da Cunha DA, Boschero AC, Collares-Buzato CB. Histomorphology and ultrastructure of pancreatic islet tissue during in vivo maturation of rat pancreas. Ann Anat 188: 221–234, 2006. [DOI] [PubMed] [Google Scholar]
- 8.De Blasio MJ, Blache D, Gatford KL, Robinson JS, Owens JA. Placental restriction increases adipose leptin gene expression and plasma leptin and alters their relationship to feeding activity in the young lamb. Pediatr Res 67: 603–608, 2010. [DOI] [PubMed] [Google Scholar]
- 9.De Blasio MJ, Gatford KL, Harland ML, Robinson JS, Owens JA. Placental restriction reduces insulin sensitivity and expression of insulin signaling and glucose transporter genes in skeletal muscle, but not liver, in young sheep. Endocrinology 153: 2142–2151, 2012. [DOI] [PubMed] [Google Scholar]
- 10.De Blasio MJ, Gatford KL, McMillen IC, Robinson JS, Owens JA. Placental restriction of fetal growth increases insulin action, growth, and adiposity in the young lamb. Endocrinology 148: 1350–1358, 2007. [DOI] [PubMed] [Google Scholar]
- 11.De Blasio MJ, Gatford KL, Robinson JS, Owens JA. Placental restriction of fetal growth reduces size at birth and alters postnatal growth, feeding activity, and adiposity in the young lamb. Am J Physiol Regul Integr Comp Physiol 292: R875–R886, 2007. [DOI] [PubMed] [Google Scholar]
- 12.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab Gastrointest Physiol 237: E214–E223, 1979. [DOI] [PubMed] [Google Scholar]
- 13.Eriksson M, Wallander MA, Krakau I, Wedel H, Svardsudd K. Birth weight and cardiovascular risk factors in a cohort followed until 80 years of age: the study of men born in 1913. J Intern Med 255: 236–246, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Flanagan DE, Moore VM, Godsland IF, Cockington RA, Robinson JS, Phillips DI. Fetal growth and the physiological control of glucose tolerance in adults: a minimal model analysis. Am J Physiol Endocrinol Metab 278: E700–E706, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Fowden AL. Effects of arginine and glucose on the release of insulin in the sheep fetus. J Endocrinol 85: 121–129, 1980. [DOI] [PubMed] [Google Scholar]
- 16.Gatford KL, De Blasio MJ, How TA, Harland ML, Summers-Pearce BL, Owens JA. Testing the plasticity of insulin secretion and β-cell function in vivo: responses to chronic hyperglycaemia in the sheep. Exp Physiol 97: 663–675, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Gatford KL, De Blasio MJ, Thavaneswaran P, Robinson JS, McMillen IC, Owens JA. Postnatal ontogeny of glucose homeostasis and insulin action in sheep. Am J Physiol Endocrinol Metab 286: E1050–E1059, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Gatford KL, Mohammad SN, Harland ML, De Blasio MJ, Fowden AL, Robinson JS, Owens JA. Impaired β-cell function and inadequate compensatory increases in β-cell mass after intrauterine growth restriction in sheep. Endocrinology 149: 5118–5127, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Gatford KL, Sulaiman SA, Mohammad SN, De Blasio MJ, Harland ML, Simmons RA, Owens JA. Neonatal exendin-4 reduces growth, fat deposition and glucose tolerance during treatment in the intrauterine growth-restricted lamb. PLoS One 8: e56553, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greig NH, Holloway HW, De Ore KA, Jani D, Wang Y, Zhou J, Garant MJ, Egan JM. Once daily injection of exendin-4 to diabetic mice achieves long-term beneficial effects on blood glucose concentrations. Diabetologia 42: 45–50, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter PD. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 303: 1019–1022, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 87: 1409–1439, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Jaquet D, Gaboriau A, Czernichow P, Levy-Marchal C. Insulin resistance early in adulthood in subjects born with intrauterine growth retardation. J Clin Endocrinol Metab 85: 1401–1406, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Jensen CB, Storgaard H, Dela F, Holst JJ, Madsbad S, Vaag AA. Early differential defects of insulin secretion and action in 19-year-old Caucasian men who had low birth weight. Diabetes 51: 1271–1280, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Kaempf JW, Li HQ, Groothuis JR, Battaglia FC, Zerbe GO, Sparks JW. Galactose, glucose, and lactate concentrations in the portal venous and arterial circulations of newborn lambs after nursing. Pediatr Res 23: 598–602, 1988. [DOI] [PubMed] [Google Scholar]
- 26.Kassem SA, Ariel I, Thornton PS, Scheimberg I, Glaser B. Beta-cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes 49: 1325–1333, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Kjems LL, Volund A, Madsbad S. Quantification of beta-cell function during IVGTT in Type II and non-diabetic subjects: assessment of insulin secretion by mathematical methods. Diabetologia 44: 1339–1348, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346: 393–403, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamont BJ, Andrikopoulos S. Hope and fear for new classes of type 2 diabetes drugs: is there preclinical evidence that incretin-based therapies alter pancreatic morphology? J Endocrinol 221: T43–T61, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Larsen M, Kristensen NB. Precursors for liver gluconeogenesis in periparturient dairy cows. Animal 7: 1640–1650, 2013. [DOI] [PubMed] [Google Scholar]
- 31.Lithell HO, McKeigue PM, Berglund L, Mohsen R, Lithell UB, Leon DA. Relation of size at birth to non-insulin dependent diabetes and insulin concentrations in men aged 50–60 years. BMJ 312: 406–410, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long NM, George LA, Uthlaut AB, Smith DT, Nijland MJ, Nathanielsz PW, Ford SP. Maternal obesity and increased nutrient intake before and during gestation in the ewe results in altered growth, adiposity, and glucose tolerance in adult offspring. J Anim Sci 88: 3546–3553, 2010. [DOI] [PubMed] [Google Scholar]
- 33.Look AHEAD Research Group, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, Espeland MA, Evans M, Foreyt JP, Ghazarian S, Gregg EW, Harrison B, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montez MG, Murillo A, Nathan DM, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Reboussin D, Regensteiner JG, Rickman AD, Ryan DH, Safford M, Wadden TA, Wagenknecht LE, West DS, Williamson DF, Yanovski SZ. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 369: 145–154, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mericq V, Ong KK, Bazaes R, Pena V, Avila A, Salazar T, Soto N, Iniguez G, Dunger DB. Longitudinal changes in insulin sensitivity and secretion from birth to age three years in small- and appropriate-for-gestational-age children. Diabetologia 48: 2609–2614, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Muhlhausler BS, Ritorto V, Schultz C, Chatterton BE, Duffield JA, McMillen IC. Birth weight and gender determine expression of adipogenic, lipogenic and adipokine genes in perirenal adipose tissue in the young adult sheep. Domest Anim Endocrinol 35: 46–57, 2008. [DOI] [PubMed] [Google Scholar]
- 36.National Health and Medical Research Council. Australian Code for the Care and Use of Animals for Scientific Purposes (7th ed.) Canberra, Australia: National Health and Medical Research Council, 2004. [Google Scholar]
- 37.Newsome CA, Shiell AW, Fall CH, Phillips DI, Shier R, Law CM. Is birth weight related to later glucose and insulin metabolism?—A systematic review. Diabet Med 20: 339–348, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ 320: 967–971, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owens JA, Thavaneswaran P, De Blasio MJ, McMillen IC, Robinson JS, Gatford KL. Sex-specific effects of placental restriction on components of the metabolic syndrome in young adult sheep. Am J Physiol Endocrinol Metab 292: E1879–E1889, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Pinney SE, Jaeckle Santos LJ, Han Y, Stoffers DA, Simmons RA. Exendin-4 increases histone acetylase activity and reverses epigenetic modifications that silence Pdx1 in the intrauterine growth retarded rat. Diabetologia 54: 2606–2614, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piper K, Brickwood S, Turnpenny LW, Cameron IT, Ball SG, Wilson DI, Hanley NA. Beta cell differentiation during early human pancreas development. J Endocrinol 181: 11–23, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Pyke C, Heller RS, Kirk RK, Ørskov C, Reedtz-Runge S, Kaastrup P, Hvelplund A, Bardram L, Calatayud D, Bjerre Knudsen L. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 155: 1280–1290, 2014. [DOI] [PubMed] [Google Scholar]
- 43.Raab EL, Vuguin PM, Stoffers DA, Simmons RA. Neonatal exendin-4 treatment reduces oxidative stress and prevents hepatic insulin resistance in intrauterine growth-retarded rats. Am J Physiol Regul Integr Comp Physiol 297: R1785–R1794, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr 70: 811–816, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med 295: 349–353, 1976. [DOI] [PubMed] [Google Scholar]
- 46.Reddy S, Elliott RB. Ontogenic development of peptide hormones in the mammalian fetal pancreas. Experientia 44: 1–9, 1988. [DOI] [PubMed] [Google Scholar]
- 47.Robinson JS, Kingston EJ, Jones CT, Thorburn GD. Studies on experimental growth retardation in sheep. The effect of removal of endometrial caruncles on fetal size and metabolism. J Dev Physiol 1: 379–398, 1979. [PubMed] [Google Scholar]
- 48.Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S. Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology 138: 1736–1741, 1997. [DOI] [PubMed] [Google Scholar]
- 49.Seoane JR, Warner RG, Seoane NA. Heparin-induced lipolysis and feeding behavior in sheep. Physiol Behav 9: 419–422, 1972. [DOI] [PubMed] [Google Scholar]
- 50.Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes 50: 2279–2286, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Spedale SB, Battaglia FC, Sparks JW. Hepatic metabolism of glucose, galactose, and lactate after milk feeding in newborn lambs. Am J Physiol Endocrinol Metab 262: E46–E51, 1992. [DOI] [PubMed] [Google Scholar]
- 52.Stoffers DA, Desai BM, DeLeon DD, Simmons RA. Neonatal exendin-4 prevents the development of diabetes in the intrauterine growth retarded rat. Diabetes 52: 734–740, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Summermatter S, Mainieri D, Russell AP, Seydoux J, Montani JP, Buchala A, Solinas G, Dulloo AG. Thrifty metabolism that favors fat storage after caloric restriction: a role for skeletal muscle phosphatidylinositol-3-kinase activity and AMP-activated protein kinase. FASEB J 22: 774–785, 2008. [DOI] [PubMed] [Google Scholar]
- 54.Szayna M, Doyle ME, Betkey JA, Holloway HW, Spencer RG, Greig NH, Egan JM. Exendin-4 decelerates food intake, weight gain, and fat deposition in Zucker rats. Endocrinology 141: 1936–1941, 2000. [DOI] [PubMed] [Google Scholar]
- 55.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344: 1343–1350, 2001. [DOI] [PubMed] [Google Scholar]
- 56.Valdez R, Athens MA, Thompson GH, Bradshaw BS, Stern MP. Birthweight and adult health outcomes in a biethnic population in the USA. Diabetologia 37: 624–631, 1994. [DOI] [PubMed] [Google Scholar]
- 57.van Bloemendaal L, Ten Kulve JS, la Fleur SE, Ijzerman RG, Diamant M. Effects of glucagon-like peptide 1 on appetite and body weight: focus on the CNS. J Endocrinol 221: T1–T16, 2014. [DOI] [PubMed] [Google Scholar]
- 58.van der Linden DS, Sciascia Q, Sales F, McCoard SA. Placental nutrient transport is affected by pregnancy rank in sheep. J Anim Sci 91: 644–653, 2013. [DOI] [PubMed] [Google Scholar]
- 59.Willes RF, Boda JM, Stokes H. Cytological localization of insulin and insulin concentration in the fetal ovine pancreas. Endocrinology 84: 671–675, 1969. [DOI] [PubMed] [Google Scholar]
- 60.Wooldridge AL, Bischof RJ, Meeusen EN, Liu H, Heinemann GK, Hunter DS, Giles LC, Kind KL, Owens JA, Clifton VL, Gatford KL. Placental restriction of fetal growth reduces cutaneous responses to antigen after sensitization in sheep. Am J Physiol Regul Integr Comp Physiol 306: R441–R446, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both β-cell replication and neogenesis, resulting in increased β-cell mass and improved glucose tolerance in diabetic rats. Diabetes 48: 2270–2276, 1999. [DOI] [PubMed] [Google Scholar]
- 62.Zar JH. Biostatistical Analysis. Englewood Cliffs, NJ: Prentice-Hall, 1974. [Google Scholar]


