Deletion of Rad-GTPase results in a novel adaptive cardiac phenotype that includes stable increased cardiac output and preserved function that is maintained in aged mice. Mechanistically, Rad improves Ca2+ homeostasis. These studies suggest that Rad deletion is a potential therapeutic approach for maintaining cardiac function in aging and perhaps disease.
Keywords: cardiac hypertrophy, calcium signaling, echocardiography, genetically modified mice
Abstract
Rad-GTPase is a regulator of L-type calcium current (LTCC), with increased calcium current observed in Rad knockout models. While mouse models that result in elevated LTCC have been associated with heart failure, our laboratory and others observe a hypercontractile phenotype with enhanced calcium homeostasis in Rad−/−. It is currently unclear whether this observation represents an early time point in a decompensatory progression towards heart failure or whether Rad loss drives a novel phenotype with stable enhanced function. We test the hypothesis that Rad−/− drives a stable nonfailing hypercontractile phenotype in adult hearts, and we examine compensatory regulation of sarcoplasmic reticulum (SR) loading and protein changes. Heart function was measured in vivo with echocardiography. In vivo heart function was significantly improved in adult Rad−/− hearts compared with wild type. Heart wall dimensions were significantly increased, while heart size was decreased, and cardiac output was not changed. Cardiac function was maintained through 18 mo of age with no decompensation. SR releasable Ca2+ was increased in isolated Rad−/− ventricular myocytes. Higher Ca2+ load was accompanied by sarco/endoplasmic reticulum Ca2+ ATPase 2a (SERCA2a) protein elevation as determined by immunoblotting and a rightward shift in the thapsigargan inhibitor-response curve. Rad−/− promotes morphological changes accompanied by a stable increase in contractility with aging and preserved cardiac output. The Rad−/− phenotype is marked by enhanced systolic and diastolic function with increased SR uptake, which is consistent with a model that does not progress into heart failure.
NEW & NOTEWORTHY
Deletion of Rad-GTPase results in a novel adaptive cardiac phenotype that includes stable increased cardiac output and preserved function that is maintained in aged mice. Mechanistically, Rad improves Ca2+ homeostasis. These studies suggest that Rad deletion is a potential therapeutic approach for maintaining cardiac function in aging and perhaps disease.
disruptions to calcium cycling contribute to compromised function during heart failure progression. In response to pressure overload, L-type calcium channel (LTCC) single-channel current is increased (32), and sarcolemmal channel expression decreases (6). Impaired calcium homeostasis is further attributed to changes in the expression of calcium-handling proteins in the sarcoplasmic reticulum (SR) membrane, leading to reduction of SR Ca2+ load. In particular, recent work has focused on modulation of the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA2) and SERCA-inhibitory proteins (16, 21, 30).
Rad is a member of the RGK GTPase family (including Rem, Gem, and Kir) that regulates current though the LTCC. Rad deletion or RNAi-mediated knockdown results in an increase in LTCC current (3, 23, 34). Rad protein is downregulated in the myocardium of failing human hearts (3) suggesting that Rad loss may be an integral signaling component in myocardial adaptation. Our more recent studies have demonstrated that Rad deletion enhances systolic function at the cellular and the whole organ level (23).
Here, we test the specific hypothesis that the absence of Rad-GTPase does not accompany a loss of cardiac function in vivo. Rather, we report a novel, beneficial phenotype characterized by stable morphological changes and sustained elevation of function, without decompensation or contractile failure.
METHODS
Mouse housing and generation.
Global RRAD null mice (Rad−/−) were generated as previously described (23). We were unable to use littermates as a control because of incomplete record keeping involving the strategy utilized in the generation of the original Rad knockout. Therefore age-matched in-house bred C57/Bl6 mice served as controls. Male mice ages 4, 10, 18, or 21 mo were evaluated. The experimental procedures and methods used were approved by the Animal Care and Use Committee of the University of Kentucky and conformed to the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Quantitative RT-PCR.
Male mice were anesthetized with ketamine and xylazine and hearts were quickly excised, after which the apex of the left ventricle (LV) was removed and snap frozen in liquid nitrogen. Frozen tissue was then homogenized, and RNA was isolated using RNAqueous kit (Life Technologies) and quantified using a Nanodrop (ThermoScientific). cDNA was generated from 500 ng RNA, which was then amplified via RT-PCR using Taqman probes from Life Technologies: gapdh (Mm99999915_g1), nppa (Mm01255747_g1), nppb (Mm01255770_g1), collagen isoform col3a1 (Mm01254476_m1), and Primetime probes from IDT: ppp3bc, calcineurin (Mm.PT.56a.11865879), and calcineurin-responsive rcan1 (Mm.PT.56a.41844729.g). Threshold values (CT) for nppa, nppb, col3a1, ppp3bc, and rcan1 were normalized by subtraction from gapdh, and wild type was then subtracted from Rad−/− (ΔΔCT) and fold changes were calculated as 2−ΔΔCT.
Echocardiography.
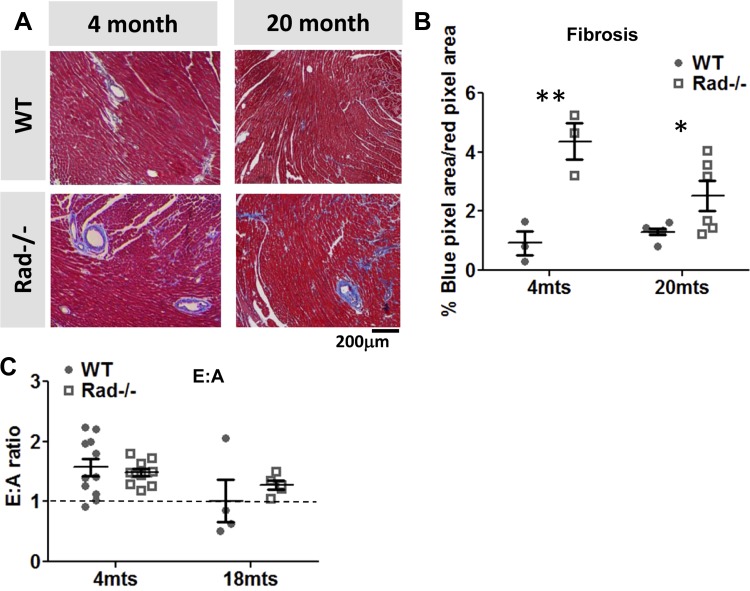
Echocardiography was conducted using a Vevo2100 high-frequency ultrasound (VisualSonics). Mice were anesthetized with isoflurane. Heart rate was monitored using surface ECG, and temperature was controlled at 37°C. A MS550D probe was used to acquire M-mode and B-mode image series along the long, short, and apical axes of the LV. Mitral valve Doppler imaging was used to determine the E:A ratio (33), which was taken as an indication for diastolic stiffness. Doppler mitral inflow pattern was acquired, and the two peaks indicating diastolic inflow were identified, followed by a negative outflow during systole. The early peak “E” was taken as a measure of passive filling, while the later peak “A” was taken as a measure of inflow due to atrial contraction. The ratio of the former to latter was measured, and an E value higher than A (i.e., E:A ratio is greater than 1) indicates normal passive stiffness. An A value higher than E (i.e., E:A ratio is less than 1) indicates elevated stiffness. Main pulmonary artery (MPA) flow imaging was done as a measure of cardiac output. Pulmonary flow is the product of the main pulmonary area times the velocity time integral of the pulsed wave Doppler of the pulmonary flow. The analysis was done using Vevo2100 software v1.4.
Adult ventricular myocyte isolation.
LV myocytes from Rad−/− and wild-type hearts were isolated as previously described (23). Briefly, mice were anesthetized with ketamine and xylazine. Hearts were excised and subjected to retrograde perfusion on a Langendorff apparatus using a modified Tyrode's solution. Five to seven milligrams of Liberase (Roche) were dissolved in perfusion buffer to digest extracellular matrix, after which the LV was dissected from the rest of the heart and was pulled apart with forceps. Digestion was stopped in 10% fetal bovine serum. Healthy cells were resuspended in stop buffer, and the calcium concentration was gradually increased in a step-wise fashion to 1 mM. Before calcium elevation, an aliquot of cells was removed and fixed in 4% paraformaldehyde.
Histology.
Mice were anesthetized as described above, and hearts were excised and perfused with saline followed by 10% formalin buffered in PBS. These hearts were cut along the short axis to reveal papillary muscles, embedded in paraffin, and cut into 5-μm sections. Sections were then deparaffinized, hydrated, and stained with Masson's trichrome. Embedding, sectioning, and staining were completed with the help of the Department of Molecular Pathology at the University of Texas, Southwest. Stained sections were then photographed at ×4. Images were analyzed using ImageJ for the ratio of blue pixels to red pixels meeting an intensity threshold, and this ratio was used to give a percent fibrosis value.
Myocyte size measurements.
Fixed myocytes were permeabilized with 1% Triton X-100, and nonselective binding was blocked with incubation with 10% BSA. Cells were then incubated with mouse anti-α-actinin (Sigma), followed by Alexa-496 conjugated anti-mouse secondary antibody. This resulted in a characteristic striation pattern that was used to visually identify myocytes. Fluorescent images of stained cells were collected using Nikon NIS-Elements, and area was measured by selecting myocytes and adjusting an intensity threshold until the entire cell was highlighted. Area was calculated automatically with Nikon NIS-Elements.
Analysis of global calcium transients.
Ventricular myocytes were isolated as described (23), loaded with cell permeable fura-2AM at room temperature for 5 min, washed, and resuspended in physiological saline solution (PSS) containing 1.8 mM calcium. Myocytes were then field stimulated at 2 Hz, and the F340/380 ratio was measured as an indicator of changes in cytosolic calcium concentration using Ion Wizard (IonOptix). For SR load evaluation, caffeine-induced calcium release measurements were performed by initially pacing cardiomyocytes for >60 s at 2 Hz to attain steady state. Caffeine (50 mM) dissolved in PSS was rapidly administered 1 s after stimulus was halted. For caffeine-induced Ca2+ decay constant measurements, bath calcium was reduced to 0 for 20 s before caffeine puff to eliminate contamination of the calcium signal decay by LTCC current. Thapsigargan dose-response curves were generated by preincubating isolated cells in increasing concentrations of thapsigargan (1.0e-10, 1.0e-9, and 1.0e-8) for at least 10 min before recording.
Spark measurement.
Ventricular myocytes were isolated as described above. Cells were loaded with cell-permeable Fluo-4 at room temperature and paced at 3 Hz to steady state and then stopped to measure sparks on a Live 5 (Zeiss) live cell scanning microscope. Line scans were acquired with a pixel size of 0.13 μm/pixel and a temporal resolution of 1 ms/line. Sparks were detected using Sparkmaster on ImageJ.
Western blotting.
Hearts were homogenized by abrasive media (1.0 mm zirconium oxide beads) in buffer (20 mM Tris·HCl pH 7.5, 250 mM sodium chloride, 10 mM magnesium chloride, 1% Triton X-100, 1 mM sodium vanadate, 50 mM β-glycerophosphate, 1× protease inhibitor cocktail 1; Calbiochem no. 539131), and 0.5 mM DTT using a Next Advance Bullet Blender at 4°C. Samples were heated to 55°C for 15 min or 95°C for 5 min (for phospholamban blots) before running on SDS-PAGE gels. Proteins were transferred to nitrocellulose membranes for 16 h at low current (0.08 mA). Membranes were blocked with casein before application of primary antibodies (mouse anti-phospholamban, Thermo Scientific; rabbit anti-SERCA 2a, Badrilla; rabbit anti-Ryanodine receptor, Santa Cruz Biotechnology; and rabbit anti-calsequestrin, Abcam). Antibodies for Rad GTPase were generated in house. Additional antibody source detail is presented in Table 1. Horseradish peroxidase-conjugated secondary antibodies (goat anti-rabbit, Invitrogen; and goat anti-mouse, Jackson ImmunoReasearch) were detected with Hyglo chemiluminescent reagent (Denville Scientific), and blots were visualized on a ChemiDoc MP (Bio-Rad). Protein bands were analyzed with Image Lab software (Bio-Rad), and values are expressed relative to the wild-type mean.
Table 1.
Antibodies used in Western blotting
| Antibody | Supplier | Catalog No. | Dilution |
|---|---|---|---|
| Mouse anti-phospholamban | Thermo Scientific | MA3-922 | 1:5,000 |
| Rabbit anti-SERCA2a | Badrilla | A010-20 | 1:500 |
| Rabbit anti-ryanodine receptor | Santa Cruz Biotechnology | ac-13942 | 1:200 |
| Rabbit anti-calsequestrin | Abcam | ab3516 | 1:500 |
| Rabbit anti-CaV1.2 | Alomone Labs | acc-003 | 1:500 |
| Rabbit anti-GAPDH | Cell Signaling Technology | 5174 | 1:1,000 |
| Goat anti-rabbit | Invitrogen | 65-6120 | 1:20,000 |
| Goat anti-mouse | Jackson ImmunoResearch | 115-035-068 | 1:20,000 |
SERCA2a, sarco/endoplasmic reticulum Ca2+ ATPase 2a.
Statistical analysis.
Significance was determined by Student's t-test when comparing wild-type and Rad−/− cells or echocardiography-derived parameters. One-way ANOVA was used when two or more variables were compared, as in RT-PCR analysis, and two-way ANOVA was used to compare wild-type and Rad−/− echocardiographic measurements in young and aged mice. Nonlinear regression was used to fit thapsigargan dose-response values. P < 0.05 was considered statistically significant.
RESULTS
Rad loss results in novel cardiac muscle morphology.
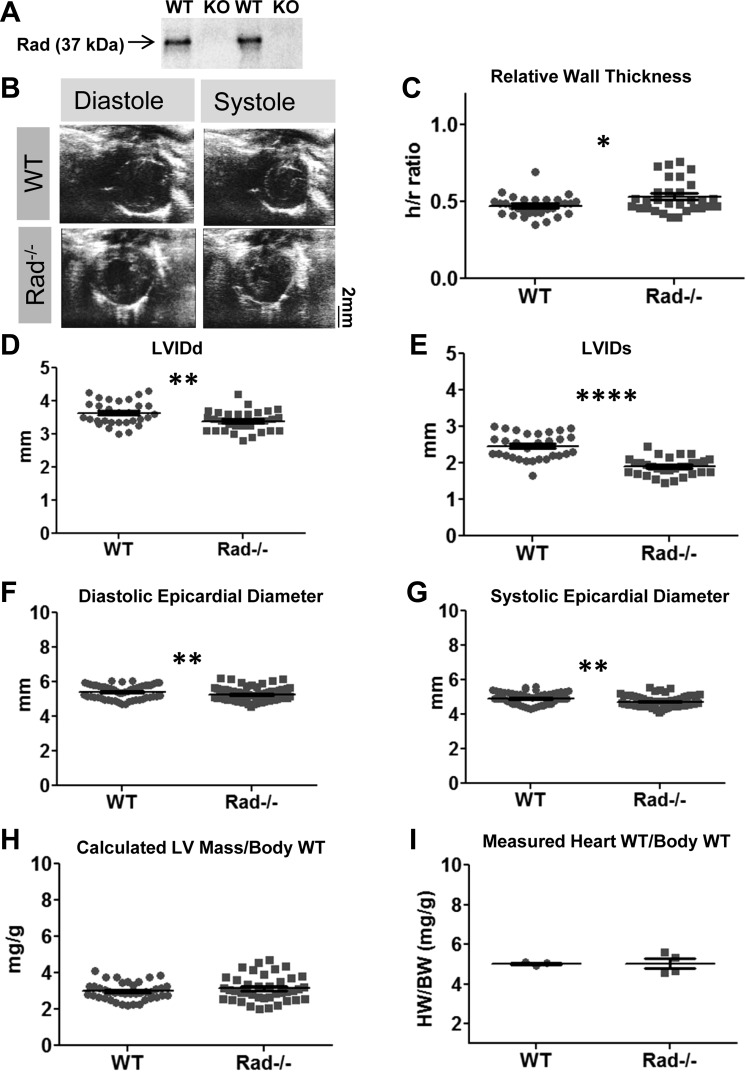
Rad protein loss was demonstrated in the Rad−/− myocardium by immunoblotting (Fig. 1A) Echocardiography revealed that Rad−/− hearts exhibited thicker walls relative to the ventricular lumen inner dimension (h/r ratio; Fig. 1, B and 1C). Rad−/− ventricular lumen was decreased at diastole and systole (Fig. 1, D and E), as was epicardial diameter (Fig. 1, F and G), suggesting that Rad−/− hearts are smaller with thicker walls. Calculated ventricle weight normalized to body weight was unchanged (Fig. 1H), which was matched by observed heart weights normalized to body weight (Fig. 1I). Normalizing heart weight to tibia length did not reveal a difference either (Fig. 1H; HW/TL: wild type, 9.65 ± 0.36; Rad−/− 9.10 ± 0.42).
Fig. 1.
Rad deletion alters cardiac morphology. A: representative immunoblot demonstrating the loss of Rad protein; n = 3 mice for each genotype. KO, knockout; WT, wild type. B: representative echocardiographs of WT and Rad−/− at diastole and systole. C: relative wall thickness (h/r ratio) of WT and Rad−/− left ventricles (LVs). D and E: LV inner dimension (LVID) is reduced in Rad−/− at both diastole (LVIDd; D) and systole (LVIDs; E). F and G: epicardial diameter is similarly reduced in Rad−/− at both diastole (F) and systole (G). H and I: calculated LV mass normalized to body weight (H) and observed heart weight/body weight measurements are not different in Rad−/− mice compared with WT (I); n = 30 mice per genotype for all echocardiographic measures and 3–4 mice per genotype for heart weight/body weight measures. *P < 0.05, **P < 0.01, ****P < 0.0001 vs. WT.
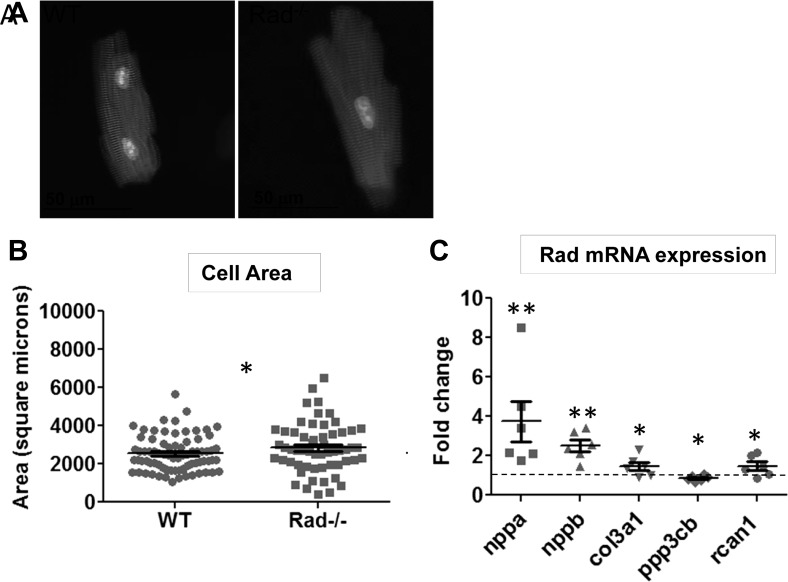
The measured area of isolated ventricular myocytes in Rad−/− is greater than that of wild type (Fig. 2, A and B). These alterations in myocyte dimensions motivated evaluation of the expression of the canonical fetal gene program associated with cardiac hypertrophy. nppa, nbbp, And col3a1 are significantly increased in expression in Rad−/− compared with wild-type hearts (Fig. 2C). Additionally, the expression of rcan1 (regulator of calcineurin 1) was evaluated to investigate a possible connection between the reported increased diastolic calcium in Rad−/− (23) and the calcium-responsive calcineurin/NFAT hypertrophic pathway. A similar increase in the expression of this gene was observed, with a corresponding decrease of ppp3cb (calcineurin; Fig. 2C). These data suggest that Rad deletion produces structural changes in the shape and size of the heart that are distinct from pathological hypertrophic remodeling.
Fig. 2.
Rad deletion increases cell size and upregulates fetal and growth markers. A: representative isolated ventricular myocytes stained for α-actinin (red) and nucleus (blue). B: an increase in area is observed in myocytes isolated from Rad−/− hearts; n = 3 hearts. *P < 0.05 vs. WT. C: upregulation of hypertrophic markers nppa (ANF), nppb (BNP), collagen isoform col3a1, pp3cb (calcineurin), and rcan1 (MCIP) in Rad−/− hearts; n = 6 hearts. *P < 0.05, **P < 0.01 vs. WT.
Rad null mice exhibit greater contractility in vivo.
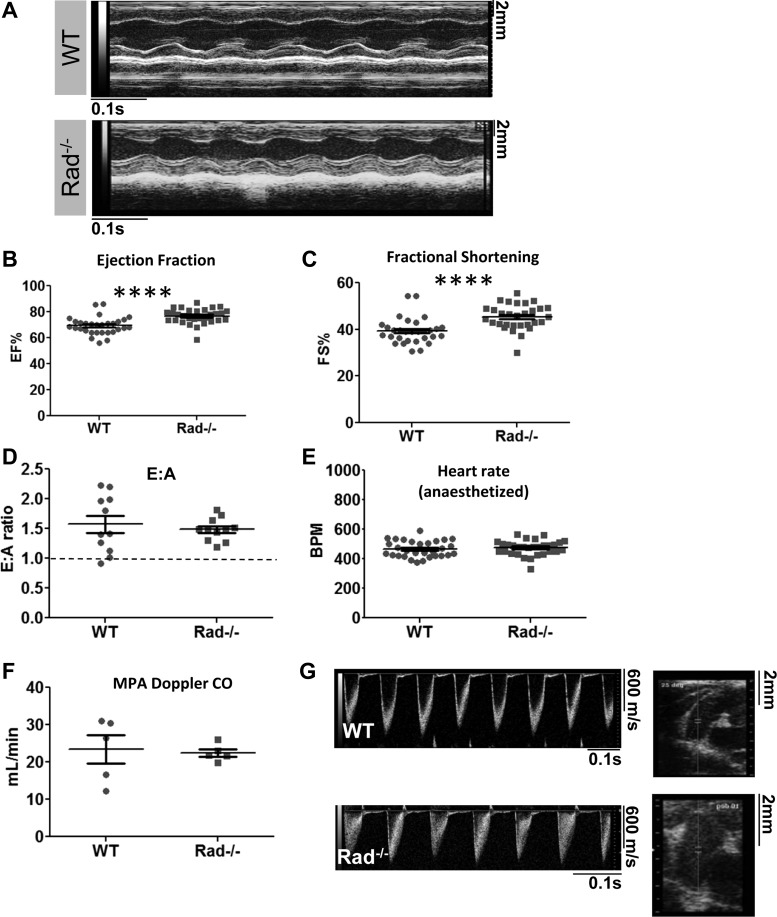
Changes in Rad−/− heart morphology were accompanied by significantly increased LV function (Fig. 3A), as expected from previously published data examining the function of individual cells and isolated working hearts (23). Rad−/− hearts displayed improved ejection fraction (Fig. 3B) and fractional shortening (Fig. 3C). Diastolic function was also measured because previous results showed that diastolic calcium and fibrosis are increased in Rad−/− (3, 23). E:A ratios were significantly above 1 for both wild-type and Rad−/− mice, suggesting that diastolic function is not impaired (Fig. 3D). To demonstrate that these differences are not due to changes in intrinsic heart rate reported previously (23), heart rate was titrated with anesthesia to similar levels for all recordings (Fig. 3E). MPA imaging revealed that cardiac output was no different between Rad−/− and wild type (Fig. 3F), suggesting that the hypercontractile state observed in Rad−/− hearts is sufficient to maintain stable output despite a smaller LV lumen. Bright, relatively cohesive traces indicate laminar flow in wild type (Fig. 3G, top left). Diffuse, grey shading is observed in the Rad−/−, consistent with turbulence from ejecting blood faster through a fixed diameter MPA (Fig. 3G, bottom left).
Fig. 3.
Rad deletion improves contractile function. A: representative M-mode traces for WT and Rad−/− 4-mo-old mice. B and C: ejection fraction (EF; B) and fractional shortening (C) are elevated in Rad−/− hearts; n = 30 mice per genotype. D: diastology is not significantly different between WT and Rad−/−; n = 9–10 mice. E:A, early-to-late peak ratio. E: heart rate of anesthetized mice is maintained at a constant rate for both genotypes. F: cardiac output as measured by main pulmonary artery (MPA) imaging is not significantly different between WT and Rad−/−. G: representative MPA cardiac output Doppler traces. ****P < 0.0001 vs. WT.
Improved function is preserved in aged Rad−/− mice.
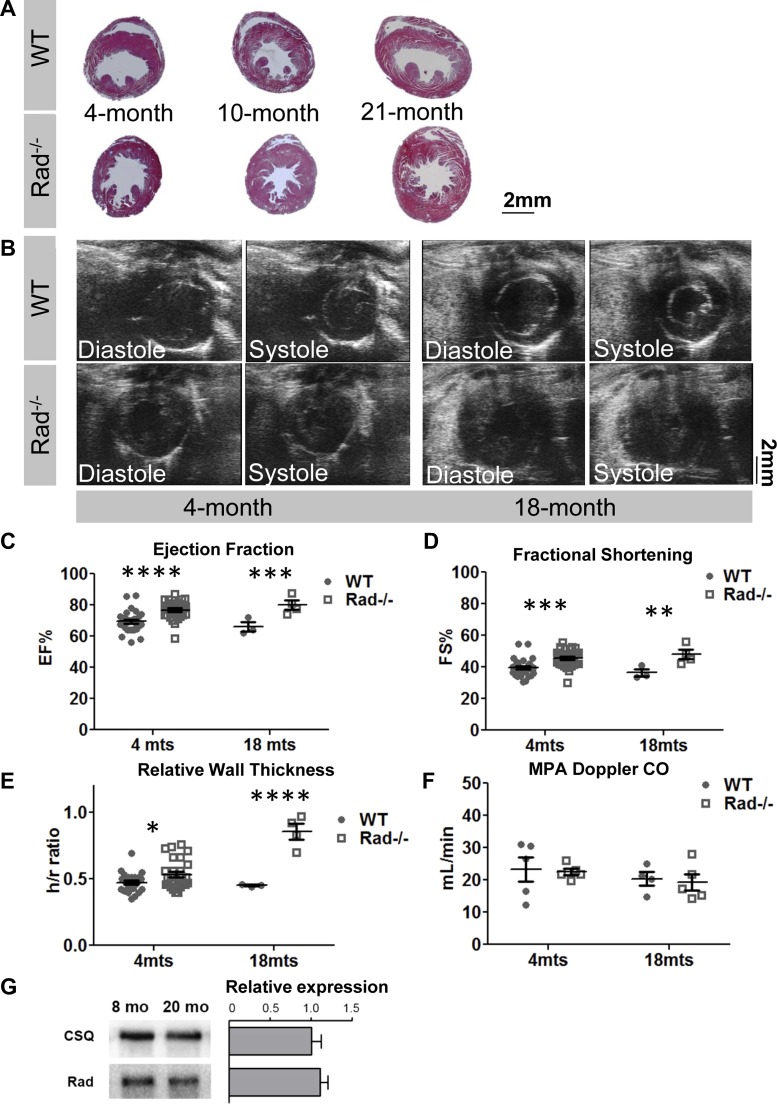
Enhanced Ca2+-entry acutely increases function, but overexpression of LTCC channels has been found in other models to lead to the development of pathological hypertrophy over a time frame of ∼8 mo (27). We therefore allowed a cohort of wild-type and Rad−/− mice to age into senescence. At the advanced age of 18 mo, histological sections and echocardiography showed no evidence of wall thinning in Rad−/− hearts (Fig. 4, A and B). Senescent LV function was significantly greater in Rad−/− compared with wild type (Fig. 4, B–D). Relative wall thickness (h/r ratio) was significantly increased in Rad−/− with aging but not wild type (Fig. 4E). MPA imaging indicated that cardiac output was maintained in Rad−/− mice, suggesting a stable phenotype with hyperdynamic LV function (Fig. 4F). Finally, immunoblotting established that Rad protein expression remained unchanged in senescent wild-type mice (Fig. 4G).
Fig. 4.
Aged Rad−/− hearts exhibit enhanced function with progressive LV wall thickening. A: representative images of Masson's trichome-stained sections showing increasing wall thickness in Rad−/− from 4, 8, and 21 mo. B: representative B-mode images from 4- and 18-mo-old mice. C and D: ejection fraction and fractional shortening are elevated in young and aged Rad−/−. E: relative wall thickness (h/r) is progressively higher in Rad−/−; n = 10–13 4-mo and 3–4 18-mo mice. *P < 0.05, **P < 0.01, ***P < 0.001,****P < 0.0001 vs. WT. F: cardiac output remains unchanged in WT and Rad−/− mice; n = 4–5 mice per genotype. G: Rad expression is not changed in old age. Data represented as expression in 20-mo-old relative to 8-mo-old mice. Calsequestrin (CSQ) is shown as a loading control; n = 3 mice per genotype.
Previously published data suggested that fibrosis was increased in Rad−/− mice compared with wild type, so we next evaluated whether this is altered in old age. We confirmed that fibrosis is increased in Rad−/−, and we have further found that this increase is maintained at 18 mo (Fig. 5, A and B). However, E:A ratios do not show a difference between Rad−/− or wild type in either young or aged animals, suggesting that this increase in fibrosis had no detectable effect on myocardial stiffness (Fig. 5C). Further measures of diastology using tissue Doppler (young mice: e' = −19.8 ± 0.9 for WT, −20.8 ± 4.0 for Rad−/−; aged mice: e' = −17.2 ± 3.4 for WT, −20.2 ± 5.2 for Rad−/−) were similarly unchanged.
Fig. 5.
Young and aged Rad−/− hearts show increased fibrosis, without an observable effect on myocardial stiffness A: representative images from of Masson's trichome-stained sections showing increasing fibrosis in Rad−/− from 4 and 20 mo. B: significantly more fibrosis is measured in trichrome stained hearts from Rad−/− compared with WT in both young and aged mice. C: E:A ratio is unchanged and remains above 1 for both WT and Rad−/− in old age. *P < 0.05, **P < 0.01 vs. WT; n = 3–6 mice.
SR load is increased in Rad−/− ventricular myocytes.
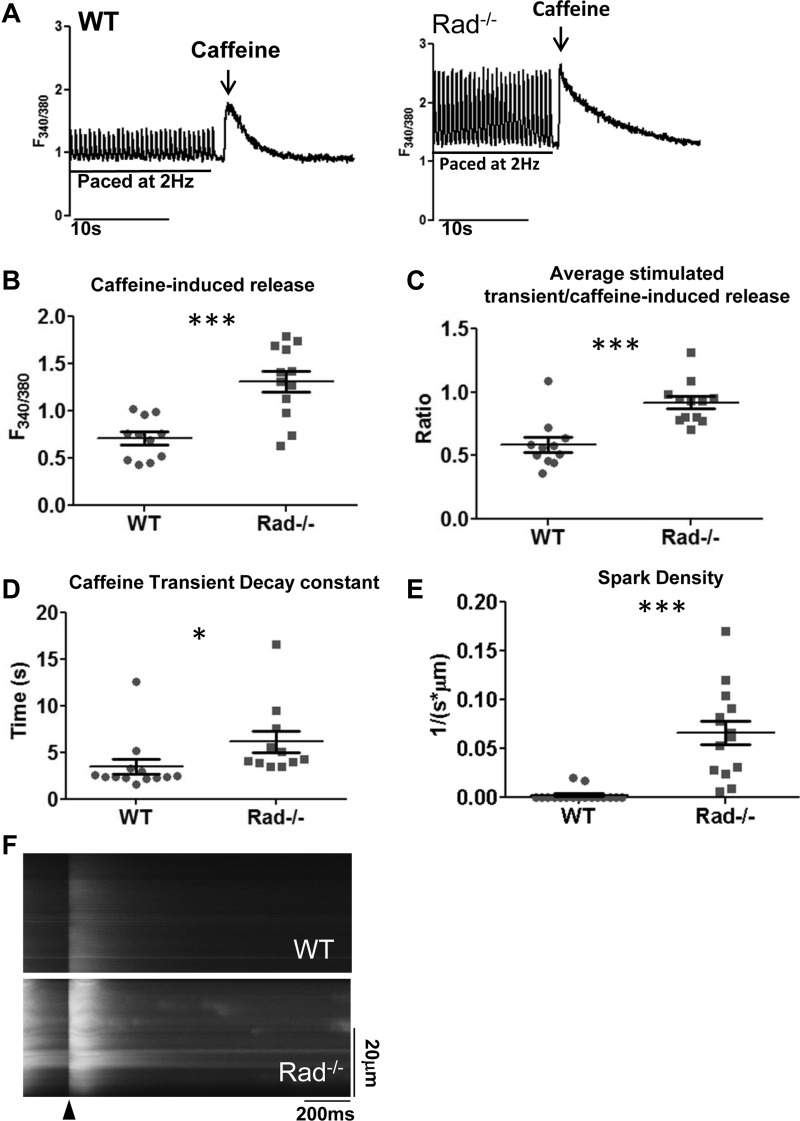
Rad−/− myocardium has increased calcium current density (23). Dogma dictates that chronic increased LTCC activity promotes heart failure. Thus a Rad−/− phenotype characterized by enhanced heart function despite persistently increased LTCC function well into old age was unexpected (26, 27). To investigate a potential mechanism by which Rad−/− hearts avoid heart failure, we examined the SR Ca2+ load. SERCA downregulation contributes to impaired SR Ca2+ loading in heart failure (14), and rescue of SERCA expression and SR loading improves heart function (17, 24). To determine the impact of Rad-deletion on SR Ca2+ load, caffeine-inducible Ca2+-release was measured from cells after pacing to steady state (Fig. 6A). In ventricular myocytes from Rad−/− hearts, the caffeine-inducible peak was increased (Fig. 6B), as was the percentage of Ca2+ available in the SR that was released during each stimulus (Fig. 6C). Thus both SR load and the extent of SR Ca2+ release were increased in Rad−/− myocytes. Interestingly, the decay constant of the caffeine-induced transient was slower in Rad−/− myocytes (Fig. 6D), suggesting that enhanced calcium extrusion from the cell by the sodium calcium exchanger (NCX) is not necessarily triggered by elevated Ca2+ influx. Given that increased SR load is associated with increased leak (4), we next measured spark release as measure of calcium release during diastole. As expected, more sparks were observed in Rad−/− cells than WT (Fig. 6, E and F).
Fig. 6.
Sarcoplasmic reticulum (SR) load is elevated in Rad−/−. A: representative paced and caffeine-stimulated Ca2+ transients from WT and Rad−/−. B and C: caffeine-induced Ca2+-release amplitude (B) and the ratio of stimulated calcium release to total caffeine-induced SR Ca2+ release (C) was greater in Rad−/− compared with WT. D: caffeine decay constant (tau, τ) is significantly prolonged in Ca2+-free media. E: spark frequency is increased in Rad−/− compared with WT. F: representative line scan showing stimulus (arrowhead) and sparks measured after a >e-fold decay of the global Ca2+ transient in WT and Rad−/−; n = 3 mice per genotype. *P < 0.05, ***P < 0.001 vs. WT.
SERCA protein is elevated in Rad−/− hearts.
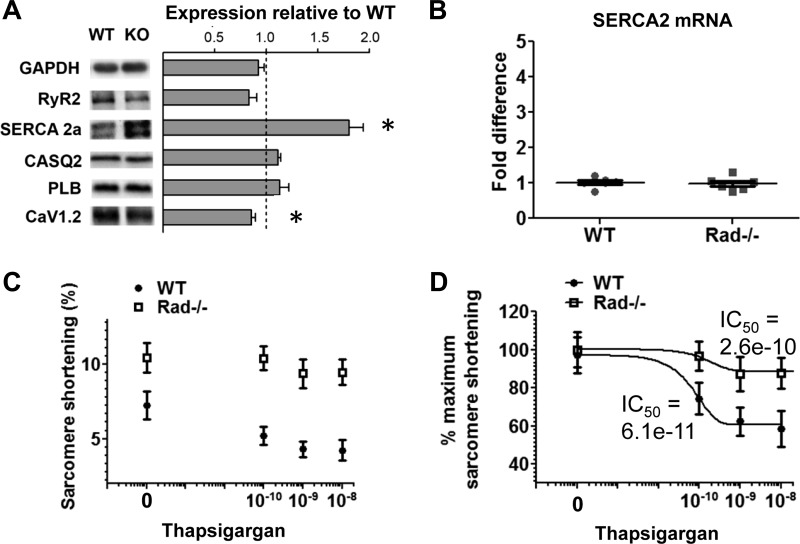
Immunoblotting Rad−/− hearts previously showed changes in the phosphorylation state of phospholamban and calcium/calmodulin-dependent protein kinase II (CaMKII) (20). This motivated the evaluation of the expression of other calcium handling proteins. Immunoblotting shows that SERCA 2a protein is significantly upregulated in Rad−/− hearts (Fig. 7A), although the absence of a difference in mRNA suggests that SERCA is not subject to differential transcriptional control in Rad−/− (Fig. 7B). Calsequestrin 2, RyR2, and phospholamban protein levels are unchanged, and CaV1.2 protein is decreased (Fig. 7A). To determine whether SERCA2 protein levels are tied to the increased function observed in Rad−/− hearts, a concentration-response curve to the SERCA inhibitor thapsigargan was generated (Fig. 7, C and D). As previously described (20), thapsigargan reduced contractility by ∼50% in wild-type ventricular myocytes with an IC50 of 61 pM. However, the response of Rad−/− cardiomyocytes was both attenuated and shifted rightward (IC50: 260 pM), indicating that more SERCA2 is present. Moreover, a thapsigargan-insensitive component may contribute to increased sarcomere shortening in Rad−/− cells, consistent with increased SERCA protein increasing SR loading and in conjunction with increased LTCC current, summing to improved cardiac function.
Fig. 7.
Sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) is increased in Rad−/− hearts. A: SERCA 2a protein is significantly upregulated, while there is no change in protein levels of ryanodine receptors (RyR2), calsequestrin 2 (CSQ2), or phospholamban (PLB). CaV1.2 is slightly but significantly downregulated. Approximate molecular weights of bands as shown: RyR2: ∼550 kDa; CaV1.2: ∼200 kDa; SERCA2a: ∼100 kDa,; CASQ2: ∼55 kDa; GAPDH: ∼37 kDa; PLB: ∼10 kDa; n = 3–4 mice. *P < 0.05. B: SERCA 2 mRNA is not significantly upregulated in Rad−/− hearts. C and D: inhibitor-response curves of thapsigargan-induced depression of sarcomere shortening (C) and concentration response normalized to maximal function (D) is shifted rightward in Rad−/− myocytes; n = 3 mice per genotype, 30 cells per concentration.
DISCUSSION
The present study of Rad−/− mice characterizes a novel hypercontractile phenotype that is preserved during aging. Rad−/− mice exhibit smaller hearts with increased heart wall thickness and larger cardiomyocytes. However, cardiac remodeling in Rad−/− mice is not hypertrophic, as cardiac mass does not differ between wild-type and Rad−/− hearts. These hearts remain hypercontractile in aged mice, suggesting that their increased systolic and diastolic function is stable and sustainable. This improved function is associated with increased SR load and upregulation of SERCA2a.
As our laboratory and others have demonstrated, the loss of Rad increases LTCC current (ICaL) and calcium transient amplitude, with an associated increase in systolic function (23, 34). We also observe a decrease in CaV1.2 protein, which is the expected result of an increase in LTCC activity due to the loss of a negative current regulator (31). Conversely, all RGK family members block LTCC activity (8–12, 22, 29). Opposite approaches that rely on overexpression models have shown profound block of ICaL. Overexpression of RGK protein Gem, for example, reduces transient amplitude and function, which supports the findings that RGK proteins like Rad act as negative regulators of function (25). Rad−/− mouse hearts mimic several characteristics of young mice overexpressing the α-subunit of the LTCC, including increased ICaL current density, a hyperpolarizing shift in LTCC activation potential, reduced sensitivity to adrenergic stimulation, increased contractility, and heart wall growth (23, 27). However, LTCC-overexpressing mice progress to decompensation after 8 mo (26). Importantly, increased function and preserved calcium handling persist in Rad−/− hearts long after this point (Fig. 4), and significant mortality was not exhibited in Rad−/−, even after 1.5 yr. It should also be noted that both the α-subunit overexpressing model and Rad−/− model appear to be distinct from genetic modifications that target expression of the β-subunit of the LTCC directly, such as β2a-overexpression or knockdown, which results in severe heart failure (5) or protection from transverse aortic constriction-induced hypertrophy (7), respectively. Rather, Rad deletion seems to produce a sustained stable compensatory phenotype. This appears to occur despite gradual and continuous wall thickening that occurs into old age. These studies assess global Rad loss; therefore, we cannot exclude an effect of Rad loss in noncardiomyocytes and the potential long-term effect of neural and humoral impacts on cardiac function. However, single cell and isolated working heart measures presented both here and in previously published data (23) mirror in vivo parameters consistent with cardiac specific effects.
SERCA2a has been shown to play a role in the preservation of function under conditions that otherwise would be expected to produce failure (2, 13, 18, 19). SERCA regulatory proteins such as phospholamban and sarcolipin are also associated with improved contractility and mitigation of heart failure (1). We have shown previously that phospholamban is phosphorylated in vivo to a higher degree in Rad−/− hearts (23), which would be expected to result in higher SERCA activity. Furthermore, we show here that SERCA protein itself is upregulated. These data together point to a phenotype whereby increased calcium sequestration via SERCA balances an increase in calcium influx via the LTCC. This increase in uptake may explain the increase of caffeine-induced SR load in Rad−/− myocardium. Increases of SERCA and SR calcium load are observed in models of nonfailing hypertrophy (physiological hypertrophy) such as with exercise or pregnancy (15). These changes do not compromise myocardial contractility and are associated with increased SERCA2a expression (35), similar to Rad−/−. SERCA2a protein, but not mRNA, is also upregulated in a nonpathological model of hypertrophy induced by AKT overexpression (20). Perhaps most importantly, SERCA2a expression levels have been shown to play a key role in restoring efficient loading of the SR in human heart failure patients, including trials that increase expression of SERCA2a and restore the capacity of the SR to sequester and subsequently release calcium (18, 19). These studies have found that adeno-associated virus-mediated SERCA2a expression is associated with reduced mortality and increased systolic function in patients with heart failure. These studies underscore the clinical relevance of exploring models that increase SERCA2a protein levels. Both immunoblotting and a rightward shift in the IC50 following thapsigargan treatment suggest that SERCA2a protein is upregulated in the Rad−/− myocardium, which provides a potential explanation for the preserved function observed in Rad−/− hearts despite increased calcium current. Importantly, maximal thapsigargan concentrations fail to return sarcomere shortening to the same levels observed in wild-type mice, which is consistent with previously published studies by our laboratory and others that elevated L-type calcium channel current density contributes to increased contractile function (20).
The present study demonstrates stable basal changes in function imposed by Rad deletion. Our studies complement and expand on an earlier report of the dimensional changes of the Rad−/− myocardium in response to pressure overload (3). In the earlier study it was proposed that Rad−/− hearts show an enhanced hypertrophic response to pathological stimuli; however, measures were limited to cell size from histological sections (subject to error imposed by helical arrangement of myocardial fibers) and quantitative RT-PCR. No echocardiography or other measure of cardiac function was reported. Basal changes in cell size, fetal gene program biomarkers, or heart function were not previously noted. We observe elevated ANF (nppa) expression in Rad knockout mice that was not previously seen, although it should be noted that the previous report failed to detect the expected ANF induction in response to thoracic aortic constriction in wild-type mice (3). Consistent with increased Ca2+ homeostasis reflexively decreasing CaV1.2 expression (31), Rad−/− exhibits downregulation of CaV1.2. We also present in vivo functional data for Rad−/− mice, revealing the unexpected insight that contractility remains elevated in Rad−/− mice well into senescence, with a corresponding preservation of cardiac output. In view of these findings, the downregulation of Rad in long-term human heart failure (3) may be recontextualized from playing a pathological role to a compensatory one. This is particularly important as novel therapies to promote systolic function must take into account the possibility that prevention of Rad loss in the heart, rather than slowing decompensation, will reduce systolic function and produce further injury. A critically important question is whether SERCA2a upregulation and enhanced SR loading caused by Rad deletion will protect against pathological hypertrophic stimulus, such as pressure overload. Studies are ongoing to determine the answer to this question.
In summary, we demonstrate an in vivo characterization of the effects of Rad deletion on the morphology and function of the heart. We demonstrate significant changes in relative ventricular wall thickness without a corresponding change in overall heart size, accompanied by an increase in contractility and preserved cardiac output. These changes are preserved into old age and do not result in loss of either systolic or diastolic function. This is consistent with an increase in myocyte SR load and increased expression of SERCA2a protein. These data suggest that Rad loss plays a novel role in calcium homeostasis of the heart. Deletion of Rad results in a heart with reduced LV volume compensated by faster ejection. The net outcome is that Rad deletion safely maintains cardiac output despite increased Ca2+ homeostasis.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-072936 (to D. A. Andres and J. Satin), HL-074091 (to J. Satin), F32-HL-126300-01 (to J. R. Manning), and T32-HL-072743 (to C. N. Withers); University of Kentucky 2012–2013 Research Professorship (to D. A. Andres); American Heart Association Grant 14POST20460224 (to J. R. Manning); and National Science Foundation Grant DGE-1247392 (to C. N. Withers). Additionally, research reported in this publication was supported by an Institutional Development Award (IdeA) from National Institute of General Medical Sciences Grant P20-GM-103527-05.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.R.M., C.N.W., B.L., D.A.A., and J.S. conception and design of research; J.R.M., C.N.W., B.L., J.D.S., and J.S. performed experiments; J.R.M., B.L., J.D.S., and J.S. analyzed data; J.R.M., B.L., D.A.A., and J.S. interpreted results of experiments; J.R.M., C.N.W., B.L., J.D.S., and J.S. prepared figures; J.R.M., D.A.A., and J.S. drafted manuscript; J.R.M., C.N.W., B.L., D.A.A., and J.S. edited and revised manuscript; J.R.M., C.N.W., B.L., J.D.S., D.A.A., and J.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank John Shelton and the University of Texas Southwest Molecular Pathology Core for assistance with the histology presented in this manuscript.
REFERENCES
- 1.Bai Y, Jones PP, Guo J, Zhong X, Clark RB, Zhou Q, Wang R, Vallmitjana A, Benitez R, Hove-Madsen L, Semeniuk L, Guo A, Song LS, Duff HJ, Chen SR. Phospholamban knockout breaks arrhythmogenic Ca2+ waves and suppresses catecholaminergic polymorphic ventricular tachycardia in mice. Circ Res 113: 517–526, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bupha-Intr T, Laosiripisan J, Wattanapermpool J. Moderate intensity of regular exercise improves cardiac SR Ca2+ uptake activity in ovariectomized rats. J Appl Physiol 107: 1105–1112, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Chang L, Zhang J, Tseng YH, Xie CQ, Ilany J, Bruning JC, Sun Z, Zhu X, Cui T, Youker KA, Yang Q, Day SM, Kahn CR, Chen YE. Rad GTPase deficiency leads to cardiac hypertrophy. Circulation 116: 2976–2983, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen W, Wang R, Chen B, Zhong X, Kong H, Bai Y, Zhou Q, Xie C, Zhang J, Guo A, Tian X, Jones PP, O'Mara ML, Liu Y, Mi T, Zhang L, Bolstad J, Semeniuk L, Cheng H, Zhang J, Chen J, Tieleman DP, Gillis AM, Duff HJ, Fill M, Song LS, Chen SR. The ryanodine receptor store-sensing gate controls Ca2+ waves and Ca2+-triggered arrhythmias. Nat Med 20: 184–192, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X, Nakayama H, Zhang X, Ai X, Harris DM, Tang M, Zhang H, Szeto C, Stockbower K, Berretta RM, Eckhart AD, Koch WJ, Molkentin JD, Houser SR. Calcium influx through Cav1.2 is a proximal signal for pathological cardiomyocyte hypertrophy. J Mol Cell Cardiol 50: 460–470, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, Piacentino V, Furukawa S 3rd, Goldman B, Margulies KB, Houser SR. L-type Ca2+ channel density and regulation are altered in failing human ventricular myocytes and recover after support with mechanical assist devices. Circ Res 91: 517–524, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Cingolani E, Ramirez Correa GA, Kizana E, Murata M, Cho HC, Marban E. Gene therapy to inhibit the calcium channel beta subunit: physiological consequences and pathophysiological effects in models of cardiac hypertrophy. Circ Res 101: 166–175, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Correll RN, Botzet GJ, Satin J, Andres DA, Finlin BS. Analysis of the Rem2-voltage dependant calcium channel beta subunit interaction and Rem2 interaction with phosphorylated phosphatidylinositide lipids. Cell Signal 20: 400–408, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crump SM, Correll RN, Schroder EA, Lester WC, Finlin BS, Andres DA, Satin J. L-type calcium channel α-subunit and protein kinase inhibitors modulate Rem-mediated regulation of current. Am J Physiol Heart Circ Physiol 291: H1959–H1971, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Finlin BS, Correll RN, Pang C, Crump SM, Satin J, Andres DA. Analysis of the complex between Ca2+ channel beta-subunit and the Rem GTPase. J Biol Chem 281: 23557–23566, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Finlin BS, Crump SM, Satin J, Andres DA. Regulation of voltage-gated calcium channel activity by the Rem and Rad GTPases. Proc Natl Acad Sci USA 100: 14469–14474, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finlin BS, Mosley AL, Crump SM, Correll RN, Ozcan S, Satin J, Andres DA. Regulation of L-type Ca2+ channel activity and insulin secretion by the Rem2 GTPase. J Biol Chem 280: 41864–41871, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Freeman K, Lerman I, Kranias EG, Bohlmeyer T, Bristow MR, Lefkowitz RJ, Iaccarino G, Koch WJ, Leinwand LA. Alterations in cardiac adrenergic signaling and calcium cycling differentially affect the progression of cardiomyopathy. J Clin Invest 107: 967–974, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasenfuss G. Alterations of calcium-regulatory proteins in heart failure. Cardiovasc Res 37: 279–289, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med 358: 1370–1380, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Hovnanian A. SERCA pumps and human diseases. Subcell Biochem 45: 337–363, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Ito K, Yan X, Feng X, Manning WJ, Dillmann WH, Lorell BH. Transgenic expression of sarcoplasmic reticulum Ca(2+) ATPase modifies the transition from hypertrophy to early heart failure. Circ Res 89: 422–429, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, Borow K, Dittrich H, Zsebo KM, Hajjar RJ; Calcium Up-Regulation by Percutaneous Administration of Gene Therapy In Cardiac Disease (CUPID) Trial Investigators. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail 15: 171–181, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, Yaroshinsky A, Zsebo KM, Dittrich H, Hajjar RJ; Calcium Up-Regulation by Percutaneous Administration of Gene Therapy In Cardiac Disease (CUPID) Trial Investigators. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation 124: 304–313, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim YK, Kim SJ, Yatani A, Huang Y, Castelli G, Vatner DE, Liu J, Zhang Q, Diaz G, Zieba R, Thaisz J, Drusco A, Croce C, Sadoshima J, Condorelli G, Vatner SF. Mechanism of enhanced cardiac function in mice with hypertrophy induced by overexpressed Akt. J Biol Chem 278: 47622–47628, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Louch WE, Vangheluwe P, Bito V, Raeymaekers L, Wuytack F, Sipido KR. Phospholamban ablation in hearts expressing the high affinity SERCA2b isoform normalizes global Ca2+ homeostasis but not Ca2+-dependent hypertrophic signaling. Am J Physiol Heart Circ Physiol 302: H2574–H2582, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Magyar J, Kiper CE, Sievert G, Cai W, Shi GX, Crump SM, Li L, Niederer SA, Smith NP, Andres DA, Satin J. Rem-GTPase regulates cardiac myocyte L-type calcium current. Channels (Austin) 6: 1–8, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manning JR, Yin G, Kaminski CN, Magyar J, Feng HZ, Penn J, Sievert G, Thompson K, Jin JP, Andres DA, Satin J. Rad GTPase deletion increases L-type calcium channel current leading to increased cardiac contraction. J Am Heart Assoc 2: e000459, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyamoto MI, del Monte F, Schmidt U, DiSalvo TS, Kang ZB, Matsui T, Guerrero JL, Gwathmey JK, Rosenzweig A, Hajjar RJ. Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proc Natl Acad Sci USA 97: 793–798, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murata M, Cingolani E, McDonald AD, Donahue JK, Marban E. Creation of a genetic calcium channel blocker by targeted gem gene transfer in the heart. Circ Res 95: 398–405, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Muth JN, Bodi I, Lewis W, Varadi G, Schwartz A. A Ca(2+)-dependent transgenic model of cardiac hypertrophy: a role for protein kinase Calpha. Circulation 103: 140–147, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Muth JN, Yamaguchi H, Mikala G, Grupp IL, Lewis W, Cheng H, Song LS, Lakatta EG, Varadi G, Schwartz A. Cardiac-specific overexpression of the alpha(1) subunit of the L-type voltage-dependent Ca(2+) channel in transgenic mice. Loss of isoproterenol-induced contraction. J Biol Chem 274: 21503–21506, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Pang C, Crump SM, Jin L, Correll RN, Finlin BS, Satin J, Andres DA. Rem GTPase interacts with the proximal CaV1.2 C-terminus and modulates calcium-dependent channel inactivation. Channels (Austin) 4: 192–202, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Periasamy M, Bhupathy P, Babu GJ. Regulation of sarcoplasmic reticulum Ca2+ ATPase pump expression and its relevance to cardiac muscle physiology and pathology. Cardiovasc Res 77: 265–273, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Schroder E, Byse M, Satin J. L-type calcium channel C terminus autoregulates transcription. Circ Res 104: 1373–1381, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schroder F, Handrock R, Beuckelmann DJ, Hirt S, Hullin R, Priebe L, Schwinger RH, Weil J, Herzig S. Increased availability and open probability of single L-type calcium channels from failing compared with nonfailing human ventricle. Circulation 98: 969–976, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Tournoux F, Petersen B, Thibault H, Zou L, Raher MJ, Kurtz B, Halpern EF, Chaput M, Chao W, Picard MH, Scherrer-Crosbie M. Validation of noninvasive measurements of cardiac output in mice using echocardiography. J Am Soc Echocardiogr 24: 465–470, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang G, Zhu X, Xie W, Han P, Li K, Sun Z, Wang Y, Chen C, Song R, Cao C, Zhang J, Wu C, Liu J, Cheng H. Rad as a novel regulator of excitation-contraction coupling and beta-adrenergic signaling in heart. Circ Res 106: 317–327, 2010. [DOI] [PubMed] [Google Scholar]
- 35.Wisloff U, Loennechen JP, Falck G, Beisvag V, Currie S, Smith G, Ellingsen O. Increased contractility and calcium sensitivity in cardiac myocytes isolated from endurance trained rats. Cardiovasc Res 50: 495–508, 2001. [DOI] [PubMed] [Google Scholar]