Abstract
In a recent study (Huang YH et al. Respir Physiol Neurobiol 143: 1–8, 2004), we showed that prenatal nicotine exposure (PNE) increased the frequency of spontaneous apneic events on the first 2 days of life in unanesthetized neonatal rats. Here we test the hypothesis that PNE blunts chemoreceptor reflexes. Ventilatory responses to three levels each of hypoxia (inspired O2 fraction: 16, 12, and 10%) and hypercapnia (3, 6, and 9% inspired CO2 fraction, all in 50% O2, balance N2), and one level each of combined hypoxia-hypercapnia (H/H; 12% inspired O2 fraction/5% inspired CO2 fraction) and hyperoxia (50% O2, 50% N2) were recorded with head-out plethysmography in neonatal rats exposed to either nicotine (N = 12) or physiological saline (N = 12) in the prenatal period. Recordings were made on postnatal day 1 (P1), P3, P6, P9, P12, and P18, in each animal. The change in ventilation in response to hypoxia was blunted in PNE animals on P1 and P3, but there were no other treatment effects. Hyperoxia significantly depressed ventilation in both groups from P3–P18, but there were no significant treatment effects. The ventilatory response to 3, 6, and 9% inspired CO2 was significantly blunted in PNE animals at all ages studied, due exclusively to a blunted tidal volume response. PNE also blunted the ventilatory response to H/H at all ages, due primarily to blunting of the tidal volume response. PNE had no significant effect on body mass or metabolic rate, except that PNE animals had a slightly higher mass on P18 and a lower metabolic rate on P1. As shown by others, PNE has small and inconsistent effects on hypoxic ventilatory responses, but here we show that responses to hypercapnia and H/H are consistently blunted by PNE due to a diminished tidal volume response. The combination of reduced hypoxic and hypercapnic sensitivity over the first 3 days of life may define an especially vulnerable developmental period.
Keywords: breathing, chemoreceptor, neonate, plethysmography
at least 20% of women continue to smoke during pregnancy, a habit that is associated with a variety of physiological and behavioral abnormalities in their neonates (41). Of the various chemicals in tobacco smoke, nicotine readily crosses the blood-brain barrier and has a marked impact on neuronal growth and differentiation (52, 53). Thus, understanding how prenatal nicotine exposure (PNE) alters the development of neurons that control key homeostatic systems, such as the respiratory control system, is a public health imperative.
The response of the respiratory system to changes in blood oxygen and carbon dioxide levels is among the most powerful and important of the homeostatic reflexes in mammals. It is essential that these ventilatory reflexes are functional so that oxygen supply to vital organs is preserved and blood pH regulated when neonates are challenged with hypoxia and/or hypercapnia (e.g., as occurs when the face is submerged in bedding).
Several investigators, with a variety of animal models and methods, have studied the influence of PNE on the ventilatory response to hypoxia and, to a lesser extent, hypercapnia (for review, see Ref. 23). However, the results are equivocal because most studies have examined ventilatory responses on only one or a few postnatal ages, few studies have examined multiple levels of hypoxia and hypercapnia, and fewer still have studied the same animals on each of several days after birth. Moreover, differences in the temperature at which the pups are studied, the rat strain, and the details of the plethysmography methods also contribute to different results throughout the literature. As a result of these differences in approach and findings, it has been difficult to arrive at a consensus about the influence of PNE on chemoreceptor reflexes in mammals.
Here we compare ventilatory responses to three levels of hypoxia, one level of hyperoxia, three levels of hyperoxic hypercapnia, and one level of hypoxia/hypercapnia in neonatal rats that were exposed to either saline (control) or nicotine throughout the prenatal period. Each animal was subjected to each of the stimulus conditions on postnatal day 1 (P1), P3, P6, P9, P12, and P18, and their ventilatory responses were determined with head-out plethysmography. Our results show that PNE consistently blunts the ventilatory response to hypercapnia at all ages studied and is associated with a small but significant reduction in hypoxic sensitivity on the first 3 days of life, but not thereafter.
METHODS
Animals.
All data presented here were derived from experiments approved by the Institutional Animal Care and Use Committee at The University of Arizona. Neonatal rats were derived from four saline-exposed and four nicotine-exposed dams; two to four animals were used from each litter. We measured ventilatory responses in 12 saline-exposed and 12 nicotine-exposed neonates. Measurements were made on P1, P3, P6, P9, P12, and P18 in each animal. We also measured developmental changes in metabolic rate in normoxia and hypoxia in a separate group of animals (see below).
PNE.
A 28-day osmotic mini-pump (2ML4, Alzet, Cupertino, CA) was implanted into eight pregnant rats on day 5 of gestation, as described in detail previously (26, 33, 34, 43). In Sprague-Dawley rats, implantation of the embryo in the uterine wall is complete by the 7th day of gestation; implantation at day 5 ensures that exposure to nicotine begins in the early embryonic period. Pumps were set to deliver nicotine (6 mg·kg−1·day−1 at a rate of 2.5 μl/h) in four rats, and physiological saline (also at a rate of 2.5 μl/h) in four rats.
Measurements.
Ventilation was measured in neonates using head-out body plethysmography, as described previously (26). Studies were carried out between 8:00 AM and 3:00 PM. Pups studied on P1, P3, P6, and P9 were placed in a chamber with a volume of 32 ml (diameter = 2.6 cm, length = 6.5 cm), while the chamber used for the 12- and 18-day-old pups had a volume of 70 ml (diameter = 3.7 cm, length = 6.5 cm). Both chambers were homemade from plastic tubing using a design similar to that described by Saetta and Mortola (45). The pups were inserted tail end into the chamber with the head out. A neck seal was made by placing the animal's head through a small hole cut into the middle of a square piece of parafilm. The parafilm was secured around the chamber with rubber bands. In some cases, two or three pieces of parafilm were required to obtain a tight seal. If leaks were present, they were sealed with putty (North Coast Thera-putty, NC52340-03).
The rate of respiratory airflow was estimated from recordings of gas flow into and out of the chamber with a pneumotachometer (Hans-Rudolph model 8431, Kansas City, MO; range, 0–3 l/min). Each chamber had three ports: a volume injection port used for calibration; a port used for insertion of a thermocouple probe for regulation of chamber temperature; and a port that was connected to the pneumotachometer. As described previously (26), an increase in lung volume forced air from the chamber and across the pneumotachometer, while a decrease in lung volume caused opposite changes in flow. The pneumotachometer was connected to the positive and negative ports of a pressure transducer with a working range of ±2 cmH2O (Validyne DP45–16, Northridge, CA). The resultant changes in airflow were recorded on a polygraph (Grass model 7, Quincy, MA) after the signal was conditioned with a Validyne Carrier Demodulator and a Grass DC amplifier (model 7P122). The area under the inspiratory flow (V̇i)-time curve was electronically integrated (Grass model 7DA) to derive tidal volume (Vt), as shown in Fig. 1.
Fig. 1.
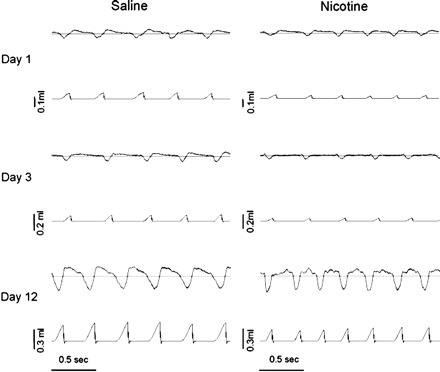
Sample recordings showing airflow rate (top traces in each panel; inspiration represented by downward deflections) and inspired tidal volume (Vt; bottom trace in each panel) from representative neonatal rat pups studied on postnatal day 1 (P1), P3, and P12. All recordings were taken while the animal was breathing compressed, humidified air. Recordings for both prenatal saline-exposed (left) and prenatal nicotine-exposed (PNE; right) pups are shown for comparison. Horizontal lines through the flow recordings represent zero flow. The plethysmographs were calibrated by injecting known volumes of gas and digitally integrating the inspiratory flow (V̇i) signal with respect to time, yielding inspired Vt (described in detail in methods); thus the flow trace is uncalibrated. Small differences in frequency and volume between saline-exposed and nicotine-exposed pups under these conditions were not a consistent finding, as the average data demonstrate (Fig. 2).
For calibration, we used a graduated 1-ml syringe and injected volumes of 0.1, 0.2, and 0.3 ml of air before and after each recording session; the average of the two curves was used to compute Vt (see below). Chamber temperature was maintained between 32 and 34°C, as the thermoneutral zone for neonatal rats has been estimated to range from 30 to 34°C (37, 39, 46). A thermocouple (Thermalert TH-5, Physitemp, Clifton, NJ) was inserted into a chamber port, and the excess space was sealed with Thera-putty. The thermocouple was connected to a control unit (TCAT-1A Temperature Controller, Physitemp, Clifton, NJ) that turned on an infrared heat lamp when the temperature dropped below the set point, and turned the lamp off when the temperature exceeded the set point.
Experimental protocol.
To deliver gas to the animal, cylinders containing CO2, N2, and O2 were connected to a precision Matheson rotameter. The outflow port of the rotameter was connected in series to a water-filled flask for humidification and a plastic cone that was placed loosely around the animal's head. Recording did not begin until the neonates were still and breathing regularly. The animal first breathed humidified compressed air until 3–4-min of continuous, stable breathing without movement artifact was obtained. Animals were then subjected to the following eight inspired gas conditions: hypoxia (16, 12, and 10% O2 in N2), hypercapnia (3, 6, and 9% CO2 in 50% O2, balance N2), hypoxic hypercapnia (12% O2, 5% CO2, balance N2), and hyperoxia (50% O2, balance N2). The protocol was fully randomized, and each condition was maintained for 3 min. A 20- to 30-min air-breathing recovery period was interposed between each of the gas conditions. A string of 15–20 breaths in the last minute of each condition was analyzed for the computation of Vt and breathing frequency (f). Although we typically obtained a continuous string of breaths, this was not always possible due to movement artifacts in the flow signal.
Measurement of metabolic rate in normoxia and hypoxia.
We also measured oxygen consumption (V̇o2) and carbon dioxide production (V̇co2) rates in saline-exposed and nicotine-exposed neonates, in normoxia and in severe hypoxia (inspired O2 fraction = 0.10), on P1 (N = 12 saline, 12 PNE), P3 (12 saline, 11 PNE), P6 (9 saline, 6 PNE), P9 (9 saline, 8 PNE), P12 (11 saline, 8 PNE), P18 (12 saline, 8 PNE), and P21 (6 saline, 6 PNE). These studies were done to determine if the well-described drop in metabolic rate in hypoxia (37, 40) differs in nicotine-exposed neonates compared with controls. Experiments on P21 were included because this time point was included in our companion study of prolonged hypoxic responses (unpublished observations). Two to three neonates were placed in a cylindrical Plexiglas chamber, with the number of neonates and the chamber size (50, 110, or 235 ml) chosen such that the animal-to-chamber volume ratio was optimized. Animals were allowed to acclimate to the chamber for 10 min. Compressed air was passed through a rotameter to control airflow (250–300 ml/min) and a water-filled flask before entering the chamber. Effluent flow was measured continuously with a pneumotachometer (Hans-Rudolph model 8431, Kansas City, MO) placed in the outflow port of the chamber. The effluent flow passed through a drying column before being sampled by CO2 and O2 analyzers arranged in parallel (Raytech). Ambient temperature was maintained in the thermoneutral range for neonates, as described above. Animals underwent two 10-min exposures to normoxia and one 10-min exposure to hypoxia (10% O2, balance N2). Data were recorded using Spike II hardware and software (Cambridge Electronic Design, London); the last 5 min of each trial were used for data analysis. V̇o2 and V̇co2 were calculated using standard equations, and expressed under stpd conditions.
Data analysis and statistics.
Body weight, V̇i, Vt, and f were measured in all animals before and during each of the inspired gas conditions. For statistical comparisons of ventilatory responses to changing inspired O2 and CO2 levels, we measured the change (from the baseline level breathing humidified compressed air) in V̇i, Vt, and f evoked by each of the inspired gas conditions. We chose to analyze changes in ventilatory outcome variables rather than their absolute values, because our primary goal was to examine the reflex response to changing inspired gas concentrations, independently of the baseline ventilatory drive and/or metabolic rate. Nonetheless, the absolute resting values for all variables are provided in Fig. 2, so the interested reader can easily make the conversion to absolute values.
Fig. 2.
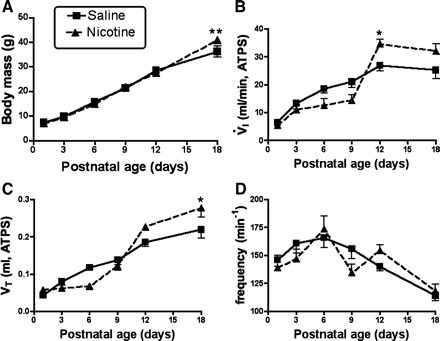
Body mass and ventilatory parameters measured under baseline conditions. A: growth curves in saline-exposed control animals and animals that were exposed to nicotine in utero. Body mass changed at the same rate, with the exception of a higher body mass in PNE pups on P18. B and C: the developmental time course of baseline minute ventilation (V̇i) and Vt, respectively, while the animals breathed compressed air (see methods). Note that V̇i and Vt rose with age at the same rate up to P9; thereafter, the nicotine-exposed pups showed a trend for higher V̇i and Vt, with significantly greater values on P12 for V̇i and on P18 for Vt. D: frequency declined significantly as a function of age in both groups, with no differences between groups (see results). *Difference between groups at the indicated postnatal age, P < 0.05. **P < 0.01.
Thus changes in V̇i, Vt, and f in response to changes in inspired oxygen concentration were subjected to a three-factor, mixed-model ANOVA, with two within-factors (age and gas concentration) and one between-group factor (saline exposed or PNE). Overall effects of each of the main factors are reported in results. If age × treatment interactions were significant, differences between saline-exposed and PNE animals within an age group were tested with Bonferroni post hoc tests. Because the presentation of each of the gas conditions was fully randomized (see above), we treated each condition as if it were independent of the others, and thus analyzed each gas condition separately for the purpose of post hoc analysis. Thus, since animals were subjected to each of the gas conditions at six different ages, there are six contrasts for each of the conditions, and the P value was adjusted as follows: Bonferroni corrected P = 0.05/6 = 0.0083. We also performed a three-factor, mixed-model ANOVA for the three-hypercapnia conditions, and a two-factor ANOVA (age and treatment group) for the hypoxia/hypercapnia experiments, for the V̇co2 and V̇o2 measurements (which were done in separate groups of saline-exposed and PNE animals), for the body mass measurements, and for the resting V̇i, Vt, and f measurements. For all experiments, data are presented as means ± SE in the text and Figs. 2–9. In all cases, comparisons were considered statistically significant if P < 0.05.
Fig. 3.
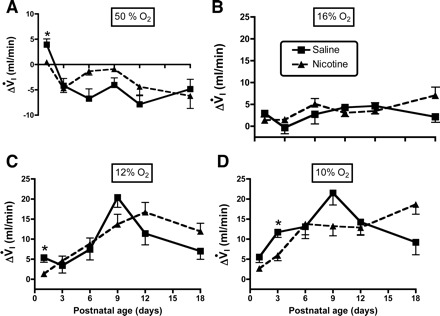
A–D: influence of age and PNE on the ventilatory response to changing inspired oxygen levels: 50, 16, 12, and 10% O2, respectively. Each panel shows the change in V̇i (ΔVi) on the y-axis, and postnatal age on the x-axis. The results of statistical analyses are given in Table 1 and in the text of results. Differences between treatment groups were few and, when present, small. *Difference between groups at the indicated postnatal age, P < 0.05.
Fig. 4.
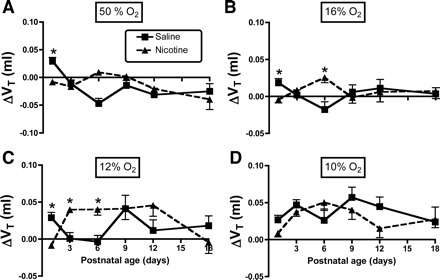
A–D: influence of age and PNE on the Vt response to changing inspired oxygen levels: 50, 16, 12, and 10% O2, respectively. Each panel shows the ΔVt on the y-axis, and postnatal age on the x-axis. The results of statistical analyses are given in Table 1 and in the text of results. Differences between treatment groups, when present, vary as a function of age. *Difference between groups at the indicated postnatal age, P < 0.05.
Fig. 5.
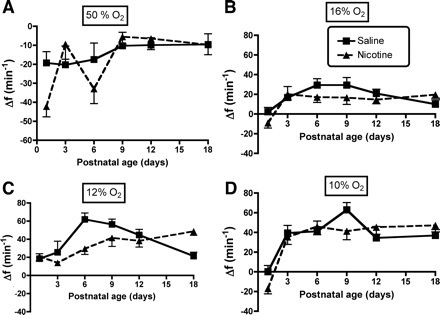
A–D: influence of age and PNE on the breathing frequency (f) response to changing inspired oxygen levels: 50, 16, 12, and 10% O2, respectively. Each panel shows the Δf on the y-axis, and postnatal age on the x-axis. The results of statistical analyses are given in Table 1 and in the text of results. There were no significant between-group effects, nor age-treatment group interactions.
Fig. 6.
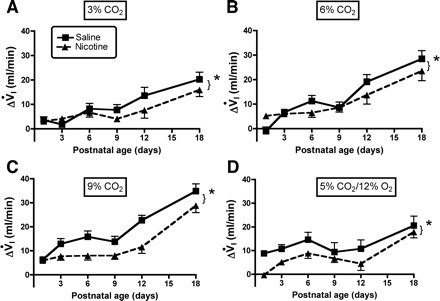
A–C: influence of age and PNE on the ventilatory response to different levels of hyperoxic hypercapnia are shown: 3, 6, and 9% CO2, respectively. The results of statistical analyses are given in Table 2 and in the text of results. As above, each panel shows the ΔVi on the y-axis, and postnatal age on the x-axis. *Significant treatment effect at each level of hypercapnia; however, there was not a significant age-treatment interaction at any level of hypercapnia, indicating that the effects were proportional across the age range studied (refer to Table 2). D: the ΔVi in response to combined hypercapnia/hypoxia; as with hyperoxic hypercapnia, the response was blunted in PNE animals (refer to Table 3 and results).
Fig. 7.
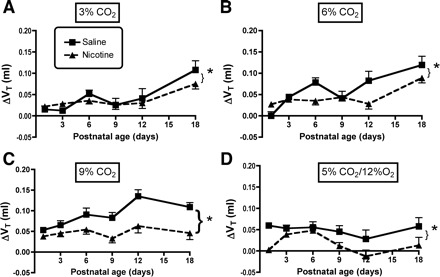
A–C: influence of age and PNE on the Vt response to different levels of hyperoxic hypercapnia are shown: 3, 6, and 9% CO2, respectively. The results of statistical analyses are given in Table 2 and in the text of results. As above, each panel shows the ΔVt on the y-axis, and postnatal age on the x-axis. *Significant treatment effect at each level of hypercapnia. As with V̇i, there were no significant age-treatment interactions at any level of hypercapnia, indicating that the effects were proportional across the age range studied (refer to Table 2). Moreover, since frequency did not change with hypercapnia (Fig. 8), alterations in the control of Vt explain the blunted ventilatory response to hypercapnia in the PNE animals. D: the ΔVt in response to combined hypercapnia/hypoxia. As with hyperoxic hypercapnia, the response was blunted in PNE animals (refer to Table 3 and results).
Fig. 8.
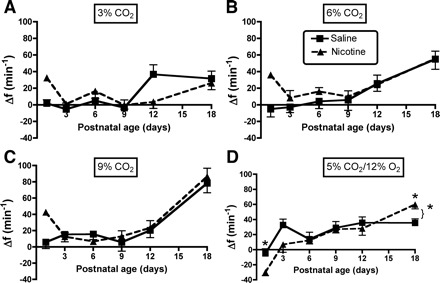
A–C: influence of age and PNE on the f response to different levels of hyperoxic hypercapnia are shown: 3, 6, and 9% CO2, respectively. The results of statistical analyses are given in Table 2 and in the text of results. There were no significant treatment effects at any level of hyperoxic hypercapnia. D: the Δf in response to combined hypercapnia/hypoxia. *There was a significant treatment effect (bracket + asterisk) and a significant age-treatment interaction; the lone asterisks indicate a blunted Δf response in PNE pups on P1, but an enhanced response on P18 (refer to Table 3 and results).
Fig. 9.
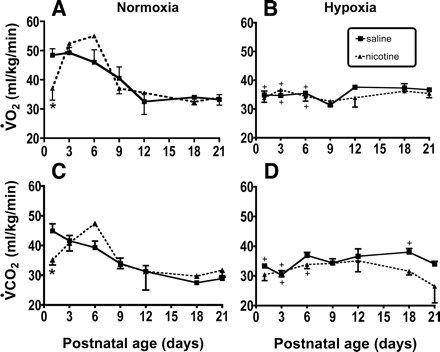
Changes in mass-specific O2 consumption (V̇o2) and CO2 production (V̇co2) in normoxia and hypoxia (10% O2/90% N2). A and B: developmental changes in V̇o2 under normoxic and hypoxic conditions, respectively. C and D: the developmental change in V̇co2 in normoxia and hypoxia, respectively. Experiments were done in up to 12 control and 12 nicotine-exposed pups, with each neonate studied on each of the indicated postnatal days, as described in methods. +Hypoxia significantly different than normoxia within a treatment group; *saline different than PNE at indicated postnatal age: P < 0.05.
RESULTS
Age-dependent changes in body mass and baseline ventilatory responses in normoxia.
Baseline data for body mass, V̇i, Vt, and f are shown in Fig. 2, A–D, respectively. As shown in Fig. 2A, neonates gained weight at the same rate up to P12, but PNE animals were significantly heavier on P18. Figure 2B shows baseline V̇i values as a function of age. V̇i increased with age in both groups (F = 46.3, P < 0.0001), consistent with our laboratory's earlier work on similarly aged pups (26). V̇i increased in a fairly linear way up to P12, but we did not observe a further increase at P18. Although there was no overall treatment effect, the age-treatment interaction was significant (F = 4.75, P = 0.0005). Post hoc analysis showed that V̇i was significantly greater in PNE animals on P12. Vt also increased significantly with age in both groups, with no treatment effect and a significant age-treatment interaction, and with post hoc tests revealing a higher Vt in PNE animals on P18 (Fig. 2C). Baseline f declined significantly after P9 in both groups (F = 9.71, P < 0.0001, Fig. 2D), but there were no treatment or interaction effects.
Ventilatory responses to hypoxia and hyperoxia.
The influence of PNE on ventilatory responses to hyperoxia and hypoxia are shown in Figs. 3–5, with the results of the three-factor ANOVA presented in Table 1. As expected, there were significant effects of inspired oxygen content and age on the changes in V̇i, Vt, and f in both groups (Table 1). There were no effects of treatment for any of the variables, although there was a significant age-treatment interaction for the change in V̇i (P = 0.012, Table 1) and Vt (P < 0.001, Table 1). As shown in Fig. 3, post hoc analysis revealed that the source of the age-treatment interaction for the change in V̇i, was on P1 during hyperoxia (Fig. 3A), on P1 during the 12% inspired oxygen challenge (Fig. 3C), and on P3 in the 10% oxygen condition (Fig. 3D). Measurement of the change in Vt in response to hyperoxia revealed significant treatment effects on P1 (Fig. 4A), on P1 and P6 during 16% oxygen breathing (Fig. 4B), and on P1, P3, and P6 with 12% oxygen breathing (Fig. 4C). Note that, for both V̇i and Vt, the effects of changing inspired oxygen level were variable, with both larger and smaller responses observed in the PNE pups, depending on age and oxygen level. Although f appeared to differ between groups in hyperoxia and with 12% oxygen breathing (Fig. 5, A and C), the treatments effects for the change in f did not reach significance (P = 0.063, Table 1).
Table 1.
Results of three-factor analysis of variance for hyperoxia and hypoxia experiments
| Dependent Variable | Factor | Degrees of Freedom | F | P |
|---|---|---|---|---|
| ΔV̇i | [Inspired oxygen] | 3 | 252.8 | <0.001 |
| Age | 5 | 7.35 | <0.001 | |
| Treatment | 1 | 0.11 | 0.75 | |
| Age × treatment | 5 | 3.1 | 0.012 | |
| ΔVt | [Inspired oxygen] | 3 | 69.9 | <0.001 |
| Age | 5 | 1.6 | 0.18 | |
| Treatment | 1 | 0.28 | 0.6 | |
| Age × treatment | 5 | 5.1 | <0.001 | |
| Δf | [Inspired oxygen] | 3 | 277.7 | <0.001 |
| Age | 5 | 11.4 | <0.001 | |
| Treatment | 1 | 3.8 | 0.063 | |
| Age × treatment | 5 | 1.92 | 0.096 |
ΔV̇i, change in inspiratory flow; ΔVt, change in tidal volume; Δf, change in breathing frequency; [Inspired oxygen], concentration of inspired oxygen.
Ventilatory responses to hyperoxic hypercapnia.
The influence of PNE on ventilatory responses to hyperoxic hypercapnia is shown in Figs. 6–8, with the results of the three-factor ANOVA presented in Table 2. ANOVA revealed significant effects of inspired CO2 concentration and age on the changes in V̇i, Vt, and f in both groups (Table 2). There were also highly significant treatment effects for V̇i and Vt, but not f (Table 2). There were no significant age-treatment interactions, indicating that the treatment effects were statistically the same at all ages studied. This is clear when looking at the changes in V̇i and Vt in Figs. 6, A–C and 7, A–C, respectively. The treatment effects were significant at all three levels of hypercapnic exposure (Table 2 and Figs. 6, A–C, and 7, A–C). Although f appeared to differ between groups on P1 with 3 and 9% inspired CO2 (Fig. 8, A–C), and on P12 with 3% CO2 (Fig. 8A), the treatments effects for the change in f did not reach significance (P = 0.062, Table 2).
Table 2.
Results of three-factor analysis of variance for hyperoxic hypercapnia experiments
| Dependent Variable | Factor | Degrees of Freedom | F | P |
|---|---|---|---|---|
| ΔV̇i | [Inspired carbon dioxide] | 2 | 85.7 | <0.001 |
| Age | 5 | 31.41 | <0.001 | |
| Treatment | 1 | 9.7 | 0.005 | |
| Age × treatment | 5 | 1.4 | 0.23 | |
| ΔVt | [Inspired carbon dioxide] | 2 | 37.5 | <0.001 |
| Age | 5 | 9.87 | <0.001 | |
| Treatment | 1 | 14.9 | <0.001 | |
| Age × treatment | 5 | 2.1 | 0.067 | |
| Δf | [Inspired carbon dioxide] | 2 | 60.2 | <0.001 |
| Age | 5 | 13.1 | <0.001 | |
| Treatment | 1 | 3.8 | 0.062 | |
| Age × treatment | 5 | 2.2 | 0.059 |
[Inspired carbon dioxide], concentration of inspired carbon dioxide.
Ventilatory responses to combined hypoxia/hypercapnia.
Ventilatory responses to hypoxia/hypercapnia were modest in both groups (Figs. 6D, 7D, and 8D), but there were significant age and treatment effects for V̇i, Vt, and f (Table 3). As with hyperoxic hypercapnia, there were no interactions between age and treatment for V̇i or Vt (Figs. 6D and 7D, and Table 3), indicating that PNE depressed the increase in V̇i and Vt at all ages. The f response was more complex, with a relatively weak treatment effect (P = 0.045), but a strong age-treatment interaction (P = 0.008; Table 3, Fig. 8D). Post hoc analyses revealed that the age-treatment interaction for f was significant on P1 and P18 (Fig. 8D). On P1, the PNE pups dropped their f, while the saline-exposed control pups showed no change; on P18 the PNE animals had a larger f response than the controls.
Table 3.
Results of two-factor analysis of variance for the combined hypoxia/hypercapnia experiments
| Dependent Variable | Factor | Degrees of Freedom | F | P |
|---|---|---|---|---|
| ΔV̇i | Age | 5 | 8.2 | <0.001 |
| Treatment | 1 | 17.5 | <0.001 | |
| Age × treatment | 5 | 0.45 | 0.81 | |
| ΔVt | Age | 5 | 2.84 | 0.019 |
| Treatment | 1 | 26.1 | <0.001 | |
| Age × treatment | 5 | 1.1 | 0.38 | |
| Δf | Age | 5 | 18.4 | <0.001 |
| Treatment | 1 | 4.48 | 0.045 | |
| Age × treatment | 5 | 3.3 | 0.008 |
Age-dependent changes in metabolic rate in normoxia and hypoxia.
Three-factor ANOVA (i.e., age, treatment, inspired gas) revealed that mass-specific V̇o2 and V̇co2 changed significantly as a function of age, treatment, and gas (P < 0.0001 for all factors). There was also a significant age-treatment interaction (P < 0.0001). Post hoc analyses revealed that V̇o2 and V̇co2 measured in normoxia declined up to P12 in control pups, leveling off thereafter (Fig. 9, A and B). The PNE pups had a more complex response, inasmuch as metabolic rate was significantly lower in PNE pups compared with controls on P1 (asterisks in Fig. 9, A and B), and then increased markedly on P3 and P6, followed by the age-dependent decline and subsequent leveling off observed in the saline-exposed controls. Hypoxia (10% inspired O2) depressed V̇o2 and V̇co2 significantly on P1, P3, and P6 in the saline-exposed control animals, but only on P3 and P6 in the PNE animals (Fig. 9, A and B). Both groups of animals were able to maintain metabolic rate in hypoxia on P9 and beyond.
DISCUSSION
We examined the effects of PNE on the development of ventilatory responses to chemoreceptor stimulation. Our new contributions include the examination of multiple chemoreceptor stimuli and a longitudinal study design wherein each animal was studied at each of six postnatal ages. Our main finding is that PNE blunts the ventilatory response to hypercapnia and combined hypoxia/hypercapnia at all ages, but has only small and inconsistent effects on the response to hyperoxia and hypoxia. The blunted response to CO2 is consistent with recent data showing that PNE alters development of central chemoreceptor pathways (11), although it is possible that the carotid body contribution to CO2 sensing (8, 54) was also altered by PNE.
Influence of PNE on baseline ventilation and body mass.
The age-dependent rise in V̇i under baseline conditions was similar in control and PNE animals and was driven by a systematic increase in Vt with no significant change in f in either group. This age-dependent increase in V̇i is consistent with previous studies of ventilatory control in neonatal rats (3, 4, 10–13, 26, 35, 40, 51, 58). One exception is the significantly higher V̇i in PNE pups on P12. In our laboratory's previous study, we found no differences in eupneic V̇i between control and PNE pups, although the trend was for lower V̇i in PNE animals on P14 and P18 (26). We also found subtle differences in the development of Vt, with PNE animals showing slightly higher values on P18. Again, this contrasts with our previous findings showing that Vt was lower in PNE pups on P14 and P18 (26). Although we cannot explain the different results, it is noteworthy that the sex of the animals was not determined in either this study or our previous one. Given that others have noted small sex differences (50), perhaps the sex distributions differed in our two studies, causing systematic differences in our data.
Interestingly, PNE animals were heavier than the controls on P18, which is consistent with data reported in a recent study (35). In contrast, other investigators have shown that PNE reduces body mass in neonatal rats (3). The reason for the higher body mass in the PNE pups on P18 is not clear, although prenatal exposure to neuroteratogens, including nicotine, has been shown to disrupt the endocrine system, leading to abnormalities in body weight regulation (25).
Influence of PNE on ventilatory responses to changing inspired oxygen tension.
We used hyperoxia (50% O2) to attenuate basal peripheral chemoreceptor discharge (3, 9, 20), which should disfacilitate central respiratory neurons and decrease ventilatory output. Hyperoxia actually increased V̇i on P1 in saline-exposed control animals, while the PNE pups showed no change. The reason for the increase in V̇i with hyperoxia on P1 in the control neonates is unknown, although we note that 30-s exposures to hyperoxia has been shown to increase ventilation by 20% in healthy, 1-day-old human neonates (56).
From P3 onward, hyperoxia reduced V̇i in both groups, with no between-group differences. Examination of the change in V̇i from baseline values shows that the carotid bodies make a substantial contribution to eupneic ventilation. For example, in P3 pups, hyperoxia reduced eupneic V̇i by 32% in saline-exposed animals and 43% in PNE pups. On P18, the carotid body contribution to eupnea averaged 19% in both groups. This magnitude of hyperoxic blunting is consistent with previous data in similarly aged neonatal rats (3, 38). Although we did not demonstrate a blunting of the hyperoxic ventilatory response with PNE, a previous study did show hyperoxic blunting with PNE on P3, but not at P8 or P18 (3). We note that, in that study, 100% O2 was used, where we used only 50% O2, suggesting that the differences may be caused by dose dependence of the hyperoxic ventilatory response. Nonetheless, human infants born to smoking mothers showed no blunting of the ventilatory response to 100% O2 (57).
We also studied the ventilatory response to mild, moderate, and severe hypoxia, and, like others before us, we show that the hypoxic ventilatory response in awake neonatal animals is extremely variable, with often small and inconsistent differences between control and PNE animals, even in cases where the data are statistically significant (for review, see Ref. 23). Here, statistical comparisons between groups at a given age revealed only two significant differences: blunted ventilatory responses in the PNE pups on P1 during 12% oxygen breathing, and on P3 during 10% oxygen breathing. Others have shown that PNE reduces some aspect of the hypoxic ventilatory response in rats and mice (11, 32, 51, 58, 61), or have reported no significant difference (3, 4). Such discrepancies may be attributed to the considerable variability in hypoxic responsiveness mentioned above, to age-dependent changes in neurotransmitter receptor expression (61), or to other factors, such as the intensity and/or duration of the hypoxic stimulus, the age group chosen for study, the ambient temperature at which measures are made, the method of measurement (e.g., whole body vs. head-out plethysmography), and the technical challenges involved in measuring V̇i in awake neonatal animals (for review, see Ref. 39). Finally, it is possible that sex differences in the hypoxic ventilatory response increased variability. In this study, we did not record sex, but recent work examining the ventilatory responses to neonatal maternal separation shows enhanced hypoxic responses and tyrosine hydroxylase expression in the carotid body in males but not females (21, 27, 28). Thus it is possible that the response to other stressors, such as hypoxia, is also sex dependent.
How PNE causes these subtle alterations in development of hypoxic sensitivity in awake animals is not known, but could involve changes in carotid body oxygen sensing, central processing of the afferent input, or alterations in ventilatory output pathways. PNE increases mRNA for enzymes responsible for synthesizing dopamine and norepinephrine (19), both of which inhibit the discharge rate of carotid body afferent nerve endings. In contrast, PNE had no influence on the in vitro discharge rate of carotid sinus nerve afferents in response to severe hypercapnia (3), which was studied in a background of either normoxic gas or severe hypoxia (1% O2). From these data, the authors concluded that PNE may alter the central processing of chemoreceptor afferent discharge, or that the afferent nerves had not yet reached the nucleus of the solitary tract, where central processing of the input occurs (36). These are both intriguing possibilities that need to be explored in more detail.
Further insight may be gained from the results of recent in vitro studies showing that PNE alters central information processing and respiratory motor output. Specifically, PNE blunts the respiratory motor response to exogenous nicotine (43), while enhancing the response to exogenous agonists of GABAA, glycine, and AMPA receptors (33, 34, 43). These data suggest that PNE disrupts the complex changes in neurotransmitter receptor expression that occur in respiratory neurons over the normal course of development (61), leading to subtle and complex changes in ventilatory control (reviewed in Refs. 16, 17). Interestingly, hypoxia increases the release of both inhibitory and excitatory neurotransmitters in the brain stem (5, 24, 31, 44, 55) and increases both glutamatergic and GABAergic excitatory and inhibitory postsynaptic currents in rat neocortex (14). Taken together, these data suggest that the ventilatory response to hypoxia or hyperoxia could differ, depending on the relative release of inhibitory and excitatory neurotransmitters, as well as the postsynaptic expression of neurotransmitter receptors.
Influence of PNE on ventilatory responses to changing inspired carbon dioxide tension.
PNE significantly blunted the hypercapnic ventilatory response at every age examined and at all three levels of hyperoxic hypercapnia that we studied. These observations are consistent with recent results showing that PNE blunted the ventilatory response to severe hypercapnia (10% inspired CO2 for 10 min) in mice (11). Nevertheless, there are some important differences between the two studies. For example, Eugenin et al. (11) found an approximately twofold difference in the ventilatory response of control and nicotine-exposed pups on P0 and P1, a smaller but still significant difference on P3, but no difference between the groups on P8. In contrast, we found that the influence of PNE on the hypercapnic ventilatory response in rat pups persisted for the entire 18-day study period, at all three levels of inspired CO2. Although this could be explained by a species difference in the timing of nervous system development, predictive models suggest that rat and mouse nervous systems develop at the same rate (7). Adult mice do metabolize nicotine more effectively than adult rats (29), but it is not clear if this is true in neonates. Another factor could be species differences in the nicotine-mediated release of neurotransmitters, which greatly modulates respiratory rhythm generation in neonatal rats (16, 18, 49). Interestingly, nicotine-evoked catecholamine release from rat hippocampal slices exceeds that in the hippocampal slice from mouse brain by a factor of almost eight (48). These data suggest that differences in nicotine-mediated modulation of neurotransmitter release in the vicinity of respiration-related neurons may provide clues to the different responses in nicotine-exposed neonatal rats and mice older than 8 days of age.
An important similarity between our results and those of Eugenin et al. (11) is that the reduced hypercapnic ventilatory response in the nicotine-exposed animals is due primarily to a lower Vt, although f was also reduced in nicotine-exposed mice, unlike the rat pups studied here. These observations are consistent with other data showing that the rise in V̇i with hypercapnia in neonatal rats is largely due to increased Vt (59) as it is in adult rats (1, 2). Interestingly, PNE does not appear to alter the hypercapnic response in human neonates (30), although, in that study, the hypercapnic gases were mixed with air instead of hyperoxic gas, raising the possibility that CO2 sensing by carotid bodies and central chemoreceptors may have interacted in a complex manner that obscured a PNE effect on the hypercapnic ventilatory response (see Refs. 15, 60). As discussed above, PNE had no influence on the in vitro discharge rate of carotid sinus nerve afferents in response to severe hypercapnia (3), suggesting that the effects of PNE influence central chemosensing mechanisms (11). However, nicotine exposure also alters the lung mechanical response to hypoxia in lambs (47), suggesting that PNE may influence the ventilatory response to blood-gas alterations independently of changes in chemoreceptor reflexes. Further study will be needed to explain the significant reduction in the hypercapnic sensitivity of nicotine-exposed neonatal rats.
Caveats pertaining to the repeated-measures study design.
While repeated measures are generally considered to provide more statistical power, multiple exposures to hypoxia and hypercapnia may confound development of ventilatory responses in the neonates that are the subject of this study. However, the saline-exposed neonates that we used as the control group should correct for the influence of multiple exposure effects, since all animals were treated exactly the same, except for the contents of the osmotic minipump implanted into the pregnant dams. As a result, the probability of any systematic effect of multiple exposures to hypoxia or hypercapnia would be the same in both groups, with any remaining effects due to PNE. Another issue that could confound developmental studies in both the repeated-measures design that we used, and also in cross-sectional experimental designs, are litter-based genetic effects. However, genetic effects are more likely to be problematic in cross-sectional study designs, where measurements on several animals from a single litter are made, for example, on P1 and are compared with another group from a different litter that are studied on a different day. In this study, we used a few pups from each of four litters in both saline-exposed and nicotine-exposed cases and studied each of the pups repeatedly. Thus a systematic genetic effect is highly unlikely. Moreover, our laboratory has tracked both in vivo and in vitro responses of animals belonging to the same litter for several years now, as we use nicotine exposure routinely also in our in vitro studies (18, 33, 34, 42). To date, we have not identified any litter-related differences in baseline ventilation, body mass, or in vitro C4 ventral nerve root frequency in litters derived from either saline-exposed or nicotine-exposed dams.
Physiological relevance.
The true physiological consequence of an obstructive or central apnea is combined hypoxia and hypercapnia, with the magnitude increasing with apnea duration. Here we showed that PNE blunted the ventilatory response to combined hypoxia/hypercapnia. Interestingly, these effects were significant at all ages studied, even though the between-group differences in hypoxic sensitivity were significant only on P1 and P3. This suggests that PNE exerts its major effects on mechanisms controlling sensitivity to changes in carbon dioxide/pH in the blood and/or cerebrospinal fluid, which could occur during central or obstructive apneas, or when their face is trapped in bedding, resulting in CO2 rebreathing (6).
GRANTS
These studies were generously supported by the American Heart Association (0255970Z).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Bailey EF, Fregosi RF. Coordination of intrinsic and extrinsic tongue muscles during spontaneous breathing in the rat. J Appl Physiol 96: 440– 449, 2004. [DOI] [PubMed] [Google Scholar]
- 2. Bailey EF, Jones CL, Reeder JC, Fuller DD, Fregosi RF. Effect of pulmonary stretch receptor feedback and CO2 on upper airway and respiratory pump muscle activity in the rat. J Physiol 532: 525– 534, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bamford OS, Carroll JL. Dynamic ventilatory responses in rats: normal development and effects of prenatal nicotine exposure. Respir Physiol 117: 29– 40, 1999. [DOI] [PubMed] [Google Scholar]
- 4. Bamford OS, Schuen JN, Carroll JL. Effect of nicotine exposure on postnatal ventilatory responses to hypoxia and hypercapnia. Respir Physiol 106: 1– 11, 1996. [DOI] [PubMed] [Google Scholar]
- 5. Burton MD, Kazemi H. Neurotransmitters in central respiratory control. Respir Physiol 122: 111– 121, 2000. [DOI] [PubMed] [Google Scholar]
- 6. Carroll JL. Developmental plasticity in respiratory control. J Appl Physiol 94: 375– 389, 2003. [DOI] [PubMed] [Google Scholar]
- 7. Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience 105: 7– 17, 2001. [DOI] [PubMed] [Google Scholar]
- 8. Cummings KJ, Frappell PB. Breath-to-breath hypercapnic response in neonatal rats: temperature dependency of the chemoreflexes and potential implications for breathing stability. Am J Physiol Regul Integr Comp Physiol 297: R124– R134, 2009. [DOI] [PubMed] [Google Scholar]
- 9. Donnelly DF. Developmental aspects of oxygen sensing by the carotid body. J Appl Physiol 88: 2296– 2301, 2000. [DOI] [PubMed] [Google Scholar]
- 10. Eden GJ, Hanson MA. Maturation of the respiratory response to acute hypoxia in the newborn rat. J Physiol 392: 1– 9, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eugenin J, Otarola M, Bravo E, Coddou C, Cerpa V, Reyes-Parada M, Llona I, von Bernhardi R. Prenatal to early postnatal nicotine exposure impairs central chemoreception and modifies breathing pattern in mouse neonates: a probable link to sudden infant death syndrome. J Neurosci 28: 13907– 13917, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fewell JE, Smith FG. Perinatal nicotine exposure impairs ability of newborn rats to autoresuscitate from apnea during hypoxia. J Appl Physiol 85: 2066– 2074, 1998. [DOI] [PubMed] [Google Scholar]
- 13. Fewell JE, Smith FG, Ng VK, Wong VH, Wang Y. Postnatal age influences the ability of rats to autoresuscitate from hypoxic-induced apnea. Am J Physiol Regul Integr Comp Physiol 279: R39– R46, 2000. [DOI] [PubMed] [Google Scholar]
- 14. Fleidervish IA, Gebhardt C, Astman N, Gutnick MJ, Heinemann U. Enhanced spontaneous transmitter release is the earliest consequence of neocortical hypoxia that can explain the disruption of normal circuit function. J Neurosci 21: 4600– 4608, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Forster HV, Martino P, Hodges M, Krause K, Bonis J, Davis S, Pan L. The carotid chemoreceptors are a major determinant of ventilatory CO2 sensitivity and of PaCO2 during eupneic breathing. Adv Exp Med Biol 605: 322– 326, 2008. [DOI] [PubMed] [Google Scholar]
- 16. Fregosi R. Influence of prenatal nicotine exposure on development of neurotransmission in central respiratory neurons. In: Sleep and Breathing in Children, edited by Marcus CL, Carroll JL, Donnelly DF, Loughlin GM. New York: Informa Health Care, 2008, p. 341– 362. [Google Scholar]
- 17. Fregosi RF, Pilarski JQ. Prenatal nicotine exposure and development of nicotinic and fast amino acid-mediated neurotransmission in the control of breathing. Respir Physiol Neurobiol 164: 80– 86, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fregosi RF, Pilarski JQ. Prenatal nicotine exposure and development of nicotinic and fast amino acid-mediated neurotransmission in the control of breathing. Respir Physiol Neurobiol 164: 80– 86, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gauda EB, Cooper R, Akins PK, Wu G. Prenatal nicotine affects catecholamine gene expression in newborn rat carotid body and petrosal ganglion. J Appl Physiol 91: 2157– 2165, 2001. [DOI] [PubMed] [Google Scholar]
- 20. Gauda EB, Lawson EE. Developmental influences on carotid body responses to hypoxia. Respir Physiol 121: 199– 208, 2000. [DOI] [PubMed] [Google Scholar]
- 21. Genest SE, Gulemetova R, Laforest S, Drolet G, Kinkead R. Neonatal maternal separation and sex-specific plasticity of the hypoxic ventilatory response in awake rat. J Physiol 554: 543– 557, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gentry CL, Lukas RJ. Regulation of nicotinic acetylcholine receptor numbers and function by chronic nicotine exposure. Curr Drug Targets CNS Neurol Disord 1: 359– 385, 2002. [DOI] [PubMed] [Google Scholar]
- 23. Hafstrom O, Milerad J, Sandberg KL, Sundell HW. Cardiorespiratory effects of nicotine exposure during development. Respir Physiol Neurobiol 149: 325– 341, 2005. [DOI] [PubMed] [Google Scholar]
- 24. Hehre DA, Devia CJ, Bancalari E, Suguihara C. Brainstem amino acid neurotransmitters and ventilatory response to hypoxia in piglets. Pediatr Res 63: 46– 50, 2008. [DOI] [PubMed] [Google Scholar]
- 25. Heindel JJ, vom Saal FS. Role of nutrition and environmental endocrine disrupting chemicals during the perinatal period on the aetiology of obesity. Mol Cell Endocrinol 304: 90– 96, 2009. [DOI] [PubMed] [Google Scholar]
- 26. Huang YH, Brown AR, Costy-Bennett S, Luo Z, Fregosi RF. Influence of prenatal nicotine exposure on postnatal development of breathing pattern. Respir Physiol Neurobiol 143: 1– 8, 2004. [DOI] [PubMed] [Google Scholar]
- 27. Kinkead R, Genest SE, Gulemetova R, Lajeunesse Y, Laforest S, Drolet G, Bairam A. Neonatal maternal separation and early life programming of the hypoxic ventilatory response in rats. Respir Physiol Neurobiol 149: 313– 324, 2005. [DOI] [PubMed] [Google Scholar]
- 28. Kinkead R, Joseph V, Lajeunesse Y, Bairam A. Neonatal maternal separation enhances dopamine D2-receptor and tyrosine hydroxylase mRNA expression levels in carotid body of rats. Can J Physiol Pharmacol 83: 76– 84, 2005. [DOI] [PubMed] [Google Scholar]
- 29. Kyerematen GA, Morgan M, Warner G, Martin LF, Vesell ES. Metabolism of nicotine by hepatocytes. Biochem Pharmacol 40: 1747– 1756, 1990. [DOI] [PubMed] [Google Scholar]
- 30. Lewis KW, Bosque EM. Deficient hypoxia awakening response in infants of smoking mothers: possible relationship to sudden infant death syndrome. J Pediatr 127: 691– 699, 1995. [DOI] [PubMed] [Google Scholar]
- 31. Lin J, Suguihara C, Huang J, Hehre D, Devia C, Bancalari E. Effect of N-methyl-d-aspartate-receptor blockade on hypoxic ventilatory response in unanesthetized piglets. J Appl Physiol 80: 1759– 1763, 1996. [DOI] [PubMed] [Google Scholar]
- 32. Liu Q, Lowry TF, Wong-Riley MT. Postnatal changes in ventilation during normoxia and acute hypoxia in the rat: implication for a sensitive period. J Physiol 577: 957– 970, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Luo Z, Costy-Bennett S, Fregosi RF. Prenatal nicotine exposure increases the strength of GABA(A) receptor-mediated inhibition of respiratory rhythm in neonatal rats. J Physiol 561: 387– 393, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Luo Z, McMullen NT, Costy-Bennett S, Fregosi RF. Prenatal nicotine exposure alters glycinergic and GABAergic control of respiratory frequency in the neonatal rat brainstem-spinal cord preparation. Respir Physiol Neurobiol 157: 226– 234, 2007. [DOI] [PubMed] [Google Scholar]
- 35. Mahliere S, Perrin D, Peyronnet J, Boussouar A, Annat G, Viale JP, Pequignot J, Pequignot JM, Dalmaz Y. Prenatal nicotine alters maturation of breathing and neural circuits regulating respiratory control. Respir Physiol Neurobiol 162: 32– 40, 2008. [DOI] [PubMed] [Google Scholar]
- 36. Mifflin SW. Arterial chemoreceptor input to nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol 263: R368– R375, 1992. [DOI] [PubMed] [Google Scholar]
- 37. Mortola JP. Breathing pattern in newborns. J Appl Physiol 56: 1533– 1540, 1984. [DOI] [PubMed] [Google Scholar]
- 38. Mortola JP. Hamsters versus rats: ventilatory responses in adults and newborns. Respir Physiol 85: 305– 317, 1991. [DOI] [PubMed] [Google Scholar]
- 39. Mortola JP. Influence of temperature on metabolism and breathing during mammalian ontogenesis. Respir Physiol Neurobiol 149: 155– 164, 2005. [DOI] [PubMed] [Google Scholar]
- 40. Mortola JP, Rezzonico R, Lanthier C. Ventilation and oxygen consumption during acute hypoxia in newborn mammals: a comparative analysis. Respir Physiol 78: 31– 43, 1989. [DOI] [PubMed] [Google Scholar]
- 41. Pauly JR, Slotkin TA. Maternal tobacco smoking, nicotine replacement and neurobehavioural development. Acta Paediatr 97: 1331– 1337, 2008. [DOI] [PubMed] [Google Scholar]
- 42. Pilarski JQ, Fregosi RF. Prenatal nicotine exposure alters medullary nicotinic and AMPA-mediated control of respiratory frequency in vitro. Respir Physiol Neurobiol 169: 1– 10, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pilarski JQ, Fregosi RF. Prenatal nicotine exposure alters medullary nicotinic and AMPA-mediated control of respiratory frequency in vitro. Respir Physiol Neurobiol 169: 1– 10, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Richter DW, Schmidt-Garcon P, Pierrefiche O, Bischoff AM, Lalley PM. Neurotransmitters and neuromodulators controlling the hypoxic respiratory response in anaesthetized cats. J Physiol 514: 567– 578, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Saetta M, Mortola JP. Interaction of hypoxic and hypercapnic stimuli on breathing pattern in the newborn rat. J Appl Physiol 62: 506– 512, 1987. [DOI] [PubMed] [Google Scholar]
- 46. Saetta M, Noworaj A, Mortola JP. Cold exposure of the pregnant rat and neonatal respiration. Exp Biol 47: 177– 181, 1988. [PubMed] [Google Scholar]
- 47. Sandberg KL, Poole SD, Hamdan A, Minton PA, Sundell HW. Prenatal nicotine exposure transiently alters the lung mechanical response to hypoxia in young lambs. Respir Physiol Neurobiol 156: 283– 292, 2007. [DOI] [PubMed] [Google Scholar]
- 48. Scholze P, Orr-Urtreger A, Changeux JP, McIntosh JM, Huck S. Catecholamine outflow from mouse and rat brain slice preparations evoked by nicotinic acetylcholine receptor activation and electrical field stimulation. Br J Pharmacol 151: 414– 422, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shao XM, Feldman JL. Cholinergic neurotransmission in the preBotzinger Complex modulates excitability of inspiratory neurons and regulates respiratory rhythm. Neuroscience 130: 1069– 1081, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shi Y, Schlenker EH. Neonatal sex steroids affect ventilatory responses to aspartic acid and NMDA receptor subunit 1 in rats. J Appl Physiol 92: 2457– 2466, 2002. [DOI] [PubMed] [Google Scholar]
- 51. Simakajornboon N, Vlasic V, Li H, Sawnani H. Effect of prenatal nicotine exposure on biphasic hypoxic ventilatory response and protein kinase C expression in caudal brain stem of developing rats. J Appl Physiol 96: 2213– 2219, 2004. [DOI] [PubMed] [Google Scholar]
- 52. Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol 198: 132– 151, 2004. [DOI] [PubMed] [Google Scholar]
- 53. Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther 285: 931– 945, 1998. [PubMed] [Google Scholar]
- 54. Smith CA, Rodman JR, Chenuel BJ, Henderson KS, Dempsey JA. Response time and sensitivity of the ventilatory response to CO2 in unanesthetized intact dogs: central vs. peripheral chemoreceptors. J Appl Physiol 100: 13– 19, 2006. [DOI] [PubMed] [Google Scholar]
- 55. Soto-Arape I, Burton MD, Kazemi H. Central amino acid neurotransmitters and the hypoxic ventilatory response. Am J Respir Crit Care Med 151: 1113– 1120, 1995. [DOI] [PubMed] [Google Scholar]
- 56. Sovik S, Eriksen M, Lossius K, Grogaard J, Walloe L. A method of assessing ventilatory responses to chemoreceptor stimulation in infants. Acta Paediatr 88: 563– 570, 1999. [DOI] [PubMed] [Google Scholar]
- 57. Sovik S, Lossius K, Eriksen M, Grogaard J, Walloe L. Development of oxygen sensitivity in infants of smoking and non-smoking mothers. Early Hum Dev 56: 217– 232, 1999. [DOI] [PubMed] [Google Scholar]
- 58. St. John WM, Leiter JC. Maternal nicotine depresses eupneic ventilation of neonatal rats. Neurosci Lett 267: 206– 208, 1999. [DOI] [PubMed] [Google Scholar]
- 59. Stunden CE, Filosa JA, Garcia AJ, Dean JB, Putnam RW. Development of in vivo ventilatory and single chemosensitive neuron responses to hypercapnia in rats. Respir Physiol 127: 135– 155, 2001. [DOI] [PubMed] [Google Scholar]
- 60. Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol 572: 503– 523, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wong-Riley MT, Liu Q. Neurochemical and physiological correlates of a critical period of respiratory development in the rat. Respir Physiol Neurobiol 164: 28– 37, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


