Abstract
Using optical recording of synaptically mediated calcium transients and selective spinal lesions, we investigated the pattern of activation of spinal motoneurons (MNs) by the pontine reticulospinal projection in isolated brain stem-spinal cord preparations from the neonatal mouse. Stimulation sites throughout the region where the pontine reticulospinal neurons reside reliably activated MNs at cervical, thoracic, and lumbar levels. Activation was similar in MNs ipsi- and contralateral to the stimulation site, similar in medial and lateral motor columns that contain trunk and limb MNs, respectively, and similar in the L2 and L5 segments that predominantly contain flexor and extensor MNs, respectively. In nonlesioned preparations, responses in both ipsi- and contralateral MNs followed individual stimuli in stimulus trains nearly one-to-one (with few failures). After unilateral hemisection at C1 on the same side as the stimulation, responses had substantially smaller magnitudes and longer latencies and no longer followed individual stimuli. After unilateral hemisection at C1 on the side opposite to the stimulation, the responses were also smaller, but their latencies were not affected. Thus we distinguish two pontine reticulospinal pathways to spinal MNs, one uncrossed and the other crossed, of which the uncrossed pathway transmits more faithfully and appears to be more direct.
Keywords: motor control, descending pathways, brain stem, spinal cord, trunk, limb
the mammalian reticulospinal system, consisting of the mesencephalic, pontine, and medullary reticulospinal pathways, is crucial for the control of skeletal musculature (Baker 2011; Kuypers 1964; Lemon 2008; Perreault and Glover 2013; Peterson 1979; Pettersson et al. 2007), but the neural mechanisms by which it initiates and regulates movement are far from understood.
Electrical stimulation has been a key method in studying the organization of the mammalian reticulospinal system, revealing excitatory reticulospinal connections (mono- and polysynaptic) with axial motoneurons (MNs), proximal and distal limb MNs, and digit MNs in a variety of species (monkey: Davidson and Buford 2006; Riddle et al. 2009; cat: Drew and Rossignol 1990b; Galea et al. 2010; Grillner et al. 1968; Jankowska et al. 2003; Lloyd 1941; Peterson et al. 1979; Sprague and Chambers 1954; Wilson and Yoshida 1969; rat: Bolzoni et al. 2013; Floeter and Lev-Tov 1993; Umeda et al. 2010; and mouse: Alstermark and Ogawa 2004; Szokol et al. 2008). However, many studies have not differentiated among different areas of the reticular formation (RF) that project to the spinal cord. For example, stimulation of the medial longitudinal fasciculus (MLF), in which many (but certainly not all) reticulospinal axons project, is often used as a proxy for stimulating reticulospinal projections (Alstermark and Ogawa 2004; Edgley et al. 2004; Floeter et al. 1993; Grillner et al. 1968; Jankowska et al. 2003; Riddle et al. 2009). Although reasonable for many purposes, for instance to compare reticulospinal pathways with other descending pathways, this approach does little to address the internal organization of the reticulospinal system.
The existence of an internal organization by which reticulospinal neurons in different regions of the pontomedullary RF have different functional relationships with axial and limb musculature has been suggested by earlier electrophysiological studies in the cat (Drew and Rossignol 1990a,b; Perreault et al. 1993; Peterson et al. 1979) and has received renewed attention due to new technological opportunities that allow for high-throughput investigation of the reticulospinal connections. In recent work on the isolated brain stem and spinal cord of the neonatal mouse (Perreault and Glover 2013), we used optical recording to characterize medullary reticulospinal connections to MNs and interneurons of lumbar segments and demonstrated a previously undescribed mediolateral organization related to the activation of axial and hindlimb musculature (Szokol et al. 2008, 2011; Szokol and Perreault 2009). Here, using the same high-throughput optical recording approach in the neonatal mouse, we begin an investigation of pontine reticulospinal (pRS) connections to spinal MNs. The principal goals have been to establish appropriate parameters for selective and focal electrical stimulation of the pRS neurons and to determine the laterality and longitudinal extent of functional connections along the rostrocaudal axis of the spinal cord. We find that pRS neurons reliably activate MNs in cervical, thoracic, and lumbar segments through a faithfully transmitting uncrossed and a less faithfully transmitting, probably less direct, crossed pathway. Thus, already at birth, the pRS projection is functional and exhibits a widespread pattern of influence on spinal MNs. Parts of this work have been published previously in abstract form (Sivertsen et al. 2011, 2012).
METHODS
Brain stem-spinal cord preparation.
Experiments were performed on brain stem and spinal cord preparations (n = 83) isolated from wild-type mice [Hsd:ICR (CD-1) strain; Harlan] at postnatal (P) days 0–2. After deep anesthesia with isoflurane, pups were decerebrated by transecting the brain with a spatula inserted at a 60–65° angle from just rostral to the superior colliculus dorsally to just above the mammillary bodies on the ventral side and submerged in ice-cold (4°C), oxygenated (95% O2-5% CO2), low-calcium, “dissection” artificial cerebrospinal fluid (d-ACSF; containing in mM: 250 glycerol, 2 KCl, 11 d-glucose, 0.15 CaCl2, 2 MgSO4, 1.2 NaH2PO4, 5 HEPES, and 25 NaHCO3). Animals were then eviscerated, and the brain stem-spinal cord with the dorsal and ventral roots (VRs) attached was carefully dissected out. To maximize oxygenation of the pons, d-ACSF was exchanged every 5 min during the dissection, and the cerebellum was removed (Fig. 1A).
Fig. 1.
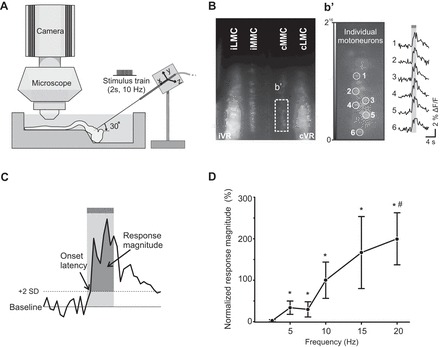
Imaging pontine reticular formation (PRF)-evoked calcium responses in spinal motoneurons (MNs) of the isolated brain stem-spinal cord mouse preparation and effective PRF stimulation parameters. A: diagram of experimental setup. Spinal MNs in the medial and lateral motor columns (MMC and LMC) of C6, T7, and/or L2 segments are labeled with the fluorescent calcium indicator CGDA in an isolated brain stem-spinal cord neonatal mouse preparation. After labeling, the preparation is transferred to a recording chamber with circulating oxygenated artificial cerebrospinal fluid, and a tungsten microelectrode is inserted in the PRF to activate pontine reticulospinal (pRS) neurons electrically. Changes in MN fluorescence before, during, and after PRF stimulation are visualized with a ×40 water-immersion objective and recorded with a charge-coupled device (CCD) camera. B: low-magnification image of calcium fluorescence in MMC and LMC MNs in the L2 segment a few hours after applying CGDA to the ipsilateral (i) and contralateral (c) ventral roots (VRs). Laterality is expressed relative to the side of the stimulation in the pons. b′, Left: high-magnification image (16-bit) of a single frame (1 out of 480 frames) showing baseline fluorescence intensity in a subset of cMMC MNs. Right: changes in fluorescence (F) during PRF stimulation (2-s train, 10 Hz, 100 μA or stimulus intensity of 2 T) during 50 consecutive frames shown as waveforms for 6 cMMC MNs (labeled 1–6). C: measurement method: a PRF-evoked response in individual MNs was defined as a positive waveform deflection that remained above the detection limit (mean prestimulation baseline + 2 SD) throughout the entire duration of the stimulation train. The area between the positive waveform deflection and the detection limit (darker shaded area) was defined as the response magnitude. D: graph displaying the magnitude-frequency curve for PRF-evoked responses in LMC MNs of the L2 segment when stimulating with 2-s trains at 2 T and 2.5 Hz (n = 8), 5 Hz (n = 8), 7.5 Hz (n = 7), 10 Hz (n = 8), 15 Hz (n = 6), and 20 Hz (n = 7). Each point represents a grand average (average across several preparations) with ±1 SE expressed as a percentage of the grand average response at 10 Hz. Asterisks and hash symbol indicate statistically significant difference (P < 0.05) from responses obtained at 2.5 and 5 Hz, respectively.
All efforts were made to minimize the number of animals used and their suffering in accordance with the European Commission Council Directive 86/609/EEC and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All procedures were approved by the National Animal Research Authority in Norway.
Retrograde labeling of pRS neurons.
pRS neurons were retrogradely labeled with tetramethylrhodamine-conjugated dextran amine (RDA; 3 kDa; Molecular Probes; Auclair et al. 1999; Glover 1995) to map their locations (n = 13) or with Calcium Green-1 dextran amine (CGDA; 3 kDa; Molecular Probes) to assess the extent of current spread during stimulation within the pontine RF (PRF; n = 4; see below). After dissecting out the brain stem and cervical spinal cord in ice-cold d-ACSF, the preparation was transferred to room temperature ACSF (containing in mM: 128 NaCl, 3 KCl, 11 d-glucose, 2.5 CaCl2, 1 MgSO4, 1.2 NaH2PO4, 5 HEPES, and 25 NaHCO3). A transverse slit was made at cervical segments C1-C2 using fine microdissection scissors. One tip of the scissors was inserted at the midline, the other tip determining the lateral extent of the slit. Immediately after making the slit, a dextran amine crystal was applied. For the 1st 5 min, a high concentration of dextran amine was maintained by applying new crystals. After 10–15 min, the remaining dextran amine was flushed away. The tracer was applied to the ventral and ventrolateral funiculi unilaterally (RDA) or the ventral funiculus alone bilaterally (CGDA). The tracer was allowed to transport retrogradely in the dark for either 9–14 h for RDA or 5 h for CGDA. In the latter case, since the preparations were used for electrophysiological experiments, the labeling time was a compromise between intensity of labeling and tissue health.
The pattern of labeling of pRS and other neuron populations was used to define operationally the extent of the PRF that was subjected to electrical stimulation (see below). We defined the PRF as the quadrangular region encompassing the full mediolateral and dorsoventral extent of the pRS neuron population and stretching from the rostral limit of labeled pRS neurons to the rostral limit of labeled raphespinal neurons. The latter limit is in register with the boundary between the 4th and 5th rhombomeric domains and thus represents a good approximation of the transition between pons and medulla (Auclair et al. 1999).
Retrograde labeling of MNs and optical recording of calcium responses.
MNs in C6, thoracic segment (T) 7, and lumbar segments (L) 2 and 5 were labeled retrogradely with CGDA. Labeling was performed at room temperature after transferring the preparation to oxygenated ACSF. MNs in both the medial and lateral motor columns (MMC and LMC) were labeled by applying crystals of CGDA to cut VRs for about 1–3 min. The VRs were cut close to the VR exit, and the CGDA was retrogradely transported in the dark for 3 h. Figure 1B shows an example of retrogradely labeled MNs in the MMC and LMC of L2.
After labeling, the preparation was transferred to a recording chamber with a Sylgard substrate (total chamber volume of 19 ml) and pinned ventral side up using minuten pins inserted through the mesencephalon, sacral spinal cord, dorsal roots, and remaining dura. As shown in Fig. 1A, the Sylgard substrate of the chamber was molded to ensure insertion of the microelectrode at the appropriate angle (see below). The chamber was perfused with oxygenated ACSF during (8 ml/min) and between (17 ml/min) Ca2+ recordings.
CGDA-labeled MNs were visualized using the ×40 water-immersion objective (LUMPlanFl; 0.8 NA; Olympus) of an epifluorescence microscope (Axioskop; Carl Zeiss, Oberkochen, Germany) equipped with a direct current-powered 100-W halogen lamp and excitation and emission filters (band pass 450–490 nm and long pass 515 nm, respectively). A video zoom (44 C 1/3“; Carl Zeiss) installed between the microscope and the charge-coupled device (CCD) camera (Cascade 650; Photometrics, Texas Instruments) was used to ensure that the camera chip was exposed to the entire field of view. Video images (16-bit) were acquired using the acquisition software MetaMorph 7.7 (Molecular Devices) at a digitization rate of either 4 frames per second (gain 2 or 3, binning 2, recording sessions of 45 s) or 96 frames per second (gain 3, binning 4, recording sessions of 20 or 30 s). Low- and high-frame-rate recordings were used to analyze response magnitudes and latencies, respectively (see Analysis of calcium responses).
Cervical spinal hemisections.
In a subset of preparations (n = 13), we made unilateral, transverse hemisections at the level of C1-C2 before calcium recording. We used fine microdissection scissors and checked for completeness in post hoc histological sections (see below).
Electrical stimulation.
Electrical current was delivered to the PRF using a monopolar tungsten microelectrode [Parylene-coated, shaft diameter 0.2 mm, tip diameter 1–2 μm, impedance 0.1 MΩ at 1 kHz; World Precision Instruments (WPI)] and a digital stimulator (DS8000; WPI) coupled to a stimulus isolation unit (ISO-Flex; A.M.P.I.). The microelectrode was mounted on a three-axis hydraulic oil micromanipulator (MO-103; Narishige) and inserted into the brain stem at a 30° angle from the frontal plane. Before electrode insertion, we removed the meninges to avoid tissue dimpling and electrode damage. In initial mapping experiments (see below), we made electrode insertions over a broad range covering most of the volume of the PRF and including nearby surrounding structures. Based on this initial mapping, we established a volume consistent with PRF-specific stimulation, defined by electrode entries lying between 500 and 600 μm from the midline and 0–600 μm caudal to the rostral margin of the pontine nuclei and with a penetration distance of 300–1,300 μm.
The stimulus intensity threshold (T) for evoking a detectable increase in fluorescence in spinal MNs (see Analysis of calcium responses) was determined at the beginning of each experiment (on obtaining the 1st effective stimulation site). One to five sites (separated by 200 μm) were stimulated along each microelectrode track. Stimulus intensity thresholds in nonhemisected preparations ranged between 20 and 60 μA (44 ± 2 μA, mean ± SE, n = 37). During data acquisition, we used in all experiments a stimulus intensity of 2 T, which consequently had a range of 40–120 μA. For all other stimulation parameters, we used as a starting point the parameters applied in our previous studies of the medullary reticulospinal system (Szokol et al. 2008; Szokol and Perreault 2009). However, concerns about synaptic fatigue [which was not observed in our previous studies but manifested here as a decrease in response magnitude at different recording intervals between consecutive recordings (n = 4)] prompted us to reduce the train duration from 5 to 2 s or to 1 s in experiments where responses in MNs were assessed in more than one segment in the same preparation.
In a series of control experiments (n = 9; Fig. 1D), we also tested the effect of stimulation frequency on the magnitude of the response evoked in MNs of the ipsilateral MMC. Response magnitude and variability increased with stimulation frequency. Response variability was noticeably larger at 15 and 20 Hz. In fact, compatible with synaptic fatigue, many MNs failed to respond after stimulating at these frequencies. Thus we chose 10 Hz as our standard stimulation frequency.
Localization of stimulation sites.
To localize electrical stimulation sites at the end of experiments, we used three different approaches. First, we coated the stimulation electrodes with a solution of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI; Molecular Probes), saturated in absolute alcohol, to mark the electrode trajectories fluorescently (DiCarlo et al. 1996). Second, we registered the coordinates of the electrode manipulator for each stimulation site. Finally, we made an electrolytic lesion (Szokol and Perreault 2009) at the last site stimulated (in experiments involving calcium recording from spinal MNs) or at each stimulation site (in experiments involving calcium recording from pRS neurons). In nearly all calcium recording experiments performed in this study (82/83 total animals), the locations of the stimulation sites were confirmed with post hoc histology. In the experiments involving recording from pRS neurons, electrode coordinates and histological assessment in overview and close-up images were used to determine the distances between each stimulation site and each individual pRS neuron recorded from, using trigonometry in three dimensions.
Assessment of current spread during PRF stimulation.
We assessed current spread by quantifying the local effects of PRF stimulation measured as calcium transients in nearby CGDA-labeled pRS neurons. We used a current intensity of 120 μA because this was the highest current strength used in the study. To assess current spread, we combined retrograde labeling of pRS neurons with CGDA and calcium imaging to measure the changes in fluorescence intensity in CGDA-labeled pRS neurons. To improve optical access to the CGDA-labeled pRS neurons, a superficial layer of tissue was shaved from the ventral surface of the brain stem using a 15° angled, 5-mm disposable knife (Fine Science Tools).
Histology.
After each experiment, the brain stem and spinal cord were fixed in 4% paraformaldehyde for 4–72 h, cryoprotected in 20% sucrose, embedded in O.C.T. (Tissue-Tek), frozen, and cryostat-sectioned serially at 50 μm in the parasagittal or transverse plane. The sections were stained with methylene blue (10 s in 0.3% wt/vol solution; Difco Laboratories, West Molesey, Surrey, United Kingdom), coverslipped in gelatin-glycerol, and photographed using a ProgRes C14 camera (Jenoptik) mounted on an AX70 microscope (Olympus). Photographs of the DiI-labeled electrode tracks in the brain stem (see above) were taken both before and after methylene blue staining.
Analysis of calcium responses.
To synchronize electrical stimulation and optical recordings, a digital pulse from a digitizer (Digidata 1320A; Molecular Devices) was sent to both the stimulator and the CCD camera. The timing markers for the PRF stimulation and the gating pulse from the CCD camera were recorded by the digitizer at 500 Hz.
Fluorescence intensity was measured within separate digital apertures or regions of interest (ROIs). The ROIs were manually positioned over the somata of individual MNs (small ROIs) or over many MNs in one motor column (large ROIs). The MN somata (small ROIs) were selected according to labeling intensity and location within the same focus plane. The selection was semirandom and designed to cover as much of the field of view as possible. Within each recording, fluorescence intensity in both small and large ROIs was averaged across all the pixels. The responses were quantified as changes in fluorescence (ΔF) divided by the mean baseline fluorescence (F0) measured before the stimulation [ΔF/F0 = (F − F0)/F0]. Data from MetaMorph were converted into text files using a custom-made program and imported to the analysis software Clampfit 9.0 (Molecular Devices) where they were expressed as waveforms. A detectable calcium response was defined as a continuous positive waveform deflection that exceeded a detection limit set at 2 SD above the mean prestimulation baseline.
Measuring response magnitudes.
To assess the magnitude of the calcium responses, we used low-frame-rate (4 Hz) recordings. Response magnitude was defined as the area under the waveform during the entire duration of the stimulus train (Fig. 1C). At each stimulation site, one recording per motor column was obtained. Six MNs per motor column were analyzed using small ROIs, and the response magnitudes were averaged to obtain a mean magnitude per motor column. Mean magnitudes from different stimulation sites (2-8) were also averaged, producing one grand mean magnitude per column per preparation.
Measuring response onset latencies.
To assess the onset latencies of the responses, we used high-frame-rate (96 Hz) recordings and only data from large ROIs (see above). Large ROI responses were averaged from 1 to 15 recordings in each preparation. Onset latencies were calculated in 2 ways: 1) as the time from the 1st stimulation pulse to the onset of the average response; or 2) as the time from the stimulation pulse that immediately preceded the onset of the average response. The latter was possible in all cases in which response waveforms exhibited discrete events that followed stimuli one-for-one, permitting an unambiguous alignment of individual stimuli and individual responses (see results).
Statistics.
Values are reported as median (range) or mean ± SE unless stated otherwise. Nonparametric statistical tests (Mann-Whitney, Wilcoxon signed-rank, and sign tests) were performed using the statistics software SPSS (IBM), and P values of 0.05 or less were considered statistically significant.
RESULTS
General overview of experiments.
The PRF was stimulated in 42 spinally intact and 13 spinally hemisected brain stem-spinal cord preparations in which calcium recordings were obtained from MNs in 1 or more of the C6 (n = 11), T7 (n = 8), L2 (n = 39), and L5 (n = 8) segments and in 4 ventrally shaved brain stem preparations (see methods). In brain stem-spinal cord preparations, recordings were obtained from MMC and/or LMC on 1 or both sides of the midline. In each motor column, recordings were made from single MNs and/or populations of MNs using small and/or large ROIs (see methods). In ventrally shaved brain stem preparations, recordings were made from single pRS neurons using small ROIs. Unless stated otherwise, sample sizes (n) refer to the number of preparations.
Threshold for MN activation by electrical stimulation of the PRF.
The mean threshold current (T) for a detectable activation of the MNs by electrical microstimulation of the PRF was 44 ± 2 μA, similar to the threshold current previously reported for electrical microstimulation of the medullary RF (Szokol et al. 2008). To ensure clearly detectable and stable responses, we routinely stimulated at 2 T (mean = 87 ± 3 μA).
Assessment of current spread in the PRF.
To get an estimate of the effective stimulation volume affected by a microelectrode placed within the PRF, we labeled pRS neurons retrogradely with CGDA in shaved brain stem preparations and assessed their calcium responses while stimulating at different distances from their somata (Fig. 2, A and a′; 4 animals, 7 electrode trajectories, 5–10 stimulation sites per trajectory, total of 38 pRS neurons). For each pRS neuron analyzed, we plotted the magnitudes of the evoked calcium responses as a function of the distance between the tip of the electrode and the soma of the neuron. Using a current strength of 120 μA (the highest 2-T current strength used in this study), we found that the magnitudes of the calcium responses in single pRS neurons were inversely related to the distance from the soma to the electrode tip. As an example, the neuron marked by the gray asterisk in Fig. 2a' and inset in Fig. 2B responded with the largest calcium response when stimulation was applied closest to the soma (black lines in graph of Fig. 2B). The relationship between distance and response magnitude for most neurons resembled the curve of the inverse square of the distance (gray curves in Fig. 2B). The average distance beyond which responses could no longer be evoked was 486 μm (range 245–726 μm, n = 31 pRS neurons). Direct comparison with previously published estimates of current spread is difficult because of differences in recording method (electrophysiological vs. optical), current stimulation protocol (threshold vs. suprathreshold), brain region studied, and animal species (reviewed in: Ranck 1975; Tehovnik et al. 2006). Nevertheless, if we use our average threshold current of 44 μA for synaptic MN activation (see methods) as the threshold current for direct activation of pRS neurons, we can calculate based on the mean value of the excitability constant provided by Hentall et al. (1984) from the medulla of adult rats that the volume of PRF directly excited would be encompassed by a sphere of average radius of ∼226 μm, that is, a little less than half of the average distance of 486 μm at 120 μA.
Fig. 2.
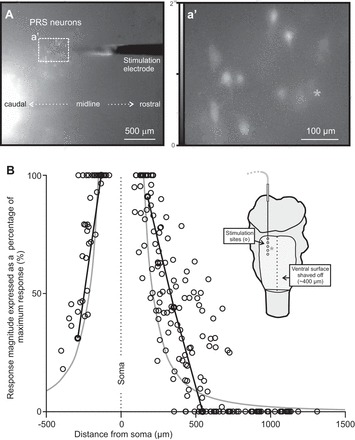
Local activation of pRS neurons by PRF stimulation. A: low-magnification image (ventral view) of a stimulation microelectrode (tip) inserted in the PRF of a brain stem preparation for which the ventral surface had been shaved and in which pRS neurons (and other spinally projecting neurons) were retrogradely labeled with CGDA. The microelectrode entered the tissue ∼750 μm rostral to the rostral edge of the field of view, which shows the CGDA-labeled pRS neurons from which calcium recordings were obtained (dotted rectangle). a′: High-magnification image (single frame) showing baseline calcium fluorescence in some of the CGDA-labeled pRS neurons surrounding the microelectrode tip shown in A. A gray asterisk is placed above the soma of pRS neuron 3 in this experiment. The same neuron is singled out in B as well (black lines in graph and gray asterisk in schematic). B: relationship between response magnitude and distance from microelectrode tip for 38 pRS neurons (4 animals, 1–2 electrode tracks per animal). For each pRS neuron, the magnitudes of responses to a 2-s stimulus train of 120 μA at 10 Hz (1 response for each site stimulated along the electrode track) are plotted as a percentage of the largest response evoked in that neuron. The distances between pRS somata and stimulation sites in 3-dimensional space are calculated with basic trigonometry using distances obtained from overview pictures similar to the 1 shown in A and histological assessments of the stimulation sites (see methods). The 2 black lines join the responses of pRS neuron 3 evoked at stimulation sites 1–3 and 4–5, respectively. The similarly orderly behavior of the other pRS neurons is concealed in the broad point-cloud distribution. Solid gray curves follow a function that describes the inverse of the squared distance (see text). The inset shows a cartoon of the experimental setup with the approximate position of pRS neuron 3 (gray asterisk) relative to the 5 sites stimulated along the electrode track (open circles).
Overlapping distributions of pRS neurons and effective stimulation sites.
The distribution of pRS neurons was determined by unilateral application of the fluorescent tracer RDA at C1/C2 (n = 13). Retrogradely labeled pRS neurons were found within a narrow volume along both the mediolateral axis (200–300 μm wide) and dorsoventral axis (250–350 μm long). pRS neurons were found mostly ipsilaterally (ipsilateral side in Fig. 3, A and B, indicated by solid black or white arrows, respectively), but a population of contralaterally labeled pRS neurons (numbering <30% of the ipsilateral pRS neurons) was also found (left of the midline in Fig. 3, A–C). The contralateral pRS neurons lay within the same domain of the PRF as the ipsilateral pRS neurons but skewed toward the more lateral, ventral, and rostral region. Thus the distribution of pRS neurons in the neonatal mouse is similar to that reported in the mouse embryo (Auclair et al. 1999) and in the adult mouse, demonstrated using different retrograde tracers (Liang et al. 2011; Vanderhorst and Ulfhake 2006). A more comprehensive account of the anatomic organization and relationships of the ipsilateral and contralateral pRS neuron populations is in preparation (M. S. Sivertsen, M.-C. Perreault, and J. C. Glover, unpublished observations).
Fig. 3.
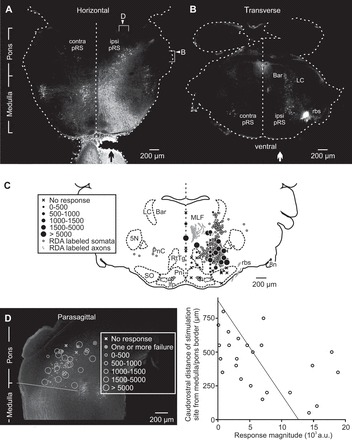
Spatial distributions of pRS neurons and effective stimulation sites overlap. A: horizontal section (50 μm) showing the spatial distribution of reticulospinal neurons retrogradely labeled by application of tetramethylrhodamine-conjugated dextran amine (RDA) to the right VF at C2 level (vertical black arrow). Reticulospinal neurons were found bilaterally both in the medulla and the pons, but the contralateral population was sparser in the pons. Right arrowhead: approximate level of the section shown in B. Right bracket: approximate range covered by the 5 consecutive sections used in C. Top arrowhead: approximate level of the section shown in D. Top bracket: approximate range covered by the 4 consecutive sections from which stimulation sites were gathered and shown in D. B: the relatively restricted and predominantly ipsilateral spatial distribution of RDA-labeled pRS neurons shown in a single 50-μm transverse section taken in the middle of the pons. On the side of the RDA application (vertical white arrow), a dorsal population of locus coeruleus (LC) and Barrington's nucleus (Bar) neurons and a ventrolateral bundle of rubrospinal axons are also labeled. C: locations of RDA-labeled pRS neurons (solid closed gray circles) and stimulation sites in 5 consecutive 50-μm transverse sections encompassed by the right bracket in A plotted on a reference section from a postnatal day 3 mouse. Stimulation sites are represented by either an X or a black circle depending on whether they evoked calcium responses in L2 MNs. The size of the black circle is proportional to response magnitude (relative units, as shown in the inset). D, left: the distribution of RDA-labeled ipsilateral pRS neurons together with the locations of stimulation sites from 4 experiments shown in a 50-μm parasagittal section. Only stimulation sites at a distance of ≤100 μm from the labeled neurons are included. The oblique solid line indicates the border between medulla and pons as used in this study. Right: graph displaying the caudorostral position of 24 stimulation sites vs. the largest response magnitude they evoked in L2 MNs. The coefficient of determination of the regression line r2 = 0.25. 5N, trigeminal motor nucleus; 8n, vestibulocochlear nerve; ipsi pRS and contra pRS, ipsilateral and contralateral pRS populations; lfp, longitudinal fasciculus of the pons; MLF, medial longitudinal fasciculus; Pn, pontine nuclei; PnC, nucleus reticularis pontis caudalis; RtTg, reticulotegmental nucleus of the pons; rbs, rubrospinal tract; SO, superior olive; a.u., arbitrary units.
We then compared the distribution of pRS neurons to the location of the stimulation sites that effectively evoked responses in lumbar MNs. For this comparison, we selected recordings from 12 experiments with identical stimulus parameters (120 μA, 2-s train, 10 Hz) and spinal segment of MN recording (L2). Figure 3C shows the effective and ineffective stimulation sites within a transverse slice of the brain stem spanning ±125 μm from the level of a standard transverse section of a P3 mouse brain stem. Nearly all effective stimulation sites were located within the area where pRS neurons were found. The large majority of the sites stimulated were within the nucleus reticularis pontis caudalis (PnC; within the caudal 2/3 of the labeled pRS population, containing predominantly large neurons; see Fig. 3C), but some sites were also in the nucleus reticularis pontis oralis (PnO; within the rostral 1/3 of the pRS population, containing predominantly smaller neurons; see Fig. 3D). Stimulation sites close to or within the MLF, where a number of pRS axons descend, also effectively evoked responses in lumbar MNs. Noneffective and less-effective sites were sometimes found within the pRS neuron population but were predominantly outside of it (Fig. 3C). No large MN responses were evoked by sites at distances of more than ∼200 μm from the pRS neuron population or from the MLF. We also observed a trend toward larger evoked responses in MNs when the electrode was placed more caudally along the rostrocaudal axis (Fig. 3D). However, this change occurred gradually, with no distinct step. Accordingly, data from stimulation sites in PnO and PnC have been pooled in subsequent analyses.
PRF stimulation activates MNs in cervical, thoracic, and lumbar segments.
PRF-evoked responses were observed in ipsilateral and contralateral MNs of C6 (ipsilateral, n = 11), T7 (ipsilateral, n = 8), L2 (ipsilateral, n = 23; contralateral, n = 12), and L5 (ipsilateral, n = 8; contralateral, n = 8) segments. Figure 4A shows responses evoked in different motor columns in C6, T7, and L2 segments while stimulating a single site in each of three different preparations. A comparison of the C6, T7, and L2 responses among the three preparations indicates that the shapes and magnitudes of the responses varied from preparation to preparation. However, in these and all other nonhemisected preparations (n = 19 in total), the PRF-evoked responses had a “sawtooth” waveform in which response steps followed individual train stimuli one-to-one. In many cases, the number of response steps equaled the number of stimuli and had equivalent temporal spacing, but in some cases the initial response step appeared to be missing. Sawtooth waveforms were common (93% of investigated motor columns) and observed in MNs of both the MMC and the LMC and on both ipsilateral and contralateral sides (see Fig. 5B for examples in contralateral MNs). The presence of individual response steps, which suggests a faithful transmission of descending signals from pRS neurons to MNs with few intercalated synaptic relays, did not appear to be related to the overall shape or magnitude of the response.
Fig. 4.
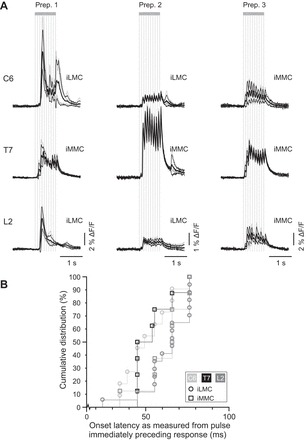
PRF-evoked responses in MNs of the cervical, thoracic, and lumbar segments. A: population responses [large regions of interest (ROIs)] evoked in motor columns of C6, T7, and L2 segments in 3 different preparations (Prep.). In each preparation, the responses in C6, T7, and L2 were evoked from the same site in the PRF. Each set of waveforms consists of a mean population response (thick black waveform) obtained by averaging the population responses from several recordings (thin gray waveforms) and ±1 SD (thin black waveforms). The high-temporal-resolution (96 Hz) recordings reveal the presence of sawtooth response patterns whereby each train stimulus (vertical gray lines) evoked a distinct calcium transient. B: cumulative distribution of response latencies measured in ipsilateral MMC (open circles) and/or LMC (open squares) in C6 (light gray), T7 (black), and L2 (dark gray) segments. Each point represents the onset latency of the mean population response in a given motor column of a given segment in a single experiment (e.g., response displayed in A). MNs in C6 and T7 often responded with shorter latencies than MNs in L2 (see main text for statistics).
Fig. 5.
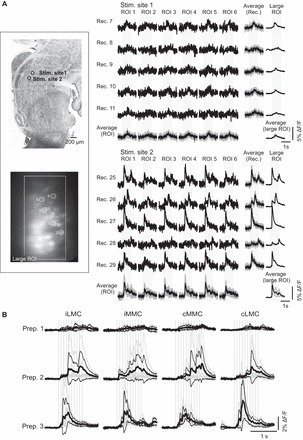
Diversity of responses elicited by different stimulation sites and similarity of responses within and between motor columns. A: example of an experiment in which responses evoked in 6 different iLMC MNs (ROI 1–6) from 2 stimulation sites are compared. Left: the location of the 2 stimulation sites is shown on a parasagittal reference section (top) and the location of the recorded L2 MNs on a background-subtracted, z-stacked image (bottom). Right: for each stimulation site, 5 recordings are shown (Rec. 7–11 and 25–29). Within each recording, the responses in the different MNs are very similar. Occasionally, the response pattern varied from 1 recording to another (for example, from Rec. 26 to 28). Even in these instances, however, the responses in the different MNs remain similar. The 2 separate sets of average responses shown at the bottom [Average (ROI)] and on the right-hand side [Average (Rec.)] show the variability across recordings for each MN and across MNs within each recording session, respectively. Average responses are shown as black waveforms, and the gray waveforms represent 1 SD above and 1 SD below these average responses. Responses across MNs within a given recording session tended to show less variability (smaller SDs). For comparison, the population response (large ROI) during each recording and the average across all recordings are also shown. B: averaged population responses (large ROIs) from a single stimulation site showing similarity of responses in iLMC, iMMC, cMMC, and cLMC in 3 different preparations. Each average was obtained from 4 to 10 individual recordings. See legend for Fig. 4A for other details.
We then compared onset latencies of the PRF-evoked responses in C6, T7, and L2 segments by measuring the time between stimulation and response onsets (n = 19). Onset latencies ranged from 24 to 170 ms with a median and range of 66 ms (34–160 ms) in C6, 50 ms (45–76 ms) in T7, and 66 ms (24–170 ms) in L2. The difference in onset latency measured in this way was statistically significant between C6 and T7 and between T7 and L2 (P = 0.04 for C6 vs. T7, P = 0.03 for T7 vs. L2, and P = 0.68 for C6 vs. L2, Wilcoxon signed-rank test). We suspected, however, that measuring latencies relative to the first stimulus of the train might underestimate the latency difference between C6 and L2, as many of the sawtooth responses in C6 evidently included failures to the first stimulus. We therefore remeasured the latencies using the time from the stimulus pulse that immediately preceded the first detectable response step in the sawtooth waveform rather than from the onset of the stimulus train. The difference in onset latency measured in this way (Fig. 4B) was significant between C6 and L2 and between T7 and L2, with medians and ranges of 49 ms (34–76 ms) in C6, 49 ms (45–76 ms) in T7, and 66 ms (24–76 ms) in L2 (P = 0.5 for C6 vs. T7, P = 0.05 for T7 vs. L2, and P = 0.01 for C6 vs. L2, Wilcoxon-signed rank test).
Altogether, these findings indicate that PRF stimulation evokes widespread, multisegmental activation of spinal MNs and that, despite substantial overlap, the onset latencies of the responses in cervical and thoracic MNs are on average shorter than in lumbar MNs.
Similarity of responses among MNs of individual motor columns and between MNs of different motor columns.
As shown in Fig. 1b′, PRF-evoked responses in MNs within a given motor column appear to have similar waveforms. To investigate this further, we compared PRF-evoked responses among MNs in individual motor columns in the C6, T7, and L2 segments using high-frequency recordings (n = 19). As illustrated in Fig. 5, we found that responses in MNs recorded in a given motor column were remarkably consistent in shape and magnitude for any given stimulation site and recording session but varied substantially as a function of stimulation site and recording session. Figure 5A shows the responses evoked in six different ipsilateral (i) LMC MNs in L2 (ROI 1–6) following stimulation at two different sites (Stim. sites 1 and 2) and in five different recording sessions at each site (Rec. 7–11 and 25–29, respectively). It can first be appreciated that the responses elicited by the two stimulation sites were quite different, those elicited by Stim. site 1 having consistently smaller amplitude, more gradual rise to peak, and simpler waveform than those elicited by Stim. site 2. The same difference was seen in responses recorded with large ROIs (Fig. 5A, last column), supporting a similarity of responses among many MNs in a given motor column when stimulating at a given site in the PRF. In addition, for each stimulation site, responses among the six MNs (ROI 1–6) within a given recording session were more similar than responses in a given MN across recording sessions. This was reflected by a lower SD of responses averaged across MNs in a given recording session than of responses averaged across recording sessions in a given MN [Average (Rec.) vs. Average (ROI)], a feature that is particularly obvious for Stim. site 2 due to the larger response amplitudes. This also held when the SDs during stimulation were normalized to the SDs before stimulation (baseline). Overall (n = 56 sets of recordings from 16 preparations), the Average (Rec.) SDs were 11% (range 1–103%) greater than baseline SDs, whereas the Average (ROI) SDs were 35% greater (range 2–185%). The differential increase was statistically significant (P < 0.001, Wilcoxon signed-rank test).
Similarity of responses was observed also across motor columns. Figure 5B compares population responses (large ROIs) in the different motor columns at the L2 level in three different preparations. The overall impression is that responses vary much more across preparations in any given motor column than they do across motor columns in any given preparation. This was true both for response waveform and magnitude. We therefore assessed response magnitude quantitatively across motor columns in segments L2 and L5. In L2 (n = 7; Fig. 5B), we found no statistically significant difference between response magnitudes in MMC and LMC MNs [iMMC vs. iLMC: P = 0.2, n = 7; contralateral (c) MMC vs. cLMC: P = 1.0, n = 8; Wilcoxon signed-rank test]. In L5 (responses not shown), differences were barely significant on the ipsilateral side (LMC responses larger) and not significant on the contralateral side (iMMC vs. iLMC: P = 0.05, n = 8; cMMC vs. cLMC: P = 0.7, n = 8; Wilcoxon signed-rank test). Because of the preferential involvement of L2 and L5 segment in the control of hindlimb flexors and extensors, respectively, we also compared column for column the response magnitudes between L2 and L5. Again, the differences were not statistically significant (L2 vs. L5: P = 0.5, U = 22 for iLMC; P = 0.07, U = 12 for iMMC; P = 0.07, U = 12 for cMMC; P = 0.3, U = 18 for cLMC; Mann-Whitney U test; n = 15).
Finally, we assessed response latency as a function of motor column in the subset of preparations in which recordings were obtained in all four motor columns (n = 5 in L2). Differences in onset latency were not statistically significant among the motor columns, suggesting that at a given segmental level the aggregate pRS inputs to ipsilateral vs. contralateral MNs and to the MMC vs. the LMC involve similar numbers of synaptic relays (P = 0.1, z = −1.60 for iMMC vs. cMMC; P = 0.6, z = −0.55 for iMMC vs. iLMC; P = 0.2, z = −1.36 for iMMC vs. cLMC; P = 0.4, z = −0.92 for cMMC vs. cLMC; P = 0.3, z = −1.09 for cMMC vs. iLMC; P = 0.1, z = −1.60 for iLMC vs. cLMC; Wilcoxon signed-rank test).
Altogether, these findings support the conclusion that whereas the magnitude and dynamics of the responses vary from one stimulation site to another, each stimulation site in the PRF exerts a widespread and similar activation of MNs, encompassing MNs innervating axial and limb muscles and flexor and extensor muscles within a limb.
Contribution of ipsilaterally and contralaterally projecting pRS neurons.
To investigate the relative contributions of the ipsilateral (uncrossed) and contralateral (crossed) pRS projections (represented differentially in the leftmost schematic of Fig. 6A), we compared PRF-evoked responses in the L2 segment in control and hemisected preparations (n = 13). Hemisections were made in the transverse plane at C1-C2 either on the same side as the PRF stimulation (middle schematic of Fig. 6A) or on the opposite side (rightmost schematic of Fig. 6A). Hemisection on the same side allowed us to study the contribution of the crossed pRS projection in isolation, whereas hemisection on the opposite side allowed us to study the contribution of the uncrossed pRS projection. All hemisections were verified histologically at the end of the experiment (see inset in Fig. 6A). In two preparations, there was uncertainty about the completeness of the hemisection; some axons appeared to be spared immediately adjacent to the midline. However, since the results from these preparations did not differ from the rest, they have been included here.
Fig. 6.
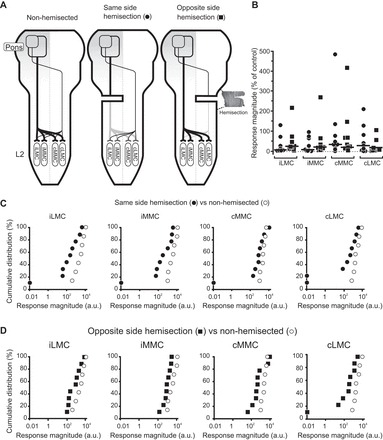
PRF-evoked responses are reduced after unilateral transverse hemisection. A: cartoons depicting the 2 pathways deemed necessary to explain our results in nonhemisected (leftmost) and hemisected (2 rightmost) preparations. The 2 pathways consist of an uncrossed pathway (thick line) and a crossed pathway (thin line) through which pRS axons contact MNs either directly or via spinal interneurons (not illustrated for the sake of simplicity). In preparations where pRF stimulation was on the same side as the hemisection, the uncrossed pathway is interrupted. In preparations where pRF stimulation was on the opposite side of the hemisection, the crossed pathway is interrupted. A horizontal section from the upper cervical cord is shown on the right-hand side of the rightmost cartoon to illustrate how the completeness of hemisections was confirmed histologically. Right: graph displaying response magnitude after same-side hemisection (closed circles) or opposite-side hemisection (solid squares) as a percentage of control responses in nonhemisected preparations for each of the 4 motor columns in L2. Each point represents the mean response in a single preparation and each thick horizontal line the median across all preparations. All individual values have been normalized to the median value from the nonhemisected preparations. The large degree of variation is partly due to variation among preparations but also to a few very eccentric outliers. B and C: graphs comparing the cumulative distributions of the magnitudes of the responses evoked in iLMC, iMMC, cMMC, and cLMC in hemisected (solid symbols) vs. nonhemisected (open circles) preparations. Again, each point represents the mean response in a single preparation. A logarithmic scale was used for the x-axis.
Comparing response magnitudes (4-Hz recordings, graph in Fig. 6A and cumulative plots in Fig. 6, B and C), PRF-evoked responses in hemisected preparations (n = 9) had smaller magnitudes than PRF-evoked responses in nonhemisected preparations (n = 7) irrespective of whether the hemisection was on the same (Fig. 6B) or opposite (Fig. 6C) side of the PRF stimulation. With hemisection on the same side as the PRF stimulation, the magnitudes of the PRF-evoked responses in the iLMC, iMMC, cMMC, and cLMC were at only 6, 8, 33, and 38% (Fig. 6A) of those evoked in nonhemisected, control preparations (P = 0.02, U = 9 for iLMC; P = 0.02, U = 10 for iMMC; P = 0.09, U = 15 for cMMC; and P = 0.04, U = 12 for cLMC; Mann-Whitney U test; n = 16). This suggests a strong contribution from the uncrossed projection to the responses in iMMC and iLMC and a smaller but still substantial contribution to the responses in cMMC and cLMC. The responses in the contralateral motor columns must be mediated either by commissural pRS axon collaterals or commissural spinal interneurons.
The fact that there were still remaining responses after same-side hemisection also suggests a contribution from the spared crossed projection. Compatible with this suggestion, we found that after opposite-side hemisection the magnitudes of the PRF-evoked responses in the iLMC, iMMC, cMMC, and cLMC were at 25, 22, 20, and 18% (Fig. 6A) of those evoked in nonhemisected, control preparations (P = 0.1, U = 16 for iLMC; P = 0.02, U = 10 for iMMC; P = 0.07, U = 14 for cMMC; and P = 0.01, U = 6 for cLMC; Mann-Whitney U test; n = 16). These findings are consistent with the conclusion that in all four motor columns the responses arise from a combination of uncrossed and crossed pRS inputs. However, the similarly large decrements in responses after removal of either input indicate that these inputs do not summate linearly. Rather, as argued previously for the medullary reticulospinal projections (Szokol et al. 2008), it is likely that the various inputs to any given motor column interact, resulting in nonlinear summation. Attempting a strict additive analysis is therefore of limited value. It is noteworthy that the current thresholds in the hemisected preparations were noticeably higher than in nonhemisected preparations (56 ± 3 vs. 40 ± 5 μA, respectively; P = 0.003, U = 16, Mann-Whitney U test; n = 16), suggesting the possibility of additional, mutual influences between the pRS neurons and the spinal cord. The implication is that the response magnitudes in the hemisected preparations may actually have been smaller (since we had to stimulate more strongly to evoke the responses).
The uncrossed and crossed pRS projections differed with regard to the waveforms of the responses they evoked in MNs. In preparations with same-side hemisection (spared crossed projection), we never observed sawtooth waveforms (Fig. 7A, top row). In contrast, in all preparations with opposite-side hemisection (spared uncrossed projection), responses had sawtooth waveforms as in nonhemisected preparations (Fig. 7A, bottom row). Sawtooth waveforms were observed in all L2 motor columns examined (iLMC 4/4, iMMC 3/3, cMMC 2/2, and cLMC 2/2). This is compatible with a more direct uncrossed pRS projection and a less direct crossed pRS projection (as shown schematically in Fig. 6A).
Fig. 7.
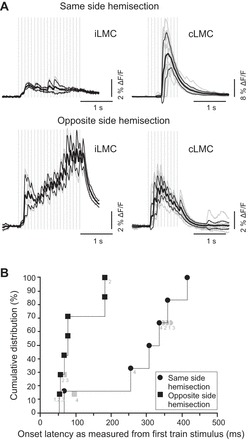
Sawtooth responses may be the result of more faithful and direct transmission in the uncrossed pRS pathway. A: high-temporal-resolution recordings of population responses (large ROIs) evoked in iLMC and cLMC by stimulating the PRF on the same side (top row) or the opposite side (bottom row) of a cervical hemisection. Recordings for the 2 conditions were obtained from the same preparation after moving the electrode from 1 side of the brain stem to the other. Activation of the uncrossed pathway, but not the crossed pathway, produced sawtooth responses. For other details, see the legend for Fig. 4A. B: cumulative distributions of response latencies in iLMC (points unlabeled or marked with light gray label 1), iMMC (points marked with gray label 2), cMMC (points marked with light gray label 3), or cLMC (points marked with light gray label 4) on the same side of hemisection (solid black circles) and opposite side of hemisection (solid black squares) recording configurations. In 3 experiments, response latencies were measured in >1 motor column (indicated by light gray symbols). In all cases, the response latencies were included in the same cumulative distribution with the shortest 1 displayed with a solid black symbol. In the 1 experiment where the latency values in different motor columns were identical (due to finite temporal resolution of the recordings), the overlapping points were identified with light gray labels separated by a comma. The data clearly show that activation of the uncrossed pRS pathway produced shorter response latencies than activation of the crossed pRS pathway.
The onset latencies of the responses in the preparations with only the crossed pRS projection intact ranged from 66 to 414 ms (median of 320 ms), whereas the onset latencies in the preparations with only the uncrossed pRS projection intact ranged from 53 to 181 ms (median of 76 ms; Fig. 7B). Response latencies in preparations with only the crossed pRS projection intact were significantly longer than those in nonhemisected preparations (P = 0.01, U = 6.5, Mann-Whitney U test), which was not the case for preparations with only the uncrossed pRS projection intact (P = 0.4, U = 30, Mann-Whitney U test). This provides additional support to the suggestion that the uncrossed pRS projection is more direct than the crossed pRS projection.
DISCUSSION
General overview of salient results.
In this study, we have begun a physiological characterization of the pRS projection in the newborn mouse using high-throughput optical recording of synaptically evoked calcium transients to obtain a broad picture of the organization of inputs to MNs at different segmental levels. We find that 1) focal electrical stimulation of the PRF where the pRS neurons reside leads to consistent activation of MNs at cervical, thoracic, and lumbar levels, 2) the pRS projection includes ipsilaterally descending (uncrossed) and contralaterally descending (crossed) axons, 3) similar activation occurs in MMC and LMC both ipsilateral and contralateral to the side of stimulation, 4) calcium transients in all recorded MNs typically exhibit a sawtooth pattern in which individual components relate one-to-one to individual stimuli in a stimulus train, and 5) the sawtooth pattern is lost, and response latencies become longer, if uncrossed pRS axons are cut, leaving only the crossed axons. Our interpretation of these results is that the pRS system in newborn mice has already established excitatory synaptic connections with spinal neurons at multiple levels through parallel uncrossed and crossed projections and that transmission along the uncrossed pathway is more direct than along the crossed pathway.
Technical considerations.
Our results have been obtained in an ex vivo preparation of the isolated brain stem and spinal cord. Although such preparations are widely used, they do present challenges when attempting to extrapolate to normal physiology in an intact organism. For example, the preparations are decerebrated, and the recordings are obtained at subphysiological temperatures and in a physiological saline that almost certainly does not perfectly replicate CSF. Moreover, sensory feedback is eliminated. Whether the pRS projections actually function in the intact mouse according to the connectivity patterns we report in the isolated brain stem-spinal cord preparation remains to be determined.
Our approach utilizes high-throughput optical recording of synaptically mediated calcium transients. This has the advantage of providing information about inputs to many MNs simultaneously at single-neuron resolution and the ability to record from several different spinal segmental levels while stimulating the same site in the brain stem. However, the method also involves certain technical limitations. Calcium transients depend on the influx of extracellular calcium or the release of calcium from intracellular stores. These two alternative sources cannot be discriminated without pharmacological intervention, which we have not performed. Calcium signals involving influx of extracellular calcium are dependent on depolarization, meaning that inhibitory inputs are not visible. Thus our records are most likely dominated by depolarizing synaptic events. Moreover, irrespective of the source, the time courses of all calcium signals are dependent on multiple intracellular and transmembrane processes with slow kinetics relative to electrical signals. Thus temporal resolution is intrinsically limited. On the other hand, as we have noted in earlier studies, due to the fact that many descending axons in the neonatal mouse are unmyelinated and therefore slowly conducting, response latencies are long enough that resolution of monosynaptic connections is not unlikely (Szokol et al. 2011).
A related issue is that optical recording is easily performed at neonatal stages but much less so at adult stages due to the intrinsically favorable optical properties at neonatal stages. Optical recording of descending evoked responses in spinal neurons in the intact spinal cord of adult mammals has yet to be attained. Thus we can only compare our results obtained using optical recording with those obtained in adult mammals using electrophysiological recording (see Functional considerations). An important issue here is that synaptic connectivity in the neonate may not be the same as in the adult. However, it is likely that the broad strokes of connectivity in descending systems, including the pathways and regional termination areas of specific categories of descending axons, are established during development through stereotyped genetic mechanisms of neuron specification and target recognition. Thus there is strong reason to believe that certain principal features of connectivity are the same in neonates and adults (Di Bonito et al. 2013a,b; Kasumacic et al. 2012; Narita and Rijli 2009; Philippidou and Dasen 2013; Szokol et al. 2011).
We also used optical recording to investigate the pattern of direct activation of pRS neurons by electrical stimulation (Fig. 2), an application for which the approach is especially well-suited. Retrograde labeling with CGDA ensures a definitive identification of reticulospinal and other spinally projecting neurons, and many can be selectively recorded from within a single field of view, providing a rapid assessment of the spread of effective stimulating current. Moreover, the fact that this is an optical method avoids the problem of electrical stimulation artifacts, which is a major obstacle to short-range activation experiments using electrical recording (Histed et al. 2009). It is challenging, however, to interpret the relationship between calcium transients and electrical events (see below).
Origin and internal organization of the functional pRS projection.
The area of the PRF from which MN responses could be elicited was well-delineated and corresponded to the area occupied by the pRS neurons as revealed by retrograde tracing. Moreover, our local activation experiments indicate a limited volume of effective current density beyond which activation of pRS neurons falls to low levels or failures along a typical electrode track. Plotting the response magnitudes of individual pRS neurons against the distance from the stimulating electrode resulted in curves that conformed roughly to the inverse of the squared distance (1/x2), which would be expected in a homogeneous conducting medium. An inverse quadratic relationship vs. distance has also been shown for current thresholds in various mammalian species and brain regions (Tehovnik et al. 2006), although at least one study suggests that the relationship may be linear at distances below 50 μm (Rattay and Wenger 2010). An inverse quadratic relationship implies that even though pRS neurons within a relatively large radius from the electrode tip will be depolarized to some extent, pRS neurons close to the electrode will be activated much more strongly than more distant pRS neurons. It is important to realize that we cannot translate this pattern of local activation of pRS neurons seen with focal stimulation to a pattern of impulse initiation effective in activating spinal MNs. The calcium transients may not arise from suprathreshold depolarizations (spiking) in all activated pRS neurons, or, if they do, they may not represent the minimum firing rate or the minimum number of firing pRS neurons necessary to activate spinal MNs. An indication of this is that the effective stimulation radius for eliciting calcium responses in the pRS neurons (mean 486 μm, range 245–726 μm; Fig. 2) is larger than the effective stimulation radius within the pRS population for activating lumbar MNs (100–200 μm judging from Fig. 3).
Thus our findings suggest that activation of pRS neurons within a limited volume surrounding the electrode tip is sufficient for eliciting MN responses. However, activation of axons of passage cannot be discounted, as illustrated by the fact that stimulation points outside of the pRS neuron population but close to the MLF [which at these neonatal ages contain interstitiospinal, reticulospinal, and medial vestibulospinal tract axons (Kudo et al. 1993)] could elicit responses as strong as those elicited by stimulation points within the pRS neuron population. Nevertheless, stimulation of axons in the MLF or the rubrospinal tract (ventrolateral to the pRS neurons; Fig. 3C) seems highly unlikely to explain more than a few of the effective stimulation sites we found since stimulation needed to be quite close to such tracts to be effective (points >200 μm away were noneffective or barely effective).
On the other hand, activation of pRS axons is likely to be a contributing factor. These run caudally within most of the mediolateral extent of the pRS neuron population. Stimulation at any point within the pRS neuron population could therefore also affect large numbers of pRS axons originating from pRS neurons at more rostral levels. This has implications for how our results can be interpreted with respect to the internal organization of the pRS neuron population. We first emphasize that effective stimulation sites were located primarily in the caudal 2/3 of the PRF in the region corresponding roughly to the PnC. In contrast to what we have reported for the MRF (Szokol et al. 2008; Szokol and Perreault 2009), we found no sign of a mediolateral regionalization within this region of the PRF related to the motor column location of target MNs. Stimulation at diverse sites within the PRF in different preparations elicited responses of similar magnitude in MMC and LMC on both sides of the spinal cord. However, we hesitate to conclude from these results that there is no internal organization in the PRF with respect to motor column targeting in the neonatal mouse because it is possible that the electrical stimulation used here is simply not sufficiently precise to reveal one.
Parallel uncrossed and crossed projections.
pRS neurons projecting to a given side of the spinal cord are found on both sides of the PRF, albeit with ipsilateral predominance. Ipsilateral hemisection at C1, interrupting all uncrossed axons, and contralateral hemisection at C1, interrupting all crossed axons, both reduced individual MN response magnitudes considerably and to about the same extent. In both cases, reduction was seen in MNs on both sides of the spinal cord. MN responses in the nonhemisected preparation thus reflect inputs mediated by both pathways, each to both sides of the spinal cord (Fig. 6A). We have noted that the reductions do not add linearly and suggest that this reflects functional interactions between converging inputs from the two pathways. For example, the activation of either pathway could exert a permissive effect (through gating of intercalated interneurons) or a facilitatory effect (through heterosynaptic interactions on the same target interneurons) on the synaptic transmission in the other pathway.
Although the two pathways each produced similar response magnitudes in isolation, high-temporal-resolution recordings revealed important differences. The responses elicited solely by the uncrossed pathway had latencies within the range observed in nonhemisected preparations, whereas responses elicited solely by the crossed pathway had significantly longer latencies. More strikingly, the responses elicited solely by the uncrossed pathway exhibited the sawtooth pattern, seen in the nonhemisected preparation, in which individual response components faithfully follow individual stimuli one-to-one. This suggests a relatively direct response that rarely fails in the face of repetitive stimulation. By contrast, responses elicited solely by the crossed pathway displayed no sawtooth pattern. This, combined with the longer latency, suggests a lower safety factor for transmission with more intercalated synapses in the crossed pathway. However, the differences in latency and response pattern seen between responses elicited by the two pathways could be due to any combination of differences in numbers of projecting neurons, numbers of intercalated synapses, and the conduction velocity of each axon involved.
Although contralaterally projecting pRS neurons are the most likely source of the contralateral responses elicited in preparations with a same-side hemisection, two other possibilities should be considered. The stimulated pRS neurons could have supraspinal connections to 1) ipsilaterally projecting pRS or medullary reticulospinal neurons located on the opposite side of the brain stem, or 2) medullary reticulospinal neurons on the same side that project contralaterally into the spinal cord. A reticulo-reticular component would introduce at least one more synapse in the contralateral pathway. Although anatomic studies have demonstrated reticulo-reticular connections at many levels of the brain stem (Jones and Yang 1985; Walberg 1974), evidence of such connections onto reticular neurons identified as reticulospinal neurons is still lacking.
It is also important to emphasize that we have labeled both pRS neuron populations retrogradely from C1-C2, and this does not tell us how far either population actually projects down the spinal cord. Another retrograde labeling study in the neonatal rat (Leong et al. 1984) suggests that at least some pRS axons project to lumbar levels, but no information on this point is available for the neonatal mouse. Accordingly, we cannot exclude a contribution to either pathway from propriospinal interneurons. Nonetheless, the fidelity of the responses evoked by the ipsilateral pathway (sawtooth pattern) is suggestive of a monosynaptic component.
Another aspect concerning the laterality of connectivity is the similarity of the responses in MNs on either side of the midline. Comparing nonhemisected preparations to those with only the uncrossed pathway intact, we found that response latencies were indistinguishable and that the sawtooth pattern was present in all four motor columns at lumbar levels. The similarity on ipsilateral and contralateral sides seems to be at odds with the termination patterns of most uncrossed pRS axons in the adult cat, which target only the ipsilateral side, whereas a minority targets both sides (Matsuyama et al. 1999). Two alternative explanations are: 1) in the neonatal mouse, a greater proportion of uncrossed axons extend collaterals to target both sides of the cord; and 2) inputs to ipsilateral MNs and to contralateral MNs both involve intercalated lumbar interneurons. The latter alternative would fit with the observation that uncrossed pRS axons in the adult cat terminate predominantly in the ipsilateral laminae VII and VIII (Mitani et al. 1988; Nyberg-Hansen 1965), which contain ipsilaterally projecting and commissural interneurons, respectively. In the adult cat, a pRS input to contralateral MNs mediated by commissural interneurons in lamina VIII has been demonstrated electrophysiologically (Jankowska et al. 2003), and there is general agreement that although the reticulospinal neurons do have monosynaptic connections to MNs, they exert their major effect polysynaptically (Alstermark and Ogawa 2004; Floeter and Lev-Tov 1993).
Functional considerations.
The pRS projection to spinal MNs has been implicated in many motor behaviors in mammals, including the stereotyped movement pattern of the startle reflex (Yeomans and Frankland 1995), locomotion (Prentice and Drew 2001), and reaching (Schepens and Drew 2004). A common denominator of many of these behaviors is that they require postural adjustments through coordinated actions of limb and trunk muscles. In this paper, we demonstrate in newborn mice that focal stimulation of a limited number of pRS neurons anywhere within the PRF, and particularly within the caudal 2/3, can reliably activate MNs at cervical, thoracic, and lumbar levels on each side of the spinal cord. Such widespread connectivity from a limited number of projecting neurons seems well-suited to produce the concerted postural adjustments involved in the behaviors listed above. Of particular interest in this regard is our demonstration that both MMC and LMC are activated similarly, consistent with a coordination of limb and trunk musculature.
Most electrophysiological studies of reticulospinal pathways in the cat and monkey have focused on the MRF and the caudalmost parts of the PRF without much distinction within this large region (Davidson and Buford 2006; Drew and Rossignol 1990a,b; Peterson et al. 1975; Soteropoulos et al. 2012). Moreover, the practice of stimulating the MLF as a means of activating the reticulospinal pathway (Alstermark and Ogawa 2004; Edgley et al. 2004; Floeter et al. 1993; Grillner et al. 1968; Jankowska et al. 2003; Riddle et al. 2009) contributes little information about regional differences within the RF. Thus our results provide insight into the topography and types of responses elicited specifically by the pRS projection. It will be important in future studies to extend the scope and precision of the analysis to obtain a more detailed picture of how the mammalian pRS projection targets spinal circuitry and interacts with the medullary reticulospinal projection.
We have shown that at birth the mouse pRS projection, potentially at the level of individual pRS neurons, has a topography that involves substantial divergence both across segments and across motor columns. Widespread segmental effects of pRS stimulation have been demonstrated also in the adult cat (Drew and Rossignol 1984) and are consistent with the extensive collateralization made by individual pRS axons (Matsuyama et al. 1997, 1999). The similar divergence in neonatal mouse and adult cat suggests a conserved projection pattern that is likely to mediate a crucial function in mammalian motor control. Although the divergence is indicative of a rather generalized pattern of MN activation, our results also highlight heterogeneity in response amplitude and waveform elicited by different PRF stimulation sites that could contribute to a more finely tuned activation pattern. Being such a large neuron population, it seems unlikely that pRS neurons are functionally uniform. In the adult cat, individual neurons within the medullary RS and pRS populations have been shown to differ in MN connectivity (Drew and Rossignol 1990a,b; Peterson et al. 1979) and activity patterns during movement (Schepens and Drew 2006). These studies have, however, only showed minor differences in terms of topographical position, noting that overlap of regions containing neurons with different functionality is more prominent than segregation. It is therefore unclear to what extent projection or function is topographically organized in the pRS neuron population.
Reticulospinal neurons in lower vertebrates are also involved in regulating behaviors such as startle responses and locomotion, and functional homologies between pRS subpopulations in mammals and nonmammalian species are likely to exist. For example, reticular neurons in rhombomeres (r) 1–4 in larval zebrafish, including large, ipsilaterally projecting reticulospinal neurons in r4 that are likely to be homologous to pRS neurons in the most caudal portion of the mammalian PRF, have been shown to be involved in regulating the spinal locomotor network (Kimura et al. 2013). Startle responses in the zebrafish, however, are abolished by eliminating collectively the contralaterally projecting Mauthner neuron in r4 and its contralaterally projecting medullary segmental homologs, the MiD2cm and MiD3cm neurons in r5 and r6 (Liu and Fetcho 1999). This suggests that startle behavior in fish is primarily a medullary reticulospinal function. A deeper analysis of the evolutionary relationships of mammalian and nonmammalian reticulospinal neurons should shed light on the degree of functional conservation that prevailed in these descending systems as the more extensive behavioral repertoire of mammals developed.
Developmental considerations.
Rodents have limited motor abilities at birth, and substantial behavioral development occurs postnatally. Weight-bearing locomotion, for instance, matures significantly between the 1st and 2nd postnatal week (Geisler et al. 1993; Gramsbergen 1998; see, however, Jamon and Clarac 1998). Despite the protracted development of the behavior, neural networks can already at birth produce left-right and flexor-extensor alternation and interlimb coordination, all of which can be manifested under nonweight-bearing conditions such as air-stepping and swimming (Clarac et al. 2004; Gramsbergen 1998; Jamon 2006). That the pRS projection already by birth has established functional connections with MNs innervating trunk and limb muscles from cervical to lumbar levels indicates that this potential substrate for initiating movement is in place very early. To address the early functionality of the pRS projection, it will be important in future studies to investigate the degree of coordination (intersegmental, flexor vs. extensor) in pRS-elicited motor activity and the extent to which the pRS projection can be activated by cortical and sensory inputs during the 1st postnatal week.
Summary.
We have shown that electrical stimulation of pRS neurons in the newborn mouse elicits responses in cervical, thoracic, and lumbar MNs through a relatively direct ipsilateral pathway and a less direct contralateral pathway. The ipsilateral pathway distributes inputs to the MMC and LMC on each side of the cord, probably through a combination of monosynaptic and polysynaptic inputs. The contralateral pathway also distributes inputs to all four motor columns at lumbar levels through a pathway that likely involves at least one additional synapse. Further studies using these and complementary techniques should shed light on the precise synaptic connectivity involved in the pRS projection.
GRANTS
This work was supported by grants from the Research Council of Norway to J. C. Glover and grants from the South East Norway Regional Health Authority (HSØ), the International Foundation for Research on Paraplegia, and the Craig H. Neilsen Foundation to M.-C. Perreault.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.S.S., J.C.G., and M.-C.P. conception and design of research; M.S.S. performed experiments; M.S.S., J.C.G., and M.-C.P. analyzed data; M.S.S., J.C.G., and M.-C.P. interpreted results of experiments; M.S.S. and M.-C.P. prepared figures; M.S.S. and M.-C.P. drafted manuscript; M.S.S., J.C.G., and M.-C.P. edited and revised manuscript; M.S.S., J.C.G., and M.-C.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Bruce Piercey for writing the file conversion program (FileConvert) and Kobra Sultani for technical assistance with breeding of mouse colonies.
REFERENCES
- Alstermark B, Ogawa J. In vivo recordings of bulbospinal excitation in adult mouse forelimb motoneurons. J Neurophysiol 92: 1958–1962, 2004. [DOI] [PubMed] [Google Scholar]
- Auclair F, Marchand R, Glover JC. Regional patterning of reticulospinal and vestibulospinal neurons in the hindbrain of mouse and rat embryos. J Comp Neurol 411: 288–300, 1999. [DOI] [PubMed] [Google Scholar]
- Baker SN. The primate reticulospinal tract, hand function and functional recovery. J Physiol 589: 5603–5612, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolzoni F, Baczyk M, Jankowska E. Subcortical effects of transcranial direct current stimulation in the rat. J Physiol 591: 4027–4042, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarac F, Pearlstein E, Pflieger JF, Vinay L. The in vitro neonatal rat spinal cord preparation: a new insight into mammalian locomotor mechanisms. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 190: 343–357, 2004. [DOI] [PubMed] [Google Scholar]
- Davidson AG, Buford JA. Bilateral actions of the reticulospinal tract on arm and shoulder muscles in the monkey: stimulus triggered averaging. Exp Brain Res 173: 25–39, 2006. [DOI] [PubMed] [Google Scholar]
- Di Bonito M, Glover JC, Studer M. Hox genes and region-specific sensorimotor circuit formation in the hindbrain and spinal cord. Dev Dyn 242: 1348–1368, 2013a. [DOI] [PubMed] [Google Scholar]
- Di Bonito M, Narita Y, Avallone B, Sequino L, Mancuso M, Andolfi G, Franze AM, Puelles L, Rijli FM, Studer M. Assembly of the auditory circuitry by a Hox genetic network in the mouse brainstem. PLoS Genet 9: e1003249, 2013b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCarlo JJ, Lane JW, Hsiao SS, Johnson KO. Marking microelectrode penetrations with fluorescent dyes. J Neurosci Methods 64: 75–81, 1996. [DOI] [PubMed] [Google Scholar]
- Drew T, Rossignol S. Functional organization within the medullary reticular formation of intact unanesthetized cat. I. Movements evoked by microstimulation. J Neurophysiol 64: 767–781, 1990a. [DOI] [PubMed] [Google Scholar]
- Drew T, Rossignol S. Functional organization within the medullary reticular formation of intact unanesthetized cat. II. Electromyographic activity evoked by microstimulation. J Neurophysiol 64: 782–795, 1990b. [DOI] [PubMed] [Google Scholar]
- Drew T, Rossignol S. Phase-dependent responses evoked in limb muscles by stimulation of medullary reticular formation during locomotion in thalamic cats. J Neurophysiol 52: 653–675, 1984. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Hammar I. Ipsilateral actions of feline corticospinal tract neurons on limb motoneurons. J Neurosci 24: 7804–7813, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floeter MK, Lev-Tov A. Excitation of lumbar motoneurons by the medial longitudinal fasciculus in the in vitro brain stem spinal cord preparation of the neonatal rat. J Neurophysiol 70: 2241–2250, 1993. [DOI] [PubMed] [Google Scholar]
- Floeter MK, Sholomenko GN, Gossard JP, Burke RE. Disynaptic excitation from the medial longitudinal fasciculus to lumbosacral motoneurons: modulation by repetitive activation, descending pathways, and locomotion. Exp Brain Res 92: 407–419, 1993. [DOI] [PubMed] [Google Scholar]
- Galea MP, Hammar I, Nilsson E, Jankowska E. Bilateral postsynaptic actions of pyramidal tract and reticulospinal neurons on feline erector spinae motoneurons. J Neurosci 30: 858–869, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler HC, Westerga J, Gramsbergen A. Development of posture in the rat. Acta Neurobiol Exp (Warsz) 53: 517–523, 1993. [PubMed] [Google Scholar]
- Glover JC. Retrograde and anterograde axonal tracing with fluorescent dextran-amines in the embryonic nervous system. Neurosci Prot 30: 1–13, 1995. [Google Scholar]
- Gramsbergen A. Posture and locomotion in the rat: independent or interdependent development? Neurosci Biobehav Rev 22: 547–553, 1998. [PubMed] [Google Scholar]
- Grillner S, Hongo T, Lund S. Reciprocal effects between two descending bulbospinal systems with monosynaptic connections to spinal motoneurones. Brain Res 10: 477–480, 1968. [DOI] [PubMed] [Google Scholar]
- Hentall ID, Zorman G, Kansky S, Fields HL. Relations among threshold, spike height, electrode distance, and conduction velocity in electrical stimulation of certain medullospinal neurons. J Neurophysiol 51: 968–977, 1984. [DOI] [PubMed] [Google Scholar]
- Histed MH, Bonin V, Reid RC. Direct activation of sparse, distributed populations of cortical neurons by electrical microstimulation. Neuron 63: 508–522, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamon M. The early development of motor control in neonate rat. C R Palevol 5: 657–666, 2006. [Google Scholar]
- Jamon M, Clarac F. Early walking in the neonatal rat: a kinematic study. Behav Neurosci 112: 1218–1228, 1998. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Slawinska U, Maleszak K, Edgley SA. Neuronal basis of crossed actions from the reticular formation on feline hindlimb motoneurons. J Neurosci 23: 1867–1878, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE, Yang TZ. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J Comp Neurol 242: 56–92, 1985. [DOI] [PubMed] [Google Scholar]
- Kasumacic N, Glover JC, Perreault MC. Vestibular-mediated synaptic inputs and pathways to sympathetic preganglionic neurons in the neonatal mouse. J Physiol 590: 5809–5826, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Satou C, Fujioka S, Shoji W, Umeda K, Ishizuka T, Yawo H, Higashijima S. Hindbrain V2a neurons in the excitation of spinal locomotor circuits during zebrafish swimming. Curr Biol 23: 843–849, 2013. [DOI] [PubMed] [Google Scholar]
- Kudo N, Furukawa F, Okado N. Development of descending fibers to the rat embryonic spinal cord. Neurosci Res 16: 131–141, 1993. [DOI] [PubMed] [Google Scholar]
- Kuypers HG. The descending pathways to the spinal cord, their anatomy and function. Prog Brain Res 11: 178–202, 1964. [DOI] [PubMed] [Google Scholar]
- Lemon RN. Descending pathways in motor control. Annu Rev Neurosci 31: 195–218, 2008. [DOI] [PubMed] [Google Scholar]
- Leong SK, Shieh JY, Wong WC. Localizing spinal-cord-projecting neurons in neonatal and immature albino rats. J Comp Neurol 228: 18–23, 1984. [DOI] [PubMed] [Google Scholar]
- Liang H, Paxinos G, Watson C. Projections from the brain to the spinal cord in the mouse. Brain Struct Funct 215: 159–186, 2011. [DOI] [PubMed] [Google Scholar]
- Liu KS, Fetcho JR. Laser ablations reveal functional relationships of segmental hindbrain neurons in zebrafish. Neuron 23: 325–335, 1999. [DOI] [PubMed] [Google Scholar]
- Lloyd DP. Activity in neurons of the bulbospinal correlation system. J Neurophysiol 4: 115–134, 1941. [Google Scholar]
- Matsuyama K, Mori F, Kuze B, Mori S. Morphology of single pontine reticulospinal axons in the lumbar enlargement of the cat: a study using the anterograde tracer PHA-L. J Comp Neurol 410: 413–430, 1999. [PubMed] [Google Scholar]
- Matsuyama K, Takakusaki K, Nakajima K, Mori S. Multi-segmental innervation of single pontine reticulospinal axons in the cervico-thoracic region of the cat: anterograde PHA-L tracing study. J Comp Neurol 377: 234–250, 1997. [PubMed] [Google Scholar]
- Mitani A, Ito K, Mitani Y, McCarley RW. Descending projections from the gigantocellular tegmental field in the cat: cells of origin and their brainstem and spinal cord trajectories. J Comp Neurol 268: 546–566, 1988. [DOI] [PubMed] [Google Scholar]
- Narita Y, Rijli FM. Hox genes in neural patterning and circuit formation in the mouse hindbrain. Curr Top Dev Biol 88: 139–167, 2009. [DOI] [PubMed] [Google Scholar]
- Nyberg-Hansen R. Sites and mode of termination of reticulo-spinal fibers in the cat. An experimental study with silver impregnation methods. J Comp Neurol 124: 71–99, 1965. [DOI] [PubMed] [Google Scholar]
- Perreault MC, Drew T, Rossignol S. Activity of medullary reticulospinal neurons during fictive locomotion. J Neurophysiol 69: 2232–2247, 1993. [DOI] [PubMed] [Google Scholar]
- Perreault MC, Glover JC. Glutamatergic reticulospinal neurons in the mouse: developmental origins, axon projections, and functional connectivity. Ann NY Acad Sci 1279: 80–89, 2013. [DOI] [PubMed] [Google Scholar]
- Peterson BW. Reticulospinal projections to spinal motor nuclei. Annu Rev Physiol 41: 127–140, 1979. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Maunz RA, Pitts NG, Mackel RG. Patterns of projection and braching of reticulospinal neurons. Exp Brain Res 23: 333–351, 1975. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Pitts NG, Fukushima K. Reticulospinal connections with limb and axial motoneurons. Exp Brain Res 36: 1–20, 1979. [DOI] [PubMed] [Google Scholar]
- Pettersson LG, Alstermark B, Blagovechtchenski E, Isa T, Sasaski S. Skilled digit movements in feline and primate–recovery after selective spinal cord lesions. Acta Physiol (Oxf) 189: 141–154, 2007. [DOI] [PubMed] [Google Scholar]
- Philippidou P, Dasen JS. Hox genes: choreographers in neural development, architects of circuit organization. Neuron 80: 12–34, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice SD, Drew T. Contributions of the reticulospinal system to the postural adjustments occurring during voluntary gait modifications. J Neurophysiol 85: 679–698, 2001. [DOI] [PubMed] [Google Scholar]
- Ranck JB., Jr. Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res 98: 417–440, 1975. [DOI] [PubMed] [Google Scholar]
- Rattay F, Wenger C. Which elements of the mammalian central nervous system are excited by low current stimulation with microelectrodes? Neuroscience 170: 399–407, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci 29: 4993–4999, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepens B, Drew T. Descending signals from the pontomedullary reticular formation are bilateral, asymmetric, and gated during reaching movements in the cat. J Neurophysiol 96: 2229–2252, 2006. [DOI] [PubMed] [Google Scholar]
- Schepens B, Drew T. Independent and convergent signals from the pontomedullary reticular formation contribute to the control of posture and movement during reaching in the cat. J Neurophysiol 92: 2217–2238, 2004. [DOI] [PubMed] [Google Scholar]
- Sivertsen MS, Glover JC, Perreault MC. Functional organization of pontine reticulospinal synaptic connections to lumbar motoneurons in the neonatal mouse as revealed by calcium imaging. Program no. 809.02 (Online). In: 2011 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience, 2011. [Google Scholar]
- Sivertsen MS, Glover JC, Perreault MC. Segmental comparison of motoneuron responses to pontine reticulospinal inputs in the neonatal mouse. Program no. 788.08 (Online) In: 2012 Neuroscience Meeting Planner New Orleans, LA: Society for Neuroscience, 2012. [Google Scholar]
- Soteropoulos DS, Williams ER, Baker SN. Cells in the monkey ponto-medullary reticular formation modulate their activity with slow finger movements. J Physiol 590: 4011–4027, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague JM, Chambers WW. Control of posture by reticular formation and cerebellum in the intact, anesthetized and unanesthetized and in the decerebrated cat. Am J Physiol 176: 52–64, 1954. [DOI] [PubMed] [Google Scholar]
- Szokol K, Glover JC, Perreault MC. Differential origin of reticulospinal drive to motoneurons innervating trunk and hindlimb muscles in the mouse revealed by optical recording. J Physiol 586: 5259–5276, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szokol K, Glover JC, Perreault MC. Organization of functional synaptic connections between medullary reticulospinal neurons and lumbar descending commissural interneurons in the neonatal mouse. J Neurosci 31: 4731–4742, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szokol K, Perreault MC. Imaging synaptically mediated responses produced by brainstem inputs onto identified spinal neurons in the neonatal mouse. J Neurosci Methods 180: 1–8, 2009. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Tolias AS, Sultan F, Slocum WM, Logothetis NK. Direct and indirect activation of cortical neurons by electrical microstimulation. J Neurophysiol 96: 512–521, 2006. [DOI] [PubMed] [Google Scholar]
- Umeda T, Takahashi M, Isa K, Isa T. Formation of descending pathways mediating cortical command to forelimb motoneurons in neonatally hemidecorticated rats. J Neurophysiol 104: 1707–1716, 2010. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VG, Ulfhake B. The organization of the brainstem and spinal cord of the mouse: relationships between monoaminergic, cholinergic, and spinal projection systems. J Chem Neuroanat 31: 2–36, 2006. [DOI] [PubMed] [Google Scholar]
- Walberg F. Crossed reticulo-reticular projections in the medulla, pons and mesencephalon. An autoradiographic study in the cat. Kidney Int 5: 127–134, 1974. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Yoshida M. Comparison of effects of stimulation of Deiters' nucleus and medial longitudinal fasciculus on neck, forelimb, and hindlimb motoneurons. J Neurophysiol 32: 743–758, 1969. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Frankland PW. The acoustic startle reflex: neurons and connections. Brain Res Brain Res Rev 21: 301–314, 1995. [DOI] [PubMed] [Google Scholar]


