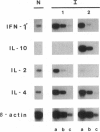
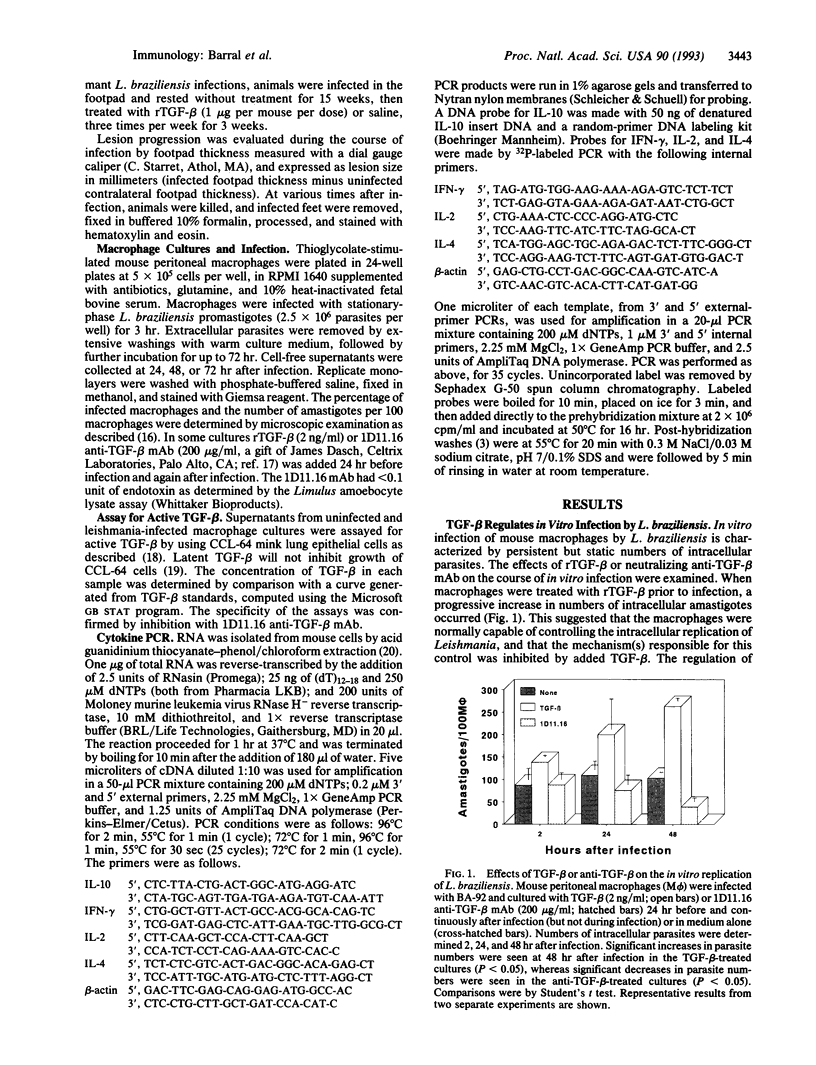
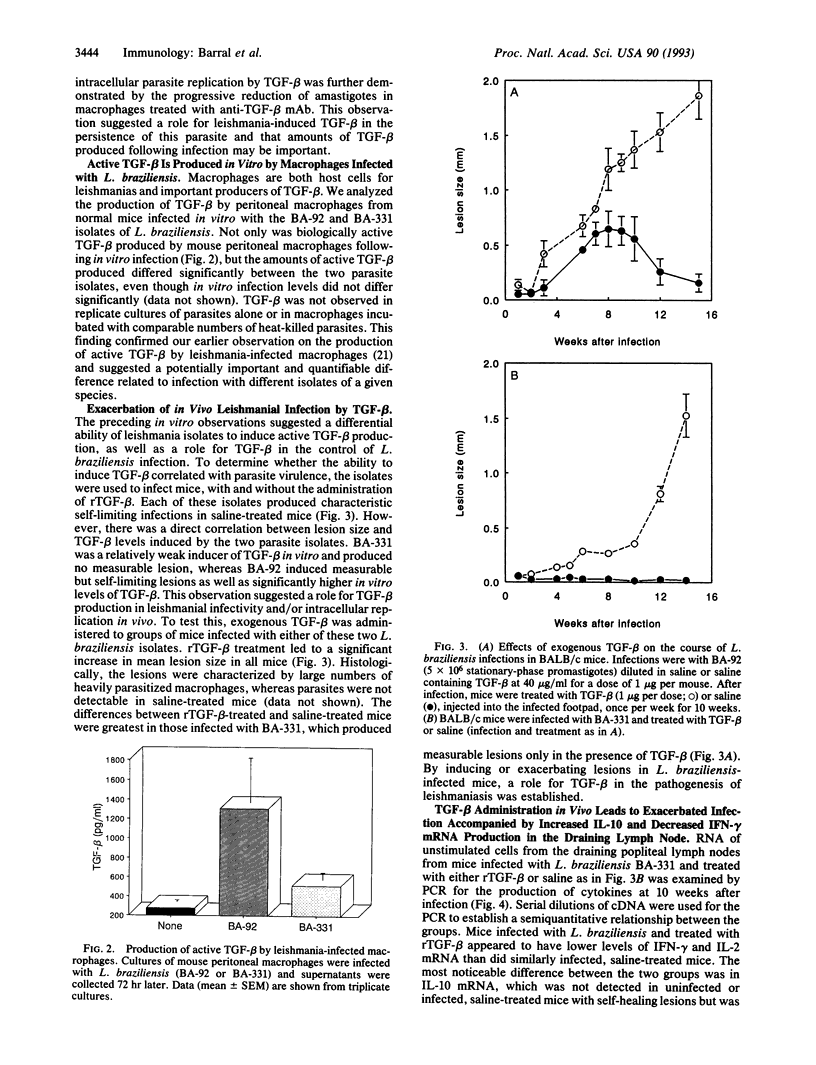
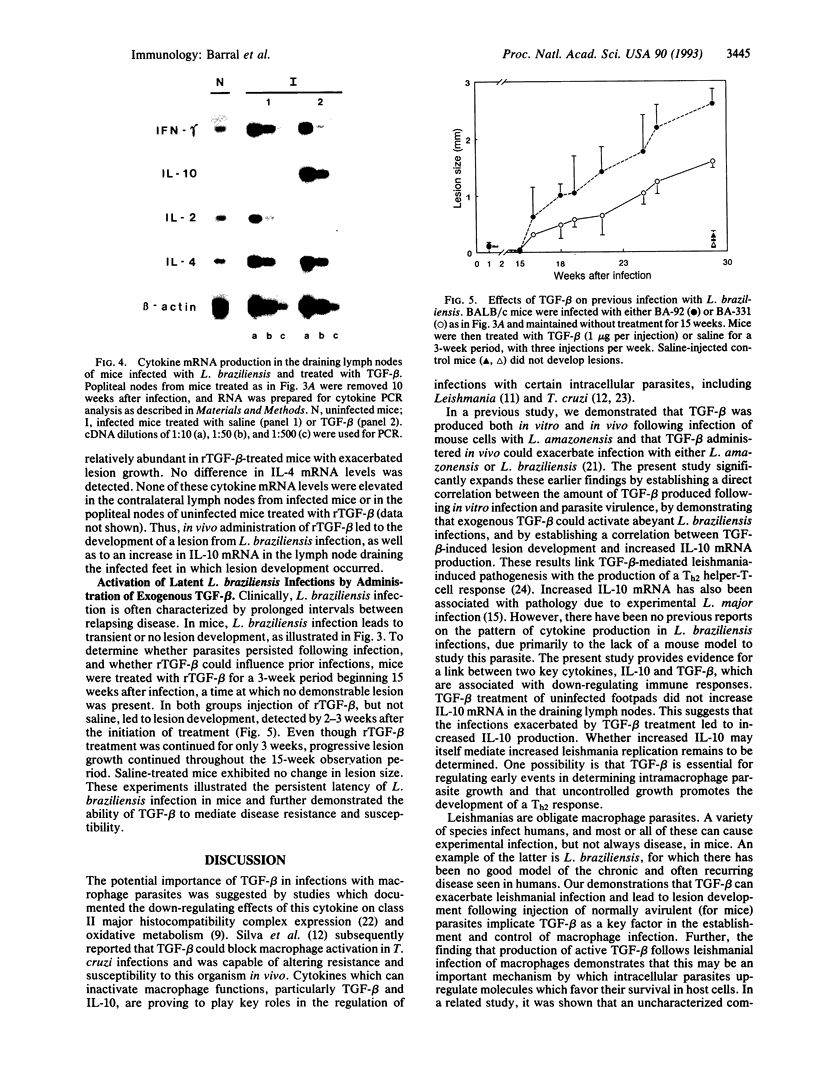
Abstract
Transforming growth factor beta (TGF-beta) has potent down-regulating effects on macrophages and is thus capable of influencing the fate of intramacrophage parasites, including leishmanias. We report the development of a mouse model for the study of the human pathogen Leishmania braziliensis and demonstrate, both in vitro and in vivo, a key regulatory role for TGF-beta in the pathogenesis of infection with this parasite. Recombinant TGF-beta added to cultures of murine peritoneal macrophages led to increased intracellular L. braziliensis replication, whereas addition of neutralizing anti-TGF-beta monoclonal antibody decreased levels of infection. Macrophages infected with L. braziliensis produced biologically active TGF-beta, with a direct correlation between amounts of TGF-beta induced by two parasite isolates and their relative virulence. In vivo, treatment with recombinant TGF-beta rendered avirulent parasites virulent and activated latent L. braziliensis infection. Activation of parasite replication was observed in mice which had been infected with L. braziliensis 15 weeks previously but had not developed lesions or had healed lesions, depending on the parasite isolate used to infect the mice. The exacerbation of L. braziliensis infection in vivo was associated with an increase of interleukin 10 mRNA in the draining lymph node. These results demonstrate that TGF-beta is able to alter the course of in vitro and in vivo infections with L. braziliensis, the latter being characterized by an increase in interleukin 10, an important Th2 helper-T-cell cytokine.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Fleurdelys B. E., Stevenson H. C., Miller P. J., Madtes D. K., Raines E. W., Ross R., Sporn M. B. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral-Netto M., Barral A., Brownell C. E., Skeiky Y. A., Ellingsworth L. R., Twardzik D. R., Reed S. G. Transforming growth factor-beta in leishmanial infection: a parasite escape mechanism. Science. 1992 Jul 24;257(5069):545–548. doi: 10.1126/science.1636092. [DOI] [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Czarniecki C. W., Chiu H. H., Wong G. H., McCabe S. M., Palladino M. A. Transforming growth factor-beta 1 modulates the expression of class II histocompatibility antigens on human cells. J Immunol. 1988 Jun 15;140(12):4217–4223. [PubMed] [Google Scholar]
- Dasch J. R., Pace D. R., Waegell W., Inenaga D., Ellingsworth L. Monoclonal antibodies recognizing transforming growth factor-beta. Bioactivity neutralization and transforming growth factor beta 2 affinity purification. J Immunol. 1989 Mar 1;142(5):1536–1541. [PubMed] [Google Scholar]
- Ding A., Nathan C. F., Graycar J., Derynck R., Stuehr D. J., Srimal S. Macrophage deactivating factor and transforming growth factors-beta 1 -beta 2 and -beta 3 inhibit induction of macrophage nitrogen oxide synthesis by IFN-gamma. J Immunol. 1990 Aug 1;145(3):940–944. [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Mutha S. S., Locksley R. M. Production of interferon gamma, interleukin 2, interleukin 4, and interleukin 10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7011–7015. doi: 10.1073/pnas.88.16.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Johnson W. D., Jr, Barretto A. C., Lago E., Badaro R., Cerf B., Reed S. G., Netto E. M., Tada M. S., Franca T. F. Epidemiology of American cutaneous leishmaniasis due to Leishmania braziliensis braziliensis. J Infect Dis. 1987 Jul;156(1):73–83. doi: 10.1093/infdis/156.1.73. [DOI] [PubMed] [Google Scholar]
- Kehrl J. H., Roberts A. B., Wakefield L. M., Jakowlew S., Sporn M. B., Fauci A. S. Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J Immunol. 1986 Dec 15;137(12):3855–3860. [PubMed] [Google Scholar]
- Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden P. D. Selective primary health care: strategies for control of disease in the developing world. XIV. Leishmaniasis. Rev Infect Dis. 1984 Sep-Oct;6(5):736–744. doi: 10.1093/clinids/6.5.736. [DOI] [PubMed] [Google Scholar]
- Mulé J. J., Schwarz S. L., Roberts A. B., Sporn M. B., Rosenberg S. A. Transforming growth factor-beta inhibits the in vitro generation of lymphokine-activated killer cells and cytotoxic T cells. Cancer Immunol Immunother. 1988;26(2):95–100. doi: 10.1007/BF00205600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacy C. A., Fortier A. H., Meltzer M. S., Buchmeier N. A., Schreiber R. D. Macrophage activation to kill Leishmania major: activation of macrophages for intracellular destruction of amastigotes can be induced by both recombinant interferon-gamma and non-interferon lymphokines. J Immunol. 1985 Nov;135(5):3505–3511. [PubMed] [Google Scholar]
- Nelson B. J., Ralph P., Green S. J., Nacy C. A. Differential susceptibility of activated macrophage cytotoxic effector reactions to the suppressive effects of transforming growth factor-beta 1. J Immunol. 1991 Mar 15;146(6):1849–1857. [PubMed] [Google Scholar]
- Ranchalis J. E., Gentry L., Ogawa Y., Seyedin S. M., McPherson J., Purchio A., Twardzik D. R. Bone-derived and recombinant transforming growth factor beta's are potent inhibitors of tumor cell growth. Biochem Biophys Res Commun. 1987 Oct 29;148(2):783–789. doi: 10.1016/0006-291x(87)90944-2. [DOI] [PubMed] [Google Scholar]
- Reed S. G. In vivo administration of recombinant IFN-gamma induces macrophage activation, and prevents acute disease, immune suppression, and death in experimental Trypanosoma cruzi infections. J Immunol. 1988 Jun 15;140(12):4342–4347. [PubMed] [Google Scholar]
- Reed S. G., Nathan C. F., Pihl D. L., Rodricks P., Shanebeck K., Conlon P. J., Grabstein K. H. Recombinant granulocyte/macrophage colony-stimulating factor activates macrophages to inhibit Trypanosoma cruzi and release hydrogen peroxide. Comparison with interferon gamma. J Exp Med. 1987 Dec 1;166(6):1734–1746. doi: 10.1084/jem.166.6.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D. L., Perkins P. V. Identification of an infective stage of Leishmania promastigotes. Science. 1984 Mar 30;223(4643):1417–1419. doi: 10.1126/science.6701528. [DOI] [PubMed] [Google Scholar]
- Samuelson J., Lerner E., Tesh R., Titus R. A mouse model of Leishmania braziliensis braziliensis infection produced by coinjection with sand fly saliva. J Exp Med. 1991 Jan 1;173(1):49–54. doi: 10.1084/jem.173.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988 Nov 1;168(5):1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. S., Morrissey P. J., Grabstein K. H., Mohler K. M., Anderson D., Reed S. G. Interleukin 10 and interferon gamma regulation of experimental Trypanosoma cruzi infection. J Exp Med. 1992 Jan 1;175(1):169–174. doi: 10.1084/jem.175.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. S., Twardzik D. R., Reed S. G. Regulation of Trypanosoma cruzi infections in vitro and in vivo by transforming growth factor beta (TGF-beta). J Exp Med. 1991 Sep 1;174(3):539–545. doi: 10.1084/jem.174.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunawaki S., Sporn M., Ding A., Nathan C. Deactivation of macrophages by transforming growth factor-beta. Nature. 1988 Jul 21;334(6179):260–262. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- Twardzik D. R., Mikovits J. A., Ranchalis J. E., Purchio A. F., Ellingsworth L., Ruscetti F. W. Gamma-interferon-induced activation of latent transforming growth factor-beta by human monocytes. Ann N Y Acad Sci. 1990;593:276–284. doi: 10.1111/j.1749-6632.1990.tb16119.x. [DOI] [PubMed] [Google Scholar]
- Weiser W. Y., Van Niel A., Clark S. C., David J. R., Remold H. G. Recombinant human granulocyte/macrophage colony-stimulating factor activates intracellular killing of Leishmania donovani by human monocyte-derived macrophages. J Exp Med. 1987 Nov 1;166(5):1436–1446. doi: 10.1084/jem.166.5.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]