Abstract
Rationale
India reports the largest number of multidrug-resistant tuberculosis cases in the world; yet, no longitudinal study has assessed factors related to treatment outcomes under programmatic conditions in the public sector.
Objectives
To describe demographic, clinical, and risk characteristics associated with treatment outcomes for all patients with multidrug-resistant tuberculosis registered in the Revised National Tuberculosis Control Programme, Kerala State, India from January 1, 2009 to June 30, 2010.
Methods
Cox regression methods were used to calculate adjusted hazard ratios with 95% confidence intervals (CIs) to assess factors associated with an unsuccessful treatment outcome.
Measurements and Main Results
Of 179 patients with multidrug-resistant tuberculosis registered, 112 (63%) had successful treatment outcomes (77 bacteriologically cured, 35 treatment completed) and 67 (37%) had unsuccessful treatment outcomes (30 died, 26 defaulted, 9 failed treatment, 1 stopped treatment because of drug-related adverse events, and 1 developed extensively drug-resistant tuberculosis). The hazard for unsuccessful outcome was significantly higher among patients who consumed alcohol during treatment (adjusted hazard ratio, 4.3; 95% CI, 1.1–17.6) than those who did not. Persons who consumed alcohol during treatment, on average, missed 18 more intensive-phase doses (95% CI, 13–22) than those who did not. Although many patients had diabetes (33%), were ever smokers (39%), or had low body mass index (47%), these factors were not associated with outcome.
Conclusion
Overall treatment success was greater than global and national averages; however, outcomes among patients consuming alcohol remained poor. Integration of care for multidrug-resistant tuberculosis and alcoholism should be considered to improve treatment adherence and outcomes.
Keywords: multidrug-resistant tuberculosis treatment outcome, multidrug-resistant tuberculosis, alcohol, tuberculosis, India
In 2012, the estimated incidence of multidrug-resistant tuberculosis was 310,000 globally, of which 66,000 (21%) were in India (1–3). Multidrug-resistant tuberculosis, defined as tuberculosis caused by Mycobacterium tuberculosis resistant to at least both isoniazid and rifampicin, has become a major barrier to achieving tuberculosis control. Multidrug-resistant tuberculosis therapy is less effective, is associated with more adverse events, and is more costly to treat when compared with standard first-line therapy (2, 4, 5). Inadequate treatment of multidrug-resistant tuberculosis, poor adherence to short-course chemotherapy, poor clinical management practices, and underuse of diagnostic services has exacerbated the epidemiology, developing further resistance to second-line and even tertiary antituberculosis drugs, resulting in extensively and “totally” drug-resistant tuberculosis (3–10).
In India, the prevalence of multidrug-resistant tuberculosis is estimated to be 3% among new cases and 17% among previously treated cases (2). The Revised National Tuberculosis Control Program has established a program for the diagnosis, treatment, and management of multidrug-resistant tuberculosis using high-quality, second-line antituberculosis drugs at no cost to patients (3). Despite this accomplishment, only a small fraction (5%) of all multidrug-resistant tuberculosis cases in India were treated according to these standards in 2011 (11). With the rapid and ambitious plan to scale up multidrug-resistant tuberculosis services by 2017 (12), understanding the effectiveness of this program is urgent. However, no longitudinal study has either assessed or described treatment outcomes of multidrug-resistant tuberculosis cases in the public sector of India. Thus, we sought to investigate potential factors that might affect treatment outcomes using routinely collected programmatic data in India—a country with the most multidrug-resistant tuberculosis cases in the world (1, 11). Some of the results of these studies have been previously reported in the form of an abstract (13).
Methods
Study Population
We abstracted clinical records and tuberculosis program records and reports of all culture-confirmed multidrug-resistant tuberculosis cases receiving treatment from the Revised National Tuberculosis Control Programme in the state of Kerala from January 1, 2009 through June 30, 2010. Kerala is India’s southernmost state, with a population of approximately 33 million people.
Diagnosis, Treatment, and Follow-Up
In Kerala, persons suspected of multidrug-resistant tuberculosis (previously treated tuberculosis cases, persons who failed tuberculosis treatment, persons with sputum-smear positive test results who had known exposure to multidrug-resistant tuberculosis) sought care at the Peripheral Health Institutions. All sputum specimens were collected and transported to the tuberculosis reference laboratories in Chennai and Trivandrum. Culture and drug susceptibility tests for first-line drugs (i.e., isoniazid, rifampicin, ethambutol, and streptomycin) were performed by conventional solid culture method using Lowenstein-Jensen medium. Although all patients received drug susceptibility tests on the initial positive culture, repeat follow-up drug susceptibility tests are performed only for default patients who have completed initial 3 months of intensive-phase treatment and then return for treatment (3).
Diagnosed multidrug-resistant tuberculosis cases are then referred to tertiary care centers called Drug Resistant Tuberculosis Centres for initiation of treatment. Pretreatment clinical examinations are a prerequisite before the initiation of multidrug-resistant tuberculosis treatment. These examinations include chest radiograph; complete hemogram; liver, renal and thyroid function tests; HIV serology; screening for diabetes mellitus; calculation of body mass index; and pregnancy test for all women. All cases are then required to complete at least 7 days of inpatient treatment at Drug Resistant Tuberculosis Centres at the initiation of treatment (3). During this time, early-onset drug-related adverse events and drug intolerance are monitored and, if needed, dosage and/or drugs prescribed are modified. After 1 week of treatment at Drug Resistant Tuberculosis Centres, patients are discharged to continue the treatment at their residence with daily supervised directly observed therapy by trained health care providers. All patients with multidrug-resistant tuberculosis were treated with a standardized, World Health Organization–recommended treatment regimen composed of an intensive phase for 6 months with kanamycin, levofloxacin, cycloserine, ethionamide, pyrazinamide, and ethambutol, and continuation phase of 18 months with levofloxacin, cycloserine, ethionamide, and ethambutol (3).
Routine follow-up clinic visits to monitor adverse drug-related events (e.g., nausea or vomiting, neuropathy, ototoxicity, psychosis, renal insufficiency, seizures, and suicidal ideation) occurred monthly throughout treatment and whenever indicated. The decision to stop treatment because of adverse drug-related events was based on the clinician’s discretion, and these events were not coded for severity. Bacteriological monitoring was done using sputum cultures at months 3, 4, 5, and 6 during the intensive phase. At the end of 6 months of treatment, if the fourth-month culture remained positive, the intensive phase was extended for an additional 3 months. Additional sputum culture examinations continued every third month through month 24 (e.g., months 9, 12, 15, 18, 21, and 24). Patients who failed multidrug-resistant tuberculosis treatment underwent drug susceptibility tests using a modified proportion sensitivity method for liquid culture for second-line drugs like ofloxacin and kanamycin at the Tuberculosis Research Centre, Chennai for a diagnosis of extensively drug-resistant tuberculosis.
Time to multidrug-resistant tuberculosis treatment initiation was calculated as the difference in number of days between sputum collection and multidrug-resistant tuberculosis treatment start date. Alcohol consumption and tobacco smoking were based on self-report and were documented in routine medical examinations and follow-up clinic visits. Patients self-reporting a history of diabetes mellitus or those with fasting blood glucose greater than or equal to 126 mg/dl were defined as diabetic.
Treatment Outcome Definitions
Treatment outcomes were defined as successful or unsuccessful. Successful treatment outcomes were defined as cured (completed treatment with at least five consecutive negative culture results in the last 12–15 months) or completed (completed treatment as per national guidelines (3), but did not have all bacteriological results available). All other treatment outcomes were considered unsuccessful. This included treatment failure as determined by having two of five or the final three cultures positive, death from any cause during treatment, treatment interruptions for 2 or more consecutive months, transfer to another Drug Resistant Tuberculosis Centre and treatment outcome remained unknown; treatment having been stopped because of severe adverse drug reaction events; or the development of additional resistance and was subsequently prescribed a regimen for extensively drug-resistant tuberculosis.
Statistical Analysis
Frequencies and proportions were calculated for all variables. Pearson’s Chi-square test and Fisher exact test (when appropriate) were used to compare differences in proportions between groups (i.e., successful vs. unsuccessful outcome). For continuous variables, we calculated medians, interquartile ranges, means, and standard deviations. To test for differences in means between groups, we used t test or Kruskal-Wallis test as applicable. We calculated time-to-event (i.e., outcome) in months using the difference between the treatment start date and treatment end date. Deaths included death of any cause during treatment; otherwise, individuals were censored at the treatment end date, or date of outcome, as outlined above. We used Kaplan-Meier curves to compare unadjusted time-to-event ratios among tuberculosis cases for both successful and unsuccessful treatment outcomes. Differences across strata were examined using the log-rank test. A Cox proportional hazards model with a stepwise backward elimination approach was used to assess the effect of select clinical and demographic variables on time-to-event during treatment. Hazard ratios (HRs) were used as the measure of association with 95% confidence intervals (CIs). The proportionality of risks in the Cox model was verified using a Schoenfeld residuals plot. Relative risk and corresponding 95% CI were calculated to measure the association between consuming alcohol during treatment and missing more than seven doses during the intensive phase. All statistical tests were considered to be significant at an a less than 0.05.
Ethics Considerations
The study protocol was reviewed and approved by the Ethics Advisory Group of the International Union Against tuberculosis and Lung disease (Paris, France) and the Institutional Ethics Committee of the National Tuberculosis Institute, Bangalore, India. These data were collected and analyzed as part of routine public health activities, so no informed consent was required. All data were safeguarded to protect patient confidentiality and no individual patient identifiers were retained. Participation of the United States Centers for Disease Control and Prevention in this project did not meet the definition of engagement in human subjects research because the U.S. Centers for Disease Control and Prevention investigators did not interact with study subjects or have access to patient identifiable data; thus, a separate institutional review board approval was not required.
Results
During January 1, 2009 through June 30, 2010, 1,207 persons in Kerala sought care for suspected multidrug-resistant tuberculosis. Among these, 202 (16.7%) had M. tuberculosis isolates that were resistant to at least isoniazid and rifampin. Nearly 90% (n = 179) of the multidrug-resistant tuberculosis patients initiated treatment during the study period; 139 (77.7%) were men and 40 (22.3%) were women. The median age was 45 years (interquartile range, 35–53 yr). The majority of patients (68.7%) reported a daily income below the international poverty line (United States $1.25).
At the time of pretreatment clinical assessment, 60 (33.5%) patients had diabetes, 5 (2.8%) had evidence of cardiovascular disease, 2 (1.1%) had hypothyroidism, and 1 (0.6%) was HIV seropositive. Nearly one-half of the patients (n = 85) had a body mass index less than 18.5 before the start of multidrug-resistant tuberculosis treatment. Nearly all (98.9%) were previously treated with first-line antituberculosis drugs (Table 1).
Table 1.
Clinical and demographic characteristic of patients with multidrug-resistant tuberculosis by treatment outcome in Kerala, India, 2009–2010
| Variable | Treatment Outcome
|
Total (N = 179) | HR | (95% CI) | Adjusted HR | (95% CI) | |||
|---|---|---|---|---|---|---|---|---|---|
| Unsuccessful*
|
Successful†
|
||||||||
| n | (%) | n | (%) | ||||||
| Sex | |||||||||
| Male | 59 | (42.4) | 80 | (57.6) | 139 | 0.9 | (0.6–1.4) | — | — |
| Female | 8 | (20.0) | 32 | (80.0) | 40 | Referent | — | — | |
| Age, yr | |||||||||
| 15–24 | 3 | (23.1) | 10 | (76.9) | 13 | 1.0 | (0.5–1.9) | — | — |
| 25–44 | 26 | (34.7) | 49 | (65.3) | 75 | Referent | — | — | |
| >44 | 38 | (41.8) | 53 | (58.2) | 91 | 1.0 | (0.7–1.5) | — | — |
| Living below poverty line (<United States $1.25 per day) | |||||||||
| Yes | 45 | (36.6) | 78 | (63.4) | 123 | 1.1 | (0.7–1.7) | — | — |
| No | 22 | (39.3) | 34 | (60.7) | 56 | Referent | — | — | |
| Self-reported alcohol consumption before treatment | |||||||||
| Yes | 32 | (53.3) | 28 | (46.7) | 60 | 0.9 | (0.6–1.5) | — | — |
| No | 31 | (27.2) | 83 | (72.8) | 114 | Referent | — | — | |
| Self-reported alcohol consumption during multidrug-resistant tuberculosis treatment | |||||||||
| Yes | 14 | (87.5) | 2 | (12.5) | 16 | 4.9 | (1.2–20.3) | 4.3 | (1.1–17.6) |
| No | 49 | (30.8) | 110 | (69.2) | 159 | Referent | — | — | |
| Self-reported tobacco smoker before treatment | |||||||||
| Yes | 32 | (45.1) | 39 | (54.9) | 71 | 0.9 | (0.6–1.4) | 0.6 | (0.2–1.7) |
| No | 31 | (30.4) | 71 | (69.6) | 102 | Referent | — | — | |
| Self-reported tobacco smoker during multidrug-resistant tuberculosis treatment | |||||||||
| Yes | 16 | (64.0) | 9 | (36.0) | 25 | 1.7 | (0.9–3.5) | 1.2 | (0.3–5.0) |
| No | 47 | (31.3) | 103 | (68.7) | 150 | Referent | — | — | |
| HIV seropositive | |||||||||
| Yes | 1 | (100) | 0 | 1 | 20.3 | (0.0–∞) | — | — | |
| No | 66 | (37.1) | 112 | (62.9) | 178 | Referent | — | — | |
| Diabetes mellitus | |||||||||
| Yes | 20 | (33.3) | 40 | (66.7) | 60 | 0.9 | (0.6–1.5) | — | — |
| No | 47 | (39.5) | 72 | (60.5) | 119 | Referent | — | — | |
| Cavitary chest radiograph | |||||||||
| Yes | 34 | (36.2) | 60 | (63.8) | 94 | 0.7 | (0.3–1.9) | — | — |
| No | 30 | (36.6) | 52 | (63.4) | 82 | Referent | — | — | |
| Number of previous tuberculosis episodes | |||||||||
| 1–2 | 24 | (39.3) | 37 | (60.7) | 61 | 0.9 | (0.6–1.5) | — | — |
| ≥3 | 43 | (36.4) | 75 | (63.6) | 118 | Referent | — | — | |
| At least one adverse drug-related event during treatment‡ | |||||||||
| Yes | 20 | (28.6) | 50 | (60.2) | 70 | 1.0 | (0.7–1.5) | — | — |
| No | 46 | (42.6) | 62 | (57.4) | 108 | Referent | — | — | |
| Hospitalization required during treatment§,‖ | |||||||||
| Yes | 34 | (60.7) | 22 | (39.3) | 56 | 1.7 | (1.1–2.7) | 1.5 | (1.0–2.5) |
| No | 33 | (26.8) | 90 | (56.6) | 123 | Referent | — | — | |
Definition of abbreviations: CI = confidence interval; HR = hazard ratio.
Boldface indicates statistically significant at α = 0.05.
Includes treatment failure, default, death, adverse drug reactions, and development of extensively drug-resistant tuberculosis treatment.
Includes cured and treatment completed.
Adverse drug reactions include gastrointestinal, neuropathy, ototoxicity, psychosis, renal insufficiency, seizures, suicidal ideation.
Does not include 7 days of initial admission period of treatment.
Excluded one patient who was defined as unsuccessful because of stopping treatment due to adverse drug-related event (i.e., risk factor and outcome are the same).
Of 179 patients with multidrug-resistant tuberculosis treatment outcome, 77 (43.0%) were classified as cured per bacteriology, 35 (19.6%) completed treatment, 30 (16.8%) died, 26 (14.5%) defaulted, 9 (5%) failed treatment, 1 (0.6%) stopped treatment because of a severe adverse drug-related event, and 1 (0.6%) developed extensively drug-resistant tuberculosis. There was no meaningful or statistically significant difference between the time to multidrug-resistant tuberculosis treatment initiation between those with successful outcomes and unsuccessful outcomes (P = 0.67); however, on average, it took 145 days to start multidrug-resistant tuberculosis treatment (SD, ±58 days).
Sex, age group, living below the poverty line, self-reported alcohol consumption before treatment, self-reported smoking before treatment, smoking during treatment, HIV seropositivity, diabetes mellitus, pulmonary cavities, low body mass index, and the number of prior tuberculosis episodes were all not associated with outcome based on crude HR calculations (Table 1).
Treatment-related adverse events were identified in 71 (39.6%) patients during the course of the treatment, but these events were not associated with outcome. Of note, 29 (16.2%) were psychiatric events, including one suicidal ideation. Gastrointestinal upset (13.4%), arthralgia (10.1%), ototoxicity, (6.1%), nephrotoxicity (5.0%), neuropathy (2.8%), and jaundice (1.1%) were also identified during treatment. Overall, those requiring hospitalization during treatment had higher hazard for unsuccessful outcome (HR, 1.7; 95% CI, 1.1–2.7) than those not hospitalized. Those consuming alcohol during treatment (HR, 4.9; 95% CI, 1.2–20.3) (Table 1, Figure 1) had higher hazard for poor outcome than those who did not. After adjusting for hospitalization during treatment, persons who consumed alcohol during treatment had a higher hazard (adjusted HR, 4.3; 95% CI, 1.1–17.6) for poor outcome when compared with those who did not. Alcohol consumption during treatment was not independently associated with death (HR, 0.9; 95% CI, 0.2–3.1) or default from treatment (HR, 0.8; 95% CI, 0.2–3.3).
Figure 1.
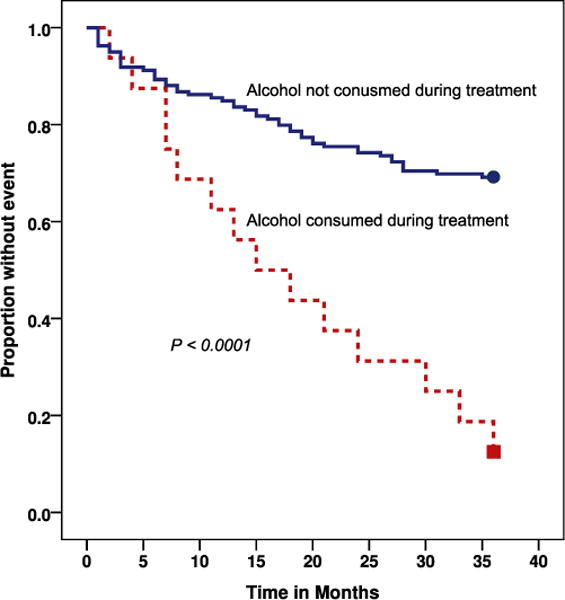
Kaplan-Meier survival curve of time survival versus alcohol usage during multidrug-resistant tuberculosis (MDR-TB) treatment in 179 patients in Kerala, India. The P value reflects the results of the log-rank test of the equality of the two survival curves. Survival is measured in months after starting MDR-TB treatment.
Of note, when testing for potential confounding, we discovered that persons who consumed alcohol during treatment were nearly seven times more likely to miss more than seven intensive-phase doses than those who did not consume alcohol during treatment (relative risk, 6.6; 95% CI, 3.2–13.6). A closer examination of the mean number of doses missed during treatment stratified by outcome and alcohol consumption are presented in Table 2. Persons with unsuccessful treatment outcomes, on average, missed seven more intensive-phase doses (95% CI, 3.8–9.3) than those with successful outcomes (Table 2). Persons who consumed alcohol during treatment, on average, missed 18 more intensive-phase doses (95% CI, 13.2–21.8) than those who did not (Table 2).
Table 2.
Mean number of antituberculosis drug doses missed during intensive phase and continuation phase of treatment of multidrug-resistant tuberculosis patients in Kerala, India, 2009–2010
| N = 179 | Mean No. of Missed Doses (SD) | Mean Difference* (95% CI) | P Value | |
|---|---|---|---|---|
| Intensive phase | ||||
| Unsuccessful treatment outcome† | ||||
| Yes | 67 | 7.2 (14.2) | 6.6 (3.8 to 9.3) | <0.0001 |
| No | 112 | 0.6 (3.3) | ||
| Alcohol consumption during treatment | ||||
| Yes | 16 | 19.1 (22.0) | 17.5 (13.2 to 21.8) | <0.0001 |
| No | 159 | 1.6 (5.4) | ||
| Continuation phase | ||||
| Unsuccessful treatment outcome† | ||||
| Yes | 67 | 7.6 (22.1) | 4.2 (−0.7 to 9.1) | 0.09 |
| No | 112 | 3.4 (11.0) | ||
| Alcohol consumption during treatment | ||||
| Yes | 16 | 12.5 (28.5) | 8.1 (−0.3 to 16.5) | 0.06 |
| No | 159 | 4.4 (14.5) | ||
Boldface indicates statistically significant at α = 0.05.
Equal variances assumed.
Includes treatment failure, default, death, adverse drug reactions, and development of extensively drug-resistant tuberculosis treatment.
Discussion
In this retrospective cohort study of patients treated for multidrug-resistant tuberculosis in Kerala, we found that the majority (63%) of patients had a successful outcome. This success rate was higher than reported at the national (47%) and global levels (53%) (2, 3), but similar to recent metaanalyses or large cohort studies conducted elsewhere (14–16).
Despite a high prevalence of diabetes mellitus (33.5%), more than three tuberculosis treatment episodes (36.6%), low body mass index at diagnosis (47.5%), and pulmonary cavities at the time of diagnosis (52.5%), these factors, although previously found to be associated with unsuccessful outcomes in other studies (14–16), were not associated with outcome in our cohort. Consuming alcohol during treatment was the only factor independently associated with unsuccessful outcome.
Kerala has the highest per capita rate of alcohol consumption in India, with more than 8 L per person per annum—a rate nearly three times higher than the national rate (17, 18). Over the past 4 years, alcohol sales have doubled and contribute to major revenues in Kerala’s state annual budget (19). These statistics are especially concerning considering that alcohol use during treatment of multidrug-resistant tuberculosis appears to be the most important risk factor for unsuccessful treatment outcomes in Kerala. Alcohol abuse has long been associated with unsuccessful treatment compliance and clinical outcomes for a variety of illnesses, including tuberculosis (20–24). Numerous studies have demonstrated that persons who abuse alcohol were more likely to default from tuberculosis treatment (21–24).
The relationship between alcohol consumption and treatment outcomes is complex. First, from a treatment adherence perspective, treatment interruptions are associated with an increased risk for treatment default, failure, or death (22, 25). Moreover, several studies have reported that persons who drink alcohol in excess are more likely to have treatment interruptions (25, 26). In Kerala, those who drank alcohol during treatment missed more doses during both intensive-phase and continuation-phase of tuberculosis treatment than those who did not. Therefore, missed doses probably are responsible for the majority of unsuccessful outcomes. However, some patients consumed alcohol while they were adherent and still suffered unsuccessful treatment outcome. Alcohol consumption detracts from general health and may impair immune responses against M. tuberculosis, which thus may lead to treatment failure or delayed response to treatment (27). In immunocompetent persons, more than 90% of inhaled M. tuberculosis bacteria are eradicated from the body by alveolar macrophages (28). Several studies have demonstrated that alcohol improves in vitro intracellular survival of mycobacteria within human macrophages by suppressing mobilization, adherence, phagocytosis, and superoxide production (28, 29). Alcohol has been shown to decrease antigen-specific T-cell activation by disrupting the capacity to present mycobacterial antigens to lymphocytes (30). Moreover, chronic alcohol exposure may suppress cytokine production, which has an essential role in cellular communication, activation, proliferation, and migration, and in regulating inflammation and other healing mechanisms (31). Among patients who failed to convert on sputum culture, three of nine (33%) consumed alcohol during treatment, one of whom missed only two doses during the intensive phase (data not shown), suggesting failure may have been attributed to the effects of alcohol as opposed to effective treatment and adherence.
Despite the well-recognized implications of alcohol consumption on treatment adherence and outcome, few programs have been implemented that specialize in the simultaneous treatment of both tuberculosis and alcoholism. To our knowledge, only one program has been implemented that specifically focused on an integrative approach (32). This program in Tomsk, Russia, increased the proportion of favorable tuberculosis treatment outcomes among persons with a history of attempting to abstain from drinking alcohol by 18% (33). Although it is unclear if this approach would be effective in other communities, a recent study that assessed the feasibility of integrated alcoholism–tuberculosis treatment and care suggested the immediate need in India (34). It has been suggested that national tuberculosis programs, such as the Revised National Tuberculosis Control Program, could provide the infrastructure for an integrative approach where specialized alcoholism treatment services are almost nonexistent in the public sector (32). Moreover, given that alcohol abuse is a psychiatric disorder and that some patients experience other psychiatric drug-related adverse events during multidrug-resistant tuberculosis treatment, pretreatment clinical examinations might benefit from the inclusion of psychological assessments to screen for alcohol abuse disorders and other psychological conditions. However, the decision to invest in an integrative program should be approached with caution, especially among multidrug-resistant tuberculosis treatment programs—balancing current resources for effective tuberculosis program delivery offset by the additional training and other requirements that may be needed to take on new and potentially demanding responsibilities.
Notably in this cohort, all patients received the same standardized multidrug-resistant tuberculosis regimen, without regard for drug susceptibility test results. Individualized treatment regimens tailored to all drug susceptibility test results are recommended but require substantial clinical management that may be resource intensive (35, 36).
This study has several limitations. First, our findings were based on data that were abstracted from routinely collected records and reports designed to capture clinical encounters and surveillance information. Because of the retrospective nature of the study and because all of the source records and reports were not designed for study purposes, some information may be incomplete or contain errors. Second, follow-up time was limited to the completion of treatment. Although this time frame is sufficient for documenting surveillance-based treatment outcomes, it may not be sufficient to assess long-term clinical outcomes. It is possible that some of the patients may have recurrent episodes in the future. Third, our cohort was limited to patients treated in one state in India during one time period, and our findings should be explored in other settings if they are to be generalized. Fourth, it is possible that some important factors previously associated with unsuccessful treatment outcomes (e.g., HIV, diabetes mellitus, delayed treatment initiation, previous treatment episodes, and malnutrition) may become statistically significant with a larger cohort that is followed for a longer period of time. Fifth, we did not qualify the amount of alcohol consumed. However, because each patient was counseled to abstain from consuming alcohol during treatment to avoid potential harmful hepatotoxic effects of concomitant alcohol use with antituberculosis drugs, we believe any alcohol consumption may be an indicator for more serious alcohol-related behavioral disorders or at the very least a hazardous risk factor. Finally, second-line drug susceptibilities were not considered when determining treatment regimens; it is possible that some patients had unsuccessful outcomes because of ineffective treatment owing to resistance to one or more second-line antituberculosis drugs.
Conclusions
Kerala has the highest per capita rates of alcohol consumption in India. Among patients with multidrug-resistant tuberculosis in Kerala, consumption of alcohol during treatment was associated with poor treatment outcomes. The Revised National Tuberculosis Control Program should consider implementing pretreatment clinical examinations that include psychological assessments to screen for alcohol abuse disorders and other psychological conditions and develop an integrative approach to managing drug-resistant tuberculosis and alcohol use disorders simultaneously in this state.
Supplementary Material
Acknowledgments
The authors thank the staff of the State TB Cell, District TB Officers, and their staff of the state of Kerala in the process of data collection for their unreserved assistance. The study was conducted as a part of the “TB Operations Research Training Project” aimed to build operational research capacity within the Government of India’s Revised National Tuberculosis Control Programme. This training project was conceived and implemented jointly by Central TB Division (Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India), The National TB Institute (Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India Bangalore, India), World Health Organization (India Country Office), The International Union Against Tuberculosis and Lung Diseases (The Union, South-East Asia Regional Office, New Delhi, India), and U.S. Centers for Disease Control and Prevention (Division of TB Elimination, Atlanta, GA). The authors also thank the patients with tuberculosis of the state whose participation in the study made this research possible.
Footnotes
Author Contributions: K.D., S.M., S.G., S.A.N., and A.M.V.K. conceived and designed the experiments; K.D., S.M., S.B., and J.S. performed the experiments; K.D., S.G., P.K.M., and A.M.V.K. analyzed the data; K.D., S.M., S.G., S.A.N., S.B., J.S., J.E.O., P.K.M., and A.M.V.K. contributed materials/analysis tools; K.D., S.G., J.E.O., P.K.M., and A.M.V.K. wrote the paper; K.D., S.M., S.G., S.A.N., S.B., J.S., J.E.O., P.K.M., and A.M.V.K. provided comments and input to revise manuscript.
The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions or policies of the World Health Organization and US Centers for Disease Control and Prevention.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.World Health Organization. Global tuberculosis report 2013. Geneva, Switzerland: WHO/HTM/TB/2013.11. [Google Scholar]
- 2.World Health Organization. Guidelines for the programmatic management of drug-resistanttuberculosis:2011Update. Geneva, Switzerland: WHO; 2011. [Google Scholar]
- 3.Revised National Tuberculosis Control Programme. Guidelines on programmatic management of drug resistant TB (PMDT) in India-May 2012 Central TB Division, Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India, New Delhi. [accessed 2012 Jun 14]. Available from: http://www.tbcindia.nic.in/documents.html.
- 4.Marks SM, Flood J, Seaworth B, Hirsch-Moverman Y, Armstrong L, Mase S, Salcedo K, Oh P, Graviss EA, Colson PW, et al. TB Epidemiologic Studies Consortium Treatment practices, outcomes, and costs of multidrug-resistant and extensively drug-resistant tuberculosis, United States, 2005–2007. Emerg Infect Dis. 2014;20:812–821. doi: 10.3201/eid2005.131037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Resch SC, Salomon JA, Murray M, Weinstein MC. Cost-effectiveness of treating multidrug-resistant tuberculosis. PLoS Med. 2006;3:e241. doi: 10.1371/journal.pmed.0030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrews JR, Shah NS, Weissman D, Moll AP, Friedland G, Gandhi NR. Predictors of multidrug-and extensively drug-resistant tuberculosis in a high HIV prevalence community. PLoS ONE. 2010;5:e15735. doi: 10.1371/journal.pone.0015735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flor de Lima B, Tavares M. Risk factors for extensively drug-sistant tuberculosis: a review. Clin Respir J. 2014;8:11–23. doi: 10.1111/crj.12044. [DOI] [PubMed] [Google Scholar]
- 8.Shin SS, Keshavjee S, Gelmanova IY, Atwood S, Franke MF, Mishustin SP, Strelis AK, Andreev YG, Pasechnikov AD, Barnashov A, et al. Development of extensively drug-resistant tuberculosis during multidrug-resistant tuberculosis treatment. Am J Respir Crit Care Med. 2010;182:426–432. doi: 10.1164/rccm.200911-1768OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim DH, Kim HJ, Park S-K, Kong S-J, Kim YS, Kim T-H, Kim EK, Lee KM, Lee S-S, Park JS, et al. Treatment outcomes and survival based on drug resistance patterns in multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2010;182:113–119. doi: 10.1164/rccm.200911-1656OC. [DOI] [PubMed] [Google Scholar]
- 10.Udwadia ZF, Amale RA, Ajbani KK, Rodrigues C. Totally drug-resistant tuberculosis in India. Clin Infect Dis. 2012;54:579–581. doi: 10.1093/cid/cir889. [DOI] [PubMed] [Google Scholar]
- 11.Falzon D, Jaramillo E, Wares F, Zignol M, Floyd K, Raviglione MC. Universal access to care for multidrug-resistant tuberculosis: an analysis of surveillance data. Lancet Infect Dis. 2013;13:690–697. doi: 10.1016/S1473-3099(13)70130-0. [DOI] [PubMed] [Google Scholar]
- 12.Sachdeva KS, Kumar A, Dewan P, Kumar A, Satyanarayana S. New vision for Revised National Tuberculosis Control Programme (RNTCP): universal access - reaching the un-reached. Indian J Med Res. 2012;135:690–694. [PMC free article] [PubMed] [Google Scholar]
- 13.Dsa K, Mrithyunjayan S, Ghosh S, Sreenivas A, Balakrishnan S, Subramoniapillai J, Moonan P, Kumar A. Treatment outcomes of persons with multidrug resistant tuberculosis, Kerala, India, 2009–2010. Int J Tuberc Lung Dis. 2013;17:5483–5484. [abstract] [Google Scholar]
- 14.Orenstein EW, Basu S, Shah NS, Andrews JR, Friedland GH, Moll AP, Gandhi NR, Galvani AP. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis. 2009;9:153–161. doi: 10.1016/S1473-3099(09)70041-6. [DOI] [PubMed] [Google Scholar]
- 15.Johnston JC, Shahidi NC, Sadatsafavi M, Fitzgerald JM. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLoS ONE. 2009;4:e6914. doi: 10.1371/journal.pone.0006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurbatova EV, Taylor A, Gammino VM, Bayona J, Becerra M, Danilovitz M, Falzon D, Gelmanova I, Keshavjee S, Leimane V, et al. Predictors of poor outcomes among patients treated for multidrug-resistant tuberculosis at DOTS-plus projects. Tuberculosis (Edinb) 2012;92:397–403. doi: 10.1016/j.tube.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das SK, Balakrishnan V, Vasudevan DM. Alcohol: its health and social impact in India. Med Soc. 2006;19:94A9. [PubMed] [Google Scholar]
- 18.Sugathan TN, Soman CR, Sankaranarayanan K. Behavioural risk factors for non communicable diseases among adults in Kerala, India. Indian J Med Res. 2008;127:555–563. [PubMed] [Google Scholar]
- 19.Kerala State Beverages Corporation (KSBC) 2013 Apr; [accessed 2014 May 22]. Available from: http://www.ksbc.kerala.gov.in/
- 20.Oeltmann JE, Kammerer JS, Pevzner ES, Moonan PK. Tuberculosis and substance abuse in the United States, 1997–2006. Arch Intern Med. 2009;169:189–197. doi: 10.1001/archinternmed.2008.535. [DOI] [PubMed] [Google Scholar]
- 21.Rehm J, Baliunas D, Borges GLG, Graham K, Irving H, Kehoe T, Parry CD, Patra J, Popova S, Poznyak V, et al. The relation between different dimensions of alcohol consumption and burden of disease: an overview. Addiction. 2010;105:817–843. doi: 10.1111/j.1360-0443.2010.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jakubowiak WM, Bogorodskaya EM, Borisov SE, Danilova ID, Kourbatova EV. Risk factors associated with default among new pulmonary TB patients and social support in six Russian regions. Int J Tuberc Lung Dis. 2007;11:46–53. [PubMed] [Google Scholar]
- 23.Parry C, Rehm J, Poznyak V, Room R. Alcohol and infectious diseases: an overlooked causal linkage? Addiction. 2009;104:331–332. doi: 10.1111/j.1360-0443.2008.02500.x. [DOI] [PubMed] [Google Scholar]
- 24.Rehm J, Samokhvalov AV, Neuman MG, Room R, Parry C, Lönnroth K, Patra J, Poznyak V, Popova S. The association between alcohol use, alcohol use disorders and tuberculosis (TB): a systematic review. BMC Public Health. 2009;9:450. doi: 10.1186/1471-2458-9-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Driver CR, Matus SP, Bayuga S, Winters AI, Munsiff SS. Factors associated with tuberculosis treatment interruption in New York City. J Public Health Manag Pract. 2005;11:361–368. doi: 10.1097/00124784-200507000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Podewils LJ, Gler MT, Quelapio MI, Chen MP. Patterns of treatment interruption among patients with multidrug-resistant TB (MDR TB) and association with interim and final treatment outcomes. PLoS ONE. 2013;8:e70064. doi: 10.1371/journal.pone.0070064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dannenberg AM., Jr Immune mechanisms in the pathogenesis of pulmonary tuberculosis. Rev Infect Dis. 1989;11:S369–S378. doi: 10.1093/clinids/11.supplement_2.s369. [DOI] [PubMed] [Google Scholar]
- 28.Bermudez LE, Wu M, Martinelli J, Young LS. Ethanol affects release of TNF and GM-CSF and membrane expression of TNF receptors by human macrophages. Lymphokine Cytokine Res. 1991;10:413–419. [PubMed] [Google Scholar]
- 29.Rimland D. Mechanisms of ethanol-induced defects of alveolar macrophage function. Alcohol Clin Exp Res. 1984;8:73–76. [PubMed] [Google Scholar]
- 30.Szabo G, Mandrekar P, Catalano D. Inhibition of superantigen-induced T cell proliferation and monocyte IL-1 beta, TNF-alpha, and IL-6 production by acute ethanol treatment. J Leukoc Biol. 1995;58:342–350. doi: 10.1002/jlb.58.3.342. [DOI] [PubMed] [Google Scholar]
- 31.Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, Qin L, Szabo G, Wheeler M, Zou J. Cytokines and alcohol. Alcohol Clin Exp Res. 2006;30:720–730. doi: 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- 32.Greenfield SF, Shields A, Connery HS, Livchits V, Yanov SA, Lastimoso CS, Strelis AK, Mishustin SP, Fitzmaurice G, Mathew TA, et al. Integrated management of physician-delivered alcohol care for tuberculosis patients: design and implementation. Alcohol Clin Exp Res. 2010;34:317–330. doi: 10.1111/j.1530-0277.2009.01094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin S, Livchits V, Connery HS, Shields A, Yanov S, Yanova G, Fitzmaurice GM, Nelson AK, Greenfield SF, Tomsk Tuberculosis Alcohol Working Group Effectiveness of alcohol treatment interventions integrated into routine tuberculosis care in Tomsk, Russia. Addiction. 2013;108:1387–1396. doi: 10.1111/add.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas B, Suhadev M, Mani J, Ganapathy BG, Armugam A, Faizunnisha F, Chelliah M, Wares F. Feasibility of an alcohol intervention programme for TB patients with alcohol use disorder (AUD)-a qualitative study from Chennai, South India. PLoS ONE. 2011;6:e27752. doi: 10.1371/journal.pone.0027752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leimane V, Riekstina V, Holtz TH, Zarovska E, Skripconoka V, Thorpe LE, Laserson KF, Wells CD. Clinical outcome of individualised treatment of multidrug-resistant tuberculosis in Latvia: a retrospective cohort study. Lancet. 2005;365:318–326. doi: 10.1016/S0140-6736(05)17786-1. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. Management of MDR-TB: a field guide A companion document to Guidelines for the Programmatic Management of Drug-resistant Tuberculosis. Geneva, Switzerland: World Health Organization; 2009. (WHO/HTM/TB/2008.402a) [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


