Abstract
Islet xenotransplantation is a potential treatment for diabetes without the limitations of tissue availability. Although successful experimentally, early islet loss remains substantial and attributed to an instant blood mediated inflammatory reaction (IBMIR). This syndrome of islet destruction has been incompletely defined and characterization in pig-to-primate models has been hampered by logistical and statistical limitations of large animal studies. To further investigate IBMIR, we developed a novel in vivo dual islet transplant model to precisely characterize IBMIR as proof-of-concept that this model can serve to properly control experiments comparing modified xenoislet preparations. Wild-type (WT) and α1,3-galactosyltransferase knockout (GTKO) neonatal porcine islets (NPIs) were studied in non-immunosuppressed rhesus macaques. Inert polyethylene microspheres served as a control for the effects of portal embolization. Digital analysis of immunohistochemistry targeting IBMIR mediators was performed at one and 24 hours after intraportal islet infusion. Early findings observed in transplanted islets include complement and antibody deposition, and infiltration by neutrophils, macrophages, and platelets. Insulin, complement, antibody, neutrophils, macrophages, and platelets were similar between GTKO and WT islets, with increasing macrophage infiltration at 24 hours in both phenotypes. This model provides an objective and internally controlled study of distinct islet preparations and documents the temporal histology of IBMIR.
Introduction
Islet transplantation is a treatment option for selected patients with type 1 diabetes mellitus. Marginal long-term islet function and the adverse effects of immunosuppression have limited the growth of this field, however, the most significant hindrance has been the limited availability of quality donor organs. This obstacle could be overcome through porcine islet xenotransplantation. Indeed, preclinical models have demonstrated that porcine islets can engraft, survive, and achieve glucose homeostasis in diabetic non-human primates (NHPs) (1–12).
During intraportal infusion, islet allografts and porcine xenografts undergo rapid destruction attributed in part to a process that has been broadly termed the instant blood mediated inflammatory reaction (IBMIR) (13–16). IBMIR has been connected to a variety of inflammatory mediators; binding of antibody and complement, rapid activation of the coagulation cascade, and innate cellular infiltration all contribute to islet destruction and non-engraftment (13, 17). The mechanistic understanding of IBMIR has developed over time via extensive in vitro assays measuring isolated variables suspected to play a part in this process (18–24). However, the redundancy of the immune system demands a dedicated in vivo evaluation of IBMIR performed using a rigorous and clinically relevant model. Thus far, the logistical limitations of studies in large animals have made it difficult to control for the numerous individual variations confounding the analysis of trials with small numbers of animals (12, 15, 25, 26). Furthermore, the challenges inherent in the study of primates (the necessary recipient in clinically relevant islet xenotransplant studies) have hampered efforts to combat IBMIR or subsequent rejection through islet modification, a potential strategy that is particularly relevant to xenotransplantation. Thus, there remains a need to objectively define IBMIR and identify potential targets for therapeutic modification. To accomplish this a method is required that is controlled but still sensitive to the logistical challenges of pig-to-primate investigations, allowing for the evaluation of potential islet modifications, both to mollify the effects of IBMIR and xenograft rejection.
Several groups have used transgenic modifications of porcine tissue with hopes of improving islet engraftment. A fundamental example is the use of islets from α1,3-galactosyltransferase total knockout (GTKO) (9), human CD46 transgenic pigs (12), and a recent study of porcine islets with multiple genetic modifications (27). Indeed, as clinical trials in islet xenotransplantation are contemplated (28–31) the use of transgenic porcine tissue is generally felt to be an essential component for meaningful engraftment with acceptable degrees of immunosuppression. However, preclinical work in the pig to primate model has made controlled studies of specific transgenes difficult, and conclusions based on numerous protocol variations have made it difficult to quantify the benefit of specific transgenic modifications (32). As islet graft survival studies require the investment of substantial time and resources, a more definitive understanding of the potential benefits of a specific transgenic modification in the early engraftment phase is required to select more favorable islet phenotypes for long-term preclinical studies.
Therefore, we have developed a unique in vivo model in which to rigorously compare islet phenotypes within a single NHP recipient, and herein present a proof-of-concept study using this model to define early engraftment and evaluate a distinct genetic modification, GTKO, in neonatal porcine islets (NPIs). GTKO neonatal islet preparations have been shown to engraft and function better than wild-type (WT) preparations (9). The potential role of IBMIR in this observation has not been elucidated, thus, we chose to define and directly compare IBMIR in both islet types. The “dual islet transplant model” exploits the independent blood supply of the two anatomic lobes of the liver into which phenotypically distinct islet preparations can be infused via the portal vein. This model removes many of the inter-recipient variables present in NHP work by creating a direct comparison within a single recipient. This initial application of the dual transplant model was utilized to directly compare IBMIR amongst intraportally infused GTKO and WT NPIs at 1 and 24 hour time intervals without immunosuppression. To segregate islet-specific inflammatory events from those associated with the mechanical effects of portal vein embolization, biologically inert material control consisting of polyethylene microspheres (MS) were also studied (33).
Materials and Methods
Neonatal porcine islet procurement, isolation, and culture
Neonatal piglets, either α1,3-galactosyl transferase nullizygous (GTKO) or hemizygous (phenotypic wild-type), were obtained from Fios, Inc. (Rochester, MN) and bred at the University of Georgia Department of Animal and Dairy Science (Athens, GA). Piglets underwent terminal pancreatectomy 3–6 days after birth followed by immediate collagenase digestion and islet isolation via a previously described modified Korbutt technique (34). Phenotypically similar islets were maintained in culture using NPI culture media (Corning, Corning, NY) supplemented with 2.5mL penicillin streptomycin solution (Corning) for 6–8 days and were quantified in islet equivalents (IEQs) on day of transplantation.
Pretransplant islet characterization
Phenotypic analysis by peripheral blood flow cytometry was used to confirm the presence or absence of galactose-α1,3-galactose(Gal) epitope (isolectin B4-FITC, #L2895, Sigma-Aldrich) Peripheral blood was collected from piglets soon after birth and Ficoll separation utilized to isolate peripheral blood mononuclear cells. These cells were then stained using isolectin-B4 conjugated with fluorescein (Sigma-Aldrich, St. Louis, MO). Flow cytometry was performed using a BD LSR II® flow cytometer and analyzed using FlowJo, version 10 (Tree Star, Ashland, Oregon). On the day of transplantation, islets were assessed for quantity by dithizone (Sigma-Aldrich), for viability by fluorescein diacetate (Sigma-Aldrich) and propidium iodide (Sigma-Aldrich), for bacterial contamination by Gram stain and culture, and for in vitro function by static incubation assay and glucose stimulation index (GSI).
Dual transplant model of islet transplantation
Rhesus macaques (Macaca mulatta - Yerkes National Primate Center, Atlanta, GA) weighing approximately 3–10 kgs were selected as NPI xenograft recipients (Table 1). No immunosuppression was given in this study. All procedures and care of animals was performed in accordance with the Guide for the Care and Use of Laboratory Animals (35) and approved by the Emory University Institutional Animal Care and Use Committee. To correlate results from these experiments with our previous data, individual islet infusions were prepared identical with our prior WT and GTKO islet xenotransplant study using 20mL CMRL 1066 without phenol red (Corning),100 units of heparin sodium, and 1.5mg/kg of etanercept (Enbrel Immunex Corp, Thousand Oaks, CA). Two islet preparations were made for each transplant recipient, one for each islet phenotype, and balanced to provide comparable IEQ infusions to each hemiliver. Polyethylene microspheres (Cospheric, Santa Barbara, CA) were similarly quantified into equivalent IEQ dosing. Recipient macaques underwent induction of general anesthesia, midline laparotomy, and isolation of the portal structures. The main portal vein was dissected in the hilum to identify and control the left and right portal venous branches entering the liver. The left or right portal venous branch was independently occluded while one phenotypically distinct islet preparation was infused into the contralateral hemiliver. The second islet preparation was then infused into the remaining hemiliver in an analogous manner (Figure 1A). The side of the infusion was randomized so that differences segregating with lateralization were controlled. Ten animals underwent dual transplant of GTKO and WT NPIs, and 6 animals were transplanted with GTKO islets and MS as outlined in Table 1.
Table 1. Dual islet transplant recipients.
A. There were 10 recipients of WT and GTKO NPIs, 5 for each endpoint. IEq/kg was matched as closely as possible for transplant. Glucose simulation index (GSI) was over 1.0 for all islets confirming glucose sensitive insulin production.
B. There were 6 recipients of GTKO NPIs and MS, 3 for each endpoint. IEq/kg of MS was determined by counting of spheres using the same technique as islets and matching for infused islet volume.
| a) WT vs GTKO NPI recipients
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Recipient ID | Weight | Endpoint | Left lobe | IEq/kg | GSI | Right Lobe | IEq/kg | GSI |
| WTGTKO1 | 4.5 kg | 1 hour | WT NPI | 50,822 | 1.69 | GTKO | 52,792 | 2.43 |
| WTGTKO2 | 4.94 kg | 1 hour | GTKO | 26,895 | 2.29 | WT NPI | 25,641 | 2.82 |
| WTGTKO3 | 9.3 kg | 1 hour | WT NPI | 25,228 | 5.7 | GTKO | 24,912 | 3.77 |
| WTGTKO4 | 3.57 kg | 1 hour | GTKO | 52,822 | 1.46 | WT NPI | 58,272 | 1.69 |
| WTGTKO5 | 8.35 kg | 1 hour | WT NPI | 26740 | 2.68 | GTKO | 26,734 | 3.52 |
| WTGTKO6 | 7.55 kg | 24 hour | GTKO | 25,320 | 2.92 | WT NPI | 25,296 | 2.82 |
| WTGTKO7 | 6.14 kg | 24 hour | WT NPI | 24,868 | 2.82 | GTKO | 25,317 | 2.29 |
| WTGTKO8 | 7.15 kg | 24 hour | GTKO | 26,381 | 3.07 | WT NPI | 24,604 | 2.29 |
| WTGTKO9 | 9.5 kg | 24 hour | GTKO | 25,976 | 4.11 | WT NPI | 25,976 | 3.11 |
| WTGTKO10 | 3.2 kg | 24 hour | GTKO | 31,172 | 8.26 | WT NPI | 32,203 | 7.97 |
|
| ||||||||
| b) GTKO NPI vs MS recpients
| ||||||||
| Recipient ID | Weight | Endpoint | Left lobe | IEq/kg | GSI | Right Lobe | IEq/kg | GSI |
|
| ||||||||
| MSGTKO1 | 8.7 kg | 1 hour | GTKO | 26,000 | 2.3 | Sphere | matched | N/A |
| MSGTKO2 | 7.0 kg | 1 hour | GTKO | 26,244 | 7.23 | Sphere | matched | N/A |
| MSGTKO3 | 10.0 kg | 1 hour | GTKO | 19,835 | 4.92 | Sphere | matched | N/A |
| MSGTKO4 | 4.05 kg | 24 hour | Sphere | matched | N/A | GTKO | 20,903 | 1.67 |
| MSGTKO5 | 8.8 kg | 24 hour | Sphere | matched | N/A | GTKO | 25,568 | 2.96 |
| MSGTKO6 | 9.97 kg | 24 hour | GTKO | 25,075 | 5.17 | Sphere | matched | N/A |
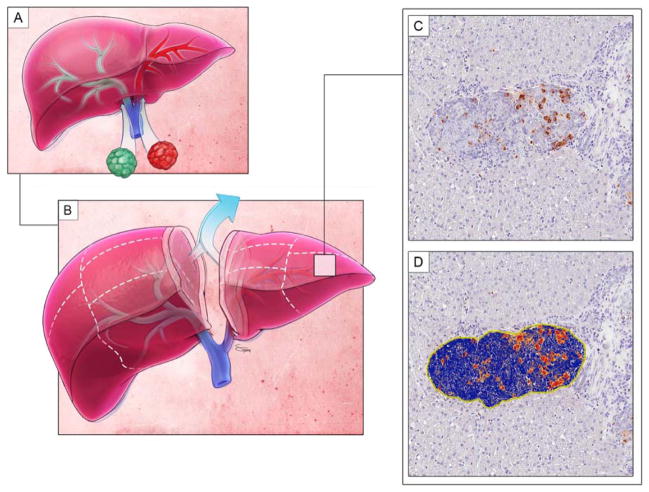
Figure 1. The dual islet transplant model.
A. Islets of a different phenotype (denoted by colors) are infused into contralateral hemilivers. Islets are separated by the independent right and left portal venous distribution.
B. At the experimental time point, the liver is divided by lobe and sectioned by predetermined margins. A small central margin is eliminated to maintain phenotypic purity for analysis.
C. Sections then are processed and converted into digital format.
D. Digital pathology software analyzes the staining of the image and computes a quantitative value for the positivity of each immunohistochemical stain. In this representative image, red means strong staining positivity, orange medium staining positivity, yellow weak staining positivity, and blue is negative.
Tissue collection and immunohistochemistry
Livers were procured from euthanized islet recipients at 1 and 24 hours post infusion time points for detailed immunohistochemical analysis. Each transplant group described above was evenly distributed into 1 hour and 24 time points. The explanted liver was divided into right and left lobes along the portal venous distribution with 1.0cm of central tissue excluded from analysis in each hemiliver to preclude crossover contamination between islet phenotypes. Hemilivers were divided into 5 sections (Figure 1B), each undergoing independent staining for standard hematoxylin and eosin, as well as galactose-α1,3-galactose (#801-090-1, Enzo Life Sciences, Farmingdale, NY), insulin (#I2018, Sigma-Aldrich), C3d ((#ab15981, Abcam, Cambridge, MA, USA), C4d (#12-5000, American Research Products, Waltham, MA, USA), IgG (#A2054, Sigma-Aldrich), IgM (#071-11-031, KPL, Gaithersburg, MD), neutrophil (neutrophil elastase, #M0752, Dako, Carpinteria, CA), macrophage (CD68, #M0814, Dako), and platelet (CD61, #M0753, Dako). The signal was visualized using 3,3 diaminobenzidine peroxidation and counterstained with hematoxylin. Whole slide digital images were captured using the Aperio ScanScope XT (Leica Biosystems, Vista, CA) slide scanner system with 20× objective magnification.
Digital analysis of immunohistochemistry
Quantitative immunohistochemical analysis was performed using Aperio Imagescope (Leica Biosystems) digital pathology software. Individual slides were scanned and islets were manually selected and analyzed using an optimized positive pixel algorithm to obtain a percent pixel positivity of the individual stain per islet (Figure 1C and 1D). The sum of individual islets within a hemiliver was compiled to compute a percent positive staining representative of each hemiliver, termed the positivity index. Data for a specific stain were excluded from an animal if the total number of islets found in a hemiliver was less than five.
Statistical analysis
Positivity was calculated within each hemiliver and paired against the contralateral hemiliver positivity for each animal. As positivity was measured in a percentage format, a paired t-test with logarithmic data transformation was performed to analyze stain differences at the study time points of 1 and 24 hours. An unpaired t-test was performed to measure differences in positivity from 1 to 24 hours for each distinct islet phenotype. Statistical analysis was performed using GraphPad Prism (GraphPad Software Inc., LaJolla, CA) version 6 statistical software. A p ≤ 0.05 was determined to be statistically significant.
Results
Assessment of Islet Segregation in the Model, and Evaluation of IBMIR in WT and GTKO Neonatal Porcine Islet Xenografts
To confirm separation of GTKO and WT NPIs via contralateral portal vein infusion, Gal staining was compared between hemilivers of each animal (Figure 2). Histological sections from each hemiliver demonstrated homogenous islet phenotypes of either GTKO or WT, therefore pixel positivity was used to objectively confirm separation in addition to a control stain to determine the presence of Gal. At 1 and 24 hours the difference in Gal positivity was statistically significant (1h p=0.05, 24h p=0.01) validating the model’s principal tenet of anatomical separation of each islet preparation within their respective hemiliver. Comparison of Gal expression from 1 to 24 hours demonstrated no change in positivity index over time (1h p=0.97, 24h p=0.96), indicating stable separation and consistency between 1 and 24 hour experimental time points.
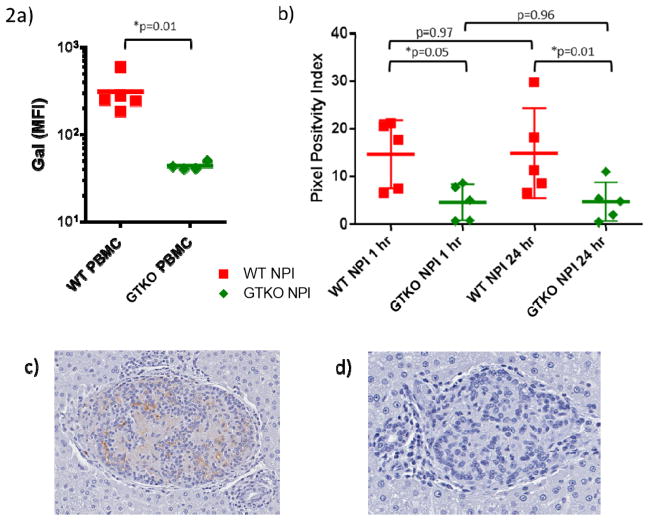
Figure 2. Islets preparations are separated by the anatomic distribution of portal venous blood flow.
A. PBMC flow cytometry confirming loss of Gal expression in GTKO animals.
B. Immunohistochemical staining of galactose-alpha-1,3-galactose was analyzed to determine adequate segregation of islets by hemiliver. At both 1 hour (p=0.05) and 24 hours (p=0.01) staining between islet phenotypes was significantly different. There was no difference in the positivity of staining from 1 to 24 hours within each phenotype (WT p=0.97, GTKO p=0.96).
C. Representative Image of α-Gal positive WT islet cluster at 24 hours.
D. Representative image of α-Gal negative GTKO islet cluster at 24 hrs.
Neonatal porcine islets are incompletely mature at implantation and require approximately 2 weeks to gain full function. However, some islet development begins at birth and insulin production can be detected immediately after transplantation. An in vitro standardized glucose stimulation index was obtained from a subset of islets from the same batch on the day of transplantation confirming glucose sensitive insulin production as depicted in Table 1. Insulin staining between islet phenotypes trended higher in the GTKO islets at 1 hour (p=0.09), an advantage that was lost by 24 hours (p=0.29). Comparison of staining between 1 and 24 hours within a given islet type revealed no significant difference for WT (p=0.90) or GTKO (p=0.18) preparations.
Antibody staining (Figure 3) did not differ significantly between the two islet types for either IgG or IgM at 1 hour (IgG: p=0.21, IgM: p=0.36) or 24 hours (IgG: p=0.68, IgM: p=0.49). Over the time course of the experiment, IgG staining significantly decreased in both WT (p=0.01) and GTKO islets (p=0.05). A similar finding was present in IgM, both in WT (p<0.01) and GTKO islets (p=0.01).
Figure 3. Antibody binding similar in both islet phenotypes.
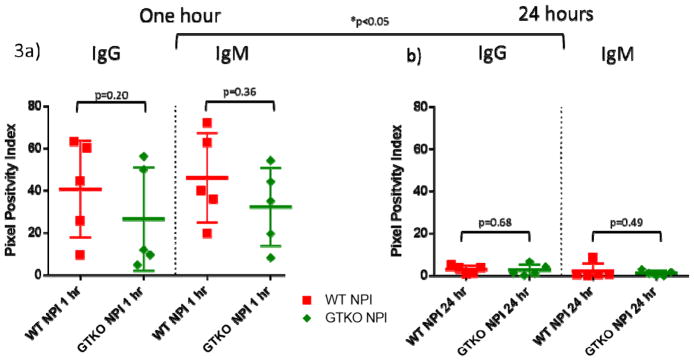
A. At 1 hour there was no difference in the presence of IgG (p=0.20) or IgM (p=0.36) between WT and GTKO NPIs.
B. At 24 hours there was no difference in the presence of IgG (p=0.68) or IgM (p=0.49) between WT and GTKO NPIs. Over 24 hours, there was a significant decrease in IgG (WT: p=0.01, GTKO: p=0.05) and IgM (WT: p<0.01, GTKO: p=0.01) in both phenotypes.
Complement deposition and activation have been proposed as significant factors in early xenoislet loss (17, 36, 37). C4d staining was applied to measure antibody mediated complement activation via the classical pathway. There was no significant difference in C4d staining between WT and GTKO islets at 1 hour (p=0.14) or 24 hours (p=0.62). C4d staining did not significantly change over time in either WT (p=0.99) or GTKO (p=0.38) islets. C3d staining was applied as a measure of alternative pathway complement activation. Similar to C4d results, there was no difference in staining between WT and GTKO islets at 1 (p=0.16) or 24 hours (p=0.98). C3d also did not change over time in WT (p=0.09) and GTKO islets (p=0.79).
There was no difference seen in platelet positivity index at 1 (p=0.63) or 24 hours (0.71). There was no significant change over 24 hours in both WT (p= 0.89) and GTKO islets (p=0.65). Neutrophil positivity was similar between WT and GTKO at 1 hour (p= 0.74) and 24 hours (p=0.43). Overall there was a trend towards decreased neutrophil presence over 24 hours in WT (p=0.09) and GTKO islets (p=0.07).
There was no significant difference in macrophage positivity between WT and GTKO islets at 1 hour (p=0.52) and 24 hours (p=0.36). However, macrophage infiltration was significantly increased in both WT and GTKO islets (p<0.01) over the 24-hour observation period (Figure 4). A summary of experimental findings from 1 to 24 hours after dual islet transplantation of WT and GTKO NPIs is illustrated in Figure 5.
Figure 4. Increasing macrophage infiltration over 24 hours is independent of gal presence.
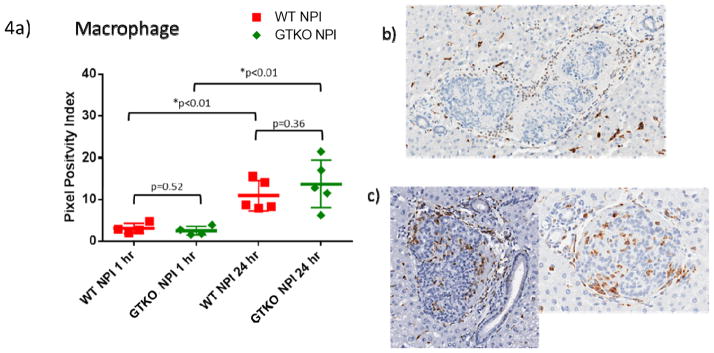
A. At 1 and 24 hours, there is no significant difference in macrophage presence between islet phenotypes (1h p=0.52, 24h p=0.36). However, there was a significant increase in both WT (p<0.01) and GTKO (p<0.01) islets from 1 to 24 hours.
B. Representative group of GTKO islets surrounded by macrophages with minimal infiltration at one hour.
C. GTKO islets from two different animals demonstrating infiltration of macrophages within the islets clusters by 24 hours.
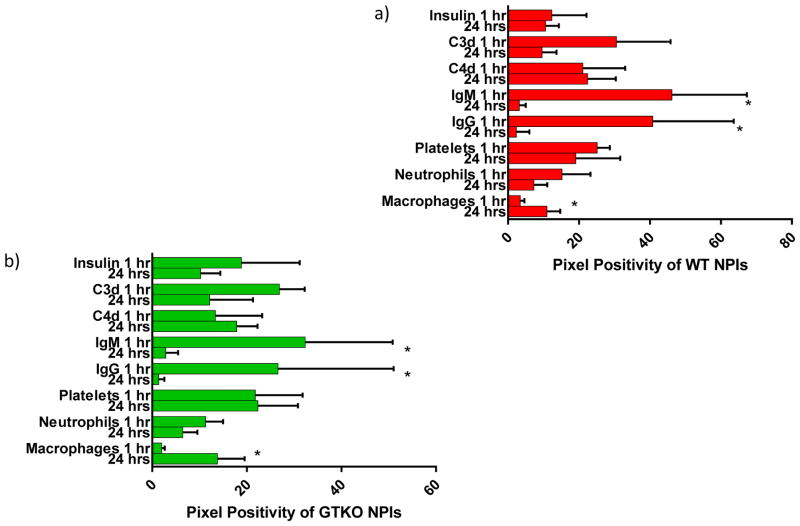
Figure 5. Temporal summary of WT and GTKO NPIs within the dual islet transplantation model.
Findings over 24 hours for WT (a) and GTKO (b) NPIs, an asterisk (*) denotes a statistically significant difference observed from 1 to 24 hours. Both islet phenotypes demonstrated slightly diminished insulin release, decrease in antibody binding, minimal changes in complement deposition and platelet aggregation, a slight decrease in neutrophil infiltration and an increase in macrophage infiltration.
Comparison of GTKO NPI and inert polyethylene microspheres (MS)
Inflammatory responses observed after intraportal islet infusion could be attributed to either a graft specific inflammatory response or a reaction evoked by the mechanical effect of portal embolization. To delineate the contribution of this embolic phenomenon, we used the dual transplant model to examine differences between GTKO NPIs and inert polyethylene MS.
There was no difference in antibody binding between GTKO islets and MS at 1 hour (IgG: p=0.55, IgM: p=0.55) or 24 hours (IgG: p=0.23, IgM: p=0.25). We observed a platelet rich thrombus surrounding all islets immediately after infusion limiting their exposure to free circulation.
The positivity of C3d was significantly greater in GTKO islets than MS at 1 hour (p<0.01) and 24 hours (p=0.02). The relative staining of C3d was maintained from 1 to 24 hours in both GTKO islets (p=0.98) and MS (0.65). GTKO islets trended towards greater C4d deposition at both 1 (p=0.08) and 24 hours (p=0.06). Overall positivity did not change from 1 to 24 hours in either GTKO islets (p=0.97) or MS (p=0.65).
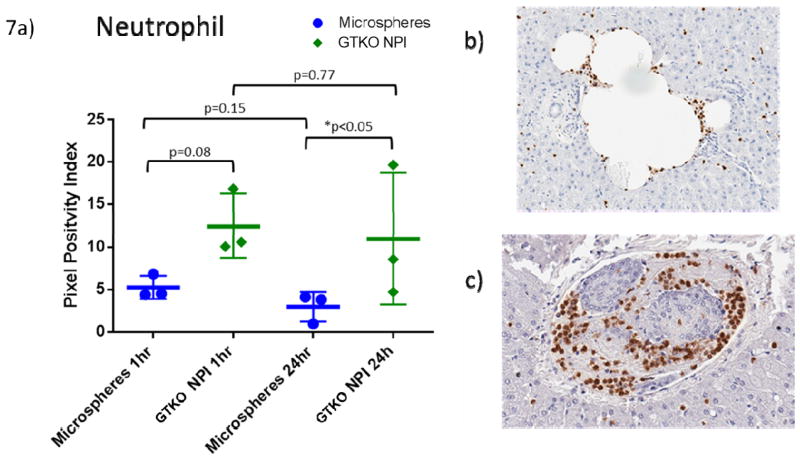
GTKO islets produced greater platelet positivity than MS at 1 hour (p=0.03) and 24 hours (p=0.02) (Figure 6). Over 24 hours, no significant temporal change was seen in MS (p=0.13) or GTKO NPIs (p=0.74). Neutrophils trended towards higher positivity in GTKO islets versus MS in the first hour (p=0.08), which became a significant difference at 24 hours (p=0.05) (Figure 7). There was minimal change in neutrophil presence around MS (p=0.15) and GTKO NPIs (0.77) over the 24 hour experiment.
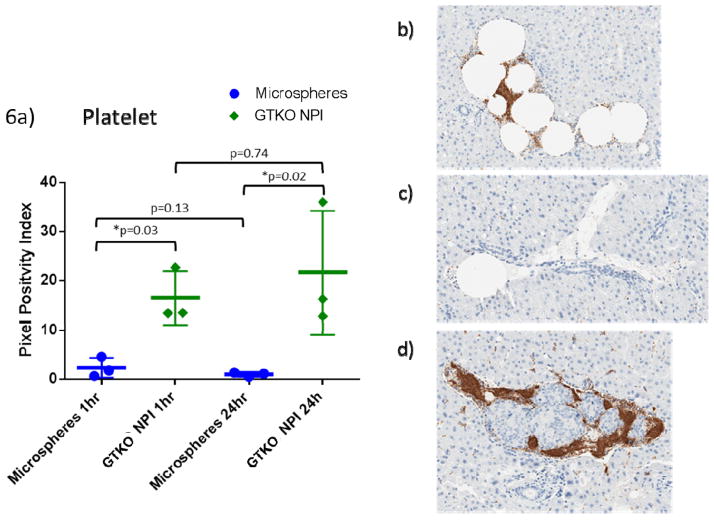
Figure 6. GTKO NPIs initiate coagulation.
A. Platelet deposition around microspheres was significantly lower compared to GTKO islets (1hr p=0.03, 24h p=0.02). Over the 24 hour period there was little change in both microspheres (p= 0.13) and GTKO islets (p=0.74).
B. Obstructive embolus consisting of multiple microspheres with platelet clot formation at one hour.
C. A single microsphere at the proximal end of a sinusoid with minimal platelet involvement at 24 hours.
D. GTKO islet cluster with surrounding platelets at one hour.
Figure 7. GTKO NPIs promote neutrophil adhesion.
A. Neutrophils are increased in GTKO islets (1h p=0.08, 24h p=0.05). There was no significant change over 24 hours: MS (p=0.15), GTKO (p=0.77).
B. Representative histology depicting neutrophils around a microsphere embolus at one hour.
C. Neutrophils surrounding a GTKO islet cluster at one hour.
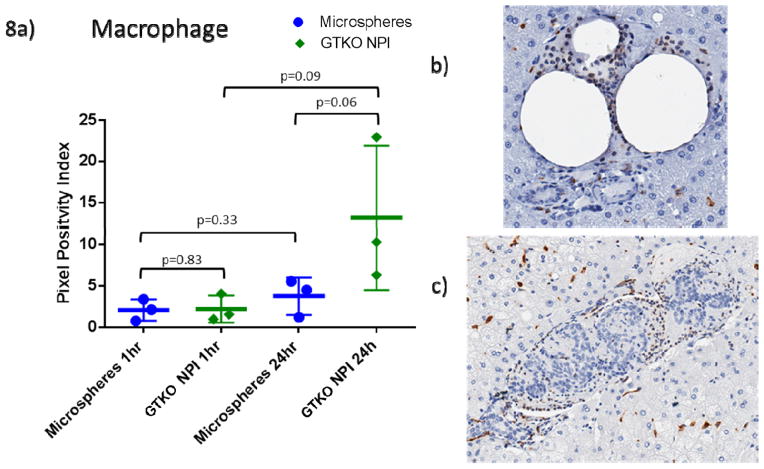
Macrophage positivity at 1 hour was similar between MS and GTKO islets (p=0.83), with the islets approaching significantly higher macrophage presence at 24 hours (p=0.06) (Figure 8). Over 24 hours, there was no significant change in MS (p=0.32) and GTKO NPIs (p=0.10).
Figure 8. GTKO NPIs stimulate macrophage infiltration.
A. Macrophage staining is similar at one hour (p=0.83), at 24 hours GTKO islets have an increased involvement of macrophages over MS that approach significance (p=0.06). There is minimal change in microsphere staining of macrophages (p=0.33) with GTKO islets approaching a significant increase over 24 hours (p=0.09).
B. Representative histology of macrophages around a microsphere embolus at one hour.
C. Macrophages surrounding a GTKO islet cluster at one hour.
Discussion
This series of experiments details a novel in vivo dual islet transplantation model’s capacity to rigorously examine two separate islet preparations within the same large animal recipient, allowing for highly controlled experiments to be performed in a clinically relevant large animal model. Indeed, continued development of novel transgenics provides impetus for continued objective evaluation of porcine islet modifications improving xenoislet engraftment. An example of this is provided by a recent report by Hawthorne et al. (27), illustrating advantages of novel islet xenograft modification utilizing an in vivo NHP model with conventional control and experimental groups. The strength of the dual transplant model lies in intrinsic, controlled comparisons within a single animal, increasing experimental power and therefore requiring fewer animals. We suggest this platform is a suitable testing ground to examine specific porcine islet modifications, including those induced by transient transfection or chemical treatment, thereby conserving the logistical and economic investment in transgenic animals until the modification itself can be validated with compelling evidence for the intended effect. Furthermore, with the ease and pace of successful transgenic modification, this model can ultimately test the end product of these alterations and evaluate whether an objective measurable benefit has been achieved in the important early stages of islet engraftment.
To explore the utility of this model, we have further defined the immunology of IBMIR after intraportal xenotransplantation of NPIs and elucidated the contribution of portal embolization to the inflammatory milieu of islets. We investigated whether the survival advantage previously observed in GTKO neonatal porcine islets could be explained by differences in the early engraftment phase. The WT NPIs used in this experiment are of hemizygous genetic background consistent with the prior study. Therefore, we hypothesized that these WT NPIs would elicit greater inflammation which contributed to their poor outcome relative to GTKO islets; however, we observed no significant differences between GTKO and WT NPIs within 24 hours after islet infusion. As this finding remains contradictory to our previously reported long term outcomes, objective considerations may suggest potential limitations in this model. We intended the exclusion of immunosuppression to unmask marginal differences between the two islet preparations. In contrast, the observations reported may be more related to a robust unmodified inflammatory response leading to an imperceptible difference, possibly specifying a need to incorporate immunosuppression in this model. The limited window in which these experiments were performed may have preceded a Gal specific response, therefore future experiments will examine intrahepatic inflammation at more relevant time points. The primary measure in this model is specific for direct comparison of IBMIR at the histologic level. However, we must acknowledge that systemic inflammatory factors such as cytokines could drive these processes in a manner that would overcome any benefits of transgenic modifications examined in the context of this model. We suggest that these systemic factors are inherent to the process of intraportal islet xenotransplantation in general (38); modification of the systemic reaction to xenoislets would exceed the influence of the islet phenotype alone. The dual transplant model offers a method to control for recipient variables such as these systemic inflammatory mediators by focusing on the comparison of localized responses surrounding the individual islets. Therefore, we present this report to introduce an innovative model to study xenoislet immunity in the context of IBMIR; these data presented herein enable us to adapt future studies to include additional methods of interrogation ultimately maximizing its utility. Regardless, this model provides an alternative and efficient use of animals where preclinical NHP study is warranted for direct clinical relevance (39).
Consistent with the studies of others (15, 23, 40–46), IBMIR encompasses a wide spectrum of innate immune components including complement, macrophages, neutrophils, and platelet aggregation. We show that some portion of this is in response to portal embolization, but that islet-specific factors contribute substantially to the augmentation of this cascade. The breadth of the innate response seen suggests that while targeting individual pathways may lead to some benefit against IBMIR, the immune system’s redundancy may circumvent many specific interventions.
Natural antibodies specific for the Gal antigen have long been known to be a major barrier to porcine xenotransplantation (47–50) leading to the development of GTKO pigs (51–53). The principal evidence supporting the use of GTKO organs is the exclusion of the Gal-epitope, which is targeted by naturally occurring Gal-specific antibodies (54). Although Gal expression is not thought to be evident on adult islets (55), neonatal pancreatic tissue does express Gal and thus differences in this expression could lead to an advantage for GTKO preparations. We have shown that GTKO NPIs have an engraftment advantage that can minimize early rejection and improve engraftment in islet xenotransplantation (9). One well-described potential benefit is likely due to the avoidance of a Gal-specific antibody response (23). However, in this series of experiments antibody presence was similar between WT and GTKO NPIs suggesting this response at hour 1 to be largely non-specific, perhaps evoked by the general adhesiveness of damaged islets that abated over 24 hours. Thus, we did not delineate differences attributable to the Gal epitope with regard to antibody binding when systemic immunosuppression was not given.
Biologically inert microsphere control experiments support the finding that there was no significant change in IgG or IgM over 24 hours. Although more subtle effects could perhaps require larger numbers of animals to discern, this supports the initial assumption that the bulk of the antibody binding seen after portal islet infusion and concomitant portal venous embolization was non-specific. Nevertheless, no parameter examined was obviously different between the GTKO and WT preparations. This indicates that the advantage of GTKO islets involves a variable not examined, one that was not differentiated due to the lack of immunosuppression, or one evolving to present itself outside the timeframe of this study.
Complement has been identified as an important contributing factor to IBMIR in both islet allotransplantation (56) and xenotransplantation (15, 20). Specifically, porcine tissue has been shown to be particularly susceptible to complement-mediated destruction due to lack of human complement regulatory factors (57). Complement activation was clearly noted in both the WT and GTKO NPIs. Although measurements were not significantly different between the preparations, comparison of GTKO NPIs to MS illustrated that complement activation was enhanced beyond that attributable to embolization alone.
Prior studies have demonstrated that complement binding in both the alternative and classical pathway are major components in the acute rejection of xenograft tissues (58), and disruption of the complement cascade can abrogate some of the effects of IBMIR on xenogeneic porcine islets (17). In this model without the use of systemic immunosuppression, the Gal antigen does not appear to influence antibody or complement during the first 24 hours after intraportal islet infusion. These data support complement specific therapeutic agents or transgenic modifications such as CD46, CD55 or CD59 (27, 36, 59, 60). This mechanism may in part explain the successful engraftment beyond one year with the transplantation of transgenic porcine islets with human CD46 (12).
Platelet aggregation observed between GTKO and WT islets did not reveal a Gal-dependent difference in the absence of immunosuppression. Platelet aggregates were present on microsphere histology, however, the increased thrombus in islet clusters suggest that surface or secreted factors play a significant role to propagate the coagulation cascade rather than a primary endothelial or hematologic response to sinusoidal embolism alone. Indeed, exposed collagen from the digestion and isolation process of islets can initiate the intrinsic coagulation pathway (43). Islets, and not pancreatic exocrine tissue, can express tissue factor, which has been implicated as a stimulus in the initiation of coagulation (41, 61, 62). Inhibition of tissue factor expression has demonstrated reduction in the magnitude of coagulation and subsequent consumption of neutrophils, macrophages, and complement in vitro (42, 63). Studies demonstrate measurable benefit after delivery of targeted anticoagulation either concomitantly (25, 40, 64–67) or when bound to islets (24, 68, 69). Transgenic modifications, such as the addition of human CD39, endothelial protein C receptor, complement regulatory proteins, thrombomodulin, or tissue factor pathway inhibitor, may also provide some protection against deleterious thrombus formation (18, 19, 27, 32). Our study supports the concept of islet tissue driven coagulation; however, it is yet to be determined whether a targeted transgenic modification, drug treatment, or a combination of both will provide the optimal outcome.
Although similar in the first hour, macrophage and neutrophil infiltration was greater in GTKO islets than MS at 24 hours, suggesting that this innate cellular response is propagated by islet-specific factors. Human neutrophils are able to adhere and migrate to porcine endothelium (70–72) likely due to cross species interactions of adhesion molecules. The contribution of anti-Gal antibodies in that process is not clearly defined (73, 74). Our data do not support neutrophil adhesion as a process dependent on the Gal epitope, with the inclusion of immunosuppression likely affecting this response toward both islet preparations in a similar manner. The macrophage response appeared to be the most dynamic component in the first 24 hours after intraportal islet infusion and also did not appear to change in the presence or absence of Gal. The inclusion of the resident liver Kupffer cells when using an in vivo model may contribute to our observations (75). Porcine MCP-1 produced by xenogeneic islets has been shown to recruit human macrophages in vitro (76) that may be a contributing factor. These data support macrophages as a key component in the early injurious response after xenogeneic islet infusion, although the lack of immunosuppression may have contributed to the increasing infiltration observed at 24 hours. As no differences were observed between the two islet phenotypes in either macrophage or neutrophil infiltration at both time points, the Gal epitope may not be a key antigenic determinant for the innate cellular response in this early timeframe.
The dual islet transplant model offers a novel and rigorous means to compare the effects of specific islet modification. We propose this as a useful means of examining agents and modifications with comparatively low cost and high throughput when compared to the current standard of practice. This initial report describes the model’s ability to segregate islets and directly observe the individual islet microenvironment in order to objectively compare two different islet phenotypes. Future considerations include the addition of immunosuppression, extension of the experimental endpoint, and measurement of serum inflammatory factors that may influence the outcomes. This platform will enable the rapid and efficient comparison of potential islet modifications prior to substantial investment in preclinical long-term graft outcome studies.
Acknowledgments
The authors wish to acknowledge Linda Stempora from the Duke University Medical Center and Johanna Moreno of the Emory Transplant Center for their excellent technical assistance, and Satyen Tripathi for his illustration. This study was supported by the National Institute of Health grant #AI090956.
Abbreviations
- NHPs
Non-human primates
- IBMIR
Instant blood mediated inflammatory reaction
- Gal
galactose-α1,3-galactose
- GTKO
α1,3-galactosyltransferase total knockout
- WT
Wild-type
- NPIs
Neonatal porcine islets
- MS
Microspheres
- GSI
glucose stimulation index
- IEQs
Islet equivalents
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Marigliano M, Bertera S, Grupillo M, Trucco M, Bottino R. Pig-to-nonhuman primates pancreatic islet xenotransplantation: an overview. Current diabetes reports. 2011;11(5):402–12. doi: 10.1007/s11892-011-0213-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hering BJ, Walawalkar N. Pig-to-nonhuman primate islet xenotransplantation. Transplant immunology. 2009;21(2):81–6. doi: 10.1016/j.trim.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Buhler L, Deng S, O’Neil J, Kitamura H, Koulmanda M, Baldi A, Rahier J, Alwayn IP, Appel JZ, Awwad M, et al. Adult porcine islet transplantation in baboons treated with conventional immunosuppression or a non-myeloablative regimen and CD154 blockade. Xenotransplantation. 2002;9(1):3–13. doi: 10.1034/j.1399-3089.2002.1o044.x. [DOI] [PubMed] [Google Scholar]
- 4.Cardona K, Korbutt GS, Milas Z, Lyon J, Cano J, Jiang W, Bello-Laborn H, Hacquoil B, Strobert E, Gangappa S, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nature medicine. 2006;12(3):304–6. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- 5.Cardona K, Milas Z, Strobert E, Cano J, Jiang W, Safley SA, Gangappa S, Hering BJ, Weber CJ, Pearson TC, et al. Engraftment of adult porcine islet xenografts in diabetic nonhuman primates through targeting of costimulation pathways. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007;7(10):2260–8. doi: 10.1111/j.1600-6143.2007.01933.x. [DOI] [PubMed] [Google Scholar]
- 6.Hering BJ, Wijkstrom M, Graham ML, Hardstedt M, Aasheim TC, Jie T, Ansite JD, Nakano M, Cheng J, Li W, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nature medicine. 2006;12(3):301–3. doi: 10.1038/nm1369. [DOI] [PubMed] [Google Scholar]
- 7.Russell MCCK, Olivia VL, Korbutt G, Cano J, Jiang W, et al. Abstracts of the Joint Meeting of the International Xenotransplantation Association (IXA), the International Pancreas and Islet Transplant Association (IPITA), and the Cell Transplant Society (CTS), Minneapolis, Minnesota, USA, September 15–20, 2007. Xenotransplantation. 2007;14(5):375–549. doi: 10.1111/j.1399-3089.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- 8.Thomas FT, Ricordi C, Contreras JL, Hubbard WJ, Jiang XL, Eckhoff DE, Cartner S, Bilbao G, Neville DM, Jr, Thomas JM. Reversal of naturally occuring diabetes in primates by unmodified islet xenografts without chronic immunosuppression. Transplantation. 1999;67(6):846–54. doi: 10.1097/00007890-199903270-00011. [DOI] [PubMed] [Google Scholar]
- 9.Thompson P, Badell IR, Lowe M, Cano J, Song M, Leopardi F, Avila J, Ruhil R, Strobert E, Korbutt G, et al. Islet xenotransplantation using gal-deficient neonatal donors improves engraftment and function. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(12):2593–602. doi: 10.1111/j.1600-6143.2011.03720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson P, Badell IR, Lowe M, Turner A, Cano J, Avila J, Azimzadeh A, Cheng X, Pierson RN, 3rd, Johnson B, et al. Alternative immunomodulatory strategies for xenotransplantation: CD40/154 pathway-sparing regimens promote xenograft survival. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(7):1765–75. doi: 10.1111/j.1600-6143.2012.04031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson P, Cardona K, Russell M, Badell IR, Shaffer V, Korbutt G, Rayat GR, Cano J, Song M, Jiang W, et al. CD40-specific costimulation blockade enhances neonatal porcine islet survival in nonhuman primates. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(5):947–57. doi: 10.1111/j.1600-6143.2011.03509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Windt DJ, Bottino R, Casu A, Campanile N, Smetanka C, He J, Murase N, Hara H, Ball S, Loveland BE, et al. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9(12):2716–26. doi: 10.1111/j.1600-6143.2009.02850.x. [DOI] [PubMed] [Google Scholar]
- 13.Bennet W, Groth CG, Larsson R, Nilsson B, Korsgren O. Isolated human islets trigger an instant blood mediated inflammatory reaction: implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Upsala journal of medical sciences. 2000;105(2):125–33. doi: 10.1517/03009734000000059. [DOI] [PubMed] [Google Scholar]
- 14.Nilsson B. The instant blood-mediated inflammatory reaction in xenogeneic islet transplantation. Xenotransplantation. 2008;15(2):96–8. doi: 10.1111/j.1399-3089.2008.00460.x. [DOI] [PubMed] [Google Scholar]
- 15.Goto M, Tjernberg J, Dufrane D, Elgue G, Brandhorst D, Ekdahl KN, Brandhorst H, Wennberg L, Kurokawa Y, Satomi S, et al. Dissecting the instant blood-mediated inflammatory reaction in islet xenotransplantation. Xenotransplantation. 2008;15(4):225–34. doi: 10.1111/j.1399-3089.2008.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirchhof N, Shibata S, Wijkstrom M, Kulick DM, Salerno CT, Clemmings SM, Heremans Y, Galili U, Sutherland DE, Dalmasso AP, et al. Reversal of diabetes in non-immunosuppressed rhesus macaques by intraportal porcine islet xenografts precedes acute cellular rejection. Xenotransplantation. 2004;11(5):396–407. doi: 10.1111/j.1399-3089.2004.00157.x. [DOI] [PubMed] [Google Scholar]
- 17.Bennet W, Sundberg B, Lundgren T, Tibell A, Groth CG, Richards A, White DJ, Elgue G, Larsson R, Nilsson B, et al. Damage to porcine islets of Langerhans after exposure to human blood in vitro, or after intraportal transplantation to cynomologus monkeys: protective effects of sCR1 and heparin. Transplantation. 2000;69(5):711–9. doi: 10.1097/00007890-200003150-00007. [DOI] [PubMed] [Google Scholar]
- 18.Iwase H, Ekser B, Hara H, Phelps C, Ayares D, Cooper DK, Ezzelarab MB. Regulation of human platelet aggregation by genetically modified pig endothelial cells and thrombin inhibition. Xenotransplantation. 2013 doi: 10.1111/xen.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwase H, Ezzelarab MB, Ekser B, Cooper DK. The role of platelets in coagulation dysfunction in xenotransplantation, and therapeutic options. Xenotransplantation. 2014 doi: 10.1111/xen.12085. [DOI] [PubMed] [Google Scholar]
- 20.Omori T, Nishida T, Komoda H, Fumimoto Y, Ito T, Sawa Y, Gao C, Nakatsu S, Shirakura R, Miyagawa S. A study of the xenoantigenicity of neonatal porcine islet-like cell clusters (NPCC) and the efficiency of adenovirus-mediated DAF (CD55) expression. Xenotransplantation. 2006;13(5):455–64. doi: 10.1111/j.1399-3089.2006.00335.x. [DOI] [PubMed] [Google Scholar]
- 21.Akima S, Hawthorne WJ, Favaloro E, Patel A, Blyth K, Mudaliar Y, Chapman JR, O’Connell PJ. Tirofiban and activated protein C synergistically inhibit the Instant Blood Mediated Inflammatory Reaction (IBMIR) from allogeneic islet cells exposure to human blood. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9(7):1533–40. doi: 10.1111/j.1600-6143.2009.02673.x. [DOI] [PubMed] [Google Scholar]
- 22.Cabric S, Sanchez J, Lundgren T, Foss A, Felldin M, Kallen R, Salmela K, Tibell A, Tufveson G, Larsson R, et al. Islet surface heparinization prevents the instant blood-mediated inflammatory reaction in islet transplantation. Diabetes. 2007;56(8):2008–15. doi: 10.2337/db07-0358. [DOI] [PubMed] [Google Scholar]
- 23.van der Windt DJ, Marigliano M, He J, Votyakova TV, Echeverri GJ, Ekser B, Ayares D, Lakkis FG, Cooper DK, Trucco M, et al. Early islet damage after direct exposure of pig islets to blood: has humoral immunity been underestimated? Cell transplantation. 2012;21(8):1791–802. doi: 10.3727/096368912X653011. [DOI] [PubMed] [Google Scholar]
- 24.Luan NM, Iwata H. Inhibition of instant blood-mediated inflammatory responses by co-immobilization of sCR1 and heparin on islets. Biomaterials. 2013;34(21):5019–24. doi: 10.1016/j.biomaterials.2013.03.041. [DOI] [PubMed] [Google Scholar]
- 25.Rood PP, Bottino R, Balamurugan AN, Smetanka C, Ayares D, Groth CG, Murase N, Cooper DK, Trucco M. Reduction of early graft loss after intraportal porcine islet transplantation in monkeys. Transplantation. 2007;83(2):202–10. doi: 10.1097/01.tp.0000250680.36942.c6. [DOI] [PubMed] [Google Scholar]
- 26.Goto M, Johansson H, Maeda A, Elgue G, Korsgren O, Nilsson B. Low molecular weight dextran sulfate prevents the instant blood-mediated inflammatory reaction induced by adult porcine islets. Transplantation. 2004;77(5):741–7. doi: 10.1097/01.tp.0000114872.26990.4f. [DOI] [PubMed] [Google Scholar]
- 27.Hawthorne WJ, Salvaris EJ, Phillips P, Hawkes J, Liuwantara D, Burns H, Barlow H, Stewart AB, Peirce SB, Hu M, et al. Control of IBMIR in neonatal porcine islet xenotransplantation in baboons. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(6):1300–9. doi: 10.1111/ajt.12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hering BJ, Cooper DKC, Cozzi E, Schuurman H-J, Korbutt GS, Denner J, O’Connell PJ, Vanderpool HY, Pierson RN., III Executive summary. Xenotransplantation. 2009;16(4):196–202. doi: 10.1111/j.1399-3089.2009.00547.x. [DOI] [PubMed] [Google Scholar]
- 29.van der Windt DJ, Bottino R, Kumar G, Wijkstrom M, Hara H, Ezzelarab M, Ekser B, Phelps C, Murase N, Casu A, et al. Clinical islet xenotransplantation: how close are we? Diabetes. 2012;61(12):3046–55. doi: 10.2337/db12-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooper DK, Bottino R, Satyananda V, Wijkstrom M, Trucco M. Toward clinical islet xenotransplantation - are revisions to the IXA guidelines warranted? Xenotransplantation. 2013;20(2):68–74. doi: 10.1111/xen.12015. [DOI] [PubMed] [Google Scholar]
- 31.Samy KP, Martin BM, Turgeon NA, Kirk AD. Islet cell xenotransplantation: a serious look toward the clinic. Xenotransplantation. 2014;21(3):221–9. doi: 10.1111/xen.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagaraju S, Bottino R, Wijkstrom M, Hara H, Trucco M, Cooper DK. Islet xenotransplantation from genetically engineered pigs. Current opinion in organ transplantation. 2013;18(6):695–702. doi: 10.1097/MOT.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 33.Lamblin A, Tournoys A, Gmyr V, Jourdain M, Lefebvre J, Kerr-Conte J, Proye C, Pattou F. Coagulation activation with intraportal islets of Langerhans transplantation in swine. Annales de chirurgie. 2001;126(8):743–50. doi: 10.1016/s0003-3944(01)00594-6. [DOI] [PubMed] [Google Scholar]
- 34.Korbutt GS, Elliott JF, Ao Z, Smith DK, Warnock GL, Rajotte RV. Large scale isolation, growth, and function of porcine neonatal islet cells. The Journal of clinical investigation. 1996;97(9):2119–29. doi: 10.1172/JCI118649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan PT, Leong KH. The use of biological agents in the treatment of rheumatoid arthritis. Annals of the Academy of Medicine, Singapore. 2007;36(2):128–34. [PubMed] [Google Scholar]
- 36.Schuurman HJ, Pino-Chavez G, Phillips MJ, Thomas L, White DJ, Cozzi E. Incidence of hyperacute rejection in pig-to-primate transplantation using organs from hDAF-transgenic donors. Transplantation. 2002;73(7):1146–51. doi: 10.1097/00007890-200204150-00024. [DOI] [PubMed] [Google Scholar]
- 37.Zhou CY, McInnes E, Copeman L, Langford G, Parsons N, Lancaster R, Richards A, Carrington C, Thompson S. Transgenic pigs expressing human CD59, in combination with human membrane cofactor protein and human decay-accelerating factor. Xenotransplantation. 2005;12(2):142–8. doi: 10.1111/j.1399-3089.2005.00209.x. [DOI] [PubMed] [Google Scholar]
- 38.Kanak MA, Takita M, Kunnathodi F, Lawrence MC, Levy MF, Naziruddin B. Inflammatory Response in Islet Transplantation. International Journal of Endocrinology. 2014;2014(13) doi: 10.1155/2014/451035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooper DKC, Casu A. Chapter 4: Pre-clinical efficacy and complication data required to justify a clinical trial. Xenotransplantation. 2009;16(4):229–38. doi: 10.1111/j.1399-3089.2009.00543.x. [DOI] [PubMed] [Google Scholar]
- 40.Goto M, Johansson H, Maeda A, Elgue G, Korsgren O, Nilsson B. Low-molecular weight dextran sulfate abrogates the instant blood-mediated inflammatory reaction induced by adult porcine islets both in vitro and in vivo. Transplantation proceedings. 2004;36(4):1186–7. doi: 10.1016/j.transproceed.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 41.Ji M, Yi S, Smith-Hurst H, Phillips P, Wu J, Hawthorne W, O’Connell P. The importance of tissue factor expression by porcine NICC in triggering IBMIR in the xenograft setting. Transplantation. 2011;91(8):841–6. doi: 10.1097/TP.0b013e3182106091. [DOI] [PubMed] [Google Scholar]
- 42.Ma X, Ye B, Gao F, Liang Q, Dong Q, Liu Y, Rong P, Wang W, Yi S. Tissue factor knockdown in porcine islets: an effective approach to suppressing the instant blood-mediated inflammatory reaction. Cell transplantation. 2012;21(1):61–71. doi: 10.3727/096368911X580563. [DOI] [PubMed] [Google Scholar]
- 43.van der Windt DJ, Bottino R, Casu A, Campanile N, Cooper DK. Rapid loss of intraportally transplanted islets: an overview of pathophysiology and preventive strategies. Xenotransplantation. 2007;14(4):288–97. doi: 10.1111/j.1399-3089.2007.00419.x. [DOI] [PubMed] [Google Scholar]
- 44.Wang S, Zhao Z, Cong Z, Suo G. Thrombin-activatable fibrinolysis inhibitor is activated in an instant blood-mediated inflammatory reaction after intraportal islet transplant. Experimental and clinical transplantation: official journal of the Middle East Society for Organ Transplantation. 2014;12(1):62–6. doi: 10.6002/ect.2013.0077. [DOI] [PubMed] [Google Scholar]
- 45.Marquet RL, Bonthuis F, van IM, Bouwman E, Wolvekamp MC, van Rooijen N, Scheringa M, JNIJ Primary nonfunction of islet xenografts: the role of macrophages. Transplant international: official journal of the European Society for Organ Transplantation. 1994;7(Suppl 1):S660–2. doi: 10.1111/j.1432-2277.1994.tb01467.x. [DOI] [PubMed] [Google Scholar]
- 46.Nilsson B, Ekdahl KN, Korsgren O. Control of instant blood-mediated inflammatory reaction to improve islets of Langerhans engraftment. Current opinion in organ transplantation. 2011;16(6):620–6. doi: 10.1097/MOT.0b013e32834c2393. [DOI] [PubMed] [Google Scholar]
- 47.Sandrin MS, McKenzie IF. Gal alpha (1,3)Gal, the major xenoantigen(s) recognised in pigs by human natural antibodies. Immunological reviews. 1994;141:169–90. doi: 10.1111/j.1600-065x.1994.tb00877.x. [DOI] [PubMed] [Google Scholar]
- 48.Sandrin MS, Vaughan HA, Dabkowski PL, McKenzie IF. Anti-pig IgM antibodies in human serum react predominantly with Gal(alpha 1-3)Gal epitopes. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(23):11391–5. doi: 10.1073/pnas.90.23.11391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cooper DK, Ye Y, Kehoe M, Niekrasz M, Rolf LL, Jr, Martin M, Baker J, Kosanke S, Zuhdi N, Worsley G, et al. A novel approach to “neutralization” of preformed antibodies: cardiac allotransplantation across the ABO blood group barrier as a paradigm of discordant transplantation. Transplantation proceedings. 1992;24(2):566–71. [PubMed] [Google Scholar]
- 50.Good AH, Cooper DK, Malcolm AJ, Ippolito RM, Koren E, Neethling FA, Ye Y, Zuhdi N, Lamontagne LR. Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in humans. Transplantation proceedings. 1992;24(2):559–62. [PubMed] [Google Scholar]
- 51.Kolber-Simonds D, Lai L, Watt SR, Denaro M, Arn S, Augenstein ML, Betthauser J, Carter DB, Greenstein JL, Hao Y, et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(19):7335–40. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, Samuel M, Bonk A, Rieke A, Day BN, et al. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002;295(5557):1089–92. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- 53.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, Ball S, Specht SM, Polejaeva IA, Monahan JA, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299(5605):411–4. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li S, Waer M, Billiau AD. Xenotransplantation: role of natural immunity. Transplant immunology. 2009;21(2):70–4. doi: 10.1016/j.trim.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 55.Komoda H, Miyagawa S, Kubo T, Kitano E, Kitamura H, Omori T, Ito T, Matsuda H, Shirakura R. A study of the xenoantigenicity of adult pig islets cells. Xenotransplantation. 2004;11(3):237–46. doi: 10.1111/j.1399-3089.2004.00121.x. [DOI] [PubMed] [Google Scholar]
- 56.Tjernberg J, Ekdahl KN, Lambris JD, Korsgren O, Nilsson B. Acute antibody-mediated complement activation mediates lysis of pancreatic islets cells and may cause tissue loss in clinical islet transplantation. Transplantation. 2008;85(8):1193–9. doi: 10.1097/TP.0b013e31816b22f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Capey S, van den Berg CW. Porcine complement regulators protect aortic smooth muscle cells poorly against human complement-induced lysis and proliferation: consequences for xenotransplantation. Xenotransplantation. 2005;12(3):217–26. doi: 10.1111/j.1399-3089.2005.00217.x. [DOI] [PubMed] [Google Scholar]
- 58.Miyagawa S, Yamamoto A, Matsunami K, Wang D, Takama Y, Ueno T, Okabe M, Nagashima H, Fukuzawa M. Complement regulation in the GalT KO era. Xenotransplantation. 2010;17(1):11–25. doi: 10.1111/j.1399-3089.2010.00569.x. [DOI] [PubMed] [Google Scholar]
- 59.Cowan PJ, Aminian A, Barlow H, Brown AA, Chen CG, Fisicaro N, Francis DM, Goodman DJ, Han W, Kurek M, et al. Renal xenografts from triple-transgenic pigs are not hyperacutely rejected but cause coagulopathy in non-immunosuppressed baboons. Transplantation. 2000;69(12):2504–15. doi: 10.1097/00007890-200006270-00008. [DOI] [PubMed] [Google Scholar]
- 60.Byrne GW, McCurry KR, Martin MJ, McClellan SM, Platt JL, Logan JS. Transgenic pigs expressing human CD59 and decay-accelerating factor produce an intrinsic barrier to complement-mediated damage. Transplantation. 1997;63(1):149–55. doi: 10.1097/00007890-199701150-00027. [DOI] [PubMed] [Google Scholar]
- 61.Johansson H, Lukinius A, Moberg L, Lundgren T, Berne C, Foss A, Felldin M, Kallen R, Salmela K, Tibell A, et al. Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes. 2005;54(6):1755–62. doi: 10.2337/diabetes.54.6.1755. [DOI] [PubMed] [Google Scholar]
- 62.Moberg L, Johansson H, Lukinius A, Berne C, Foss A, Kallen R, Ostraat O, Salmela K, Tibell A, Tufveson G, et al. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet. 2002;360(9350):2039–45. doi: 10.1016/s0140-6736(02)12020-4. [DOI] [PubMed] [Google Scholar]
- 63.Moberg L, Olsson A, Berne C, Felldin M, Foss A, Kallen R, Salmela K, Tibell A, Tufveson G, Nilsson B, et al. Nicotinamide inhibits tissue factor expression in isolated human pancreatic islets: implications for clinical islet transplantation. Transplantation. 2003;76(9):1285–8. doi: 10.1097/01.TP.0000098905.86445.0F. [DOI] [PubMed] [Google Scholar]
- 64.Johansson H, Goto M, Dufrane D, Siegbahn A, Elgue G, Gianello P, Korsgren O, Nilsson B. Low molecular weight dextran sulfate: a strong candidate drug to block IBMIR in clinical islet transplantation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6(2):305–12. doi: 10.1111/j.1600-6143.2005.01186.x. [DOI] [PubMed] [Google Scholar]
- 65.Koh A, Senior P, Salam A, Kin T, Imes S, Dinyari P, Malcolm A, Toso C, Nilsson B, Korsgren O, et al. Insulin-heparin infusions peritransplant substantially improve single-donor clinical islet transplant success. Transplantation. 2010;89(4):465–71. doi: 10.1097/TP.0b013e3181c478fd. [DOI] [PubMed] [Google Scholar]
- 66.Ozmen L, Ekdahl KN, Elgue G, Larsson R, Korsgren O, Nilsson B. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes. 2002;51(6):1779–84. doi: 10.2337/diabetes.51.6.1779. [DOI] [PubMed] [Google Scholar]
- 67.Contreras JL, Eckstein C, Smyth CA, Bilbao G, Vilatoba M, Ringland SE, Young C, Thompson JA, Fernandez JA, Griffin JH, et al. Activated protein C preserves functional islet mass after intraportal transplantation: a novel link between endothelial cell activation, thrombosis, inflammation, and islet cell death. Diabetes. 2004;53(11):2804–14. doi: 10.2337/diabetes.53.11.2804. [DOI] [PubMed] [Google Scholar]
- 68.Hwang JW, Jung HS, Lee DY. Inhibition of platelet adhesion onto intrahepatically transplanted islets using PEGylation for attenuating instant blood-mediated inflammatory reaction (IBMIR) Journal of controlled release: official journal of the Controlled Release Society. 2011;152(Suppl 1):e213–4. doi: 10.1016/j.jconrel.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 69.Im BH, Jeong JH, Haque MR, Lee DY, Ahn CH, Kim JE, Byun Y. The effects of 8-arm-PEG-catechol/heparin shielding system and immunosuppressive drug, FK506 on the survival of intraportally allotransplanted islets. Biomaterials. 2013;34(8):2098–106. doi: 10.1016/j.biomaterials.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 70.Holgersson J, Ehrnfelt C, Hauzenberger E, Serrander L. Leukocyte endothelial cell interactions in pig to human organ xenograft rejection. Veterinary immunology and immunopathology. 2002;87(3–4):407–15. doi: 10.1016/s0165-2427(02)00094-6. [DOI] [PubMed] [Google Scholar]
- 71.Cardozo LA, Rouw DB, Ambrose LR, Midulla M, Florey O, Haskard DO, Warrens AN. The neutrophil: the unnoticed threat in xenotransplantation? Transplantation. 2004;78(12):1721–8. doi: 10.1097/01.tp.0000147341.40485.b4. [DOI] [PubMed] [Google Scholar]
- 72.Gilli UO, Schneider MK, Loetscher P, Seebach JD. Human polymorphonuclear neutrophils are recruited by porcine chemokines acting on CXC chemokine receptor 2, and platelet-activating factor. Transplantation. 2005;79(10):1324–31. doi: 10.1097/01.tp.0000155429.44902.44. [DOI] [PubMed] [Google Scholar]
- 73.Ehrnfelt C, Serrander L, Holgersson J. Porcine endothelium activated by anti-alpha-GAL antibody binding mediates increased human neutrophil adhesion under flow. Transplantation. 2003;76(7):1112–9. doi: 10.1097/01.TP.0000079305.60271.96. [DOI] [PubMed] [Google Scholar]
- 74.Ehrnfelt C, He Z, Holgersson J. No role of alpha-Gal in human monocyte-endothelial cell interactions in vitro. Scandinavian journal of immunology. 2005;62(5):445–52. doi: 10.1111/j.1365-3083.2005.01689.x. [DOI] [PubMed] [Google Scholar]
- 75.Carlsson PO. Influence of microenvironment on engraftment of transplanted beta-cells. Upsala journal of medical sciences. 2011;116(1):1–7. doi: 10.3109/03009734.2010.548609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ehrnfelt C, Kumagai-Braesch M, Uzunel M, Holgersson J. Adult porcine islets produce MCP-1 and recruit human monocytes in vitro. Xenotransplantation. 2004;11(2):184–94. doi: 10.1046/j.1399-3089.2003.00104.x. [DOI] [PubMed] [Google Scholar]