Abstract
Background
Previous studies show significant within-person changes in binge eating and emotional eating across the menstrual cycle, with substantial increases in both phenotypes during post-ovulation. Increases in both estradiol and progesterone levels appear to account for these changes in phenotypic risk, possibly via increases in genetic effects. However, to date, no study has examined changes in genetic risk for binge phenotypes (or any other phenotype) across the menstrual cycle. The goal of the present study was to examine within-person changes in genetic risk for emotional eating scores across the menstrual cycle.
Methods
Participants were 230 female twin pairs (460 twins) from the Michigan State University Twin Registry (MSUTR) who completed daily measures of emotional eating for 45 consecutive days. Menstrual cycle phase was coded based on dates of menstrual bleeding and daily ovarian hormone levels.
Results
Findings revealed important shifts in genetic and environmental influences, where estimates of genetic influences were two times higher in post- as compared to pre-ovulation. Surprisingly, pre-ovulation was marked by a predominance of environmental influences, including shared environmental effects which have not been previously detected for binge eating phenotypes in adulthood.
Conclusions
Our study was the first to examine within-person shifts in genetic and environmental influences on a behavioral phenotype across the menstrual cycle. Results highlight a potentially critical role for these shifts in risk for emotional eating across the menstrual cycle and underscore the need for additional, large-scale studies to identify the genetic and environmental factors contributing to menstrual cycle effects.
Keywords: Emotional eating, genetic, environmental, menstrual cycle, ovarian hormones
Substantial within-person changes in risk for emotional eating (e.g., overeating in response to negative emotions) and binge eating have been observed across the menstrual cycle. Studies revealed peaks in both behaviors during post-ovulation (particularly the mid-luteal phase) (Lester et al., 2003; Edler et al., 2007; Klump et al., 2008; Klump et al., 2013b) that are driven by higher levels of both estradiol and progesterone (Edler et al., 2007; Klump et al., 2008; Klump et al., 2013b). These hormone/emotional/binge eating associations are independent of body mass index (BMI) (Edler et al., 2007; Klump et al., 2008; Klump et al., 2013a; Klump et al., 2013b), negative affect (Edler et al., 2007; Klump et al., 2008; Klump et al., 2013a; Klump et al., 2013b; Racine et al., 2013), dietary restraint (Klump et al., 2013a), weight concerns (Hildebrandt et al., 2014), and impulsive traits (Racine et al., 2009), and they have been observed in community samples (Edler et al., 2007; Klump et al., 2008; Klump et al., 2013b) and women with clinical binge eating (Edler et al., 2007; Klump et al., 2014).
However, to date, nothing is known about the mechanisms driving these associations and changes in risk. One mechanism that has been proposed (Klump et al., 2013b; Klump et al., 2014) is changes in genetic risk. Menstrual cycle changes in ovarian hormones have been proposed to lead to changes in gene expression that translate into differential phenotypic risk for emotional eating and binge eating across the cycle. The known function of ovarian hormones within the central nervous system (CNS) (i.e., to regulate gene transcription via nuclear receptors) has served as the foundation for this hypothesis, as: 1) ovarian hormones regulate neural systems that are disrupted in eating disorders (e.g., the serotonin system; Becker, 1999; Ostlund et al., 2003; Hildebrandt et al., 2010); and 2) changes in gene expression drive ovarian hormone-induced changes in food intake (i.e., decreased intake with higher estradiol levels; increased intake when both estradiol and progesterone are high) in a variety of species. Interestingly, these changes in gene expression have been shown to occur within 1–2 days in rodents (Graves et al., 2011) and likely account for changes in food intake across the rat estrous cycle (Asarian & Geary, 2013). Emerging data suggest that ovarian hormones can induce gene expression changes in human (non-brain) tissue in 1–10 days (Logan et al., 2012).
Nonetheless, no study has investigated whether changes in gene expression underlie ovarian hormone/emotional eating/binge eating associations across the menstrual cycle. The absence of such data is likely due to difficulties measuring gene expression in human brain tissue and the lack of identified risk genes for binge eating (Scherag et al., 2010; Wade et al., 2013). One indirect method for examining changes in gene expression and changes in emotional eating/binge eating is to investigate changes in heritability across the menstrual cycle. Because changes in heritability reflect changes in the influence of genetic factors, this approach allows for a straightforward way to rule in (or out) changes in genetic risk (and potentially gene expression) for binge phenotypes before embarking on more expensive and invasive procedures for directly indexing gene expression. In addition, changes in heritability index genetic risk at the latent, aggregate level, and thus, analyses are not contingent upon selection of a particular candidate gene(s) or system for analysis. Finally, menstrual cycle phases are coded based on estrogen and progesterone profiles (e.g., high estradiol and low progesterone during pre-ovulation; high levels of both hormones following ovulation); consequently, changes in genetic risk across the cycle likely reflect changing profiles of hormones and their effects on emotional eating.
Given the above, the aim of the current study was to examine within-person changes in the heritability of emotional eating in a community based sample of female twins using daily measures of emotional eating and ovarian hormones across the menstrual cycle. We focused on emotional eating rather than binge eating due to the small number of twins (N = 33) with clinical binge eating. However, past data suggest that the phenotypic effects of ovarian hormones are similar across community and clinical samples, and that the pattern of hormone effects are identical for emotional eating scores and clinically-diagnosed binge eating (Edler et al., 2007; Klump et al., 2008; Klump et al., 2013b; Klump et al., 2014). Thus, although we cannot say for certain that changes in heritability for emotional eating reflect changes for binge eating, our study provides an important first step in identifying shifts in genetic risk for binge-related phenotypes that can be examined more fully in clinical populations.
Methods
Participants
Participants were 230 (138 monozygotic (MZ), 92 dizygotic (DZ)) same-sex female twin pairs ages 16–25 years (M = 17.74, SD = 1.83) who participated in the Twin Study of Hormones and Behavior across the Menstrual Cycle (HBMC) project (Klump et al., 2013b) within the Michigan State University Twin Registry (MSUTR; see Klump & Burt, 2006 and; Burt & Klump, 2013 for MSUTR details). The primary aim of the HMBC is to examine phenotypic and genetic associations between changes in ovarian hormones and changes in binge eating across the menstrual cycle. Study inclusion/exclusion criteria were: 1) menstruation every 22–32 days for past 6 months; 2) no hormonal contraceptive use within the past 3 months; 3) no psychotropic or steroid medications within the past 4 weeks; 4) no pregnancy/lactation within the past 6 months; and 5) no genetic or medical conditions known to influence hormones or appetite/weight. Despite these criteria, the HBMC twins are representative of the recruitment region (79.5% White, 14.3% Black, 0.6% Asian, 0.3% Native American, 5.3% more than one race; 7.8% Hispanic), and they do not differ from other MSUTR twins on measures of emotional eating, binge eating, or other symptoms (e.g., weight preoccupation) (average d = .12).
The current study included a subsample of the HBMC twins with daily emotional eating data for one menstrual cycle (N = 460 twins, 230 pairs; 78% of the HBMC sample (N = 585)). We excluded 84 pairs in which one or both co-twins had missing emotional eating scores or menstrual cycle phase data (15/84 pairs; 18%), and pairs in which at least one co-twin was anovulatory (69/84; 82% of those excluded). Although some of our analyses (e.g., twin models) could account for missing data in one co-twin, other analyses could not (e.g., twin correlations). To ensure that all results included the same pairs, we focused on pairs that had full data for both co-twins. Notably, results were identical when we included incomplete pairs in the models (data not shown), and our final sample was highly representative of the larger HBMC group in terms of racial/ethnic diversity (83.9% White, 10.9% Black, 0.4% Asian, 0.4% Native American, 4.3% more than one race; 7.4% Hispanic). Twins from complete pairs also did not significantly differ from those from incomplete pairs in emotional eating or other disordered eating characteristics (e.g., weight preoccupation; average d = .12).
Procedures
Participants collected data for 45 consecutive days. Ratings of emotional eating were made each evening after 5:00 pm. Participants completed three in-person visits: one at the start of data collection, one mid-way through (~day 23), and one at the end (~day 45). Each visit included a re-assessment of eligibility and collection of samples. Staff also contacted participants 1x/week to answer questions and confirm adherence. These procedures were effective for minimizing drop-outs (7%) and identifying twins who were no longer eligible (3%).
Measures
Emotional Eating
We used the emotional eating scale of the Dutch Eating Behavior Questionnaire (DEBQ) (Van Strien et al., 1986) to assess eating in response to negative emotions (e.g., “Did you have a desire to eat when you were depressed?”) on a 5-point scale (i.e., “not at all” to “very often”). Eating in response to negative emotions is a core feature of binge eating (McManus & Waller, 1995), and the emotional eating scale has demonstrated validity in differentiating between individuals who binge eating versus those who are overweight versus college students (Wardle, 1987; Deaver et al., 2003). Emotional eating scores correlate with established measures of binge eating (r’s = .55–.69) (Van Strien, 2000; Racine et al., 2009) as well as with palatable food intake (i.e., ice cream) (Van Strien, 2000) in adults as well as in adolescents (Lluch et al., 2000; Nguyen-Michel et al., 2007; Snoek et al., 2007; van Strien et al., 2010; Laghi et al., 2015). The instructions for the scale were modified with permission to ask about emotional eating over the current day (45-day average α = .90) (Klump et al., 2008).
Menstrual Cycle Phase
Participants recorded days of menstrual bleeding in a daily log book (see Lester et al., 2003). Participants provided daily saliva samples within 30 minutes of waking, using published methods (Klump et al., 2008; Klump et al., 2013b). Saliva samples were assayed for estrogen and progesterone by Salimetrics, LLC (State College, PA) using enzyme immunoassays that show excellent intra- and inter-assay coefficients of variation (see Klump et al., 2013b).
Menstrual cycle phase was coded by trained raters (see training procedures below) after aggregating each twin’s cycle and hormone data into a single graph. The first day of bleeding served as the graph anchor, and phase days were coded based on this anchor, hormone levels, and the overall length of each cycle. Raters began by examining hormone levels ~15 days prior to the anchor to find each twin’s peak in estradiol and ovulatory phase. The day with the highest estradiol peak, as well as the days prior to and after, were coded as the ovulatory phase (~3 days). The mid-luteal phase (~7 days) was coded after ovulation based on a secondary peak in estradiol and rising (and then falling) progesterone levels. The premenstrual phase (~4 days) was coded based on falling levels of both progesterone and estradiol. The follicular phase (~10 days) was coded based on low progesterone levels and low (and then rising) levels of estradiol that occurred after menstruation but before ovulation.
All raters underwent extensive training, including review of coding rules and practice coding sessions. Each rater had to achieve an inter-rater reliability of ≥.80 with senior raters. All graphs were coded by two raters, and the codes were compared for consistency. Discrepancies were resolved via weekly meetings.
Statistical Analyses
Emotional eating scores were averaged and log transformed (to account for positive skew) within each phase prior to analyses. We calculated twin intraclass correlations within phase to provide an initial indication of additive genetic (A; genetic influences that add across genes), shared environmental (C; environmental influences that are shared by twins and are a source of similarity), and nonshared environmental (E; environmental influences that are not shared by twins and are a source of dissimilarity, including measurement error) influences on emotional eating scores. We then fit biometric models to the raw data using the maximum likelihood option in Mx (Neale et al., 2003). Comparisons of fit between different models were made by taking the difference in minus twice the log-likelihood (−2lnL) (for nested models) and by comparing Akaike’s information criterion (Akaike, 1987) and Bayesian information criterion (BIC) (Schwarz, 1978). Large (statistically significant) differences in −2lnL values led to a rejection of the nested model in favor of the full model, and models with lower AIC and BIC values were preferred. Following previous recommendations (Purcell, 2002), we present standardized parameter estimates in tables and unstandardized estimates in figures.
Results
Twin correlations by phase are presented in Table 1. In all cases, the individual MZ and DZ twin correlations (see “MZ” and “DZ” columns) were statistically significant, suggesting that there is familial resemblance for emotional eating scores across all phases of the menstrual cycle.
Table 1.
Twin Correlations for Emotional Eating Scores by Menstrual Cycle Phase.
| Phases | MZ (n = 138 pairs) | DZ (n = 92 pairs) | Z test of independence | p |
|---|---|---|---|---|
| Follicular | .33*** | .21*** | 1.18 | .17 |
| Ovulatory | .30*** | .24*** | 0.47 | .31 |
| Mid-Luteal | .45*** | .26*** | 1.52 | .05 |
| Premenstrual | .40*** | .16*** | 1.92 | .03 |
|
| ||||
| Pre-Ovulation | .36*** | .25** | 0.89 | .19 |
| Post-Ovulation | .47*** | .26** | 1.79 | .04 |
Note. MZ = monozygotic; DZ = dizygotic; Pre-Ovulation = the follicular and ovulatory phases combined; Post-Ovulation = the mid-luteal and premenstrual phases combined; “Z test of independence” = tests for significant differences between the MZ and DZ twin correlations. Emotional Eating scores were log transformed prior to analysis. All p values are one-sided, as we would expect MZ twin correlations to be greater than DZ twin correlations.
p < .01,
p < .001. The twin correlation is significantly different from zero.
Nonetheless, the magnitude of the difference between the MZ and DZ twin correlations varied across phase (see the Z test of independence and p values in Table 1). During the follicular and ovulatory phases, the MZ and DZ twin correlations were not significantly different from each other (i.e., p’s = .17 and .31), suggesting that shared environmental influences play an important role in emotional eating during the follicular and ovulatory phases of the menstrual cycle. However, additive genetic and nonshared environmental influences were likely present as well, given that the MZ twin correlation was larger than the DZ correlation, and the MZ twin correlation was less than 1.00.
By contrast, in the mid-luteal and premenstrual phases, the MZ twin correlation was almost double the DZ twin correlation, and the correlation differences were statistically significant (p = .03) or of trend-level significance (p = .05). This pattern suggested genetic and nonshared environmental influences, with little evidence of shared environmental effects. Analyses examining pre-ovulation (follicular and ovulatory) and post-ovulation (mid-luteal and premenstrual) phase variables produced very similar results with significant differences in MZ/DZ twin correlations in post-ovulation only.
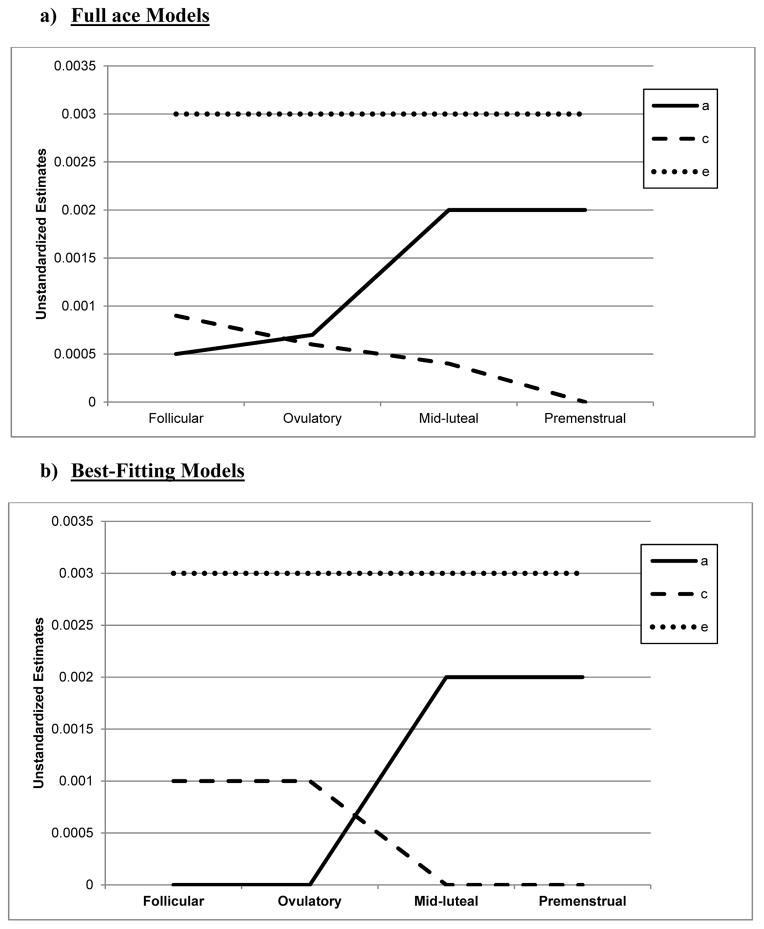
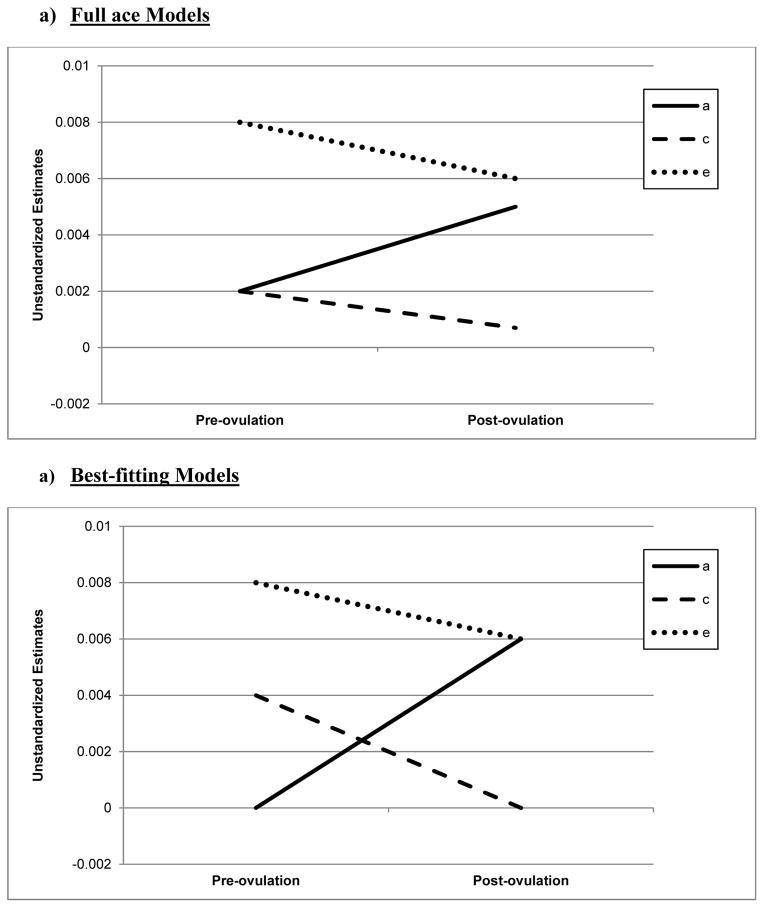
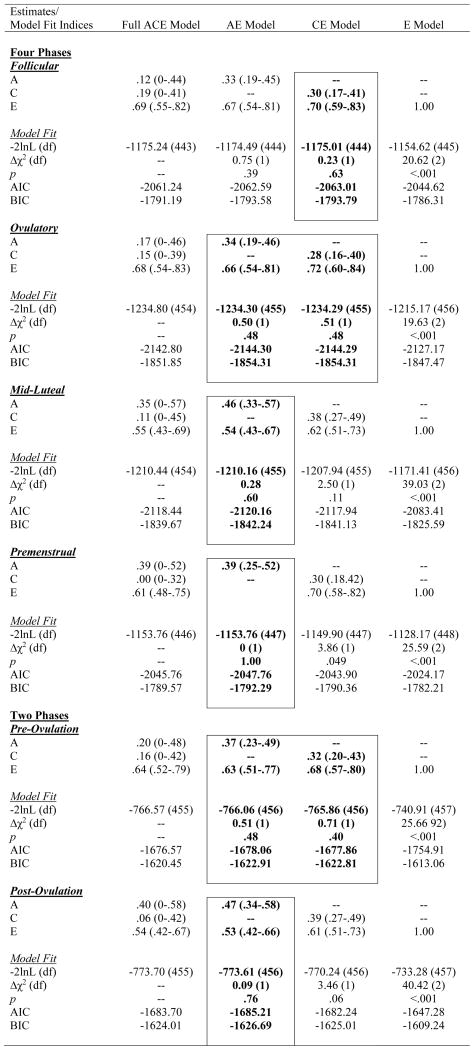
Biometric models confirmed these preliminary results. We initially fit the full model containing all three etiologic effects (i.e., A, C and E) and then compared this model to reduced models that contained A and C only, C and E only, or E. Standardized estimates from these models are presented in the A, C, and E rows in Table 2, while the unstandardized estimates (i.e., a, c, e) are depicted in Figures 1 and 2.1 For the full ACE models, there were strong nonshared environmental influences (61–69%) and more modest levels of additive genetic (12–20%) and shared environmental (15–19%) influences in the pre-ovulatory phases (i.e., follicular phase, ovulatory phase, and combined pre-ovulation phase). By contrast, in the post-ovulatory phases (i.e., mid-luteal phase, premenstrual phase, and combined post-ovulation phase), genetic influences were much more substantial (35–40%) and were much larger than the shared environmental contributions (0–11%). Perhaps not surprisingly, model fit comparisons showed that the best fitting models during post-ovulation were the AE models that contained only additive genetic and nonshared environmental influences. These models had non-significant changes in −2lnL values and low AIC and BIC values. Overall, these models showed substantial additive genetic (39–47%) and nonshared environmental influences (53–61%) on emotional eating in the post-ovulatory phases.
Table 2.
Parameter Estimates and Model Fit Indices from the Univariate Models.
|
Note. A = additive genetic effects; C = shared environmental effects; E = nonshared environmental effects; Pre-Ovulation = the follicular and ovulatory phases combined; Post-Ovulation = the mid-luteal and premenstrual phases combined; −2lnL (df) = minus twice the log-likelihood,; Δχ2 (df) = the difference in −2lnL values between the full model and the nested model, which is chi-square distributed under the null hypothesis implied by the reduced model; AIC = Akaike’s information criterion; BIC = Bayesian information criterion. Emotional Eating scores were log transformed prior to analysis. The best-fitting model(s) is noted in bolded and outlined text.
Figure 1. Unstandardized Parameter Estimates from the a) Full ace Models and b) Best-Fitting Models for the Four Phases Separately.
a = additive genetic effects; c = shared environmental effects; e = nonshared environmental effects; Full Models = models with a, c, and e. The best-fitting model for the mid-luteal and premenstrual phase was the AED model, while the CE model was best fitting for the follicular phase. Model-fitting results were equivocal for the ovulatory phase, with the ae and ce models showing equivalent fits. Because the ce model showed a slightly better fit for the other pre-ovulatory phases (i.e., the follicular phase and the combined pre-ovulation variable), we show the ce model for the ovulatory phase as well.
Figure 2. Unstandardized Parameter Estimates from the a) Full ace Models and b) Best-Fitting Models for the Pre-Ovulation (i.e., follicular and ovulatory) versus Post-Ovulation (i.e., mid-luteal and premenstrual) Phases.
a = additive genetic effects; c = shared environmental effects; e = nonshared environmental effects; Full Models = models with a, c, and e. The best-fitting model for the pre-ovulation was the ce model, while the ae model was best fitting for post-ovulation.
Model comparisons for the pre-ovulatory phases were more complicated. In most cases, the AE and CE models provided equal fits to the data, with non-significant changes in −2lnL values and AIC/BIC values that were nearly identical (see Table 2). This situation often occurs when you have similar degrees of additive genetic and shared environmental influences (like we have here – see A, C, and E estimates for the full ACE model in Table 2, and a, c, and e unstandardized estimates in Figures 1a and 2a). In these cases, very large sample sizes (in the thousands - see Martin et al., 1978) are needed to detect significant A and C parameters and/or to differentiate between the two. Although our sample of 230 pairs is quite large for a study of 45 days, it is insufficient for detecting significant A and C effects, and we therefore obtained equivocal evidence for the best-fitting model. In this case, the most conservative interpretation is that there is familial aggregation of emotional eating scores, but the models are unable to determine if the aggregation is due to A, C, or both. However, differences in twin correlations (see Table 1) and results from the full ACE models make it clear that both genetic and shared environmental factors likely contribute to familial aggregation in pre-ovulation and that the degree of genetic influence is less than in post-ovulation.
Discussion
This study was the first to examine within-person shifts in etiologic risk for disordered eating across the menstrual cycle. Results highlighted changes in both the magnitude and type of genetic and environmental influences on emotional eating scores. Twin correlations and univariate models provided consistent evidence for stronger genetic influences during post-ovulation and greater environmental (particularly shared environmental) influences during pre-ovulation. The identification of these shifts is rather remarkable given that they occur over span of just days and correspond with distinct and robust changes in the reproductive axis that occur on a monthly basis. Taken together, results highlight a critical role for changes in genetic and environmental effects on recurring phenotypic risk for emotional eating across the menstrual cycle.
Although our longitudinal results are unique, they were nonetheless constrained by (unexpected) power limitations. When this study was designed, power analyses assumed that there would be no shared environmental influences on emotional eating scores in our sample of late adolescent/early adult twins. This assumption was based on extensive twin data suggesting no shared environmental influences on binge eating, binge-related disorders (e.g., BN, BED) or any other form of eating pathology or symptom after puberty (Bulik et al., 1998; Sullivan et al., 1998; Bulik et al., 2000; Klump et al., 2000, 2003; Klump et al., 2007; Klump et al., 2010a). The finding of shared environmental influences was thus entirely unexpected. However, it did lead to several intriguing hypotheses about etiologic influences on binge eating in adulthood and across the menstrual cycle. Broadly, these data suggest that the complete lack of shared environmental effects observed in past studies of post-pubertal twins may be due to the failure to control for menstrual cycle phase at the time of assessment. Twins would have been assessed randomly across the different menstrual cycle days, and would therefore produce genetic and environmental estimates that are an average of the effects across the cycle. Given that we observed genetic effects in both pre- and post-ovulation (they were just lower in pre-ovulation), and shared environmental effects were only observed in pre-ovulation, this averaging would produce significant estimates of genetic effects but non-significant and low estimates of the shared environment. Although on average, such results broadly reflect the type of etiologic influences on emotional eating, they do not reflect the factors that may drive emotional eating on a daily basis and at different points of the menstrual cycle. It is often these proximal, daily triggers that are critical treatment targets (Fairburn, 2008) and should be targets of etiological research aimed at understanding specific risk factors for emotional eating and binge eating. Clearly, additional research is needed to replicate our findings, and a good start to this process would be to control for menstrual cycle phase in twin studies of binge-related phenotypes.
Our data also provide new leads in thinking about phenotypic risk for emotional eating. Rates of emotional eating and binge eating are lowest in the pre-ovulatory phase (Edler et al., 2007; Klump et al., 2008; Klump et al., 2013b; Klump et al., 2014) when, according to our findings, environmental influences exert a stronger influence on emotional eating risk than genetic effects. This suggests either that environmental factors decrease/protect against emotional eating during the pre-ovulatory phase, and/or that phenotypic risk is lower because genetic factors are less prominent. We think that both of these processes are likely at play, and that changes in ovarian hormones contribute to these protective and risky effects. The pre-ovulatory phase is marked by high levels of estradiol and near absent levels of progesterone. As shown in our previous work (Klump et al., 2008; Klump et al., 2013b) and decades of animal research (Asarian & Geary, 2006), high levels of estradiol are associated with decreased food intake, emotional eating, and binge eating, particularly when progesterone levels are low. Although we were unable to directly model ovarian hormones in the current study (see more on this below), collectively, our findings suggest that estrogen’s protective effects during pre-ovulation may be environmentally, rather than genetically, mediated.
Precisely how or why these effects would be environmentally mediated remains unknown, but one possibility is that estrogen acts through membrane receptors that affect behavior through non-genomic pathways. Membrane receptor activation produces rapid molecular signals that change the excitability of neurons within seconds to minutes via processes that do not require changes in gene expression (Santollo et al., 2012). It is possible that high levels of estradiol during pre-ovulation activate these membrane receptors and decrease food intake and emotional eating via non-genomic pathways in all women, regardless of genetic risk. Mechanisms underlying hormone membrane receptor effects on behavior are still poorly understood, however new data in animals suggest that their effects can be produced via non-genomic and genomic pathways (Roepke, 2009; Roepke et al., 2009). For example, activation of membrane estrogen receptors decrease food intake in ovariectomized rats, but the longer time course of the behavioral changes (i.e., 11 hours after administration of estrogen agonists) suggests that rapid membrane effects on intra-cellular signaling pathways may have ultimately led to changes in gene expression (Graves et al., 2011; Santollo et al., 2012). To date, no other studies have differentiated genomic versus non-genomic effects of estrogen on food intake. Clearly, more research is needed to understand the effects of nuclear and membrane hormone receptor effects on phenotypic and genetic risk for food intake and binge-related phenotypes across species.
However, if estrogen is protective via environmental pathways, than the increased phenotypic and genetic risk for binge eating during the mid-luteal phase (when both estradiol and progesterone are high) would seemingly contradict these results. Interestingly, there was some indication of shared environmental influences during the mid-luteal phase (see full model results in Table 2), suggesting that higher estradiol may have increased shared environmental risk during this phase. Nonetheless, genetic effects were still more prominent, and our phenotypic data (Edler et al., 2007; Klump et al., 2008; Klump et al., 2013b; Klump et al., 2014) and decades of animal research (Asarian & Geary, 2006) suggest that this might be due to the high levels of progesterone that are present during the mid-luteal phase. Extant data show that progesterone antagonizes the effects of estrogen and leads to increased food intake (Asarian & Geary, 2006), emotional eating (Klump et al., 2008; Klump et al., 2013b), and binge eating (Klump et al., 2013b; Klump et al., 2014) in humans and animals. Our findings add to this literature by suggesting that these antagonizing effects may be genetically mediated, although again, the how and why of these potential processes need to be elucidated. Progesterone has genomic effects within the CNS via its regulation of gene transcription (Wilson et al., 1998), and these genomic effects have been shown to drive changes in food intake in animals (Asarian & Geary, 2013). Unfortunately, progesterone is studied less frequently than estrogen, and so very little is known about the particular neurobiological systems involved or how the genomic effects of progesterone may antagonize/interact with estrogen. Regardless of the precise mechanisms, our data suggest that the presence of progesterone during the second half of the cycle increases phenotypic and genetic risk for binge eating via processes that are in need of additional exploration.
One final note is warranted on hormone effects. We previously found estrogen to be associated with increased genetic risk for disordered eating during puberty, with little-to-no contribution from progesterone (Klump et al., 2010b). The key difference between our current findings and this past study is developmental stage and the likely organizational versus activational effects of hormones. We (and others- see Sisk & Zehr, 2005) have argued that estrogen’s effects during puberty are primarily organizational in nature (Klump, 2013), whereby estrogen causes permanent changes in brain structure/function that then set the stage for the brain to respond to the activational effects of hormones in adulthood. By contrast, the hormonal effects that are present in adulthood and are studied herein would be considered activational in nature, i.e., transient effects that are due to circulating and changing levels of both estrogen and progesterone in adulthood. It is not uncommon for the mechanisms (i.e., genetic, non-genetic) underlying organizational versus activational effects of hormones to be different (Romeo, 2003; Sisk & Zehr, 2005), or for the two hormones to operate differently across developmental stage (Schwarz et al., 2010; Klump, 2013). In the case of emotional eating, our data suggest that estrogen activation during puberty may lead to genetically mediated (Klump et al., 2010b), organizational changes in risk that are then differentially activated in adulthood by changes in both estrogen and progesterone across the menstrual cycle – changes that are environmentally and genetically mediated. Clearly, additional data are needed to confirm these hypotheses, but they provide one framework for conceptualizing the differential effects of ovarian hormones on eating disorder risk across development.
Although this study had many strengths (e.g., the within-person, longitudinal design), the study also had limitations. Our sample was underpowered to detect both genetic and shared environmental effects. The decreased power somewhat limited the strength of conclusions that could be drawn and also prohibited us from fitting more sophisticated twin models (e.g., constraint and Cholesky decomposition models). We attempted to fit these models in post hoc analyses, but in all cases, results produced non-significant parameter estimates (particularly for shared environment and genetic/environmental correlations) and no significant phase changes in etiologic effects. These results do not align with the twin correlations or results from the biometric models described above and are likely due to difficulties with detecting all three etiologic effects in pre-ovulation. Clearly, much larger samples are needed to replicate our results and confirm the presence of significant differences in genetic and environmental effects across menstrual cycle phase.
We focused on changes across cycle phase rather than ovarian hormone levels. Because phases were defined by hormone levels, our results implicate ovarian hormones in patterns of effects. Nonetheless, confirmation awaits the development of twin models that can examine daily couplings of hormones with changes in emotional eating. These types of multivariate, repeated measures models do not exist for twin data, although our investigative team is in the process of developing analytic tools (see Boker et al., 2014). We should note that we attempted to address this issue by individually coding high/low hormone phases (using median splits, tertile splits, etc.) and examining changes across hormonal “phases” rather than menstrual cycle phases. Perhaps not surprisingly, these high/low hormone phases completely overlapped with the cycle phases already examined. This would be expected given that menstrual cycle phases were coded based on high/low hormone values across the menstrual cycle (see Methods). Consequently, these analyses were neither informative nor illustrative for confirming that menstrual cycle changes were due to ovarian hormone effects. However, the complete replication of menstrual cycle phase from hormone phases further supports our belief that the changes in genetic effects are due to changing levels of ovarian hormones across the cycle.
Finally, our sample was community-based, and we examined a continuous measure of emotional eating instead of clinical binge eating. Although past data strongly suggest that phenotypic effects of ovarian hormones are similar across community and clinical samples, and results are identical for emotional eating and clinically-diagnosed binge eating (Edler et al., 2007; Klump et al., 2008; Klump et al., 2013b; Klump et al., 2014), confirmation of changes in etiologic effects in women with clinical pathology awaits additional research.
Acknowledgments
Financial Support
This work was supported by the National Institute of Mental Health (KLK, PKK, MM, CLS, SB, SAB, R01 MH082054). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH.
Footnotes
To ensure that results were not unduly influenced by changes in body weight, we also included BMI (collected during the three in-person study visits) as a covariate in all models. There were minimal changes in weight across the study (M = −0.20 lb change, SD = 3.39), and the pattern of significant parameter estimates and best-fitting models were identical to those presented herein.
Conflict of Interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2006;361:1251–63. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarian L, Geary N. Sex differences in the physiology of eating. American Journal of Physiology: Regulatory, Integrative, and Comparative Physiology. 2013;305 doi: 10.1152/ajpregu.00446.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacology, Biochemistry and Behavior. 1999;64:803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Boker SM, Neale MC, Klump KL. A differential equations model for the ovarian hormone cycle. In: Molenaar PC, Lerner R, Newell K, editors. Handbook of Relational Developmental Systems: Emerging Methods and Concepts. John Wiley & Sons; New York: 2014. [Google Scholar]
- Bulik CM, Sullivan PF, Kendler KS. Heritability of binge-eating and broadly defined bulimia nervosa. Biological Psychiatry. 1998;44:1210–1218. doi: 10.1016/s0006-3223(98)00280-7. [DOI] [PubMed] [Google Scholar]
- Bulik CM, Sullivan PF, Wade TD, Kendler KS. Twin studies of eating disorders: A review. International Journal of Eating Disorders. 2000;17:251–261. doi: 10.1002/(sici)1098-108x(200001)27:1<1::aid-eat1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Burt SA, Klump KL. The Michigan State University Twin Registry (MSUTR): An Update. Twin Research and Human Genetics. 2013;16:344–350. doi: 10.1017/thg.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaver CM, Miltenberger RG, Symth J, Meidinger A, Crosby R. An evaluation of affect and binge eating. Behavior Modification. 2003;27 doi: 10.1177/0145445503255571. [DOI] [PubMed] [Google Scholar]
- Edler C, Lipson SF, Keel PK. Ovarian hormones and binge eating in bulimia nervosa. Psychological Medicine. 2007;37:131–141. doi: 10.1017/S0033291706008956. [DOI] [PubMed] [Google Scholar]
- Fairburn CG. Cognitive behavior therapy and eating disorders. Guilford Press; 2008. [Google Scholar]
- Graves NS, Hayes H, Fan L, Curtis KS. Time course of behavioral, physiological, and morphological changes after estradiol treatment of ovariectomized rats. Physiology & Behavior. 2011;103:261–267. doi: 10.1016/j.physbeh.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt BA, Racine SE, Keel PK, Burt SA, Neale M, Boker S, Sisk CL, Klump KL. The effects of ovarian hormones and emotional eating on changes in weight preoccupation across the menstrual cycle. International Journal of Eating Disorders. 2014 doi: 10.1002/eat.22326. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt T, Alfano L, Tricamo M, Pfaff DW. Conceptualizing the role of estrogens and serotonin in the development and maintenance of bulimia nervosa. Clinical Psychology Review. 2010;30:655–668. doi: 10.1016/j.cpr.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, McGue M. Minnesota twin family study. Twin Research. 2002;5:482–487. doi: 10.1375/136905202320906327. [DOI] [PubMed] [Google Scholar]
- Klump KL. Puberty as a critical risk period for eating disorders: A review of human and animal studies. Hormones and Behavior. 2013;64:399–410. doi: 10.1016/j.yhbeh.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Burt SA. The Michigan State University Twin Registry (MSUTR): Genetic, environmental and neurobiological influences on behavior across development. Twin Research and Human Genetics. 2006;9:971–977. doi: 10.1375/183242706779462868. [DOI] [PubMed] [Google Scholar]
- Klump KL, Burt SA, McGue M, Iacono WG. Changes in genetic and environmental influences on disordered eating across adolescence: A longitudinal twin study. Archives of General Psychiatry. 2007;64:1409–1415. doi: 10.1001/archpsyc.64.12.1409. [DOI] [PubMed] [Google Scholar]
- Klump KL, Burt SA, McGue M, Iacono WG, Wade TM. Age differences in genetic and environmental influences on weight and shape concerns. International Journal of Eating Disorders. 2010a;43:679–688. doi: 10.1002/eat.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Burt SA, Racine SE, Neale MC, Sisk CL, Boker S. Ovarian hormones and emotional eating across the menstrual cycle: An examination of the potential moderating effects of body mass index and dietary restraint. International Journal of Eating Disorders. 2013a;46:256–263. doi: 10.1002/eat.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Culbert KM, Edler C. Ovarian hormones and binge eating: Exploring associations in community samples. Psychological Medicine. 2008;38:1749–1757. doi: 10.1017/S0033291708002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Racine SE, Burt SA, Neale M, Sisk CL, Boker S, Hu JY. The interactive effects of estrogen and progesterone on changes in emotional eating across the menstrual cycle. Journal of Abnormal Psychology. 2013b;122:131–137. doi: 10.1037/a0029524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Sisk CL, Burt SA. Preliminary evidence that estradiol moderates genetic influences on disordered eating attitudes and behaviors during puberty. Psychological Medicine. 2010b;40:1745–1753. doi: 10.1017/S0033291709992236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, McGue M, Iacono WG. Age differences in genetic and environmental influences on eating attitudes and behaviors in female adolescent twins. Journal of Abnormal Psychology. 2000;109:239–251. [PubMed] [Google Scholar]
- Klump KL, McGue M, Iacono WG. Differential heritability of eating attitudes and behaviors in prepubertal versus pubertal twins. International Journal of Eating Disorders. 2003;33:287–292. doi: 10.1002/eat.10151. [DOI] [PubMed] [Google Scholar]
- Klump KL, Racine SE, Hildebrandt B, Burt SA, Neale M, Sisk CL, Boker S, Keel PK. Ovarian hormone influences on dysregulated eating: A comparison of associations in women with versus without binge episodes. Clinical Psychological Science. 2014;2:545–559. doi: 10.1177/2167702614521794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laghi F, Pompili S, Baumgartner E, Baiocco R. The role of sensation seeking and motivations for eating in female and male adolescents who binge eat. Eating Behaviors. 2015;17:119–124. doi: 10.1016/j.eatbeh.2015.01.011. [DOI] [PubMed] [Google Scholar]
- Lester NA, Keel PK, Lipson SF. Symptom fluctuation in bulimia nervosa: Relation to menstrual-cycle phase and cortisol levels. Psychological Medicine. 2003;33:51–60. doi: 10.1017/s0033291702006815. [DOI] [PubMed] [Google Scholar]
- Lluch A, Herbert B, Mejean L, Siest G. Dietary intakes, eating style and overweight in Stanilas Family Study. International journal of Obesity. 2000;24:1493–1499. doi: 10.1038/sj.ijo.0801425. [DOI] [PubMed] [Google Scholar]
- Logan PC, Ponnampalam AP, Steiner M, Mitchell MD. Effect of cyclic AMP and estrogen/progesterone on the transcription of DNA methyltraferases during the decidualization of human endometrial stromal cells. Molecular Human Reproduction. 2012;19:302–312. doi: 10.1093/molehr/gas062. [DOI] [PubMed] [Google Scholar]
- Martin MG, Eaves LJ, Kearsey MJ, Davies P. The power of the classical twin study. Heredity. 1978;40:97–116. doi: 10.1038/hdy.1978.10. [DOI] [PubMed] [Google Scholar]
- McManus F, Waller GI. A functional analysis of binge-eating. Clinical Psychology Review. 1995;15:845–863. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. Department of Psychiatry; Richmond, VA 23298: 2003. [Google Scholar]
- Nguyen-Michel ST, Unger JB, Spruijt-Metz D. Dietary correlates of emotional eating in adolescence. Appetite. 2007;49:494–499. doi: 10.1016/j.appet.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund H, Keller E, Hurd YL. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Annals of the New York Academy of Sciences. 2003;1007:54–63. doi: 10.1196/annals.1286.006. [DOI] [PubMed] [Google Scholar]
- Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Research. 2002;5:554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Racine SE, Culbert KM, Larson CL, Klump KL. The possible influences of impulsivity and dietary restraint on associations between serotonin genes and binge eating. Journal of Psychiatric Research. 2009;43:1278–1286. doi: 10.1016/j.jpsychires.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine SE, Keel PK, Burt SA, Sisk CL, Neale M, Boker S, Klump KL. Exploring the relationship between negative urgency and dysregulated eating: Etiologic associations and the role of negative affect. Journal of Abnormal Psychology. 2013;122:433–444. doi: 10.1037/a0031250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA. Oestrogen modulates hypothalamic control of energy homeostasis through multiple mechanisms. Journal of Neuroendocrinology. 2009;21:141–150. doi: 10.1111/j.1365-2826.2008.01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA, Qiu J, Bosch MA, Ronnekleiv OK, Kelly MJ. Cross-talk between membrane-initiated and nuclear-initiated oestrogen signalling in the hypothalamus. Journal of Neuroendocrinology. 2009;21:263–270. doi: 10.1111/j.1365-2826.2009.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD. Puberty: a period of both organizational and activational effects of steroid hormones on neurobehavioural development. Journal of Neuroendocrinology. 2003;15:1185–1192. doi: 10.1111/j.1365-2826.2003.01106.x. [DOI] [PubMed] [Google Scholar]
- Santollo J, Marshall A, Daniels D. Activation of membrane associated estrogen receptors decreases food and water intake in ovariectomized rats. Endocrinology. 2012;154:320–329. doi: 10.1210/en.2012-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherag S, Hebebrand J, Hinney A. Eating disorders: The current status of molecular genetic research. European Child and Adolescent Psychiatry. 2010;19:211–226. doi: 10.1007/s00787-009-0085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz GE. Estimating the dimension of a model. Annals of Statistics. 1978;6:461–464. [Google Scholar]
- Schwarz JM, Nugent BM, McCarthy MM. Developmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life span. Endocrinology. 2010;151:4871–4881. doi: 10.1210/en.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Puberty hormones organize the adolescent brain and behavior. Frontiers in Neuroendocrinology. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Snoek HM, van Strien T, Janssens JMAM, Englels RCME. Emotional, external, restrained eating and overweight in Dutch adolescents. Scandinavian Journal of Psychology. 2007;48:23–32. doi: 10.1111/j.1467-9450.2006.00568.x. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Bulik CR, Kendler K. Genetic epidemiology of binging and vomiting. British Journal of Psychiatry. 1998;173:75–79. doi: 10.1192/bjp.173.1.75. [DOI] [PubMed] [Google Scholar]
- Van Strien T. Ice-cream consumption, tendency toward overeating, and personality. Appetite. 2000;52:380–387. doi: 10.1002/1098-108x(200012)28:4<460::aid-eat16>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Van Strien T, Frijters JE, Bergers GP, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. International Journal of Eating Disorders. 1986;5:295–315. [Google Scholar]
- van Strien T, Snoek HM, van der Zwaluw CS, Engles RCME. Parental control and the dopamine D2 receptor gene (DRD2) interaction on emotional eating in adolescence. Appetite. 2010;54:255–261. doi: 10.1016/j.appet.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Wade TD, Gordon S, Medland S, Bulik CM, Heath AC, Montgomery GW, Martin NG. Genetic variants associated with disordered eating. International Journal of Eating Disorders. 2013;46:594–608. doi: 10.1002/eat.22133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle J. Eating style: A validation study of the Dutch Eating Behaviour Questionnaire in normal subjects and women with eating disorders. Journal of Psychosomatic Research. 1987;31:161–169. doi: 10.1016/0022-3999(87)90072-9. [DOI] [PubMed] [Google Scholar]
- Wilson JD, Foster DW, Kronenberg HM, Larsen PR. Williams Textbook of Endocrinology. 9. W.B. Saunders Company; Philadelphia, PA: 1998. [Google Scholar]