Abstract
Objective
To investigate the role of polymorphonuclear leukocytes (PMNs) in a mouse model of E. coli-induced labor.
Study Design
Intraperitoneal injection of rabbit anti-mouse PMN antiserum or control was performed in CD-1 mice 29 hours and 5 hours prior to laparotomy and intrauterine injection of either killed E. coli or phosphate buffered saline on Day 14.5 of pregnancy. Preterm delivery was defined as delivery of at least one pup within 48 hours. Circulating leukocyte counts were determined manually or by flow cytometry at the time of surgery and 8, 24 and 48 hours afterwards. Maternal and fetal tissues were analyzed in a separate group of animals 8 hours after surgery.
Results
Pre-treatment with anti-PMN antiserum significantly decreased the numbers of circulating leukocytes and the proportion of neutrophils among all leukocytes by 70–80% at surgery and at least 8 hours thereafter. Neutrophil depletion significantly reduced two markers of neutrophil activation in uterus and placenta (neutrophil elastase and myeloperoxidase activity) and neutrophil infiltration into gestational tissues in bacterially treated animals to baseline (control) levels, but did not affect preterm birth rates. The large E. coli-induced increases in uterine inflammatory markers (interleukin 1β, tumor necrosis factor, CCL5, cyclooxygenase 2) were not affected or were only minimally affected by neutrophil depletion.
Conclusions
Although PMN antiserum reduces both neutrophil number and activity, it does not diminish sensitivity to bacterially induced delivery or meaningfully alter the expression of inflammatory markers in the mouse model. Preterm birth and inflammation in this model are not likely to depend on neutrophil function.
Keywords: infection, mouse, neutrophils, polymorphonuclear leukocytes, preterm labor
INTRODUCTION
Preterm birth is the most common cause of neonatal morbidity and mortality in the developed world. Up to 50% of cases of preterm labor are associated with acute bacterial colonization of the gestational compartment1. The role of inflammation per se in the genesis of labor in women is not always clear. In multiple animal models, both intrauterine and systemic sterile inflammation can induce preterm birth2–9. In humans, intraamniotic inflammation (i.e. the presence of elevated inflammatory cytokines and other markers) is found approximately twice as often as are cultivable bacteria in preterm labor and preterm premature rupture of the membranes (PPROM)6, 8, suggesting that sterile inflammation or inflammation resulting from infection in remote compartments may play a role in the pathophysiology of preterm birth.
Polymorphonuclear leukocytes (PMNs), also called granulocytes, include three main types of cells: neutrophils, eosinophils and basophils. These three types of PMN have roles in host responses to different kinds of threats. Neutrophils are the most common type of PMN, representing 50–70% of circulating leukocytes in humans and 10–25% in mice, with the great majority of the remainder being lymphocytes10, 11. Neutrophils are both prototypical ‘first responders’ to inflammatory foci and a source of pro-inflammatory signals. They are responsible for eliminating pathogens, for helping to coordinate the acute inflammatory response, for participating in tissue remodeling, and for other processes.
Peripheral blood leukocytes become primed as labor approaches12, 13. Leukocyte infiltration into gestational tissues has a role in the initiation, maintenance and resolution of parturition, with major roles ascribed to macrophages and neutrophils7, 14–16. Other functions are ascribed to resident cell types (e.g. myometrium, decidua, amnion, chorion) and various other immune cells that reside in or infiltrate these tissues, such as uterine NK cells17, 18, invariant NKT (iNKT) cells19, and memory T cells20. Such cells may play different roles in infection/inflammation-induced preterm labor than in spontaneous parturition at term. In a recent study a massive influx of neutrophils into myometrial tissues occurred in association with endotoxin-induced but not other forms of labor (i.e. spontaneous or progesterone withdrawal-induced labor)21, suggesting the existence of different pathways to parturition. Nonetheless, the question remains as to whether, during infection and inflammation, these leukocytes cause labor, are a consequence of labor, or play an unrelated role.
Given the importance of PMN function in the response to acute bacterial infection, we sought to characterize the role of PMNs in a model of infection-induced preterm labor using intrauterine killed E. coli as the stimulating agent.
MATERIALS AND METHODS
Anti-PMN serum and control injections
A rabbit polyclonal anti-mouse PMN antiserum and a control rabbit serum were purchased from Accurate Chemical Corporation, Westbury, NY (Cat # AIA31140 and AIS403). Serum was diluted 1:10 in PBS and administered intraperitoneally as recommended by the supplier in a volume of 0.5ml (i.e. approximately 1.9% of total body water of a typical 40 gram pregnant CD-1 mouse). In pilot experiments modeled after published mouse models22–28, it was determined that two injections administered 29 hours and 5 hours prior surgery resulted in the greatest reduction in circulating PMN counts at the time of surgery. Control animals were treated with either the control serum or vehicle (PBS) in a similar regimen. Control serum was compared with PBS to verify that the control serum did not on its own cause observable changes (see Table 1 and related text). Because no differences were found, results for both were combined.
Table 1.
Comparison of circulating leukocyte counts and neutrophil proportions in two pre-treatment control regimens (IP injection of either PBS or control rabbit serum) 29 and 5 hours prior to intrauterine injection of either E. coli or PBS.
| Total WBC/µl blood | % neutrophils | ||||||
|---|---|---|---|---|---|---|---|
| Pretreatment (IP) | Pretreatment (IP) | ||||||
| IU injection |
Control serum |
PBS | P value | Control serum |
PBS | P value | |
| At surgery (Time 0) | --- | 6428 ± 1908 (n=10) | 5281 ± 1406 (n=9) | 0.2 | 24 ± 6.7 | 25 ± 10.1 | 0.8 |
| 24 hours after surgery | PBS | 6105 ± 2536 | 5258 ± 1876 | 0.64 | 22 ± 7.8 | 31 ± 15.9 | 0.3 |
| E. coli | 3560 ± 1607 | 4650 ± 2092 | 0.38 | 43 ± 13.9 | 51 ± 18.3 | 0.4 | |
| P value | 0.1 | 0.7 | 0.02 | 0.2 | |||
| 48 hours after surgery a | PBS | 5680 ± 1818 | 8506 ± 2967 | 0.1 | 30 ± 2.8 | 32 ± 5.9 | 0.6 |
| E. coli | --- | --- | --- | --- | |||
Blood leukocyte counts determined at surgery (Time 0), 24h and 48h after surgery by manual counts after retro-orbital sinus puncture.
Leukocyte counts 48 hours after surgery available only for mice injected IU with PBS, as the majority of animals injected with E. coli delivered and underwent necropsy prior to 48 hours. Numbers of animals per IU treatments: 4–6 per group.
Preparation of bacteria
Bacteria were freshly grown, heat-killed, concentrated to 2 × 109 organisms per ml, and frozen in aliquots at −80°C, as previously described5. Aliquots were thawed and diluted as needed prior to each experiment.
Animals
All procedures involving animals were approved by the NorthShore University HealthSystem Animal Care and Use Committee and conform to the Guide for Care and Use of Laboratory Animals (1996, National Academy of Sciences).
CD-1 female mice (housed in groups separately from males) were identified as being in estrus by the gross appearance of the vaginal epithelium as previously described29, 30. Each receptive female was placed individually with a male CD-1 stud in the afternoon and removed the following morning. Presence of a vaginal plug was considered evidence of copulation (morning of plugging = Day 0.5 of pregnancy). Intraperitoneal injections of either rabbit anti-mouse PMN antiserum or the same volume of control solution (either PBS or normal rabbit serum) were performed on the mornings of Days 13.5 and 14.5 of pregnancy. Five hours after the second injection, animals were anesthetized with 0.015 ml/g body weight of Avertin (2.5% tribromoethyl alcohol and 2.5% tert-amyl alcohol in PBS), as previously described5.
At surgery a 1.5 cm midline incision was made in the lower abdomen. Mice underwent intrauterine injection of either 2 × 107 or 6 × 107 heat-killed E. coli bacteria (an inoculum sufficient to cause preterm delivery in >80% of animals) or an equivalent volume (100 µl) of PBS in the midsection of the right uterine horn at a site between two adjacent fetuses. The abdomen was closed in two layers, with 4-0 polyglactin sutures at the peritoneum and wound clips at the skin.
Animals recovered in individual cages in the animal facility. Observations were made twice-daily for preterm delivery (defined as at least one fetus in the cage or lower vagina within 48 hours of surgery). Maternal blood was collected in heparinized capillary tubes from the right retro-orbital sinus at the time of surgery (while the animal was anesthetized), from the left retro-orbital sinus 24 hours after surgery (under isofluorane anesthesia) and from the right side again 48 hours after surgery upon euthanasia and just prior to necropsy. Additional blood samples were collected by cardiac puncture in a separate group of animals 8 hours after surgery immediately upon euthanasia, at which time necropsies were performed and tissues collected for analysis (see below). At necropsy the number of fetuses delivered or remaining in utero and the survival status of these retained fetuses (as determined by cardiac or vascular pulsations in the fetal bodies and membranes) were recorded.
Tissue harvest
For tissue harvests 8 hours after surgery the inoculated uterine horn was incised longitudinally along the anti-mesenteric border. Gestational tissues (uteri, fetal membranes, fetuses and placentas) were collected, washed in ice-cold PBS, and either flash-frozen in liquid nitrogen and stored at −80°C or fixed in 10% buffered formalin for later analysis.
Cell counts
Total white blood cell (WBC) and leukocyte differential counts were performed manually in a hemacytometer after lysis of red blood cells and nuclear staining using the Leuko-TIC kit (bioanalytic GmbH, Freiburg, Germany) according to the instructions of the manufacturer. Flow cytometry was conducted on 100µl whole blood equivalents after lysing red blood cells, centrifugation and re-suspension in PBS. Cells were blocked with rat anti-mouse CD16/CD32 (Mouse BD Fc Block buffer; BD Pharmingen, San Diego) for 10 minutes and then stained for CD45 FITC, rat anti-mouse CD11b-PE, and rat anti-mouse Ly6G PerCP-Cy5.5 (BD Pharmingen) for 45 minutes at room temperature. Appropriate isotype control antibodies were used to exclude false-positive signals. CD45+CD11b+Ly6G+ cells were considered neutrophils. Flow cytometry was performed with BD FACSCalibur and FlowJo software (BD Biosciences, San Jose, CA).
Assays of neutrophil function
Myeloperoxidase (MPO) activity (mu/gm tissue) was determined using the MPO Fluorometric Kit (Enzo Life Sciences, Plymouth Meeting, PA) according to the manufacturer’s instructions. Neutrophil elastase (ng/gm tissue) was measured using a specific ELISA kit (LSBio™ Mouse HNE/Neutrophil Elastase, Cat # LS-F11364, LifeSpan BioSciences, Seattle, WA). A set of standards was run concurrently with the samples for each of these assays.
Histologic analysis
Tissue samples were embedded in paraffin, sectioned and stained with hematoxylin and eosin (H&E). Sections (4–5 µm thick) were assessed for neutrophil infiltration.
Real-Time PCR
Total RNA was extracted from homogenized tissues with TRIzol reagent (Invitrogen, Carlsbad, CA). Two µg of total RNA were used as a template for cDNA synthesis using qScript™ cDNA Synthesis Kit (Quanta biosciences, Gaithersburg USA). TaqMan PCR primers and probes were purchased from Applied Biosystems (Foster City, CA): IL-1β Mm00434228; CCL5 (RANTES) Mm01302428; TNF Mm00443258; cyclooxygenase-2 (COX-2) Mm00478374; connexin 43 (GJA1) Mm01179639; glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (20×) Mm4452339E. Duplex reactions were performed in a 10µL mixture containing 1 µL cDNA, with one primer pair amplifying the gene of interest and the other an internal reference (GAPDH). Thermocycler parameters were 50°C for 2 minutes, 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds, and 60°C for 1 minute. Semi-quantitative analysis of gene expression was done using the comparative CT (ΔΔCT) method, normalizing expression of the gene of interest to GAPDH. PCR assays were performed in triplicate.
Statistical Analysis
Categorical data were analyzed by Fisher exact test. Continuous variables were analyzed by t-test or Mann-Whitney U test (for non-normally distributed data) or ANOVA or Kruskal-Wallis with Dunn’s post-hoc testing (for comparison of more than two groups).
RESULTS
Depletion of PMNs does not alter bacterially induced preterm delivery There were four treatment groups: 1) Pre-operative intraperitoneal (IP) injection of control solution followed by intrauterine (IU) PBS (‘IP Ctrl/IU PBS’); 2) Pre-operative IP PMN antiserum followed by IU PBS (‘IP α-PMN/IU PBS’); 3) Pre-operative IP control injections followed by IU killed E. coli (‘IP Ctrl/IU E. coli’); and 4) Pre-operative IP PMN antiserum followed by IU killed E. coli (‘IP α-PMN/IU E. coli’).
Compared to IP injection with PBS, injection with control serum produced no changes in circulating leukocyte counts, regardless of whether E. coli were injected IU (Table 1). Therefore, PBS and control serum results were combined for subsequent analyses.
Intraperitoneal injection of anti-PMN antiserum 29 and 5 hours prior to surgery resulted in large (70 – 80%) reductions in both the total number of circulating leukocytes and the proportion of these leukocytes that were neutrophils at the time of surgery (Table 2, Time 0), with the remainder almost exclusively lymphocytes. Given the fact that in the mouse, unlike the human, the predominating circulating leukocyte is the lymphocyte, this observation indicates that in addition to a specific anti-PMN effect, the antiserum also depletes lymphocytes. This non-specific lymphocyte depletion, presumably mediated through Gr-1 antigens, has been reported previously for this antiserum26, 27.
Table 2.
Circulating leukocyte counts and neutrophil proportions in animals pre-treated with intraperitoneal PMN antiserum or controls (PBS/norma l serum) followed by intrauterine killed E. coli or PBS.
| IU injection |
Total WBC/µl blood | % neutrophils | |||||
|---|---|---|---|---|---|---|---|
| IP anti-PMN antiserum |
IP Control | P value | IP anti-PMN antiserum |
IP Control | P value | ||
| At surgery a (Time 0) | --- | 1628 ± 1053 | 5884 ± 1745 | <0.0001 | 6% | 24% | <0.0001 |
| 8h after surgery b | PBS | --- | --- | --- | 3% | 48% | <0.001 |
| E. coli | --- | --- | --- | 6% | 53% | <0.0001 | |
| P value | 0.24 | 0.63 | |||||
| 24 h after surgery a | PBS | 4694 ± 1376 | 5788 ±2208 | 0.4 | 16% | 25% | 0.1 |
| E. coli | 1183 ± 199 | 4105 ±1850 | 0.002 | 23% | 47% | 0.007 | |
| P value | <0.001 | 0.1 | 0.3 | 0.005 | |||
| 48h after surgery a,c | Control | 4325 ± 1219 | 6936 ± 2678 | 0.1 | 32% | 31% | 0.7 |
Blood leukocyte counts determined at surgery (Time 0), 24h and 48h after surgery by manual counts;
leukocyte counts 8 hours after surgery determined by flow cytometry (thus, only proportions are reported);
leukocyte counts 48 hours after surgery available only for mice injected IU with PBS, as the majority of animals injected with E. coli delivered and underwent necropsy prior to 48 hours. Blood at surgery and 24/48h after surgery obtained via retro-orbital sinus puncture. Blood at 8h obtained via cardiac puncture upon euthanasia. Intraperitoneal controls (PBS or control serum) were combined. Numbers of animals per IU treatments: Controls – 4; E. coli – 5–9 per group.
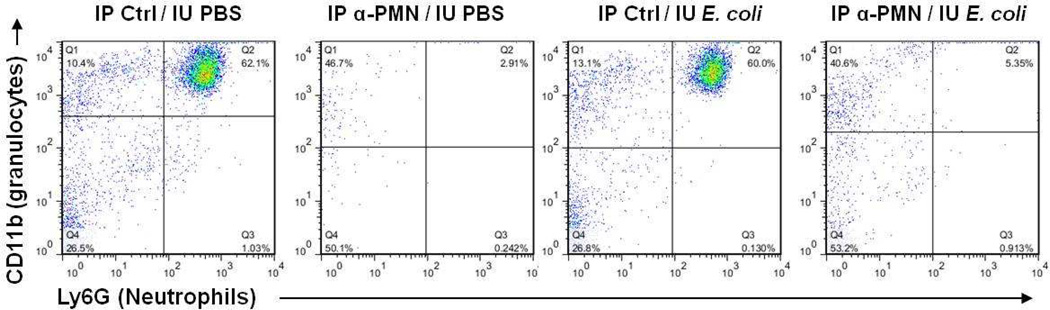
Performance of general anesthesia, laparotomy and IU injection produced a significant increase in neutrophil proportion (from 24% up to about 50%) 8 hours after surgery in animals not pre-treated with anti-serum, regardless of whether the intrauterine injections contained bacteria or only PBS. Pre-treatment with antiserum completely eliminated this 8-hour increase in neutrophil proportion (Figure 1 and Table 1). The suppression of both circulating total leukocyte count and neutrophil proportion persisted through 24 hours after surgery in animals treated with IU E. coli, but not in animals treated with IU PBS.
Figure 1.
Representative flow cytometry dot plot of CD45+ white blood cell populations 8 hours after surgery, gated for the granulocyte marker CD11b and the neutrophil-specific marker Ly6G. IP Ctrl = intraperitoneal injection of PBS; IP α-PMN = IP injection of anti-PMN antiserum; IU PBS = intrauterine injection of PBS; IU E. coli = intrauterine injection of killed E. coli.
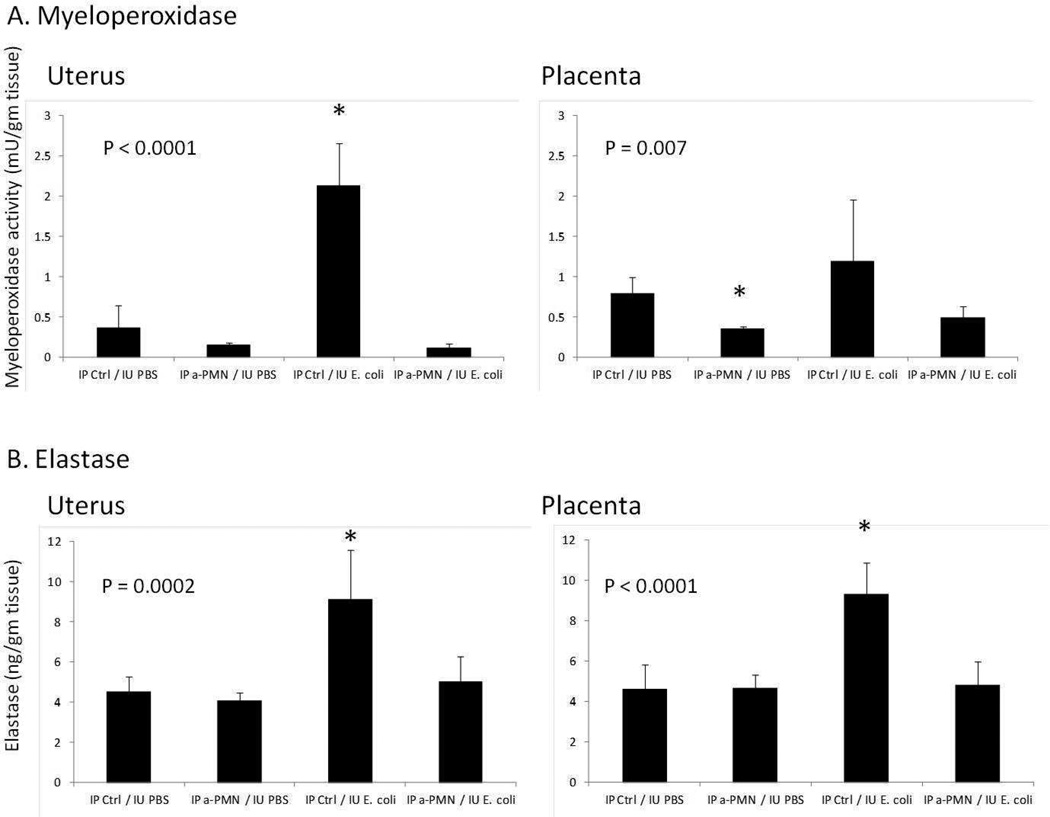
To correlate between circulating PMN numbers and function within gestational tissues, an activity assay for myeloperoxidase (MPO) and an ELISA for neutrophil elastase, markers of neutrophil activation) were conducted in uteri and placentas (Figure 2). MPO activity was increased by IU E. coli treatment by 500% in uterus and 50% in placentas (the latter not a significant increase), responses that were eliminated by pre-treatment with PMN antiserum. A similar effect was seen on levels of neutrophil elastase, which were significantly elevated by bacterial exposure in both uteri and placentas.
Figure 2.
Assays of neutrophil function in uterus and placenta 8 hours after laparotomy with intrauterine injection of either PBS or E. coli. A) Myeloperoxidase (MPO) activity (mu/gm tissue); B) Neutrophil elastase (ng/gm tissue). Animals were pre-treated with either IP anti-PMN antiserum or control injections, followed by IU E. coli or control (PBS). N = 5–9 per group. P values determined by ANOVA. Post-hoc analysis showing significantly different treatment groups indicated by asterisk (*), with all other groups no different from each other.
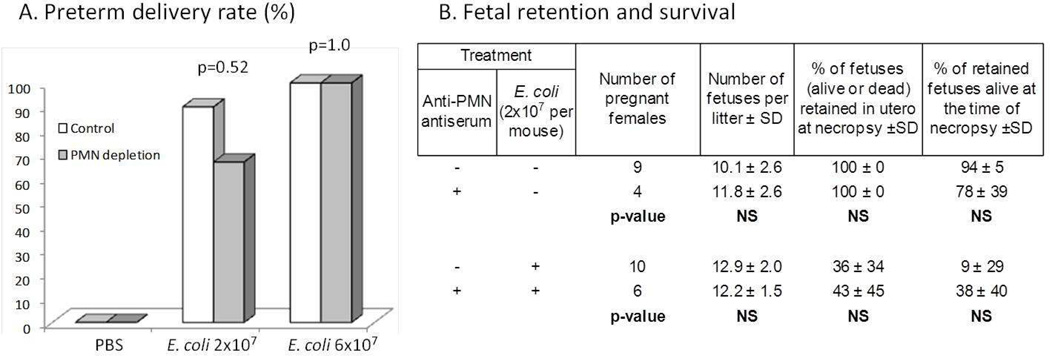
Thus, anti-PMN antiserum induced large and significant drops in both circulating neutrophil counts and neutrophil activity within tissues to levels at or below baseline during the critical time between the performance of the surgical procedure and delivery 9–13 hours later. Despite these reductions, there were no differences in either the occurrence or timing of E. coli-induced preterm delivery or in the number and survival status of fetuses retained in utero (Figure 3).
Figure 3.
Effect of PMN depletion on outcomes in bacterially induced preterm labor. A) Preterm delivery rates following intrauterine injection of the indicated quantities of bacteria with or without prior PMN depletion. B) Fetal retention and survival based on treatment groups. Note that PMN depletion produced no discernible effect on preterm delivery rates, number of fetuses delivered/retained in utero or the proportion of retained fetuses that were alive at necropsy. Necropsies were performed 48 hours after surgery in animals who did not deliver prematurely, or upon evidence of delivery in animals who delivered in < 48 hours. Intraperitonal pre-treatments with PBS and control serum were combined. P values determined by Fisher Exact test (A) and t-test (B).
Inflammation within tissues
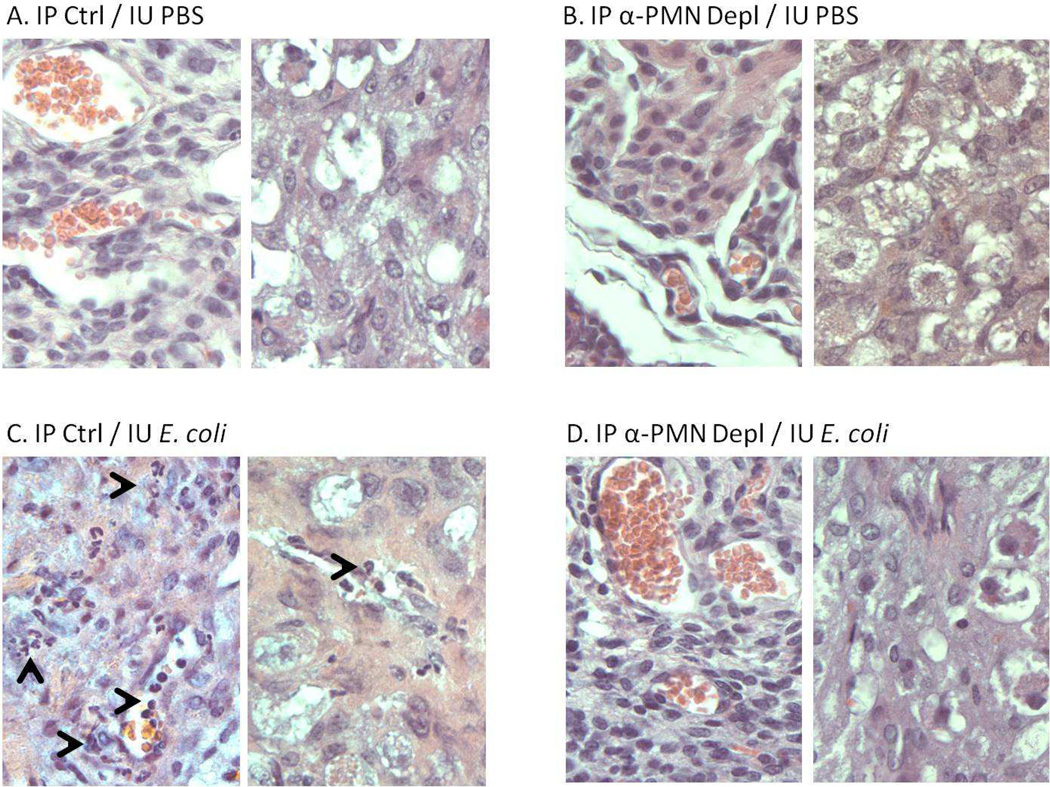
Intrauterine injection of killed E. coli but not PBS induces patchy mild neutrophil infiltration of uterine stromal and decidual tissue (compare Figures 4 A and C). These infiltrates are eliminated by PMN depletion with PMN antiserum (Figure 4D). There are no discernible changes attributable to E. coli exposure in placentas, fetal membranes or fetuses (not shown), or to anti-PMN treatment alone (Figure 4B). Thus, the relatively minor inflammatory changes induced by intrauterine injection of deliveryinducing inocula of killed E. coli are abolished by PMN depletion.
Figure 4.
Infiltration of neutrophils in gestational tissues. Uteri harvested 8 hours after surgery in animals treated with A) No PMN depletion/IU PBS; B) PMN depletion/ IU PBS; C) No PMN depletion / IU E. coli; D) PMN depletion/IU E. coli. Uterine stroma depicted on the left side and decidua depicted on the right side of each panel. Note scattered infiltration of vessels and tissues with neutrophils (arrowheads) following IU bacterial inoculation (C), suppressed by prior administration of antiserum (D). Original magnification: 400×.
Gene expression
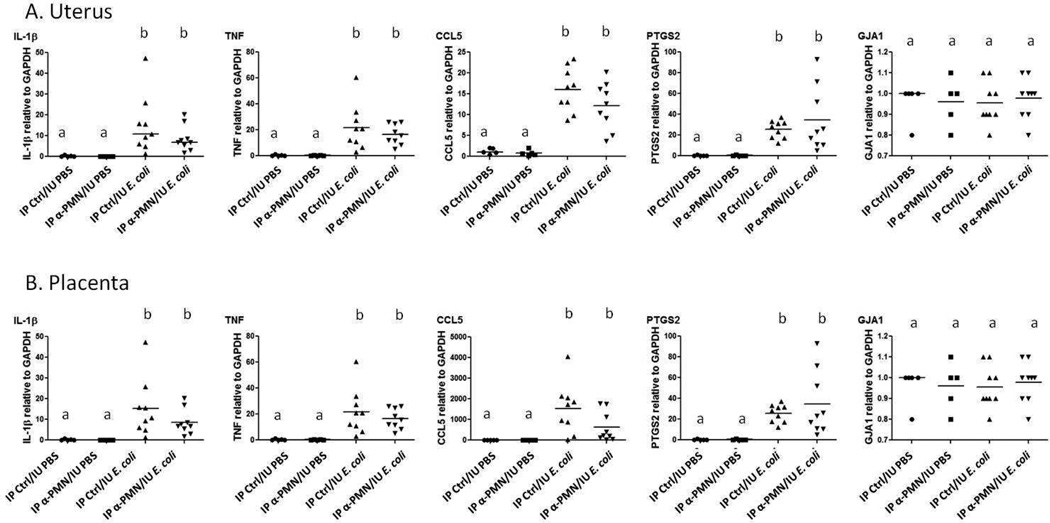
To assess the impact of intrauterine injection of E. coli with and without PMN depletion on expression of inflammatory markers, RT-PCR was performed in uterine and placental tissues (Figure 5). Inflammatory markers were chosen that represent the MyD88-dependent and MyD88-independent signaling pathways for toll-like receptors (IL-1β and CCL5 (RANTES), respectively) and both pathways (TNF), as well as inducible prostaglandin synthase (PTGS2, or COX-2). The mRNA transcripts for these genes have been demonstrated to correlate closely with protein production31. In addition, we assessed gap junction formation by measuring mRNA for connexin 43, an important component of myometrial preparation for coordinated, synchronous contraction. Intrauterine injection of E. coli led to significant increases in the expression of IL-1β, TNF, CCL5 and COX-2 in uterus and placenta. Connexin 43 was not altered by any of the treatments in either uterus or placenta compared to controls. Depletion of PMNs did not affect the expression of these genes compared to no PMN depletion.
Figure 5.
Cytokine gene expression in uterine (A) and placental (B) tissues 8 hours after surgery. Each gene of interest was amplified by RT-PCR and expression determined using the comparative CT (ΔΔCT) method, normalizing to GAPDH. N = 5–9 per treatment group. PCR assays were performed in triplicate (data points represent averages of these triplicates for each sample). Analysis by Kruskall-Wallis test with post-hoc testing for differences among groups. Treatment groups with the same letter designation (“a” or “b”) are statistically no different from each other. Groups with different letter designations are statistically different from each other (p < 0.05).
COMMENT
The literature supports the concept that the acquired and innate immune systems play central roles in both pregnancy maintenance remote from term (e.g. suppression of rejection of the fetal allograft and of uterine contractile activity) and the initiation and maintenance of labor as the time for delivery approaches32, 33. In the specific case of infection- and inflammation-induced labor, the roles of immune cells have not yet been fully defined. The present study, in which both the number and activation of polymorphonuclear leukocytes (primarily neutrophils) were reduced to ≤30% of normal levels (along with a non-specific and smaller decrease in lymphocytes), suggests that these cells do not have an essential role in preterm labor induced by a bacterially derived inflammatory stimulus. Similarly, neutrophils do not appear to have a major role in the generation of the large increases in inflammatory mediators within gestational tissues characteristically seen in animal models of bacterially induced labor. These observations are consistent with an additional finding of the present study, namely that although the intrauterine injection of a large bolus of killed bacteria produces a dramatic phenotype of both preterm delivery and increased production of inflammatory markers, there is only minor histological evidence of inflammation (i.e. neutrophil infiltration into uterine tissues). In contrast to this observation using bacteria, others have reported substantial neutrophil infiltration into myometrium and decidua with endotoxin (LPS) -induced labor21, 34, 35.
It is possible that other types of leukocyte are necessary for inflammation-induced labor. One study showed that antibody-based macrophage depletion prevented preterm delivery due to intravaginal placement of LPS in mice16. In other studies, depletion of uterine natural killer (uNK) cells led to significant reductions in preterm birth induced by intraperitoneal LPS in both wild type BALB/c17 and IL-10-deficient mice18. In a different report, a broad spectrum chemokine inhibitor used in the intraperitoneal LPS-induced mouse preterm labor model led to a significant decrease in preterm birth and cytokine/chemokine expression while at the same time diminishing neutrophil accumulation in myometrium (but not decidua)35. Together with the results of the present report and studies reviewed below, we believe the sum total of the evidence suggests that neutrophil accumulation is a consequence, rather than a cause of the processes leading to inflammation-induced labor.
A different group (Rinaldi et al.) has recently published a report similar to the present study, in which two different neutrophil-depleting antibodies directed against Ly6G and Gr-1 had no effect on preterm delivery induced by intrauterine injection of LPS34. The expression of a panel of inflammatory cytokines and chemokines showed diminishment only for IL-1β in uterus and placenta with one antibody and for IL-1β and TNFα in uterus only with the other antibody. The other markers examined (IL-6, CXCL1, CXCL2 and CXCL5) were unaffected by neutrophil depletion in these tissues or were in fact increased in fetal membranes. This relatively limited scope of decline in inflammatory markers whose expression is increased dramatically by intrauterine injection of LPS is qualitatively consistent with the present study, in which neutrophil depletion did not result in significant reductions in local expression of IL-1β, TNF, CCL5 and COX-2. The evidence thus suggests that cells other than PMNs are primarily responsible for coordinating the inflammatory cytokine response within the gestational compartment after a pro-inflammatory stimulus. It remains unclear whether these local increases in inflammatory markers in and of themselves lead to labor or are an epiphenomenon. However, we have previously noted that when one uterine horn is physically isolated from the contralateral horn and injected with bacteria in the preterm period, the uninjected horn evacuates its contents rapidly without concomitant increased local expression of inflammatory cytokines36, suggesting that increases in these cytokines represent an epiphenomenon that is not directly responsible for labor. It is notable that anesthesia followed by intrauterine injection of PBS produced a low-level inflammatory profile (see Table 2, increased neutrophil proportion 8 hours after bacterial exposure), an observation made in the Rinaldi study as well34.
We believe that the two papers demonstrating that neutrophil depletion fails to affect inflammation-induced preterm birth (the current report and Rinaldi et al.34) show that local increases in neutrophils in pregnancy tissues are a consequence of the inflammatory stimulus but not a cause of labor. In a paper focusing on neutrophil function in the cervix during normal labor (i.e. not induced), Timmons and Mahendroo also showed that neutrophil depletion using a monoclonal antibody directed against Ly6G did not affect timing or success of parturition.
A limitation of the present study is the relative non-specificity of neutrophil depletion using the commercially available antiserum, which was raised in rabbits against murine PMNs (see Table 2). We report a reduction in circulating neutrophil proportion from 24% to 6% due to pretreatment with the antiserum. However, given that there was a reduction in overall circulating leukocyte counts to 28% that of controls, it is clear that lymphocytes (the predominating circulating leukocyte in mice) were also depleted by the antiserum. As noted above, this phenomenon has been observed previously and is attributable to cross reactivity with Gr-1 antigens26, 27. However, we believe that this non-specificity does not alter the conclusion that neutrophils are unlikely to be essential for preterm delivery in this model.
In a sense, the breadth of leukocyte depletion by the PMN antiserum may represent a strength of the present study over more specific neutrophil depleting antibodies. The mouse remains the most commonly used organism used to model human immunology, including the innate immune system as it relates to parturition. Although the difference in distribution of circulating leukocytes between mice and humans (approximately 25% neutrophils in mice versus 50–70% in humans) is well known, its functional significance is not clear37. However, it has been demonstrated that in the two species similar tasks are performed by different cell types. One example of this occurs in intestinal epithelial mucosal immunity, where functional equivalence is accomplished through differential mechanisms of effector cells38. Thus, our use of a non-specific leukocyte depletion strategy in inflammation-induced preterm delivery is more likely to account for such compensatory mechanisms than a more neutrophil-specific strategy.
It should be noted that significant effects on neutrophil-dependent pathological states have been demonstrated with the antiserum used in the present study, including the severity of airway disease22, 26, 27, impairment of gut barrier function after hemorrhagic shock28, cerebral stroke volume and neurological deficit in an ischemia/reperfusion model23 and diminished severity of cerulein-induced pancreatitis24. In contrast, in other model systems the same antiserum failed to impact pathology, including fibrin deposition and thrombosis associated with oxygen deprivation25,
Other limitations of the current and other studies include the fact that in contrast to the gradual increase of live bacteria that occurs during spontaneous infection in human gestations, mouse models use a bolus of a large number of killed bacteria or LPS. However, previous observations support the suitability of the model for bacterially induced labor5, 39, 40. These observations include the production of inflammatory markers, the fact that progesterone withdrawal is not the cause of labor, and other features. The importance of these leukocytes in human pregnancies infected with live bacteria remains to be determined.
ACKNOWLEDGMENT
We thank Irene Patel, Technical Specialist, for technical assistance with manual leukocyte counting methods.
Sources of Funding: NIH 1R01 HD056118; March of Dimes 21-FY10-202; The Satter Foundation Fund in Perinatal Research
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors report no conflict of interest
Presented in part at the Society for Maternal-Fetal Medicine 34th Annual Meeting,, February 3–8, 2014, New Orleans, Louisiana.
REFERENCES
- 1.Klein LL, Gibbs RS. Use of microbial cultures and antibiotics in the prevention of infection-associated preterm birth. Am J Obstet Gynecol. 2004;190:1493–1502. doi: 10.1016/j.ajog.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal V, Hirsch E. Intrauterine infection and preterm labor. Seminars in fetal & neonatal medicine. 2012;17:12–19. doi: 10.1016/j.siny.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elovitz MA, Mrinalini C. Animal models of preterm birth. Trends in endocrinology and metabolism: TEM. 2004;15:479–487. doi: 10.1016/j.tem.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch E, Saotome I, Hirsh D. A model of intrauterine infection and preterm delivery in mice. Am J Obstet Gynecol. 1995;172:1598–1603. doi: 10.1016/0002-9378(95)90503-0. [DOI] [PubMed] [Google Scholar]
- 5.Mussalli GM, Blanchard R, Brunnert SR, Hirsch E. Inflammatory cytokines in a murine model of infection-induced preterm labor: cause or effect? J Soc Gynecol Invest. 1999;6:188–195. doi: 10.1016/s1071-5576(99)00013-1. [DOI] [PubMed] [Google Scholar]
- 6.Shim SS, Romero R, Hong JS, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2004;191:1339–1345. doi: 10.1016/j.ajog.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 7.Yellon SM, Mackler AM, Kirby MA. The role of leukocyte traffic and activation in parturition. J Soc Gynecol Investig. 2003;10:323–338. doi: 10.1016/s1071-5576(03)00116-3. [DOI] [PubMed] [Google Scholar]
- 8.Yoon BH, Romero R, Moon JB, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 9.Mussalli GM, Brunnert SR, Hirsch E. Preterm delivery in mice with renal abscess. Obstet Gynecol. 2000;95:453–456. doi: 10.1016/s0029-7844(99)00571-2. [DOI] [PubMed] [Google Scholar]
- 10.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 11.Roscoe B, Jackson Memorial Laboratory. Green EL. Biology of the laboratory mouse. New York: Blakiston Division; Number of pages. [Google Scholar]
- 12.Gomez-Lopez N, Tanaka S, Zaeem Z, Metz GA, Olson DM. Maternal circulating leukocytes display early chemotactic responsiveness during late gestation. BMC pregnancy and childbirth. 2013;13(Suppl 1):S8. doi: 10.1186/1471-2393-13-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan M, Jordan F, McInnes IB, Harnett MM, Norman JE. Leukocytes are primed in peripheral blood for activation during term and preterm labour. Mol Hum Reprod. 2009;15:713–724. doi: 10.1093/molehr/gap054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osman I, Young A, Ledingham MA, et al. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9:41–45. doi: 10.1093/molehr/gag001. [DOI] [PubMed] [Google Scholar]
- 15.Timmons BC, Mahendroo MS. Timing of neutrophil activation and expression of proinflammatory markers do not support a role for neutrophils in cervical ripening in the mouse. Biol Reprod. 2006;74:236–245. doi: 10.1095/biolreprod.105.044891. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez JM, Franzke CW, Yang F, Romero R, Girardi G. Complement activation triggers metalloproteinases release inducing cervical remodeling and preterm birth in mice. The American journal of pathology. 2011;179:838–849. doi: 10.1016/j.ajpath.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin Y, Xie M, Chen Y, Di J, Zeng Y. Preterm delivery induced by LPS in syngeneically impregnated BALB/c and NOD/SCID mice. Journal of reproductive immunology. 2006;71:87–101. doi: 10.1016/j.jri.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Murphy SP, Hanna NN, Fast LD, et al. Evidence for participation of uterine natural killer cells in the mechanisms responsible for spontaneous preterm labor and delivery. Am J Obstet Gynecol. 2009;200:308 e1–308 e9. doi: 10.1016/j.ajog.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li LP, Fang YC, Dong GF, Lin Y, Saito S. Depletion of invariant NKT cells reduces inflammation-induced preterm delivery in mice. J Immunol. 2012;188:4681–4689. doi: 10.4049/jimmunol.1102628. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Lopez N, Vega-Sanchez R, Castillo-Castrejon M, Romero R, Cubeiro-Arreola K, Vadillo-Ortega F. Evidence for a role for the adaptive immune response in human term parturition. Am J Reprod Immunol. 2013;69:212–230. doi: 10.1111/aji.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shynlova O, Nedd-Roderique T, Li Y, Dorogin A, Lye SJ. Myometrial immune cells contribute to term parturition, preterm labour and post-partum involution in mice. Journal of cellular and molecular medicine. 2013;17:90–102. doi: 10.1111/j.1582-4934.2012.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abraham E, Carmody A, Shenkar R, Arcaroli J. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1137–L1145. doi: 10.1152/ajplung.2000.279.6.L1137. [DOI] [PubMed] [Google Scholar]
- 23.Connolly ES, Jr, Winfree CJ, Springer TA, et al. Cerebral protection in homozygous null ICAM-1 mice after middle cerebral artery occlusion. Role of neutrophil adhesion in the pathogenesis of stroke. J Clin Invest. 1996;97:209–216. doi: 10.1172/JCI118392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frossard JL, Saluja A, Bhagat L, et al. The role of intercellular adhesion molecule 1 and neutrophils in acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology. 1999;116:694–701. doi: 10.1016/s0016-5085(99)70192-7. [DOI] [PubMed] [Google Scholar]
- 25.Lawson CA, Yan SD, Yan SF, et al. Monocytes and tissue factor promote thrombosis in a murine model of oxygen deprivation. J Clin Invest. 1997;99:1729–1738. doi: 10.1172/JCI119337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reino DC, Palange D, Feketeova E, et al. Activation of toll-like receptor 4 is necessary for trauma hemorrhagic shock-induced gut injury and polymorphonuclear neutrophil priming. Shock. 2012;38:107–114. doi: 10.1097/SHK.0b013e318257123a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savov JD, Gavett SH, Brass DM, Costa DL, Schwartz DA. Neutrophils play a critical role in development of LPS-induced airway disease. Am J Physiol Lung Cell Mol Physiol. 2002;283:L952–L962. doi: 10.1152/ajplung.00420.2001. [DOI] [PubMed] [Google Scholar]
- 28.Zhang HY, James I, Chen CL, Besner GE. Heparin-binding epidermal growth factor-like growth factor (HB-EGF) preserves gut barrier function by blocking neutrophil-endothelial cell adhesion after hemorrhagic shock and resuscitation in mice. Surgery. 2012;151:594–605. doi: 10.1016/j.surg.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Champlin AK, Dorr DL, Gates AH. Determining the stage of the estrous cycle in the mouse by the appearance of the vagina. Biol Reprod. 1973;8:491–494. doi: 10.1093/biolreprod/8.4.491. [DOI] [PubMed] [Google Scholar]
- 30.Filipovich Y, Lu SJ, Akira S, Hirsch E. The adaptor protein MyD88 is essential for E coli-induced preterm delivery in mice. Am J Obstet Gynecol. 2009;200:e1–e8. doi: 10.1016/j.ajog.2008.08.038. [DOI] [PubMed] [Google Scholar]
- 31.Zhao TZ, Xia YZ, Li L, et al. Bovine serum albumin promotes IL-1beta and TNF-alpha secretion by N9 microglial cells. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2009;30:379–383. doi: 10.1007/s10072-009-0123-x. [DOI] [PubMed] [Google Scholar]
- 32.Gomez-Lopez N, StLouis D, Lehr MA, Sanchez-Rodriguez EN, Arenas-Hernandez M. Immune cells in term and preterm labor. Cellular & molecular immunology. 2014 doi: 10.1038/cmi.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu P, Nanan RK. Innate and adaptive immune interactions at the fetal-maternal interface in healthy human pregnancy and pre-eclampsia. Frontiers in immunology. 2014;5:125. doi: 10.3389/fimmu.2014.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rinaldi SF, Catalano RD, Wade J, Rossi AG, Norman JE. Decidual neutrophil infiltration is not required for preterm birth in a mouse model of infection-induced preterm labor. J Immunol. 2014;192:2315–2325. doi: 10.4049/jimmunol.1302891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shynlova O, Dorogin A, Li Y, Lye S. Inhibition of infection-mediated preterm birth by administration of broad spectrum chemokine inhibitor in mice. Journal of cellular and molecular medicine. 2014 doi: 10.1111/jcmm.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirsch E, Muhle RA, Mussalli GM, Blanchard R. Bacterially induced preterm labor in the mouse does not require maternal interleukin 1 signaling. Am J Obstet Gynecol. 2002;186:523–530. doi: 10.1067/mob.2002.120278. [DOI] [PubMed] [Google Scholar]
- 37.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 38.Gibbons DL, Spencer J. Mouse and human intestinal immunity: same ballpark, different players; different rules, same score. Mucosal immunology. 2011;4:148–157. doi: 10.1038/mi.2010.85. [DOI] [PubMed] [Google Scholar]
- 39.Hirsch E, Blanchard R, Mehta S. Differential fetal and maternal contributions to the cytokine milieu in a murine model of infection-induced preterm birth. Am J Obstet Gynecol. 1999;180(2 Pt 1):429–434. doi: 10.1016/s0002-9378(99)70227-9. [DOI] [PubMed] [Google Scholar]
- 40.Hirsch E, Muhle R. Intrauterine bacterial inoculation induces labor in the mouse by mechanisms other than progesterone withdrawal. Biol Reprod. 2002;67:1337–1341. doi: 10.1095/biolreprod67.4.1337. [DOI] [PubMed] [Google Scholar]