Abstract
Current first-line drugs for the treatment of neonatal seizures have limited efficacy and are associated with side effects. Uncontrolled seizures may exacerbate brain injury and contribute to later-life neurological disability. Therefore, it is critical to develop a treatment for neonatal seizures that is effective and safe. In early-life, when the γ-aminobutyric acid (GABA) inhibitory system is not fully developed, potassium channels play an important role in controlling excitability. An earlier study demonstrated that flupirtine, a KCNQ potassium channel opener, is more efficacious than diazepam and phenobarbital for the treatment of chemoconvulsant-induced neonatal seizures. In newborns, seizures are most commonly associated with hypoxicischemic encephalopathy (HIE). Thus, in the present study, we examined the efficacy of flupirtine to treat neonatal seizures in an animal model of global hypoxia. Our results showed that flupirtine dose dependently blocks the occurrence of behavioral seizures in pups during hypoxia. Additionally, flupirtine inhibits the development of hypoxia-induced clinical seizures and associated epileptiform discharges, as well as purely electrographic (subclinical) seizures. These results suggest that flupirtine is an effective anti-seizure drug, and that further studies should be conducted to determine the time window within which it's administration can effectively treat neonatal seizures.
Keywords: Neonatal Seizures, Video- EEG monitoring, hypoxia, flupirtine, Potassium channel opener
1. Introduction
Seizures are common in human neonates and are most frequently associated with hypoxia-ischemia [38]. Survivors of neonatal hypoxic-ischemic encephalopathy (HIE) often experience neurodevelopmental disabilities and seizures in later life [4, 11, 26]. Studies in both human neonates and animal models suggest that seizures themselves may independently contribute to brain injury and poor neurological outcome [5, 18, 29, 31] (but also see [25, 41]). Unfortunately, neonatal seizures are often resistant to treatment with approved antiepileptic drugs [15]. Throughout the world phenobarbital, an agonist of γ-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the adult brain, is the most commonly used drug for treating neonatal seizures [6, 10]. The GABAergic system of the immature brain is underdeveloped compared to that of the mature brain, making it a sub-optimal target for the treatment of neonatal seizures [3, 7, 17, 21]. Evidence from clinical and basic science research studies suggests that KCNQ potassium channels play a very important role in controlling excitation in early-life [33, 35, 36, 39]. Flupirtine, a KCNQ channel opener [12, 23, 28, 42], has been used clinically as an analgesic in Europe for over two decades with a good safety record. Our earlier study demonstrated that flupirtine is more efficacious than diazepam and phenobarbital for the treatment of chemoconvulsant-induced neonatal seizures [37]. In the current study, we evaluated the efficacy of flupirtine in the treatment of hypoxia-induced neonatal seizures in an animal model. The results suggest that flupirtine is effective in preventing development of both electroclinical and purely electrographic seizures during hypoxia.
2. Materials and methods
All animal procedures were performed in accordance with the NIH guidelines for the care and use of laboratory animals and according to the protocol approved by the Institutional Animal Care and use Committee (IACUC) of the University of Colorado Anschutz Medical Campus (UC-AMC). All efforts were made to reduce animal suffering and the number of animals used. Timed pregnant Sprague-Dawley rats were obtained from Charles River laboratories (Wilmington, MA). The pregnant rats were at the 14th day of gestation (E14) on arrival at the vivarium and delivered the pups at E22 or E23. The pups from both sexes were used for the study.
2.1. Hypoxia protocol
Ten day old (P10) rat pups were exposed to global hypoxia according to a published protocol [24]. The oxygen concentration of the chamber was maintained at 7% for 8 minutes, 6% for 4 minutes, 5% for 2 minutes and 4% for 1 minute. The oxygen was balanced with nitrogen and the concentration of oxygen in the chamber was monitored using an oxygen sensor (Drager Pac 7000, Pittsburg, PA). The total hypoxia time varied slightly between the experiments, instead of exactly 15 minutes, as it took some time to equilibrate the chamber with the desired concentration of oxygen. During hypoxia, the temperature of the chamber was maintained at 36 - 37 °C, and the humidity at ~80%.
2.2. Flupirtine treatment protocol
In order to minimize the number of rats with electrodes for electroencephalography (EEG), as surgery for electrode implantation can cause short-term pain and distress to pups, a two-step approach was taken to examine the efficacy of flupirtine on behavioral and electrographic seizures. First, we identified a smallest drug dose that was most effective in preventing development of behavioral seizures during hypoxia (rats were not implanted to record EEG). For this, the rat pups were treated by intraperitoneal (i.p.) injection with either 25, 35, 45, and 50 mg/kg body weight flupirtine, or the vehicle (dimethyl sulfoxide and saline; 3:7 vol/vol) 15 to 30 minutes before exposure to hypoxic environment, and monitored for signs of behavioral seizures during hypoxia. The flupirtine doses were selected based on our previous study where we observed that the 50 mg/kg flupirtine was effective in preventing chemoconvulsant seizures [37]. The smallest flupirtine dose that was most efficacious in preventing behavioral seizure was then chosen (35 mg/kg, i.p.) to determine if it also treats EEG correlate of behavioral (clinical) seizure, and purely electrographic (subclinical) seizures.
2.3. Electrode implantation for the Video-EEG recording
At P9, the rat pups were implanted with the electrodes to record the electrical activity of the brain. One silver wire electrode (0.01” outer diameter; AM systems, Carlsborg, WA) was placed in each hemisphere of the brain over the parietal cortex. A silver electrode placed over the right and left side of the brain behind the lambdoid suture served as reference and ground electrodes respectively. The electrode assembly was held in place on the rat skull with tissue adhesive (3M Vetbond, St Paul MN) and dental acrylic cement. The entire implantation procedure was performed under isoflurane anesthesia (2 - 4% for induction and 1 - 1.5% for maintenance). After the surgery, the rats were returned to the dam and treated with analgesic (0.1 mg/kg buprenorphine hydrochloride) once every 12 hours for 48 hours.
2.4. Seizure monitoring and analysis
Behavioral seizures
The P10 rats were continuously monitored during hypoxia by an investigator (Yogendra Raol) and the occurrences of behavioral seizures were noted manually. The behavioral seizures (the rats were not implanted with electrodes to record EEG) consisted of head shakes, and clonic and tonic limb movements.
Electroclinical seizures
At P10, the rats were connected to an EEG monitoring unit (Stellate Harmonie system, Natus Medical, San Carlos, CA) to record EEG signals time-locked with digital video. The EEG signal was digitized at 1000 Hz and stored on a hard disk for offline analysis. Following a 20 to 30 minutes of baseline recording, the pups were given either vehicle or 35 mg/kg flupirtine by i.p. injection. After 15 minutes of video-EEG recording following the treatment, the pups were exposed to graded hypoxia. The pups were continuously monitored by video-EEG during the hypoxia. The video-EEG records were reviewed by a board certified clinical epileptologist (Andrew White) who was blinded to the treatment paradigm. Electroclinical seizures were defined by an EEG pattern that differed from background in either amplitude, frequency, or both, evolved over time, and contained spikes or sharp waves lasting for 10 s or more and were associated with a change in the rat's behavior. Electrographic seizures were defined as seizures observed in the EEG record that were not associated with a behavioral correlate on video.
2.5. Statistical analysis
GraphPad Prism statistical software (GraphPad Software Inc., San Diego, CA) was used for statistical analysis. Fisher's exact test and the Mann Whitney test were used to determine the statistical significance of effects of flupirtine treatment on the frequency of rats developing seizures, and the amount of time spent in electroclinical seizures, respectively during hypoxia. P values ≤ 0.05 were considered statistically significant.
3. Results
3.1. Flupirtine blocks development of behavioral seizures during hypoxia
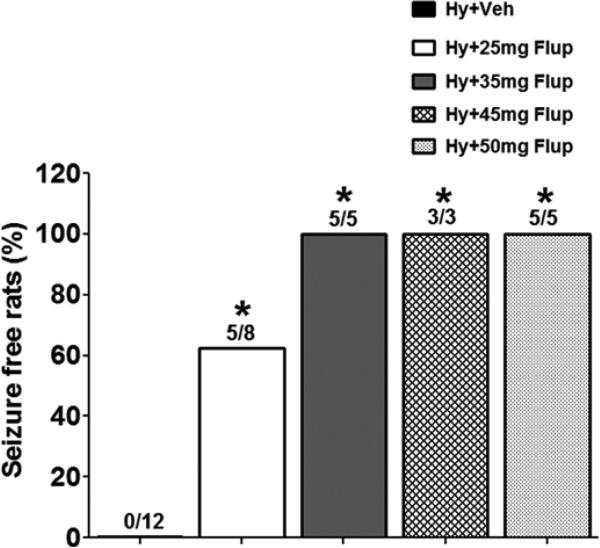
To study the effect of flupirtine on hypoxia-induced seizures, the P10 rats were given various doses of the drug or vehicle 15-30 minutes before exposure to hypoxia. All of the pups that were treated with vehicle (n = 12; males (M) = 7, females (F) = 5) developed behavioral seizures during hypoxia (Figure 1). The behavioral seizures consisted of head shakes, and clonic and tonic limb movements. Five out of eight pups (n = 8; 3M, 5F) treated with 25 mg/kg flupirtine did not exhibit any type of behavioral seizure; the remaining three rats developed head shakes during hypoxia (Figure 1). None of the rats treated with 35 (n = 5; 3M, 2F), 45 (n = 3; 2M, 1F) or 50 (n = 5; 3M, 2F) mg/kg flupirtine developed behavioral seizures during exposure to the graded hypoxia (Figure 1).
Figure 1.
Flupirtine pretreatment effectively blocks hypoxia-induced behavioral seizures. All the P10 rat pups pretreated with 50 (5/5), 45 (3/3) or 35 (5/5) mg/kg flupirtine that were exposed to graded global hypoxia remained seizure free, i.e., none of them developed behavioral seizures during hypoxia. None of the rats pretreated with vehicle (12/12) and 60% rats pretreated with 25mg/kg flupirtine (5/8) remained seizure free. Statistical comparisons were made individually between the vehicle treated group and each of the flupirtine treated groups. *p<0.005, Fisher's exact test. Hy = Hypoxia, Veh = Vehicle, Flup = Flupirtine.
3.2. Flupirtine prevents development of electroclinical seizures during hypoxia
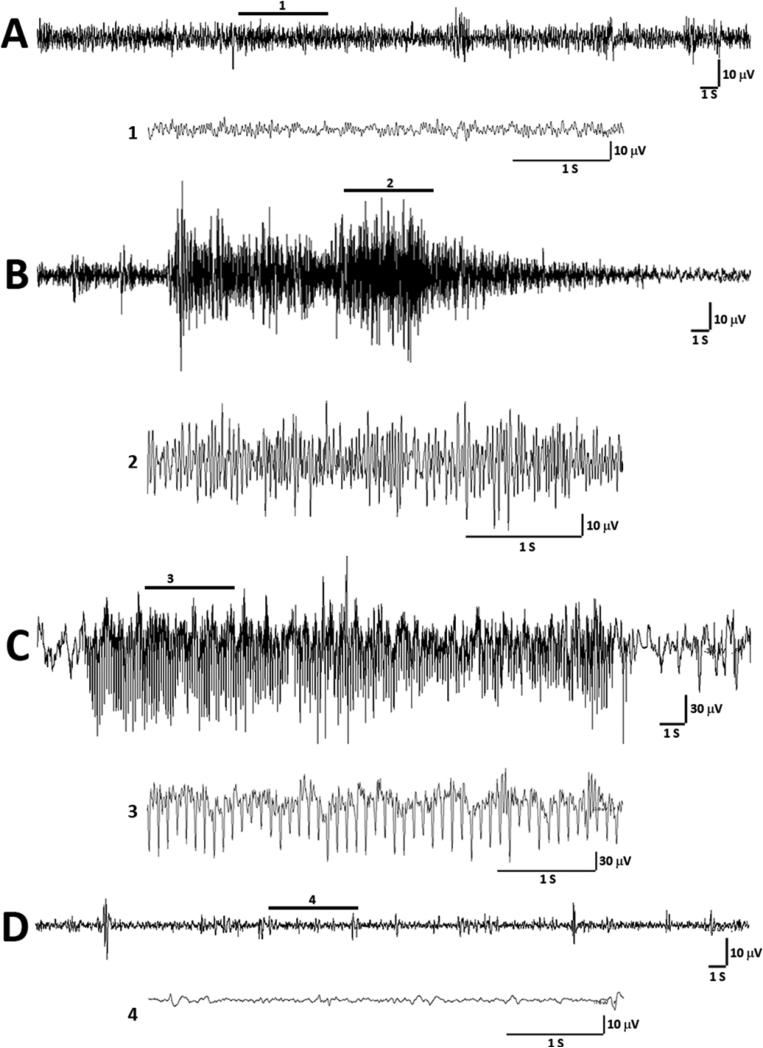
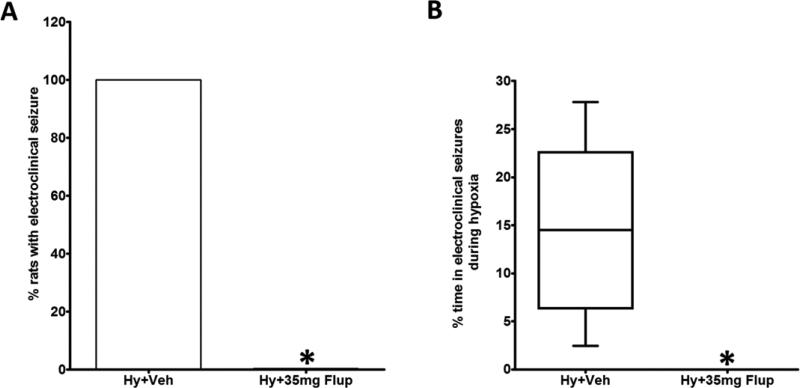
In neonates there is often dissociation between clinical (behavioral) seizures and EEG phenomena, known as electroclinical uncoupling [19]. Because of this phenomenon, some drugs effectively treat the behavioral component of the neonatal seizure without stopping the corresponding electrographic seizure. To determine the effects of flupirtine on electroclinical seizures (behavioral seizures with an EEG correlate), the P10 rats were treated with either 35 mg/kg flupirtine, the lowest dose that prevented behavioral seizures in the maximum number of rats in the current study (Figure 1), or vehicle 15 minutes before hypoxia. All vehicle treated rats experienced electroclinical seizures (Figure 2B) at varying frequency (n = 6; 4F, 2M; range = 1 – 10; mean ± SD = 0.33 ± 0.18 seizures/minute of hypoxia) during hypoxia that lasted on average for 19 minutes. Purely electrographic seizures (EEG seizures without a behavioral correlate; Figure 2C) were observed in three out of six vehicle treated rats during hypoxia (range = 0 – 6; mean ± SD = 0.076 ± 0.13 EEG only seizures/minute of hypoxia). The mean total duration of these electrographic seizures was 56.83 seconds (± SD = 128 seconds). None of the 35 mg/kg flupirtine treated rats (n = 6; 3F, 3M) developed electroclinical seizures during hypoxia (Figure 3A, the average hypoxia duration was 18.2 minutes). In contrast, vehicle treated rats were seizing on an average for 14.61% time (calculated as (time in seizure × 100) ÷ total duration of hypoxia) they were in hypoxic environment (Figure 3B). Further, none of the flupirtine treated rats developed purely electrographic seizures during hypoxia. A Behavioral seizure, as defined in the Materials and Methods Section, without an electrographic correlate was not observed in any rats.
Figure 2.
Representative EEG tracings from P10 rats during exposure to graded hypoxia. (A) The tracing shows an example of inter-ictal EEG during hypoxia. A magnified excerpt of a part of the EEG, marked by a bar above the tracing (1), is provided under the compressed EEG tracing. (B) Electroclinical seizure during the hypoxia. The EEG ictal activity was associated with a brief clonus and tonic extension of hindlimbs. The enlarged excerpt of a part of the EEG during tonic component of the behavioral seizure activity (2) is provided under the compressed EEG tracing. (C) The representative EEG from a different rat shows purely electrographic seizure (no behavioral component associated) during the hypoxia. The magnified part of the electrographic seizure (3) shows synchronized spike activity during the seizure. (D) The representative tracing shows EEG during hypoxia from a rat treated with 35 mg/kg flupirtine. The magnified excerpt (4) shows very low voltage background activity. S = second, V = volts.
Figure 3.
Flupirtine pretreatment effectively blocks hypoxia-induced electroclinical seizures. (A) All the P10 rat pups treated with vehicle 15 minutes before exposure to hypoxic environment developed electroclinical seizures during hypoxia. In contrast none of the rats treated with 35 mg/kg of flupirtine exhibited electroclinical seizures. (B) The box and whisker plot shows the percent of time spent by the rats treated with vehicle or 35 mg/kg of flupirtine in electroclinical seizures during hypoxia. n = 6 for both vehicle and flupirtine treatment groups. *Statistically different from vehicle treated rats exposed to hypoxia. *P<0.005, Fisher's exact test for the data presented in A, and Mann Whitney test for the data presented in B was used to determine the statistical significance. Hy = Hypoxia, Veh = Vehicle, Flup = Flupirtine.
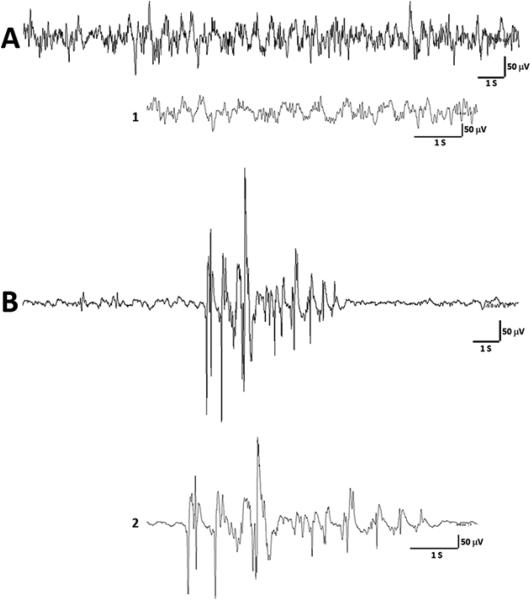
Flupirtine treatment in rats (given before hypoxia) resulted in visually obvious suppression in EEG activities that was interspersed with burst-like activity (Figure 4B). These burst suppression-like patterns appeared within seconds of flupirtine injection. The burst-like activity lasted on an average for 5 seconds, occurred approximately every 15 seconds, and continued to appear even during hypoxia. The burst suppression-like pattern appeared similar to what has been observed with the use of anesthetics such as isoflurane [1, 14], and high doses of anticonvulsants [27].
Figure 4.
Representative EEG tracings from a P10 rat injected with flupirtine. (A) The tracing shows baseline EEG obtained before the rat was injected with a drug and exposed to hypoxia. A magnified trace of EEG (1) is provided under the compact EEG tracing for a better clarity. (B) The EEG shows a burst suppression-like pattern following flupirtine (35 mg/kg) treatment that appeared before the rat was exposed to hypoxia. The EEG trace at the slower time scale (2) provides a magnified view of the burst suppression-like pattern. S = second, V = volts.
4. Discussion
The current study demonstrates that flupirtine, a potassium channel opener, effectively blocks hypoxia-induced neonatal seizures. In neonates it is often observed that some drugs effectively treat the behavioral (clinical) component of the seizure without stopping the electrographic discharge [19]. Using time-locked video-EEG recording technique we show that flupirtine not only prevents the occurrence of behavioral seizures during hypoxia, it also treats the electrographic (subclinical) component. Further, unlike 50% of vehicle treated rats that developed purely electrographic seizures (at very low frequency), none of the flupirtine treated rats experienced such seizures. These observations are in agreement with the results of our previous study, wherein flupirtine effectively blocked chemoconvulsant-induced neonatal seizures [37]. Another study has shown that pre-administration of flupirtine increases the latency to first seizure, and reduces the duration and severity of febrile seizures [43].
Flupirtine shifts the voltage dependent activation of KCNQ channels towards hyperpolarized potentials [28, 42] resulting in an increased threshold for generating neuronal action potentials. Recent studies suggest that flupirtine also shifts the gating of GABAA receptors to lower GABA concentrations [22, 23]. KCNQ channels (KCNQ1-5) are voltage-gated, depolarization activated potassium channels and are expressed in the nervous system [8, 20]. Mutations in KCNQ2/3 channels have been associated with benign familial neonatal seizures, a syndrome in which seizures resolve spontaneously within a few weeks after the onset, and KCNQ2 encephalopathy, a type of epilepsy in which seizures are usually pharmacoresistant and in which patients have an epileptic encephalopathy with moderate to severe intellectual disability. Certain mutations observed in patients with KCNQ2 encephalopathy result in an increase in threshold potential for the channel opening, slowing of the activation kinetics, and reduction in current amplitude [34]. Retigabine, an analogue of flupirtine, shifted the voltage activation of mutated KCNQ2 channels to more hyperpolarized potentials, and also increased the current amplitude [34]. Retigabine has been approved by the United States Food and Drug Administration (FDA) for the treatment of partial epilepsies in adult patients. However, retigabine has been associated with side effects such as blue discoloration in the skin and retinal abnormalities. Since flupirtine has a similar mechanism of action on KCNQ channels as retigabine, we believe that flupirtine will also effectively treat seizures observed in infants with KCNQ2 encephalopathy.
Flupirtine was first approved for use as a non-opioid analgesic in Europe in the 1980's. In the United States, flupirtine has not been approved for any indication by the FDA. Flupirtine is usually associated with mild side-effects, such as dizziness, dry mouth, nausea, pruritis, fatigue, and heartburn. In recent years, however, concerns have been raised regarding association of long-term use of flupirtine and hepatotoxicity [13, 30]. In the present study, we observed that 35 mg/kg of flupirtine induced a burst suppression-like EEG pattern in the normal rats. Burst suppression is an EEG pattern in which high voltage activity alternates with the periods of severely attenuated EEG, and is often observed with deep levels of anesthesia or high doses of anticonvulsants such as phenobarbital [1, 14, 27]. In fact, induction of the burst suppression pattern by intravenous volatile anesthetics has been used in clinics to terminate refractory status epilepticus. Sedatives and anesthetics that severely reduce neuronal activity have been shown to cause apoptosis and behavioral impairments in later life in normal animals [40], however, in animal models of neonatal HIE, anesthetics such as isoflurane reduce brain injury and improve neurological outcome [9].
One of the limitations of the current study is the use of a protocol in which the animal is given the drug prior to the insult. In the case of a hypoxic-ischemic incident in a neonate, it is likely that the drug would have to be administered subsequent to the insult. There are other instances, such as during infant cardiac surgery, where prophylactic drugs could be given to reduce occurrence of seizures and brain injury. EEG seizures have been observed in 14 to 20% neonates following cardiac surgery with cardiopulmonary bypass [16, 32], and the use of benzodiazepines during the surgery may reduce seizure occurrence [2]. Further, this study provides a strong proof that flupirtine effectively treats hypoxia-induced neonatal seizures, and hence further studies should be carried out to determine its efficacy when given after the injury or insult has occurred, for example, during the reperfusion period following HI.
5. Conclusion
Our data clearly suggest that flupirtine is effective in treating hypoxia-induced neonatal seizures. Studies to determine the therapeutic window within which flupirtine can stop hypoxiaischemia induced neonatal seizures and the comparison of its efficacy with that of phenobarbital are currently underway in our laboratory. Further, it will be both interesting and important to study the effects of treatment of neonatal seizures on hypoxia-ischemia induced brain injury and adverse neurodevelopmental outcome.
HIGHLIGHTS.
We examined efficacy of flupirtine to treat hypoxia-induced neonatal seizures.
Flupirtine blocks development of behavioral seizures during hypoxia.
Flupirtine treats clinical seizures and associated epileptiform activity.
Acknowledgements
This research was supported by CCTSI Child and Maternal Health pilot grant (NIH/NCRR Colorado CTSI Grant Number UL1 RR025780; YHR), Epilepsy Foundation of America Award (YHR), NICHD R01 HD065534 grant (YHR), and CURE Prevention of Acquired Epilepsy award (YHR). We thank the University of Colorado Anschutz Medical Campus Rodent In Vivo Neurophysiology Core for providing facilities to acquire and review video-EEG data. We thank Dr. Michael Hall and the Neuroscience Core Machine Shop for help with the construction of hypoxia chamber. We also thank Dr. Frances Jensen for help with graded hypoxia model, and Dr. Amy Brooks-Kayal for providing critical feedback during the development of this project. The funders had no role in study design, data collection and analysis, in the writing of the manuscript, and in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of conflicts of interest
None of the authors have any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Amzica F. Basic physiology of burst-suppression. Epilepsia. 2009;50(Suppl 12):38–39. doi: 10.1111/j.1528-1167.2009.02345.x. [DOI] [PubMed] [Google Scholar]
- 2.Andropoulos DB, Mizrahi EM, Hrachovy RA, Stayer SA, Stark AR, Heinle JS, McKenzie ED, Dickerson HA, Meador MR, Fraser CD., Jr. Electroencephalographic seizures after neonatal cardiac surgery with high-flow cardiopulmonary bypass. Anesth Analg. 2010;110:1680–1685. doi: 10.1213/ANE.0b013e3181dd5a58. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 4.Bergamasco B, Benna P, Ferrero P, Gavinelli R. Neonatal hypoxia and epileptic risk: a clinical prospective study. Epilepsia. 1984;25:131–136. doi: 10.1111/j.1528-1157.1984.tb04168.x. [DOI] [PubMed] [Google Scholar]
- 5.Bjorkman ST, Miller SM, Rose SE, Burke C, Colditz PB. Seizures are associated with brain injury severity in a neonatal model of hypoxia-ischemia. Neuroscience. 2010;166:157–167. doi: 10.1016/j.neuroscience.2009.11.067. [DOI] [PubMed] [Google Scholar]
- 6.Blume HK, Garrison MM, Christakis DA. Neonatal seizures: treatment and treatment variability in 31 United States pediatric hospitals. J Child Neurol. 2009;24:148–154. doi: 10.1177/0883073808321056. [DOI] [PubMed] [Google Scholar]
- 7.Brooks-Kayal AR, Pritchett DB. Developmental changes in human gamma-aminobutyric acidA receptor subunit composition. Ann Neurol. 1993;34:687–693. doi: 10.1002/ana.410340511. [DOI] [PubMed] [Google Scholar]
- 8.Brown DA, Passmore GM. Neural KCNQ (Kv7) channels. Br J Pharmacol. 2009;156:1185–1195. doi: 10.1111/j.1476-5381.2009.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burchell SR, Dixon BJ, Tang J, Zhang JH. Isoflurane provides neuroprotection in neonatal hypoxic ischemic brain injury. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. 2013;61:1078–1083. doi: 10.231/JIM.0b013e3182a07921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carmo KB, Barr P. Drug treatment of neonatal seizures by neonatologists and paediatric neurologists. J Paediatr Child Health. 2005;41:313–316. doi: 10.1111/j.1440-1754.2005.00638.x. [DOI] [PubMed] [Google Scholar]
- 11.de Vries LS, Jongmans MJ. Long-term outcome after neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2010;95:F220–224. doi: 10.1136/adc.2008.148205. [DOI] [PubMed] [Google Scholar]
- 12.Dhar S, Bitting RL, Rylova SN, Jansen PJ, Lockhart E, Koeberl DD, Amalfitano A, Boustany RM. Flupirtine blocks apoptosis in batten patient lymphoblasts and in human postmitotic CLN3- and CLN2-deficient neurons. Ann Neurol. 2002;51:448–466. doi: 10.1002/ana.10143. [DOI] [PubMed] [Google Scholar]
- 13.Douros A, Bronder E, Andersohn F, Klimpel A, Thomae M, Orzechowski HD, Kreutz R, Garbe E. Flupirtine-induced liver injury--seven cases from the Berlin Case-control Surveillance Study and review of the German spontaneous adverse drug reaction reporting database. European journal of clinical pharmacology. 2014;70:453–459. doi: 10.1007/s00228-013-1631-4. [DOI] [PubMed] [Google Scholar]
- 14.Ferron JF, Kroeger D, Chever O, Amzica F. Cortical inhibition during burst suppression induced with isoflurane anesthesia. J Neurosci. 2009;29:9850–9860. doi: 10.1523/JNEUROSCI.5176-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster M, Lewis A. The treatment of neonatal seizures: a critical review of the evidence. Neonatal Paediatr Child Health Nurs. 2007;10:11–19. [Google Scholar]
- 16.Gaynor JW, Nicolson SC, Jarvik GP, Wernovsky G, Montenegro LM, Burnham NB, Hartman DM, Louie A, Spray TL, Clancy RR. Increasing duration of deep hypothermic circulatory arrest is associated with an increased incidence of postoperative electroencephalographic seizures. J Thorac Cardiovasc Surg. 2005;130:1278–1286. doi: 10.1016/j.jtcvs.2005.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibbs JW, 3rd, Schroder GB, Coulter DA. GABAA receptor function in developing rat thalamic reticular neurons: whole cell recordings of GABA-mediated currents and modulation by clonazepam. J Neurophysiol. 1996;76:2568–2579. doi: 10.1152/jn.1996.76.4.2568. [DOI] [PubMed] [Google Scholar]
- 18.Glass HC, Glidden D, Jeremy RJ, Barkovich AJ, Ferriero DM, Miller SP. Clinical Neonatal Seizures are Independently Associated with Outcome in Infants at Risk for Hypoxic-Ischemic Brain Injury. J Pediatr. 2009;155:318–323. doi: 10.1016/j.jpeds.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glykys J, Dzhala VI, Kuchibhotla KV, Feng G, Kuner T, Augustine G, Bacskai BJ, Staley KJ. Differences in cortical versus subcortical GABAergic signaling: a candidate mechanism of electroclinical uncoupling of neonatal seizures. Neuron. 2009;63:657–672. doi: 10.1016/j.neuron.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldman AM, Glasscock E, Yoo J, Chen TT, Klassen TL, Noebels JL. Arrhythmia in heart and brain: KCNQ1 mutations link epilepsy and sudden unexplained death. Science translational medicine. 2009;1:2ra6. doi: 10.1126/scitranslmed.3000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kapur J, Macdonald RL. Postnatal development of hippocampal dentate granule cell γ-aminobutyric acidA receptor pharmacological properties. Mol Pharmacol. 1999;55:444–452. [PubMed] [Google Scholar]
- 22.Klinger F, Bajric M, Salzer I, Dorostkar MM, Khan D, Pollak DD, Kubista H, Boehm S, Koenig X. δ subunit-containing GABA-A receptors are preferred targets for the centrally acting analgesic flupirtine. Br J Pharmacol. 2015 doi: 10.1111/bph.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klinger F, Geier P, Dorostkar MM, Chandaka GK, Yousuf A, Salzer I, Kubista H, Boehm S. Concomitant facilitation of GABAA receptors and KV7 channels by the nonopioid analgesic flupirtine. Br J Pharmacol. 2012;166:1631–1642. doi: 10.1111/j.1476-5381.2011.01821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koh S, Tibayan FD, Simpson JN, Jensen FE. NBQX or topiramate treatment after perinatal hypoxia-induced seizures prevents later increases in seizure-induced neuronal injury. Epilepsia. 2004;45:569–575. doi: 10.1111/j.0013-9580.2004.69103.x. [DOI] [PubMed] [Google Scholar]
- 25.Kwon JM, Guillet R, Shankaran S, Laptook AR, McDonald SA, Ehrenkranz RA, Tyson JE, O'Shea TM, Goldberg RN, Donovan EF, Fanaroff AA, Poole WK, Higgins RD, Walsh MC. Clinical seizures in neonatal hypoxic-ischemic encephalopathy have no independent impact on neurodevelopmental outcome: secondary analyses of data from the neonatal research network hypothermia trial. J Child Neurol. 2011;26:322–328. doi: 10.1177/0883073810380915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Legido A, Clancy RR, Berman PH. Neurologic outcome after electroencephalographically proven neonatal seizures. Pediatrics. 1991;88:583–596. [PubMed] [Google Scholar]
- 27.Lin JJ, Lin KL, Wang HS, Hsia SH, Wu CT. Effect of topiramate, in combination with lidocaine, and phenobarbital, in acute encephalitis with refractory repetitive partial seizures. Brain & development. 2009;31:605–611. doi: 10.1016/j.braindev.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Martire M, Castaldo P, D'Amico M, Preziosi P, Annunziato L, Taglialatela M. M channels containing KCNQ2 subunits modulate norepinephrine, aspartate, and GABA release from hippocampal nerve terminals. J Neurosci. 2004;24:592–597. doi: 10.1523/JNEUROSCI.3143-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McBride MC, Laroia N, Guillet R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology. 2000;55:506–513. doi: 10.1212/wnl.55.4.506. [DOI] [PubMed] [Google Scholar]
- 30.Michel MC, Radziszewski P, Falconer C, Marschall-Kehrel D, Blot K. Unexpected frequent hepatotoxicity of a prescription drug, flupirtine, marketed for about 30 years. Br J Clin Pharmacol. 2012;73:821–825. doi: 10.1111/j.1365-2125.2011.04138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller SP, Weiss J, Barnwell A, Ferriero DM, Latal-Hajnal B, Ferrer-Rogers A, Newton N, Partridge JC, Glidden DV, Vigneron DB, Barkovich AJ. Seizure-associated brain injury in term newborns with perinatal asphyxia. Neurology. 2002;58:542–548. doi: 10.1212/wnl.58.4.542. [DOI] [PubMed] [Google Scholar]
- 32.Newburger JW, Jonas RA, Wernovsky G, Wypij D, Hickey PR, Kuban KC, Farrell DM, Holmes GL, Helmers SL, Constantinou J, Carrazana E, Barlow JK, Walsh AZ, Lucius KC, Share JC, Wessel DL, Hanley FL, Mayer JE, Costaneda AR, Ware JH, et al. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. N Engl J Med. 1993;329:1057–1064. doi: 10.1056/NEJM199310073291501. [DOI] [PubMed] [Google Scholar]
- 33.Okada M, Zhu G, Hirose S, Ito KI, Murakami T, Wakui M, Kaneko S. Age-dependent modulation of hippocampal excitability by KCNQ-channels. Epilepsy Res. 2003;53:81–94. doi: 10.1016/s0920-1211(02)00249-8. [DOI] [PubMed] [Google Scholar]
- 34.Orhan G, Bock M, Schepers D, Ilina EI, Reichel SN, Loffler H, Jezutkovic N, Weckhuysen S, Mandelstam S, Suls A, Danker T, Guenther E, Scheffer IE, De Jonghe P, Lerche H, Maljevic S. Dominant-negative effects of KCNQ2 mutations are associated with epileptic encephalopathy. Ann Neurol. 2014;75:382–394. doi: 10.1002/ana.24080. [DOI] [PubMed] [Google Scholar]
- 35.Pena F, Alavez-Perez N. Epileptiform activity induced by pharmacologic reduction of M-current in the developing hippocampus in vitro. Epilepsia. 2006;47:47–54. doi: 10.1111/j.1528-1167.2006.00369.x. [DOI] [PubMed] [Google Scholar]
- 36.Peters HC, Hu H, Pongs O, Storm JF, Isbrandt D. Conditional transgenic suppression of M channels in mouse brain reveals functions in neuronal excitability, resonance and behavior. Nat Neurosci. 2005;8:51–60. doi: 10.1038/nn1375. [DOI] [PubMed] [Google Scholar]
- 37.Raol YH, Lapides DA, Keating JG, Brooks-Kayal AR, Cooper EC. A KCNQ channel opener for experimental neonatal seizures and status epilepticus. Ann Neurol. 2009;65:326–336. doi: 10.1002/ana.21593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ronen GM, Buckley D, Penney S, Streiner DL. Long-term prognosis in children with neonatal seizures: a population-based study. Neurology. 2007;69:1816–1822. doi: 10.1212/01.wnl.0000279335.85797.2c. [DOI] [PubMed] [Google Scholar]
- 39.Singh NA, Westenskow P, Charlier C, Pappas C, Leslie J, Dillon J, Anderson VE, Sanguinetti MC, Leppert MF. KCNQ2 and KCNQ3 potassium channel genes in benign familial neonatal convulsions: expansion of the functional and mutation spectrum. Brain. 2003;126:2726–2737. doi: 10.1093/brain/awg286. [DOI] [PubMed] [Google Scholar]
- 40.Stratmann G, Sall JW, May LD, Loepke AW, Lee MT. Beyond anesthetic properties: the effects of isoflurane on brain cell death, neurogenesis, and long-term neurocognitive function. Anesth Analg. 2010;110:431–437. doi: 10.1213/ANE.0b013e3181af8015. [DOI] [PubMed] [Google Scholar]
- 41.Towfighi J, Housman C, Mauger D, Vannucci RC. Effect of seizures on cerebral hypoxic-ischemic lesions in immature rats. Brain Res Dev Brain Res. 1999;113:83–95. doi: 10.1016/s0165-3806(99)00004-8. [DOI] [PubMed] [Google Scholar]
- 42.Wladyka CL, Kunze DL. KCNQ/M-currents contribute to the resting membrane potential in rat visceral sensory neurons. J Physiol. 2006;575:175–189. doi: 10.1113/jphysiol.2006.113308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu F, Liu Y, Wang Y, Yin J, Wang H, Liu W, Peng B, He X. Protective effect of the KCNQ activator flupirtine on a model of repetitive febrile seizures. Epilepsy Res. 2011;97:64–72. doi: 10.1016/j.eplepsyres.2011.07.005. [DOI] [PubMed] [Google Scholar]