Abstract
Introduction
The motor symptoms of Parkinson's disease (PD) present with pathological neuronal activity in the basal ganglia. Although neuronal firing rate changes in the globus pallidus internus (GPi) and externus (GPe) are reported to underlie the development of PD motor signs, firing rates change inconsistently, vary confoundingly with some therapies, and are poor indicators of symptom severity.
Methods
We explored the relationship between parkinsonian symptom severity and the effectiveness with which pallidal neurons transmit information. We quantify neuronal entropy and information – alternatives to firing rate and correlations respectively – in and between GPe and GPi neurons using a progressive, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, non-human primate model of PD.
Results
Neuronal entropy and symptom severity were not linearly correlated: in both pallidal segments, entropy increased from naive to moderate parkinsonism, but decreased with further progression to the severely parkinsonian condition. In contrast, information transmitted from GPe to GPi increased consistently with symptom severity. Furthermore, antidromic information from GPi to GPe increased substantially with symptom severity. Together, these findings suggest that as parkinsonian severity increases, more and more information enters GPe and GPi from common sources, diminishing the relative importance of the orthodromic GPe to GPi connection.
Conclusions
With parkinsonian progression, the direct and indirect pathways lose their independence and start to convey redundant information. We hypothesize that a loss of parallel processing impairs the ability of the network to select and implement motor commands, thus promoting the hypokinetic symptoms of PD.
Keywords: Basal Ganglia, Globus Pallidus, Parkinson's disease, Neural Information
Introduction
The motor symptoms of Parkinson’s disease (PD) are hypothesized to correlate with changes in neural activity that results from the progressive degeneration of dopamine-producing neurons in the basal ganglia. The nature of these electrophysiological changes however, and how they relate to motor symptom severity, is poorly understood. In the present study, we quantify these electrophysiological changes with respect to the information transmitted between neurons in two nuclei: the globus pallidus internus (GPi) and externus (GPe). Extracellular unit activity was recorded from a progressive, non-human primate (NHP) model of PD at increasing levels of parkinsonian severity through staged, serial exposure to the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). We then quantified the relationship between symptom severity and information transmission between GPe and GPi.
Basal ganglia efferents project from GPi to the pallidal receiving area of motor thalamus with GABAergic, inhibitory connections (Fig. S1). The standard rate model hypothesized that these inhibitory efferents are hyperactive in the parkinsonian state, over-inhibiting thalamic neurons, and thus suppressing movements [1]. This model has been refined to posit that changes in firing rate are less consequential than changes in firing patterns; increases in oscillatory patterning [2,3], neuronal bursting [2,4,5], and spike-train irregularity [4,6] all correlate with parkinsonian symptom severity. Supporting this refinement, therapeutic deep brain stimulation (DBS) of the subthalamic nucleus (STN) or GPi exacerbates PD-associated firing rate changes [7,8], but reverses PD-associated increases in oscillatory [9,10], bursty [7,11], and irregular [8,12] firing patterns.
Thus, some characteristics of neuronal firing patterns co-vary with parkinsonian symptom severity. In previous work we have summarized these characteristics with neuronal entropy [13], an optimal measure of firing pattern disorder that bounds the information transmitted in a spike train. Information processing in basal ganglia relies on changes in pallidal entropy, which is less than expected from the wide range of inter-spike intervals [14], and decreases with therapeutically effective high-frequency DBS but increases with therapeutically ineffective low-frequency DBS [15]. From a rodent model of PD, we reported that neuronal entropy in globus pallidus and substantia nigra pars reticulata increases with parkinsonian onset, and decreases with therapeutic DBS of the STN [6]. These prior efforts compared entropy between discrete cases: PD or no PD, DBS or no DBS. In the present work, we take advantage of a progressive model of PD to ascertain how firing pattern entropy varies with parkinsonian motor severity.
While firing pattern entropy may be an esoteric metric, the information transmitted between neurons is a concrete concept that might frame our understanding of how impaired neural processing drives neurological symptoms. Work in a rodent model reports that information from STN to GPe increases with parkinsonism onset [16], suggesting that excess information through the basal ganglia correlates with symptoms. Computational studies support this finding, suggesting that low-entropy pallidal activity improves information processing in thalamic neurons [17].
For the present work, we recorded neural activity simultaneously in GPe and GPi, from an NHP progressive model of PD. In a recent study on the same animals, we explored the relationship between oscillatory activities in particular bandwidths and severity of disease [18]. We observed the presence of beta activity in normal animals with no consistent relationship in the incidence of such activity to the disease severity. However, an emergence of coupling between the phase of beta and the amplitude of high frequency oscillations (256–362 Hz) in the mild state suggested a significant relationship between the phase-amplitude coupling and disease severity. We concluded that rather than the emergence of oscillatory activity in one frequency spectrum or the other, parkinsonian motor signs may relate more to the development of altered coupling across multiple frequency bands [18].
In the present study, we report that directed information increased from neurons in GPe to those in GPi with parkinsonian severity, but also increased in the antidromic direction from neurons in GPi to those in GPe. This severity-dependent increased functional connectivity between neurons is consistent with the previously observed severity-dependent increased phase-amplitude coupling of the local field activity. Further, bi-directional increases in information may derive from increasingly redundant signals arriving via common inputs from STN or the striatum.
Methods
Analyses were performed on data collected during previously published experiments [18]. Surgical and experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Minnesota, and complied with United States Public Health Service policy on care and use of laboratory animals.
Data Acquisition
Briefly, data were collected from two rhesus monkeys (female, 5.2 kg; male, 9.4 kg) acclimated to the laboratory environment and to passive manipulation of the limbs for determination of neuronal receptive fields. Motor severity was assessed twice per week with a modified UPDRS that was used to rate rigidity, bradykinesia, akinesia, and tremor of the upper and lower extremities bilaterally, as well as six global motor features of parkinsonism (i.e., gait, posture, balance, turning, defense reaction and food retrieval). Scoring for each feature (0–3) was performed during observation of spontaneous behavior, investigator interaction, and passive limb manipulation. The maximum possible score was 42; composite scores within the ranges of 3–13, 18–28, and 32–42 were used to define the mild, moderate, and severe parkinsonian conditions, respectively. Once data collection was complete for the control condition, each animal was made progressively hemiparkinsonian through staged, unilateral intracarotid injections of the neurotoxin MPTP (0.2–0.8 mg/kg, 1 mg/ml solution, 15-minute infusion) using aseptic surgical procedures under isoflurane anesthesia.
Paired, extracellular recordings were made from the GPi and GPe using epoxylite-insulated tungsten microelectrodes (~1.0 MΩ at 1 kHz; FHC Inc., Bowdoin, ME USA) lowered through permanently implanted cephalic chambers on the awake, head-fixed subjects [18]. Neuronal activity was transduced acoustically, allowing on-line evaluation of isolated neurons for somatosensory responsiveness through passive limb manipulation. Each recording lasted at least 30 seconds, with data amplified (x10,000), filtered (0.3–6.0 kHz), and digitized (25 kHz) for offline analysis. Neural activity was segmented into single-unit spikes with a supervised valley-seek algorithm in Offline Sorter (Plexon Inc., Dallas TX). Spike times for each unit were stored for subsequent information analyses. In control, mild, moderate, and severe conditions, we analyzed data from 211, 78, 42 and 61 neurons in GPe, and 47, 40, 30 and 55 neurons in GPi, respectively. Subsequent to all recordings, electrode locations were confirmed histologically.
Data Analyses
Information theoretic and statistical analyses were performed in MATLAB (Mathworks, Natick MA).
Firing pattern entropy was calculated from the distribution of inter-spike intervals (ISIs) [19,20], and binned logarithmically to help differentiate categorically distinct patterns [13,15], e.g., bursting from tonic firing [21]. In short, ISI probability distributions were generated by rounding ISIs into bins of logarithmic time divided into 5 bins per decade. Maximum likelihood estimates of the entropy, in bits per spike, were calculated for each neuron y as: Hy = − Σi P (ISIi) log2 P(ISIi), where the sum is over all ISI bins. Neither increasing the dimension to ISI pairs nor decreasing the bin width affected the qualitative results. We restrict our presented results to the one dimensional, 5 bin per decade case, to ensure enough data for reliable estimates of information from all cell pairs (Fig. 1).
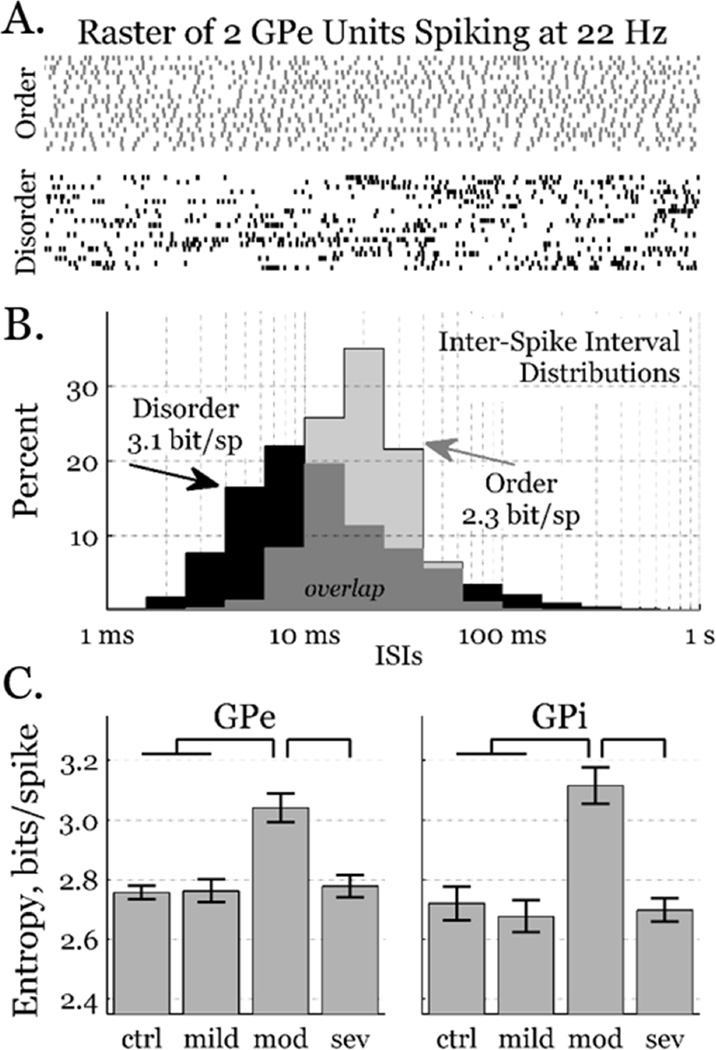
Figure 1.
Firing pattern entropy. A) Rastergrams depict twenty consecutive 1.0 s intervals of two GPe neurons with the same firing rate. Although they both spike at ~22 Hz, the grey spike pattern is fairly ordered, whereas the black spike pattern is highly disordered with many high-frequency bursts and low-frequency pauses. B) Logarithmically-binned ISI distributions from 3.0 min of the neurons depicted above. Over 80% of the ISIs from the ordered pattern (grey) are between 10 and 40 ms, yielding a firing pattern entropy of ~2.3 bits/spike. The disordered pattern (black) includes more very short (<10 ms) and very long (>60 ms) ISIs, yielding a firing pattern entropy of ~3.1 bits/spike. C) Entropy across all neurons in both GPe and GPi as a function of condition (mean±sem). Condition contributed to pattern entropy (ANOVA, p < 0.05); entropy was greater in the moderately parkinsonian condition than in more or less severely symptomatic conditions (Tukey HSD, p < 0.05).
Information was estimated from the entropy of logarithmically binned interval distributions [22]. Direct information between neuronal pairs was calculated from the conditioned entropy (Hy|x) and single unit entropy (Hy). The conditioned entropy depends on the cross-spike interval (CSI), the time between a spike in output neuron y and the most recent spike in input neuron x. For the present work: Hy|x = − Σj P(CSIj) Σi P(ISIi | CSIj) log2 P(ISIi | CSIj), where the inner sum is over all ISI bins for neuron y, and the outer sum is over the CSIs binned with the same resolution. The direct information was found as the information about the input spike train present in the output spike train: Idir = Hy – Hy|x, (Fig. 2, left).
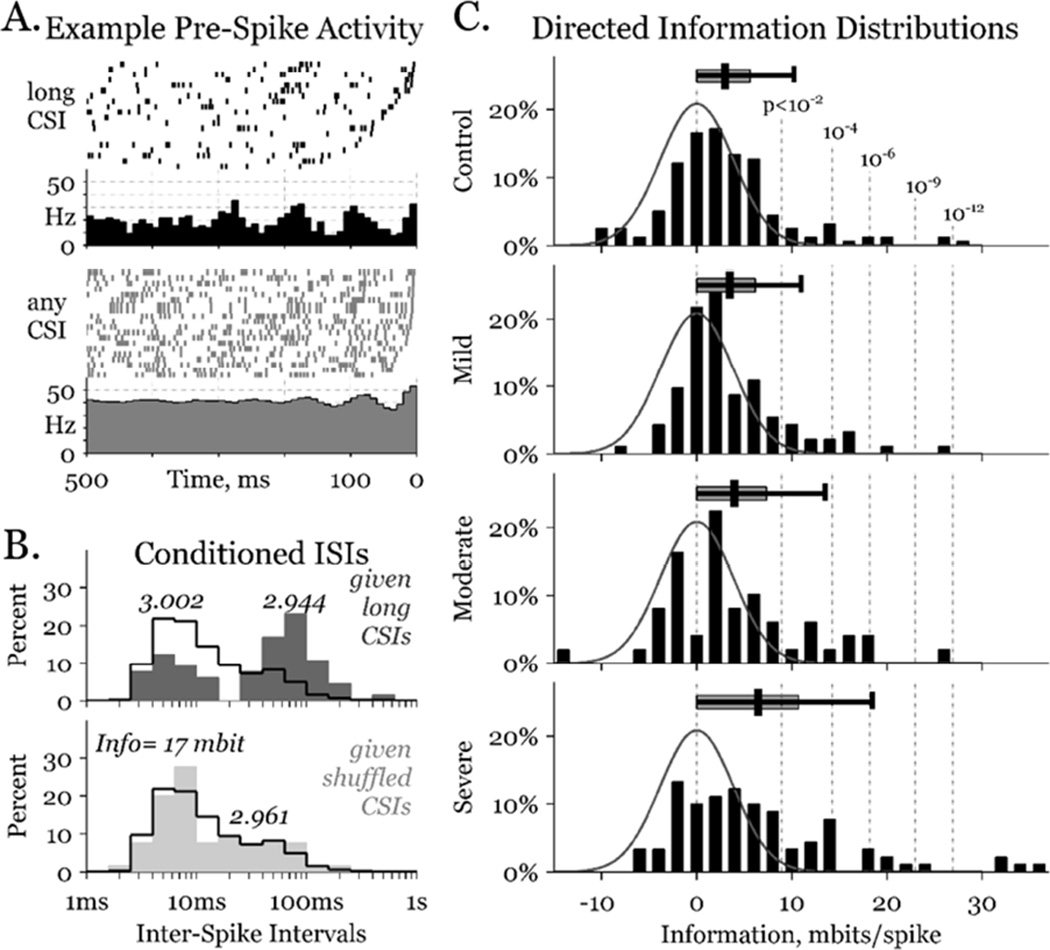
Figure 2.
Neuronal information estimates. A) Rastergrams (above) of twenty 0.5 s intervals aligned to a spike at time zero in one GPi cell, when the most recent spike in a particular GPe cell (i.e., the CSI) was 4.0–6.3 s ago (black) or chosen at random (grey). Rate histograms (below) show periodicity lasting 2–3 times longer in the long CSI case. B) ISI distributions in the long CSI (above, dark grey) and a shuffled CSI (below, light grey) cases, overlying the unconditioned distributions (black outlines). The bimodal distribution in the long CSI case carries substantial information about the CSI. Numbers above each distribution peak denote the entropy of that distribution. C) Information distributions (black bars) of all pairs – including GPe-GPe, GPe-GPi, GPi-GPe, and GPi-GPi – in each parkinsonian condition. Gaussian curves (grey line) depict the expected distribution in the truly independent, zero-information case, and are identical on all 4 graphs. Vertical dashed lines identify the probability of a given pair exceeding the corresponding information, in the zero information case. Overhanging distribution bars starts at zero, and show the median, 75th and 95th percentile distribution interval for each condition.
Inherent bias in this measure can be estimated by shuffling the CSIs and finding the entropy that would have been calculated given that redistribution of CSIs, Hy|s. We repeated this shuffling process 1000 times for each neuronal pairing, and found the expected information bias as: Ibias = Hy – <Hy|s>, where <Hy|s> is the mean value of Hy|s over all shuffles. Subtracting that bias from the direct information, we found our estimate of the true information in spike train x about spike train y: Ix→y = Idir – Ibias. While a neuron cannot transmit negative information, Ix→y may be negative; the population of pairs sharing no true information will have a mean Ix→y of zero. Thus, we fit the negative side of a zero-mean Gaussian curve to the population of negative Ix→y values. We divided each Ix→y by the standard deviation of that curve to find a standard score for each pairing, and inverted that score to the normal probability of finding such an information estimate from truly uninformative pairs (Fig. 2, right).
A one-way analysis of variance was used to test if parkinsonian condition was a factor in neuronal entropy (p<0.05), and inter-condition differences were found with the Tukey range test (p<0.05). The peculiar distributions of the information estimates – largely overlapping yet dominated by positive skewness and substantial kurtosis – make standard statistics inappropriate. We calculated boot-strapped confidence intervals for the mean information between nuclei from 10,000 repetitions for each condition. Significant differences were identified between two conditions when one yielded the greater average information in at least 95% of repetitions (p<0.05). Finally, ratios and/or proportions were fit with logistic regression across conditions, and a chi-square test was used to assess significance (p<0.05), including the fraction of significantly informative pairs, and the direction of information transmission.
Results
Behavioral states were categorized by symptom severity condition, assessed on the modified UPDRSIII, as: control, mild, moderate, or severe. From recordings of unit activity within GPe and GPi, firing pattern entropy was estimated for every neuron in each condition. Firing patterns ranged considerably, from fairly ordered (tonic firing) to fairly disordered (bursting and pausing), with entropies ranging from 2.0 to 3.5 bits/spike (Fig. 1, top). In both pallidal segments, entropy was significantly greater in the moderately parkinsonian condition than in any of the other conditions (Fig. 1, bottom).
The information that neuron x transmits to neuron y is the reduction in entropy of neuron y given the activity of neuron x. To illustrate how the activity of an arbitrary neuron x can affect the activity of arbitrary neuron y, we present rastergrams of twenty, randomly selected intervals from the same GPi neuron (y) in two cases (Fig. 2, upper-left). Individual trials are all aligned so that neuron y spikes at time zero, and all y spikes in the preceding 500 ms are depicted. In the top case, the most recent spike from neuron x was a long time ago (4.0 s < CSI < 6.3 s); in the bottom case, the trials were chosen independent of neuron x. Histograms below the rastergrams show the average firing rate for all times before each y spike in both the long CSI (N=65) and unconditioned (N>8000) cases. The unconditioned case has a mean firing rate of ~40 Hz, with weak 12–15 Hz oscillations beginning ~150 ms before a spike. In contrast, the long CSI case shows a mean firing rate of ~20 Hz, with much stronger 12–15 Hz oscillations beginning ~350 ms before a spike.
Examining the final ISIs from both cases (Fig. 2, lower-left), we discern a bimodal distribution for long CSIs (dark-grey); the most recent ISI was either ~5 ms or ~100 ms. This bimodality can be observed in the rastergrams as well, where the right-most spike either resides very close to time 0, or clusters in a broader window from ~50 to ~200 ms in the past. The unconditioned ISI distribution is shown overlapping with a black line. From these overlapping distributions, an ISI of 50–200 ms was much more likely to happen when the most recent x spike was 4.0–6.3 s ago. Thus, long ISIs in neuron y provided substantial information about recent activity in neuron x. The difference between the entropy of the unconditioned case (Hy = 3.002 bit/spike) and the mean entropy of all conditioned cases (Hy|x = 2.944 bit/spike) is the direct information estimate for this neuronal pairing, Idir = 58 mbit/spike.
However, the unconditioned case was constructed from >8000 trials, whereas the conditioned cases were constructed from substantially fewer (e.g., 65 trials in the 4.0–6.3 s case), biasing the direct information. To correct for this bias, we imposed independence by reshuffling the CSIs 1000 times, and found the mean shuffled entropy (Hy|s = 2.961 bit/spike), and the corresponding information bias (Ibias = 41 mbit/spike). The direct information minus this bias yields an estimate of the true information in neuron y about neuron x: Ix→y = 17 mbit/spike.
Information was estimated for all 396 neuronal pairs recorded simultaneously. A gaussian curve was fit to the population of negatively informative pairs, to model the standard deviation of information estimates introduced by the unbiasing procedure (Fig. 2 right, grey lines). That standard deviation was used to map the probability of finding positive information values of different size, assuming true independence (Fig. 2 right, dashed lines). The distribution of information between all pairs is plotted on the same graphs, separated by parkinsonian severity. The high information pairs appear to increase with symptom severity. Overhanging horizontal bars summarize each distribution, with the 50th, 75th and 95th percentiles (vertical black line, grey box and error-bar, respectively) trending higher with increasing parkinsonian severity.
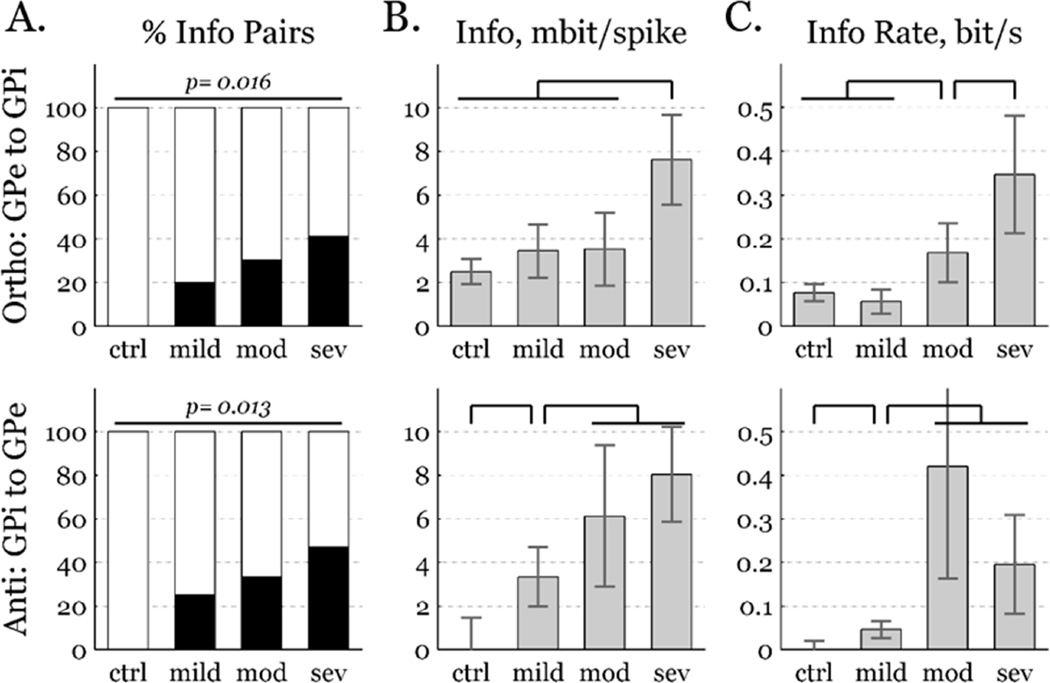
Restricting the analysis to inter-segmental pairs, information increased with parkinsonian severity. In no pairs from the control condition did information exceed the 95th chance percentile, in either the orthodromic (i.e., GPe to GPi) or antidromic (i.e., GPi to GPe) directions. However, a substantial fraction of GPe-GPi pairs had significant levels of directed information in the parkinsonian conditions (Fig. 3, left), in both directions. This analysis identified only highly informative pairs, ignoring a likely majority that were real but less informative. To include moderately informative pairs, we calculated the mean information of all pairs in both directions (Fig. 3, middle). Mean transmission in the control condition was significantly informative in the orthodromic direction, but not in the antidromic direction. Orthodromic information trended toward increasing with parkinsonian severity, but was significantly greater only in the most severe state. Antidromic information was significantly non-zero in the mildly parkinsonian condition, and increased again with the transition to moderately parkinsonian. Trends were qualitatively similar between information in units of bits per spike and information rate (Fig. 3, right).
Figure 3.
Pallidal information increases with parkinsonian severity in the orthodromic (top, GPe to GPi) and antidromic (bottom, GPi to GPe) directions. A) Percentage of GPe-GPi pairs with predictive information exceeding 95% chance level (black bars), increased with symptom severity. B) Information per spike increased with symptom severity, mean±sem. C) Information rate increased with symptom severity, mean±sem. Comparison bars denote p < 0.05 significance.
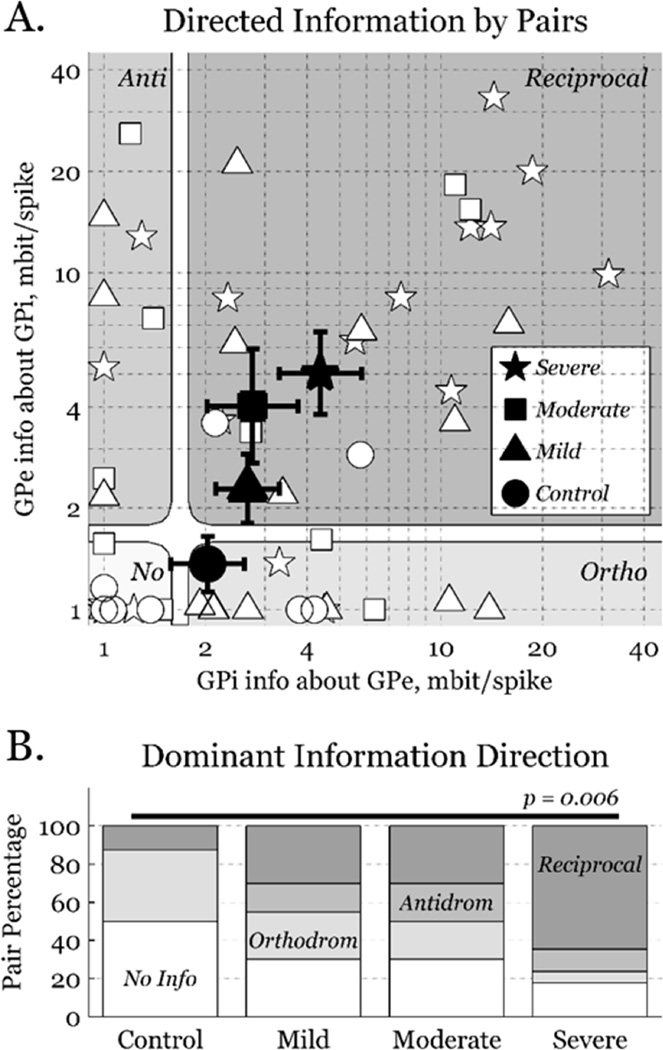
We quantified antidromic information versus orthodromic information for each pair (Fig. 4, top). We set a minimum on all pairings of 1.0 mbit per spike, and one-half of the aforementioned standard deviation as a threshold between informative and non-informative pairs. All pairings were assigned to one of four classes, based on whether their orthodromic and/or antidromic information was above or below threshold. The mean pairing was orthodromically directed in controls, but reciprocally directed in all parkinsonian conditions, with information in both directions increasing with parkinsonian severity (Fig. 4, bottom). Since there is no major GPi to GPe projection, elevated antidromic information suggests that common inputs to GPe and GPi strengthen with parkinsonian severity. Thus, apparent increases from GPe to GPi could be artifacts of the same common inputs, rather than reflect increased information through the pallido-pallidal portion of the indirect pathway.
Figure 4.
Bidirectional information. A) A marker (white) for each recorded pair, where the shape denotes the parkinsonian condition. Large markers (black) indicate geometric mean±sem for each condition, as is natural for this logarithmic representation. Information greater than one-half of a standard deviation was considered significant (1.9 mbit per spike). Connections are divided into 4 classes by information direction: no information, orthodromic, antidromic, and reciprocal. B) Distributions of information direction for the four conditions. Uninformative (white) and orthodromic (light grey) pairs decrease, while reciprocal (dark grey) pairs increase, with parkinsonian severity.
Discussion
While firing rate models provide an attractive framework to explain parkinsonian symptom severity, they fail to accommodate available data, particularly those collected during therapeutic DBS [7,8]. A modern interpretation is that parkinsonian symptoms are generated by qualitative changes in neural firing patterns that include prominent oscillations, irregularity, and bursting [23]. The present work is predicated on the notion that these qualitative behaviors constitute entropy that subserves information transmission. Supporting the idea that neurons constrain their entropy to communicate, the entropy of actual firing patterns from pallidal neurons is substantially less than the entropy of shuffled firing patterns [14]. Using information metrics to classify neural activity as normal versus pathological may provide biomarkers for diagnosis and therapeutic dosing in other neurological disorders [15,24].
Some studies suggest that loss of striatal dopamine initiates parkinsonian symptoms through increased neuronal entropy in efferent basal ganglia [16,25]. Computational work supports this model, reporting that increased pallidal entropy induces thalamic relay errors [17,26]. Firing pattern entropy of pallidal and nigral neurons in a rat model increased with parkinsonism onset, but was decreased by therapeutic DBS [6]. That study supported earlier NHP work, in which pallidal entropy was reduced by symptom alleviating DBS, but was increased by non-therapeutic DBS [15]. However, the present study reveals greater nuance; entropy in both GPe and GPi neurons increased in the moderate parkinsonian relative to the control condition, but returned to baseline levels in the severe condition (Fig. 1).
While the potential for anomalous findings exists, the similarity of entropy recovery in two targets (GPe and GPi) suggests an underlying reversal in the severe parkinsonian condition. That reversal supports a prediction from computational work, which hypothesized an initial loss of action selectivity followed by widespread neuronal synchronization as striatal dopamine becomes increasingly depleted [27]. A study from the same group in a different NHP model strongly supports that the pallidal activity responsible for action selection is disrupted by moderate dopamine depletion, but that pallidal neurons are synchronized by severe dopamine depletion [28]. Integrating those results with the present work, the pallidal activity interfering with action-selection signals in the moderately parkinsonian condition constitutes elevated neuronal entropy levels, while the regularity introduced by widespread synchrony in the severely parkinsonian condition lowers neuronal entropy back toward healthy levels.
Despite limited changes in neuronal entropy, intra-pallidal information increased robustly with symptom severity. Although somewhat inconsistent with an earlier rodent study [6], we recently reported that parkinsonian onset is associated with directed information increases between basal ganglia and thalamus in the orthodromic and antidromic directions [29]. Similarly, worsening of motor symptom severity in the present work accompanied increased information directed orthodromically from GPe to GPi, and antidromically from GPi to GPe. In the orthodromic direction, neuronal pairs in a severely parkinsonian condition had two to four times the information of those in the control condition. Thus, it appears that neuronal pairs throughout efferent nuclei of basal ganglia contain substantially more mutual information in parkinsonian states.
One interpretation of this widespread information increase is that it could represent a loss of independent information channels. That perspective is supported by the finding that antidromic information transmission increased even more steeply with parkinsonian severity than orthodromic transmission. A GPi neuron conveying more information about all GPe neurons indicates that those GPe neurons convey largely redundant information. This interpretation is consistent with observations that pallidal neurons have broader receptive fields in the parkinsonian condition [30].
The ultimate source of this increased information remains unknown. Likely candidates include increasingly common inputs from the cortico-subthalamo-pallidal or cortico-striato-pallidal pathways. Work from rodent models suggests that basal ganglia efferent activity is biased toward the striato-pallido-subthalamo-nigral pathway in the cataleptic state, and moves toward the striato-nigral pathway with therapeutic stimulation of STN [31]. Our interpretation is that common inputs to the indirect and direct pathway strengthen the apparent importance of the connection from the indirect pathway to basal ganglia efferent nuclei, while simultaneously making the connection itself irrelevant. This view supports recent findings that subthalamo-pallidal information increases in the parkinsonian relative to control condition [16], and decreases with therapeutic STN stimulation [32].
Naïvely, one might expect that more neural information is generally advantageous to individual well-being. However, recent work in the NHP demonstrated that pallidal information about motor activity may be reduced by symptom-alleviating DBS [30], and that the eliminated information may relate exclusively to parkinsonian symptoms and not to intentioned motor commands [33]. Excess pallidal information reflecting a loss of motor selectivity may convey the very instructions that dictate motor symptoms. We hypothesize that successful therapies reduce this excess information, e.g., by anatomical lesion or stimulation-driven entrainment. The roles of information from the trans-striatal, inhibitory afferents versus the trans-subthalamic, excitatory afferents should be examined in future work.
In conclusion, pallido-pallidal information increased with parkinsonian severity and its directionality was broadened. Accepting that this increased pallidal information is pathological, we hypothesize that it likely originates in the altered firing patterns of medium spiny neurons, driven by the insufficient dopamine they receive in the parkinsonian striatum. Misdirected and increased information dominates pallidal activity as muddled instructions that block and/or over-writing intended motor commands, leading to bradykinesia, akineisa, tremor and rigidity.
Supplementary Material
Highlights.
Quantified information between pallidal neurons in primate model of progressive PD.
Information transmitted from GPe to GPi increased with symptom severity.
Antidromic information from GPi to GPe increased with symptom severity.
With progression, direct & indirect pathways start to convey redundant information.
Hypothesize loss of parallel processing promotes hypokinetic symptoms of PD.
Acknowledgments
This work was supported by funding from the National Institutes of Health, NINDS-R01-058945 (J.L.V.) and NINDS-R01-037019 (J.L.V.); the National Science Foundation, CBET-CAREER-1351112 (A.D.D.); and a post-doctoral fellowship from the Parkinson's Disease Foundation (A.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests.
References
- 1.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 2.Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- 3.Raz A, Vaadia E, Bergman H. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of parkinsonism. J Neurosci. 2000;20:8559–8571. doi: 10.1523/JNEUROSCI.20-22-08559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang JKH, Moro E, Lozano AM, Lang AE, Hutchison WD, Mahant N, et al. Firing rates of pallidal neurons are similar in Huntington’s and Parkinson’s disease patients. Exp Brain Res. 2005;166:230–236. doi: 10.1007/s00221-005-2359-x. [DOI] [PubMed] [Google Scholar]
- 5.Hahn PJ, Russo GS, Hashimoto T, Miocinovic S, Xu W, McIntyre CC, et al. Pallidal burst activity during therapeutic deep brain stimulation. Exp Neurol. 2008;211:243–251. doi: 10.1016/j.expneurol.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorval AD, Grill WM. Deep brain stimulation of the subthalamic nucleus reestablishes neuronal information transmission in the 6-OHDA rat model of parkinsonism. J Neurophysiol. 2014;111:1949–1959. doi: 10.1152/jn.00713.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson ME, Postupna N, Ruffo M. Effects of high-frequency stimulation in the internal globus pallidus on the activity of thalamic neurons in the awake monkey. J Neurophysiol. 2003;89:1150–1160. doi: 10.1152/jn.00475.2002. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003;23:1916–1923. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown P, Mazzone P, Oliviero A, Altibrandi MG, Pilato F, Tonali PA, et al. Effects of stimulation of the subthalamic area on oscillatory pallidal activity in Parkinson’s disease. Exp Neurol. 2004;188:480–490. doi: 10.1016/j.expneurol.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Meissner W, Leblois A, Hansel D, Bioulac B, Gross CE, Benazzouz A, et al. Subthalamic high frequency stimulation resets subthalamic firing and reduces abnormal oscillations. Brain. 2005;128:2372–2382. doi: 10.1093/brain/awh616. [DOI] [PubMed] [Google Scholar]
- 11.Grill WM, Snyder AN, Miocinovic S. Deep brain stimulation creates an informational lesion of the stimulated nucleus. Neuroreport. 2004;15:1137–1140. doi: 10.1097/00001756-200405190-00011. [DOI] [PubMed] [Google Scholar]
- 12.Bar-Gad I, Elias S, Vaadia E, Bergman H. Complex locking rather than complete cessation of neuronal activity in the globus pallidus of a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primate in response to pallidal microstimulation. J Neurosci. 2004;24:7410–7419. doi: 10.1523/JNEUROSCI.1691-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorval AD. Probability distributions of the logarithm of inter-spike intervals yield accurate entropy estimates from small datasets. J Neurosci Methods. 2008;173:129–139. doi: 10.1016/j.jneumeth.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darbin O, Soares J, Wichmann T. Nonlinear analysis of discharge patterns in monkey basal ganglia. Brain Res. 2006;1118:84–93. doi: 10.1016/j.brainres.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 15.Dorval AD, Russo GS, Hashimoto T, Xu W, Grill WM, Vitek JL. Deep brain stimulation reduces neuronal entropy in the MPTP-primate model of Parkinson’s disease. J Neurophysiol. 2008;100:2807–2818. doi: 10.1152/jn.90763.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cruz AV, Mallet N, Magill PJ, Brown P, Averbeck BB. Effects of dopamine depletion on information flow between the subthalamic nucleus and external globus pallidus. J Neurophysiol. 2011;106:2012–2023. doi: 10.1152/jn.00094.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo Y, Rubin JE, McIntyre CC, Vitek JL, Terman D. Thalamocortical relay fidelity varies across subthalamic nucleus deep brain stimulation protocols in a data-driven computational model. J Neurophysiol. 2008;99:1477–1492. doi: 10.1152/jn.01080.2007. [DOI] [PubMed] [Google Scholar]
- 18.Connolly AT, Jensen AL, Bello EM, Netoff TI, Baker KB, Johnson MD, et al. Modulations in oscillatory frequency and coupling in globus pallidus with increasing parkinsonian severity. J Neurosci. 2015;35:6231–6240. doi: 10.1523/JNEUROSCI.4137-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rieke F, Warland D, Bialek W. Coding Efficiency and Information Rates in Sensory Neurons. Europhys Lett. 1993;22:151–156. [Google Scholar]
- 20.Strong SP, Koberle R, de Ruyter van Steveninck RR, Bialek W. Entropy and Information in Neural Spike Trains. Phys Rev Lett. 1998;80:197. [Google Scholar]
- 21.Selinger JV, Kulagina NV, O’Shaughnessy TJ, Ma W, Pancrazio JJ. Methods for characterizing interspike intervals and identifying bursts in neuronal activity. J Neurosci Methods. 2007;162:64–71. doi: 10.1016/j.jneumeth.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Dorval AD. Estimating Neuronal Information: Logarithmic Binning of Neuronal Inter-Spike Intervals. Entropy. 2011;13:485–501. doi: 10.3390/e13020485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wichmann T, DeLong MR. Pathophysiology of Parkinson’s disease: the MPTP primate model of the human disorder. Ann N Y Acad Sci. 2003;991:199–213. doi: 10.1111/j.1749-6632.2003.tb07477.x. [DOI] [PubMed] [Google Scholar]
- 24.Trevelyan AJ, Bruns W, Mann EO, Crepel V, Scanziani M. The information content of physiological and epileptic brain activity. J Physiol (Lond) 2013;591:799–805. doi: 10.1113/jphysiol.2012.240358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mallet N, Pogosyan A, Sharott A, Csicsvari J, Bolam JP, Brown P, et al. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J Neurosci. 2008;28:4795–4806. doi: 10.1523/JNEUROSCI.0123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dorval AD, Kuncel AM, Birdno MJ, Turner DA, Grill WM. Deep brain stimulation alleviates parkinsonian bradykinesia by regularizing pallidal activity. J Neurophysiol. 2010;104:911–921. doi: 10.1152/jn.00103.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leblois A, Boraud T, Meissner W, Bergman H, Hansel D. Competition between Feedback Loops Underlies Normal and Pathological Dynamics in the Basal Ganglia. J Neurosci. 2006;26:3567–3583. doi: 10.1523/JNEUROSCI.5050-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leblois A, Meissner W, Bioulac B, Gross CE, Hansel D, Boraud T. Late emergence of synchronized oscillatory activity in the pallidum during progressive parkinsonism. European Journal of Neuroscience. 2007;26:1701–1713. doi: 10.1111/j.1460-9568.2007.05777.x. [DOI] [PubMed] [Google Scholar]
- 29.Anderson CJ, Sheppard DT, Huynh R, Anderson DN, Polar CA, Dorval AD. Subthalamic deep brain stimulation reduces pathological information transmission to the thalamus in a rat model of parkinsonism. Front Neural Circuits. 2015;9:31. doi: 10.3389/fncir.2015.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agnesi F, Connolly AT, Baker KB, Vitek JL, Johnson MD. Deep Brain Stimulation Imposes Complex Informational Lesions. PLoS ONE. 2013;8:e74462. doi: 10.1371/journal.pone.0074462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Degos B, Deniau J-M, Thierry A-M, Glowinski J, Pezard L, Maurice N. Neuroleptic-induced catalepsy: electrophysiological mechanisms of functional recovery induced by high-frequency stimulation of the subthalamic nucleus. J Neurosci. 2005;25:7687–7696. doi: 10.1523/JNEUROSCI.1056-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenbaum R, Zimnik A, Zheng F, Turner RS, Alzheimer C, Doiron B, et al. Axonal and synaptic failure suppress the transfer of firing rate oscillations, synchrony and information during high frequency deep brain stimulation. Neurobiol Dis. 2013;62C:86–99. doi: 10.1016/j.nbd.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimnik AJ, Nora GJ, Desmurget M, Turner RS. Movement-related discharge in the macaque globus pallidus during high-frequency stimulation of the subthalamic nucleus. J Neurosci. 2015;35:3978–3989. doi: 10.1523/JNEUROSCI.4899-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.