Abstract
UVB radiation contributes to both direct and indirect damage to the skin including the generation of free radicals and reactive oxygen species (ROS), inflammatory responses, immunosuppression, and gene mutations, which can ultimately lead to photocarcinogenesis. A plant-derived flavonoid, baicalin, has been shown to have antioxidant, anti-inflammatory, and free radical scavenging activities. Previous studies from our laboratory have shown that in murine skin, Toll like receptor-4 (TLR4) enhanced both UVB-induced DNA damage and inflammation. The aim of the current study is to investigate the efficacy of baicalin against TLR4-mediated processes in the murine keratinocyte PAM 212 cell line. Our results demonstrate that treating keratinocytes with baicalin both before and after UV radiation (100 mJ/cm2) significantly inhibited the level of intracellular ROS and decreased cyclobutane pyrimidine dimers (CPDs) and 8-Oxo-2′-deoxyguanosine (8-oxo-dG)—markers of DNA damage. Furthermore, cells treated with baicalin demonstrated an inhibition of TLR4 and its downstream signaling molecules, MyD88, TRIF, TRAF6, and IRAK4. TLR4 pathway inhibition resulted in NF-κB inactivation and down-regulation of iNOS and COX-2 protein expression. Taken together, baicalin treatment effectively protected keratinocytes from UVB-induced inflammatory damage through TLR pathway modulation.
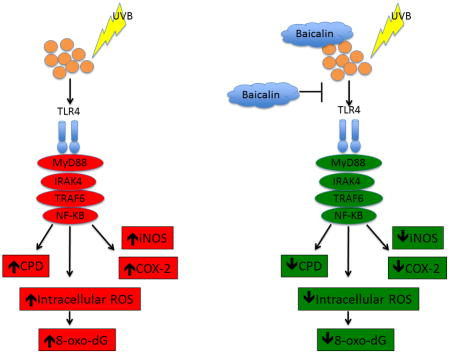
Graphical abstract
INTRODUCTION
The skin is the first barrier to defend the body from environmental pathogens and damage, specifically including ultraviolet (UV) irradiation (1). Continuous and chronic exposure to UV radiation is known to result in skin damage, skin aging and skin cancer (2,3). Skin cancer represents a major, and growing, public health problem. It has been estimated that more than one million new cases of skin cancers are diagnosed each year in the United States alone, which is equivalent to the incidence of malignancies in all other organs combined (4). The major cell type in the epidermis is the keratinocyte, comprising more than 90% of the cells of the epidermal layer. The epidermis is most intensely exposed to solar UV radiation, especially its UVB (290~320 nm) component, which causes the generation of free radicals and reactive oxygen species (ROS), secretion of cytokines, immunosuppression, DNA damage and mutations, which all contribute to photocarcinogenesis (5–7). The formation of photoproducts, predominantly cis-syn cyclobutane pyrimidine dimers (CPDs), is critical to the initiation of UV-induced skin cancer (8,9). Further, there have been many studies supporting an important role for UV-induced ROS in the development of skin cancers (10,11). UV exposure has been shown to suppress a wide variety of immune responses. The immunosuppressive mediators derived from keratinocytes and induced by UV may enter the circulation and inhibit immune reactions at skin sites that are not exposed directly to UV radiation (12,13).
Toll-like receptors (TLRs) initiate innate immune responses and direct subsequent adaptive immunity that play important roles in defense against pathogens via recognition of a wide variety of pathogen-associated molecular patterns (PAMPs) (14,15). TLRs also regulate cell proliferation and survival by expanding immune cells and integrating inflammatory responses and tissue repair processes (16–18). Many recent studies have shown that TLRs are widely expressed on tumor cells and are critical for the initiation and progression of cancer. Increasing evidence suggests that TLRs act as a double-edged sword in cancer cells. On the one hand, uncontrolled TLR signaling provides a microenvironment that is necessary for tumor cells to proliferate and evade the immune response. In contrast, TLRs can induce an antitumor immune response in order to inhibit tumor progression (19–21). Our previous studies have shown that TLR4 mediates UVB-induced DNA damage and inflammation in murine skin (22, 23).
There has been great interest in cancer chemoprevention, whereby the use of naturally occurring agents is considered a less toxic and more effective approach to reduce the risk of both melanoma and nonmelanoma skin cancers (24–26). Baicalin is the predominant flavonoid isolated from the roots of Scutellaria lateriflora Georgi (Huang Qin), which is one kind of Chinese herbal medicine. It has been reported that baicalin has many anti-bacterial, antiviral, anti-inflammatory, anti-oxidative and anticarcinogenic activities (27–29). In addition, our previous studies have shown the photoprotective effects of baicalin against UV-induced photodamage, including reducing apoptotic cells, oxidative stress and IL-6 secretion levels (30–33). However, whether baicalin could protect skin cells from UVB irradiation-induced oxidative stress and DNA damage through the TLR pathway remains to be clarified. The objective of the present study is to investigate the efficacy of baicalin against TLR4-mediated photodamage processes in the murine keratinocyte PAM 212 cell line.
MATERIALS AND METHODS
Cell line and reagents
The murine epidermal keratinocyte cell line, PAM212, was obtained from Lonza Walkersville, Inc. (Walkersville, MD). Baicalin and N-acetyl-L-cysteine (NAC) were supplied by Sigma Aldrich (St. Louis, MO). Anti-8-oxo-dG antibody was purchased from Millipore (Billerica, MA) and anti-CPD antibody was purchased from Kamiya Biomedical Company (Tukwila, WA). Secondary Alexafluor 488 antibody and Alexafluor 594 antibody were purchased from Invitrogen (Carlsbad, CA). The primary antibodies TLR4 and the secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies for TRAF6, TRIF, myd88, NF-κB, iNOS, COX-2, IRAK4 and β-actin were procured from Cell Signaling Technology, Inc. (Danvers, MA). The DC protein assay kit was obtained from Bio-Rad Laboratories (Hercules, CA) and the enhanced chemiluminescence Western blotting detection reagents were purchased from Amersham Pharmacia Biotech (Piscataway, NJ).
Cell culture and UVB irradiation
PAM212 cells were cultured as monolayers in DMEM, which contained 25 mM glucose, 10% heat-inactivated fetal bovine serum (FBS), and 1% penicillin–streptomycin (Invitrogen), and maintained in an atmosphere of 5% CO2 at 37°C. Baicalin was initially dissolved to a final concentration of 50 mg/ml using the serum-free culture medium and filter-sterilized by 0.22 mm syringe filter unit (Millipore) before use. The UV irradiation was performed with a band of four UVB lamps (Daavlin; UVA/UVB Research Irradiation Unit, Bryan, OH) equipped with an electronic controller to regulate UV dosage. The majority of the resulting wavelengths were in the UVB (290–320 nm; about 80%) and UVA (about 20%) range and the peak emission was recorded at 314 nm. The cells were irradiated at a fixed distance of 24 cm from the lamps to the surface of the cell culture plates. The UVB dose was chosen based on the previous study of our lab (34).
Treatment of PAM212 cells
When the cultured cells reached sub-confluency (70–80%), they were harvested and divided into different subgroups for the following experiments. N-acetyl-L-Cysteine (NAC), a well-known antioxidant, was used for the positive control at a concentration of 1 mM and BA was used at 50μg/ml. Subconfluent cells were treated with BA and NAC for 24 h before UVB irradiation, then cells were washed three times with PBS and exposed to 100 mJ/cm2 UVB irradiation. After UVB exposure, cells were incubated again with BA or NAC and harvested at the desired time points.
MTT detection for cellular viability
Cell death was assayed by MTT detection as previously described (34). Different concentrations (10, 20, 50, 100 and 200 μg/ml) of BA were added to subconfluent PAM212 cells in a 96-well plate and incubated for 24 hours then cells were washed three times with PBS and exposed to 100 mJ/cm2 UVB irradiation. After UVB exposure, cells were incubated again with different concentrations of BA for 24 hours. MTT was added (0.5 mg/ml) to the cells and incubated for 4 hours. The media was aspirated and DMSO (200 μl/well) was added. Cell density was determined by measuring the values of absorbance at 490 nm.
Immunocytochemical detection of CPD or 8-oxo-dG positive cells
The CPD and 8-oxo-dG positive cells induced by UVB irradiation were detected using the protocol described previously (34). Briefly, the cells were adhered on coverslips overnight and the culture medium was replaced with fresh assay medium supplemented with 2.0% fetal bovine serum. PAM212 cells were treated for 24h with 50μg/ml BA and 1mM NAC before UVB irradiation and subsequently irradiated with 100mJ/cm2 UVB. After UVB exposure, the cells were incubated again for 30 min and 24 hours with 50μg/ml BA and 1mM NAC, to assay CPD and 8-oxo-dG generation. Thereafter, cells were fixed in 1% paraformaldehyde for 10 min and washed with PBS and subsequently permeabilized with 0.5% Triton-X 100 for 5 min on ice. 2.0 M HCl was used for DNA denaturation at room temperature for 20 min. Afterwards, cells were incubated in blocking buffer, 10% anti-goat normal serum, at room temperature for 1 hour. Then cells were incubated with either CPD-specific or 8-oxo-G-specific mouse monoclonal antibody for 1 hour at room temperature and incubated with anti-mouse secondary IgG antibody Alexa Fluor 488 or Alexa Fluor 594 respectively for 45 min. The coverslips were counterstained with DAPI and the CPD and 8-oxo-dG positive cells were counted in five fields of view using an Olympus BX41.
Detection of ROS level
The intracellular ROS level was determined by 2′7′-dichlorofluorescin diacetate (DCFH-DA) assay. Briefly, the PAM212 cells were seeded in 35 mm dishes (Nunc, Denmark) and incubated with BA or NAC for 24h before UVB irradiation. After 100 mJ/cm2 UVB exposure, the cells were washed three times with PBS and then incubated with culture medium containing 10 μM DCFH-DA (Sigma, Sigma-Aldrich Co, U.S.A) for 30 min at 37°C in the dark. Thereafter, the cells were washed with PBS three times and collected for observation and flow cytometry detection. The ROS-positive cells were visualized and counted using fluorescence microscopy with excitation at 485 nm and emission at 538 nm. For flow cytometry detection, the cells were analyzed with a FACS flow cytometer.
Western blotting analysis
The PAM212 cells were plated in 100 mm cell culture dishes (Nunc, Denmark) and after confluency incubated with 50μg/ml BA or 1 mM NAC for 24h before UVB irradiation. After irradiation, the cells were washed twice with ice-cold PBS and the total protein was extracted with lysis buffer containing 62.5 mM Tris–HCl (pH 6.8), 1% Triton X-100, 1% sodium deoxycholate and 0.1% sodium dodecyl sulfate (SDS). The protein concentrations were measured by BCA assay. 50 μg proteins was loaded in each well and resolved on 10% or 12% SDS polyacrylamide gels and transferred onto nitrocellulose membranes. The membranes were blocked in blocking buffer (Tris-buffered saline with 5% nonfat dry milk) and then incubated with various primary antibodies overnight at 4°C. The membrane was then washed with TBS-T and incubated with secondary antibody conjugated with horseradish peroxidase. After washing three times in TBST, protein bands were visualized using the enhanced chemiluminescence detection system (Amersham Life Science, Inc.). The band density was analyzed using Quantity One software (Bio-Rad) and the values were normalized to the β-actin band density.
Statistical analysis
Each experiment was repeated at least three times. The data were expressed as mean ± SD. The differences in the data among groups were statistically analyzed with one-way analysis of variance (ANOVA) using the SPSS statistical software version 11.0 (SPSS Inc, Chicago, USA). P-values less than 0.05 were considered to be statistically significantly different.
RESULTS
The effect of BA and UVB on PAM212 cell proliferation
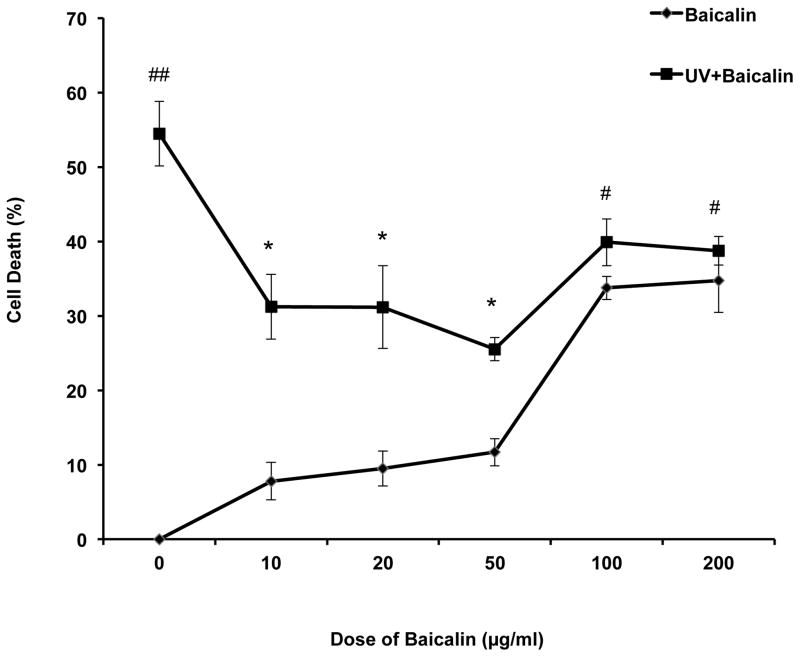
After UVB exposure, the PAM212 cells were incubated for 24h. Percent cell death was assessed by MTT detection. The results showed that treatment of BA from 10 to 50μg/ml did not result in significant cell death after 24 h incubation. However, 100 and 200μg/ml BA treatment induced a notable cell death more than 30%. At the same time, 10, 20, 50 μg/ml BA treatment remarkably reduced the percent cell death induced by UVB irradiation therefore for further experiments, the dose of 50μg/ml was selected for the assessment of chemopreventive potential of BA on PAM212 cells. (Fig. 1). A closer look at the effects of the selected dose of Baicalin on PAM212 cell proliferation following UVB exposure can be found in Figure S1.
Figure 1. Effect of BA and UVB on the proliferative activity of PAM212 cells.
Dose-dependent effect of BA on the cell death of PAM212 keratinocytes was determined by MTT assay. The cells were treated with 0, 10, 20, 50, 100 and 200 μg/ml BA for 24 hours before and after, with or without 100 mJ/cm2 UVB irradiation. There was no significant cell death induced by treatment of BA alone from 10 to 50 μg/ml after 24 h incubation. However, 100 and 200 μg/ml BA alone treatment induced more than 30% cell death. 10, 20, 50 μg/ml BA treatment remarkably reduced the percent cell death induced by UVB irradiation. The values are represented as the percentage of cell death. The data represent the mean ± SD of three independent experiments each conducted in triplicate. (#P<0.05 and ##P <0.01 vs control; *P > 0.05 vs UVB).
The effect of BA on UVB-induced CPD and 8-oxo-dG formation
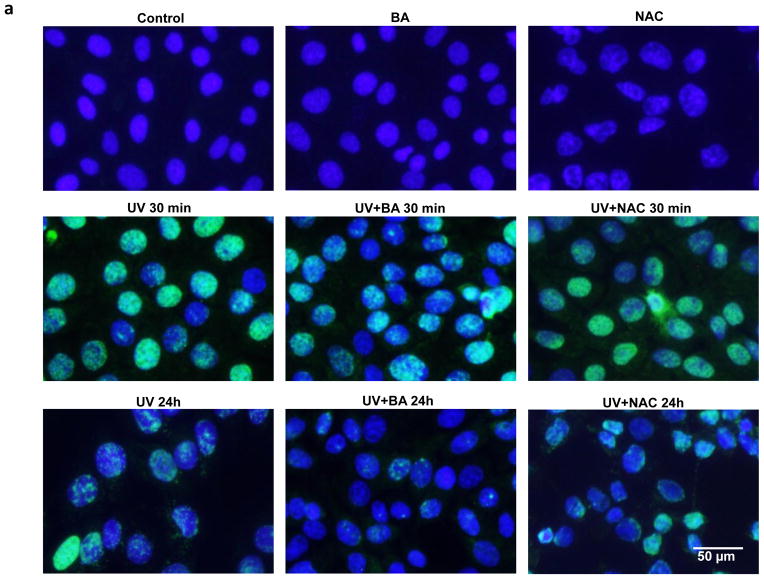
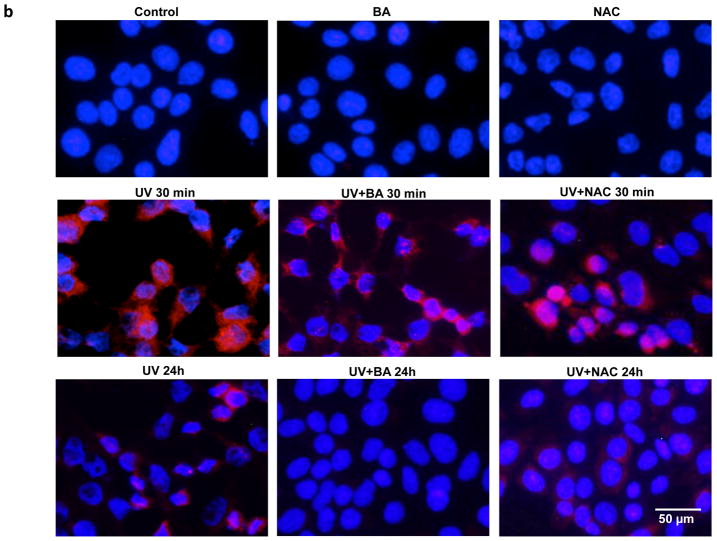
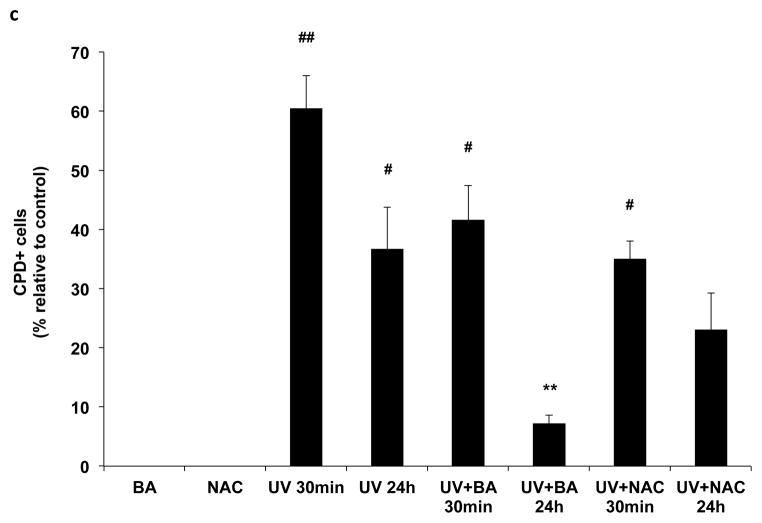
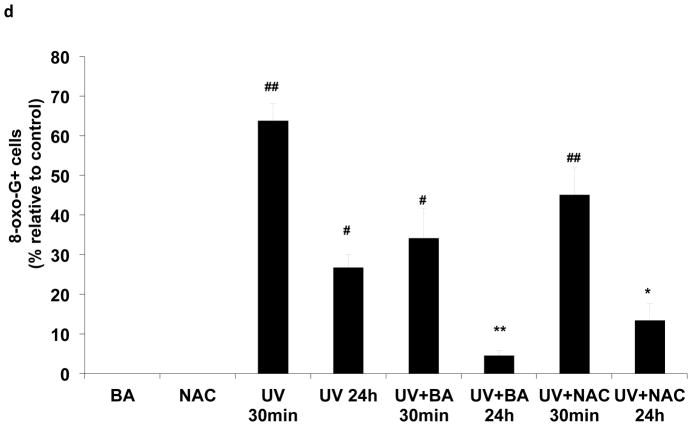
The photodamage induced by UVB irradiation include photoproducts and ROS production, measured by CPD and 8-oxo-dG formation. To determine whether BA could reduce UVB-induced photodamage in PAM212 cells, the CPD and 8-oxo-dG positive cells induced by UVB irradiation were assayed using immunocytochemical detection. After UVB exposure, cells were cultured with or without BA and NAC treatment for either 30 min or 24 h, harvested, and examined for CPD and 8-oxo-G positive cells (Fig. 2a,2b). Neither CPD nor 8-oxo-G positive cells were detected in untreated cells or cells treated with BA or NAC alone. Furthermore, the amount of CPD or 8-oxo-G positive cells largely increased immediately following UVB exposure (30 min) compared to unexposed cells (Fig 2c, 2d, #p<0.01). Even at 24 h after UVB irradiation, there were still about 36% positive cells detected. With BA treatment, the percentages of CPD-positive cells were decreased upto 7.26% at 24 h after UVB irradiation, which were significantly decreased compared to untreated cells (Fig 2c, **p<0.01). 8-oxo-dG positive cells were also significantly decreased, with 4.51% after 24 h (Fig 2d, **p<0.01). NAC treatment also significantly reduced 8-oxo-dG positive cells upto 11.04% after 24 h (Fig 2d, *p<0.05)., but had no much influence on UVB-induced CPD formation. These results suggest that BA treatment may reduce UVB-induced photodamage or accelerate its repair in PAM212 cells.
Figure 2. The inhibitory effect of BA on UVB-induced formation of CPDs and 8-oxo-dG in PAM212 cells.
The cells were treated with 50 μg/ml BA and exposed to 100 mJ/cm2 UVB as described in the Materials and Methods section. Immunocytochemical staining for CPDs (A) and 8-oxo-dG (B) was performed using appropriate antibodies. The number of CPDs-positive cells (C) and 8-oxo-dG-positive cells (D) after immunostaining were counted in five different areas of the sections under microscope. The number of CPDs and 8-oxo-dG positive cells are represented as the percent of CPDs- and 8-oxo-dG-positive cells, respectively. The data represent the mean ± SD of cells in triplicate (#P<0.05 and ##P <0.01 vs control; *P > 0.05 and **P > 0.01 vs UVB). The representative micrographs are shown from three independent experiments.
The effect of BA on intracellular ROS levels
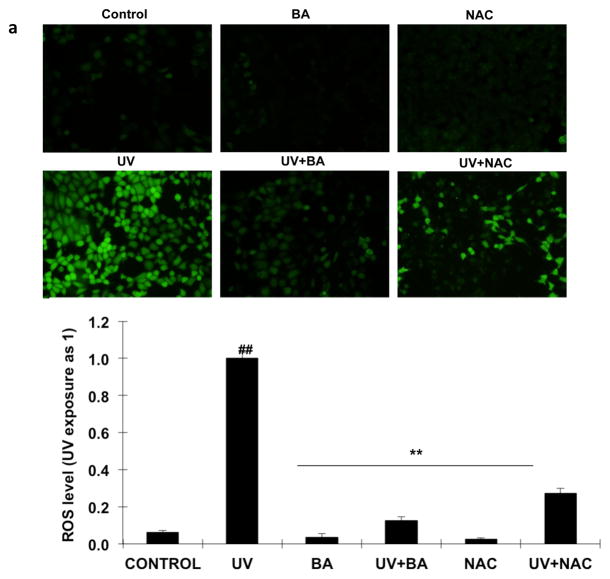
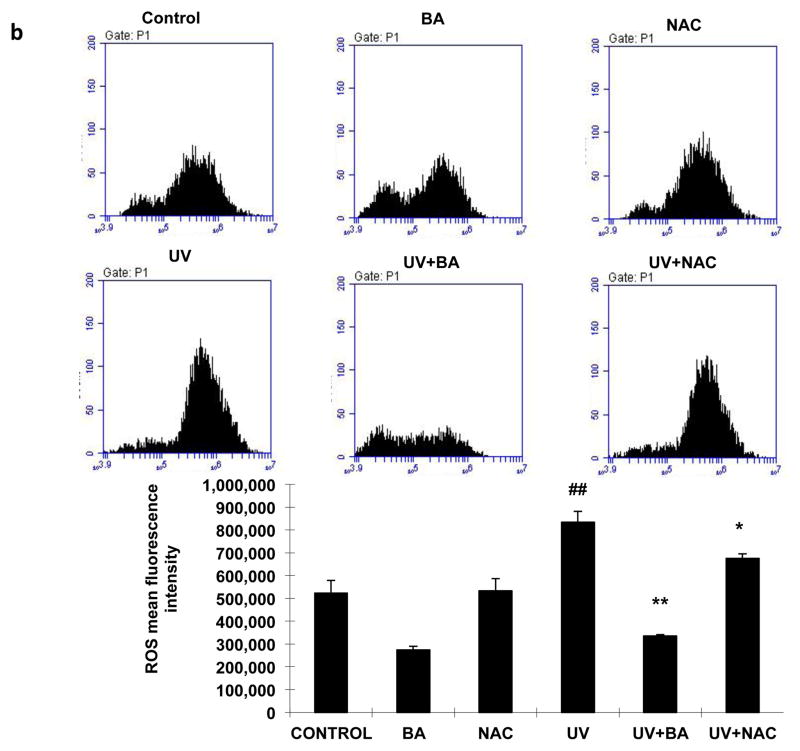
We examined intracellular ROS levels by DCFH-DA assay at 24 h following 100 mJ/cm2 UVB exposure. The DCFH-DA-positive fluorescence intensity significantly increased in UVB irradiated cells compared to the untreated/unexposed cells and cells treated with BA or NAC alone (Fig 3a, p<0.05). However, both BA and NAC treatment markedly decreased the fluorescence intensity for 73% and 87% respectively (p<0.01) after UVB exposure, which suggested the ROS levels of the PAM212 cells treated with BA or NAC were much lower than UVB-exposure cells. In addition, these results were further confirmed by FACS analysis. The ROS fluorescence intensity declined with BA and NAC treatment, while it decreased more significantly with BA treatment (Fig 3b, p<0.05). These results suggested that BA effectively reduced the production of intracellular ROS induced by UVB irradiation.
Figure 3. Effect of BA on UVB-induced ROS generation in PAM212 cells.
PAM212 cells were treated with 50 μg/ml BA or 1 mM NAC concentration with/without exposure to 100 mJ/cm2 UVB. Then, ROS levels were detected by DCFH-DA assay for observation using fluorescence microscopy (A) and flow cytometry detection (B). Both BA and NAC treatment significantly decreased the fluorescence intensity for 73% and 87% respectively (p<0.01) after UVB exposure. The representative data are shown as mean ± SD (n = 3). (##P <0.01 vs control; *P > 0.05 and **P > 0.01 vs UVB)
The effects of BA and UVB on TLR4 pathway proteins’ expression
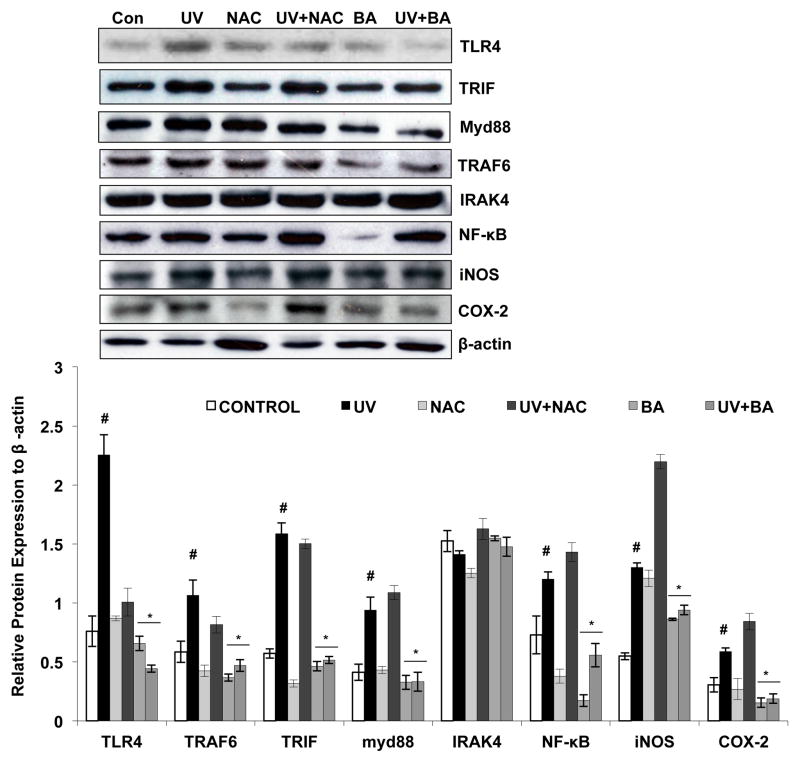
In order to clarify the pharmacological effect and mechanism of BA on UVB-induced photodamage, the protein levels of TLR4, TRIF, myd88, TRAF6, IRAK4, NF-κB, iNOS and COX-2 were analyzed via Western blotting. The results showed that UVB exposure up-regulated TLR4, TRIF, myd88, TRAF6 and NF-κB, protein levels (Fig 4 p<0.05), demonstrating that UVB can activate the TLR4 pathway and DNA damage process. However, BA treatment inhibited the up-regulation of the above proteins in the physiological condition and after UVB exposure (Fig 4 p<0.05). In addition to this BA also inhibits the downstream effectors of NF-kB such as iNOS and COX-2 and therefore it leads inhibition of UVB mediated inflammatory response (Fig 4 p<0.05). These data suggest that BA may offer protection to PAM212 cells against UVB-induced photodamage by suppression of the TLR4-mediated inflammatory signaling pathway. Further, we found that NAC has no significant influence on the TLR4 pathway as an antioxidant.
Figure 4. Effect of BA on UVB-induced TLR4 pathway proteins expression in PAM212 cells.
Cells were treated with either BA or with NAC and exposed to 100 mJ/cm2 UVB as described in the Materials and Methods section. Western blot analysis was performed to determine the protein expression using TLR4 pathway proteins specific antibodies, including TLR4, MyD88, TRIF, TRAF6, NF-κB, iNOS and COX-2. There was an up-regulation of TLR4 and its downstream targets after UVB exposure (p<0.05). However, BA treatment inhibited the up-regulation of the above proteins induced by UVB exposure (p<0.05). The representative blots are shown from three independent experiments. Values are given as mean ± SD (n = 3). (#P<0.05 vs control; *P > 0.05 and **P > 0.01 vs UVB).
DISCUSSION
Human skin directly suffers from the deleterious effects of UVB radiation, such as ROS production and DNA damage, which induce inflammatory responses and contribute to skin cancer development. The incidence of skin cancer, including melanoma and nonmelanoma skin cancer (NMSC), has increased dramatically worldwide in the last several decades (35). The DNA and inflammatory damage induced by UVB is also considered an important event in skin carcinogenesis. TLRs have been reported to play a key role in the innate immune system and participate in the regulation of the cellular response to UV exposure (36, 37). There are also reports indicating that the TLR signaling pathways have important effects on cancer progression, whereby cancer cells may suppress the immune system through the TLRs. Our previous study indicated that TLR4 plays an important role in UVB-induced DNA damage and cutaneous photoimmunosuppression in murine skin (22, 23). However, the relationship between TLRs and photocarcinogenesis is still obscure.
Baicalin has been reported to exhibit a wide range of pharmacological actions such as antioxidant, anti-inflammatory, and anti-tumor activity, as well as free radical scavenging ability. Our previous study demonstrated that baicalin effectively inhibits UV-induced damage to human skin cells (including keratinocytes and fibroblasts) and mouse skin, by reducing inflammatory cytokine secretion and inhibiting cell cycle arrest induced by UV (30–33). But more studies still need to be carried out to clarify the photoprotective mechanisms of baicalin. In the current study, we assessed the photoprotective effect of baicalin against UVB irradiation in the mouse keratinocyte PAM212 cell line. Our results showed that treatment with baicalin affords significant protection from cell death following UVB exposure. In addition, the data also demonstrated that specific doses of baicalin (less than 50μg/ml) had no obvious toxicity on PAM212 cells.
UVB irradiation induces both direct and indirect DNA damage, the later is mainly through the generation of ROS. Both CPDs and 8-oxo-dG are considered as important biomarkers of DNA damage following UVB irradiation (38, 39). Our results showed that baicalin treatment significantly reduced the number of CPD and 8-oxo-dG-positive cells, which may be partly due to DNA repair capacity strengthened by baicalin. ROS generation induced by UV irradiation initiates molecular responses in the skin and plays a key role in the immunosuppression and photocarcinogenesis processes (40). Though skin cells contain networks of antioxidants to eliminate ROS or decrease their harmful effects, long-term UVB irradiation continuously induces ROS production and accumulation in keratinocytes, which overwhelms the antioxidant networks and contributes to the carcinogenic process (41, 42). In this study, we assessed the antioxidant effects of baicalin. Our data showed that baicalin remarkably reduced UVB-induced ROS generation in PAM212 cells. We also compared the antioxidant effect of baicalin to NAC, a potent free radical scavenger, which demonstrated that baicalin has a strong ability to scavenge ROS and protect cells from photodamage. Previous literature has shown that Baicalin has strong antioxidant properties. Baicalin demonstrates its antioxidant capabilities by enhancing the activity and expression level of the endogenous antioxidant network, whereas NAC shows prompt antioxidant activity by replenishing the glutathione (GSH) level.
It has been reported that the immuno-inflammatory responses play a key role in UV-related tumorigenesis, suggesting that prevention of inflammatory pathways and genes could provide molecular targets for skin cancer prevention (43, 44). TLRs have emerged as a key component in the cellular response to UV exposure. Upon the cell damage-specific factor, such as UVR, the stimulation of TLRs would recruit adaptor molecules, including MyD88, TRAF6 and TRIF leading to activation of NF-κB, which results in inflammatory gene expression and reactions (47–51). TLR signaling has also been reported to induce the activation of cyclooxygenase-2 (COX-2) and nitric oxide synthase (iNOS), which have been implicated in UVB-induced skin inflammation and photocarcinogenesis, including enhanced cell proliferation, induction of angiogenesis, regulation of apoptosis, cancer cell invasion and metastasis (52–54). Although how UV radiation activates TLR4 is still under investigation, a few studies have shown that UV radiation activates several endogenous TLR4 ligands such as heat-shock proteins (55), S100 family proteins (56, 57), HMGB1 (58), and hyaluronic acid (59) that leads to TLR4 activation. In the present study, we assessed the influence of baicalin on TLR4 pathway protein expression following UVB irradiation. Our results demonstrate baicalin treatment inhibited TLR4 and its downstream signaling molecules, MyD88, TRIF and TRAF6. And TLR4 pathway inhibition also resulted in NF-κB inactivation and down-regulation of iNOS and COX-2 protein expression. In contrast, NAC treatment had no significant inhibitory effects on protein expression, indicating that baicalin may directly affect the TLR signaling pathway to inhibit UV-induced inflammation, in addition to acting as an antioxidant. The experimental design from this study cannot rule out whether these ligands are proximate targets of baicalin treatment which lead to the TLR4 inhibition. Therefore, futher studies are needed to clarify the exact mechanism.
In conclusion, our data demonstrate that baicalin treatment effectively protected keratinocytes from UVB-induced oxidative stress, DNA damage and inflammatory damage by modulating the TLR pathway. Thus, the application of baicalin may exert photochemopreventive effects against skin carcinogenesis.
Supplementary Material
Choice of BA dose based on the proliferative activity of PAM212 cells. Dose-dependent effect of BA on the cell death of PAM212 keratinocytes was determined by MTT assay, resulting in the selection of 50 μg/ml BA for further experimentation. The cells were treated with 0 or 50 μg/ml BA for 24 hours before and after, with or without 100 mJ/cm2 UVB irradiation. 50 μg/ml BA treatment remarkably reduced the percent cell death induced by UVB irradiation. The values are represented as the percentage of cell death. The data represent the mean ± SD of three independent experiments each conducted in triplicate. (#P<0.05 and ##P <0.01 vs control; *P > 0.05 vs UVB).
Acknowledgments
This work was supported by a Pilot & Feasibility Program Grant to NY from the NIH-funded UAB Skin Diseases Research Center (#P30AR050948), we well as funding from the Traditional Chinese Medicine Bureau of Jiangsu Province (LZ11095) and the Jiangsu National Natural Science Foundation (BK2012170). This work was also partially supported by an NIH Cancer Prevention and Control Training Grant (R25CA47888) to EMB.
Footnotes
Additional Supporting Information may be found in the online version of this article:
References
- 1.Biniek K, Levi K, Dauskardt RH. Solar UV radiation reduces the barrier function of human skin. Proc Natl Acad Sci U S A. 2012;109:17111–17116. doi: 10.1073/pnas.1206851109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovach BT, Sams HH, Stasko T. Systemic strategies for chemoprevention of skin cancers in transplant recipients. Clin Transplant. 2005;19:726–734. doi: 10.1111/j.1399-0012.2005.00412.x. [DOI] [PubMed] [Google Scholar]
- 3.Tsatsou F, Trakatelli M, Patsatsi A, Kalokasidis K, Sotiriadis D. Extrinsic aging: UV-mediated skin carcinogenesis. Dermatoendocrinol. 2012;4:285–297. doi: 10.4161/derm.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Orazio J, Jarrett S, Amaro-Ortiz A, Scott T. UV Radiation and the Skin. Int J Mol Sci. 2013;14:12222–12248. doi: 10.3390/ijms140612222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinberg J. Overall increasing incidence of nonmelanoma skin cancer. Rev Recent Clin Trials. 2012;7:1. doi: 10.2174/157488712799363244. [DOI] [PubMed] [Google Scholar]
- 6.Perluigi M, Di Domenico F, Blarzino C, Foppoli C, Cini C, Giorgi A, Grillo C, De Marco F, Butterfield DA, Schinina ME, Coccia R. Effects of UVB-induced oxidative stress on protein expression and specific protein oxidation in normal human epithelial keratinocytes: a proteomic approach. Proteome Sci. 2010;8:13. doi: 10.1186/1477-5956-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Gruijl FR. Photocarcinogenesis: UVA vs. UVB radiation. Skin Pharmacol Appl Skin Physiol. 2002;15:316–320. doi: 10.1159/000064535. [DOI] [PubMed] [Google Scholar]
- 8.Swalwell H, Latimer J, Haywood RM, Birch-Machin MA. Investigating the role of melanin in UVA/UVB- and hydrogen peroxide-induced cellular and mitochondrial ROS production and mitochondrial DNA damage in human melanoma cells. Free Radic Biol Med. 2012;52:626–634. doi: 10.1016/j.freeradbiomed.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Pfeifer GP, Besaratinia A. UV wavelength-dependent DNA damage and human non-melanoma and melanoma skin cancer. Photochem Photobiol Sci. 2012;11:90–97. doi: 10.1039/c1pp05144j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mabruk MJ, Toh LK, Murphy M, Leader M, Kay E, Murphy GM. Investigation of the effect of UV irradiation on DNA damage: comparison between skin cancer patients and normal volunteers. J Cutan Pathol. 2009;36:760–5. doi: 10.1111/j.1600-0560.2008.01164.x. [DOI] [PubMed] [Google Scholar]
- 11.Allouche Y, Warleta F, Campos M, Sanchez-Quesada C, Uceda M, Beltran G, Gaforio JJ. Antioxidant, antiproliferative, and pro-apoptotic capacities of pentacyclic triterpenes found in the skin of olives on MCF-7 human breast cancer cells and their effects on DNA damage. J Agric Food Chem. 2011;59:121–30. doi: 10.1021/jf102319y. [DOI] [PubMed] [Google Scholar]
- 12.Tulah AS, Birch-Machin MA. Stressed out mitochondria: the role of mitochondria in ageing and cancer focussing on strategies and opportunities in human skin. Mitochondrion. 2013;13:444–453. doi: 10.1016/j.mito.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Yarosh DB. DNA repair, immunosuppression, and skin cancer. Cutis. 2004;74:10–13. [PubMed] [Google Scholar]
- 14.Ullrich SE. Sunlight and skin cancer: lessons from the immune system. Mol Carcinog. 2007;46:629–633. doi: 10.1002/mc.20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delneste Y, Beauvillain C, Jeannin P. Innate immunity: structure and function of TLRs. Med Sci (Paris) 2007;23:67–73. doi: 10.1051/medsci/200723167. [DOI] [PubMed] [Google Scholar]
- 16.Chalifour A, Jeannin P, Gauchat JF, Blaecke A, Malissard M, N’Guyen T, Thieblemont N, Delneste Y. Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers alpha-defensin production. Blood. 2004;104:1778–83. doi: 10.1182/blood-2003-08-2820. [DOI] [PubMed] [Google Scholar]
- 17.Chang XT, Nian XF, Wang ZH. Progress of research on TLRs-mediated signaling pathway. Sheng Li Ke Xue Jin Zhan. 2011;42:340–346. [PubMed] [Google Scholar]
- 18.Shcheblyakov DV, Logunov DY, Tukhvatulin AI, Shmarov MM, Naroditsky BS, Gintsburg AL. Toll-Like Receptors (TLRs): The Role in Tumor Progression. Acta Naturae. 2010;2:21–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Monie TP, Bryant CE, Gay NJ. Activating immunity: lessons from the TLRs and NLRs. Trends Biochem Sci. 2009;34:553–561. doi: 10.1016/j.tibs.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Kaczanowska S, Joseph AM, Davila E. TLR agonists: our best frenemy in cancer immunotherapy. J Leukoc Biol. 2013;93:847–863. doi: 10.1189/jlb.1012501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ray A, Chakraborty K, Ray P. Immunosuppressive MDSCs induced by TLR signaling during infection and role in resolution of inflammation. Front Cell Infect Microbiol. 2013;3:52. doi: 10.3389/fcimb.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis W, Simanyi E, Li H, Thompson CA, Nasti TH, Jaleel T, Xu H, Yusuf N. Regulation of ultraviolet radiation induced cutaneous photoimmunosuppression by toll-like receptor-4. Arch Biochem Biophys. 2011 Apr 15;508(2):171–7. doi: 10.1016/j.abb.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmad I, Simanyi E, Guroji P, Tamimi IA, delaRosa HJ, Nagar A, Nagar P, Katiyar SK, Elmets CA, Yusuf N. Toll-like receptor-4 deficiency enhances repair of UVR-induced cutaneous DNA damage by nucleotide excision repair mechanism. J Invest Dermatol. 2014 Jun;134(6):1710–7. doi: 10.1038/jid.2013.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saint-Jean M, Knol AC, Nguyen JM, Khammari A, Dreno B. TLR expression in human melanoma cells. Eur J Dermatol. 2011;21:899–905. doi: 10.1684/ejd.2011.1526. [DOI] [PubMed] [Google Scholar]
- 25.Chen AC, Halliday GM, Damian DL. Non-melanoma skin cancer: carcinogenesis and chemoprevention. Pathology. 2013;45:331–341. doi: 10.1097/PAT.0b013e32835f515c. [DOI] [PubMed] [Google Scholar]
- 26.Campbell RM, DiGiovanna JJ. Skin cancer chemoprevention with systemic retinoids: an adjunct in the management of selected high-risk patients. Dermatol Ther. 2006;19:306–314. doi: 10.1111/j.1529-8019.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- 27.Qian YP, Cai YJ, Fan GJ, Wei QY, Yang J, Zheng LF, Li XZ, Fang JG, Zhou B. Antioxidant-based lead discovery for cancer chemoprevention: the case of resveratrol. J Med Chem. 2009;52:1963–74. doi: 10.1021/jm8015415. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Li H, Zhao Y, Gao Z. Dietary supplementation of baicalin and quercetin attenuates iron overload induced mouse liver injury. Eur J Pharmacol. 2006;535:263–269. doi: 10.1016/j.ejphar.2006.01.067. [DOI] [PubMed] [Google Scholar]
- 29.Chu ZY, Chu M, Teng Y. Effect of baicalin on in vivo anti-virus. Zhongguo Zhong Yao Za Zhi. 2007;32:2413–2415. [PubMed] [Google Scholar]
- 30.Xiping Z, Hua T, Hanqing C, Li C, Binyan Y, Jing M. Effects of Baicalin on inflammatory mediators and pancreatic acinar cell apoptosis in rats with sever acute pancreatitis. J Res Med Sci. 2009;14:19–27. [PMC free article] [PubMed] [Google Scholar]
- 31.Min W, Lin XF, Miao X, Wang BT, Yang ZL, Luo D. Inhibitory effects of Baicalin on ultraviolet B-induced photo-damage in keratinocyte cell line. Am J Chin Med. 2008;36:745–60. doi: 10.1142/S0192415X0800620X. [DOI] [PubMed] [Google Scholar]
- 32.Zhou BR, Luo D, Wei FD, Chen XE, Gao J. Baicalin protects human fibroblasts against ultraviolet B-induced cyclobutane pyrimidine dimers formation. Arch Dermatol Res. 2008;300:331–334. doi: 10.1007/s00403-008-0851-4. [DOI] [PubMed] [Google Scholar]
- 33.Xu Y, Zhou B, Wu D, Yin Z, Luo D. Baicalin modulates microRNA expression in UVB irradiated mouse skin. J Biomed Res. 2012;26:125–134. doi: 10.1016/S1674-8301(12)60022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmad I, Jimenez H, Yaacob NS, Yusuf N. Tualang honey protects keratinocytes from ultraviolet radiation-induced inflammation and DNA damage. Photochem Photobiol. 2012;88:1198–204. doi: 10.1111/j.1751-1097.2012.01100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou BR, Liu WL, Luo D. Protective effect of baicalin against multiple ultraviolet B exposure-mediated injuries in C57BL/6 mouse skin. Arch Pharm Res. 2011;34:261–268. doi: 10.1007/s12272-011-0212-2. [DOI] [PubMed] [Google Scholar]
- 36.Wei-Passanese EX, Han J, Lin W, Li T, Laden F, Qureshi AA. Geographical variation in residence and risk of multiple nonmelanoma skin cancers in US women and men. Photochem Photobiol. 2012;88:483–9. doi: 10.1111/j.1751-1097.2012.01077.x. [DOI] [PubMed] [Google Scholar]
- 37.Harberts E, Gaspari AA. TLR signaling and DNA repair: are they associated? J Invest Dermatol. 2013;133:296–302. doi: 10.1038/jid.2012.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruscanu S, Pascale F, Bourge M, Hemati B, Elhmouzi-Younes J, Urien C, Bonneau M, Takamatsu H, Hope J, Mertens P, Meyer G, Stewart M, Roy P, Meurs EF, Dabo S, Zientara S, Breard E, Sailleau C, Chauveau E, Vitour D, Charley B, Schwartz-Cornil I. The double-stranded RNA bluetongue virus induces type I interferon in plasmacytoid dendritic cells via a MYD88-dependent TLR7/8-independent signaling pathway. J Virol. 2012;86:5817–28. doi: 10.1128/JVI.06716-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mouret S, Baudouin C, Charveron M, Favier A, Cadet J, Douki T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc Natl Acad Sci U S A. 2006;103:13765–70. doi: 10.1073/pnas.0604213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foksinski M, Gackowski D, Rozalski R, Siomek A, Guz J, Szpila A, Dziaman T, Olinski R. Effects of basal level of antioxidants on oxidative DNA damage in humans. Eur J Nutr. 2007;46:174–80. doi: 10.1007/s00394-006-0642-7. [DOI] [PubMed] [Google Scholar]
- 41.Grether-Beck S, Wlaschek M, Krutmann J, Scharffetter-Kochanek K. Photodamage and photoaging--prevention and treatment. J Dtsch Dermatol Ges. 2005;3(Suppl 2):S19–25. doi: 10.1111/j.1610-0387.2005.04394.x. [DOI] [PubMed] [Google Scholar]
- 42.Aitken GR, Henderson JR, Chang SC, McNeil CJ, Birch-Machin MA. Direct monitoring of UV-induced free radical generation in HaCaT keratinocytes. Clin Exp Dermatol. 2007;32:722–727. doi: 10.1111/j.1365-2230.2007.02474.x. [DOI] [PubMed] [Google Scholar]
- 43.Poljsak B, Dahmane R. Free radicals and extrinsic skin aging. Dermatol Res Pract. 2012;2012:135206. doi: 10.1155/2012/135206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muthusamy V, Piva TJ. The UV response of the skin: a review of the MAPK, NFkappaB and TNFalpha signal transduction pathways. Arch Dermatol Res. 2010;302:5–17. doi: 10.1007/s00403-009-0994-y. [DOI] [PubMed] [Google Scholar]
- 45.Wolfle U, Esser PR, Simon-Haarhaus B, Martin SF, Lademann J, Schempp CM. UVB-induced DNA damage, generation of reactive oxygen species, and inflammation are effectively attenuated by the flavonoid luteolin in vitro and in vivo. Free Radic Biol Med. 2011;50:1081–93. doi: 10.1016/j.freeradbiomed.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 46.Harberts E, Fishelevich R, Liu J, Atamas SP, Gaspari AA. MyD88 mediates the decision to die by apoptosis or necroptosis after UV irradiation. Innate Immun. 2013 doi: 10.1177/1753425913501706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kodys K, Kurt-Jones E, Szabo G. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224–31. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Q, Nagarkar DR, Bowman ER, Schneider D, Gosangi B, Lei J, Zhao Y, McHenry CL, Burgens RV, Miller DJ, Sajjan U, Hershenson MB. Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. J Immunol. 2009;183:6989–97. doi: 10.4049/jimmunol.0901386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fejer G, Drechsel L, Liese J, Schleicher U, Ruzsics Z, Imelli N, Greber UF, Keck S, Hildenbrand B, Krug A, Bogdan C, Freudenberg MA. Key role of splenic myeloid DCs in the IFN-alphabeta response to adenoviruses in vivo. PLoS Pathog. 2008;4:e1000208. doi: 10.1371/journal.ppat.1000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yogianti F, Kunisada M, Ono R, Sakumi K, Nakabeppu Y, Nishigori C. Skin tumours induced by narrowband UVB have higher frequency of p53 mutations than tumours induced by broadband UVB independent of Ogg1 genotype. Mutagenesis. 2012;27:637–43. doi: 10.1093/mutage/ges029. [DOI] [PubMed] [Google Scholar]
- 51.Shatz M, Menendez D, Resnick MA. The human TLR innate immune gene family is differentially influenced by DNA stress and p53 status in cancer cells. Cancer Res. 2012;72:3948–3957. doi: 10.1158/0008-5472.CAN-11-4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sutherland AM, Walley KR, Nakada TA, Sham AH, Wurfel MM, Russell JA. A nonsynonymous polymorphism of IRAK4 associated with increased prevalence of gram-positive infection and decreased response to toll-like receptor ligands. J Innate Immun. 2011;3:447–58. doi: 10.1159/000323880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joseph T, Zalenskaya IA, Yousefieh N, Schriver SD, Cote LC, Chandra N, Doncel GF. Induction of cyclooxygenase (COX)-2 in human vaginal epithelial cells in response to TLR ligands and TNF-alpha. Am J Reprod Immunol. 2012;67:482–90. doi: 10.1111/j.1600-0897.2011.01099.x. [DOI] [PubMed] [Google Scholar]
- 54.Agrawal T, Bhengraj AR, Vats V, Salhan S, Mittal A. Expression of TLR 2, TLR 4 and iNOS in cervical monocytes of Chlamydia trachomatis-infected women and their role in host immune response. Am J Reprod Immunol. 2011;66:534–543. doi: 10.1111/j.1600-0897.2011.01064.x. [DOI] [PubMed] [Google Scholar]
- 55.Gaspari AA, Fleisher TA, Kraemer KH. Impaired interferon production and natural killer cell activation in patients with the skin cancer-prone disorder, xeroderma pigmentosum. J Clin Invest. 1993;92:1135–42. doi: 10.1172/JCI116682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gläser R, Navid F, Schuller W, et al. UV-B radiation induces the expression of antimicrobial peptides in human keratinocytes in vitro and in vivo. J Allergy Clin Immunol. 2009;123:1117–23. doi: 10.1016/j.jaci.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 57.Grimbaldeston MA, Geczy CL, Tedla N, et al. 100A8 induction in keratinocytes by ultraviolet A irradiation is dependent on reactive oxygen intermediates. J Invest Dermatol. 2003;121:1168–74. doi: 10.1046/j.1523-1747.2003.12561.x. [DOI] [PubMed] [Google Scholar]
- 58.Barkauskaite V, Ek M, Popovic K, et al. Translocation of the novel cytokine HMGB1 to the cytoplasm and extracellular space coincides with the peak of clinical activity in experimentally UV-induced lesions of cutaneous lupus erythematosus. Lupus. 2007;16:794–802. doi: 10.1177/0961203307081895. [DOI] [PubMed] [Google Scholar]
- 59.Hiramoto K, Kobayashi H, Yamate Y, et al. Intercellular pathway through hyaluronic acid in UVB-induced inflammation. Exp Dermatol. 2012;21:911–4. doi: 10.1111/exd.12032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Choice of BA dose based on the proliferative activity of PAM212 cells. Dose-dependent effect of BA on the cell death of PAM212 keratinocytes was determined by MTT assay, resulting in the selection of 50 μg/ml BA for further experimentation. The cells were treated with 0 or 50 μg/ml BA for 24 hours before and after, with or without 100 mJ/cm2 UVB irradiation. 50 μg/ml BA treatment remarkably reduced the percent cell death induced by UVB irradiation. The values are represented as the percentage of cell death. The data represent the mean ± SD of three independent experiments each conducted in triplicate. (#P<0.05 and ##P <0.01 vs control; *P > 0.05 vs UVB).