Abstract
Purpose
To investigate the involvement of hsa-microRNA-195-5p (miR-195) in progression and prognosis of human prostate cancer (PCa).
Experimental Design
qRT-PCR was performed to detect miR-195 expression in both PCa cell lines and clinical tissue samples. Its clinical significance was statistically analyzed. The roles of miR-195 and its candidate target gene ribosomal protein S6 kinase, 70kDa, polypeptide 1 (RPS6KB1) in PCa progression were confirmed based on both in vitro and in vivo systems.
Results
MiR-195 downregulation in PCa tissues was significantly associated with high Gleason score (P=0.001), positive metastasis failure (P<0.001) and biochemical recurrence (BCR, P<0.001). Survival analysis identified miR-195 as an independent prognostic factor for BCR-free survival of PCa patients (P=0.022). Then, we confirmed the tumor suppressive role of miR-195 through PCa cell invasion, migration and apoptosis assays in vitro, along with tumor xenografts growth, angiogenesis and invasion in vivo according to both gain-of-function and loss-of-function experiments. Additionally, RPS6KB1 was identified as a novel direct target of miR-195 through proteomic expression profiling combined with bioinformatic target prediction and luciferase reporter assay. Moreover, the re-expression and knockdown of RPS6KB1 could respectively rescue and imitate the effects induced by miR-195. Importantly, RPS6KB1 expression was closely correlated with aggressive progression and poor prognosis in PCa patients as opposed to miR-195. Furthermore, we identified MMP-9, VEGF, BAD and E-cadherin as the downstream effectors of miR-195-RPS6KB1 axis.
Conclusion
The newly identified miR-195-RPS6KB1 axis partially illustrates the molecular mechanism of PCa progression and represents a novel potential therapeutic target for PCa treatment.
Keywords: prostate cancer, microRNA-195, ribosomal protein S6 kinase, 70kDa, polypeptide 1, tumor suppressor, prognosis
Introduction
Clinical behavior of prostate cancer (PCa) ranges from indolent tumors with no or little clinical significance to aggressive metastatic and lethal diseases (1). Treatment options for PCa depend on clinicopathological features of the disease, such as TNM stage, serum prostate-specific antigen (PSA), surgical margin status and Gleason score. However, these factors explain only a moderate proportion of the observed heterogeneity in treatment outcome (2–4). Therefore, it is urgent to better understand the molecular mechanism underlying the progressive process of PCa and to identify more accurate predictors for stratifying patients into indolent and aggressive cases, so that the optimal therapeutic strategies can be decided.
MicroRNAs (miRNAs) represent the best characterized class of small (19–25 nt in length) non-coding RNA transcripts that are endogenously expressed in animal and plant cells (5–7). Due to their important roles in the regulation of genes that are involved in many physiological processes, including cell development, proliferation, differentiation, and apoptosis, it is not of a surprise that miRNAs are also involved in the initiation and progression of various human diseases such as cancer (8). miRNAs can either serve as a tumor suppressor or as an oncogene, depending on the genes they target (9–10). According to the Taylor dataset, we noticed that hsa-miR-195-5p (miR-195) has a potential as a marker for biomedical recurrence of PCa patients. It is located from 6881953 bp to 6862065 bp on chromosome 17p13.1 and belongs to the miR-15/16/195/424/497 family (11). It has been reported to be upregulated as an oncogene in malignant melanoma, while down-regulated as a strong tumor suppressor in breast cancer, hepatocellular carcinoma, adrenocortical carcinoma, squamous cell carcinoma of tongue and esophageal cancer (12–17). However, up to now, its roles in PCa are still unclear.
To address this problem, we performed the current study to evaluate the associations of miR-195 dysregulation with cancer progression and patients' prognosis of PCa, and to further investigate the underlying molecular mechanisms according to the technical strategy shown in Fig. 1.
Fig. 1.
A schematic diagram of the systematic strategies for uncovering the involvement of miR-195 in human prostate cancer (PCa)
Materials and methods
Patients and Tissue Samples
The study was approved by the human study ethics committees at MGH, Boston, MA and the Ministry of Public Health of P.R. China. Written informed consent was obtained from all patients. All specimens were handled and made anonymous according to the ethical and legal standards.
Detailed information on patients and tissue samples was summarized in Supplementary File S1.
Cell culture
Normal human prostate epithelial cells (PrEC) were purchased from Lonza Company in 2012 and were cultured in PrEGM Bullet kit (Lonza, USA) with antibiotics. Human PCa cell lines, PC-3, LNCaP and DU145 were purchased from the American Type 6 Culture Collection (Manassas, VA, USA) in 2012 and were cultured in RPMI 1640 medium (Hyclone, USA) supplemented with 10% fetal bovine serum (Gibico, USA), 2 mM L-glutamine, and antibiotics. The companies performed cell lines characterizations and passaged in the laboratory for about 3 months after resuscitation. Therefore, we did not carry out the reauthentication of the cell lines. They used Short Tandem Repeat (STR) profiling to detect the misidentified, cross-contaminated, or genetically drifted cells. Promega’s PowerPlex® 18D System was used to amplify 17 STR loci plus Amelogenin. All cell lines were maintained at 37 □ in a humidified chamber supplemented with 5% CO2.
Animals
Animal experiments in this study were performed in compliance with the guidelines of the Institute for Laboratory Animal Research at Guangzhou Medical University, Guangzhou, P.R.China. Twenty BALB/c nude mice (4~5-week-old males) were purchased from Guangdong Medical Laboratory Animal Center and were housed five per cage in wire-top cages with sawdust bedding in an isolated, clean, air-conditioned room at a temperature of 25–26 □ and a relative humidity of ~50%, lit 12 h/day.
Cell lines construction and transfection
The miR-195 coding sequence was cloned into the pMIRNA1 lentivectors (Human pre-microRNA Expression Construct Lenti-miR-195 MI0000489, Cat No: PMIRH195PA-1, SBI, USA) for expressing the miR-195 precursor (miR-195). Precursor Scramble control hairpin in pCDH-CMV-Scramble hairpin-EF1-copGFP (CD511B-1) was purchased from the same vendor (Cat No: PMIRH000PA-1, SBI, USA) for negative controls (miR-NC). The miRZip anti-microRNA Constructs (miRZip-195 anti-miR-195 microRNA construct, Cat No: MZIP195-PA-1) and miRZip control vectors (pGreenPuro Scramble Hairpin Control-Construct, Cat No: MZIP000-PA-1) were designed and cloned by SBI (USA). To package the construct, 293TN cells were transfected with miR-195/miR-NC or anti-195/anti-NC by pPACKH1 Packaging Plasmid Mix (Cat No: LV500A-1, SBI, USA), and then after 3 days, the virus particles were collected according to the packaging protocol of SBI with the Lenti-Concentin Virus Precipitation Solution (Cat No: LV810A-1, SBI, USA). DU145 and LNCaP cells were infected with TransDux virus transduction reagent (Cat No: LV850A-1, SBI, USA). The infected cells were isolated with a flow cytometer and cultured in 96-well plates.
Oligonucleotide and plasmid transfection
The miRNA mimics (Cat.No: miR10000461-1–5) and miRNA mimics negative control (Cat.No: miR01201-1–5) that were used for transient transfection were designed and synthesized by RiboBio (Guangzhou, China). The RPS6KB1 coding sequence (without 3’-UTR) was cloned into pCDNA3.1(+)-Vector (Cat.No: V790-20, Invitrogen, USA). The blank vector was used as negative control. Short interfering RNA (siRNA) against RPS6KB1 (si-RPS6KB1) and negative control (si-NC) with non-specific sequences was synthesized by Sigma (USA). The sequence of siRNA targeting RPS6KB1 was as follow: 5’-CCACAAUCGUGCUGUGGAUdTdT-3’ (sense), 5’-AUCCACAGCACGAUUGUGGdTdT-3’ (antisense). The sequence of siRNA negative control was as follow: 5’-UUCUCCGAACGUGUCACGUTT-3’ (sense), 5’-ACGUGACACGUUCGGAGAATT-3’ (antisense). Cells were transfected with miRNA mimic, siRNA and pCDNA3.1(+)-RPS6KB1 using Lipofectamine 2000 Reagent (Cat.No: 11668019, Invitrogen, USA) according to the manufacturer’s protocol. For migration, invasion, apoptosis, proliferation, RNA extraction and western blot assays, cells were used 48 hours after transfection.
Proteomics expression profiling by mass spectrometric analysis using an isobaric tagging reagent iTRAQ
Identification of novel targets for miR-195 by proteomic expression profiling with iTRAQ tagging was performed according to the protocol as described in Supplementary File S2.
Bioinformatic miRNA target prediction
Three online programs Target-Scan (release 6.2) (18), miRWalk (Last Update: March 29, 2011) (19), and miRanda (August 2010 Release, Last Update: November 01, 2010) (20) were used to predict potential target genes for miR-195.
qRT-PCR
Expression levels of miR-195, SMAP2, DCUN1D1, SMAD4, IPO9, RPS6KB1, CAPZA2, and CPNE1 mRNA in PCa cell lines, xenograft tumors and clinical PCa tissues were detected by qRT-PCR analysis according to the protocol of our previous studies (21, 22). The sequences of all the primers used in this study were shown in Supplementary Tab. S2.
Western blot analysis
Expression levels of RPS6KB1, MMP-9, VEGF, BAD and E-cadherin proteins in PCa cell lines, xenograft tumors and clinical PCa tissues were detected by Western blot analysis according to the protocol of our previous studies (21, 22). The antibodies used in this study were shown in Supplementary Tab. S3.
Immunohistochemistry
Expression pattern and subcellular localization of RPS6KB1 protein in clinical PCa tissues, and those of CD31 and Vimentin proteins in subcutaneous tumor xenografts of nude mice were detected by immunohistochemistry and the immunoreactivity scores (IRS) of Vimentin were calculated according to the protocol of our previous studies (21, 22). Vasculature density in tumor xenografts was determined by the number of CD31 positive vessels. For Immunohistochemistry evaluation of RPS6KB1 in clinical PCa tissues, tumor specimens were scored as positive if greater than 10% of the tumor cells exhibited immunoreactivity. The antibodies used in this study were shown in Supplementary Tab. S3.
Generation of the in vivo xenograft model
For the in vivo tumor formation assays, DU145 or LNCaP cells transfected with the miR-195, miR-NC, anti-195 or anti-NC lentivectors were trypsinized and suspended in Phosphate-buffered saline (PBS). Then, the cells were subcutaneously injected into the flanks of each nude mouse (5 per group). DU145 cells were subcutaneously injected at a concentration of 1 × 106 cells. LNCaP cells were subcutaneously injected as a mixture of 2 × 106 cells and an equal volume of Matrigel (Cat No: 356234, BD Biosciences), reaching a total concentration of 10 mg/mL. The tumor sizes were measured at 4-day intervals as soon as the tumors were measurable and the tumor volumes were calculated: V(mm3)=width2(mm2) ×length (mm) / 2. On day 44 for LNCaP and day 36 for DU145 groups, the mice were sacrificed. The mice were manipulated and housed according to protocols approved by the Institute for Laboratory Animal Research at Guangzhou Medical University.
Luciferase reporter assay
The expression of miR-195-targeted gene was evaluated by using a luciferase reporter assay in LNCaP cells. The putative miR-195 complementary site in the 3'-UTR of RPS6KB1 mRNA (NM_001272060; 3'-UTR: 1624–1630) or its mutant sequence was cloned into the psiCHECK-2 luciferase reporter vector (Promega, USA). LNCaP cells were co-transfected with 50 nM miR-195 mimic (Cat No: miR10000461-1–5, RiboBio, Guangzhou, China) or the negative control (Cat No: miR01201-1–5, RiboBio, Guangzhou, China) and 0.5µg of psiCHECK-2-RPS6KB1–3’UTR-WT or psiCHECK-2-RPS6KB1–3’UTR-MUT. Cells were collected 48 h after transfection and analysed with the Dual-Luciferase Reporter Assay System (Promega, CA, USA). The firefly and Renilla luciferase signals were generated by the GloMax fluorescence reader (Promega), the Renilla luciferase signal normalized to the firefly luciferase signal.
Cell invasion and migration assays
Cell invasion and migration were respectively detected by the Transwell and the scratch wound-healing motility assay according to the protocol of our previous studies (21, 22).
Apoptosis assay
Cell apoptosis was detected using APC-conjugated Annexin V (Annexin V-APC) Kit (Cat No: 550474, BD Biosciences, USA) and 7-aminoactinomycin D (7-AAD) (Cat No: AP104-60-AAD, Multisciences, China) according to the protocol of our previous studies (21, 22).
Statistical analysis
The version13.0 SPSS for Windows (SPSS Inc, IL, USA) and SAS 9.1 (SAS Institute, Cary, NC) softwares were used for statistical analysis. Continuous variables were expressed as X̅ ± s. Statistical analyses of qRT-PCR and Western blot were conducted using Wilcoxon signed-rank test. Statistical analysis was performed independently by two biostatisticians with Fisher’s exact test for any 2×2 tables and Pearson χ2 test for non-2×2 tables. Kolmogorov-Smirnov (K–S) test was used to test for normality of the distribution of miR-195 expression level. Mann-Wnitney U and Kruskal-Wallis H tests were performed to examine the associations between miR-195 expression and clinicopathological characters of PCa patients in Taylor dataset. Kaplan-Meier method was used for the survival analysis and Cox regression analysis was used for the univariate and multivariate analysis. The Spearman correlation was calculated between the expression levels of miR-195 and RPS6KB1 in PCa tissues. Differences were considered statistically significant when the p value was less than 0.05.
Results
Decreased expression of miR-195 in human PCa cells and tissues
Expression levels of miR-195 in three human PCa cell lines (LNCaP, DU145, and PC-3) and PCa tissues were respectively lower than those in a non-malignant prostate epithelial cell line (PrEC) and adjacent non-cancerous prostate tissues (Supplementary Fig. S1A and B). Among the three PCa cell lines, DU145 cells had the lowest miR-195 expression. LNCaP cells are androgen-sensitive human PCa cells, while DU145 cells are not hormone sensitive. Thus, DU145 and LNCaP cells were chosen for further studies.
Decreased expression of miR-195 associates with the aggressive progression of human PCa
To investigate whether the expression of miR-195 was associated with clinicopathological features of PCa patients, a publicly available data set (Taylor dataset) consisting of miRNAs expression profiles for 113 primary PCa tissues was used (23). The data shown in Supplementary Tab. S4 revealed that miR-195 downregulation was frequently found in PCa tissues with high Gleason score (P=0.001), positive metastasis failure (P<0.001) and biochemical recurrence (P<0.001).
Decreased expression of miR-195 predicts poor prognosis of human PCa
To evaluate the prognostic value of miR-195 expression in PCa, the Kaplan-Meier method was performed to analyze the correlations between miR-195 expression with BCR-free survival, overall survival, non-metastatic BCR-free survival and non-metastatic survival of PCa patients in Taylor dataset. Pairwise comparisons showed a significant difference in the BCR-free survival (Supplementary Fig. S2A) and non-metastatic BCR-free survival (Supplementary Fig. S2B) between patients with high and low miR-195 expression. However, the data revealed that there were no significant differences in overall survival and non-metastatic survival between high and low miR-195 expression group (Supplementary Fig. S2C and D). Multivariate analysis revealed that down-regulation of miR-195 had the potential to serve as an independent predictor for shorter BCR-free survival (Supplementary Tab. S5).
miR-195 suppresses invasion and migration, but promotes apoptosis of PCa cells in vitro
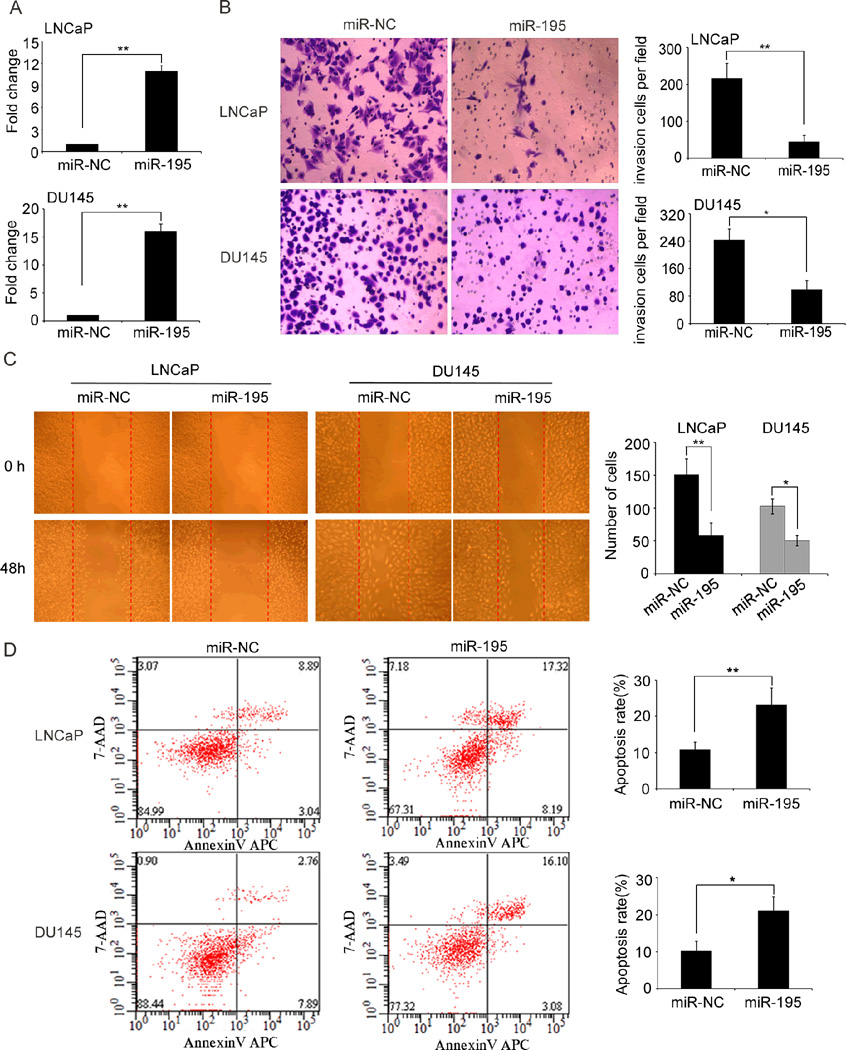
To determine the suppressive role of miR-195 in prosate cancer in vitro, we first constructed a lentiviral vector expressing miR-195 and established stable cell lines LNCaP and DU145 expressing miR-195 after lentivectors transduction. qRT-PCR analysis confirmed that the cell lines were successfully established (Fig. 2A). Transwell assays clearly revealed that enforced expression of miR-195 significantly reduced the invasive activities of both DU145 and LNCaP cells compared with those of control cells (Fig. 2B). Wound-healing assays demonstrated that miR-195 upregulation markedly weakened the migratory abilities of both DU145 and LNCaP cells (Fig. 2C). In contrast, the apoptotic rates of miR-195-transfected DU145 and LNCaP cells were significantly higher than those of control cells (Fig. 2D). Moreover, we also stably suppressed the miR-195 expression in LNCaP and DU145 cell lines via lentivectors transduction (Supplementary Fig. S3A). Intriguingly, the knockdown of miR-195 expression with lentivectors in LNCaP and DU145 cell lines could dramatically enhance the abilities of cellular invasion, motility and reduce cellular apoptosis (Supplementary Fig. S3B–D).
Fig. 2. The re-expression of miR-195 inhibits invasion and migration, but promotes apoptosis of LNCaP and DU145 cells in vitro.
(A). miR-195 was re-expressed in the miR-195-stably transfected cell lines LNCaP and DU145. miR-195 levels were determined by qRT–PCR after transfection. miR-195 expression (miR-195/U6) was calculated as the fold change relative to the negative control (NC). (B). Transwell analysis showed that miR-195 overexpression suppressed invasive abilities of LNCaP and DU145 cells. Statistical analysis was performed with three independent experiments. (C). Woundhealing assays indicated that miR-195 upregulation inhibited migration of LNCaP and DU145 cells. Statistical analysis was performed with three independent experiments. (D). Enforced expression of miR-195 promoted the cell apoptosis of LNCaP and DU145 cells. Statistical analysis was performed with three independent experiments. Data were presented as Mean ± SD. *P<0.05, **P<0.01 compared with negative control.
miR-195 suppresses tumor growth, angiogenesis and invasion in vivo
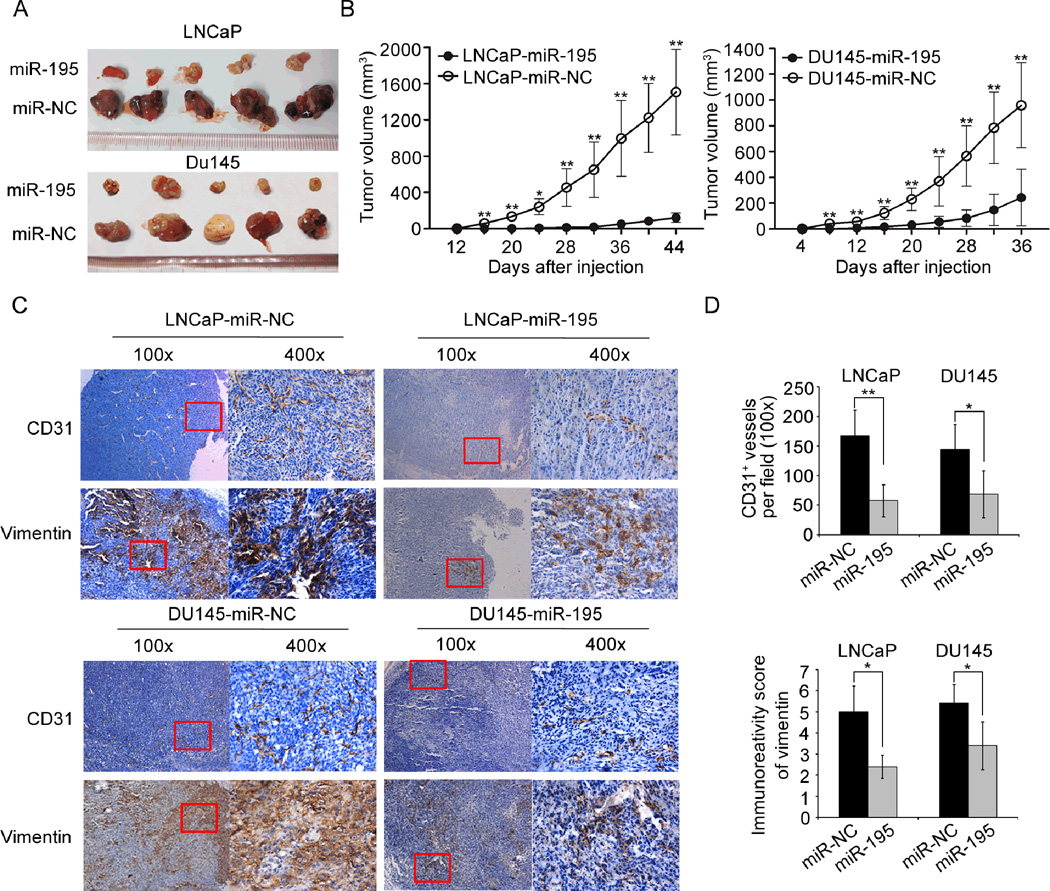
To better evaluate the biological functions of miR-195 in vivo, LNCaP and DU145 PCa cell lines stably expressing miR-195 or vector control via lentivectors transduction were used. The miR-195-overexpressed cell lines (miR-195) were subcutaneously injected into the flank of each male nude mice, simultaneously, the vector control PCa cell lines (miR-NC) were subcutaneously injected into the other flank of the same mice. The LNCaP and DU145 cells stably expressing miR-195 formed significantly smaller tumor nodules (Fig. 3A) and remarkably slowed tumor xenografts growth compared with the controls (Fig. 3B). On the other hand, we further found that the PCa cells that permanently suppressed the expression of miR-195 with lentivectors could enhance tumor growth compared with the controls (Supplementary Fig. S4A and B). Next, immunohistochemical analysis using pan-endothelial marker CD31 and Vimentin antibody, which is a marker of mesenchymally-derived cells or cells undergoing an epithelial-to-mesenchymal transition (EMT) (24), were employed to evaluate the angiogenesis and invasive tendency of the tumor xenografts. The results indicated that the expression level of CD31 and Vimentin protein in the tumor xenografts established by LNCaP or DU145 cells stably expressing miR-195 was remarkably lower than that in the xenografts established by cells transfected with control vectors (Fig. 3C–D). Moreover, the tumor xenografts established by LNCaP or DU145 cells with low miR-195 expression presented significantly more CD31 and Vimentin protein than the control xenografts (Supplementary Fig. S4C–D). These results strongly demonstrated that miR-195 could significantly inhibit tumor growth, angiogenesis and invasion in vivo.
Fig. 3. miR-195 suppresses PCa growth, angiogenesis and invasion in vivo.
(A). Lentivectors mediated overexpression of miR-195 in LNCaP and DU145 cells significantly retarded subcutaneous tumor growth. Tumor growth was followed for 44 days (LNCaP) or 36 days (DU145) after tumor cells injection. (B). The tumor growth curve is shown. LNCaP (n=5) and DU145 (n=5) cells transfected with lentivectors expressing miR-195 or the mock control were subcutaneously injected into nude mice. The tumor sizes were measured at 4-day intervals as soon as the tumors were measurable. *P<0.05 and **P<0.01 by Independent-Samples t test. Data were presented as Mean ± SD. (C). Immunochemistry analysis of the tumor xenografts. CD31 stained the cytomembrane or cytoplasm of the pan-endothelial cells of angiogenesis. Vimentin stained the cytoplasm of the mesenchymally-derived or undergoing an EMT PCa cells (shown in the fields at a magnification of ×400). CD31 staining results indicated that the number of angiogenesis in tumor xenografts established by cells overexpressing miR-195 was significantly reduced compared with that in control tumor xenografts. Vimentin staining results indicated that the tumor xenografts established by cells overexpressing miR-195 expressed less Vimentin. The representative fields used for statistical analysis were presented at a magnification of ×100. (D). Vasculature density in tumor xenografts and Immunoreativity score of Vimentin, as determined by immunohistochemistry (at a magnification of ×100). The results were presented as Mean ± SD. *P<0.05, **P<0.01 compared with negative control.
Proteomics analysis of miR-195-induced changes in protein synthesis
To identify direct targets of miR-195 in PCa, we performed differential tagging with iTRAQ followed by nano-reversed phase liquid chromatography (nano-LC) and tandem mass spectrometry (MS/MS) analysis on LNCaP cells (miR-195 vs miR-NC) to detect miR-195-induced changes in protein synthesis. As a result, a total of 3194 proteins were identified, when detection of at least two matching peptides per protein was set as a requirement for unambiguous identification. All proteins were reliably quantified in at least two experiments. Of note, ectopic expression of miR-195 caused only moderate changes in overall protein synthesis, with the majority of proteins having miR-195/miR-NC ratios between −1.5 and 1.5. Among the 3194 proteins quantified in at least two experiments, only 78 (2.44 %) were differentially regulated with fold changes ≤-1.5 or ≥1.5 (miR-195 vs miR-NC), including 50 down-regulated proteins and 28 up-regulated proteins (Supplementary Tab. S6). Consistent with these findings, recent studies have indicated that ectopic miRNA expression often exerts overall moderate effects on global protein synthesis (25).
Since miRNAs are non-coding RNA transcripts and they can only execute their biological functions through their direct and indirect targets which are coding genes, we then investigated the molecular role of all the differentially-expressed proteins induced by miR-195. Pathway and biological functional enrichment analyses respectively based on Ingenuity Pathways Analysis (IPA) and GO annotation system gave us a global clue of their functional roles in PCa progression. Among the pathways enriched by candidate targets of miR-195, we concentrated on tumor-related pathways, such as mTOR signaling, IL-8 signaling, AMPK and IGF-1 signaling pathways (Supplementary Fig. S5 and Supplementary Tab. S7), which all have broad effects on cell behavior. On the other hand, GO annotation system uses a controlled and hierarchical vocabulary to assign function to genes or gene products in any organism. The changed proteins induced by miR-195 significantly controlled many biological processes directly relevant to cancers, such as cell cycle, cell morphology, cell assembly and organization, and cellular movement (Supplementary Tab. S8), which are involved in multiple steps of cell viability, and evidences had shown that dysregulations of the cell viability components may lead to tumor formation. These results suggested that the candidate targets of miR-195 may control broad biological functions associated with PCa.
RPS6KB1 is the direct target of miR-195
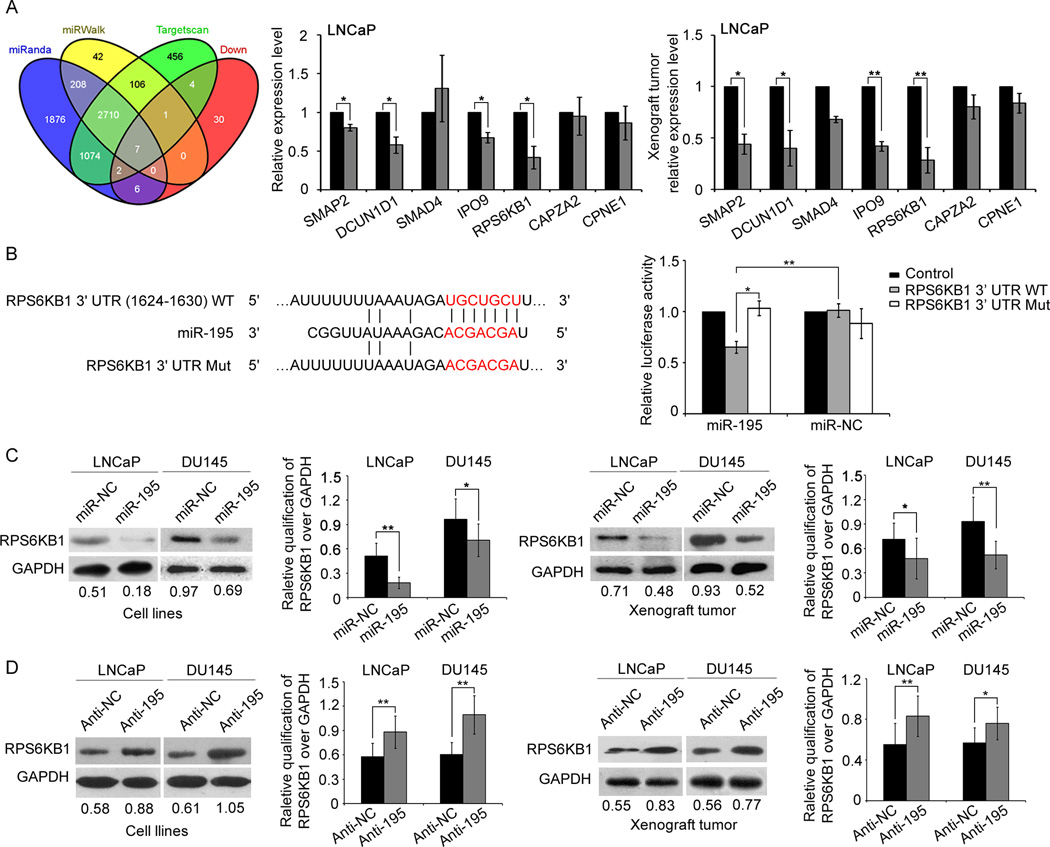
We also used three miRNA target predicting programs (Target-Scan, miRWalk, and miRanda) to identify the candidate targets of miR-195. As a result, the three programs all predicted RPS6KB1, SMAP2, DCUN1D1, SMAD4, IPO9, CAPZA2, and CPNE1 as candidate targets of miR-195. Moreover, they also encoded the corresponding down-regulated proteins according to the results of proteomics analysis of miR-195-induced changes in protein synthesis (Fig. 4A). To verify this prediction, qRT-PCR analysis was performed and the results showed that the endogenous SMAP2, RPS6KB1, IPO9 and DCUN1D1 expression in cells and established tumors associated with LNCaP cells stably expressing miR-195 were all significantly reduced at mRNA levels (Fig. 4A). Among them, RPS6KB1 was the most significantly down-regulated gene. More importantly, RPS6KB1 is an important component in mTOR signaling pathway, which was one of the significantly enriched pathways involved by miR-195-induced differentially-expressed proteins (Supplementary Fig. S5A and Supplementary Tab. S7). Therefore, we would like to focus on the biological role of miR-195-RPS6KB1 axis during PCa progression in further experiments.
Fig. 4. miR-195 downregulates RPS6KB1 expression by directly targeting its 3′-UTR.
(A). Overlap of three miRNA target bioinformatic prediction methods and miR-195-induced down-regulated proteins. qRT-PCR analysis shown that the endogenous SMAP2, RPS6KB1, IPO9 and DCUN1D1 expression in cells and established tumors associated with LNCaP cells stably expressing miR-195 were all significantly reduced at mRNA levels. Data were presented as Mean ± SD. *P<0.05, **P<0.01. (B). RNA sequence alignment showing the 3'-UTR of RPS6KB1 mRNA contains a complementary site for the seed region of miR-195. RPS6KB1 mut is a mutant with substitutions in the complementary region as a negative control. Dual luciferase reporter assay was performed to confirm the miR-195 binding target. The luciferase activity was detected after transfection of psiCHECK-2 luciferase reporter vector (psiCHECK-2-RPS6KB1-3’UTR-WT or psiCHECK-2-RPS6KB1-3’UTR-MUT) into the miR-195 or miR-NC transfected LNCaP cells. Data were presented as Mean ± SD. *P<0.05, **P<0.01. (C). Enforced expression of miR-195 suppressed the expression of RPS6KB1 protein in both PCa cell lines and the corresponding tumor xenografts. (D). Expression of RPS6KB1 protein was increased in both PCa cell lines and the corresponding tumor xenografts following the knockdown of miR-195. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was measured as internal control. The semi-quantification of RPS6KB1 protein was measured relative to GAPDH. *P<0.05, **P<0.01 compared with control. Data were presented as Mean ± SD.
To confirm RPS6KB1 being targeted by miR-195, the luciferase reporter containing the complimentary seed sequence of miR-195 at the 3'-UTR region of RPS6KB1 mRNA was constructed (Fig. 4B). Luciferase activity assay showed that the expression of the reporter of RPS6KB1 was significantly reduced by co-transfection with hsa-miR-195 mimics. In contrast, the expression of the reporter of RPS6KB1 containing the mutated sequence of the same fragment was not affected by co-transfection with hsa-miR-195 mimics (Fig. 4B). The result indicated that the fragment at the 3'-UTRs of the RPS6KB1 mRNA was the complementary site for the miR-195 seed region, suggesting that RPS6KB1 was the direct target of miR-195.
We performed western blot to detect the expression levels of RPS6KB1 protein in LNCaP and DU145 cells transfected by lentivectors and in the tumor xenografts established by these PCa cells. RPS6KB1 protein levels were remarkably down-regulated in LNCaP or DU145 cells stably overexpressing miR-195 (Fig. 4C). The similar findings were observed in the corresponding tumor xenografts established by cell lines overexpressing miR-195 (Fig. 4C). In line with the above gain-of-function experiments, we also knocked down the expression of miR-195 in LNCaP or DU145 cells using anti-miR-195 lentivectors and found that the RPS6KB1 protein levels in cell lines and corresponding tumor xenografts were all significantly up-regulated (Fig. 4D). Overall, miR-195 negatively regulated RPS6KB1 expression in vitro and in vivo.
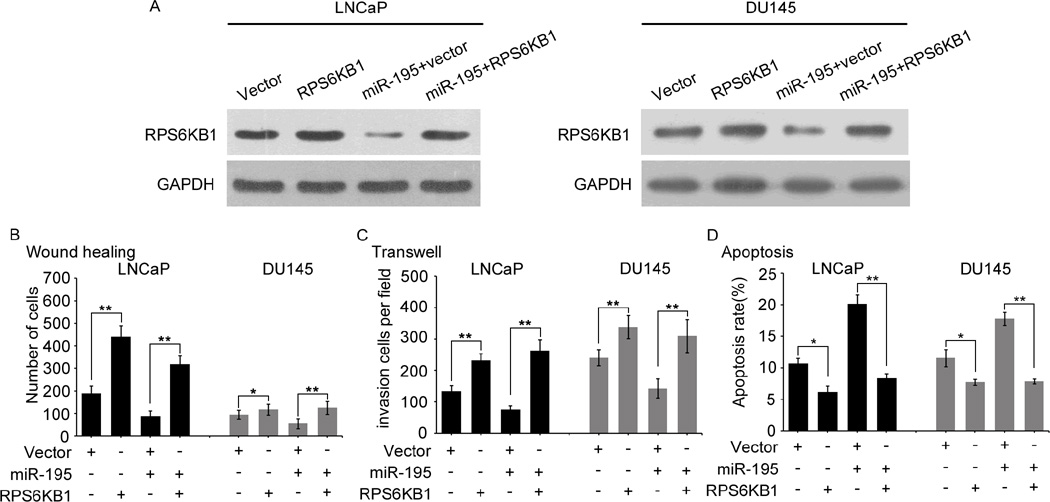
RPS6KB1 is a critical downstream mediator of miR-195 suppressive effects in PCa progression
To clarify whether the role of miR-195 in PCa was mediated through suppressing RPS6KB1 expression, pCDNA3.1(+)-Vectors expressing RPS6KB1 without its 3’-UTR were conducted. As shown in the Fig. 5A, the endogenous RPS6KB1 expression levels detected by western blot in LNCaP and DU145 cells transfected with the miR-195 mimics in the presence of RPS6KB1 or vector control for 48h. Migration (Fig. 5B and Supplementary Fig. S7A), invasion (Fig. 5C and Supplementary Fig. S7B), and apoptosis (Fig. 5D and Supplementary Fig. S7C) assays all indicated that restoration of RPS6KB1 expression dramatically attenuated the effects induced by miR-195. Further, to verify whether inhibition of RPS6KB1 expression could generate similar in vitro phenotypes induced by miR-195 overexpression, we knocked down the endogenous RPS6KB1 protein expression in LNCaP and DU145 cells with RPS6KB1 siRNA (Supplementary Fig. S6A). In vitro results showed that the knockdown of endogenous RPS6KB1 expression could imitate the effects associated with miR-195 overexpression in PCa cells, including inhibiting the abilities of migration (Supplementary Fig. S6B and Supplementary Fig. S7A) and invasion (Supplementary Fig. S6C and Supplementary Fig. S7B), as well as promoting cellular apoptosis (Supplementary Fig. S6D and Supplementary Fig. S7C). Collectively, these findings indicated that RPS6KB1 was a critical mediator in the tumor suppressive roles of miR-195 in PCa.
Fig. 5. The re-expression of RPS6KB1 could rescue the tumor suppressive effects of miR-195.
(A). The endogenous RPS6KB1 expression levels were detected by western blot in LNCaP and DU145 cells transfected with the miR-195 mimic in the presence of RPS6KB1 or vector control for 48 h. (B–D). Expression of RPS6KB1 using a construct lacking its 3′-UTR rescued the biological effects associated with re-introduction of miR-195, through cell migration, invasion and apoptosis assays. These experiments were carried out after the co-transfection of miR-195 mimics and/or the RPS6KB1 3′-UTR (−) for 48h. The data were from at least three independent experiments and presented as Mean ± SD. *P<0.05, **P<0.01 compared with control.
Reverse correlation between miR-195 and RPS6KB1 expression in human PCa tissues
We have observed that the expression of miR-195 was down-regulated in PCa tissues (Supplementary Fig. S1B). In contrast to miR-195, western blot and qRT-PCR analyses revealed that RPS6KB1 expression at protein and mRNA levels were both up-regulated in the same human PCa tissues compared with adjacent non-cancerous prostate tissues (Supplementary Fig. S8A–C). Importantly, the Spearman Correlation analysis clearly presented a negative correlation between RPS6KB1 mRNA and miR-195 expression in PCa tissues (Supplementary Fig. S8D).
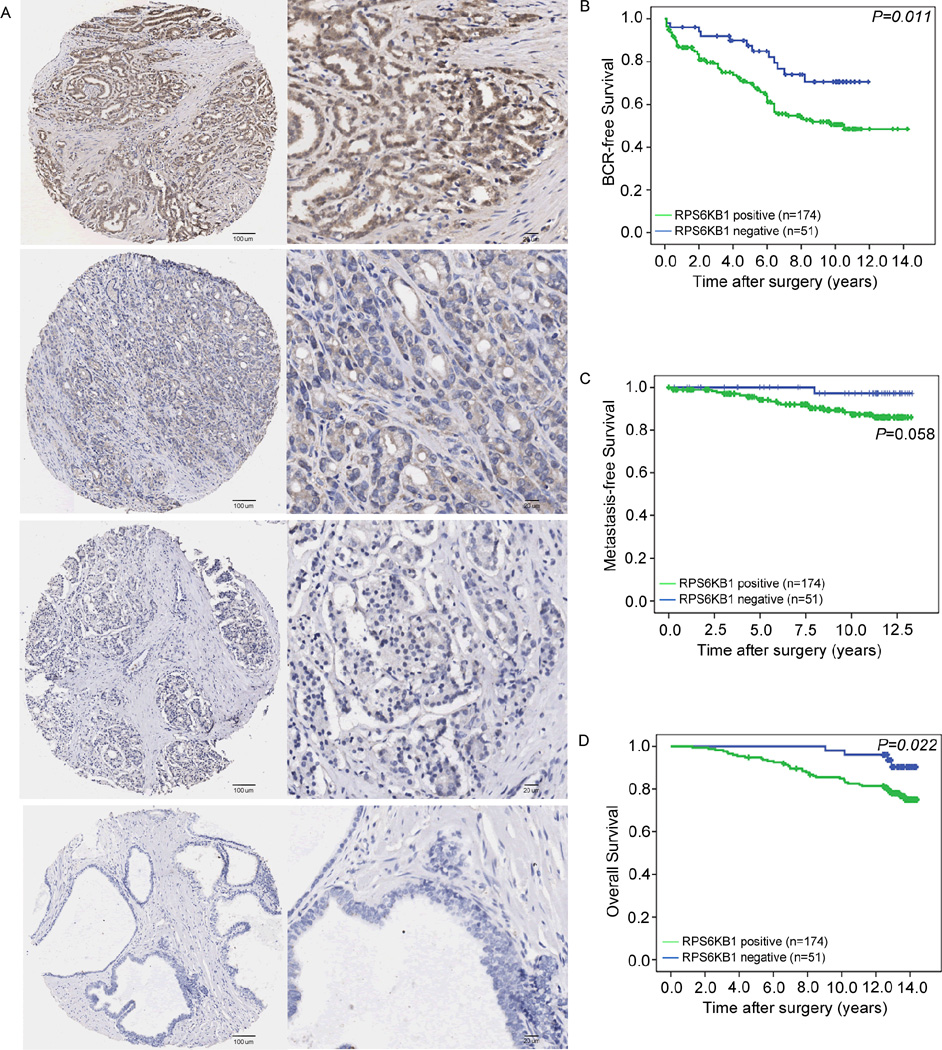
RPS6KB1 positive expression associates with the aggressive progression and poor prognosis of human PCa
To investigate whether RPS6KB1 expression could be linked to the clinicopathological features of human PCa, the immunohistochemical staining using the antibody that specifically recognizes RPS6KB1 was employed to detect the expression pattern and subcellular localization of RPS6KB1 expression in 225 PCa and 25 adjacent non-cancerous prostate tissues. In general, we found that this antibody stained the cytoplasm and cellular membrane of PCa cells and gave evenly distributed staining pattern with various intensities (Fig. 6A). The positive expression rate of RPS6KB1 protein in PCa clinical samples [174/225 (77.3%)] was significantly higher than that in adjacent benign tissues [10/25 (40%), (P<0.001)].
Fig. 6. Immunohistochemical staining of RPS6KB1 in PCa and adjacent benign prostate tissues and its prognostic implications.
(A). Positive RPS6KB1 staining with weak, moderate and strong expression levels in PCa was found in cell membrane and cytoplasm. Besides, negative RPS6KB1 staining was presented in benign prostate tissues. (Left panel: magnification x50; right panel: magnification x200). (B–D). RPS6KB1 protein expression showed a prognostic value in BCR-free and overall survival, but not in metastasis-free survival, as indicated by Kaplan–Meier analysis.
Then, we analyzed 225 radical prostatectomy specimens represented in TMA from PCa patients. The data shown in Supplementary Tab. S4 revealed that RPS6KB1 positive expression was significantly associated with advanced pathological stage (P=0.036), positive surgical margin status (P=0.02), positive BCR (P=0.017) and shorter overall survival (P=0.02) of PCa patients. Kaplan-Meier analysis was conducted to assess the prognostic value of RPS6KB1 expression in human PCa. We found that there were significant differences in the BCR-free survival (P=0.011, Fig. 6B) and overall survival (P=0.022, Fig. 6D), but not in metastasis-free survival (P=0.058, Fig. 6C), between patients with positive and negative RPS6KB1 expression.
Univariate analysis [(a) in Supplementary Tab. S9)] revealed that RPS6KB1 expression (P=0.014 and 0.03, respectively) and Gleason score (P<0.001 and P=0.001, respectively) were significant prognostic factors for BCR-free survival and overall survival in patients with PCa. Our analysis also demonstrated that pathological tumor stage (P=0.002) and surgical margin (P=0.001) were significant prognostic factors for BCR-free survival but did not show any correlative relationship with overall survival in patients with PCa [(a) in Supplementary Tab. S9]. Besides, PSA failure (P=0.006) was a significant prognostic factor for overall survival. We then used the Cox proportional hazards multivariate model to examine the association of clinicopathological factors and RPS6KB1 staining status with BCR-free survival and overall survival. The results indicated that only RPS6KB1 expression and Gleason score were independent predictors of BCR-free survival and overall survival in PCa patients [(b) in Supplementary Tab. S9].
MMP-9, VEGF, BAD and E-cadherin function as the downstream effectors of miR-195-RPS6KB1 axis
Growing evidences suggest that RPS6KB1 may be required for tumor cells survival, tumor invasion and angiogenesis through inactivating the pro-apoptotic molecule BAD and the epithelial marker E-cadherin, inducing expression of MMP-9 and VEGF, respectively (26–29), which prompted us to determine whether MMP-9, VEGF, BAD and E-cadherin were downstream effectors of miR-195-RPS6KB1 axis in human PCa. As shown in Supplementary Fig. S9A, we found that enforced expression of miR-195 and knockdown of RPS6KB1 protein in two PCa cell lines LNCaP and DU145 both significantly reduced the protein expression levels of MMP-9 and VEGF, but increased those of E-cadherin and BAD, implying the four proteins might function as the downstream effectors of miR-195-RPS6KB1 axis in human PCa and be implicated in various pathological events during PCa progression (Supplementary Fig. S9B).
Discussion
Extensive evidences show that the PSA screening worldwide has brought an over-diagnosis of PCa, which subsequently leading to an overtreatment of patients with indolent disease (4). Therefore, it is extremely necessary to identify novel and more efficient biomarkers which can discriminate between indolent and aggressive PCa, so that patients with low risk of progression may better benefit from avoiding unnecessary treatments. miRNAs have been recognized to be promising biomarkers for cancer patients due to their deregulated expression in a variety of human cancer types as well as their tissue-specific expression patterns in cancer and high stabilities in blood (30). In our study, miR-195 was identified as a novel tumor suppressor of PCa. We further determined that miR-195 could directly target RPS6KB1 by binding to its 3'-UTR and be implicated into the progression of PCa via regulating RPS6KB1 signaling. More interestingly, our clinical evidences revealed that both miR-195 and RPS6KB1 could be used to predict prognosis of PCa. These findings provided us with abundant information to further decode the functional implications of miR-195-RPS6KB1 axis in the progression of human PCa.
Altered expression of the miR-15/16/195/424/497 family has been reported to exert diverse effects in tumor cells. Similar with other members of this family, miR-195 expression is down-regulated frequently in multiple cancer types and implicates its fundamental role in cell cycle regulation and carcinogenesis (31). In the current study, we found that the reduced expression of miR-195 was a characteristic molecular change in both PCa cell lines and clinical tissue samples. miR-195 is localized to the segment of chromosome 17p13.1, which has been reported to be frequently deleted in cancer cells (32), implying that miR-195 downregulation in PCa cells and tissues might be related to gene copy number reduction. Besides, the methylation of CpG islands upstream of the miR-195 might be another cause contributing to the downregulation of miR-195 (13). In the current study, we analyzed the CGH array information in the Taylor dataset, but failed to find any copy number variations (CNVs) of miR-195, suggesting the CNVs is not the reason for the downregulation of miR-195 here. Then, we also confirmed the significant associations of miR-195 downregulation with aggressive clinicopathological characteristics, shorter BCR-free and non-metastatic BCR-free survivals of PCa patients, which prompted us to determine the roles of miR-195 in malignant phenotypes of PCa in vitro systems and in vivo models. Our data confirmed the tumor suppressive role of miR-195 through PCa cells invasion, migration and apoptosis assays in vitro, along with tumor xenografts growth, angiogenesis and invasion in vivo according to both gain-of-function and loss-of-function experiments.
To further disclose the molecular mechanisms underlying the involvement of miR-195 in PCa, we combined iTRAQ-based nano-LC-MS/MS and three miRNA target prediction algorithms to identify the potential targets of miR-195. iTRAQ-based nano-LC-MS/MS provides a potential platform for simultaneously identifying and quantifying target proteins of miRNAs (33). Compared to the conventional proteomics methods, such as 2-dimensional gel electrophoresis and the peptide mass fingerprint method via matrix assisted laser desorption/ionization—time-of-flight MS, the iTRAQ-based nano-LC-MS/MS methods can undertake high-throughput tasks and identify proteins more accurately. Then, we also integrated the prediction results of bioinformatic methods. As a result, RPS6KB1, SMAP2, DCUN1D1, SMAD4, IPO9, CAPZA2, and CPNE1 were predicted as candidate targets of miR-195. Numerous genes have been identified as the direct targets of miR-195. For example, miR-195 targeted the TNF-a/NF-κB pathway by down-regulating IκB Kinase Alpha and TAB3 in Hepatocellular Carcinoma (14). Cell cycle checkpoint kinase WEE1 was targeted by miR-195 in malignant melanoma (12). Here, we showed great interest in RPS6KB1 biological role as a downstream target of miR-195, because it is a crucial component of the mTOR signaling pathway which was one of the significantly enriched pathways involved by miR-195-induced differentially-expressed proteins and plays an important role in PCa, as reported many times (34, 35). Consequently, RPS6KB1 was identified as a novel direct target of miR-195 confirmed by luciferase report assay. RPS6KB1 is an evolutionarily conserved serine/threonine kinase and has been recognized as an integrator of nutrient and growth factor signals for fundamental cellular processes, such as cell growth and proliferation (36). It plays an important role in protein synthesis, via interacting with or activating other transcriptional factors (37, 38), as well as being a transcriptional factor (28). Growing evidences suggest that the alteration of RPS6KB1 may be a crucial driver of tumor initiation and progression (39, 40), and that the inactivation of RPS6KB1 may be therapeutically effective in many cancers (41, 42). Our data here suggested that the enforced expression and the loss of miR-195 could respectively cause the reduced and the increased expression of RPS6KB1 at both mRNA and protein levels in PCa cells and tumor xenografts dramatically. qRT-PCR analysis in human PCa tissues also showed an adverse relationship between miR-195 and RPS6KB1 expression. Of note, the re-expression and knockdown of RPS6KB1 could respectively rescue and imitate the effects associated with miR-195. Moreover, the prognostic impact of RPS6KB1 expression in PCa was also determined based on a cohort of 225 PCa patients. We further identified the downstream effectors of miR-195-RPS6KB1 axis. Our data showed that the decreased expression of miR-195 might upregulate RPS6KB1, leading to the increasing expression of MMP-9 and VEGF proteins and the decreasing expression of E-cadherin and BAD proteins, which together trigger a series of tumor reactions in PCa, including tumor invasion, angiogenesis, EMT and survival (Supplementary Fig. S9B).
In conclusion, our data offered the convincing evidences that miR-195 may function as a tumor suppressor with prognostic impact in human PCa. miR-195 deregulation may confer proliferative advantage and promote PCa migration and invasion by regulating RPS6KB1 signaling. The newly identified miR-195-RPS6KB1 axis partially illustrates the molecular mechanism of PCa progression and represents a novel potential therapeutic target for PCa treatment.
Supplementary Material
Statement of translational relevance.
Optimal therapeutic strategies for prostate cancer (PCa) patients are still challenging due to its highly variable natural history. Growing evidences show that genetic and epigenetic alterations are involved in PCa progression, however, the underlying molecular mechanisms have not been fully elucidated. Here, we identified hsa-microRNA-195-5p (miR-195) as a critical tumor suppressor in PCa progression via reducing the expression of RPS6KB1. More interestingly, both miR-195 and RPS6KB1 could be used to predict prognosis of PCa. Collectively, the newly identified miR-195-RPS6KB1 axis partially illustrates the molecular mechanism of PCa progression and represents a novel potential therapeutic target for PCa treatment.
Acknowledgments
Grant Support
This work was supported by grants from National Natural Science Foundation of China (81170699, 81272813, 81200550, 81470983), Projects of Guangdong Key Laboratory of Clinical Molecular Medicine and Diagnostics, Guangzhou Municipal Science and Technology Project (201501010135), NIH/NCI P01 CA120964 and Beijing Nova program (Z1511000003150126).
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
Authors’ Contributions
WZ and CW: participated in study design and coordination, analysis and interpretation of data, material support for obtained funding, and supervised study. CC, QC, ZH and YZ: performed most of the experiments and statistical analysis and drafted the manuscript. Other author: carry out the experiment and sample collection. All authors read and approved the final manuscript.
Reference
- 1.Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2013. CA CANCER J CLIN. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003;169:517–523. doi: 10.1097/01.ju.0000045749.90353.c7. [DOI] [PubMed] [Google Scholar]
- 3.Lapointe J, Li C, Higgins JP, van de Rijn M, Bair E, Montgomery K, et al. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci U S A. 2004;101:811–816. doi: 10.1073/pnas.0304146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Amico AV, Moul J, Carroll PR, Sun L, Lubeck D, Chen MH. Cancer-specific mortality after surgery or radiation for patients with clinically localized prostate cancer managed during the prostate-specific antigen era. J Clin Oncol. 2003;21:2163–2172. doi: 10.1200/JCO.2003.01.075. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayub SG, Kaul D, Ayub T. Microdissecting the role of microRNAs in the pathogenesis of prostate cancer. Cancer Genet. 2015 Mar 2; doi: 10.1016/j.cancergen.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Schmittgen TD. Regulation of microRNA processing in development, differentiation and cancer. J Cell Mol Med. 2008;12:1811–1819. doi: 10.1111/j.1582-4934.2008.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porkka KP, Pfeiffer MJ, Waltering KK, Vessella RL, Tammela TL, Visakorpi T. MicroRNA expression profiling in prostate cancer. Cancer Res. 2007;67:6130–6135. doi: 10.1158/0008-5472.CAN-07-0533. [DOI] [PubMed] [Google Scholar]
- 11.Xu T, Zhu Y, Xiong Y, Ge YY, Yun JP, Zhuang SM. MicroRNA-195 suppresses tumorigenicity and regulates G1/S transition of human hepatocellular carcinoma cells. Hepatology. 2009;50:113–121. doi: 10.1002/hep.22919. [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharya A, Schmitz U, Wolkenhauer O, Schonherr M, Raatz Y, Kunz M. Regulation of cell cycle checkpoint kinase WEE1 by miR-195 in malignant melanoma. Oncogene. 2013;32:3175–3183. doi: 10.1038/onc.2012.324. [DOI] [PubMed] [Google Scholar]
- 13.Li D, Zhao Y, Liu C, Chen X, Qi Y, Jiang Y, et al. Analysis of MiR-195 and MiR-497 expression, regulation and role in breast cancer. Clin Cancer Res. 2011;17:1722–1730. doi: 10.1158/1078-0432.CCR-10-1800. [DOI] [PubMed] [Google Scholar]
- 14.Ding J, Huang S, Wang Y, Tian Q, Zha R, Shi H, et al. Genome-wide screening reveals that miR-195 targets the TNF-α/NF-κB pathway by down-regulating IκB kinase alpha and TAB3 in hepatocellular carcinoma. Hepatology. 2013;58:654–666. doi: 10.1002/hep.26378. [DOI] [PubMed] [Google Scholar]
- 15.Soon PS, Tacon LJ, Gill AJ, Bambach CP, Sywak MS, Campbell PR, et al. miR-195 and miR-483-5p Identified as Predictors of Poor Prognosis in Adrenocortical Cancer. Clin Cancer Res. 2009;15:7684–7692. doi: 10.1158/1078-0432.CCR-09-1587. [DOI] [PubMed] [Google Scholar]
- 16.Jia LF, Wei SB, Gong K, Gan YH, Yu GY. Prognostic implications of micoRNA miR-195 expression in human tongue squamous cell carcinoma. PLoS One. 2013;8:e56634. doi: 10.1371/journal.pone.0056634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu MG, Li S, Yu TT, Qian LJ, Cao RS, Zhu H, et al. Differential expression of miR-195 in esophageal squamous cell carcinoma and miR-195 expression inhibits tumor cell proliferation and invasion by targeting of Cdc42. FEBS Lett. 2013;587:3471–3479. doi: 10.1016/j.febslet.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 18.Maragkakis M, Alexiou P, Papadopoulos GL, Reczko M, Dalamagas T, Giannopoulos G, et al. Accurate microRNA target prediction correlates with protein repression levels. BMC Bioinformatics. 2009;10:295. doi: 10.1186/1471-2105-10-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dweep H, Sticht C, Pandey P, Gretz N. miRWalk--database: prediction of possible miRNA binding sites by "walking" the genes of three genomes. J Biomed Inform. 2011;44:839–847. doi: 10.1016/j.jbi.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Betel D, Koppal A, Agius P, Sander C, Leslie C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome. Biol. 2010;11:R90. doi: 10.1186/gb-2010-11-8-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin ZY, Huang YQ, Zhang YQ, Han ZD, He HC, Ling XH, et al. MicroRNA-224 inhibits progression of human prostate cancer by downregulating TRIB1. Int J Cancer. 2014;135:541–550. doi: 10.1002/ijc.28707. [DOI] [PubMed] [Google Scholar]
- 22.Chen G, Liang YX, Zhu JG, Fu X, Chen YF, Mo RJ, et al. CC Chemokine Ligand 18 Correlates with Malignant Progression of Prostate Cancer. Biomed Res Int. 2014;2014:230183. doi: 10.1155/2014/230183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Havel LS, Kline ER, Salgueiro AM, Marcus AI. Vimentin regulates lung cancer cell adhesion through a VAV2-Rac1 pathway to control focal adhesion kinase activity. Oncogene. 2015 Apr 9;34(15):1979–1990. doi: 10.1038/onc.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 26.Harada H, Andersen JS, Mann M, Terada N, Korsmeyer SJ. p70S6 kinase signals cell survival as well as growth, inactivating the pro-apoptotic molecule BAD. Proc Natl Acad Sci U S A. 2001;98:9666–9670. doi: 10.1073/pnas.171301998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou HY, Wong AS. Activation of p70S6K induces expression of matrix metalloproteinase 9 associated with hepatocyte growth factor-mediated invasion in human ovarian cancer cells. Edocrinology. 2006;147:2557–2566. doi: 10.1210/en.2005-1404. [DOI] [PubMed] [Google Scholar]
- 28.Pon YL, Zhou HY, Cheung AN, Ngan HY, Wong AS. p70 S6 kinase promotes epithelial to mesenchymal transition through snail induction in ovarian cancer cells. Cancer Res. 2008;68:6524–6532. doi: 10.1158/0008-5472.CAN-07-6302. [DOI] [PubMed] [Google Scholar]
- 29.Bian CX, Shi Z, Meng Q, Jiang Y, Liu LZ, Jiang BH. P70S6K1 regulation of angiogenesis through VEGF and HIF-1alpha expression. Biochem Biophys Res Commun. 2010;398:395–399. doi: 10.1016/j.bbrc.2010.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahn R, Heukamp LC, Rogenhofer S, von Ruecker A, Müller SC, Ellinger J. Circulating microRNAs (miRNA) in serum of patients with prostate cancer. Urology. 2011;77:1265. doi: 10.1016/j.urology.2011.01.020. e9–16. [DOI] [PubMed] [Google Scholar]
- 31.He JF, Luo YM, Wan XH, Jiang D. Biogenesis of MiRNA-195 and its role in biogenesis, the cell cycle, and apoptosis. J Biochem Mol Toxicol. 2011;25:404–408. doi: 10.1002/jbt.20396. [DOI] [PubMed] [Google Scholar]
- 32.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ou M, Zhang X, Dai Y, Gao J, Zhu M, Yang X, et al. Identification of potential microRNA-target pairs associated with osteopetrosis by deep sequencing, iTRAQ proteomics and bioinformatics. Eur J Hum Genet. 2014;22:625–632. doi: 10.1038/ejhg.2013.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang L, Graham PH, Hao J, Ni J, Bucci J, Cozzi PJ, et al. PI3K/Akt/mTOR pathway inhibitors enhance radiosensitivity in radioresistant prostate cancer cells through inducing apoptosis, reducing autophagy, suppressing NHEJ and HR repair pathways. Cell Death Dis. 2014;5:e1437. doi: 10.1038/cddis.2014.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edlind MP, Hsieh AC. PI3K–AKT-mTOR signaling in prostate cancer progression and androgen deprivation therapy resistance. Asian J Androl. 2014;16:378–386. doi: 10.4103/1008-682X.122876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khotskaya YB, Goverdhan A, Shen J, Ponz-Sarvise M, Chang SS, Hsu MC, et al. S6K1 promotes invasiveness of breast cancer cells in a model of metastasis of triple-negative breast cancer. Am J Transl Res. 2014;6:361–376. [PMC free article] [PubMed] [Google Scholar]
- 37.Fleckenstein DS, Dirks WG, Drexler HG, Quentmeier H. Tumor necrosis factor receptor-associated factor (TRAF) 4 is a new binding partner for the p70S6 serine/threonine kinase. Leuk Res. 2003;27:687–694. doi: 10.1016/s0145-2126(02)00325-9. [DOI] [PubMed] [Google Scholar]
- 38.de Groot RP, Ballou LM, Sassone-Corsi P. Positive regulation of the cAMP-responsive activator CREM by the p70 S6 kinase: an alternative route to mitogen-induced gene expression. Cell. 1994;79:81–91. doi: 10.1016/0092-8674(94)90402-2. [DOI] [PubMed] [Google Scholar]
- 39.Ip CK, Cheung AN, Ngan HY, Wong AS. p70 S6 kinase in the control of actin cytoskeleton dynamics and directed migration of ovarian cancer cells. Oncogene. 2011;30:2420–2432. doi: 10.1038/onc.2010.615. [DOI] [PubMed] [Google Scholar]
- 40.Li PD, Zhang WJ, Zhang MY, Yuan LJ, Cha YL, Ying XF, et al. Overexpression of RPS6KB1 predicts worse prognosis in primary HCC patients. Med Oncol. 2012;29:3070–3076. doi: 10.1007/s12032-012-0268-y. [DOI] [PubMed] [Google Scholar]
- 41.Kim EK, Kim JH, Kim HA, Seol H, Seong MK, Lee JY, et al. Phosphorylated S6 kinase-1: a breast cancer marker predicting resistance to neoadjuvant chemotherapy. Anticancer Res. 2013;33:4073–4079. [PubMed] [Google Scholar]
- 42.Ben-Hur V, Denichenko P, Siegfried Z, Maimon A, Krainer A, Davidson B, et al. S6K1 alternative splicing modulates its oncogenic activity and regulates mTORC1. Cell Rep. 2013;3:103–115. doi: 10.1016/j.celrep.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.