Abstract
Overwhelming data indicate that individuals with even mildly elevated blood pressure (BP) are at great risk for developing clinical hypertension and future cardiovascular disease (CVD). There remains a lack of consensus regarding treatment strategies for mildly elevated BP, termed prehypertension, and the knowledge of pathophysiology and mechanisms of its clinical outcomes remains limited. Our primary aim was to investigate βAR-mediated inflammation control (BARIC) responses of blood monocytes to isoproterenol (Iso) in relation to BP and CVD risk factors, including obesity, depressive mood, fasting glucose, triglycerides, and cholesterol levels in the 64 prehypertensive compared to 84 individuals with normal BP. BARIC was determined by measuring the degree of inhibition in lipopolysaccharides-stimulated monocytic intracellular TNF production by ex vivo Iso treatment (10−8 M). Depressive mood was assessed by Beck Depression Inventory (BDI). Fasting metabolic and lipid panels were assessed, and plasma levels of inflammatory cytokines TNF, IL-1β, IL-6 were measured in a subset to confirm proinflammatory state of prehypertensive participants. Prehypertensive participants were older, heavier, included more men, and presented higher levels of fasting glucose, triglycerides, cholesterol, and plasma TNF compared to normotensive participants (p’s< .05). BARIC was significantly attenuated in the prehypertensive compared to normotensive group (p< .05). BARIC was negatively associated with systolic BP, diastolic BP, age, BMI, fasting glucose, triglycerides, total and low density cholesterol levels, and somatic depressive symptoms in all participants (p’s< .0001 to .05). However, among the prehypertensive individuals BARIC was positively associated with SBP even after controlling for the covariates (age, gender, race, BMI, glucose and lipid panel, somatic BDI scores) (p< .05). This differing nature of the BARIC-SBP relationship between the two BP groups may be attributed to moderating factors such as cardiorespiratory fitness or depressive symptoms that could not be clearly deciphered in this current study. Nonetheless, our findings indicate the associations between inflammation dysregulation mediated by sympathoadrenal activation and BP that is observable even among individuals with normal to mildly elevated BP. BARIC may be a useful and sensitive indicator of elevated risk for vascular inflammatory disease that can be detected even at lower BP levels, especially given its associations with traditional CVD risk factors and the critical role of monocytes in atherogenic processes.
Keywords: Beta adrenergic receptor, Blood pressure, Depression, Fasting glucose, Intracellular TNF levels, Lipids, Monocytes, Prehypertension
1. Introduction
It has been over a decade since the 7th Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) has emphasized that “…beginning at 115/75 mm Hg, cardiovascular disease (CVD) risk doubles for each increment of 20/10 mm Hg” and that individuals with even mildly elevated blood pressure (BP; systolic BP, SBP 121 – 139 mm Hg and/or diastolic BP, DBP 81 – 89 mm Hg termed “prehypertension”) are prone to a progressive rise in BP and CVD risk (Chobanian et al., 2003). Population studies worldwide report high prevalence of prehypertension: 30–50% of adult populations with a higher rate for men (Chockalingam et al., 2005; Choi et al., 2001; Greenlund et al., 2004; Julius et al., 2006; Lee et al., 2006; Tsai et al., 2005), as high as 57% of adolescents (Israeli et al., 2006), and almost 30% of young children (Grotto et al., 2006). Forty percent of prehypertensive, middle-aged individuals developed hypertension within two years, and nearly two thirds of them progressed to hypertension in four years when left untreated (Julius et al., 2006). Progression rates from prehypertension to hypertension in older age are higher among African Americans (Greenlund et al., 2004). Prehypertension is also related to future CVD, even after controlling for other risk factors (Liszka et al., 2005) and specifically associated with an increased risk (relative risk of 3.5) for myocardial infarction and developing coronary artery disease within 9–10 years (Qureshi et al., 2005). Clinical guidelines based on the JNC 7 strongly recommended treatment or intervention even for mild BP elevation based on these findings, including a pharmacological intervention if necessary. Clinicians also agree that classifying individuals as prehypertensive is of little value without clarification of justified and individualized intervention plans (Moser et al., 2006). While, the recently-released JNC 8 report (James et al., 2014) updated the guidelines that set BP goals at higher levels for management of high BP (e.g., to treat hypertensive persons over 60 years of age to a BP goal of less than 150/90 mm Hg). Without clear characterization of pathophysiology of prehypertension and uncovering its underlying mechanisms, implementation of efficacious therapeutics is difficult.
The associations of hypertension with markers of systemic and vascular inflammation have been reported by our group (Hong et al., 2004; Hong et al., 2005; Hong & Mills, 2008; Mills et al., 2003) and many others (Kop, 2003; Kop & Gottdiener, 2005; Hwang et al., 1997). Elevated inflammation is linked to current and future cardiovascular pathology (see Frostegard, 2013; Ross, 1999 for review). Prehypertensive individuals exhibited 7% lower total antioxidant capacity levels and 15% higher oxidized LDL levels compared to normotensive participants, implying atherosclerotic processes that may initiate at the level of mild BP elevation (Chrysohoou et al., 2004). In spite of many epidemiological studies, reporting an increasing incidence of prehypertension worldwide and risk for future CVD development, studies of underlying vascular inflammatory process and its implications in future CVD in prehypertension remain sparse.
More studies are needed to uncover the degree and pathways of the impact of modest BP elevations on vascular inflammatory and atherogenic processes in order for better risk assessment for future CVD. Migration and infiltration of inflammatory immune cells, such as monocytes into the vascular endothelium, is a key process in the pathogenesis of vascular inflammatory disease (Savoia & Schiffrin, 2007). Monocyte mobilization, recruitment, infiltration, and inflammatory cytokine production (such as tumor necrosis factor, TNF) are prominently involved in atherosclerotic lesions (Branen et al., 2004; McKellar et al., 2009; Skoog et al., 2002). The impact of sympathetic activation in accelerating hypertension and cardiovascular pathology is well-documented (Abboud, 1982; Goldstein, 1983; Weber & Drayer, 1982). Thus, investigations of sympathoadrenal regulation of monocytes/macrophages would shed light on pathophysiology of vascular inflammation. Beta 2 (β2) adrenergic receptor (AR) numbers on circulating lymphocytes are greater in hypertensive individuals, and AR density is positively correlated with BP (Brodde et al., 1984a, 1984b; Fitzgerald et al., 1983; Middeke et al., 1983). But, βARs are also shown to be desensitized in hypertensive individuals (Grassi et al., 2010), which points to the complexity in sympathoadrenal regulatory mechanisms involved in end-organ responses.
Adrenergic receptors (α1, α2, β1, and β2 subtypes) are shown to be expressed on various immune cell types, including monocytes and play a major role in regulating diverse cellular activities, including inflammatory responses (Dimitrov et al., 2013; Heine et al., 2012; Hong et al., 2014). The literature on leukocytes’ inflammatory responses upon AR engagement or catecholamine stimulation is largely variable such that catecholamines induce both pro- (e.g., Flierl et al., 2008; Kavelaars et al., 1997; Torres et al., 2005) and anti-inflammatory (e.g., Hu et al., 1991; van der Poll et al., 1994) responses by immune cells. These seemingly contradictory effects of catecholamines on inflammatory responses appear to depend on a number of factors (see Padro & Sanders, 2014 for review), including AR subtype (Hanke et al., 2012; Heijnen et al., 1996), AR agonist concentrations (e.g., Szelenyi et al., 2000), and the timing of AR engagement in relation to antigen stimulation (Sanders, 2012). We have previously reported that βAR-mediated mobilization responses of proinflammatory monocytes was attenuated among prehypertensive individuals (Dimitrov et al., 2013). We also reported a significant associations of diminished monocytes’ βAR responsivity to isoproterenol (Iso) in TNF production with elevated systemic inflammatory cytokine levels and adiposity (Hong et al., 2014).
The primary aim of the present study was to investigate the extent to which blood monocytes suppressed LPS-stimulated TNF production under β-ARs engagement using Iso among individuals with normal and prehypertensive BP. We hypothesized that prehypertensive individuals would exhibit diminished monocytic responses to the inhibitory effect of Iso in TNF production compared to normotensive individuals. Furthermore, we aimed to investigate and hypothesized that this β-AR-mediated inflammation control (namely, “BARIC”) would be associated with BP and traditional CVD risk factors, including obesity, lipid profile, and fasting glucose levels. As the secondary investigation of the study, the association between β-AR-mediated inflammation control and depressive mood was examined, given the well-documented depression-CVD association. Given that there has been limited neuro-immune characterization of prehypertension, our study to gain insight into the sympathoadrenal-immune link in relation to traditional CVD risk factors in prehypertesion would shed light on risk for future CVD and potential therapeutics.
2. Materials and Methods
2.1. Participants
All participants gave informed consent to the protocol, which was approved by the University of California, San Diego Human Research Protection Program. One hundred forty eight healthy, non-smoking men and women between the ages of 18 to 65 years with blood pressure range of normal to prehypertension (SBP< 145 and/or DBP< 95 mm Hg to account for BP elevations during office visits) were included in this study from a parent trial of prehypertension and neuro-immune activation. To confirm eligibility, all subjects underwent clinical laboratory blood tests for liver, metabolic, lipid, and thyroid panels. Individuals who reported a current diagnosis or a history of heart, liver, or renal disease, diabetes, psychiatric and mood disorders, severe asthma, ongoing inflammatory diseases (e.g., rheumatoid arthritis, multiple sclerosis, lupus), acute illness, and current pregnancy were excluded. Criteria for exclusion also included current use of any anti-hypertensive medications, anti-inflammatory medications, or other medications that are known to influence the immune or neuroendocrine parameters of interest (e.g., beta blockers), current drug or alcohol abuse, and smoking within 6 months of the enrollment in the study.
2.2. Procedure
Blood was collected between 8 and 10 am through an intravenous catheter inserted into an antecubital vein using minimal tourniquet. Participants fasted for 12 hours except for drinking plain water prior to the blood sampling. The lipid profile (triglycerides and low-density, high-density and total cholesterol levels) and glucose levels were assessed at UCSD Medical Center Clinical Laboratory. Standard anthropometric measurements (i.e. height, weight, and waist circumference) were obtained. Blood pressure was measured using a Dinamap Compact BP® monitor (Critikon, Tempa, FL) and defined as the average of two sets of three seated BP measures taken minimum an hour apart or on two different days. The mood and fatigue questionnaires were also completed.
2.3. LPS-stimulated monocytic intracellular TNF production by flow cytometry
Whole blood was analyzed for LPS-stimulated intracellular monocytic production of TNF, which is the primary cytokine produced by activated monocytes/macrophages (see Dimitrov et al., 2013; Hong et al., 2014 for detailed methods). The dose of 200 pg/mL LPS (E. coli 0111:B4, catalog # L4391, Sigma-Aldrich, St. Louis, MO) was pre-determined to be appropriate for significant activation of monocytes in preliminary experiments, with 30 to 90% of cells producing TNF across a number of healthy individuals. Peripheral blood cells were incubated in sterile polypropylene plates with or without LPS for 3.5 hours at 37°C with 5% CO2. To stop cytokine excretion (allowing intracellular detection), brefeldin A (10 µg/mL, Sigma-Aldrich) was added for the last 3 hours of LPS incubation.
Intracellular TNF production of monocytes was evaluated by multiparametric flow cytometry using fluorochrome-conjugated antibodies. Briefly, erythrocytes were lysed using ammonium chloride solution followed by centrifugation (5 min at 500 × g). The cell pellet was washed once with PBS, containing 0.1% azide and 0.5% bovine serum albumin, prior to incubation with monoclonal antibodies (15 min) for the monocytes identification: HLA-DR/PE (BD Biosciences, San Jose, CA), and CD14/APC (Biolegend, San Diego, CA). After fixation and permeabilization according to the manufacturer’s instructions (Cytofix/Cytoperm Kit, BD Biosciences), cells were stained intracellularly with TNF/FITC antibody (Biolegend). At least 10,000 gated monocytes were collected for each tube on a dual-laser FACSCalibur (BD Biosciences). Monocytes were distinguished from lymphocytes and granulocytes by means of their forward and side scatter (FSC and SSC) characteristics and were identified as CD14+/dim HLA-DR+ cells as shown previously (Dimitrov et al., 2013). The percentage of the CD14+/dimHLA-DR+ cells that were positive for TNF (“% TNF+ monocytes”) was assessed. The analysis of the flow cytometric data were performed using FlowJo (Tree star, Ashland, OR).
2.4. β-AR-mediated inflammation control (BARIC) using LPS-stimulated monocytic intracellular TNF levels
Monocytic βAR sensitivity to agonist was evaluated based on the inhibitory effect of Iso, a non-specific β1/2-AR agonist, on the monocytic intracellular TNF production in whole blood stimulated with LPS as previously described. Systematic assay optimization steps were previously taken, using blood cells from six healthy donors, to ensure the internal validity of this method of our cellular model, including the LPS dosage and stimulation time, establishing response curves to various agonists and antagonists, timing of the agonist treatment, and testing mediation by the cAMP-PKA pathway. The primary dose of Iso (10−8 M; Sigma-Aldrich) was determined from the response curve generated from a range of Iso concentrations (10−11-10−6 M) and based on our findings that % TNF expression by monocytes was suppressed by about 55% of that of the control condition in response to Iso at 10−8 M, which was also close to maximum suppression in our cellular model. The whole blood was simultaneously incubated with Iso in a range of final concentrations of 10−8, 10−9, and 10−10 M. Monocytes’ βAR-mediated responsivity in TNF inhibition was calculated as the difference in % monocytes for TNF production between LPS only and LPS plus Iso. For the purpose of this study, this assessment of β-AR-mediated suppression of LPS-stimulated intracellular expression of TNF by blood monocytes was termed β-AR-mediated inflammation control, “BARIC”. The greater values of BARIC indicate greater βAR responsivity and thus, better βAR-mediated inflammation regulation.
2.5. Cytokine levels in plasma
Blood for plasma TNF, IL-1β and IL-6 measurement was drawn in EDTA-treated vacutainers and placed on ice. After centrifugation in a refrigerated centrifuge, plasma was stored at –80°C until the assays were performed. Plasma cytokine levels were measured using commercially available immunoassay kits (Meso Scale Discovery, Gaithersburg, MD). The intra-and inter-assay variations were 6.8% and 6.4% for TNF, 8.4% and 6.0% for IL-1β, and 8.5% and 7.8% for IL-6, respectively. As secondary to this investigation, plasma cytokine levels of TNF, IL-1β and IL-6 were assessed in about 50% of the participant mainly to document an overall systemic inflammatory state of the study participants.
2.6. Depressive mood
As depression has been shown as a risk factor and/or co-morbid condition of CVD (Frasure-Smith & Lesperance, 2010; Rutledge et al., 2006) and for its impact on β-AR-associated immunomodulation in CVD (Feldman et al., 2005; Redwine et al., 2010; Redwine et al., 2014), depressive mood was assessed by self-reports, using the 21-item Beck Depression Inventory (BDI-1A) (Beck, 1993). In addition to the calculation of a total score two sub-scales of BDI were calculated: cognitive/affective depressive mood (BDI-C) and somatic depressive mood (BDI-S).
2.7. Statistical analysis
Statistical analyses were performed using SPSS Statistical Software (v 22.0). Descriptive data are presented as means ± SD. Results were considered statistically significant if p≤ 0.05, and all tests were two-tailed. In case of missing data, cases were excluded listwise. Normality of the data was determined by the Kolmogorov-Smirnov test, and variables that were not normally distributed were transformed as appropriate: age, BMI, SBP, fasting glucose, triglycerides, total, high-density, and low-density cholesterol, inflammatory cytokine and BDI values were log transformed. Collinearity statistics were examined for regression analyses.
As the first step of deciphering simple associations amongst BP, risk factors, and β-AR-mediated inflammatory control, univariate correlations among demographic characteristics (age, gender, and race), BP (SBP and DBP), obesity indices (BMI and waist circumference), fasting glucose and lipid panel (triglycerides, cholesterol) levels, inflammatory cytokines, and β-AR-mediated TNF inhibition (Iso dose 10−8 M), and depressive mood (BDI total, somatic and cognitive scores) were measured by Pearson’s r in all 148 participants and also in normotensive and prehypertensive participants separately. Next, a series of multiple regression analysis were performed to further investigate the role of β-AR-mediated TNF inhibition in predicting BP, controlling for covariates (age, gender, race, BMI, glucose, triglycerides, LDL, and somatic BDI score). Monocytic β-AR-mediated TNF inhibition was compared between normotensive and prehypertensive individuals using Student’s t-test.
3. Results
3.1. Demographic and basic clinical characteristics of participants
Of the 148 participants 43% (n= 64) presented with prehypertension (SBP> 120 mm Hg and/or DBP> 80 mm Hg). Based on this BP categorization prehypertensive individuals were older and heavier with greater BMI and waist circumference on average and included a higher number of men compared to the normotensive group (all p’s ≤ .001; Table 1). Among the prehypertensive individuals, 52% were obese according to the BMI-based weight/obesity classification compared to 20% obese among the normotensive individuals. The racial distribution of Caucasian (majority) vs. non-Caucasian participants was similar in the two BP groups with 53–55% being Caucasian. Fasting levels of blood glucose, triglycerides, and total, high density, and low density cholesterol were significantly greater in the prehypertensive compared to normotensive group (p’s ranging < .001 to .05).
Table 1.
Demographic, basic physical characteristics, and blood glucose, lipid, and cytokine levels of 148 study participants according to the BP categories.
| Variable | Prehypertension (N= 64, 43%) |
Normal BP (N= 84, 57%) |
t/ χ2 | p |
|---|---|---|---|---|
| Age (years) | 44.0 ± 11.9 | 36.1 ± 11.8 | 4.2 | <0.001 |
| Gender (men/women) | 42/22 | 31/53 | 12.02 | 0.001 |
| Race (white/others) | 34/30 | 47/37 | 6.82 | 0.34 |
| Systolic BP (mmHg) | 135.7 ± 4.5 | 108.2 ± 7.7 | 27.2 | <0.001 |
| Diastolic BP (mmHg) | 79.8 ± 7.5 | 66.5 ± 6.9† | 11.2 | <0.001 |
| Body mass index (kg/m2) | 31.7 ± 5.8 | 29.8 ± 7.0 | 5.1 | <0.001 |
| Waist circumference (cm) | 105.2 ± 14.9 | 89.2 ± 15.6 | 6.1 | <0.001 |
| Glucose (mg/dL) | 88.4 ± 15.5 | 78.5 ± 16.8 | 3.4 | 0.001 |
| Triglycerides (mg/dL) | 147.6 ± 149.3 | 91.0 ± 46.0 | 4.1 | <0.001 |
| Total Cholesterol (mg/dL) | 197.4 ± 40.3 | 180.0 ± 30.7 | 2.7 | <0.01 |
| HDL | 53.2 ± 17.2 | 59.1 ± 17.0 | 2.3 | <0.05 |
| LDL | 115.7 ± 32.9 | 102.5 ± 29.6 | 2.4 | <0.05 |
| BDI Total score | 6.08 ± 5.85 | 5.42 ± 6.63 | −0.6 | 0.53 |
| Somatic | 2.81 ± 2.48 | 2.73 ± 3.39 | −0.2 | 0.88 |
| Cognitive/affective | 3.27 ± 3.79 | 2.69 ± 3.67 | −0.9 | 0.35 |
| Plasma TNF (pg/ml)# | 7.0 ± 4.4 | 5.0 ± 2.9 | 1.1 | <0.05 |
| Plasma IL-6 (pg/ml)# | 1.1 ± 0.8 | 1.5 ± 0.8 | 0.2 | 0.81 |
| Plasma IL-1β (pg/ml)# | 0.4 ± 0.6 | 0.6 ± 0.6 | 0.9 | 0.37 |
Values are presented as mean ± SD; F/ χ2 and p-values are derived from ANOVA or non-parametric χ2 test;
Denotes non-parametric χ2 test; BP, blood pressure;
plasma levels of cytokines were assessed in a subgroup of the participants (n= 28 (52%) and 39 (53%) for the prehypertension and normal BP groups, respectively) mainly for an exploratory comparison of overall systemic inflammatory states. Although descriptive statistics (mean and standard deviation) are based on raw values, statistical tests were done on transformed values for the variables that were not normally distributed (see Statistical analysis).
Secondary to this investigation, plasma cytokine levels of TNF, IL-1β and IL-6 were assessed in a subset (44% and 46% of the prehypertensive and normotensive groups, respectively) to confirm an overall systemic inflammatory state of the prehypertensive participants: levels of TNF were greater among the prehypertensive compared to normotensive individuals (p< .05), but the differences of IL-6 and IL-1β levels between the two groups were not significant (Table 1). Neither group exhibited the cytokine levels that are indicative of active clinical inflammatory conditions or outside the range of those seen among asymptomatic individuals. Demographic and clinical characteristics did not differ between the participants for which cytokines were and were not assessed.
3.2. Univariate associations of demographic and CVD risk factors to BP
Male sex (r= −.34, p< .0001 for SBP; r= −.18, p< .05 for DBP) and older age (r= −.36, p< .0001 for SBP; r= .42, p< .0001 for DBP) were associated with higher BP in all participants. Additionally, obesity/adiposity indices BMI (r= .40, p< .0001) and waist circumference (r= .50, p< .0001) and CVD risk factors triglycerides (r= .37, p< .0001), and total (r= .24, p< .01), HDL (r= .−.23, p< .01), and LDL cholesterol (r= .23, p< .01) levels were associated with SBP in all participants. These traditional risk factors for CVD were also correlated with DBP: triglycerides (r= .34, p< .0001), and total (r= .23, p< .01), HDL (r= .−.22, p< .01), and LDL cholesterol (r= .25, p< .01) levels. Curiously, when these associations were examined among the individuals with prehypertension the effect sizes of the relations between the risk factors and BP were diminished except the associations of age (r= .33) and LDL (r= .21) to DBP.
Of note, plasma levels of cytokines were associated with neither SBP nor DBP. The correlation coefficient between IL-1β levels and SBP (r= .20, p= .10) indicated the effect size between small and medium.
3.3. Beta adrenergic receptor mediated control of monocytic inflammatory responses (BARIC)
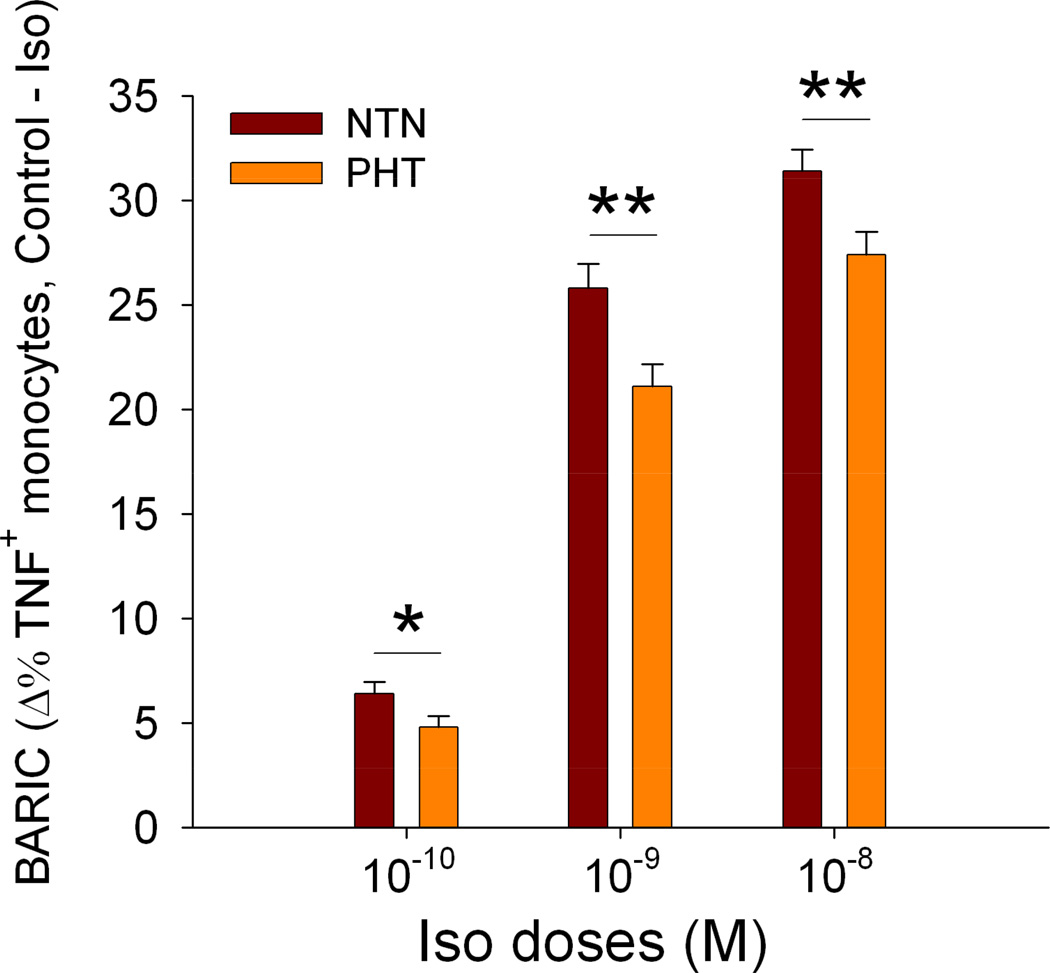
Beta-AR-mediated (in response to Iso 10−8 M) suppression of LPS-stimulated TNF expression by monocytes was negatively correlated with age (r= −.24, p< .01) and BP: SBP (r= −.23, p< .01) and DBP (r= −.28, p= .001). BARIC was also associated with CVD risk factors BMI (r= −.28, p= .001), waist (r= −.25, p< .01), fasting glucose (r= −.25, p< .01), triglycerides (r= −.24, p< .01), total cholesterol (r= −.29, p< .001), and LDL (r= −.24, p< .01) in all participants. BARIC was negatively correlated with plasma levels of IL-1β (r= −.40, p= .001) and marginally with TNF (r= −.20, p= .10). In BP group comparisons the prehypertension group showed significantly smaller BARIC compared to the individuals with normal BP, which was also evident at lower Iso doses [t(146)= 2.59, p= .01 for 10−8M; t(145)= 2.94, p< .01 (10−9M); t(146)= 2.04, p< .05 for 10−10M; Figure 1].
Figure 1.
Beta AR-mediated inflammation control (BARIC), assessed by the degree of the inhibition of monocytic TNF production by 10−8, 10−9, or 10−10 M Iso in the normotensive (NTN) vs. prehypertensive (PHT) BP groups. BARIC of two BP groups for all three Iso concentrations are depicted to showcase the reliability of the group difference and internal validity. Values are presented as mean ± SEM. Overall between group differences were significant for all Iso concentrations (p’s< 0.05).
These associations between BARIC and demographic characteristics, BP, and CVD risk factors remained similar in general among the participants with normal BP when analyzed separately from the prehypertensive group. However, among the prehypertensive participants the association of BARIC to BMI and LDL disappeared and the effect of its association to age (r= −.19), waist (r= −.14), glucose (r= −.18), triglycerides (r= −.15), and total cholesterol (r= −.15) were smaller. Curiously, among the prehypertensive participants the association between BARIC and DBP was similar to that in all (r= −.23) or greater than that among the normotensive participants, yet the relationship between BARIC and SBP was smaller in its effect size and opposite in nature (r= .18) compared to that in normotensive participants.
Multivariate analyses using regression to further investigate the association between BARIC and BP in the prehypertensive participants confirmed the associations found in simple correlation analyses. After controlling for demographic, BMI, glucose and lipid panel levels, and somatic BDI scores greater BARIC was predictive of higher SBP in the final model among the prehypertensive participants (Table 2). Among the participants with normal BP BARIC was no longer predictive of SBP after controlling for the aforementioned covariates. In the final model male gender (p< .05), and greater triglycerides at a marginal level (p< .10), remained only predictors of greater SBP among the normotensive participants.
Table 2.
Multiple regression analyses examining associations between monocytic BARIC (Δ %TNF by Iso10−8, Step 3) and SBP or DBP in individuals with prehypertension (the top half of the table) and normal BP (the bottom half of the table), after controlling for age, gender, race, and BMI in Step 1 and glucose, triglyceride, LDL cholesterol and somatic BDI levels in Step 2. Results of predictors in the final model are presented if p< 0.10.
| Dependent Variables |
Significant Predictors |
Standardized β-coefficient |
t | Final model F |
R2adjusted | ΔR2 | ΔF |
|---|---|---|---|---|---|---|---|
| Prehypertension | |||||||
| SBP | BARIC | 0.30 | 2.09** | 0.95 | 0.15 | 0.09 | 4.37** |
| DBP | BARIC | −0.25 | −1.85* | 2.08 | 0.14 | 0.06 | 3.42* |
| Normotension | |||||||
| SBP | Gender | −0.29 | −2.46** | 2.08* | 0.20 | 0.00 | 0.03 |
| Triglycerides | 0.20 | 1.76* | |||||
| DBP | Age | 0.30 | 2.42** | 1.90* | 0.09 | 0.02 | 0.22 |
| Race | 0.20 | 1.75* | |||||
Predictors of coefficients that were at p< 0.01***, p< 0.05**, and p< 0.10* are shown in the table.
3.4. Somatic depressive symptoms, BARIC, and BP
Firstly, SBP or DBP was not associated with total, somatic, or cognitive BDI scores in all participants or the separate BP groups. However, somatic BDI scores were positively associated with BMI (r= 31, P< .0001), waist (r= .27, p= .001), total (r= .23, p< .01) and LDL cholesterol (r= .20, p< .05), and marginally with glucose levels (r= .16, p= .06). Importantly, somatic but not cognitive BDI scores were negatively associated with BARIC (r= −.20, p< .05). When examined separately in the BP groups the nature and effect sizes of most aforementioned associations were similar in the normotensive group but disappeared in the prehypertensive group. When included in the multiple regression model in predicting SBP and DBP BDI scores were not significant predictors.
4. Discussion
In this investigation we report that blood monocytes’ ability to suppress intracellular TNF production through βAR upon agonist stimulation (i.e., 10−8 M Iso) is associated with BP among asymptomatic men and women with normal or mildly elevated BP. Firstly, BARIC was significantly diminished in the prehypertensive compared to normotensive group. This may indicate dysfunction at the level of βARs and diminished receptor sensitivity to agonists, but it should be made clear that BARIC itself does not directly reflect receptor sensitivity. Rather, smaller BARIC may equally likely indicate impairment in cellular pathways that regulate inflammatory responses mediated by βARs. It is also possible that cellular immune functions, including inflammatory responses upon immunologic challenge, are impaired in prehypertensive individuals, given the link between chronic low-grade inflammation and compromised immunity (Karlsson & Beck, 2010; Milner & Beck, 2012). Indeed, a post-hoc analysis revealed that % TNF+ monocytes in response to an LPS challenge at baseline (without Iso) was marginally lower in the prehypertensive compared to the normotensive group (51% vs. 56%; p= .10). This may suggest an overall “immune exhaustion” phenomenon among the prehypertensive participants with chronic low grade inflammation.
Importantly, smaller BARIC was also associated with risk factors for BP elevation and CVD: older age, both overall and central obesity, and elevated levels of fasting glucose and cholesterol. Blunted BARIC was also associated with higher levels of a systemic inflammatory marker IL-1β (and TNF at a marginal level) but not with IL-6, which also suggests that the BARIC-cytokine associations may differ, depending on one’s cytokine profile, including a pro-and anti-inflammation balance, which warrants a follow-up investigation. In our other experiments, this range of Iso produced similar TNF suppression effects to those that were induced by an endogenous β2AR agonist (epinephrine) of concentrations under physiological conditions rather than pharmacological doses (manuscript in preparation). These findings indicate that even among healthy individuals whose BP levels were below clinical hypertension, there are indications of sympathoadrenal dysregulation observed in immune cell activities which were also associated with well-documented risk factors for CVD. Thus, it is highly plausible that there may exist a reasonably linear association between one’s βAR-mediated regulatory ability of inflammatory responses of immune cells and BP, extending to a “normal” range of BP levels rather than a clear BP threshold at which sympathoadrenal dysregulation of inflammation surfaces. It is unknown, however, whether these findings truly indicate elevated risk for future CVD for individuals of normal to high normal BP levels who exhibit blunted BARIC. Nonetheless, BARIC may be a useful and sensitive indicator of elevated risk for vascular inflammatory disease that can be detected even at lower BP levels, especially given its associations with traditional CVD risk factors. It is also noteworthy that the significant associations between BARIC and blood levels of CVD risk factors (glucose, triglycerides, and cholesterol) were observed in spite of the ranges of the blood factors that were below those of clinically defined thresholds. In the literature the negative association between βAR responsivity to Iso infusion in HR responses and blood inflammatory marker (i.e., CRP) levels was shown also in healthy individuals (Euteneuer et al., 2012). Also of note, in spite of the existing evidence of both pro- and anti-inflammatory effects of βAR agonists in the literature our data clearly demonstrated suppressive effects of Iso in stimulated monocytic TNF production, which may be attributed to a relatively low dose of Iso used, given the aforementioned differing effects of βAR agonists based on their concentrations (Padro & Sanders, 2014).
The aforementioned associations were either smaller in effect sizes or disappeared in the prehypertensive group when analyzed separately. Curiously, greater BARIC was predictive of higher SBP independent of the covariates among the prehypertensive individuals in spite of the negative association between BARIC and DBP. These unexpected results may have biologically plausible sympathoadrenal-immune-vascular explanations but beyond what the data from the current investigation can offer. One can only speculate that other moderating factors such as obesity and physical fitness may be in play. As shown in our previous report (Hong et al., 2014), BARIC is significantly associated with obesity and cardiorespiratory fitness. In this investigation, obesity but not fitness was controlled for due to a lack of cardiorespiratory fitness assessments as a limitation. It is plausible that in our group of prehypertensive individuals cardiorespiratory fitness confounded the BARIC-SBP relationship such that many of prehypertensive individuals in our study may have possessed relatively higher cardiorespiratory fitness either due to genetics or physical training. Another potential explanation is the possibility that depressive symptoms moderated this relationship, given that the negative association between BARIC and somatic BDI scores seen in our normotensive participants was not observed in the prehypertensive participants. We have previously reported that SBP was positively associated with peripheral blood mononuclear cells’ (PBMC) βAR-mediated chemotactic responses to Iso among prehypertensive and hypertensive individuals with higher depressive mood (Redwine et al., 2011). We also reported that heart failure patients with major depression showed higher PBMC βAR hyperactivity in chemotactic responses to Iso in which moderate to severe depressive symptoms were associated with greater βAR hyperactivity (Redwine et al., 2014). Clearly, downstream cellular and molecular pathways involved in inflammatory molecule production (as in this current study) and chemotactic responses in our previous reports differ. But, given our previous immune-cellular findings that were also mediated by βARs, the potential effect of depressive symptoms may have implications in our current study.
We found that traditional CVD risk factors, lipid and glucose metabolism and BARIC were associated with depressive mood in individuals with normal to mildly elevated BP, although there were no strong associations between BP and depressive mood indices. Based on our results these associations were mainly driven by somatic symptoms rather than cognitive/affective components of BDI. These findings are in agreement with the literature, showing the inflammation-depression link (Dantzer et al., 2008; Dowlati et al., 2010; Miller et al., 2009) and given our previous findings of negative associations between BARIC and blood levels of inflammatory cytokines (Hong et al., 2014). Somatic but not cognitive/affective symptoms were associated with higher levels of inflammatory markers in blood in a large population study (Duivis et al., 2013) and with the greatest inflammatory bowel disease activity (Szigethy et al., 2014). Somatic symptoms also predicted increased TNF levels prospectively in women with major depression (Dannehl et al., 2014). In addition, somatic but not cognitive/affective depressive symptoms are associated with increased risk of CVD or CVD-related mortality and morbidity (de Jonge et al., 2007; Linke et al., 2009). Similar to our findings of the associations between BARIC and CVD risk factors, blunted BARIC was significantly associated with more somatic complaints even if our group of individuals reported BDI scores below clinical levels.
There exist a number of future considerations in light of the findings from this investigation. In spite of the ample data, indicating the risk of prehypertension for developing hypertension and CVD, there remains limited clear mechanistic evidence of the mild BP elevation and vascular inflammation pathophysiology. Our findings indicate associations between inflammation regulation mediated by sympathoadrenal dysregulation and BP that is observable even among individuals with normal to mildly elevated BP. Future studies that include other CV outcome measures and end organ responses such as vascular endothelial function in relation to BARIC in prehypertension would further provide insight into the role of sympathoadrenal system in vascular functions and inflammation in conditions of mild BP elevation. In addition, future studies should expand our findings to a larger sample of mild to moderate hypertensive individuals and also on and off antihypertensive medications to further decipher the nature and clinical significance of BP-BARIC and BARIC-inflammation relationships, as the current findings were confined to a small range of BP to focus on prehypertension. Prehypertensive populations of carefully selected metabolic profile would also offer insight into relative importance of BP vs. metabolic factors in cellular inflammatory responses mediated by sympathoadrenal regulatory mechanisms. Ultimately, longitudinal investigations that examine time-dependent relationships among BARIC, BP progression, and vascular inflammation will provide the knowledge that would inform efficacious treatment strategies. Lastly, in addition to the associations shown between BARIC and systemic inflammatory marker levels in our investigations it would be of benefit to confirm the associations with additional systemic inflammatory markers that are of particular relevance to CV pathology with established ranges that pose increased clinical risk such as CRP and soluble intercellular adhesion molecule 1 (sICAM-1). The BARIC-CV risk factor association can be also confirmed by assessing other intracellular cytokines in which anti-inflammatory cytokine production may provide insights into pro- vs. anti-inflammatory regulation mediated by βARs.
The new clinical guidelines released by the JNC 8 put its emphasis on treatment strategies to achieve BP levels that are higher than previously advocated (James et al., 2014). However, given its prevalence and a high probability of developing future hypertension and CVD, early detection of prehypertension and intervention are a critical and urgent issue in public health. As aforementioned, efficacious and justified intervention strategies that consider more individual-specific risk factors and subtle signs of pathophysiology may be the key in management and desirably, reversal of mild elevation of BP.
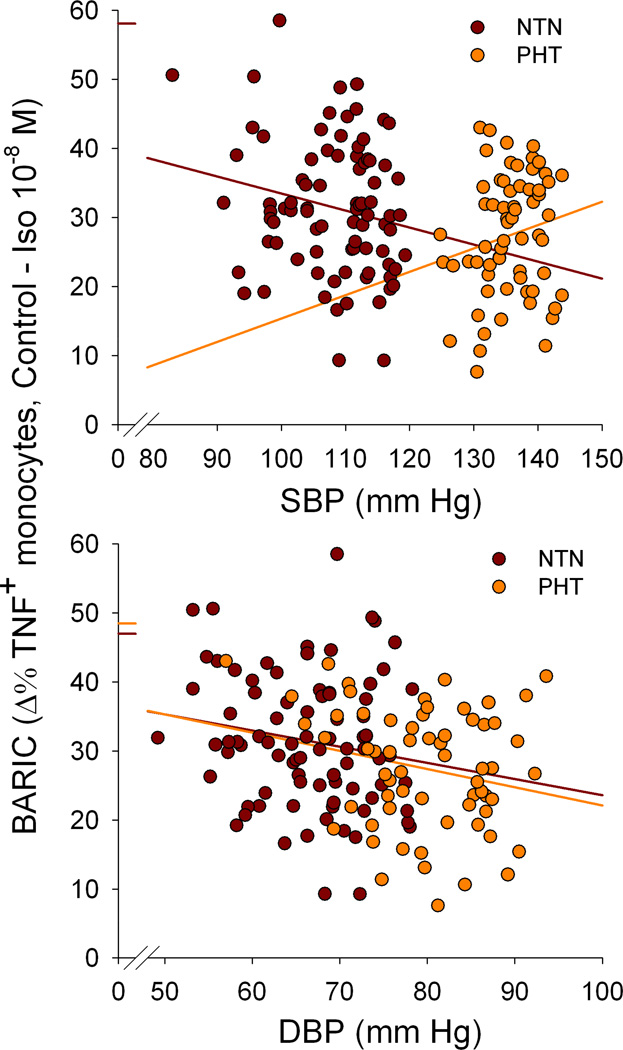
Figure 2.
Beta AR-mediated inflammation control (BARIC) at Iso 10−8 was positively correlated with both SBP (r=−.23, p< .01) and DBP (r= −.28, p= .001). The normotensive (NTN) group showed similar associations with smaller effect sizes (r= −.20, p= .07 for SBP; r= −.17, p= .13 for DBP) when analyzed separately. However, BARIC was positively associated with SBP (r= .18, p= .16) in prehypertensive (PHT) participants in spite of the negative correlation between BARIC and DBP (r= −.23, p= .07). Although none of the correlations were significant at p< .05 level, multivariate regression analyses confirmed that BARIC was independently predictive of SBP among prehypertensive participants after controlling for the covariates (p< .05). Scatter plots are presented using raw (pre-transform) SBP values for immediately interpretable illustration, although the analyses were done in log-transformed SBP values.
Highlights.
Monocytic βAR-mediated inflammation control (BARIC) is related to blood pressure (BP).
BARIC is negatively correlated with CVD risk factors in ones with normal to mildly elevated BP.
BARIC is negatively correlated with somatic depressive complaints.
Acknowledgments
This work was supported by the research grants R01HL090975 (SH), HL090975S1 (American Recovery and Reinvestment Act grant; SH), and UL1RR031980 for the UCSD Clinical and Translational Science Awards from the NIH and UC San Diego Academic Senate Research Grant (SH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: All authors declare that there are no conflicts of interest.
References
- Abboud FM. The sympathetic system in hypertension. Hypertension. 1982;4(suppl. II):11-208–11-225. [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Branen L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 2004;24:2137–2142. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- Brodde OE, Daul A, O’Hara N, Bock KD. Increased density and responsiveness of alpha 2 and beta-adrenoceptors in circulating blood cells of essential hypertensive patients. J Hypertens. 1984;2(3):S111–S114. [PubMed] [Google Scholar]
- Brodde O-E, Prywarra A, Anlauf M, Daul A, Brock KD. Increased number of β2-adrenoceptor in circulating lymphocytes of patients with essential hypertension. J Hypertens. 1984;1(suppl 2):263–266. [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Chockalingam A, Ganesan N, Venkatesan S, Gnanavelu G, Subramaniam T, Jaganathan V, Elangovan S, Alagesan R, Dorairajan S, Subramaniam A, Rafeeq K, Elangovan C, Rajendran V. Patterns and predictors of prehypertension among "healthy" urban adults in India. Angiology. 2005;56:557–563. doi: 10.1177/000331970505600506. [DOI] [PubMed] [Google Scholar]
- Choi KM, Park HS, Han JH, Lee JS, Lee J, Ryu OH, Lee KW, Cho KH, Yoon D, Baik SH, Choi DS, Kim SM. Prevalence of prehypertension and hypertension in a Korean population: Korean National Health and Nutrition Survey 2001. J Hypertens. 2006;24(8):1515–1521. doi: 10.1097/01.hjh.0000239286.02389.0f. [DOI] [PubMed] [Google Scholar]
- Chrysohoou C, Pitsavos C, Panagiotakos DB, Skoumas J, Stefanadis C. Association between prehypertension status and inflammatory markers related to atherosclerotic disease: the ATTICA study. Am J Hypertens. 2004;17(7):568–573. doi: 10.1016/j.amjhyper.2004.03.675. [DOI] [PubMed] [Google Scholar]
- Dannehl K, Rief W, Schwarz MJ, Hennings A, Riemer S, Selberdinger V, Stapf T, Euteneuer F. The predictive value of somatic and cognitive depressive symptoms for cytokine changes in patients with major depression. Neuropsychiatr Dis Treat. 2014;10:1191–1197. doi: 10.2147/NDT.S61640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge P, Mangano D, Whooley MA. Differential association of cognitive and somatic depressive symptoms with heart rate variability in patients with stable coronary heart disease: findings from the Heart and Soul Study. Psychosom Med. 2007;69(8):735–739. doi: 10.1097/PSY.0b013e31815743ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov S, Shaikh F, Pruitt C, Green M, Wilson K, Beg N, Hong S. Differential TNF production by monocyte subsets under physical stress: blunted mobilization of proinflammatory monocytes in prehypertensive individuals. Brain Behav Immun. 2013;27(1):101–108. doi: 10.1016/j.bbi.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Duivis HE, Vogelzangs N, Kupper N, de Jonge P, Penninx BW. Differential association of somatic and cognitive symptoms of depression and anxiety with inflammation: findings from the Netherlands Study of Depression and Anxiety (NESDA) Psychoneuroendocrinology. 2013;38(9):1573–1585. doi: 10.1016/j.psyneuen.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Euteneuer F, Mills PJ, Rief W, Ziegler MG, Dimsdale JE. Association of in vivo β-adrenergic receptor sensitivity with inflammatory markers in healthy subjects. Psychosom Med. 2012;74(3):271–277. doi: 10.1097/PSY.0b013e318245d762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS. Plasma catecholamines essential hypertension An analytical review. Hypertension. 1983;5(1):86–99. doi: 10.1161/01.hyp.5.1.86. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Quarti-Trevano F. The 'neuroadrenergic hypothesis' in hypertension: current evidence. Exp. Physiol. 2010;95:581–586. doi: 10.1113/expphysiol.2009.047381. [DOI] [PubMed] [Google Scholar]
- Greenlund KJ, Croft JB, Mensah GA. Prevalence of heart disease and stroke risk factors in persons with prehypertension in the United States, 1999–2000. Arch Intern Med. 2004;164:2113–2118. doi: 10.1001/archinte.164.19.2113. [DOI] [PubMed] [Google Scholar]
- Grotto I, Grossman E, Huerta M, Sharabi Y. Prevalence of prehypertension and associated carduivascular risk profiles among young Israeli adults. Hypertension. 2006;48(2):254–259. doi: 10.1161/01.HYP.0000227507.69230.fc. [DOI] [PubMed] [Google Scholar]
- Feldman DS, Carnes CA, Abraham WT, Bristow MR. Mechanisms of disease: beta-adrenergic receptors--alterations in signal transduction and pharmacogenomics in heart failure. Nat Clin Pract Cardiovasc Med. 2005;2(9):475–483. doi: 10.1038/ncpcardio0309. [DOI] [PubMed] [Google Scholar]
- Fitzgerald OJ, Doyle V, O'Brain ET, Kelly JG, O'Malley K. Beta-adrenoceptor density and responsiveness in borderline hypertension. J Hypertens. 1983;1(suppl 2):260–262. [Google Scholar]
- Flierl MA, Rittirsch D, Huber-Lang M, Sarma JV, Ward PA. Catecholamines-crafty weapons in the inflammatory arsenal of immune/inflammatory cells or opening pandora's box? Mol Med. 2008;14(3–4):195–204. doi: 10.2119/2007-00105.Flierl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasure-Smith N, Lespérance F. Depression and cardiac risk: present status and future directions. Heart. 2010;96(3):173–176. doi: 10.1136/hrt.2009.186957. [DOI] [PubMed] [Google Scholar]
- Frostegård J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013;11:117–130. doi: 10.1186/1741-7015-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijnen CJ, Rouppe van der Voort C, Wulffraat N, van der Net J, Kuis W, Kavelaars A. Functional alpha 1-adrenergic receptors on leukocytes of patients with polyarticular juvenile rheumatoid arthritis. J Neuroimmunol. 1996;71(1–2):223–226. doi: 10.1016/s0165-5728(96)00125-7. [DOI] [PubMed] [Google Scholar]
- Heine GH, Ortiz A, Massy ZA, Lindholm B, Wiecek A, Martínez-Castelao A, Covic A, Goldsmith D, Süleymanlar G, London GM, Parati G, Sicari R, Zoccali C, Fliser D. European Renal and Cardiovascular Medicine (EURECA-m) working group of the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) Monocyte subpopulations and cardiovascular risk in chronic kidney disease. Nat Rev Nephrol. 2012;8(6):362–369. doi: 10.1038/nrneph.2012.41. [DOI] [PubMed] [Google Scholar]
- Hong S, Dimitrov S, Pruitt C, Shaikh F, Beg N. Benefit of physical fitness against inflammation in obesity: role of beta adrenergic receptors. Brain Behav Immun. 2014;39:113–120. doi: 10.1016/j.bbi.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Farag N, Nelesen R, Ziegler M, Mills PJ. Effects of regular exercise on lymphocyte subsets and CD62L after psychological vs. physical stress. J Psychosom Res. 2004;56(3):363–370. doi: 10.1016/S0022-3999(03)00134-X. [DOI] [PubMed] [Google Scholar]
- Hong S, Johnson T, Farag N, Guy H, Matthews S, Mills PJ. The effect of fitness on leukocyte trafficking and adhesion molecule expression in response to moderate exercise challenge. J Appl Physiol. 2005;98:1057–1063. doi: 10.1152/japplphysiol.00233.2004. [DOI] [PubMed] [Google Scholar]
- Hong S, Mills PJ. Preferential Demargination of CD16+ Monocytes and Cell Adhesion Molecule Expression in response to Exercise in Hypertensive Individuals. Brain Behav Immun. 2008;22:590–599. doi: 10.1016/j.bbi.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XX, Goldmuntz EA, Brosnan CF. The effect of norepinephrine on endotoxin-mediated macrophage activation. J Neuroimmunol. 1991;31(1):35–42. doi: 10.1016/0165-5728(91)90084-k. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Ballantyne CM, Sharrett AR, Smith LC, Davis CE, Gotto AM, Jr, Boerwinkle E. Circulating Adhesion Molecules VCAM-1, ICAM, and E-selectin in Carotid Atherosclerosis and Incident Coronary Heart Disease Cases. Circulation. 1997;96:4219–4225. doi: 10.1161/01.cir.96.12.4219. [DOI] [PubMed] [Google Scholar]
- Israeli E, Schochat T, Korzets Z, Tekes-Manova D, Bernheim J, Golan E. Prehypertension and obesity in adolescents: a population study. Am J Hypertens. 2006;19:708–712. doi: 10.1016/j.amjhyper.2006.01.012. [DOI] [PubMed] [Google Scholar]
- James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr, Narva AS, Ortiz E. Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- Julius S, Nesbitt SD, Egan BM, Weber MA, Michelson EL, Kaciroti N, Black HR, Grimm RH, Jr, Messerli FH, Oparil S, Schork MA. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med. 2006;354:1685–1697. doi: 10.1056/NEJMoa060838. [DOI] [PubMed] [Google Scholar]
- Karlsson EA, Beck MA. The burden of obesity on infectious disease. Exp Biol Med (Maywood) 2010;235(12):1412–1424. doi: 10.1258/ebm.2010.010227. [DOI] [PubMed] [Google Scholar]
- Kavelaars A, van de Pol M, Zijlstra J, Heijnen CJ. Beta 2-adrenergic activation enhances interleukin-8 production by human monocytes. J Neuroimmunol. 1997;77(2):211–216. doi: 10.1016/s0165-5728(97)00076-3. [DOI] [PubMed] [Google Scholar]
- Kop WJ. The integration of cardiovascular behavioral medicine and psychoneuroimmunology: new developments based on converging research fields. Brain behave Immun. 2003;17:233–237. doi: 10.1016/s0889-1591(03)00051-5. [DOI] [PubMed] [Google Scholar]
- Kop WJ, Gottdiener JS. The role of immune system parameters in the relationship between depression and coronary artery disease. Psychosom Med. 2005;67(Suppl 1):S37–S41. doi: 10.1097/01.psy.0000162256.18710.4a. [DOI] [PubMed] [Google Scholar]
- Landmann R, B Orgisser E, B Ohler FR. Human lymphocytes as a model for beta-adrenoceptors in clinical investigation. J Recept Res. 1983;3:71–88. doi: 10.3109/10799898309041924. [DOI] [PubMed] [Google Scholar]
- Lee JH, Hwang SY, Kim EJ, Kim MJ. Comparison of risk factors between prehypertension and hypertension in korean male industrial workers. Public Health Nurs. 2006;23:314–323. doi: 10.1111/j.1525-1446.2006.00567.x. [DOI] [PubMed] [Google Scholar]
- Linke SE, Rutledge T, Johnson BD, Vaccarino V, Bittner V, Cornell CE, Eteiba W, Sheps DS, Krantz DS, Parashar S, Bairey Merz CN. Depressive symptom dimensions and cardiovascular prognosis among women with suspected myocardial ischemia: A report from the National Heart, Lung, and Blood Institute-sponsored Women's Ischemia Syndrome Evaluation. Arch Gen Psychiatry. 2009;66(5):499–507. doi: 10.1001/archgenpsychiatry.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liszka HA, Mainous AG3rd, King DE, Everett CJ, Egan BM. Prehypertension and cardiovascular morbidity. Ann Fam Med. 2005;3(4):294–299. doi: 10.1370/afm.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKellar GE, McCarey DW, Sattar N, McInnes IB. Role for TNF in atherosclerosis? Lessons from autoimmune disease. Nat. Rev. Cardiol. 2009;6:410–417. doi: 10.1038/nrcardio.2009.57. [DOI] [PubMed] [Google Scholar]
- Middeke M, Remien J, Block LH, Kirzinger S, Landrock A, Holzgreve H. Beta2-Adrenoceptor Density on Membranes and on intact mononuclear cells in essential hypertension. Res Exp Med. 1983:227–232. doi: 10.1007/BF01855645. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills PJ, Farag N, Hong S, Barry C, Ziegler MG. Leukocyte CD62L and CD11a expression in response to a psychological stressor in human hypertension. Brain Behav Immun. 2003;17:260–267. doi: 10.1016/s0889-1591(03)00055-2. [DOI] [PubMed] [Google Scholar]
- Milner JJ, Beck MA. The impact of obesity on the immune response to infection. Proc Nutr Soc. 2012;71(2):298–306. doi: 10.1017/S0029665112000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M, Giles TD, Izzo JL, Black HR. Prehypertension- what is it and should it be treated? J. Clin Hypertens. 2006;8:812–818. [PubMed] [Google Scholar]
- Padro CJ, Sanders VM. Neuroendocrine regulation of inflammation. Semin Immunol. 2014;26(5):357–368. doi: 10.1016/j.smim.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi AI, Suri MF, Kirmani JF, Divani AA, Mohammad Y. Is prehypertension a risk factor for cardiovascular diseases? Stroke. 2005;36:1859–1863. doi: 10.1161/01.STR.0000177495.45580.f1. [DOI] [PubMed] [Google Scholar]
- Redwine L, Hong S, Wilson KS, Mills PJ. Elevated depressive symptoms are associated with altered leukocyte beta-adrenergic sensitivity in hypertensive and prehypertensive patients. Psychosom Med. 2013;75:A83. [Abstract] [Google Scholar]
- Redwine LS, Hong S, Rutledge T, Wentworth B, Pung M, Ziegler MG, Maisel A, Greenberg B, Mills PJ. Leukocyte β-adrenergic receptor sensitivity and depression severity in patients with heart failure. Psychosom Med. 2014;76(9):726–731. doi: 10.1097/PSY.0000000000000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redwine LS, Wirtz PH, Hong S, Bosch JA, Ziegler MG, Greenberg B, Mills PJ. Depression as a potential modulator of Beta-adrenergic-associated leukocyte mobilization in heart failure patients. J Am Coll Cardiol. 2010;16: 56(21):1720–1727. doi: 10.1016/j.jacc.2010.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;14;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48(8):1527–1537. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- Sanders VM. The beta2-adrenergic receptor on T and B lymphocytes: do we understand it yet? Brain Behav Immun. 2012;26(2):195–200. doi: 10.1016/j.bbi.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoia C, Schiffrin EL. Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci (Lond) 2007;112(7):375–384. doi: 10.1042/CS20060247. [DOI] [PubMed] [Google Scholar]
- Skoog T, Dichtl W, Boquist S, Skoglund-Andersson C, Karpe F, Tang R, Bond MG, de FU, Nilsson J, Eriksson P, Hamsten A. Plasma tumour necrosis factor-alpha and early carotid atherosclerosis in healthy middle-aged men. Eur. Heart J. 2002;23:376–383. doi: 10.1053/euhj.2001.2805. [DOI] [PubMed] [Google Scholar]
- Szigethy EM, Youk AO, Benhayon D, Fairclough DL, Newara MC, Kirshner MA, Bujoreanu SI, Mrakotsky C, Bousvaros A, Srinath AI, Keljo DJ, Kupfer DJ, DeMaso DR. Depression subtypes in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2014;58(5):574–581. doi: 10.1097/MPG.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szelényi J, Kiss JP, Vizi ES. Differential involvement of sympathetic nervous system and immune system in the modulation of TNF-alpha production by alpha2- and beta-adrenoceptors in mice. J Neuroimmunol. 2000;103(1):34–40. doi: 10.1016/s0165-5728(99)00234-9. [DOI] [PubMed] [Google Scholar]
- Torres MB, Vega VL, Bedri M, Saad D, Trentzsch H, Reeves RH, De Maio A. IL-10 plasma levels are elevated after LPS injection in splenectomized A/J mice. J Surg Res. 2005;129(1):101–106. doi: 10.1016/j.jss.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Tsai PS, Ke TL, Huang CJ, Tsai JC, Chen PL, Wang SY, Shyu YK. Prevalence and determinants of prehypertension status in the Taiwanese general population. J Hypertens. 2005;23:1355–1360. doi: 10.1097/01.hjh.0000173517.68234.c3. [DOI] [PubMed] [Google Scholar]
- Weber MA, Drayer JIM. The sympathetic nervous system in primary hypertension. Mineral Electrolyte Metab. 1982;7:57–66. [PubMed] [Google Scholar]