Abstract
There are extensive bidirectional interactions between the gut microbiota and the central nervous system (CNS), and studies demonstrate that stressor exposure significantly alters gut microbiota community structure. We tested whether oligosaccharides naturally found in high levels in human milk, which have been reported to impact brain development and enhance the growth of beneficial commensal microbes, would prevent stressor-induced alterations in gut microbial community composition and attenuate stressor-induced anxiety-like behavior. Mice were fed standard laboratory diet, or laboratory diet containing the human milk oligosaccharides 3′Sialyllactose (3′SL) or 6′Sialyllactose (6′SL) for two weeks prior to being exposed to either a social disruption stressor or a non-stressed control condition. Stressor exposure significantly changed the structure of the colonic mucosa-associated microbiota in control mice, as indicated by changes in beta diversity. The stressor resulted in anxiety-like behavior in both the light/dark preference and open field tests in control mice. This effect was associated with a reduction in immature neurons in the dentate gyrus as indicated by doublecortin (DCX) immunostaining. These effects were not evident in mice fed milk oligosaccharides; stressor exposure did not significantly change microbial community structure in mice fed 3′SL or 6′SL. In addition, 3′SL and 6′SL helped maintain normal behavior on tests of anxiety-like behavior and normal numbers of DCX+ immature neurons. These studies indicate that milk oligosaccharides support normal microbial communities and behavioral responses during stressor exposure, potentially through effects on the gut microbiota-brain axis.
1. Introduction
The body is colonized by a vast and complex mixture of microbes collectively referred to as the microbiota. These microbes reside within communities in their specific niche in the body. During homeostatic states, the structure of the bacterial communities in the gut is relatively stable (Faith et al., 2013). However, this stability can be altered through environmental and dietary manipulations. For example, research from our laboratory, as well as others, has shown that exposure to different types of stressors, ranging from social stressors to physical and physiological stressors, can impact the composition of the microbiota (Tannock and Savage, 1974, Bangsgaard Bendtsen et al., 2012, De Palma et al., 2014, Galley et al., 2014a, Galley et al., 2014b), with reductions in potentially beneficial microbes often being found (Bailey and Coe, 1999, Bailey et al., 2004, Knowles et al., 2008, Galley et al., 2014a, Galley et al., 2014b). Diet can also impact the microbiota, and different diets, such as those containing high fat contents, are associated with different microbial communities within the gut (Hildebrandt et al., 2009, Daniel et al., 2014). Changing the composition of the gut microbiota in turn changes microbial community functions (Turnbaugh et al., 2008, Albenberg and Wu, 2014), which has renewed interest in the use of diet to enhance beneficial microbial populations. One purpose of this study was to determine whether the inclusion of prebiotic human milk oligosaccharides into the diet would impact stressor-induced changes to microbial community composition.
Prebiotics are defined as nonviable food components that confer a health benefit on the host associated with modulation of the microbiota (Gibson et al., 2010); compounds that can enhance the growth of administered or commensal probiotic microbes are typically identified as prebiotics. Probiotics, in turn, are live microorganisms that, when administered in adequate amounts confer a health benefit on the host (Hill et al., 2014). Human milk oligosaccharides (HMO) can be considered prebiotics, because they have been demonstrated to play a significant role in the growth of specific bacteria including probiotic members of the genus Bifidobacterium and Lactobacillus (Coppa et al., 2006, Bindels et al., 2015). Prebiotic-induced enhancement of these beneficial microbes is thought to have multiple beneficial effects on host immunity and physiology (Vandenplas et al., 2015). Moreover, Bifidobacterium and Lactobacillus spp. strongly affect the brain-gut-axis (Dinan et al., 2013, Zhou and Foster, 2015). After ingestion, HMO pass mainly unabsorbed through the small intestine into the colon, where they are fermented to short-chain fatty acids (SCFA) and lactic acids (Ogawa et al., 1992). Sialyllactose, which is the core structure of the sialyllated HMO, is essentially sialic acid (SA) bound to a lactose molecule. The predominant forms of sialyllactose are 6′-sialyllactose (6′SL) and 3′Sialyllactose (3′SL) (Martin-Sosa et al., 2003). 3′SL is a compound where the N-acetyl-D-neuraminic acid (Neu5Ac or SA) unit is connected to the galactose unit of lactose at the 3 position; in 6′SL, this connection is at the 6 position. Levels of 3′SL in human milk remains relatively stable throughout lactation while 6′SL gradually declines (Asakuma et al., 2007). While the levels of 3′SL and 6′SL differ, the biological effects of these HMOs are very similar. Both 6′SL and 3′SL have anti-inflammatory properties that can impact opportunistic bacterial attachment in the gut (Andersson et al., 1986, Simon et al., 1997) and can support the growth of beneficial gut bacteria (Gourbeyre et al., 2011). For example, 3′SL and 6′SL promoted growth of Bifidobacterium longum in vitro (Yu et al., 2013). B. longum has been shown to improve psychological wellbeing in humans (as assessed by global hospital anxiety and depression scores on the Hopkins symptoms checklist-90) (Messaoudi et al., 2011b) and impact anxietylike behavior in laboratory animals (Bercik et al., 2011, Savignac et al., 2014). This led us to test whether the administration of 3′SL or 6′SL could impact stressor-induced anxiety-like behavior.
In addition to the effects that 3′SL and 6′SL have on the microbiota, these compounds can also have a more direct effect on the brain. Sialyllactose has been shown to be a source of SA that is particularly important for brain development and for cognitive functions (Wang and Brand-Miller, 2003). For example, rats fed sialyllactose or galactosylated SA showed improved learning ability in a swimming learning test, an effect that was associated with increased SA and ganglioside content in the brain (Sakai et al., 2006). It is now recognized that SA from dietary sources becomes available during digestion and absorption and is utilized by brain cells to form gangliosides and sialylated proteins, such as neural cell adhesion molecule (NCAM) (Wang et al., 2003). Sialic acid is part of the glycosylation process for a variety of molecules including NCAM and brain-derived neurotrophic factor (BDNF) (Janas & Janas, 2011; Sato & Katajima, 2013). Given the importance of these factors for the proliferation and development of brain-derived cells, an additional aspect of this study was to assess whether administering HMO would impact the effects of stressor exposure on brain cell proliferation and stability.
Reductions in brain cell proliferation are widely known to be associated with stress-induced behavioral changes (McEwen and Magarinos, 2001), including cognitive behaviors (Lee et al., 2015). Whether there are additional behavioral consequences of this reduction in brain cell proliferation is not as well-studied, but evidence is beginning to emerge that changes in brain cell proliferation are also involved in anxiety- and depressive-like behaviors. For example, recent studies in mice bred for comorbid high trait anxiety/depressive-like behavior show that depressive-like behavior was rescued by anti-depressants, while anxiety-like behavior and deficits in neural progenitor (i.e., Bromodeoxyuridine [BrdU] positive cells) and immature neurons (i.e., Doublecortin [DCX] positive cells) remained unaltered (Sah et al., 2012). In addition, reducing neural progenitor cells and immature neurons residing in the dentate gyrus of the hippocampus by selective ablation, resulted in marked anxiety-like behaviors in the elevated plus maze and light/dark tests (Revest et al., 2009), thus supporting a role for reduced neurogenesis in anxiety. We have previously shown that in addition to changing the commensal microbiota, exposing mice to a social disruption (SDR) stressor increases anxiety-like behavior (Kinsey et al., 2007, Wohleb et al., 2011). Thus, a final aspect of this project was to determine whether brain cell proliferation was decreased in stressor-exposed animals, and whether 3′SL or 6′SL could prevent this stressor-induced effect.
2. Methods
2.1. Subjects
Subjects were 6 to 8 week-old male C57/BL6 mice purchased from Charles River Laboratories (Wilmington, MA). Male mice were selected because of the use of the social disruption stressor that involves aggressive interactions between conspecifics. While it would be of interest to verify study results in females, these aggressive interactions do not occur between female mice. Thus, the results of the study have limited extrapolation to females. Upon arrival, animals were separated 3/cage and allowed to acclimate in the animal facility for ∼1 week. Mice were kept in standard polycarbonate mouse cages and maintained on a 12 hr light/dark cycle with lights being turned on at 0600 in an AAALAC (American Association of Accreditation of Laboratory Animal Care) facility. Food and water was available ad libitum unless experimental manipulations were being conducted. Aggressors used in the SDR protocol were individually housed CD-1 male retired breeders. Animals were treated in compliance with the Guide for the Care and Use of Laboratory Animals, and the experiments were carried out in accordance with a protocol approved by the Institutional Laboratory Animal Care and Use Committee (ILACUC) at The Ohio State University.
2.2. Diet
Mice were fed either a control AIN-93G semi-purified laboratory mouse diet, a modified AIN-93G diet supplemented with 6′SL or a modified AIN-93G diet supplemented with 3′SL (5% of the diet; Harlan Laboratories, Inc.) for 2 weeks prior to, and during stressor exposure. AIN-93G is a common experimental diet that contains essential nutrients for growing and gestational rodents.
2.3. Social Disruption (SDR) Stressor
The SDR paradigm has been previously described (Allen et al., 2012, Tarr et al., 2012). In brief, an aggressive male intruder CD-1 mouse was introduced into cages of established male cohorts (3 per cage) of naïve C57BL/6 mice for 2 hours between 1600 and 1800 for six consecutive nights. Intruder mice were outbred CD-1 retired breeders that were individually housed upon arrival to the animal facility. During SDR, resident mice were observed for subordinate behaviors such as upright posture, exposed under flank, fleeing, and cowering in the corners of the cage. If the aggressive intruder did not initiate an attack within 5 minutes or was attacked by any of the resident mice, a new intruder was introduced. At the end of each SDR cycle, intruder mice were removed and placed into their original home cage until the next SDR cycle began. Care was taken to introduce the same intruders on consecutive nights to reduce the chance of transference of multiple microbiotas through fecal contents. The health status of the mice was carefully examined throughout the paradigm. Mice that were injured or moribund were removed from the study. Consistent with previous studies using SDR, less than 5% of mice met the early removal criteria. Mice exposed to the stressor are referred to as SDR stressor mice, whereas Home Cage Control (HCC) mice were not handled throughout the experiment and were fed either the control or an experiment diet.
2.4. Experimental Design
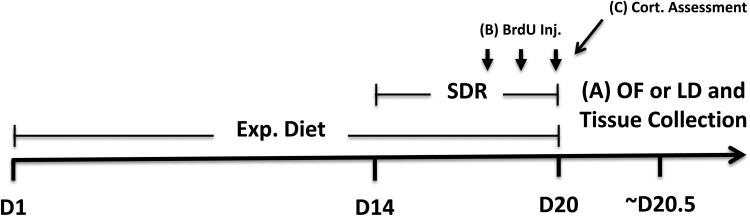
To examine the effects of the sialyllactose on SDR-induced anxiety-like behavior, upon arrival animals were fed control diets or diets supplemented with 6′SL or 3′SL for 2 weeks prior to stressor exposure (Fig. 1). Animals were then exposed to our SDR stressor while continuing the experimental diet. Body masses were collected every third day throughout dietary manipulation to assess for alterations in weight gain that the diet may have caused. Fourteen hrs after the last cycle of SDR (i.e., 6th cycle), control and experimental animals were either tested in the open field or light/dark preference tasks to assess for variations in anxiety-like behavior. Immediately following behavioral testing, animals were sacrificed by CO2 asphyxiation, and whole blood, spleens and colons were removed for biological analyses. Whole blood was used to determine serum levels of IL-6, spleens were weighed and used as a readout for SDR's effectiveness, and colons were used to assess 6′SL and 3′SL effects on the bacterial community structure of the gut. In a separate group of animals, whole blood was collected 1 hr following the 6th cycle of SDR to evaluate potential alterations to serum corticosterone that 6′SL or 3′SL may have had. Brains were also taken 14 hrs after the last cycle of SDR to evaluate the effects of SDR and 6′SL and 3′SL on brain cell proliferation and the acute impact on immature neurons.
Figure 1. Overall experimental time line.
Three separate cohorts of mice (with each cohort replicated 3 times with different shipments of mice) were used to determine: (1) presence of anxiety-like behavior in the open field and brain immunohistochemistry, (2) presence of anxiety-like behavior on the light dark preference, and (3) colonic microbiota, plasma corticosterone, plasma IL-6, and spleen mass. n=9 mice per group per diet were randomly assigned at the beginning of the experiment.
2.5. Open field and Light/Dark Preference Tests for Anxiety-like Behavior
Open field and light/dark preference apparatus were used to determine if pretreatment with prebiotics influenced SDR-induced anxiety-like behavioral deficits. The open field and light/dark preference tasks are widely used behavioral tests of anxiety-like behavior that require no “learning” of novel tasks. The open field apparatus consisted of an opaque 40×40×25 cm Plexiglas box divided into a grid pattern and is designed to measure a rodent's innate tendency to explore their environment while avoiding open spaces. The light/dark apparatus used the same box as the open field but was divided into two equal zones (i.e., light and dark) with an opening separating the two at floor level. The dark zone was enclosed by black Plexiglas and had significantly less light than the light zone (∼3 lux). Mice that show anxiety-like behavior (i.e., SDR stressed animals) have a decreased time spent in the open area of the open field and have an increased time spent in the dark portion of the light/dark box compared to control mice (i.e., non-stressed controls).
Fourteen hours following the last cycle of SDR, subjects were transported to a separate room and tested in the open field or light/dark for a total of 5min based on prior reports from our laboratory using SDR stressor paradigm (Kinsey et al., 2007, Wohleb et al., 2013, Wohleb et al., 2014a, Wohleb et al., 2014b). Between each group of animals being tested, the open field box was cleaned with H2O and diluted ETOH to reduce odor cues between subjects. Experimental subject's activities were monitored using an automated digital beam break system attached to computer containing an open field or light/dark Fusion software template (Omnitech Electronics, Inc., Columbus, OH). Dependent measures to assess anxiety-like behavior in the open field included total distance traveled, latency to enter the center, and time in the center. For the light/dark preference test the dependent measures were time spent in the dark, latency to enter the dark, light-dark transitions, and total distance travelled.
2.6. Serum Corticosterone and Interleukin-6 Enzyme-Linked Immunosorbant Assay (ELISA)
To assess serum corticosterone levels ∼100 μl of whole blood was collected retro-orbitally by disrupting the capillary bed of the eye socket 1 hr following the last cycle of SDR. To determine serum IL-6 levels, whole blood was collected by cardiac heart puncture following CO2 asphyxiation. Following serum collection, whole blood was allowed to coagulate at room temperature for 30 min, followed by centrifugation at 2500 RPM for 15 min. After collection, serum was stored at -80 °C until further analyses by ELISA. Both serum corticosterone (Abcam; Cambridge, MA) and IL-6 (R&D Systems, Inc.; Minneapolis, MN) ELISAs kits were conducted following manufacturer's protocols.
2.7. Microbiota Sequencing and Analyses
Microbiota sequencing was used to determine if our pretreatment with prebiotics influenced SDR-induced alterations in microbiota diversity. All DNA isolation, enrichment and sequencing was performed by Second Genome (South San Francisco, CA). Briefly, DNA was extracted from the colonic tissue using the MoBio UltraClean Tissue and Cells DNA Isolation Kit. The DNA was then cleaned using PowerClean DNA Clean-Up Kit and quantified with the Qubit Quant-iT dsDNA Broad-Range Kit. DNA was then amplified for 16S V4 rDNA targeting and flow-cell adaptor and indexing barcodes were integrated. Illumina MiSeq paired-end sequencing (2×250bp) was performed for 250 total cycles.
Sequences, in fastq files, were de-multiplexed by barcode. Stitching, quality filtering and sequence analysis was performed using Quantitative Insights Into Microbial Ecology (QIIME) (Caporaso et al., 2010). Paired-end sequences overlapped and were stitched using fastq-join (Aronesty, 2011). Filtering was based on default QIIME parameters, including 1.5 allowed errors in barcode, sequence quality threshold of Q20, and 3 allowed bad quality bases before truncation. OTU picking was performed based on the closed reference protocol against the GreenGenes 13_5 database with Uclust (Edgar, 2010, McDonald et al., 2012) using the 97% OTU cluster. After final filtering/curation, a mean of 139,669 sequences/sample over 47 samples remained.
To specifically analyze the sequencing data from the OTU biom table that was constructed during OTU picking, individual diets were filtered out (Control, 6′SL, 3′SL) into separate OTU tables. Control chow had a mean of 129,438 sequences/samples, 6′SL had a mean of 135,449 sequences/sample and 3′SL had a mean of 152,772 sequences/sample. Diet bioms were also joined together for analysis of the overall effect of prebiotic supplementation.
2.8. Brain Cell Proliferation and Immature Neuronal Assessment and Analyses
On the last three nights of SDR, mice were injected with 50mg/kg of BrdU (a thymidine analogue) 1 hr before each cycle of SDR. Fourteen hours after the last cycle of SDR, mice were transcardially perfused using sterile PBS followed by 4% paraformaldehyde (PFA; Sigma-Aldrich; St. Louis, MO). Brains were then post-fixed in 4% PFA for 24 hrs and then put in 20% sucrose for 72 hrs, with solution changes every 24 hrs. After post-fixiation, brains were flash frozen in ice-cold isopentane and subsequently sectioned on a cryostat at 40 μm and placed in 24-well tissue culture plates. Collection started with sections containing a fully intact dentate gyrus (DG) and ended when the DG was no longer visible. Hippocampal brain areas were identified based on anatomical landmarks referenced in a stereotaxic mouse brain atlas.
Once obtained, sections were washed, denatured in 2N HCL at 37° C for 30 min, and blocked with 3% normal donkey serum. Sections were then incubated for 24 hrs in the appropriate primary antibody and then overnight in their species matched secondary antibodies. The primary antibodies used were rat anti BrdU (1:1000; Abcam, Cambridge, MA) to stain proliferating cells and goat anti-DCX (1:200; Santa Cruz Biotechnology, Dallas, TX) to stain immature neurons. The secondary antibodies used were Alexafluor goat anti-rat 488 and Alexafluor goat anti-rabbit 594 (1:500; Life Technologies, Carlsbad, CA). Following staining, sections were placed on slides, cover-slipped using Fluoromount (Beckman Coulter, Inc., Fullerton, CA) and visualized using a wide field fluorescent microscope.
To quantify singly labeled BrdU+ cells, every 6th coronal section was counted for single positive cells and an average number of positive cells/slice was obtained. To quantify singly labeled DCX+ cells, three sections (i.e., rostral, caudal, and mid-sections) were collected and counted to obtain an average number of immature neurons/slice (i.e., DCX+ cells).
2.9. Statistical Procedures
In all studies, 9 mice per group (stress vs. non-stress) and per diet (control, 3′SL, or 6′SL) were used. Mice were removed from the study if there were any signs of wounds in control mice (as this indicates social defeat in the control mice) or if wounds exceeded our early removal criteria. The final sample sizes for each measure are listed in the Figure legends. Standard two-way ANOVAs were used to analyze light/dark preference, open field, spleen mass, serum corticosterone, serum IL-6, microbiome sequencing, and brain immunohistochemistry data with Diet and Stress as the between-subjects variables. In addition, body mass was analyzed using a repeated-measures ANOVA with Stress and Diet as the between-subjects variables and Day as the within-subject variable. When appropriate, significant interaction effects were subjected to Bonferroni corrected t-tests. An alpha level of p<0.05 was set as the rejection criteria for the null hypothesis. All data were analyzed using SPSS statistical software version 21 (IBM Corp.; Armonk, NY) and presented as treatment means ± standard error of the mean (SEM).
Beta and alpha diversity analysis were performed with QIIME in order to compare the effect of SDR on mouse colonic microbiota. Beta diversity was analyzed with unweighted Unifrac distance matrices (Lozupone and Knight, 2005) at a rarefied depth of 44,338 sequences per sample. Principle coordinate analysis (PCoA) plots were produced with QIIME to visualize clustering of samples based upon composition of the colonic microbial community. 3D PCoA plots were produced using Emperor (Vazquez-Baeza et al., 2013). The adonis statistic, provided through the vegan package of R (Development, Oksanen J) and implemented into QIIME, was used to calculate significance in variation of the distance matrices between HCC and SDR mice, in order to determine whether groups were altered in different diets.
Alpha diversity analysis included Shannon Diversity, Chao1 (estimation of richness), equitability (a measurement of evenness), and observed OTUs. All alpha diversity measurements were compared (HCC vs. SDR) using parametric T-tests with QIIME. Varying depths were used for HCC vs. SDR alpha-diversity statistical testing: 6′SL chow- 42,730 sequences; Control Chow- 42,580 sequences; 3′SL Chow- 65,690 sequences. Varying depths were also used in statistical analysis of the effect of diet upon alpha diversity: Control-3′SL, Control-6′SL, 3′SL-6′SL dichotomous analysis had 42,028 sequences, 42,730 sequences, and 42,601 sequences respectively.
For phyla and genera taxonomic comparisons, bacterial proportions were normalized by finding the square root of the proportion, then the arcsine of this square root. Parametric t-tests were performed on dichotomous variables (HCC vs. SDR) using these normalized values. Univariate ANOVA was used for taxonomic abundance comparisons of the three diets (Control Diet vs. 3′SL vs. 6′SL), and post-hoc LSD tests were used to identify the significantly different diets. The Benjamini-Hochberg correction for multiple comparisons was used with a q-value of 0.100 for all taxonomic comparisons. All taxonomic comparisons were made with SPSS and presented as proportion means ± SEM.
3. Results
3.1. Dietary sialyllactose significantly affects the colonic microbiota
Alpha diversity, which is a measure of within sample diversity, was measured using the Shannon Diversity Index (SDI) which encompasses bacterial species abundance (i.e., the total number of bacterial species) and evenness (i.e., the distribution of those species). Control-fed mice had significantly higher SDI than 6′SL-fed mice (p<0.05, t=2.21), but were unchanged compared to 3′SL-fed mice (p=0.455, t=0.778). The SDI was not significantly different in 3′SL and 6′SL-fed mice (p=0.114, t=1.648).
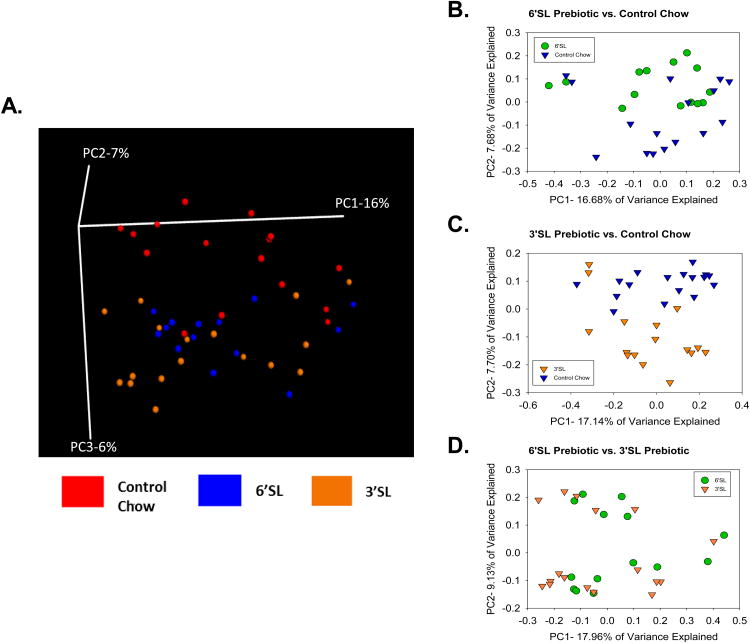
Beta-diversity was analyzed using unweighted UniFrac distances, which is a method of comparing microbial communities by calculating phylogenetic differences between sets of taxa in one sample compared to a second sample. Once all pairwise comparisons are made, a phylogenetic distance matrix can be created and plotted on either a 2-Dimensional (2D) or 3-Dimensional (3D) principal coordinate analysis (PCoA) plot depicting variability along the 2 or 3 principal components (respectively). In general, samples that cluster closer together on the PCoA plot represent samples with similar microbial community composition. Using a 3D PCoA plot, the control diet clustered separately from the prebiotic diets (Fig. 2A). The use of the multivariate adonis statistic indicated that mice that were fed 6′SL or 3′SL had an altered microbial profile when compared to mice that were fed a control diet (adonis, p<0.01, R2=0.06734). When compared dichotomously, the control diet was significantly different from 6′SL diet (p <0.05, R2=0.0562) and the 3′SL diet (p<0.005, R2=0.0648), while 3′SL and 6′SL did not differ significantly (p=0.391, R2=0.03345) (Fig. 2B-D).
Figure 2. Dietary sialyllactose shifts the beta diversity of the murine colonic microbiota community structure.
The main effects of diet on microbial community composition were assessed using sequence data collapsed across stressor-exposed and non-stressed control mice. (A) A 3D PCoA plot using unweighted UniFrac distances showed that control diet clustered separately from 6′SL or 3′SL (p<.05). Dichotomous microbiota community structure clustering using unweighted UniFrac 2D PCoA plots was also performed. Microbial communities in mice fed a control diet were significantly different than communities in mice fed 6′SL (B; p<.05) or fed 3′SL (C; p<.05). Community structure did not differ in mice fed 6′SL or 3′SL (D). Significance testing was calculated using adonis, implemented in QIIME. n=16 control diet, n=14 3′SL, n=17 6′SL.
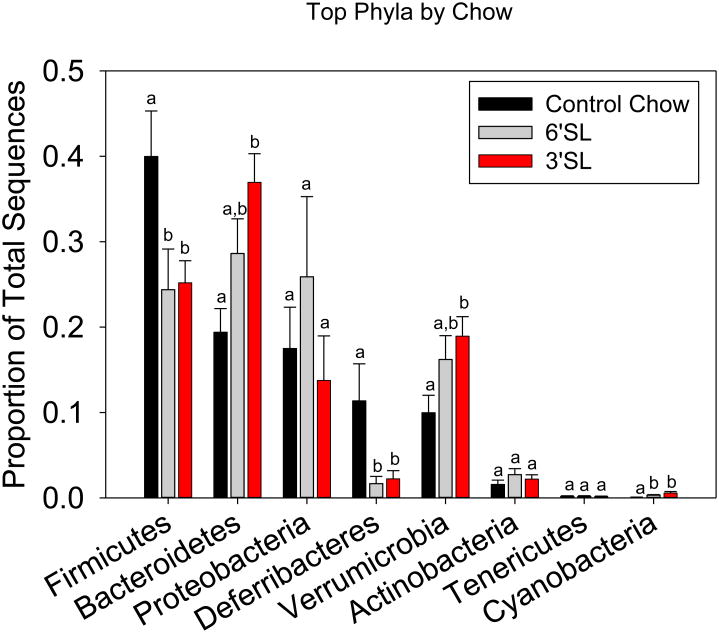
Taxonomic shifts were next examined. At the phylum level, the Firmicutes, Bacteroidetes, Verrumicrobia, Deferribacteres, and Cyanobacteria were all significantly changed by diet when analyzed using ANOVA. Firmicutes and Cyanobacteria were significantly decreased in the prebiotic groups compared to control chow (p<.05), while the Bacteroidetes were increased in 3′SL compared to control (p<.05). Verrumicrobia was also increased in 3′SL compared to control only, while Deferribacteres was increased in the control group compared to both 3′SL and 6′SL (Fig. 3). At the genus level, prebiotic supplementation affected a number of groups. Akkermansia was significantly increased in mice that had been fed 3′SL over control, while Bacteroides and Coprococcus were increased in the 3′SL mice over both 6′SL and control. Mucispirillum was significantly reduced in the prebiotic groups. Unclassified Clostridiales, unclassified Clostridiaceae and unclassified Ruminococcaceae were increased in controls over prebiotic-fed mice (Table 1).
Figure 3. Dietary sialyllactose alters the abundances of the major phyla.
Colons were removed and the proportion of total sequences was determined from the overall abundance of bacteria comprising the microbiota. Bars represent means ± SEM. Different letters represent statistical significance between groups within each bacterial phyla (p<0.05). n=16 control diet, n=14 3′SL, n=17 6′SL.
Table 1. Tarr et al., Prebiotics and anxiety.
| Top 25 Most Abundant Genera | Control Chow | 3′SL | 6′SL | |||
|---|---|---|---|---|---|---|
|
| ||||||
| HCC Control | SDR Stressor | HCC Control | SDR Stressor | HCC Control | SDR Stressor | |
| Unclassified Clostridiales b | 21.01 ± 5.04 | 15.23 ± 5.38 | 11.11 ± 2.06 | 7.57 ± 1.81 | 11.65 ± 3.33 | 5.41 ± 1.52 |
| Mucispirillum a,b | 20.03 ± 7.53 | 2.68 ± 0.95 | 3.27 ± 1.63 | 1.06 ± 0.80 | 3.33 ± 1.46 | 0.00 ± 0.00 |
| Akkermansia c | 8.49 ± 2.81 | 11.43 ± 4.04 | 21.75 ± 3.50 | 15.76 ± 2.69 | 16.15 ± 3.03 | 16.26 ± 4.96 |
| Pseudomonas | 3.57 ± 1.11 | 14.76 ± 5.22 | 5.29 ± 2.24 | 3.41 ± 1.49 | 14.23 ± 10.63 | 2.82 ± 1.46 |
| Unclassified S24-7 | 7.09 ± 2.37 | 5.53 ± 1.96 | 7.06 ± 2.31 | 5.59 ± 1.40 | 4.25 ± 2.50 | 13.45 ± 3.80 |
| Unclassified Ruminococcaceae b | 7.28 ± 1.87 | 5.31 ± 1.88 | 2.77 ± 0.44 | 2.04 ± 0.48 | 2.22 ± 0.77 | 4.01 ± 2.23 |
| Oscillospira | 5.60 ± 1.21 | 4.60 ± 1.62 | 7.83 ± 1.77 | 4.12 ± 1.30 | 3.31 ± 1.28 | 6.90 ± 2.52 |
| Unclassified Lachnospiraceae | 4.98 ± 1.51 | 4.68 ± 1.65 | 3.11 ± 0.45 | 2.07 ± 0.53 | 5.35 ± 1.73 | 1.54 ± 0.54 |
| Bacteroides d | 5.63 ± 2.65 | 3.83 ± 1.35 | 13.03 ± 1.58 | 21.41 ± 3.97 | 12.60 ± 2.69 | 4.93 ± 2.19 |
| Unclassified Rikenellaceae a | 3.28 ± 0.96 | 4.46 ± 1.58 | 3.93 ± 0.92 | 6.06 ± 1.18 | 2.40 ± 1.03 | 5.00 ± 1.29 |
| Parabacteroides | 2.57 ±0.66 | 2.92 ± 0.79 | 7.29 ± 3.10 | 5.83 ±1.91 | 8.89 ± 3.03 | 1.83 ± 1.24 |
| Bilophila | 2.43 ± 1.17 | 1.80 ± 0.81 | 1.03 ± 0.84 | 0.84 ± 0.53 | 0.00 ± 0.00 | 2.08 ± 1.24 |
| Helicobacter a | 0.00 ± 0.00 | 3.48 ± 3.48 | 0.00 ± 0.00 | 13.05 ± 8.87 | 0.00 ±0.00 | 14.51 ± 9.84 |
| Ruminococcus | 2.15 ± 0.85 | 1.08 ± 0.41 | 0.67 ± 0.11 | 0.39 ±0.08 | 1.21 ± 0.43 | 0.84 ± 0.31 |
| Unclassified Comamonadaceae | 0.25 ± 0.11 | 2.63 ± 1.81 | 0.23 ± 0.13 | 0.66 ± 0.52 | 0.51 ± 0.43 | 0.10 ± 0.04 |
| Unclassified Oxalobacteraceae | 0.36 ± 0.14 | 1.97 ± 0.86 | 0.48 ±0.27 | 0.34 ± 0.19 | 1.13 ± 0.69 | 0.47 ± 0.31 |
| Lachnospiraceae; Ruminococcus e | 0.91 ± 0.24 | 0.87 ± 0.37 | 1.81 ± 0.27 | 0.82 ±0.28 | 0.64 ± 0.37 | 0.28 ± 0.09 |
| Bifidobacterium | 0.50 ± 0.19 | 1.01 ± 0.54 | 2.76 ±0.80 | 1.22 ± 0.38 | 2.80 ±0.79 | 2.20 ± 1.22 |
| Unclassified Bacteroidales a | 0.05 ± 0.04 | 1.44 ± 0.75 | 0.00 ± 0.00 | 2.30 ± 0.78 ** | 0.00 ± 0.00 | 2.45 ± 1.05 ** |
| Staphylococcus | 0.07 ± 0.05 | 1.14 ± 0.77 | 0.25 ± 0.11 | 0.12 ± 0.10 | 0.06 ± 0.04 | 0.03 ± 0.02 |
| Acinetobacter | 0.08 ± 0.04 | 0.65 ± 0.33 | 0.21 ± 0.10 | 0.09 ± 0.05 | 0.09 ± 0.04 | 12.23 ± 12.21 |
| Lactobacillus | 0.31 ± 0.10 | 0.38 ± 0.17 | 0.35 ± 0.12 | 0.32 ± 0.07 | 1.84 ± 0.65 | 0.05 ± 0.03 ** |
| Micrococcus | 0.00 ± 0.00 | 0.64 ± 0.62 | 0.02 ± 0.02 | 0.00 ±0.00 | 0.00 ± 0.00 | 0.07 ± 0.07 |
| Coprococcus d | 0.32 ± 0.10 | 0.28 ± 0.14 | 0.78 ± 0.26 | 0.65 ± 0.21 | 0.33 ± 0.14 | 0.10 ± 0.04 |
| Unclassified Clostridiaceae b | 0.30 ± 0.16 | 0.27 ± 0.21 | 0.04 ± 0.04 | 0.00 ± 0.00 | 0.01 ± 0.01 | 0.00 ± 0.00 |
Data are the relative abundance of each bacterial taxa (calculated as the number of sequences for each taxa/total number of sequences × 100). The mean relative abundance ± standard error are shown. A two factor analysis of variance using diet and stress as the two factors, with LSD post-hoc tests, were used for significance testing. The Benjamini-Hochberg correction for multiple comparisons was used with a q-value of 0.100.
p < 0.05 vs. HCC Control fed same diet;
p < 0.05 Main Effect for Stress with ANOVA;
p < 0.05 Main Effect for Control Chow significantly different from 3′SL and 6′SL;
p < 0.05 Main Effect for 3′SL significantly different from Control Chow only;
p < 0.05 Main Effect for 3′SL significant different from Control Chow and 6′SL;
p < 0.05 Main Effect for 3′SL significantly different from 6′SL only.
All differences passed correction for multiple comparisons.
3.2. Dietary sialyllactose abolishes SDR-induced shifts to colonic microbiota community structure
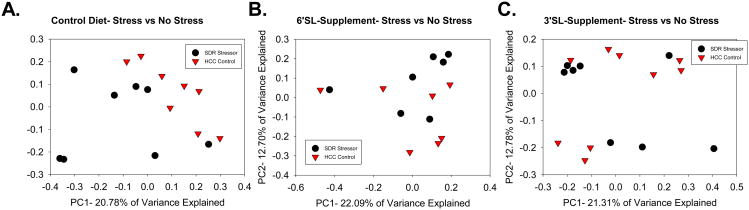
SDI used as the primary measurement of alpha diversity, was unchanged regardless of diet in SDR-exposed mice. However, beta diversity, which is community diversity compared across samples, was also assessed using unweighted UniFrac distance matrix. The Control Diet mice that underwent SDR had significant changes in community structure composition of the colonic microbiota, as measured with the adonis statistic (p<0.05, R2= 0.10247). This disruption in the colonic microbiota was further confirmed in a principal coordinate plot of unweighted UniFrac distances, which illustrated unique clustering of SDR-exposed mice separate from HCC mice (Fig. 4A). SDR exposure did not induce shifts in the composition of the colonic microbiota in mice fed chow fortified with 3′SL (p=0.142, R2=0.07677) or 6′SL (p=0.138, R2= 0.09321) prebiotics. Separate clustering of SDR-exposed mice from HCC-control mice was not seen in PCoAs of unweighted UniFrac distances of the prebiotic diets (Fig. 4B-C).
Figure 4. Dietary sialyllactose abolishes stressor-induced alterations in the beta diversity of the murine colonic microbiota community structure.
Principal coordinate plots demonstrate that exposure to the SDR stressor results in significantly different microbial community structure in mice fed a control diet (A; p<.05). However, exposure to the SDR stressor did not significantly alter microbial community structure in mice fed 6′SL (B), or 3′SL (C). Significance testing was calculated using adonis, implemented in QIIME. Control diet: n=8 HCC Control, n=8 SDR Stressor. 6′SL: n= 7 HCC Control, n=7 SDR Stressor. 3′SL: n= 9 HCC Control, n=8 SDR Stressor.
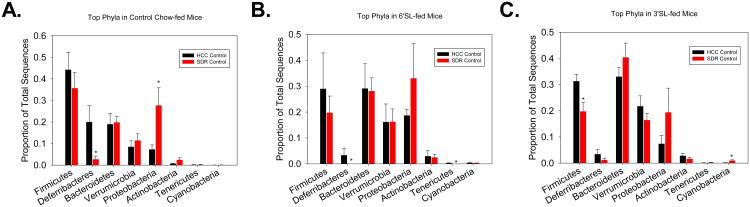
The abrogation of SDR-induced alterations in murine colonic beta-diversity by prebiotic intervention lent the impetus to analyze specific taxonomic shifts. At the phylum level, SDR-exposed Control-fed mice had a significant increase in the relative abundance of Proteobacteria (27.69% vs. 7.28%) and a significant reduction in Deferribacteres (2.68% vs. 20.02%). This effect was not evident in mice fed 3′SL or 6′SL. However, SDR-exposed mice that received 3′SL had a significant reduction in Firmicutes (19.42% vs. 30.32%) and a significant increase in Cyanobacteria (1.02% vs. 0.16%), while mice that received 6′SL had a decrease in Deferribacteres (0.00% vs. 3.34%) and an increase in Tenericutes (3.12% vs. 0.00%) in SDR-exposed mice (Fig. 5).
Figure 5. SDR exposure in control diet-fed mice alters the abundances of the major phyla.
Relative abundances of the top phyla were assessed in (A) control diet, (B) 6′SL, and (C) 3′SL fed mice. Bars represent means ± SEM. * indicates a significant difference between non-stressed and stressed animals within each bacterial phyla (p<0.05). Control diet: n=8 HCC Control, n=8 SDR Stressor. 6′SL: n= 7 HCC Control, n=7 SDR Stressor. 3′SL: n= 9 HCC Control, n=8 SDR Stressor.
At the genus level, exposure to the stressor significantly reduced the relative abundance of bacteria in the genus Mucispirillum (p<0.05), but increased the relative abundance of bacteria in the genus Helicobacter (p<0.05) and an unclassified genus in the family Rikenellaceae (p<0.05). There was also a significant increase in an unclassified genus of bacteria within the Bacteroidales order, but this was only evident in mice fed 3′SL or 6′SL.
3.3. Dietary sialyllactose ameliorated SDR-induced anxiety-like behavior in the light/dark preference and open field tests
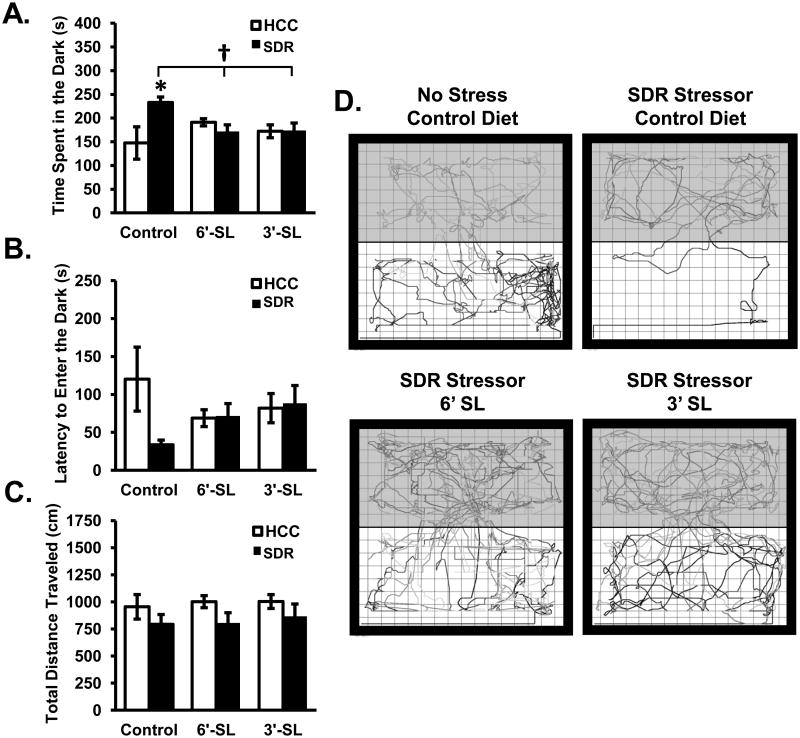
When tested on the light/dark preference test, data revealed a significant Diet × Stress interaction for the duration spent in the dark zone (F(2,42)=5.41; p<0.01; Fig. 6A). Post hoc analyses revealed that stressor-exposed mice fed the control diet had a significantly increased amount of time spent in the dark zone compared to non-stressed mice fed the control diet (p<0.05). Furthermore, stressor-exposed mice fed the control diet had a significantly increased time spent in the dark zone compared to stressor-exposed mice fed either 3′SL or 6′SL (p<0.05). Post hoc analyses showed that non-stressed control mice that received the control diet did not differ from either non-stressed or stressor-exposed mice fed 3′SL or 6′SL. The latency to enter the dark zone was also assessed. Data revealed no significant main effects of Diet or Stress; however, there was a trend for a Diet × Stress interaction (F(2,42)=2.93; p=0.06; Fig. 6B). There was also no main effect of Diet, Stress, or Diet × Stress interactions on the number of light to dark transitions (data not shown). Lastly, the total distance travelled in the light/dark preference test was assessed. Data indicated that there was no significant main effect of Diet on the total distance travelled, but a significant main effect of Stress was apparent where stressor-exposed mice spent significantly less time moving in comparison to non-stressed control mice regardless of diet (F(2,42)=5.11; p<0.05; Fig. 6C).
Figure 6. Dietary sialyllactose ameliorated SDR-induced anxiety-like behavior in the light/dark preference task.
Mice were tested in the light/dark preference test for: (A) time spent in the dark, (B) latency to enter the dark, and (C) total distance travelled. (D) Representative movement tracks made non-stressed control diet and stressed mice that received all each of the diets. Bars represent means ± SEM. * indicates a significant difference between groups stressed and non-stressed animals receiving the control diet (p<0.05). † indicates a significant difference between stressed animals fed the control diet and stressed animals fed 6′SL and 3′SL diets (p<0.05). Control diet: n=8 HCC Control, n=8 SDR Stressor. 3′SL: n=9 HCC Control, n=8 SDR Stressor. 6′SL: n=9 HCC Control, n=8 SDR Stressor.
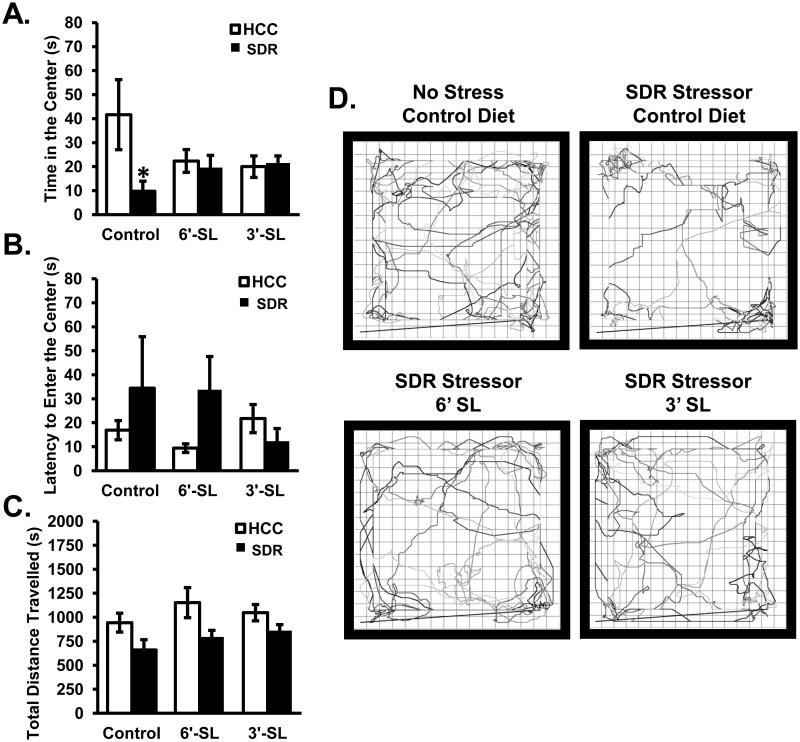
As an additional test of anxiety-like behavior an open field test was employed. Data revealed a significant Diet × Stress interaction for the time spent in the center of the maze (F(2,43)=3.60; p<0.05; Fig. 7A). Post hoc analyses indicated that non-stressed control mice spent significantly more time in the center of the open field when compared to stressor-exposed mice when all mice were fed the control diet (p<0.05). The stressor, however, did not affect the amount of time mice spent in the center of the open field if the mice were fed either the 3′SL or the 6′SL diets (Fig. 7A). There was not a statistically significant effect of stressor exposure on the latency of the mice to enter the center of the open field (Fig. 7B), but there was a tendency for 3′SL and 6′SL to reduce the latency to enter the center of the open filed (p=0.1, not considered statistically significant). The total distance travelled in the open field was also significantly reduced by stressor exposure (F(1,43)=10.35; p<0.005; Fig. 7C) indicating that mice exposed to the stressor spent significantly less time exploring the open field compared to non-stressed control mice regardless of whether their diet contained 3′SL or 6′SL.
Figure 7. Dietary sialyllactose ameliorated SDR-induced anxiety-like behavior in the open field.
Open field behavior was assessed in subjects for: (A) time spent in the center, (B) latency to enter the center, and (C) total distance travelled. (D) Representative movement tracks made non-stressed control diet and stressed mice that received all each of the diets. Bars represent means ± SEM. * indicates a significant difference between stressed animals fed the control diet and all other groups (p<0.05). Control diet: n=8 HCC Control, n=7 SDR Stressor. 3′SL: n=9 HCC Control, n=7 SDR Stressor. 6′SL: n=9 HCC Control, n=9 SDR Stressor.
3.4. Dietary 6′SL and SDR decreased overall brain cell proliferation, but sialyllactose rescued SDR-induced reduction in immature neurons
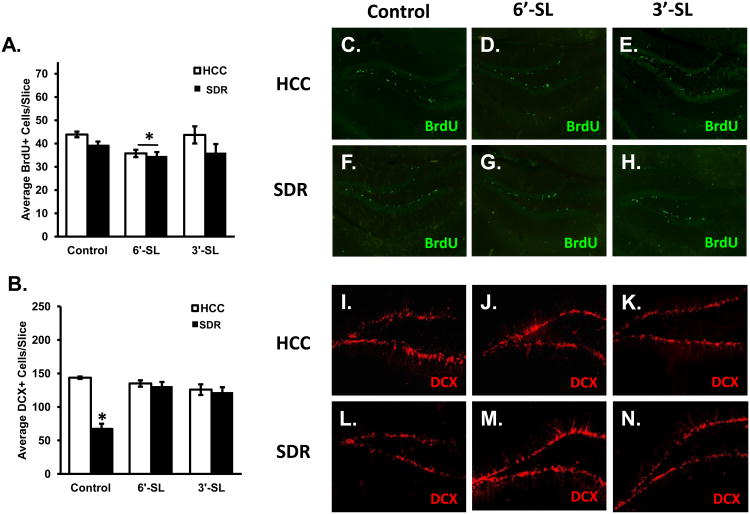
Diet significantly impacted the number of proliferating cells in the hippocampus. Data revealed a significant main effect of Diet (F(2,30)=3.45; p<0.05; Fig. 8A) where post hoc analyses indicated animals fed the 6′-SL diet had a decrease in the average number of BrdU positive cells per slice compared to animals fed the control diet regardless of stress condition (p's<0.05). In addition, data revealed a significant main effect of Stress (F(1,30)=4.95; p<0.05; Fig. 8A) showing stressed animals had an overall reduction in in the average number of BrdU positive cells in the dentate gyrus of the hippocampus. There were no significant interactions between Diet and Stress on the average number of BrdU positive cells per slice.
Figure 8. Dietary sialyllactose and SDR decreased overall brain cell proliferation, but sialyllactose rescued the SDR-induced reduction in immature neurons.
Assessment for the average number of (A) BrdU positive cells per slice in the hippocampus. * indicates a significant main effect for diet (collapsed across control and stress conditions) with mice fed 6′-SL having fewer BrdU positive cells compared to mice fed control or 3′SL diets. (B) DCX positive cells per slice in the hippocampus. * indicates a significant difference between stressed animals and all other groups (p<.05). Images of BrdU (C-H) and DCX (I-N) positive cells were taken at 10× and 20× respectively. Control diet: n=8 HCC Control, n=7 SDR Stressor. 3′SL: n=9 HCC Control, n=7 SDR Stressor. 6′SL: n=9 HCC Control, n=9 SDR Stressor.
Analyses of DCX positive cells revealed a significant Diet × Stress interaction (F(2,12)=21.89; p<0.0001; Fig. 8B). Post hoc analyses indicated that stressed animals fed the control diet had a significant reduction in the average number of DCX positive cells compared to all other groups (p's<0.05). No other groups differed.
3.5. Dietary sialyllactose did not alter body mass or SDR-induced increases in serum corticosterone, IL-6, or spleen mass
The experimental diet did not significantly affect body weight or physiological indicators of the stress response. Data revealed no significant main effects of Stress or Diet on body weight. However, there was a significant main effect of Day on body weight indicating that regardless of Diet or Stress animals gained weight when comparing weights at baseline and the day of tissue harvesting (F(1,41)=272.95; p<0.0001; Table 2). There were no significant interactions between independent variables.
Table 2. Tarr et al., Prebiotics and anxiety.
| Biological Readout | Control Chow | 6′-SL | 3′-SL | |||
|---|---|---|---|---|---|---|
|
| ||||||
| HCC Control | SDR Stressor | HCC Control | SDR Stressor | HCC Control | SDR Stressor | |
| Body Weight (g) | ||||||
| Day 1 (Baseline) | 22.13 ± 0.36 | 20.70 ± 0.0.59 | 21.00 ± 0.67 | 21.84 ± 0.33 | 21.90 ± 0.53 | 21.90 ± 0.68 |
| Day 24 (Sac Day) | 25.77 ± 0.68 | 23.80 ± 1.13 * | 25.60 ± 0.61 | 26.28 ± 0.51 * | 25.57 ± 0.99 | 25.68 ± 1.00 * |
| Spleen Mass (mg) | 77.20 ± 5.70 | 150.30 ± 13.80 | 72.00 ± 6.10 | 120.50 ± 15.70 | 70.00 ± 2.60 | 100.00 ± 17.70 |
| Serum Interleukin-6 (pg/ml) | 2.40 ± 1.51 | 162.00 ± 106.00 | 2.96 ± 1.14 | 220.00 ± 157.00 | 2.51 ± 1.35 | 88.70 ± 37.00 |
| Corticosterone (ng/ml) | 118.90 ± 36.36 | 250.00 ± 38.00 | 81.24 ± 81.24 | 377.17 ± 92.30 | 72.58 ± 8.98 | 229.9 ± 31.70 |
Da ta are represented as mean ± standard error.
Main effect of day (p < 0.05) indicating significant increases in body weight over the 24 day period. Neither diet nor stressor exposure impacted body weight.
Stressor exposure also increased spleen mass (F(1,41)=52.27; p<0.001; Table 2), serum levels of IL-6 (F(1,41)=22.29; p<0.01; Table 2), and serum corticosterone levels (F(1,41)=115.79; p<0.001; Table 2). These were main effects of stressor exposure, which were not dependent upon whether the mice were fed control chow or chow supplemented with 6′-SL or 3′-SL. Moreover, neither 6′-SL or 3′-SL significantly affected spleen mass, IL-6, or corticosterone levels.
4. Discussion
Both stressor exposure and HMO have been shown to impact the structure of mucosa-associated microbial communities (Chichlowski et al., 2011, Galley et al., 2014a, Sanders et al., 2014). However, the impact of HMO on stressor-induced alterations in the microbiota has not been studied. Here we report that prebiotics (i.e., 6′SL and 3′SL) were able to alter the microbiota community structure compared to mice fed standard laboratory control diet. Additionally, we report that microbial community structure in mice fed the 6′SL and 3′SL was similar, indicating that these HMO had similar effects on microbial community structure. Of importance to the overall scope of this report, stressor exposure did not impact microbial community structure in mice fed 6′SL or 3′SL. The PCoA analysis indicated that the community structure was altered in stressor-exposed mice fed the control diet. The differential clustering of stressor-exposed mice was abolished when animals were fed either the 3′SL or the 6′SL diet, showing that the HMO helped to maintain normal mucosa-associated microbial community structure in stressor-exposed mice. Thus, it is evident that in the absence of stress, 3′SL and 6′SL impact microbial community structure, with both having the capacity to support microbial community structure in the colon if fed prior to exposure to the stressor.
In addition to maintaining normal microbial community structure, 3′SL and 6′SL were able to support normal behavior in stressor-exposed mice. Mice fed the control diet during stressor exposure displayed anxiety-like behavior in both the open field task and the light/dark preference task, an effect that was absent in mice fed 3′SL or 6′SL. It must be acknowledged, however, that the short duration of testing on the open field also reflects the animals' responses to novel environments. Although the mechanisms by which 3′SL and 6′SL support behavior are not yet known, it is possible that effects on the microbiota are involved. There is increasing evidence that gut microbes can impact nervous system activity. This was first recognized in germfree mice that were found to have decreased anxiety-like behavior in comparison to mice that had a conventional microbiome (Diaz Heijtz et al., 2011, Neufeld et al., 2011). Colonizing the germfree mice with microbes early in life, however, prevented the anxiety-like behavior, suggesting that gut microbes impact anxiety-like behavior (Diaz Heijtz et al., 2011, Neufeld et al., 2011). More recently, the effects of exogenous probiotic microbes, such as bacteria in the genus Bifidobacterium or Lactobacillus, have been shown to attenuate anxiety in both laboratory animals and human participants (Messaoudi et al., 2011a), thus reinforcing the notion that gut microbes can impact behavior. In the current study, stressor exposed mice were found to have lower relative abundances of Mucispirillum but higher relative abundances of Rikenellaceae, Helicobacter, and Bacteroidales. It is unlikely these microbes are associated with behavior, however, because this reduction was evident in stressor-exposed mice fed 3′SL and 6′SL that did not show anxiety-like behavior. The only bacteria whose relative abundance was significantly different in stressor-exposed mice fed 3′SL or 6′SL compared to control diet was an unclassified genus within the Bacteroidales order. While it is not yet clear whether the increase in this bacterial type helped to normalize behavior, it should be noted that others have found that B. fragilis (which is a member of the Bacteroidales order) can normalize behavior in a mouse model of autism spectrum disorder (Hsiao et al., 2013). Thus, the role of the Bacteroides or other members of the Bacteroidales order on anxiety-like behavior in stressor-exposed mice given prebiotics should be further investigated.
Exposure to the SDR stressor is well known to increase spleen weight, circulating corticosterone, and IL-6, and changes in these markers have been correlated with anxiety-like behavior (Hanke et al., 2012). In addition, these physiological markers have been associated with gut microbes (Sudo et al., 2004, Bailey et al., 2011). Of interest, in a previous study we demonstrated that stressor-induced increases in IL-6 were associated with higher abundances of bacteria in the genus Coprococcus (Bailey et al., 2011). While this association was again present in this study, along with a significant association with the abundance of Mucispirillum (data not shown), 3′SL and 6′SL did not significantly affect the physiological parameters assessed, and stressor exposure still led to increased corticosterone, IL-6 and spleen mass. These data suggest that additional mechanisms, other than glucocorticoid or IL-6 involvement, mediate the effects of 3′-SL and 6′-SL on SDR-induced anxiety-like behavior in the open field and light dark preference tests.
While it is possible that the effects of 3′SL and 6′SL on stressor-induced anxiety-like behavior were due to modification of the microbiota, it is also possible that 3′SL and 6′SL had effects on the brain that were largely independent of colonic microbial community structure. Sialic acid from sialyllactose readily crosses the blood brain barrier and the highest concentrations have been found on gangliosides and glycoproteins in the grey matter of the CNS (Schnaar et al., 2014). These sialylated gangliosides are important in synaptogenesis, neurite and axonal growth, dendritogenesis, and neural transmission (Rahmann et al., 1990, Hwang et al., 1992, Ledeen et al., 1998, Rosner, 1998). Mice lacking sialylated gangliosides have impaired neurogenesis and exhibit depressive-like behaviors (Wang and Yu, 2013, Wang et al., 2014). In contrast, supplementation with SA results in increased learning and memory, as well as sialylated brain proteins (Wang et al., 2007). The effects of generalized stress on adulthood neurogenesis have been well established, showing a reduction in cell proliferation within the dentate gyrus of the hippocampus (Warner-Schmidt and Duman, 2006). There is a strong link between neurogenesis, immature neurons, and depressive- and anxiety-like behaviors, as well as cognitive deficits (Deng et al., 2009, Jessberger et al., 2009, Revest et al., 2009, de Andrade et al., 2013, Vukovic et al., 2013, Campos et al., 2014). In the current study, stressor exposure alone was able to induce a reduction in the number of BrdU positive cells. This effect of stress was not impacted by 3′SL or 6′SL, but 6′SL also led to a small reduction in BrdU staining. The importance of this small reduction in neuronal proliferation is currently not clear. Upon examination of immature neurons immunolabeled with DCX, SDR reduced the number of DCX positive cells by approximately 50%. As hypothesized both the 6′SL and 3′SL were able to prevent this deleterious effect. It is not yet known how the 3′SL and 6′SL prevent the stressor-induced reduction in DCX-positive cells, but it is possible that SA from the bacterial degradation of the 3′SL and 6′SL was involved.
This is supported by current and previous research showing that: 1) sialyllactose via sialylated gangliosides, contribute to normal brain function and plasticity (Rahmann et al., 1990, Hwang et al., 1992, Ledeen et al., 1998, Rosner, 1998), 2) DCX+ immature neurons are important in anxiety-like behaviors (Revest et al., 2009, Sah et al., 2012, Vukovic et al., 2013), and 3) anxiety-like behavior and DCX positivity, but not BrdU+ progenitor cells, was affected by our prebiotic diet. Nevertheless, the possibility that the microbiota impacted immature neurons independent of the release of SA should not be ruled out, since it is known that hippocampal neurogenesis is significantly changed when germfree mice are colonized with commensal microbes (Ogbonnaya et al., 2015). Additional studies are needed to determine how changes in the gut microbiota are related to the number of immature neurons in the brain of stressor-exposed mice.
In summary, the current study is the first to report that the prebiotic sialyllactose is able to diminish stressor-induced alterations in colonic mucosa-associated microbiota community structure, anxiety-like behavior, and immature neuron cell numbers irrespective of immune or endocrine functionality. It is not yet known whether effects on the microbiota directly contribute to protection against stressor-induced anxiety-like behavior, but others have suggested that offspring behavior might be impacted through the effepcts of maternal milk on brain development directly or indirectly through the effects on the gut microbiota (Allen-Blevins et al., 2015). Given the emerging role of the gut-brain axis in health and disease, as well as the impact of oligosaccharides on beneficial microbes, future studies will assess the possibility that the ability of HMO to prevent stressor-induced anxiety-like behavior are mediated by the commensal microbiota.
Highlights.
Exposure to the social disruption stressor resulted in significant changes to the composition of the gut microbiota, anxiety-like behavior, and the number of doublecortin-positive neurons within the hippocampus.
Feeding mice with prebiotic human milk oligosaccharides 3′Sialyllactose (3′SL) or 6′Sialyllactose (6′SL) for 2 weeks prior and during the week of stressor exposure helped maintain normal colonic microbiota community structure and non-anxious behavior.
Feeding mice with prebiotic 3′SL or 6′SL also helped maintain the number of doublecortin positive cells within the hippocampus of stressor-exposed mice.
The data support the contention that dietary interventions aimed at maintaining normal functioning of the gut-brain axis can attenuate stressor-induced anxiety-like behavior.
Acknowledgments
This work was supported in full by the Mead Johnson Nutrition granted to MTB. The authors gratefully acknowledge the technical assistance of Krysten Clark, Jake Falter, Renata Morozov, and Sami Mubarak, and helpful conversations with Dr. Tamar Gur.
Footnotes
Conflict of Interest Statement: Maciej Chichlowski and Brian M. Berg are employees of Mead Johnson Nutrition.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albenberg LG, Wu GD. Diet and the intestinal microbiome: associations, functions, and implications for health and disease. Gastroenterology. 2014;146:1564–1572. doi: 10.1053/j.gastro.2014.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen-Blevins CR, Sela DA, Hinde K. Milk bioactives may manipulate microbes to mediate parent-offspring conflict. Evolution, medicine, and public health. 2015;2015:106–121. doi: 10.1093/emph/eov007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RG, Lafuse WP, Galley JD, Ali MM, Ahmer BM, Bailey MT. The intestinal microbiota are necessary for stressor-induced enhancement of splenic macrophage microbicidal activity. Brain, behavior, and immunity. 2012;26:371–382. doi: 10.1016/j.bbi.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson B, Porras O, Hanson LA, Lagergard T, Svanborg-Eden C. Inhibition of attachment of Streptococcus pneumoniae and Haemophilus influenzae by human milk and receptor oligosaccharides. J Infect Dis. 1986;153:232–237. doi: 10.1093/infdis/153.2.232. [DOI] [PubMed] [Google Scholar]
- Aronesty E. ea-utils pp Command-line tools for processing biological sequencing data 2011 [Google Scholar]
- Asakuma S, Urashima T, Akahori M, Obayashi H, Nakamura T, Kimura K, Watanabe Y, Arai I, Sanai Y. Variation of major neutral oligosaccharides levels in human colostrum. Eur J Clin Nutr. 2007;62:488–494. doi: 10.1038/sj.ejcn.1602738. [DOI] [PubMed] [Google Scholar]
- Bailey MT, Coe CL. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev Psychobiol. 1999;35:146–155. [PubMed] [Google Scholar]
- Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain, behavior, and immunity. 2011;25:397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MT, Lubach GR, Coe CL. Prenatal stress alters bacterial colonization of the gut in infant monkeys. J Pediatr Gastroenterol Nutr. 2004;38:414–421. doi: 10.1097/00005176-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Bangsgaard Bendtsen KM, Krych L, Sorensen DB, Pang W, Nielsen DS, Josefsen K, Hansen LH, Sorensen SJ, Hansen AK. Gut microbiota composition is correlated to grid floor induced stress and behavior in the BALB/c mouse. PloS one. 2012;7:e46231. doi: 10.1371/journal.pone.0046231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D, Berger B, Huizinga JD, Kunze W, McLean PG, Bergonzelli GE, Collins SM, Verdu EF. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2011;23:1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindels LB, Delzenne NM, Cani PD, Walter J. Towards a more comprehensive concept for prebiotics. Nature reviews Gastroenterology & hepatology. 2015;12:303–310. doi: 10.1038/nrgastro.2015.47. [DOI] [PubMed] [Google Scholar]
- Campos AC, Vaz GN, Saito VM, Teixeira AL. Further evidence for the role of interferon-gamma on anxiety- and depressive-like behaviors: involvement of hippocampal neurogenesis and NGF production. Neuroscience letters. 2014;578:100–105. doi: 10.1016/j.neulet.2014.06.039. [DOI] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichlowski M, German JB, Lebrilla CB, Mills DA. The influence of milk oligosaccharides on microbiota of infants: opportunities for formulas. Annual review of food science and technology. 2011;2:331–351. doi: 10.1146/annurev-food-022510-133743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppa GV, Zampini L, Galeazzi T, Facinelli B, Ferrante L, Capretti R, Orazio G. Human Milk Oligosaccharides Inhibit the Adhesion to Caco-2 Cells of Diarrheal Pathogens: Escherichia coli, Vibrio cholerae, and Salmonella fyris. Pediatric Research. 2006;59:377–382. doi: 10.1203/01.pdr.0000200805.45593.17. [DOI] [PubMed] [Google Scholar]
- Daniel H, Moghaddas Gholami A, Berry D, Desmarchelier C, Hahne H, Loh G, Mondot S, Lepage P, Rothballer M, Walker A, Bohm C, Wenning M, Wagner M, Blaut M, Schmitt-Kopplin P, Kuster B, Haller D, Clavel T. High-fat diet alters gut microbiota physiology in mice. ISME J. 2014;8:295–308. doi: 10.1038/ismej.2013.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Andrade JS, Cespedes IC, Abrao RO, Dos Santos TB, Diniz L, Britto LR, Spadari-Bratfisch RC, Ortolani D, Melo-Thomas L, da Silva RC, Viana MB. Chronic unpredictable mild stress alters an anxiety-related defensive response, Fos immunoreactivity and hippocampal adult neurogenesis. Behavioural brain research. 2013;250:81–90. doi: 10.1016/j.bbr.2013.04.031. [DOI] [PubMed] [Google Scholar]
- De Palma G, Collins SM, Bercik P, Verdu EF. The microbiota-gut-brain axis in gastrointestinal disorders: stressed bugs, stressed brain or both? The Journal of physiology. 2014;592:2989–2997. doi: 10.1113/jphysiol.2014.273995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Development RCT. R: a language and environment for statistical computing. Coventry, United Kingdom: R Foundation for Statistical Computing; [Google Scholar]
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biological psychiatry. 2013;74:720–726. doi: 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, Rosenbaum M, Gordon JI. The long-term stability of the human gut microbiota. Science. 2013;341:1237439. doi: 10.1126/science.1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galley JD, Nelson MC, Yu Z, Dowd SE, Walter J, Kumar PS, Lyte M, Bailey MT. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol. 2014a;14:189. doi: 10.1186/1471-2180-14-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galley JD, Yu Z, Kumar P, Dowd SE, Lyte M, Bailey MT. The structures of the colonic mucosa-associated and luminal microbial communities are distinct and differentially affected by a prolonged murine stressor. Gut microbes. 2014b;5:748–760. doi: 10.4161/19490976.2014.972241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GR, Scott KP, Rastall RA, Tuohy KM, Hotchkiss A, Dubert-Ferrandon A, Gareau M, Murphy EF, Saulnier D, Loh G, Macfarlane S, Delzenne N, Ringel Y, Kozianowski G, Dickmann R, Lenoir-Wijnkoop I, Walker C, Buddington R. Dietary prebiotics: current status and new definition. Food Science and Technology Bulletin: Functional Foods. 2010;7:19. [Google Scholar]
- Gourbeyre P, Denery S, Bodinier M. Probiotics, prebiotics, and synbiotics: impact on the gut immune system and allergic reactions. J Leukoc Biol. 2011;89:685–695. doi: 10.1189/jlb.1109753. [DOI] [PubMed] [Google Scholar]
- Hanke ML, Powell ND, Stiner LM, Bailey MT, Sheridan JF. Beta adrenergic blockade decreases the immunomodulatory effects of social disruption stress. Brain, behavior, and immunity. 2012;26:1150–1159. doi: 10.1016/j.bbi.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, Knight R, Ahima RS, Bushman F, Wu GD. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137:1716–1724. e1711–1712. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature reviews Gastroenterology & hepatology. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang HM, Wang JT, Chiu TH. Effects of exogenous GM1 ganglioside on LTP in rat hippocampal slices perfused with different concentrations of calcium. Neuroscience letters. 1992;141:227–230. doi: 10.1016/0304-3940(92)90900-r. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learning & memory. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Bailey MT, Sheridan JF, Padgett DA, Avitsur R. Repeated social defeat causes increased anxiety-like behavior and alters splenocyte function in C57BL/6 and CD-1 mice. Brain, behavior, and immunity. 2007;21:458–466. doi: 10.1016/j.bbi.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles SR, Nelson EA, Palombo EA. Investigating the role of perceived stress on bacterial flora activity and salivary cortisol secretion: a possible mechanism underlying susceptibility to illness. Biological psychology. 2008;77:132–137. doi: 10.1016/j.biopsycho.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Ledeen RW, Wu G, Lu ZH, Kozireski-Chuback D, Fang Y. The role of GM1 and other gangliosides in neuronal differentiation. Overview and new finding. Annals of the New York Academy of Sciences. 1998;845:161–175. doi: 10.1111/j.1749-6632.1998.tb09669.x. [DOI] [PubMed] [Google Scholar]
- Lee W, Moon M, Kim HG, Lee TH, Oh MS. Heat stress-induced memory impairment is associated with neuroinflammation in mice. Journal of neuroinflammation. 2015;12:102. doi: 10.1186/s12974-015-0324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Sosa S, Martin MJ, Garcia-Pardo LA, Hueso P. Sialyloligosaccharides in human and bovine milk and in infant formulas: variations with the progression of lactation. Journal of dairy science. 2003;86:52–59. doi: 10.3168/jds.S0022-0302(03)73583-8. [DOI] [PubMed] [Google Scholar]
- McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012;6:610–618. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Magarinos AM. Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Human psychopharmacology. 2001;16:S7–S19. doi: 10.1002/hup.266. [DOI] [PubMed] [Google Scholar]
- Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, Bisson JF, Rougeot C, Pichelin M, Cazaubiel M, Cazaubiel JM. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. The British journal of nutrition. 2011a;105:755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- Messaoudi M, Violle N, Bisson JF, Desor D, Javelot H, Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut microbes. 2011b;2:256–261. doi: 10.4161/gmic.2.4.16108. [DOI] [PubMed] [Google Scholar]
- Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2011;23:255–264, e119. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Ben RA, Pons S, dP MI, Bustos Fernández L. Volatile fatty acids, lactic acid, and pH in the stools of breast-fed and bottle-fed infants. J Pediatr Gastroenterol Nutr. 1992;15:248–252. doi: 10.1097/00005176-199210000-00004. [DOI] [PubMed] [Google Scholar]
- Ogbonnaya ES, Clarke G, Shanahan F, Dinan TG, Cryan JF, O'Leary OF. Adult Hippocampal Neurogenesis Is Regulated by the Microbiome. Biological psychiatry. 2015 doi: 10.1016/j.biopsych.2014.12.023. [DOI] [PubMed] [Google Scholar]
- Oksanen JBF, Kindt R, Legendre R, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H. Vegan: community ecology package pp R package version 2.0-3 [Google Scholar]
- Rahmann H, Kortje KH, Seybold V, Rosner H. Ultrastructural localization of gangliosides, calcium and a high-affinity Ca(2+)-ATPase in nerve terminals: a contribution to the possible functional role of gangliosides. Indian J Biochem Biophys. 1990;27:420–424. [PubMed] [Google Scholar]
- Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV, Abrous DN. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Molecular psychiatry. 2009;14:959–967. doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- Rosner H. Significance of gangliosides in neuronal differentiation of neuroblastoma cells and neurite growth in tissue culture. Annals of the New York Academy of Sciences. 1998;845:200–214. doi: 10.1111/j.1749-6632.1998.tb09672.x. [DOI] [PubMed] [Google Scholar]
- Sah A, Schmuckermair C, Sartori SB, Gaburro S, Kandasamy M, Irschick R, Klimaschewski L, Landgraf R, Aigner L, Singewald N. Anxiety- rather than depression-like behavior is associated with adult neurogenesis in a female mouse model of higher trait anxiety-and comorbid depression-like behavior. Translational psychiatry. 2012;2:e171. doi: 10.1038/tp.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai F, Ikeuchi F, Urashima T. Effects of feeding sialyllactose and galactosylated N-acetylneuraminic acid on swimming learning ability and brain lipid composition in adult rats. J Appl Glycosci. 2006;53:249–254. [Google Scholar]
- Sanders ME, Lenoir-Wijnkoop I, Salminen S, Merenstein DJ, Gibson GR, Petschow BW, Nieuwdorp M, Tancredi DJ, Cifelli CJ, Jacques P, Pot B. Probiotics and prebiotics: prospects for public health and nutritional recommendations. Annals of the New York Academy of Sciences. 2014;1309:19–29. doi: 10.1111/nyas.12377. [DOI] [PubMed] [Google Scholar]
- Savignac HM, Kiely B, Dinan TG, Cryan JF. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2014;26:1615–1627. doi: 10.1111/nmo.12427. [DOI] [PubMed] [Google Scholar]
- Schnaar RL, Gerardy-Schahn R, Hildebrandt H. Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol Rev. 2014;94:461–518. doi: 10.1152/physrev.00033.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon PM, Goode PL, Mobasseri A, Zopf D. Inhibition of Helicobacter pylori binding to gastrointestinal epithelial cells by sialic acid-containing oligosaccharides. Infection and immunity. 1997;65:750–757. doi: 10.1128/iai.65.2.750-757.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. The Journal of physiology. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock GW, Savage DC. Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infection and immunity. 1974;9:591–598. doi: 10.1128/iai.9.3.591-598.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr AJ, Powell ND, Reader BF, Bhave NS, Roloson AL, Carson WE, 3rd, Sheridan JF. beta-Adrenergic receptor mediated increases in activation and function of natural killer cells following repeated social disruption. Brain, behavior, and immunity. 2012;26:1226–1238. doi: 10.1016/j.bbi.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell host & microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenplas Y, Zakharova I, Dmitrieva Y. Oligosaccharides in infant formula: more evidence to validate the role of prebiotics. The British journal of nutrition. 2015;113:1339–1344. doi: 10.1017/S0007114515000823. [DOI] [PubMed] [Google Scholar]
- Vazquez-Baeza Y, Pirrung M, Gonzalez A, Knight R. EMPeror: a tool for visualizing high-throughput microbial community data. Gigascience. 2013;2:16. doi: 10.1186/2047-217X-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukovic J, Borlikova GG, Ruitenberg MJ, Robinson GJ, Sullivan RK, Walker TL, Bartlett PF. Immature doublecortin-positive hippocampal neurons are important for learning but not for remembering. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:6603–6613. doi: 10.1523/JNEUROSCI.3064-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Brand-Miller J. The role and potential of sialic acid in human nutrition. Eur J Clin Nutr. 2003;57:1351–1369. doi: 10.1038/sj.ejcn.1601704. [DOI] [PubMed] [Google Scholar]
- Wang B, McVeagh P, Petocz P. Brain ganglioside and glycoprotein sialic acid in breastfed compared with formula-fed infants. Am J Clin Nutr. 2003;78:1024–1029. doi: 10.1093/ajcn/78.5.1024. [DOI] [PubMed] [Google Scholar]
- Wang B, Yu B, Karim M, Hu H, Sun Y, McGreevy P, Petocz P, Held S, Brand-Miller J. Dietary sialic acid supplementation improves learning and memory in piglets. Am J Clin Nutr. 2007;85:561–569. doi: 10.1093/ajcn/85.2.561. [DOI] [PubMed] [Google Scholar]
- Wang J, Cheng A, Wakade C, Yu RK. Ganglioside GD3 is required for neurogenesis and long-term maintenance of neural stem cells in the postnatal mouse brain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:13790–13800. doi: 10.1523/JNEUROSCI.2275-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Yu RK. Interaction of ganglioside GD3 with an EGF receptor sustains the self-renewal ability of mouse neural stem cells in vitro. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:19137–19142. doi: 10.1073/pnas.1307224110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, McKim DB, Shea DT, Powell ND, Tarr AJ, Sheridan JF, Godbout JP. Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biological psychiatry. 2014a;75:970–981. doi: 10.1016/j.biopsych.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Patterson JM, Sharma V, Quan N, Godbout JP, Sheridan JF. Knockdown of interleukin-1 receptor type-1 on endothelial cells attenuated stress-induced neuroinflammation and prevented anxiety-like behavior. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014b;34:2583–2591. doi: 10.1523/JNEUROSCI.3723-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:13820–13833. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu ZT, Chen C, Newburg DS. Utilization of major fucosylated and sialylated human milk oligosaccharides by isolated human gut microbes. Glycobiology. 2013;23:1281–1292. doi: 10.1093/glycob/cwt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Foster JA. Psychobiotics and the gut-brain axis: in the pursuit of happiness. Neuropsychiatric disease and treatment. 2015;11:715–723. doi: 10.2147/NDT.S61997. [DOI] [PMC free article] [PubMed] [Google Scholar]