Abstract
The role of glia in the development and treatment of behavioral abnormalities is understudied. Recent reports have observed glial activation in several disorders, including depression, autism spectrum disorders and self-injurious behaviors (SIB). In the current study, we examined SIB in the physiologically and anatomically relevant nonhuman primate (NHP) model. At the Tulane National Primate Research Center (TNPRC), approximately 5% of singly housed macaques develop symptoms of SIB. We have previously demonstrated that naltrexone hydrochloride can be effective in reducing SIB. We have also demonstrated that the astrocytes of animals with SIB are distinctly atrophic and display heightened innate immune activation compared with control animals. We have added a third group of animals (five macaques identified with SIB and treated with oral naltrexone at a dose of 3.2 mg/kg) to the previous cohort (six macaques with a history of SIB but not treated, and nine animals with no history of SIB) for this study. Gray and white matter astrocytes from frontal cortical tissue were examined following necropsy. Innate immune activation of astrocytes, which was increased in SIB animals, was markedly decreased in animals receiving naltrexone, as was atrophy of both grey and white matter astrocytes. This was concomitant with improved behavioral correlates. Preventing astrocyte activation in select areas of the brain to reduce injurious behavior is an innovative concept with implications for mental health studies. Differences in multiple areas of primate brain would help determine how self-injurious behavior develops. These studies suggest a stronger role for astrocytes in the cellular events associated with self-injurious behaviors.
Keywords: behavior, self-injury, glia, plasticity, astrocyte, naltrexone
Introduction
1.1
Self-injurious behavior (SIB) is recognized as a significant public health problem in the US (Jacobson and Gould, 2007). SIB is frequently associated with intellectual disabilities, genetic disease, anxiety and depressive disorders (American Psychiatric Association., 2000; Norton et al., 2008). The rhesus monkey model of SIB is informative about some types of human SIB, not only because it is a primate model but also because it arises spontaneously. We have recently initiated studies with a nonhuman primate model for self-harm: self-injurious behavior (SIB) in rhesus macaques (Lee et al., 2013b). In rhesus macaques, SIB usually takes the form of self-directed biting, hair pulling, and head banging (Ribka and Baker, 2004). These animals respond well to treatments with naltrexone and fluoxetine, further indicating the usefulness of this model (Kempf et al., 2012; Ribka and Baker, 2004).
1.2
It is known that stress can affect the plasticity of glial cells, including astrocytes (Czeh et al., 2006; Lee et al., 2013b). There are two distinct mechanisms whereby astrocytes can be activated in psychiatric disorders. First, gap junction proteins are down-regulated (Sun et al., 2012) restricting the overall syncytia of astrocytes. Reductions in the number of connecting proteins also alter the morphology of the astrocytes including the number and nature of synapses they can form with neurons and the blood-brain barrier (Czeh et al., 2006). Alternatively, dysregulation in the immune function of glial cells have been implicated in the pathogenesis of psychiatric disorders (Leonard, 2010). Our studies showed that both systems are involved (Lee et al., 2013b), unifying these areas of research.
1.3
In humans, SIB comprises of maladaptive behaviors that include skin cutting, scratching, hair-pulling, and injury to body parts and can occur alongside depression and other psychiatric disorders (Kerr et al., 2010; Langbehn and Pfohl, 1993; Nagamitsu, 1993). Critically, rhesus macaques also develop SIB, and at the Tulane National Primate Research Center (TNPRC), approximately 5% of singly-housed animals historically have exhibited this potentially debilitating syndrome (Novak, 2003). In rhesus monkeys, this pathology results in self-biting that, on occasion, can result in tissue damage and mutilation (Ribka and Baker, 2004). Although the neurobiological mechanisms of SIB are poorly characterized, we have recently demonstrated that astrocytes in the frontal cortex of macaques with SIB show significant differences in arbor, cell body size and number of processes, as well as increased expression of Toll-like receptor 2 (TLR2), when compared with control animals (Lee et al., 2013b).
1.4
There is significant evidence that highlights glial dysfunction in the pathology of mood and psychiatric disorders. Post-mortem histopathological studies have observed reduced glial cell numbers in various frontolimbic areas of depressed patients (Hercher et al., 2009). Changes in cortical astrocyte morphology have been reported in suicidal patients (Torres-Platas et al., 2011). Recently, studies examining the effects of chronic stress on astrocyte morphology and expression of astrocyte markers, GFAP and S100β, observed atrophy in astrocyte process length, branching, and volume (Tynan et al., 2013). Along with our previous studies, these results suggest that significant remodeling in the astrocyte network occurs in the setting of mental disorders. Because of morphological findings showing less GFAP in astrocytes between patients with depression (Fatemi et al., 2004; Torres-Platas et al., 2015) and monkeys with SIB, we postulate that there was a potential pathological and behavioral phenotype associated with these brain changes that may shed light on cellular mechanisms of psychiatric disorders. The mechanisms by which astrocytes are activated in self-injury and how these disrupts trophic and immune responses to neurons and endothelial cells, however, remain to be clarified in more detail.
1.5
We have recently shown that SIB macaques treated with two intramuscular injections of 20 mg/kg extended-release naltrexone (separated by four weeks) had no reversion to SIB phenotype during a seven month follow up period (Kempf et al., 2012). Rhesus macaques with SIB also exhibit persistent dysfunction in opioid and stress response systems (Tiefenbacher et al., 2005). We were therefore naturally interested in the underlying impact of this opioid inhibitor on astrocyte activation and morphology.
1.6
One hypothesis of SIB implicates the opioid system in the pathophysiology of symptoms (Roy et al., 2014; Stanley et al., 2010). In earlier studies at Tulane National Primate Research Center, we found that injectable naltrexone treatment produced a lasting decrease in SIB that persisted after the end of the treatment period. As naltrexone is effective for addressing SIB in macaques, we examined archival tissues from animals diagnosed with SIB that received oral naltrexone. We were interested in examining effects of naltrexone therapy on glial cell activation. Here we demonstrate that astrocytes of SIB macaques treated with oral naltrexone have a phenotype more similar to control macaques than those displaying the abnormal behavior. We further postulate that activation of astrocytes and concomitant retraction of processes could be a key component in the development of abnormal behaviors.
Materials and Methods
2.1 Ethics statement, Animal housing and selection of tissues
Animals were maintained in Animal Biosafety Level 2 housing with a 12:12-hour light:dark cycle, relative humidity 30% to 70%, and a temperature of 17.8 to 28.9°C. Water was available ad libitum, and a standard commercially formulated nonhuman primate diet (Lab Fiber Plus Monkey DT, 5K63, PMI Nutrition International, St. Louis, MO) was provided twice daily and supplemented daily with fresh fruit and/or forage material as part of the environmental enrichment program. All animals at Tulane National Primate Research Center (TNPRC) received environmental enrichment, widely used to improve welfare in captive macaques. Over the course of their life times, all subjects experienced some pair or group housing as well as periods of single housing. Each cage (Allentown, Inc., Allentown, NJ) measured 36 inches (91.4 centimeters) in height with 4.3–8.6 square feet (0.4–0.8 square meters) of floor space and contained a perch, a portable enrichment toy, a mirror, and a forage board for feeding enrichment. Practices in the housing and care of animals conformed to the regulations and standards of the PHS Policy on Humane Care and Use of Laboratory Animals, and the Guide for the Care and Use of Laboratory Animals. The Tulane National Primate Research Center (Animal Welfare Assurance # A4499-01) is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care-International. All animals are routinely cared for according to the guidelines prescribed by the NIH Guide to Laboratory Animal Care. The TNPRC conducts all research in accordance with the recommendations of the Weatherall report – “The use of non-human primates in research.” The Institutional Animal Care and Use Committee (IACUC) of the Tulane National Primate Research Center approved all animal-related protocols, including any treatments used with nonhuman primates. All animal procedures were overseen by veterinarians and their staff.
2.1.1
Animals were humanely euthanized by the veterinary staff at the TNPRC in accordance with endpoint policies. Euthanasia was conducted by anesthesia with ketamine hydrochloride (10 mg/kg) followed by an overdose with sodium pentobarbital and immediate necropsy. This method was consistent with the recommendation of the American Veterinary Medical Association guidelines (Lee et al., 2013b). Three brain regions approximately 1cm thick are routinely collected during necropsy of colony animals at TNPRC representing frontal lobe, parietal & temporal lobe/thalamus/basal ganglia, and cerebellum/occipital lobe. All tissues are fixed at routine necropsy by immersion in 10% neutral buffered formalin with zinc modification for 48 hours before trimming and paraffin embedding.
2.1.2
For this retrospective study, tissues were selected solely on their availability in the TNPRC tissue archive. All study subjects had been euthanized when clinical or research-related endpoints were reached. For this reason, we were not able to examine regional differences in cell morphology. None of the macaques had been used for infectious or pharmacological studies. Tissue taken from the prefrontal cortex from 9 control, 6 SIB and 5 SIB receiving naltrexone rhesus macaques (Macaca mulatta) were used for this study, for a total of 20 animals (Table 1).
Table 1.
Animals, treatment groups, behavioral status and neuropathologic findings
| Animal # | Necropsy # | # Days on Naltrexone | SIB | Age (years) | Sex | Species | Neuropathologic findings (NSL = no significant lesions) |
|---|---|---|---|---|---|---|---|
| EA12 | 10A059 | 29* | YES | 7.65 | M | Ind. M. Mulatta | NSL |
| CN74 | 04A171 | 4 | YES | 10.93 | M | Ind. M. Mulatta | NSL |
| EK05 | 10A304 | 55 | YES | 6.98 | M | Ind. M. Mulatta | Purkinje cell necrosis, neuronphagia and gliosis |
| T631 | 04A317 | 175 | YES | 8.93 | M | Ind. M. Mulatta | NSL |
| DD68 | 03A673 | 63 | YES | 2.53 | M | Ind. M. Mulatta | NSL |
| CN59 | 08A690 | 0 | YES | 19.52 | M | Ind. M. Mulatta | Spongiosis |
| GB61 | 11A128 | 0 | YES | 5.65 | M | Ind. M. Mulatta | NSL |
| N061 | 10A556 | 0 | YES | 18.31 | M | Ind. M. Mulatta | Lipofuscin |
| A999 | 91A083 | 0 | YES | 12.52 | M | Ind. M. Mulatta | Data not available |
| N539 | 01A315 | 0 | YES | 8.87 | F | Ind. M. Mulatta | Data not available |
| EH70 | 06A143 | 0 | YES | 3.00 | F | Ind. M. Mulatta | NSL |
| EI93 | 08A523 | 0 | NO | 5.31 | F | Ind. M. Mulatta | NSL |
| HT22 | 11A238 | 0 | NO | 2.91 | M | Ind. M. Mulatta | NSL |
| HM63 | 11A263 | 0 | NO | 3.04 | M | Ind. M. Mulatta | NSL |
| HN64 | 11A280 | 0 | NO | 3.03 | M | Ind. M. Mulatta | NSL |
| HP24 | 11A299 | 0 | NO | 3.03 | M | Ind. M. Mulatta | NSL |
| EB20 | 06A146 | 0 | NO | 3.82 | F | Ind. M. Mulatta | NSL |
| AV71 | 08A520 | 0 | NO | 18.42 | M | Ind. M. Mulatta | NSL |
| N142 | 11A297 | 0 | NO | 18.98 | M | Ind. M. Mulatta | NSL |
| J650 | 11A560 | 0 | NO | 22.26 | M | Ind. M. Mulatta | NSL |
Did not display behavioral response to naltrexone treatment.
2.1.3
Subjects in the current study were drawn from three populations: 1) the control population which did not exhibit SIB, 2) animals flagged for SIB but not treated pharmacologically, and 3) naltrexone-treated animals flagged for SIB. Populations could not be matched for rearing, age at first single housing, duration of lifetime single housing, or severity of self-biting or self-wounding, frequency of relocation, whether they were breeding colony or research animals, or intensity of research procedures, because these factor, as risk factors for SIB, influenced the population to which subjects were appropriately assigned (Gottlieb et al., 2013; Lutz et al., 2003; Novak, 2003; Rommeck et al., 2009) as well as feasibility of obtaining tissue. Where available, details on rearing, housing, and research use can be found in Table 2.
Table 2.
Rearing and housing conditions for animals on study.
| Animal # | Self-wound requiring veterinary care | Rearing | Age at first social housing | Age at first single housing | Singly housed as adult | Used in biomedical research projects |
|---|---|---|---|---|---|---|
| EA12 | Yes | Nursery | 6 mo | Birth | Yes | Yes |
| CN74 | Not at TNPRC | Unknown; transferred from other facility | Unknown | Unknown | Yes | No |
| EK05 | No | Mother | Birth | 3.9 y | Yes | Yes |
| T631 | Yes | Unknown; transferred | Unknown | Unknown | Yes | Yes |
| DD68 | Yes | Nursery | 1 y | Birth | N/A (euthanized as sub-adult) | Yes |
| CN59 | No | Unknown; transferred | Unknown | Unknown | Yes | Yes |
| GB61 | Yes (euthanized at presentation) | Mother | Birth | 3.8 y | Yes | Yes |
| N061 | No | Mother | Birth | 3.0 y | Yes | No |
| A999 | Unknown (predates current record-keeping) | Unknown, transferred | Unknown | Unknown | Yes | Yes |
| N539 | Unknown (predates current record-keeping) | Nursery | Unknown | Unknown | Yes | Yes |
| EH70 | No | Nursery | 1.8 y | birth | N/A/(euthanized as sub-adult) | Yes |
| EI93 | N/A | Mother | Birth | 5.3 y | No | No |
| HT22 | N/A | Mother | Birth | 2.7 y | N/A/(euthanized as sub-adult) | Yes –Normal Control |
| HM63 | N/A | Mother | Birth | 2.5 y | N/A/(euthanized as sub-adult) | Yes –Normal Control |
| HN64 | N/A | Mother | Birth | 2.6 y | N/A/(euthanized as sub-adult) | Yes –Normal Control |
| HP24 | N/A | Mother | Birth | 2.0 | N/A/(euthanized as sub-adult) | Yes –Normal Control |
| EB20 | N/A | Mother | Birth | 2.2 | N/A/(euthanized as sub-adult) | No |
| AV71 | N/A | Unknown (transferred) | Unknown | Unknown | No | No |
| N142 | N/A | Mother | Birth | 18.3 y | No | No |
| J650 | N/A | Mother | Birth | Unknown | Yes | No |
2.2 Self-injurious Behavior and Naltrexone Treatment
Historically, 15–20 animals per year (from a colony of approximately 5,000) are identified as exhibiting self-biting behavior and/or self-wounding at the TNPRC. These animals are “flagged” in the extensive records system, allowing them to be monitored prospectively and identified later for studies such as this one. Records of self-wounding and clinical intervention are maintained in the animal records system. All flagged individuals receive enhanced monitoring and implementation of inanimate enrichment. Subsets of animals are also considered for pharmacological treatment based upon the severity or frequency of self-wounding episodes or escalation over time. Among study subjects with SIB, severity ranged from a minimum of 6 self-bites per hour and no history of self-injury, to injury requiring humane euthanasia. These animals were given oral Naltrexone once daily at 3.2 mg/kg, a dose that was selected on the basis of published recommendation (Comer et al., 1993; Rowlett et al., 1998), and effective for preventing SIB in NHPs. Naltrexone was delivered in flavored syrup (10 mg/ml) or masked in fruits or other feeding enrichment items. Not all eligible animals are treated with Naltrexone due to factors such as potential confounds to the research studies to which they are assigned, short-term research plans (i.e. medication may not be pursued if euthanasia is imminent) or potential eligibility of the animal for social housing, which is the first-line treatment when possible.
2.3 Immunohistochemistry
Formalin-fixed, paraffin-embedded tissues were sectioned at 6μm and mounted onto positively charged glass slides. Sections were baked for overnight at 60°C, deparaffinized in xylene, and then rehydrated in graded concentrations of ethanol. Antigen retrieval was carried out for 20 min using a steamer and a citrate-based antigen unmasking solution (Vector Labs, Burlingame, CA). Tissues were blocked in blocking buffer (Dako) for one hour at room temperature before antibodies were applied. Tissues were incubated with TLR2 (ab24192, Abcam) and glial fibrillary acidic protein (GFAP) primary antibody (GA-5, Sigma) overnight at 4°C, washed three times with PBS with 0.2% bovine serum albumin (Santa Cruz) (PBS/BSA), and then incubated in the dark for 60 min at room temperature with secondary antibodies directly conjugated with Alexa 488 (green) or Alexa 568 (red) (Molecular Probes/Invitrogen, Carlsbad, CA). Sections were washed three times in PBS/BSA, cover-slipped with Prolong Gold with DAPI (Molecular Probes/Invitrogen), and imaged on a Nikon Eclipse TE2000-U microscope.
2.4 Quantification of Astrocyte Morphology
All samples were coded and analyzed randomly by a researcher blinded to animal number and condition. Images of non-overlapping fields in frontal cortical sections were captured by fluorescence microscopy at 40X objective (Nikon Eclipse TE2000-U) and analyzed using Neurolucida software (MBF Bioscience). Protoplasmic grey matter astrocytes reside in layers 2–6 and are complex cells with numerous fine processes (Oberheim et al., 2012). We randomly selected astrocytes from layers 3–5 for our analysis. Fibrous white matter astrocytes identified along white matter tracts are generally less complex. Selected fibrous white matter astrocytes were located significantly away from the grey-white matter interface so as not to be mistaken for Layer VI protoplasmic astrocytes. An average of 10 astrocytes from a single section for each animal with clear cell bodies and processes in both gray and white matter were chosen for reconstruction. The cells chosen were fully intact and did not have processes that touched the edges of the field. The resulting files generated by 2D reconstruction were analyzed with Neurolucida Explorer (MBF Bioscience), generating data of morphological measurements such as cell area, branching points (nodes), arbor length and volume as is routine in this lab (Lee et al., 2013a; Lee et al., 2013b; Renner et al., 2013; Snook et al., 2013).
2.5 Sholl Analyses
A linear Sholl analysis was performed on the data using Neurolucida software. As a type of quantitative analysis, Sholl analyses are used to describe changes in arbor complexity and the morphology of branching processes with respect to the overall area covered by each cell. Using this relationship, other groups have numerically distinguished between neuronal subtypes of differing neurite ramification. In this study, concentric rings 10μm apart were placed around the cell, centered at the centroid of the cell body and radiating outward, and process intersection and branching point quantities were collected. Intersections were determined as points where the astrocytic processes crossed a concentric ring, and quantified for each successive radius. Branching points (nodes) are expressed as a quantity per concentric ring area.
2.6 Quantification of TLR2 expression
Images of double-labeled (GFAP and TLR2) sections in non-overlapping fields were captured by fluorescence microscopy (Nikon Eclipse TE2000-U). An average of five fields of astrocytes were imaged for both white and gray matter at 20X, and the number of GFAP and TLR2 double-labeled astrocytes was quantified and expressed as a percentage of the total number of GFAP-labeled cells.
2.7 Statistical Analyses
Statistical analyses were performed using GraphPad Prism (version 5, GraphPad Software, La Jolla, CA). Normality was assessed by Kolmogorov-Smirnov test, and data that passed normality were analyzed by one-way ANOVA with Tukey’s multiple comparison test. Data that were not distributed normally were assessed by Kruskal-Wallace test with Dunn’s multiple comparison post-test to determine significance between groups. Results are expressed as mean ± SD. For all analyses, significance was set at p < 0.05.
Results
3.1 Oral naltrexone therapy reverses increased TLR2 expression associated with self-injurious behavior
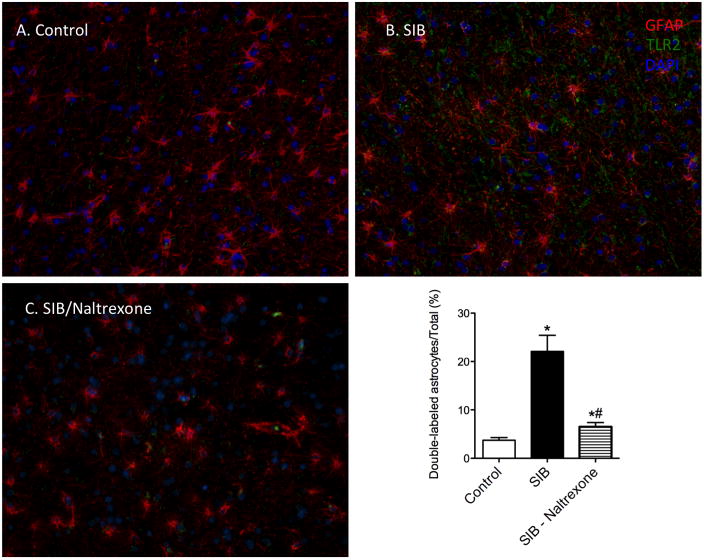
Having previously correlated increased TLR2 expression on astrocytes in macaques with SIB (Lee et al., 2013b), we postulated that naltrexone therapy would reduce the degree of glial activation. To address this question, we quantified the number of GFAP+ TLR2+ cells in white matter of three groups of macaques: controls, SIB macaques without treatment, and SIB macaques that had received oral naltrexone. The data from this study are presented in Figure 1. As previously reported, there was approximately five-fold increased expression of TLR2 on astrocytes in macaques with SIB compared with control animals (Control: 3.89% ± 2.65% vs. SIB: 22.12% ± 12.08%, p = 0.0005). Treatment with oral naltrexone produced a significant decrease in TLR2 expression to 6.41% ± 2.58% (Tukey’s: SIB-Naltrexone (6.41%, 95% CI [6.429, 24.99]). There was no significant difference between control and naltrexone-treated groups. Thus, naltrexone treatment reduces the innate inflammation associated with SIB in macaques.
Figure 1. Reversal of TLR2 upregulation following naltrexone treatment.
We hypothesized that naltrexone treatment would result in a reduction of inflammatory events that we observed in macaques with self-injurious behavior (Lee et al., 2013b). Astrocytes of control animals were generally immunonegative for TLR2 (A, and graph). There was an increase in GFAP/TLR2 double-positive white matter astrocytes in SIB animals (B). This proportion of double-positive astrocytes was significantly reduced following treatment with naltrexone, although it was still significantly higher then in control animals (C). Data is shown as shown as mean ± SD, * indicates significance from Control animals (p < 0.05), # indicates significance from SIB animals (p < 0.05).
3.1.1
To determine if the decreased TLR2 expression on astrocytes was linked to altered numbers of astrocytes, we performed a manual count of GFAP immunopositive cells per unit area and found no differences among our experimental groups (Data not shown).
3.2 Naltrexone therapy reverses atrophy associated with self-injurious behavior
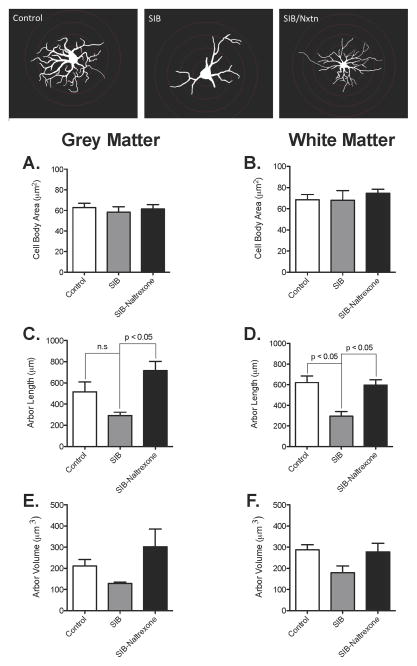
Since TLR2 expression may be linked to astrocyte activation, and TLR2 expression was reduced in naltrexone treated animals, we hypothesized that the atrophy we previously observed could also be reduced. To quantify astrocyte morphology, we stained astrocytes with GFAP and traced gray and white matter astrocytes to determine any structural changes associated with naltrexone treatment in primates. There was no change in cell body size in any of the treatment groups in either grey (Figure 2A) or white matter (Figure 2B). Naltrexone treatment in macaques displaying SIB induced a significant increase in the total length of the cytoplasmic processes of both grey (Figure 2C, Control: 493.0 ± 274.4 μm vs. SIB: 293.3 ± 75.32 μm vs. SIB-Naltrexone: 715.8 ± 195.9 μm, p = 0.0090) and white matter (Figure 2D, Control: 587.2 ± 208.5 μm vs. SIB: 295.5 ± 110.4 μm vs. SIB-Naltrexone: 597.2 ± 115.4 μm, p = 0.0072). While the volume of the processes followed the same general pattern as for length (Figures 2E & F), none of the changes was significantly different. It was also noted that there were no significant differences between the control and the naltrexone treated animals in any of the above parameters, indicating a return to normal levels. Representative tracings are included at the bottom of Figure 2. Note, the image of the astrocyte from a naltrexone-treated SIB animal has an extra Sholl ring, making the cell body appear smaller.
Figure 2. Increases in the arbor length of white matter astrocytes after naltrexone treatment in animals exhibiting SIB.
The area of the astrocyte cell body was not significantly different in macaques with SIB in grey or white matter astrocytes regardless of naltrexone treatment (a–b). The cumulative length of the astrocyte processes in animals with SIB was decreased in white matter astrocytes (d) but was not significantly decreased in grey matter astrocytes (c). In both grey and white matter, total arbor length in naltrexone-treated SIB animals was increased compared with non-treated SIB animals (c–d). However, there were no significant changes in arbor volume in SIB and naltrexone-treated SIB animals compared to controls (e–f). Data shown as mean ± SD, p < 0.05. Representative traces are included at the bottom.
3.2.1
The potential number of neurons, glia, and blood vessels that an astrocyte can interact with is a function of how many tips the cell has in addition to the length of its processes. Therefore, we analyzed the number of branching points and tips each astrocyte had. It was apparent that naltrexone reversed the loss of branching observed in animals with SIB in both grey matter (Figure 3A, Control: 31.78 ± 21.37 vs. SIB: 10.67 ± 2.244 vs. SIB-Naltrexone: 48.78 ± 7.598, p = 0.004) and white matter (Figure 3B, Control: 28.69 ± 16.34 vs. SIB: 9.633 ± 4.606 vs. SIB-Naltrexone: 38.13 ± 9.182, p = 0.0400). This was reflected in the number of tips on each astrocyte (Figure 3 C & D; Gray matter, Control: 38.63 ± 21.70 vs. SIB: 20.59 ± 5.655 vs. SIB-Naltrexone: 56.92 ± 7.146, p = 0.0073; White matter, Control: 39.93 ± 16.80 vs. SIB: 19.35 ± 5.381 vs. SIB-Naltrexone: 47.99 ± 8.851, p = 0.0037). As with the parameters in Figure 2, the degree of branching described here was not significantly different from that observed in control macaques. Overall, these results suggest significant plasticity in astrocytic processes and that there is potential for CNS recovery when macaques with abnormal behaviors are treated with opioid inhibitors.
Figure 3. Astrocyte complexity is increased in the frontal cortex of SIB animals after treatment with naltrexone.
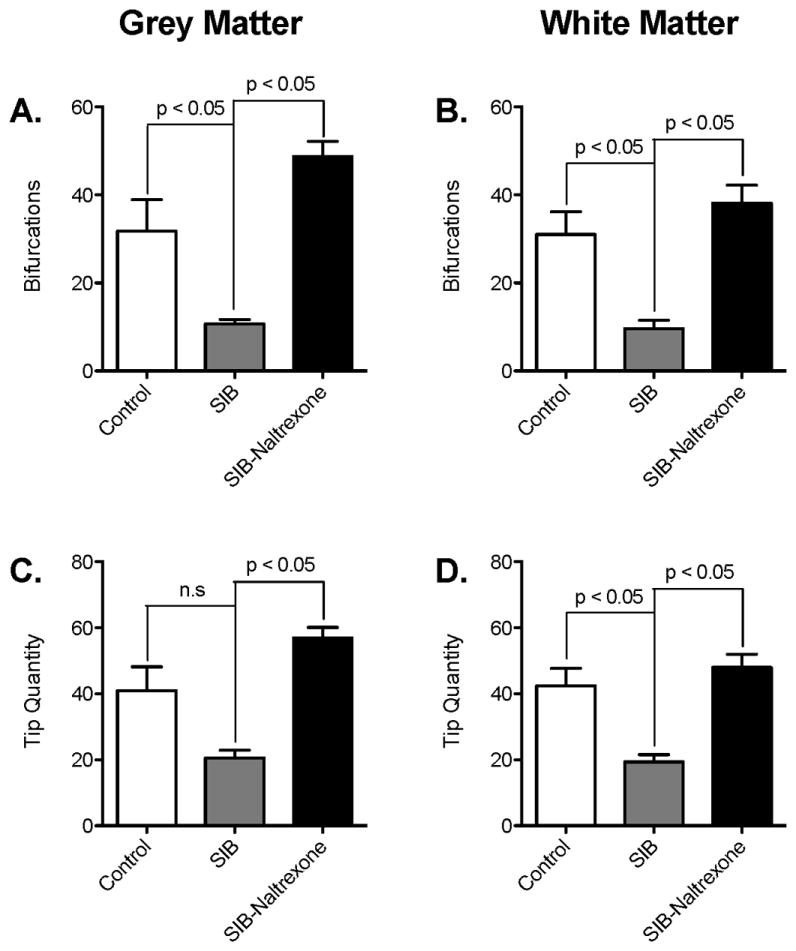
The quantity of branching points (b) and number of terminal end points (d) shown by processes in white matter astrocytes were significantly decreased in animals with SIB. These decreases were reversed with naltrexone treatment (shown as mean ± SD, p < 0.05). The numbers of bifurcations (a) and tips (c) of gray matter astrocytes in naltrexone-treated SIB animals were significantly greater than untreated SIB animals (p < 0.05). There were no differences between control and SIB-Naltrexone animals. Data shown as mean ± SD, p < 0.05.
3.2.2
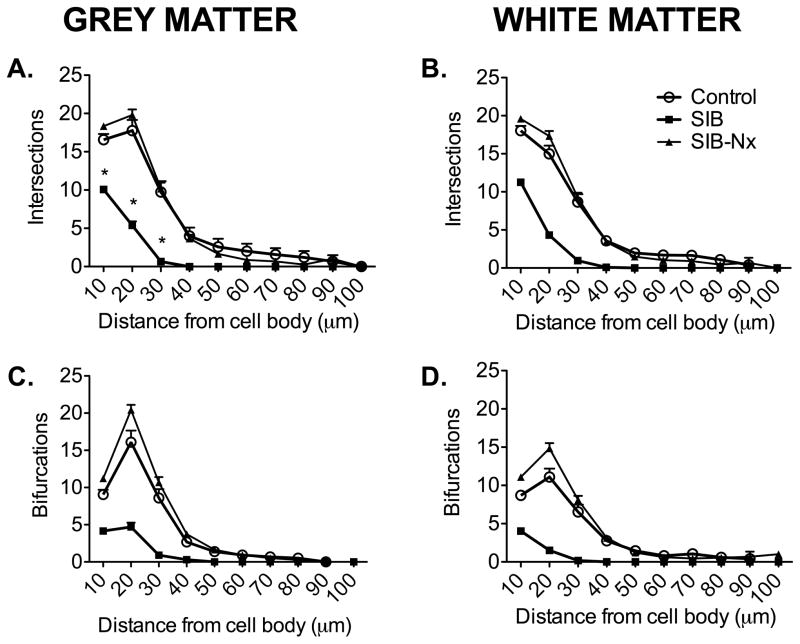
Finally, to determine where these alterations in the branch pattern were occurring, we performed modified Sholl analyses. There were significantly fewer intersections of astrocyte processes with Sholl rings in animals with SIB in both grey and white matter (Figure 4A, B). These were reversed in animals receiving naltrexone treatment. It was apparent that, in both grey and white matter the reduced branching observed in animals with SIB occurred proximal to the cell body (Figure 4C, D). This was reversed in animals receiving naltrexone treatment. Again, there were no significant differences in astrocyte morphology between control animals and SIB animals receiving naltrexone treatment.
Figure 4. Sholl analyses.
The numbers of branching points and intersections (points where astrocytic processes cross over concentric rings) significantly decreased in macaques with SIB (A–D). These changes were reversed in gray and white matter astrocytes after naltrexone treatment in animals with SIB (A–D). Such changes primarily occur in dendritic segments proximal to the cell body. Data shown as mean ± SD, p < 0.05.
Discussion
4.1
Self-injury occurs naturally in nonhuman primates and closely recapitulates self-harming behaviors in humans (Dellinger-Ness and Handler, 2006). Unlike other animal models, SIB and depressive-like behaviors in nonhuman primates can develop through social subordination and chronic social isolation (Gilmer and McKinney, 2003; Li et al., 2013). The severity of SIB in rhesus macaques ranges from biting that may not result in trauma to the skin to significant self-injury (Li et al., 2013; Willard et al., 2013). Furthermore, rhesus monkeys with SIB preferentially harm areas that are associated with acupuncture/acupressure analgesia (Marinus, 2000), an effect that has also been demonstrated in human self-injury (Symons and Thompson, 1997). SIB in macaques can be effectively managed using naltrexone (Kempf et al., 2012). As astrocytes are rapidly becoming recognized as vitally important targets of antidepressants (Czeh and Di Benedetto, 2013; Hutchinson et al., 2011), we were interested to determine if naltrexone therapy reversed the atrophy and immune dysfunction we had observed recently (Lee et al., 2013b). The current study examined changes in astrocyte morphology and Toll-like receptor expression following naltrexone treatment in rhesus macaques exhibiting SIB.
4.2
As we reported previously, protoplasmic gray matter and fibrous white matter astrocytes rhesus macaques with SIB had shorter, less complex processes compared with control animals in addition to increased expression of TLR2 (Lee et al., 2013b). The current study showed that naltrexone treatment attenuated increases in TLR2 expression in animals with SIB and also attenuated decreases seen in total process length and in the complexity of the astrocytic processes. Naltrexone has been shown to block tumor necrosis factor alpha (TNF-α) (Greeneltch et al., 2004), which in turn, induces TLR2 expression in astrocytes (Phulwani et al., 2008). Naltrexone works either through direct opioid receptor antagonism, production of unknown mediators that block TNF-α, or through the production of anti-inflammatory mediators (reviewed recently by (Hutchinson et al., 2011)).
4.3
Recently, we showed a significant retraction in astrocyte processes in nonhuman primates with depressive-like behavior (Lee et al., 2013b), and changes in astrocyte morphology have been shown in depressed human suicide (Torres-Platas et al., 2011). Here, we present findings demonstrating that these changes are reversed after the administration of naltrexone therapy (Figures 2–4), which suggests a role for astrocyte activation in the pathology of self-injurious behaviors. We have assessed astrocytic activation in macaques with neuroinflammatory diseases successfully measuring changes in astrocyte morphology in genetic (Krabbe’s diease (Snook et al., 2014)), bacterial (neurobrucellosis: (Lee et al., 2013a)) and viral (Lee et al., 2014) diseases with neuroinflammatory components. These structural changes in astrocytes are likely to impact on blood-brain barrier function (Renner et al., 2012) and neuronal homeostasis (Tasker et al., 2012).
4.4
The efficacy of extended-release injectable naltrexone in the pharmacologic treatment of rhesus macaques exhibiting SIB was recently assessed (Kempf et al., 2012). Compared to baseline values, both the frequency and the percentage of time spent displaying SIB decreased during an 8-week treatment phase. Additionally, the percentage of time displaying self-injury remained significantly lower through the end of 200-day follow-up period after termination of drug treatment. While the different behaviors of the animals in the current study were not measured, and despite differences in drug dosage and treatment duration, consistent statistically significant changes in astrocyte activation and morphological changes were observed. The continued effect of the drug on behavior suggests that the subjects had experienced a sustained change in morphology in addition to the short-term action of the drug’s affect on biochemistry. These studies demonstrate changes in astrocyte morphology and activation following long-term treatment with naltrexone. Previous work has shown astrocytes cultured from newborn rodents treated with naltrexone produce altered levels of growth factors (Mitsuo and Schwartz, 1993). Furthermore, astrocyte dysfunction has been implicated in the pathogenesis of a number of behavioral conditions, including autism-spectrum disorders. For instance, dysfunction in DNA methylation in astrocytes, implicated in Rett syndrome, can result in impaired BDNF regulation, cytokine production, and neuronal plasticity (Maezawa et al., 2009).
4.5
Biochemical imbalances have long been implicated in the etiology of self-injurious behaviors (Winchel and Stanley, 1991). Research in animals as well as humans has demonstrated that low levels of serotonin or high levels of dopamine are associated with self-injury (Kraemer and Clarke, 1990; Mueller and Nyhan, 1982). As such, pharmacological interventions targeting the serotonin system in rhesus macaques reduced self-injurious and self-directed stereotypic behavior (Fontenot et al., 2009; Fontenot et al., 2005). Opioid imbalances are also hypothesized to contribute to self-injurious behaviors. The opioid-system hypothesis of SIB posits that a portion of the population engages in self-injury in order to stimulate the release of endogenous opioids (Stanley et al., 2010). For instance, studies in adults with non-suicidal self-injurious (NSSI) behavior found significantly lower levels of CSF beta-endorphin and met-enkephalin levels in these patients than diagnostically matched controls (Stanley et al., 2010). The NSSI group did not differ from the control group in terms of serotonergic and dopaminergic CSF levels, indicating that the behaviors may arise from dysfunction in the opioid system. In the clinical setting, patients with SIB can experience an anesthesia-like effect and/or a euphoric-like feeling associated with the behaviors (Sandman et al., 1983), and studies examining the effect of opioid receptor antagonists on SIB have shown to attenuate these behaviors. Naltrexone can also function through NMDA receptors that are present on astrocytes (reviewed by (Hutchinson et al., 2011)). This would provide an alternate, or possibly complementary, pathway to target innate immune activation. Supporting this theory are early studies examining self-injury in adolescent patients that found a significant decrease in the frequency of behaviors with naltrexone treatment (Herman et al., 1987). Later case reports have noted efficacy of naltrexone treatment for impulse control disorders and self-injury, particularly in populations with developmental delay (Modesto-Lowe and Van Kirk, 2002), or in juveniles (Willemsen-Swinkels et al., 1995). Additional research suggests that elevated beta-endorphin levels following self-injury may predict response to treatment for SIB (Sandman et al., 2008). Furthermore, nonhuman primates with SIB also have reduced levels of plasma beta-endorphin immunoreactivity (Tiefenbacher et al., 2005; Tiefenbacher et al., 2004), similar to findings of reduced opioid activity in individuals with autism (Willemsen-Swinkels et al., 1996).
4.6
Our results show increased TLR2 expression in prefrontal cortical tissues coincident with previous studies in animals with compulsive behavior (Crews et al., 2011). However, attenuation of TLR2 expression after treatment with oral naltrexone suggests a possible link between behavioral changes and decreased neuroinflammation. Furthermore, the finding that altered GFAP expression was observed in both white and gray matter is perhaps unusual, as it has been speculated that oligodendrocyte pathologies account for white matter abnormalities observed previously (Czeh et al., 2008). However, self-injury is often associated with a developmental etiology (Schroeder et al., 2014). As such, the changes seen in both grey and white matter astrocytes are unique and could represent the global changes seen in these behaviors. We acknowledge that the animals used in this study were not matched for all of the variables, including housing, rearing and age. As noted above (section 2.1.2), animals were selected based on availability of tissues for this retrospective study. One of the major differences was in the age of the animals. In a separate study (Robillard et al, under revision), astrocyte process length and complexity were increased in adult animals compared with juveniles/adolescents (under 5 years of age) or eugeric aged animals (over 20). As adults were over-represented in the SIB group, this is probably not a confounding factor. However, the other factors represent potential limitations of this study.
4.7
In the present study, we examined astrocytes of macaques with SIB with and without naltrexone treatment in order to obtain a general understanding of changes that occur in the brain of SIB-affected rhesus macaques after treatment with a nonselective opioid antagonist. Previously observed changes in astrocyte morphology and TLR expression were attenuated with naltrexone treatment. These changes coincided with decreases in self-injurious behaviors in rhesus macaques. Whether the observed changes are a direct effect of naltrexone treatment – mediated by the mu-opioid receptor – or an indirect effect of opioid antagonism remains to be determined. Other novel mechanisms through which opioid antagonism could produce changes in astrocyte morphology include interactions with filament A (Wang et al., 2008) or Toll-like receptors. Recent studies have suggested that opioid antagonists can activate glia through direct interactions with TLR4 and that this receptor serves overlapping pain functions with opioids (Hutchinson et al., 2007; Hutchinson et al., 2008), inferring an antinociceptive role for TLR4 or the overlap of opioid and TLR4 signaling pathways. Future studies clarifying the effects of opioid antagonist treatment in a control population are warranted.
Highlights.
We demonstrate that naltrexone reduces TLR2 expression in brain
Naltrexone treatment reverses astrocyte atrophy associated with self-harm
The number of potential synapses is dramatically increased following naltrexone
Acknowledgments
We thank the technicians, veterinarians and pathologists at TNPRC for their efforts in identifying animals with abnormal behaviors, their treatment and ultimately, their necropsy and collection of tissues.
Funding.
This work was supported by The Office of the Director at the National Institutes for Health (grant number OD11104), Bridge Funding by Tulane University School of Medicine and Tulane University Program in Neuroscience. Miss Lee is the inaugural Tulane National Primate Research Center Biomedical Sciences Fellow.
Footnotes
The authors have no conflicts of interest in this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic criteria from DSM-IV-TR. American Psychiatric Association; Washington, D.C: 2000. [Google Scholar]
- Comer SD, Lac ST, Curtis LK, Carroll ME. Effects of buprenorphine and naltrexone on reinstatement of cocaine-reinforced responding in rats. J Pharmacol Exp Ther. 1993;267:1470–1477. [PubMed] [Google Scholar]
- Crews FT, Zou J, Qin L. Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav Immun. 2011;25(Suppl 1):S4–S12. doi: 10.1016/j.bbi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Di Benedetto B. Antidepressants act directly on astrocytes: evidences and functional consequences. Eur Neuropsychopharmacol. 2013;23:171–185. doi: 10.1016/j.euroneuro.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Czeh B, Perez-Cruz C, Fuchs E, Flugge G. Chronic stress-induced cellular changes in the medial prefrontal cortex and their potential clinical implications: does hemisphere location matter? Behavioural brain research. 2008;190:1–13. doi: 10.1016/j.bbr.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Czeh B, Simon M, Schmelting B, Hiemke C, Fuchs E. Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology. 2006;31:1616–1626. doi: 10.1038/sj.npp.1300982. [DOI] [PubMed] [Google Scholar]
- Dellinger-Ness LA, Handler L. Self-injurious behavior in human and non-human primates. Clin Psychol Rev. 2006;26:503–514. doi: 10.1016/j.cpr.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Laurence JA, Araghi-Niknam M, Stary JM, Schulz SC, Lee S, Gottesman II. Glial fibrillary acidic protein is reduced in cerebellum of subjects with major depression, but not schizophrenia. Schizophrenia research. 2004;69:317–323. doi: 10.1016/j.schres.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Fontenot MB, Musso MW, McFatter RM, Anderson GM. Dose-finding study of fluoxetine and venlafaxine for the treatment of self-injurious and stereotypic behavior in rhesus macaques (Macaca mulatta) J Am Assoc Lab Anim Sci. 2009;48:176–184. [PMC free article] [PubMed] [Google Scholar]
- Fontenot MB, Padgett EE, 3rd, Dupuy AM, Lynch CR, De Petrillo PB, Higley JD. The effects of fluoxetine and buspirone on self-injurious and stereotypic behavior in adult male rhesus macaques. Comp Med. 2005;55:67–74. [PubMed] [Google Scholar]
- Gilmer WS, McKinney WT. Early experience and depressive disorders: human and non-human primate studies. J Affect Disord. 2003;75:97–113. doi: 10.1016/s0165-0327(03)00046-6. [DOI] [PubMed] [Google Scholar]
- Gottlieb DH, Capitanio JP, McCowan B. Risk factors for stereotypic behavior and self-biting in rhesus macaques (Macaca mulatta): animal’s history, current environment, and personality. Am J Primatol. 2013;75:995–1008. doi: 10.1002/ajp.22161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeneltch KM, Haudenschild CC, Keegan AD, Shi Y. The opioid antagonist naltrexone blocks acute endotoxic shock by inhibiting tumor necrosis factor-alpha production. Brain Behav Immun. 2004;18:476–484. doi: 10.1016/j.bbi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Hercher C, Turecki G, Mechawar N. Through the looking glass: examining neuroanatomical evidence for cellular alterations in major depression. J Psychiatr Res. 2009;43:947–961. doi: 10.1016/j.jpsychires.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Herman BH, Hammock MK, Arthur-Smith A, Egan J, Chatoor I, Werner A, Zelnik N. Naltrexone decreases self-injurious behavior. Ann Neurol. 1987;22:550–552. doi: 10.1002/ana.410220419. [DOI] [PubMed] [Google Scholar]
- Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. Scientific World Journal. 2007;7:98–111. doi: 10.1100/tsw.2007.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR. Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev. 2011;63:772–810. doi: 10.1124/pr.110.004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, Rice KC, Watkins LR. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4) Eur J Neurosci. 2008;28:20–29. doi: 10.1111/j.1460-9568.2008.06321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson CM, Gould M. The epidemiology and phenomenology of non-suicidal self-injurious behavior among adolescents: a critical review of the literature. Archives of suicide research: official journal of the International Academy for Suicide Research. 2007;11:129–147. doi: 10.1080/13811110701247602. [DOI] [PubMed] [Google Scholar]
- Kempf DJ, Baker KC, Gilbert MH, Blanchard JL, Dean RL, Deaver DR, Bohm RP., Jr Effects of extended-release injectable naltrexone on self-injurious behavior in rhesus macaques (Macaca mulatta) Comp Med. 2012;62:209–217. [PMC free article] [PubMed] [Google Scholar]
- Kerr PL, Muehlenkamp JJ, Turner JM. Nonsuicidal self-injury: a review of current research for family medicine and primary care physicians. Journal of the American Board of Family Medicine: JABFM. 2010;23:240–259. doi: 10.3122/jabfm.2010.02.090110. [DOI] [PubMed] [Google Scholar]
- Kraemer GW, Clarke AS. The behavioral neurobiology of self-injurious behavior in rhesus monkeys. Progress in neuro-psychopharmacology & biological psychiatry. 1990;14(Suppl):S141–168. doi: 10.1016/0278-5846(90)90092-u. [DOI] [PubMed] [Google Scholar]
- Langbehn DR, Pfohl B. Clinical correlates of self-mutilation among psychiatric inpatients. Annals of clinical psychiatry: official journal of the American Academy of Clinical Psychiatrists. 1993;5:45–51. doi: 10.3109/10401239309148923. [DOI] [PubMed] [Google Scholar]
- Lee KM, Chiu KB, Renner NA, Sansing HA, Didier PJ, MacLean AG. Form follows function: astrocyte morphology and immune dysfunction in SIV neuroAIDS. J Neurovirol. 2014;20:474–484. doi: 10.1007/s13365-014-0267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Chiu KB, Sansing HA, Didier PJ, Ficht TA, Arenas-Gamboa AM, Roy CJ, Maclean AG. Aerosol-induced brucellosis increases TLR-2 expression and increased complexity in the microanatomy of astroglia in rhesus macaques. Frontiers in cellular and infection microbiology. 2013a;3:86. doi: 10.3389/fcimb.2013.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Chiu KB, Sansing HA, Inglis FM, Baker KC, Maclean AG. Astrocyte atrophy and immune dysfunction in self-harming macaques. PLoS One. 2013b;8:e69980. doi: 10.1371/journal.pone.0069980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard BE. The concept of depression as a dysfunction of the immune system. Curr Immunol Rev. 2010;6:205–212. doi: 10.2174/157339510791823835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Xu F, Xie L, Ji Y, Cheng K, Zhou Q, Wang T, Shively C, Wu Q, Gong W, Fang L, Zhan Q, Melgiri ND, Xie P. Depression-Like Behavioral Phenotypes by Social and Social Plus Visual Isolation in the Adult Female Macaca fascicularis. PLoS One. 2013;8:e73293. doi: 10.1371/journal.pone.0073293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz C, Well A, Novak M. Stereotypic and self-injurious behavior in rhesus macaques: a survey and retrospective analysis of environment and early experience. Am J Primatol. 2003;60:1–15. doi: 10.1002/ajp.10075. [DOI] [PubMed] [Google Scholar]
- Maezawa I, Swanberg S, Harvey D, LaSalle JM, Jin LW. Rett syndrome astrocytes are abnormal and spread MeCP2 deficiency through gap junctions. J Neurosci. 2009;29:5051–5061. doi: 10.1523/JNEUROSCI.0324-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinus LM, Chase WK, Novak MA. Self-biting behavior in rhesus macaques (Macaca mulatta) is preferentially directed to body areas associated with acupuncture analgesia. Am J Primatol. 2000;51:61–74. [Google Scholar]
- Mitsuo K, Schwartz JP. Chronic treatment of newborn rats with naltrexone alters astrocyte production of nerve growth factor. Journal of molecular neuroscience: MN. 1993;4:21–28. doi: 10.1007/BF02736687. [DOI] [PubMed] [Google Scholar]
- Modesto-Lowe V, Van Kirk J. Clinical uses of naltrexone: a review of the evidence. Exp Clin Psychopharmacol. 2002;10:213–227. doi: 10.1037//1064-1297.10.3.213. [DOI] [PubMed] [Google Scholar]
- Mueller K, Nyhan WL. Pharmacologic control of pemoline induced self-injurious behavior in rats. Pharmacol Biochem Behav. 1982;16:957–963. doi: 10.1016/0091-3057(82)90052-1. [DOI] [PubMed] [Google Scholar]
- Nagamitsu S. CSF beta-endorphin levels in pediatric neurologic disorders. The Kurume medical journal. 1993;40:233–241. doi: 10.2739/kurumemedj.40.233. [DOI] [PubMed] [Google Scholar]
- Norton PJ, Temple SR, Pettit JW. Suicidal ideation and anxiety disorders: elevated risk or artifact of comorbid depression? J Behav Ther Exp Psychiatry. 2008;39:515–525. doi: 10.1016/j.jbtep.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Novak MA. Self-injurious behavior in rhesus monkeys: new insights into its etiology, physiology, and treatment. Am J Primatol. 2003;59:3–19. doi: 10.1002/ajp.10063. [DOI] [PubMed] [Google Scholar]
- Oberheim NA, Goldman SA, Nedergaard M. Heterogeneity of astrocytic form and function. Methods Mol Biol. 2012;814:23–45. doi: 10.1007/978-1-61779-452-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phulwani NK, Esen N, Syed MM, Kielian T. TLR2 expression in astrocytes is induced by TNF-alpha- and NF-kappa B-dependent pathways. J Immunol. 2008;181:3841–3849. doi: 10.4049/jimmunol.181.6.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner NA, Redmann RK, Moroney-Rasmussen T, Sansing HA, Aye PP, Didier PJ, Lackner AA, Maclean AG. S100beta as a novel and accessible indicator for the presence of monocyte-driven encephalitis in AIDS. Neuropathol Appl Neurobiol. 2012;38:162–174. doi: 10.1111/j.1365-2990.2011.01200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner NA, Sansing HA, Inglis FM, Mehra S, Kaushal D, Lackner AA, Maclean AG. Transient acidification and subsequent proinflammatory cytokine stimulation of astrocytes induce distinct activation phenotypes. J Cell Physiol. 2013;228:1284–1294. doi: 10.1002/jcp.24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribka EP, Baker KC. Naltrexone (naltrexone hydrochloride) as a treatment to decrease incidence and severity of self-injurious behavior in rhesus macaques (Macaca mulatta) American Journal of Primatology. 2004;62:31–47. [Google Scholar]
- Rommeck I, Anderson K, Heagerty A, Cameron A, McCowan B. Risk factors and remediation of self-injurious and self-abuse behavior in rhesus macaques. J Appl Anim Welf Sci. 2009;12:61–72. doi: 10.1080/10888700802536798. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Wilcox KM, Woolverton WL. Self-administration of cocaine-heroin combinations by rhesus monkeys: antagonism by naltrexone. J Pharmacol Exp Ther. 1998;286:61–69. [PubMed] [Google Scholar]
- Roy A, Roy M, Deb S, Unwin G, Roy A. Are opioid antagonists effective in attenuating the core symptoms of autism spectrum conditions in children: a systematic review. Journal of intellectual disability research: JIDR. 2014 doi: 10.1111/jir.12122. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Datta PC, Barron J, Hoehler FK, Williams C, Swanson JM. Naloxone attenuates self-abusive behavior in developmentally disabled clients. Applied research in mental retardation. 1983;4:5–11. doi: 10.1016/s0270-3092(83)80014-9. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Touchette PE, Marion SD, Chicz-DeMet A. The role of proopiomelanocortin (POMC) in sequentially dependent self-injurious behavior. Dev Psychobiol. 2008;50:680–689. doi: 10.1002/dev.20323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder SR, Marquis JG, Reese RM, Richman DM, Mayo-Ortega L, Oyama-Ganiko R, LeBlanc J, Brady N, Butler MG, Johnson T, Lawrence L. Risk factors for self-injury, aggression, and stereotyped behavior among young children at risk for intellectual and developmental disabilities. American journal on intellectual and developmental disabilities. 2014;119:351–370. doi: 10.1352/1944-7558-119.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snook ER, Fisher-Perkins JM, Sansing HA, Lee KM, Alvarez X, Maclean AG, Peterson KE, Lackner AA, Bunnell BA. Innate Immune Activation in the Pathogenesis of a Murine Model of Globoid Cell Leukodystrophy. Am J Pathol. 2013 doi: 10.1016/j.ajpath.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snook ER, Fisher-Perkins JM, Sansing HA, Lee KM, Alvarez X, MacLean AG, Peterson KE, Lackner AA, Bunnell BA. Innate immune activation in the pathogenesis of a murine model of globoid cell leukodystrophy. Am J Pathol. 2014;184:382–396. doi: 10.1016/j.ajpath.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley B, Sher L, Wilson S, Ekman R, Huang YY, Mann JJ. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord. 2010;124:134–140. doi: 10.1016/j.jad.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JD, Liu Y, Yuan YH, Li J, Chen NH. Gap junction dysfunction in the prefrontal cortex induces depressive-like behaviors in rats. Neuropsychopharmacology. 2012;37:1305–1320. doi: 10.1038/npp.2011.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons FJ, Thompson T. Self-injurious behaviour and body site preference. Journal of intellectual disability research: JIDR. 1997;41 (Pt 6):456–468. doi: 10.1111/j.1365-2788.1997.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Tasker JG, Oliet SH, Bains JS, Brown CH, Stern JE. Glial regulation of neuronal function: from synapse to systems physiology. J Neuroendocrinol. 2012;24:566–576. doi: 10.1111/j.1365-2826.2011.02259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiefenbacher S, Novak MA, Lutz CK, Meyer JS. The physiology and neurochemistry of self-injurious behavior: a nonhuman primate model. Front Biosci. 2005;10:1–11. doi: 10.2741/1500. [DOI] [PubMed] [Google Scholar]
- Tiefenbacher S, Novak MA, Marinus LM, Chase WK, Miller JA, Meyer JS. Altered hypothalamic-pituitary-adrenocortical function in rhesus monkeys (Macaca mulatta) with self-injurious behavior. Psychoneuroendocrinology. 2004;29:501–515. doi: 10.1016/s0306-4530(03)00068-4. [DOI] [PubMed] [Google Scholar]
- Torres-Platas SG, Hercher C, Davoli MA, Maussion G, Labonte B, Turecki G, Mechawar N. Astrocytic hypertrophy in anterior cingulate white matter of depressed suicides. Neuropsychopharmacology. 2011;36:2650–2658. doi: 10.1038/npp.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Platas SG, Nagy C, Wakid M, Turecki G, Mechawar N. Glial fibrillary acidic protein is differentially expressed across cortical and subcortical regions in healthy brains and downregulated in the thalamus and caudate nucleus of depressed suicides. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.65. [DOI] [PubMed] [Google Scholar]
- Tynan RJ, Beynon SB, Hinwood M, Johnson SJ, Nilsson M, Woods JJ, Walker FR. Chronic stress-induced disruption of the astrocyte network is driven by structural atrophy and not loss of astrocytes. Acta Neuropathol. 2013;126:75–91. doi: 10.1007/s00401-013-1102-0. [DOI] [PubMed] [Google Scholar]
- Wang HY, Frankfurt M, Burns LH. High-affinity naloxone binding to filamin a prevents mu opioid receptor-Gs coupling underlying opioid tolerance and dependence. PLoS One. 2008;3:e1554. doi: 10.1371/journal.pone.0001554. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Willard SL, Riddle DR, Forbes ME, Shively CA. Cell Number and Neuropil Alterations in Subregions of the Anterior Hippocampus in a Female Monkey Model of Depression. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen-Swinkels SH, Buitelaar JK, Weijnen FG, Thijssen JH, Van Engeland H. Plasma beta-endorphin concentrations in people with learning disability and self-injurious and/or autistic behaviour. The British journal of psychiatry: the journal of mental science. 1996;168:105–109. doi: 10.1192/bjp.168.1.105. [DOI] [PubMed] [Google Scholar]
- Willemsen-Swinkels SH, Buitelaar JK, Weijnen FG, van Engeland H. Placebo-controlled acute dosage naltrexone study in young autistic children. Psychiatry Res. 1995;58:203–215. doi: 10.1016/0165-1781(95)02749-m. [DOI] [PubMed] [Google Scholar]
- Winchel RM, Stanley M. Self-injurious behavior: a review of the behavior and biology of self-mutilation. Am J Psychiatry. 1991;148:306–317. doi: 10.1176/ajp.148.3.306. [DOI] [PubMed] [Google Scholar]