Abstract
Background
To compare the mechanical performance of a rotator cuff repaired with a novel tendon-fibrocartilage-bone composite bridging patch vs the traditional Mason-Allen repair in an in vitro canine model.
Methods
Twenty shoulders and 10 bridging patches from patellar tendon were harvested. The patches were trimmed and sliced into 2 layers. An infraspinatus tendon tear was created in each shoulder. Modified Mason-Allen sutures were used to repair the infraspinatus tendon to the greater tuberosity, with or without the bridging patch (bridging patch group and controls, respectively). Shoulders were loaded to failure under displacement control at a rate of 0.5mm/sec.
Findings
The ultimate tensile load was significantly higher in the bridging patch group than control (mean [SD], 365.46 [36.45] vs 272.79 [48.88] N; P<.001). Stiffness at the greater tuberosity repair site and the patch-infraspinatus tendon repair site was significantly higher than the control repair site (93.96 [27.72] vs 42.62 [17.48] N/mm P<.001; 65.94 [24.51] vs 42.62 [17.48] N/mm P=.02, respectively).
Interpretation
The tendon-fibrocartilage-bone composite bridging patch achieved higher ultimate tensile load and stiffness at the patch–greater tuberosity repair site compared with traditional repair in a canine model. This composite tissue transforms the traditional tendon-to-bone healing interface (with dissimilar tissues) into a pair of bone-to-bone and tendon-to-tendon interfaces, which may improve healing quality and reduce retear rate.
Keywords: rotator cuff repair, bridging patch, tendon-to-bone healing
1. INTRODUCTION
Surgical rotator cuff repair is the criterion standard to relieve pain and restore shoulder function after rotator cuff tears. However, up to 17% to 94% of patients are reported to have partial or full-thickness retears when evaluated postoperatively by ultrasound or magnetic resonance imaging1–3. Postoperative retears, especially large ones, greatly affect range of motion, decrease muscle strength, and cause pain4; 5. Various mechanical and biological approaches have been developed to prevent retears, including improved suture techniques, bone substitutes, periosteum autografts, growth factors, gene therapy, stem cell transplantation, and others 6–9. However, these efforts have not been completely successful because of the unsatisfactory regeneration of the natural tendon-to-bone transition zone.
The slow healing process at the tendon-to-bone interface and the chronic tendon and muscle degeneration associated with rotator cuff injury are believed to cause poor healing after repair10–12. The natural gradual transition from tendon to uncalcified fibrocartilage to calcified fibrocartilage to bone tissue is difficult to regenerate because of differences in the 2 tissues13. Chronic tendon and muscle degeneration may cause postoperative gap formation between tendon and bone tissues, which increases the risk of rotator cuff retear. Numerous attempts to rebuild the transition zone have been unsuccessful to date. However, anterior cruciate ligament (ACL) reconstruction uses a bone–patellar tendon–bone (BPTB) graft, which strengthens healing by using a bone-to-bone healing interface instead of the weaker tendon-to-bone interface14. This concept potentially could be applied in rotator cuff repair by replacing the dissimilar-tissue (tendon-to-bone) healing interface with similar-tissue healing interfaces (tendon-to-tendon and bone-to-bone).
In the current study, we tested a novel approach that replaced the conventional tendon-to-bone repair interface with a pair of homogeneous healing interfaces (i.e., tendon-to-tendon and bone-to-bone interfaces) by using allograft augmentation sourced from a patellar tendon-fibrocartilage-bone composite (TFBC). The purpose of this study was to examine the mechanical performance of rotator cuffs and compare those repaired with the TFBC bridging patch technique vs the traditional repair method in an in vitro model. We hypothesized that rotator cuff repair with the TFBC patch would have better mechanical properties immediately after repair. If confirmed, these results would provide preliminary evidence supporting in vivo evaluation of tendon healing using this technique.
2. MATERIAL AND METHODS
2.1 Rotator Cuff Repair
Twenty shoulders and 10 TFBC were harvested from 10 mixed-breed dogs that were euthanized for other studies approved by the Mayo Clinic Institutional Animal Care and Use Committee. All muscle attachments, except for the infraspinatus tendon and the infraspinatus muscle, were detached from the humerus for each shoulder. Ten composites of the patella and patellar tendon also were harvested. Each patellar tendon was trimmed to 10×15 mm, with its bony attachment segment intact. Because the tendon was soft and slippery, it could not be securely held to be cut while not compressing the tendon. Tissue freezing medium (Tissue-Tek; Sakura, Inc., Torrance, CA) was used to embed and to fix the tendon. The patellar tendons were then cut horizontally into 2 layers. Rotator cuff tears were consistently created according to a previously described model15; briefly, the anterior and posterior extent of the infraspinatus tendon were identified and then the tendon was sharply detached in its entirety from the bone surface.
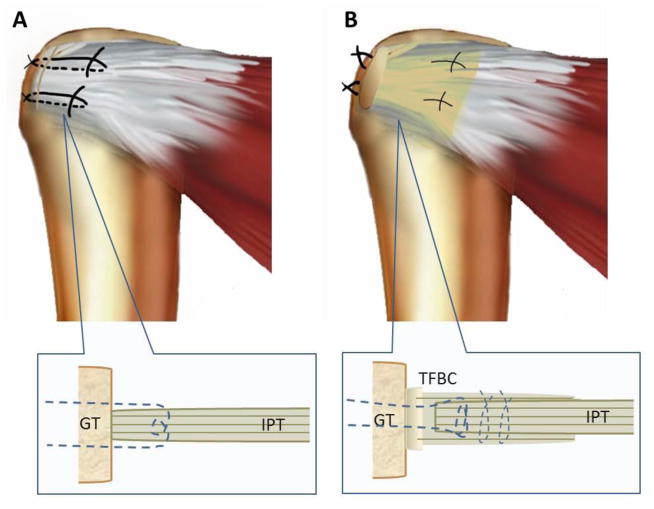
For rotator cuff repair, 10 specimens were randomly assigned to the TFBC group and 10 to the control group. A schematic of the repairs is shown in Figure 1. In the TFBC group, the infraspinatus tendon was inserted between patella tendon layers. Modified Mason-Allen sutures of polyester braid and long-chain polyethylene (Fiberwire #2; Arthrex, Inc., Naples, FL) were used to repair the patellar tendon to the greater tuberosity. Two parallel loops were sewn through the full thickness of the sandwich-like tendon interface with 3–0 polyglactin sutures (Vicryl; Ethicon, Inc., Bridgewater, NJ). The bone fragment of the TFBC was fixed to the attachment point of the infraspinatus tendon using a metal wire threaded through 2 bone tunnels introduced into the humeral head. For the control group, the infraspinatus tendons were repaired with modified Mason-Allen sutures of Fiberwire #2 sutures through 2 bone tunnels.
Figure 1.
A Repair with the Mason-Allen technique (control). B) Repair with the TFBC patch.) GT indicates greater tuberosity; IPT, infraspinatus tendon; TFBC, tendon-fibrocartilage-bone composite. (By permission of Mayo Foundation for Medical Education and Research. All rights reserved.)
2.2 Mechanical Testing
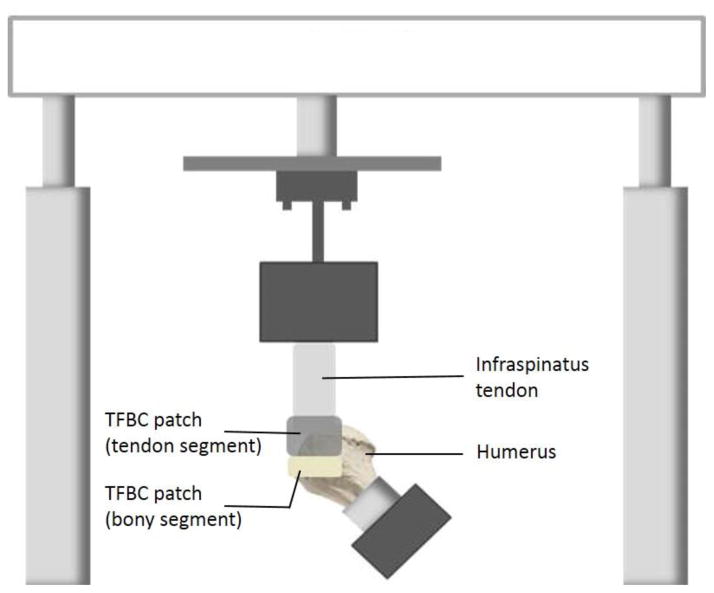
After rotator cuff repair, the specimen was mounted on a servohydraulic test machine (858 MiniBionix II; MTS Systems Corp, Eden Prairie, MN) for mechanical evaluation. A custom-made clamp gripped the infraspinatus muscle. The humerus was potted into a small block of polymethyl methacrylate, mounted, and positioned at an incline of 135° to the long axis of the tendon to model the physiologic pull of the infraspinatus tendon. For TFBC specimens, markers were placed on the greater tuberosity, the bony part of the TFBC, and the infraspinatus tendon (Figure 2). For control specimens, markers were placed on the greater tuberosity and the infraspinatus tendon (Figure 3).
Figure 2.
Diagram of specimen mounting for TFBC patches.
Figure 3.

Diagram of gap formation after mechanical testing. Repair A) with the Mason-Allen technique. A “Gap” can form between the IPT and GT. B) Repair with TFBC patch. “Gap 1” can form at the TFBC-GT repair site. “Gap 2” can form at the TFBC-IPT repair site. The asterisks indicate marker locations. GT indicates greater tuberosity; IPT, infraspinatus tendon; TFBC, tendon-fibrocartilage-bone composite.
Each repaired rotator cuff specimen was loaded to failure under displacement control at a rate of 0.5mm/sec. Load and actuator displacement were recorded at a sample rate of 50 Hz. Ultimate tensile load was defined as the peak force observed during loading. Each specimen’s failure mechanism (i.e., suture breakage, suture pullout through tendon, or bone tunnel breakage) was also recorded.
To assess local deformation for stiffness calculations, specimen loading was video recorded (frame rate, 29 frames/second) throughout the testing. Videos were processed with image-analysis software (Analyze 11.0; Mayo Clinic) to measure marker displacement and thereby determine displacement between the TFBC and greater tuberosity and between the TFBC and infraspinatus tendon. Stiffness was calculated from the slope of the linear region of the load-displacement curve.
2.3 Statistical Methods
All data are presented as the mean (SD). The ultimate tensile loads for the two methods of rotator cuff repair were compared using the Student’s t test. One-way ANOVA with a post hoc test was used to compare the stiffness at different regions. Statistical significance was set at P<.05.
To our knowledge, no prior study has reported using this novel model to reconstruct the rotator cuff, and therefore, no related data were available for sample size determination. Given this constraint, a power analysis was conducted to estimate the size of differences that could be detected with this sample size. Ma et al.16 reported the same outcomes in a biomechanical study of rotator cuff repair techniques. Using the formula (SD: standard deviation, SE: standard error, n: sample size), the standard deviations of ultimate tensile load and stiffness were estimated to be 75.9 and 53.8, respectively. If the variability in this proposed study was similar, then a sample of 20 shoulders (10 specimens per group) would provide 80% power to detect differences of at least 100.3 N in ultimate tensile load and 71.5 N/mm in stiffness.
3. RESULTS
No suture breakage was observed during testing in either group. For the TFBC group, failure in 8 specimens was due to suture pullout from the sandwich-like tendon complex. Bone tunnel breakage at the bony part of the TFBC patch was observed in 2 specimens, 1 of which was associated with suture pullout. For the control group, all 10 specimens failed by suture pullout from the tendon.
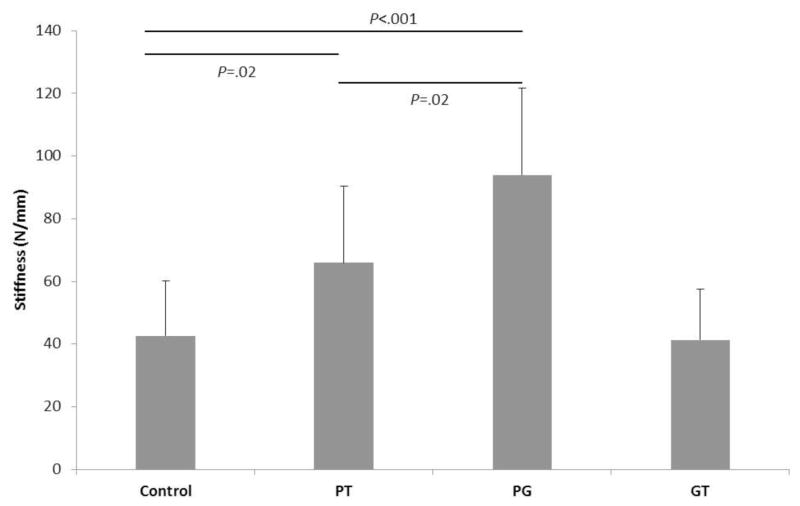
Ultimate tensile load was significantly higher in the TFBC group than the control group (mean [SD], 365.46 [36.45] vs 272.79 [48.88] N; P<.001) (Figure 4). The overall stiffness for the TFBC repair composite and conventional repair were not significantly different (41.29 [16.37] vs 42.62 [17.48] N/mm; P=.43) (Figure 5). However, stiffness at the TFBC–greater tuberosity repair site (93.96 [27.72] N/mm) was significantly higher than the control repair site (infraspinatus tendon to greater tuberosity) (P<.001), and the stiffness at the TFBC–infraspinatus tendon repair site (65.94 [24.51] N/mm) was also significantly higher than the control repair site (P=.02). The stiffness at the TFBC–greater tuberosity repair site was significantly higher than the TFBC–infraspinatus tendon repair site (P=.02).
Figure 4.

Ultimate tensile load after tendon-fibrocartilage-bone composite (TFBC) or control repair.
Figure 5.
Overall stiffness after tendon-fibrocartilage-bone composite or control repair. PT indicates stiffness of the TFBC repaired construct; GT, stiffness at the TFBC-greater tuberosity repair site; PG, stiffness at the TFBC-infraspinatus tendon repair site.
4. DISCUSSION
Two major factors have been recognized as contributing to the poor healing outcomes of rotator cuff repair. The first is the slow healing process at the tendon-to-bone interface. Native bone insertions have a unique fibrocartilage transition zone, which effectively limits flexibility and reduces stress concentration through its gradient structure, composition, and mechanical behavior13. However, numerous experimental studies have shown that this transition zone from tendon to bone is difficult to rebuild, thus leading to a repair weakness, bone resorption, and matrix degradation10; 11; 17. The second factor is that rotator cuff injury is accompanied by combined chronic tendon and muscle degeneration. Because of long-term tendon retraction, high tension occurs after tendon repair, especially for massive rotator cuff tears. Such constant tensile loading may cause repair gap formation, which prolongs tendon-to-bone healing12; 18 and is a precursor of possible future repair failures19; 20. Thus, not only does the tendon-to-bone interface not regenerate after repair, but the mechanical strength of the replacement scar tissue is lower than the native interface4; 21. Although various methods, including growth factor regulation, use of biological agents, cell therapy, gene therapy, and tissue engineering approaches have been applied to improve tendon-to-bone healing, mechanical properties of the repaired tendon are still inferior to those of normal tissues because of the impaired healing between dissimilar tissues22–24. Better methods need to be identified to reduce the retear rate of rotator cuff repair. Therefore, TFBC augmentation for rotator cuff repair not only increase the repair strength to reduce gap formation but also transplant the native fibrocartilage zone, thus converting tendon/bone healing interface into tendon/tendon and bone/bone uniform healing interface with a fibrocartilage zone in between as the Figure 3 illustrated.
Unlike tendon-to-bone healing, bone-to-bone healing is widely accepted to be strong and relatively fast25. Based on this idea, the BPTB graft is considered the best option for ACL reconstruction because it uses a bone-tendon-bone approach, which transforms the weaker tendon-to-bone healing into stronger bone-to-bone healing14. Because the BPTB graft is anchored on both sides of the bone, its biomechanical properties equal or exceed those of the native ACL. BPTB grafts can also achieve failure rates as low as6.1% to 13% and have a fast incorporating time of 4 to 6 weeks26; 27. ABPTB graft provides a bone-to-bone healing interface to promote fast and secure ACL reconstruction28.
In the current study, we designed a novel approach to rotator cuff repair based on the aforementioned concept. To transform the tendon-to-bone interface into a homogeneous tissue healing interface (i.e., tendon-to-tendon and bone-to-bone), allograft augmentation with a patellar TFBC was used. This augmentation can also increase repair strength by adopting techniques that decrease gap formation, i.e., from tendon-to-bone repair to tendon-to-tendon and bone-to-bone repairs. The results of this study support the hypothesis that the rotator cuff repair with the TFBC patch provides significantly higher ultimate tensile load to failure compared with the traditional Mason-Allen repair, which may lead to fewer postoperative failures for a given level of activity. We observed no significant differences in the overall stiffness of the TFBC repair and control repair. The stiffness of TFBC repair was influenced by 2 components, displacement between the infraspinatus tendon and the TFBC patch and displacement between the TFBC patch and greater tuberosity. Both parts showed higher stiffness compared with the simple Mason-Allen repair.
We believe that the novel structure of the TFBC patch may improve healing of the repaired rotator cuff. First, because both sides of the infraspinatus tendon are covered by the patellar tendon, this telescoping structure guarantees contact between the 2 tendon tissues, provided that the relative displacement does not exceed the length of patellar tendon (Figure 5). This setup may be able to diminish potential gap formation in the healing process from infraspinatus tendon retraction, which contributes to postoperative failure with current repair methods29; 30. Second, the higher stiffness at the TFBC–greater tuberosity repair site (i.e., the shorter displacement compared with the traditional repair) provides a more secure environment for tissue healing. In our study, all traditionally repaired specimens failed by suture pullout from the tendon, indicating that the suture-tendon interface is a weak point for repair failure. However, among the specimens repaired with TFBC patches, 2 failed by bone tunnel breakage, indicating that the TFBC patch enhanced suture retention. Third, bone-to-bone and tendon-to-tendon healing are widely accepted to be relatively easier and faster to rebuild than tendon-to-bone healing25; 31. The native transitional zone from tendon to bone is composed of fibrocartilage tissue, which reduces the stress concentration between the 2 different tissue types32; 33. Our approach converted this difficult healing interface into 2 homogeneous healing interfaces.
A reasonable conclusion is that the conversion of tissue interfaces likely will achieve better and faster postoperative healing. In addition, the TFBC–greater tuberosity repair site was associated with higher stiffness, and creation of the telescoping structure between TFBC and infraspinatus tendon assured appropriate contact between tendons. In the future, engineered tendons with transplanted cells are expected to accelerate the healing process.
We acknowledge several limitations in this study. Shoulders harvested from dogs were not paired and assigned to separate TFBC and control groups, which would have provided stronger statistical power. The use of healthy rotator cuff specimens rather than degenerative tendons did not realistically mimic the scenario of rotator cuff repair. However, the TFBC bridging patch may be even better for degenerative tendons because it increases the strength at the tendon-suture interface. Finally, this study was exclusively performed in vitro. Although the concept of altering the healing interface has the potential to improve healing, it must first be verified in vivo.
5. CONCLUSIONS
In conclusion, we have designed a novel TFBC scaffold for rotator cuff augmentation. The results of the current study indicate that the TFBC augmentation could achieve higher ultimate tensile load and higher stiffness at the TBFC–greater tuberosity repair site compared with the traditional Mason-Allen rotator cuff repair in a canine model. Future in vivo studies can be conducted to compare the effects of the TFBC bridging patch vs traditional methods on rotator cuff healing.
Highlights.
Novel tendon-fibrocartilage-bone composite bridging patch for rotator cuff repair
Transforming traditional tendon-bone healing to tendon-tendon and bone-bone healing
Higher ultimate tensile of bridging patch compared with traditional repair
Acknowledgments
This study was supported by a grant from NIH/NIAMS (AR57745); study was funded by Mayo Foundation.
Abbreviations
- ACL
anterior cruciate ligament
- BPTB
bone–patellar tendon–bone
- TFBC
tendon-fibrocartilage-bone composite
Footnotes
The authors have no conflicts of interest to declare.
Conflict of Interest Statement
The authors have no conflicts of interest related to the work presented in this manuscript. The funding resource had no involvement in the study design, the collection, analysis or interpretation of the data, nor in the writing or decision to submit the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iannotti JP, Deutsch A, Green A, et al. Time to failure after rotator cuff repair: a prospective imaging study. J Bone Joint Surg Am. 2013;95:965. doi: 10.2106/JBJS.L.00708. [DOI] [PubMed] [Google Scholar]
- 2.Kim KC, Shin HD, Cha SM, et al. Repair integrity and functional outcomes for arthroscopic margin convergence of rotator cuff tears. J Bone Joint Surg Am. 2013;95:536. doi: 10.2106/JBJS.L.00397. [DOI] [PubMed] [Google Scholar]
- 3.Galatz LM, Ball CM, Teefey SA, et al. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86-A:219. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Lubiatowski P, Kaczmarek P, Dzianach M, et al. Clinical and biomechanical performance of patients with failed rotator cuff repair. Int Orthop. 2013;37:2395. doi: 10.1007/s00264-013-2024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park JY, Lhee SH, Oh KS, et al. Clinical and ultrasonographic outcomes of arthroscopic suture bridge repair for massive rotator cuff tear. Arthroscopy. 2013;29:280. doi: 10.1016/j.arthro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Boyer MI, Ditsios K, Gelberman RH, et al. Repair of flexor digitorum profundus tendon avulsions from bone: an ex vivo biomechanical analysis. J Hand Surg Am. 2002;27:594. doi: 10.1053/jhsu.2002.33708. [DOI] [PubMed] [Google Scholar]
- 7.Lim JK, Hui J, Li L, et al. Enhancement of tendon graft osteointegration using mesenchymal stem cells in a rabbit model of anterior cruciate ligament reconstruction. Arthroscopy. 2004;20:899. doi: 10.1016/j.arthro.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 8.Martinek V, Latterman C, Usas A, et al. Enhancement of tendon-bone integration of anterior cruciate ligament grafts with bone morphogenetic protein-2 gene transfer: a histological and biomechanical study. J Bone Joint Surg Am. 2002;84-A:1123. doi: 10.2106/00004623-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Tien YC, Chih TT, Lin JH, et al. Augmentation of tendon-bone healing by the use of calcium-phosphate cement. J Bone Joint Surg Br. 2004;86:1072. doi: 10.1302/0301-620x.86b7.14578. [DOI] [PubMed] [Google Scholar]
- 10.Carofino B, Fulkerson J. Medial hamstring tendon regeneration following harvest for anterior cruciate ligament reconstruction: fact, myth, and clinical implication. Arthroscopy. 2005;21:1257. doi: 10.1016/j.arthro.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Deehan DJ, Cawston TE. The biology of integration of the anterior cruciate ligament. J Bone Joint Surg Br. 2005;87:889. doi: 10.1302/0301-620X.87B7.16038. [DOI] [PubMed] [Google Scholar]
- 12.Park MC, Bui C, Park CJ, et al. Rotator cuff tendon repair morphology comparing 2 single-anchor repair techniques. Arthroscopy. 2013;29:1149. doi: 10.1016/j.arthro.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Benjamin M, Ralphs JR. Fibrocartilage in tendons and ligaments--an adaptation to compressive load. J Anat. 1998;193(Pt 4):481. doi: 10.1046/j.1469-7580.1998.19340481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hospodar SJ, Miller MD. Controversies in ACL reconstruction: bone-patellar tendon-bone anterior cruciate ligament reconstruction remains the gold standard. Sports Med Arthrosc. 2009;17:242. doi: 10.1097/JSA.0b013e3181c14841. [DOI] [PubMed] [Google Scholar]
- 15.Smith MJ, Cook JL, Kuroki K, et al. Comparison of a novel bone-tendon allograft with a human dermis-derived patch for repair of chronic large rotator cuff tears using a canine model. Arthroscopy. 2012;28:169. doi: 10.1016/j.arthro.2011.08.296. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y, Berger EJ, Zhao C, et al. Mapping lubricin in canine musculoskeletal tissues. Connect Tissue Res. 2006;47:215. doi: 10.1080/03008200600846754. [DOI] [PubMed] [Google Scholar]
- 17.Aune AK, Hukkanen M, Madsen JE, et al. Nerve regeneration during patellar tendon autograft remodelling after anterior cruciate ligament reconstruction an experimental and clinical study. J Orthop Res. 1996;14:193. doi: 10.1002/jor.1100140205. [DOI] [PubMed] [Google Scholar]
- 18.Reilly P, Bull AM, Amis AA, et al. Passive tension and gap formation of rotator cuff repairs. J Shoulder Elbow Surg. 2004;13:664. doi: 10.1016/j.jse.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Rossouw DJ, McElroy BJ, Amis AA, et al. A biomechanical evaluation of suture anchors in repair of the rotator cuff. J Bone Joint Surg Br. 1997;79:458. doi: 10.1302/0301-620x.79b3.6983. [DOI] [PubMed] [Google Scholar]
- 20.Shea KP, Obopilwe E, Sperling JW, et al. A biomechanical analysis of gap formation and failure mechanics of a xenograft-reinforced rotator cuff repair in a cadaveric model. J Shoulder Elbow Surg. 2012;21:1072. doi: 10.1016/j.jse.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 21.Jo CH, Shin JS, Park IW, et al. Multiple Channeling Improves the Structural Integrity of Rotator Cuff Repair. Am J Sports Med. 2013;41:2650. doi: 10.1177/0363546513499138. [DOI] [PubMed] [Google Scholar]
- 22.Chen CH, Chen WJ, Shih CH, et al. Enveloping the tendon graft with periosteum to enhance tendon-bone healing in a bone tunnel: A biomechanical and histologic study in rabbits. Arthroscopy. 2003;19:290. doi: 10.1053/jars.2003.50014. [DOI] [PubMed] [Google Scholar]
- 23.Chen CH, Liu HW, Tsai CL, et al. Photoencapsulation of bone morphogenetic protein-2 and periosteal progenitor cells improve tendon graft healing in a bone tunnel. The American journal of sports medicine. 2008;36:461. doi: 10.1177/0363546507311098. [DOI] [PubMed] [Google Scholar]
- 24.Rodeo SA, Suzuki K, Deng XH, et al. Use of recombinant human bone morphogenetic protein-2 to enhance tendon healing in a bone tunnel. The American journal of sports medicine. 1999;27:476. doi: 10.1177/03635465990270041201. [DOI] [PubMed] [Google Scholar]
- 25.Guerrero TG, Makara MA, Katiofsky K, et al. Comparison of healing of the osteotomy gap after tibial tuberosity advancement with and without use of an autogenous cancellous bone graft. Vet Surg. 2011;40:27. doi: 10.1111/j.1532-950X.2010.00772.x. [DOI] [PubMed] [Google Scholar]
- 26.Stensbirk F, Thorborg K, Konradsen L, et al. Iliotibial band autograft versus bone-patella-tendon-bone autograft, a possible alternative for ACL reconstruction: a 15-year prospective randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2013;22:2094. doi: 10.1007/s00167-013-2630-9. [DOI] [PubMed] [Google Scholar]
- 27.Sun K, Tian S, Zhang J, et al. Anterior cruciate ligament reconstruction with BPTB autograft, irradiated versus non-irradiated allograft: a prospective randomized clinical study. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2009;17:464. doi: 10.1007/s00167-008-0714-8. [DOI] [PubMed] [Google Scholar]
- 28.Barrett G, Stokes D, White M. Anterior cruciate ligament reconstruction in patients older than 40 years: allograft versus autograft patellar tendon. The American journal of sports medicine. 2005;33:1505. doi: 10.1177/0363546504274202. [DOI] [PubMed] [Google Scholar]
- 29.Kim JR, Cho YS, Ryu KJ, et al. Clinical and radiographic outcomes after arthroscopic repair of massive rotator cuff tears using a suture bridge technique: assessment of repair integrity on magnetic resonance imaging. Am J Sports Med. 2012;40:8. doi: 10.1177/0363546511434546. [DOI] [PubMed] [Google Scholar]
- 30.Khazzam M, Kuhn JE, Mulligan E, et al. Magnetic resonance imaging identification of rotator cuff retears after repair: interobserver and intraobserver agreement. The American journal of sports medicine. 2012;40:1722. doi: 10.1177/0363546512449424. [DOI] [PubMed] [Google Scholar]
- 31.Lui P, Zhang P, Chan K, et al. Biology and augmentation of tendon-bone insertion repair. J Orthop Surg Res. 2010;5:59. doi: 10.1186/1749-799X-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Genin GM, Kent A, Birman V, et al. Functional grading of mineral and collagen in the attachment of tendon to bone. Biophys J. 2009;97:976. doi: 10.1016/j.bpj.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomopoulos S, Marquez JP, Weinberger B, et al. Collagen fiber orientation at the tendon to bone insertion and its influence on stress concentrations. J Biomech. 2006;39:1842. doi: 10.1016/j.jbiomech.2005.05.021. [DOI] [PubMed] [Google Scholar]