Abstract
Platelet-derived growth factor receptor-β (PDGFRβ) is expressed in the brain by vascular mural cells - brain capillary pericytes and arterial vascular smooth muscle cells (VSMCs). Recent evidence shows that blood-brain barrier (BBB) disruption and increased permeability, especially in the hippocampus, positively correlates with elevated levels of soluble PDGFRβ (sPDGFRβ) in cerebrospinal fluid (CSF) in patients with mild dementia. To determine which vascular cell type(s) contributes to increased sPDGFRβ in CSF, we compared PDGFRβ expression and sPDGFRβ shedding in response to injury in early passage primary cultures of human brain pericytes, brain arterial VSMCs, and brain endothelial cells. PDGFRβ protein was undetectable in endothelial cells, but was found both in pericytes and VSMCs. PDGFRβ relative protein abundance was by 4.2-fold (p < 0.05) higher in pericytes compared to VSMCs. Hypoxia (1% O2) or amyloid-β peptide (25 μM) compared to normoxia (21% O2) both increased over 48 h shedding of sPDGFRβ and its levels in the culture medium from pericytes cultures, but not from VSMCs cultures, by 4.3-fold and 4.6-fold, respectively, compared to the basal sPDGFRβ levels in the medium (1.43 ± 0.15 ng/ml). This was associated with the corresponding loss of cell-associated PDGFRβ from pericytes and no change in cellular levels of PDGFRβ in VSMCs. Thus, sPDGFRβ is a biomarker of pericyte injury, and elevated sPDGFRβ levels in biofluids in patients with dementia and/or other neurodegenerative disorders likely reflects pericyte injury, which supports the potential for sPDGFRβ to be developed and validated as a biomarker of brain pericyte injury and BBB dysfunction.
Keywords: Soluble platelet-derived growth factor receptor-β, Hypoxia, Pericytes, Vascular smooth muscle cells
1. Introduction
Increasing evidence supports that neurovascular dysfunction contributes to several neurodegerative disorders [1,2]. Proper neuronal functioning is accomplished in parallel with the brain’s cerebrovascular network. This highly coordinated interaction is functionally defined as the neurovascular unit (NVU), consisting of vascular cells (i.e., perivascular pericytes, vascular smooth muscle cells (VSMCs), endothelial cells), glial cells, and neurons [1]. Within the NVU, brain microvascular endothelial cells and pericytes form the blood-brain barrier (BBB), which restricts entry of blood-derived neurotoxic molecules and cells into the brain from peripheral circulation [1,3]. Pericytes are crucial for maintaining BBB integrity [4–6]. Recent studies in humans and animal models have shown that brain capillary pericyte dysfunction and/or degeneration correlates and/or results in BBB breakdown, which in turn may contribute to neurovascular dysfunction and pathogenesis of complex neurological disorders such as Alzheimer’s disease (AD) and dementia [7–10], diabetes-related microangiopathy and retinopathy [11,12], amyotrophic lateral sclerosis (ALS) [13,14], cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) [15], neurovascular dysplasia and seizures [16], as recently reviewed [17].
Pericytes and VSMCs are vascular mural cells that ensheath the endothelium of brain capillaries, and small arteries and arterioles, respectively [18]. Recruitment of pericytes and VSMCs to developing CNS vessels is critical for vascular stability and is achieved in part by signal transduction mediated by platelet-derived growth factor (PDGF)-BB secreted by endothelial cells and PDGF receptor-β (PDGFRβ) in mural cells [18,19]. PDGFRβ is highly expressed by pericytes and VSMCs during development [18,19], however cell type-specific PDGFRβ expression in the adult brain is less well understood. Unlike some murine strains where PDGFRβ expression was found to co-localize in the adult brain predominantly in pericytes as in 129S1/SvlmJ mice [20], PDGFRβ expression in the human adult brain is reported in both VSMCs and pericytes [21].
In addtion to specific location in the cerebral vascular tree within brain capillaries, brain pericytes are also identified by their expression of various contractile and cytoskeletal proteins and cell surface antigens including PDGFRβ, but generally these biomarkers have overlapping pattern of expression with VSMCs and sometime other non-mural cell types [21]. To date, it has proven difficult to definitively identify brain pericytes based solely on biomarkers, thus limiting the ability to evaluate the mechanism and degree to which injury to the brain affects specifically pericytes and/or evaluate pericytes’ response to different types of injury. To our knowledge, the relative levels of PDGFRβ expression and sPDGFRβ shedding and/or release of soluble sPDGRFβ from human brain pericytes and brain arterial VSMCs in response to divergent inducers of injury have not been compared. Thus, the goal of the present study was to elucidate which mural cell type(s) is primarily responsible for sPDGFRβ shedding after cell injury, and to inform sPDGFRβ’s ability to specifically reflect subtle microvascular insults in the brain. To address this question, we studied first PDGFRβ expression levels in cultured primary human brain pericytes and brain arterial VSMCs, and next PDGFRβ shedding and the release of soluble sPDGFRβ from these two cell types in response to hypoxia and amyloid-β (Aβ) peptide-mediated cell injury. Our findings support the potential for sPDGFRβ to be developed and validated as a sensitive, indicative biomarker of brain capillary pericyte injury response in the brain.
2. Materials and methods
2.1. Human brain endothelial cell (BEC) cultures
BECs, as well as VSMCs and pericytes were harvested from rapid brain autopsies (<3 h) of young neurologically normal individuals with no vascular risk factors (average age 31.2 years) following motor vehicle accidents, under an approved Western Instituitional Review Board (WIRB) protocol, as we previously described [22,23].
BECs were isolated from the frontal cortex (Brodmann’s areas 9/10) as we previously described [24,25]. Cells were cultured in human endothelial cell medium (Catalog no. 1201, Cell Biologics, Chicago, IL) in 5% CO2 at 37°C and then characterized. BEC were > 98% positive for endothelial markers Factor VIII and CD105, and negative for CD11b (monocyte/microglia), glial fibrillar acidic protein (GFAP) (astrocytes), and α-actin (vascular smooth muscle) [24,25]. Early passage (P2-P4) cultures were used in the present study.
2.2. Human vascular smooth muscle cell (VSMCs) cultures
VSMCs were isolated from leptomeningeal arteries (>100 μm diameter), as described earlier [22,26]. Pial arterial VSMCs were isolated and characterized as reported [27]. VSMCs were >98% positive for vascular smooth muscle cell α-actin, myosin heavy chain, calponin and SM22α and negative for von Willebrand factor (endothelial cells), GFAP (astrocytes) and CD11b (microglia). Cells were cultured in smooth muscle cell medium (Catalog no. 1101, ScienCell, Carlsbad, CA) in 5% CO2 at 37°C. Early passage (P2-P4) cultures were used in the present study.
2.3. Human brain microvascular pericyte cultures
Pericytes were isolated from cortical brain tissue after removal of leptomeninges as previously described [28]. Pericytes were derived from intraparenchymal microvessels that were completely free from leptomeningeal vessel contamination. Purified microvessels were largely brain capillaries (>97%) with diameter ≤6 μm. Cells were cultured in human pericyte medium (Catalog no. 1201, ScienCell) in 5% CO2 at 37°C and were then characterized. Pericytes were positive for the pericyte markers PDGFRβ, NG2 and CD13 and negative for von Willebrand factor (endothelial cells), GFAP (astrocytes) and CD11b (microglia). Early passage (P2-P4) cultures were used in the present study.
2.4. Hypoxia or Aβ treatment
Human VSMCs and pericytes, cultured in 6-well culture plates (Thermo Scientific), were subjected to either hypoxia (1% O2, 5% CO2 at 37°C for 48 h) in modular chambers (Billups-Rothenberg, Inc., Del Mar, CA) [9,22] or monomeric Aβ40 (25 μM for 48 h), or cultured under normoxia conditions (21% O2, 5% CO2 at 37°C for 48 h), as we described [9,29]. Soluble monomeric Aβ40 peptide was prepared as previously described [30,31] and characterized via SDS/PAGE gel electrophoresis. The shedded sPDGFRβ in the culture medium and PDGFRβ in cell lysates were determined by Western blot analysis as described below. These experiments were performed in three independent cultures from three different donors per cell type in triplicate.
2.5. Immunoblotting
Cells were lysed in radioimmunoprecipitation assay (RIPA) buffer containing 50mM Tris, pH 8.0, 150 mM NaCl, 0.1% SDS, 1% NP-40, 0.5% sodium deoxycholate and protease inhibitor cocktail (Roche). Protein concentration in cell lysate was determined with a BCA protein assay kit (Thermo Scientific, Rockford, IL). 10 μg proteins were subjected to 4–12% Bis-Tris SDS/PAGE gel electrophoresis (Thermo Scientific) for 1–2h at 150V, and subsequently transferred to a nitrocellulose membrane. The membrane was blocked for 1 h with superblock blocking buffer (Catalog no. 37537, Thermo Scientific). The membrane was incubated with primary antibodies for PDGFRβ (AF1042, R&D Systems, Inc., Minneapolis, MN) and β-actin (Catalog no. 4970, Cell Signaling Technology®, Danvers, MA). Membranes were incubated with appropriate HRP-conjugated secondary antibodies (1:5000 dilution, Thermo Scientific), treated with Western ECL detection solution (Catalog no. 34075, Thermo Scientific), exposed to CL-Xposure film (Catalog no. 34091, Thermo Scienctific), and developed in an X-Omat 3000 RA film processor (Kodak). Images were acquired, and densitometry analysis was performed using NIH Image J software. The signal intensity of protein bands were normalized to β-actin to control for variability in loading of individual samples. These experiments were performed in six independent cultures for six different donors per cell type in triplicate.
2.6. Immunocytochemistry
Immunostaining analysis was conducted for PDGFRβ protein expression. Cultured endothelial cells, VSMCs and pericytes were fixed with 4% paraformaldehyde and subsequently were permeabilized and blocked with 10% donkey serum in phosphate buffered saline and 0.1% Triton X-100 (Sigma) for 1 h. Cells were stained with a PDGFRβ-specific antibody (1:100, Catalog no. AF385, R&D Systems) and Alexa Fluor® 568 donkey anti-goat secondary IgG (1:500, Catalog no. A11057, Thermo Scientific). Nuclei were stainied with DAPI (Catalog no. 0100-20, DAPI Fluoromount, Southern Biotech, Birmingham, AL). Images were scanned using the BZ 9000 fluorescence microscope (Keyence, Osaka, Japan) and analyzed using NIH Image J software.
2.7. sPDGFRβ analysis
PDGFRβ antibody (goat anti-mouse cross reacts with human, Catalog no. AF1042, R&D Systems, Inc.) was bound to protein-G coupled Dynabeads® (Catalog no. 100.07D, Invitrogen Dynal AS, Oslo, Norway), and immunoprecipitation was performed on the pericyte and VSMCs conditioned medium as described by the manufacturer. Protein concentration (ng/ml) was determined by quantitative Western blot analysis using carrier-free human recombinant PDGFRβ as a protein standard (Catalog no. 385-PR/CF, R&D Systems, Inc.).
2.8. RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
Human VSMCs and pericytes were cultured in 6-well culture plates (Thermo Scientific) until they reached confluency. RNA extraction was performed using a total RNA extraction kit (RNAeasy® Mini Kit, Catalog no. 74104, Qiagen, Germany) as per manufacturer’s instructions. RNA concentrations were determined using a NanoDrop™ Spectrophotometer (Thermo Scientific). Total RNA (200 ng) was used to generate cDNA (QuantiTect Reverse Transcriptase Kit, Catalog no. 205311, Qiagen, Germany). PCR reactions were carried out in a final volume of 20 μl containing 10 μl PerfeCTa® SYBR® Green SuperMix (Quanta BioSciences, Inc., Catalog no. 95054), 1 μl cDNA, and 5 μM forward and 5 μM reverse human primers (hPDGFRβ-F GTGCTCACCATCATCTCCCT; hPDGFRβ-R ACTCAATCACCTTCCATCGG; hGAPDH-F CGACCACTTTGTCAAGCTCA; hGAPDH-R AGGGGTCTACATGGCAACTG). Samples were run in triplicate and PCR was performed on a CFX Connect™ Real-Time System (Bio-Rad). Cq values were obtained and the ratio of PDGFRβ:GAPDH expression was calculated. Pericyte PDGFRβ expression was normalized to 1.0, and PDGFRβ mRNA relative abundance was plotted for brain pericytes and VSMCs.
2.9. Statistical analysis
sPDGFRβ levels and PDGFRβ protein or mRNA levels in differenet cell types were compared by Student’s t-test or one-way analysis of variance (ANOVA). Significance was determined at a 2-sided α = 0.05. All values are plotted as mean ± SEM.
3. Results
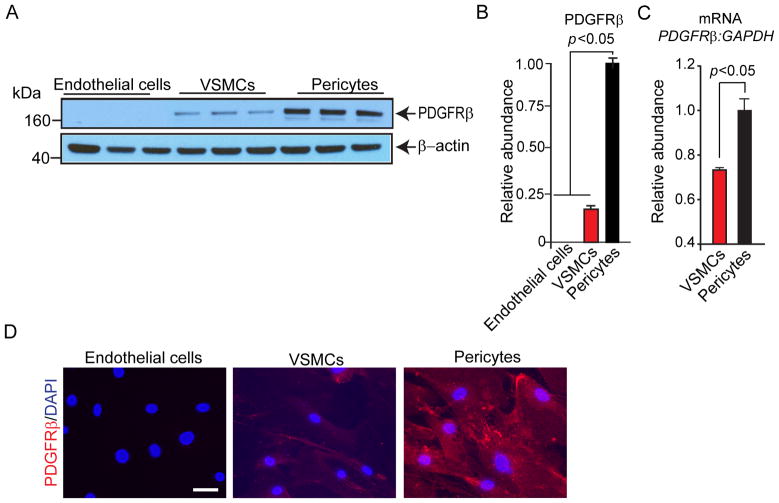
Immunobotting analysis failed to detect PDGFRβ in cultured primary human brain endothelial cells early passage P2-P4 (Fig. 1A and B) consistent with previous reports in mouse models suggesting no expression of PDGFRβ in brain endothelial cells [20, 32]. In contrast, PDGFRβ was abundantly expressed in primary cultured human pericytes early passage P2-P4. Quantitative Western blot analysis indicated that the relative PDGFRβ protein abundance normalized to β-actin was approximately 4.2-fold higher in cultured human pericytes P2-P4 compared to cultured human VSMCs P2-P4 (Fig. 1A and B). The higher relative levels of PDGFRβ expression in cultured human pericytes compared to VSMCs were confirmed by qRT-PCR analysis for PDGFRβ mRNA normalized to GAPDH (Fig. 1C). Representative immunostatining for PDGFRβ in endothelial cells, pericytes, and VSMCs corroborated the Western blot and qRT-PCR results (Fig. 1D).
Fig. 1. Abundant PDGFRβ expression in cultured primary human brain pericytes compared to other vascular cell types.
(A and B) Immunoblotting for PDGFRβ and β-actin (A) and relative abundance of PDGFRβ protein levels compared to β-actin (B) in cultured primary human brain endothelial cells, brain arterial vascular smooth muscle cells (VSMCs), and brain pericytes by Western blot analysis. (C) Quantitative real-time polymerase chain reaction (qRT-PCR) analysis of PDGFRβ mRNA levels in VSMCs and pericytes. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as an internal control. (C) Representative images of PDGFRβ immunostaining in cultured primary human brain endothelial cells, arterial VSMCs, and brain pericytes; scale bar, 20 μm. In B and C, means ± s.e.m. from 6 independent cultures from 6 donors in triplicate. P<0.05, significance by Student’s t-test (B and C).
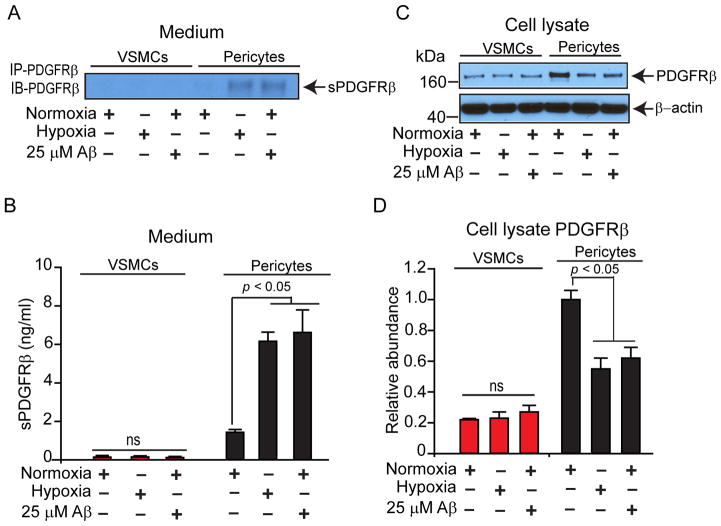
In the pericyte culture medium, the levels of shedded soluble form of PDGFRβ, sPDGFRβ, were significantly increased by 4.3-fold following treatment with severe hypoxia (1% O2) for 48 h (6.16 ± 0.47 ng/ml, p < 0.05) compared to the basal levels under normoxia (21% O2) for 48 h (1.43 ± 0.15 ng/ml), and by 4.6-fold after incubation with 25 μM Aβ40 under normoxia (6.62 ± 1.17 ng/ml, p < 0.05) compared to the basal levels under normoxia for 48 h (Fig. 2A-B). The basal levels of sPDGFRβ in VSMCs cultures under normoxic conditions were barely detectable, and approximately by 11-fold lower than in pericyte cultures. In contrast to pericyte cultures, no significant shedding of sPDGFRβ was detectable in VSMCs culture medium following treatment with severe hypoxia (0.14 ± 0.05 ng/ml) compared to normoxic conditions (0.13 ± 0.03 ng/ml) or after incubation with 25 μM Aβ40 (0.11 ± 0.06 ng/ml) compared to normoxia for 48 h (Fig. 2A and B). Consistent with these findings, the cell-associated un-shedded PDGFRβ levels determined in the cell lysates of pericyte cultures relative to β-actin were significantly decreased following hypoxia by 45% (p < 0.05) compared to normoxia, and were similarly decreased after incubation with 25 μM Aβ40 for 48 h (Fig. 2C and D). No differences in PDGFRβ levels were found in VSMCs cell lysates under different experimental conditions (Fig. 2C and D).
Fig. 2. Hypoxia and amyloid-β peptide (Aβ) lead to shedding of soluble platelet-derived growth factor receptor-β (sPDGFRβ) from cultured primary human brain pericytes, but not from brain arterial vascular smooth muscle cells (VSMCs).
(A and B) Immunoblotting for sPDGFRβ (A) and quantification of sPDGFRβ levels (ng/ml) by quantitative Western blot analysis (B) in the culture medium from primary human VSMCs and pericytes cultured under normoxic (21% O2) or hypoxic (1% O2) conditions, or incubated with human synthetic Aβ40 (25 μM) for 48 h. (C and D) Immunoblottting for cell-associated PDGFRβ (C) and relative abundance of cellular PDGFRβ levels (D) in primary human VSMCs and pericytes cultured under normoxic (21% O2) or hypoxic (1% O2) conditions, or incubated with human synthetic Aβ40 (25 μM) for 48 h. β-Actin was used as an internal loading control. Means ± s.e.m., from 3 independent cultures from 3 donors in triplicates. P<0.05, by Student’s t-test.
4. Discussion
Here, we report that PDGFRβ expression is significantly higher in cultured primary human brain pericytes compared to brain arterial VSMCs, and show that in vitro exposure to hypoxia and Aβ40 peptide results in shedding of PDGFRβ and release of soluble form of PDGFRβ, sPDGFRβ, into the medium from cultured pericytes but not from VSMCs; suggesting that pericytes are more susceptible than VSMCs to shedding of sPDGFRβ following exposure to divergent inducers of cell injury such as hypoxia and Aβ. We recently reported that injury to cultured primary human pericytes resulted in shedding of sPDGFRβ and sPDGFRβ protein levels in CSF were elevated in transgenic murine models that develop pericyte degeneration and chronic BBB breakdown, namely Pdgfrβ mutants and AD APPSwe mice [9]. Similarly, sPDGFRβ levels were increased in CSF of humans with mild cognitive impairment compared to age-matched cognitively normal subjects, and CSF sPDGFRβ positively correlated with increased BBB permeability Ktrans value in the hippocampus of individuals with mild dementia [9]. The present data support that sPDGFRβ is primarily shedded by pericytes, and therefore, increased sPDGFRβ levels in the CSF in patients with dementia and AD likely reflect brain microvascular damage due to pericyte-specific injury.
Loss of cell surface PDGFRβ due to receptor shedding promotes disrupted PDGF-BB/PDGFRβ signaling. PDGFRβ, a receptor tyrosine kinase, is functionally important for maintaining proper brain microvascular integrity and communication with neighboring NVU cell types. Downstream PDGFRβ signal transduction events regulate numerous functions including cell survival, proliferation/differentiation, migration, microvascular tone, and proinflammatory responses [5,17,20]. Transgenic animal models with Pdgfb and Pdgfrβ mutations have informed the functional effect of disrupted PDGFRβ signaling [6,20,33]. Disrupting PDGF-BB/PDGFRβ signaling in the adult brain and aging brain causes faulty signal transduction that results in pericyte loss and promotes BBB breakdown and leads to cerebral blood flow reductions and hypoxia [6]. Thus, maintenance of PDGFRβ expression on pericytes is critical for BBB integrity and prevents entry of blood-derived neurotoxins, erythrocytes, and leukocytes into the brain which harm neuronal functions [1].
The exact mechanism(s) of PDGFRβ ectodomain shedding in pericytes due to hypoxic and/or Aβ injury are currently elusive. A recent study suggested involvement of ADAM10 and ADAM17, members of a disintegrin and metalloproteinase (ADAM) family, as the principal sheddase for regulating PDGFRβ levels [34]. Moreover, elevated CSF levels of ADAM17 in subjects with mild cognitive impairment and patients with AD has been reported [35]. Whether elevated ADAM17 activity correlate with sPDGFRβ levels in CSF is currently not known. Further studies are required to elucidate mechanisms by which shedding of PDGFRβ in pericytes is initiated following an insult such as hypoxia or Aβ toxicity.
sPDGFRβ is detectable and quantifiable in CSF of human subjects and the present study illustrates that sPDGFRβ is shed in response to pericyte-specific injury, thus supporting the potential for sPDGFRβ to be developed and validated as a sensitive, indicative biomarker of microvascular pericyte injury in the brain that reflects BBB disruption. These findings will aid in elucidating pathophysiological mechanisms of vascular-related neurological and neurodegenerative conditions, including AD, and may beneficially inform diagnostic effects or novel treatment targets to aid in the delay, prevention, and/or reversal of vascular-mediated neuropathologies.
Acknowledgments
This work was supported by National Institutes of Health grants R01AG023084, R01NS090904, R01NS034467, R01AG039452 and 5P50AG005142 to B.V.Z.
Footnotes
Conflict of interest
The authors declare no financial conflict of interest.
References
- 1.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hagan N, Ben-Zvi A. The molecular, cellular, and morphological components of blood-brain barrier development during embryogenesis. Semin Cell Dev Biol. 2015;38:7–15. doi: 10.1016/j.semcdb.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 6.Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- 8.Halliday MR, Rege SV, Ma Q, Zhao Z, Miller CA, Winkler EA, et al. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2015 doi: 10.1038/jcbfm.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer’s disease. Brain Pathol Zurich Switz. 2013;23:303–310. doi: 10.1111/bpa.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall AP. Review of the pericyte during angiogenesis and its role in cancer and diabetic retinopathy. Toxicol Pathol. 2006;34:763–775. doi: 10.1080/01926230600936290. [DOI] [PubMed] [Google Scholar]
- 12.Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, Clermont A, Leitges M, Marette A, et al. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med. 2009;15:1298–1306. doi: 10.1038/nm.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winkler EA, Sengillo JD, Bell RD, Wang J, Zlokovic BV. Blood-spinal cord barrier pericyte reductions contribute to increased capillary permeability. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2012;32:1841–1852. doi: 10.1038/jcbfm.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winkler EA, Sengillo JD, Sullivan JS, Henkel JS, Appel SH, Zlokovic BV. Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol (Berl) 2013;125:111–120. doi: 10.1007/s00401-012-1039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh M, Balbi M, Hellal F, Dichgans M, Lindauer U, Plesnila N. Pericytes are involved in the pathogenesis of CADASIL. Ann Neurol. 2015 doi: 10.1002/ana.24512. [DOI] [PubMed] [Google Scholar]
- 16.Garbelli R, de Bock F, Medici V, Rousset MC, Villani F, Boussadia B, et al. PDGFRβ(+) cells in human and experimental neuro-vascular dysplasia and seizures. Neuroscience. 2015;306:18–27. doi: 10.1016/j.neuroscience.2015.07.090. [DOI] [PubMed] [Google Scholar]
- 17.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Hellström M, Kalén M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Dev Camb Engl. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 20.Winkler EA, Bell RD, Zlokovic BV. Pericyte-specific expression of PDGF beta receptor in mouse models with normal and deficient PDGF beta receptor signaling. Mol Neurodegener. 2010;5:32. doi: 10.1186/1750-1326-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Dijk CGM, Nieuweboer FE, Pei JY, Xu YJ, Burgisser P, van Mulligen E, et al. The complex mural cell: pericyte function in health and disease. Int J Cardiol. 2015;190:75–89. doi: 10.1016/j.ijcard.2015.03.258. [DOI] [PubMed] [Google Scholar]
- 22.Chow N, Bell RD, Deane R, Streb JW, Chen J, Brooks A, et al. Serum response factor and myocardin mediate arterial hypercontractility and cerebral blood flow dysregulation in Alzheimer’s phenotype. Proc Natl Acad Sci U S A. 2007;104:823–828. doi: 10.1073/pnas.0608251104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bell RD, Deane R, Chow N, Long X, Sagare A, Singh I, et al. SRF and myocardin regulate LRP-mediated amyloid-beta clearance in brain vascular cells. Nat Cell Biol. 2009;11:143–153. doi: 10.1038/ncb1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackic JB, Stins M, McComb JG, Calero M, Ghiso J, Kim KS, et al. Human blood-brain barrier receptors for Alzheimer’s amyloid-beta 1–40. Asymmetrical binding, endocytosis, and transcytosis at the apical side of brain microvascular endothelial cell monolayer. J Clin Invest. 1998;102:734–743. doi: 10.1172/JCI2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Z, Guo H, Chow N, Sallstrom J, Bell RD, Deane R, et al. Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat Med. 2005;11:959–965. doi: 10.1038/nm1287. [DOI] [PubMed] [Google Scholar]
- 26.Van Nostrand W, Rozemuller A, Chung R, Cotman C, Saporito-irwin S. Amyloid β-protein precursor in cultured leptomeningeal smooth muscle cells. Amyloid. 1994;1:1–7. [Google Scholar]
- 27.Davis J, Wagner MR, Zhang W, Xu F, Van Nostrand WE. Amyloid beta-protein stimulates the expression of urokinase-type plasminogen activator (uPA) and its receptor (uPAR) in human cerebrovascular smooth muscle cells. J Biol Chem. 2003;278:19054–19061. doi: 10.1074/jbc.M301398200. [DOI] [PubMed] [Google Scholar]
- 28.Verbeek MM, Otte-Höller I, Wesseling P, Ruiter DJ, de Waal RM. Induction of alpha-smooth muscle actin expression in cultured human brain pericytes by transforming growth factor-beta 1. Am J Pathol. 1994;144:372–382. [PMC free article] [PubMed] [Google Scholar]
- 29.Sagare AP, Bell RD, Zhao Z, Ma Q, Winkler EA, Ramanathan A, et al. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun. 2013;4:2932. doi: 10.1038/ncomms3932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 31.Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, et al. apoE isoform–specific disruption of amyloid β peptide clearance from mouse brain. J Clin Invest. 2008;118:4002. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindahl P, Johansson BR, Levéen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 33.Tallquist MD, French WJ, Soriano P. Additive effects of PDGF receptor beta signaling pathways in vascular smooth muscle cell development. PLoS Biol. 2003;1:E52. doi: 10.1371/journal.pbio.0000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendelson K, Swendeman S, Saftig P, Blobel CP. Stimulation of platelet-derived growth factor receptor beta (PDGFRbeta) activates ADAM17 and promotes metalloproteinase-dependent cross-talk between the PDGFRbeta and epidermal growth factor receptor (EGFR) signaling pathways. J Biol Chem. 2010;285:25024–25032. doi: 10.1074/jbc.M110.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang H, Hampel H, Prvulovic D, Wallin A, Blennow K, Li R, et al. Elevated CSF levels of TACE activity and soluble TNF receptors in subjects with mild cognitive impairment and patients with Alzheimer’s disease. Mol Neurodegener. 2011;6:69. doi: 10.1186/1750-1326-6-69. [DOI] [PMC free article] [PubMed] [Google Scholar]