Abstract
Diffusion Tensor Imaging (DTI) has evolved considerably over the last decade to now be knocking on the doors of wider clinical applications. There have been several efforts over the last decade to seek valuable and reliable application of DTI in different neurological disorders. The role of DTI in predicting outcomes in patients with brain tumors has been extensively studied and has become a fairly established clinical tool in this scenario. More recently DTI has been applied in mild traumatic brain injury to predict clinical outcomes based on DTI of the white matter tracts. The resolution of white matter fiber tractography based on DTI has improved over the years with increased magnet strength and better tractography post processing. The role of DTI in hemorrhagic stroke has been studied preliminarily in the scientific literature. There is some evidence that DTI may be efficacious in predicting outcomes of motor function in animal models of intracranial hemorrhage. Only a handful of studies of DTI have been performed in subarachnoid hemorrhage or intraventricular hemorrhage scenarios. In this manuscript we will review the evolution of DTI, the existing evidence for its role in hemorrhagic stroke and discuss possible application of this non-invasive evaluation technique of human cerebral white matter tracts in the future.
Keywords: cerebral hemorrhage, diffusion Tensor Imaging, subarachnoid hemorrhage
Introduction
Stroke is one of the most common causes of morbidity and mortality in the developed and the developing world (1, 2). There are two major stroke subtypes, ischemic stroke and hemorrhagic stroke. The commonest of them is ischemic stroke (IS) followed by subarachnoid hemorrhage (SAH), intraventricular hemorrhage (IVH) and then intracerebral hemorrhage (ICH) in the hemorrhagic subtype (3–6). Amongst the different types of stroke the incidence of ICH is approximately 13% and SAH is 25–30%. In the SAH group of patients, surgical treatment in the form of clipping or endovascular coil embolization is performed to preclude the risk of a subsequent fatal hemorrhage. Similarly in the ICH patients surgical evacuation is performed in large hemorrhages with mass effect. However, currently there are very few effective therapeutic options available to reduce the neurological dysfunction caused by the hemorrhagic insult. The understanding of the mechanisms of neuronal injury in these clinical settings continues to evolve. Several therapeutic options in the form of neuroprotective agents have been shown to hold promise in the animal population but have not yet translated to show benefit in human subjects.
While better and more non-human primate models are being devised to improve the understanding of the underlying pathophysiology of hemorrhagic stroke (7, 8), non-invasive imaging with MRI and CT of the brain are being utilized with a view to develop biomarkers to visualize these mechanisms of neuronal injury. Standard MRI of the brain delineates superficial and deeper brain parenchymal structures in great detail. Then there are other specialized sequences that have been developed that have not yet entered the routine clinical domain. These specialized sequences are able to shed light on the normal physiology of neuronal and axonal function. Diffusion tensor imaging or “tractography” is one such sequence that specifically looks at directionality and integrity of the axonal fibers of the brain and how they change in the event of neuronal injury due to various etiologies one of them being hemorrhagic stroke.
Conventional MRI techniques are based on registering signal from a volume of tissue by repeated rephasing and dephasing of precessing protons in the particular volume imaged. Diffusion tensor imaging relies on slightly different property of the tissues. Water molecules in most tissues diffuse equally in all directions (isotropic diffusion) but in white matter tracts the diffusion is along the direction of the tract (anisotropic). Essentially the main parameter that is measured in DTI is the degree of fractional anisotropy (FA) in a given voxel of the precessing proton and its Eigen vector in an ellipsoid domain. In each voxel imaged by DTI the Eigen vector direction in 3 dimensions has to be acquired in at least nine elements of the matrix to portray the directionality of the elaborate dataset or “tensor”. At the minimum 6 directions of DTI datasets are needed to create color maps of white fiber tracts. Mathematical calculations and data processing is then utilized to show color coded maps of different white matter fiber tracts in the brain called fiber tractography (9). In the current manuscript we will concentrate on reviewing the role of diffusion tensor imaging (DTI) in hemorrhagic stroke i.e. SAH, IVH and ICH.
Diffusion tensor imaging – history and development
Work on the tractographic sequences began in the 1990s. The first tractographic images demonstrating the multi-dimensional directional information of the curved neural tracts in the brain were obtained in 1991 by Filler et al when they submitted their patent descriptions on imaging a series of patients in the UK (7). Subsequently they were published in the World Intellectual Property Organization in 1993 ( 7). The very first images of DTI were published in the proceedings of the Society for Magnetic Resonance in Medicine annual meeting in Berlin in August 1992 (6, 7). Prior to 1992 the clinical community believed that the strategy of application of diffusion MRI to trace brain white matter tracts did not have any potential. The discoveries of Filler, Howe and Richards in London, England and Seattle demonstrated the feasibility of diffusion MRI in producing linear neural images of the white matter tracts of the brain in late 1991 and then was reaffirmed by investigators LeBihan and Basser in Bethesda in 1992 (7).
The initial interest in tract tracing was to study the evolution of higher function in the humans from the early hominoids as for example speech (10, 11). This was the original impetus to generate images of tracts in the brain. Diffusion nuclear magnetic resonance has been around for decades. It is yet another source of obtaining contrast in individual voxels based on the rate of signal decay that related to the degree of water to diffuse anisotropically (in a primary direction) as opposed to isotropically (in all directions) (12). This is the basis for diffusion weighted MRI that has been utilized to demonstrate ischemic stroke for several years now (13, 14). DTI is a fundamental modification in MRI data processing that allows each voxel to produce not only signal intensity data but also directional data that show the tensor (3D complex vector) direction in a 3 dimensional space. Each voxel represented by a single arrow is then collated into an array of directional arrows in the orientation of neural tracts. These directional arrows are then strung together by graphic techniques to produce linear images of nerve tracts (9).
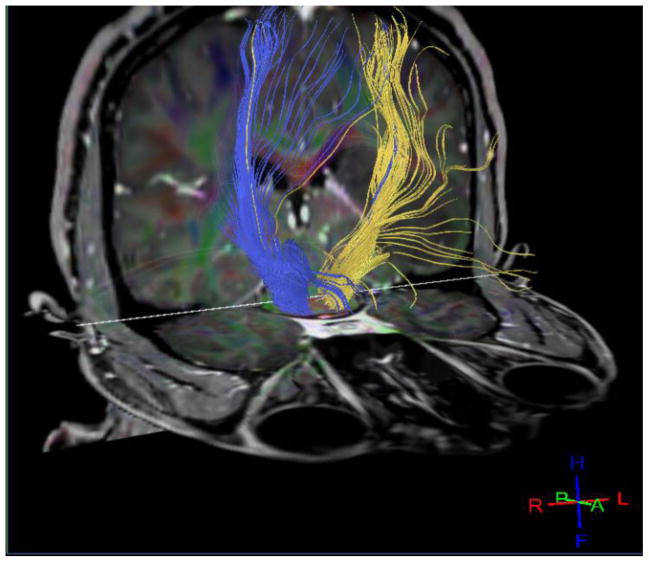
Traditionally the fiber streamlines were reconstructed utilizing deterministic (15) or probabilistic methods (16–18). These methods of white fiber analysis have several limitations. When fiber pathways are being mapped at locations of intersections the traditional analysis methods are unable to display the full dispersion of the pathways. A good example of this situation is the mapping of fibers that pass through complex white matter like the centrum semiovale, where pyramidal tract, superior longitudinal fasciculus and the corpus callosum are known to intersect. As has been demonstrated by Bucci et al these issues can be overcome by High Angular Resolution Diffusion Imaging (HARDI) technique that allows unraveling of the intersecting fibers to enable separate fiber tracking (19). They documented feasibility of this technique in 12 patients with brain tumors by showing excellent correlation of fiber tractography with intra-operative electrical stimulation (12). In a further advancement in computational abilities Presseau et al demonstrate the ability of a new compression format to process several thousands and often millions of fiber streamlines to be stored on smaller memory space (20). This is a significant advancement where this compression format has been validated in the several different DTI techniques including the HARDI technique (Figure 1).
Figure 1.
32 direction bilateral normal corticospinal tracts processed image from 3T MRI on a human subject performed at the authors’ institution. This demonstrates the current ability to perform diffusion tensor tractography on human subjects.
Clinical application of DTI
Since its inception in the 1990s there have been over 2000 studies published exploring the role of DTI tractography in the brain, although most of them have been in the last 5 years (7). The role of DTI has been explored in formal outcome studies of patients with traumatic brain injury, ICH (Figure 2), and surgical guidance in optimizing glioma resection (21–23). The other clinical scenarios where DTI has been explored are in evaluation of dementia in Alzheimer’s disease and in identifying subtle lesions that could explain the etiology of epilepsy (24, 25).
Figure 2.
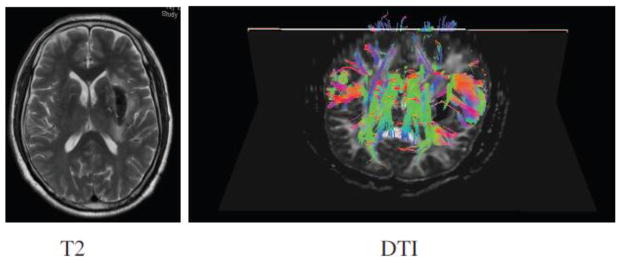
T2 and DTI MRI changes in the brain 6 days after intracerebral hemorrhage.
Application of DTI in Traumatic Brain Injury (TBI) has recently gathered strength. A pilot project has been started called TRACK TBI (transforming research and clinical knowledge in TBI) to improve understanding of mechanisms of brain injury and factors associated with improved functional outcomes (26). There are several studies that report on the changes in white matter tract DTI parameters in acute, subacute and chronic time points following mild TBI (27–35). More recently the TRACK TBI study group looked at the ability of DTI, amongst other MRI parameters, to predict functional outcomes at 3 and 6 months following mild TBI (36). They evaluated that in 76 patients with mild TBI the independent predictors of neurological function outcome were parameters identified on DTI. They demonstrate in their study that white matter fractional anisotropy (FA) was significantly reduced in CT/MRI-positive, but not in CT/MRI-negative, mild TBI patients when compared to healthy control subjects, on a group level. In addition reduced FA values in individual mild TBI patients although modest, were statistically significant predictors of unfavorable 3- and 6-month outcome. Moreover, these results remained statistically significant not only in the inclusive sample of 76 mild TBI patients but also in the subset of 37 mild TBI patients without prior history of substance abuse or other neuropsychiatric disorder (29).
Mechanisms of brain injury in hemorrhagic stroke
The mechanisms of white matter tract injuries in hemorrhagic stroke are better understood now. One of the commonest mechanisms of neuronal injury following an ICH is due to Wallerian degeneration in the corticospinal tract in the immediate vicinity and remote from the site of the hematoma. This injury is from direct injury to the neuronal structures in the deep white or grey matter. Apart from the mechanical mass effect, inflammation and edema around the periphery of the hematoma that takes place in the acute period(37, 38) there are other mechanisms mediating injury to the neural structures of the white matter (39, 40). In the early phase there is neuronal damage mediated by blood components and the hemoxygenase enzymes that cleave heme. Heme degradation products then diffuse out into the tissues causing cellular toxicity (40, 41). This mechanism of cellular injury is also applicable in patients with intraventricular hemorrhage, however, the cellular mechanisms of injury in patients with subarachnoid hemorrhage is not well understood.
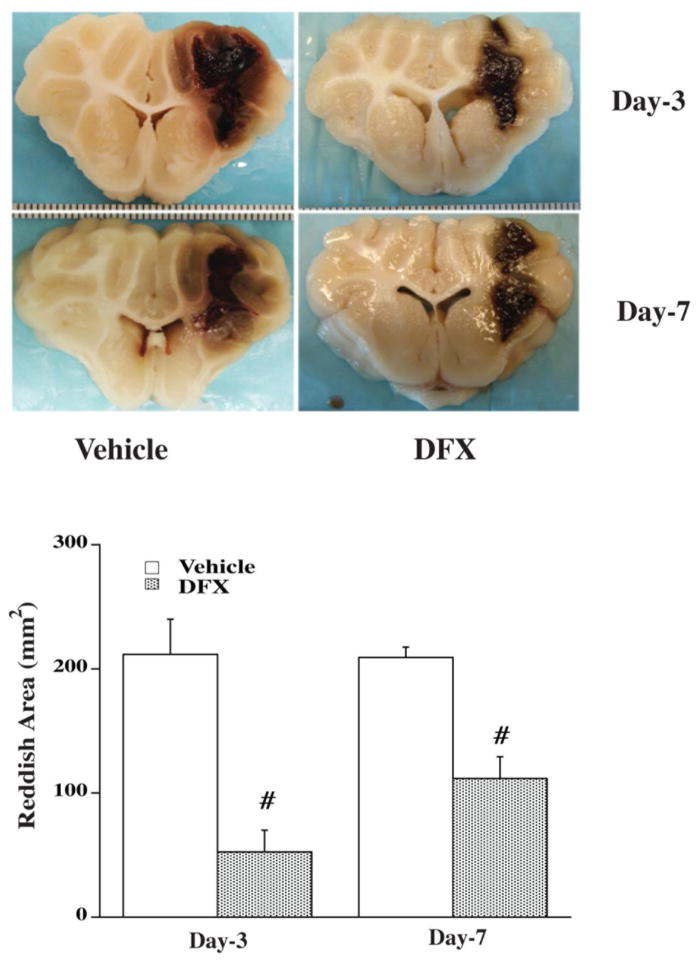
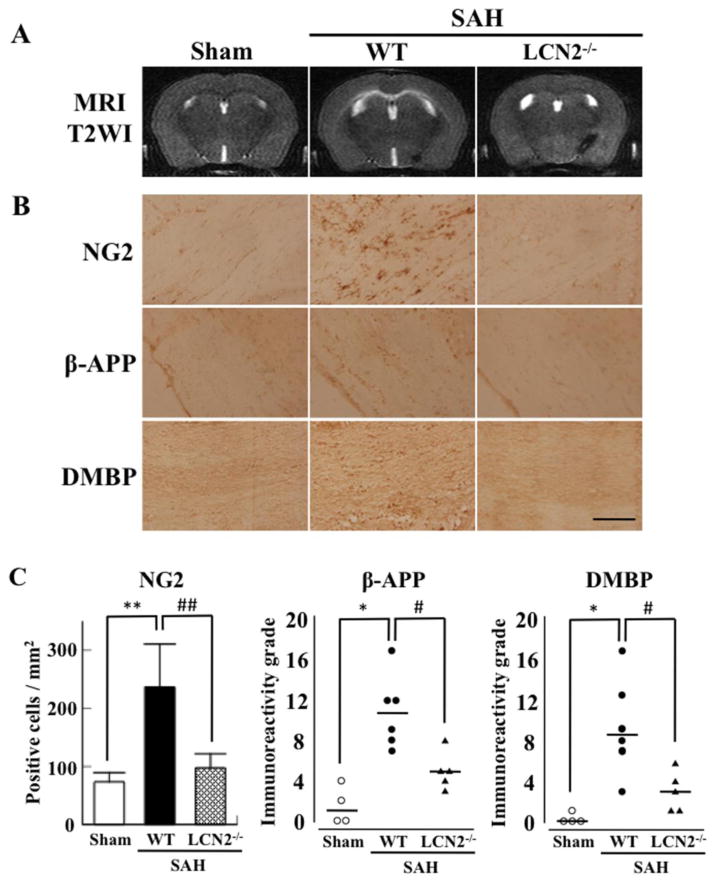
In the background of experiments on ICH animal models there is gathering body of evidence for early and delayed mechanisms of cellular injury. Currently our ability to be able to predict neuronal functional outcome in the event of a hemorrhagic stroke is suboptimal. DTI is one of the non-invasive markers which can predict functional outcome. DTI is now reaching a point when the resolution and ability to track fibers optimally is very reliable. Secondary injuries to the brain tissue at the periphery of the hematoma after primary cerebral hemorrhage are mediated by both the mass effect of the new hemorrhage and the toxicity that is associated with hematoma degradation and organization and the consequent release of inflammatory and free radical mediators. Iron has been shown to be responsible for cerebral toxicity in all types of cerebral hemorrhage including SAH (41). For example, deferoxamine, an iron chelator, reduces ICH-induced brain damage (39) (Figure 3). Egashira et al in a recent publication showed that Lipocalin 2 (LCN 2) may play an important role in the mediation of white matter tract injury following SAH in a vessel perforation wild type mouse model (42). Lipocalin 2 is an iron transport protein that has been implicated in cerebral toxicity. The authors demonstrate that the T2 signal on MRI was significantly high following SAH due to LCN 2 expression as evidenced by significant lack of T2 signal abnormality on MRI in LCN2 knockout mice (Figure 4). Given this background of developing understanding of white matter tract injury mechanisms in hemorrhagic stroke, we will now look at the existing evidence for ability of DTI to evaluate injured white matter tracts due to hemorrhagic stroke. For this purpose a comprehensive search of the literature was performed on pubmed with search criteria including hemorrhagic stroke, DTI, tractography, SAH, IVH, and ICH.
Figure 3.
Deferoxamine reduces reddish zone around hematoma at day 3 and day 7 in a pig ICH model. Pigs received an injection of autologous blood into the right frontal lobe. Deferoxamine (50 mg/kg, IM) or vehicle was administered 2 hours after ICH and then every 12 hours up to 7 days. Animals were killed 3 or 7 days later to examine brain damage after ICH. Values are means±SD, n=4, # p<0.01 vs. vehicle. (Reproduced with permission from: Gu Y et al. Stroke, 2009;40:2241–2243)
Figure 4.
Representative coronal T2 images (A) and NG2, β-amyloid precursor protein (β-APP) and degraded myelin basic protein (DMBP) immunohistochemistry in white matter of sham and WT and LCN2−/− mice 24 hours after SAH (B). Quantification of each result (C). **P<0.01, *P<0.05 vs. WT, and ##P<0.01, #P<0.05 vs. LCN2−/− animals. Values are means±SD; n=4–6. Scale bar = 100μm. (Reproduced with permission from: Egashira Y et al. Stroke 2014;45:2141–2143)
Existing evidence for DTI application in hemorrhagic stroke
DTI application in early detection of white fiber injury in animal models
ICH is the most common hemorrhagic stroke subtype that the role of DTI has been explored the most in. The ipsilateral corticospinal tract (CST) including the pyramidal tract (PY) has been increasingly studied in cases of stroke induced damage. The CST is an important white matter tract made up of PY, internal capsule and the cerebral peduncle. It has been shown that the asymmetry in fractional anisotropy (FA) values generated from DTI maps in the CST correlates with clinical signs and can predict functional outcome (43–48). Furthermore there has been a recent study that showed a decrease in FA values in the contralateral CST at 7 to 28 days following ICH, suggesting that FA asymmetry may not be a robust assessment of secondary motor pathway injury (49). Shu-Jan Fan et al suggest that our poor understanding of the contralateral PY and substantia nigra (SN) changes following ICH as demonstrated in several other ICH animal model studies can be secondary to use of different ICH sites and different imaging time points in those studies (23, 43, 46, 49). In their study they employ a robust rodent ICH model utilizing intra-striatal collagenase infusion to create the hematoma (50). They then longitudinally quantitatively assessed the changes on DTI in both contralateral and ipsilateral SN and PY tracts following ICH which were compared with histological examination. In their study they performed induction of an ICH in the striatum of 14 Sprague Dawley rats. MRI at 7 T was performed at days 1, 3, 7, 14, 42, and 120 days post ICH. The parameters assessed were FA, Mean Diffusivity (MD), Axial and radial diffusivity. The authors concluded that DTI parameters revealed early changes in both the ipsilateral and contralateral SN and PY following ICH. There was good correlation of the evolution of DTI findings with histological examination. The early reduction in radial diffusivity was surmised to be due to early axonal excitation in bilateral SN secondary to the ipsilateral excitatory injury. The authors in this study also observed initial decrease in mean diffusivity (MD) simultaneously in the ipsilateral and contralateral PY. This phenomenon has been observed in the contralateral CST in ischemic stroke, which has been correlated with poor outcome (45, 47). In other experiments decreased diffusion has been demonstrated in cerebral locations remote from the primary site of ICH (51, 52). In yet other experiments vasospasm that is usually the cause of delayed cerebra ischemia has been implicated in remote ischemia in ICH involving the ventricles (53, 54). Shu-Jan Fan et al in their study show that in those ICH rodent models which were associated with ventricular involvement showed early decreased diffusivity on DTI in bilateral PY. They explain that the combination of ventricular involvement, mass effect from the hematoma, and vasogenic edema result in raised intracranial pressure causing relative transient ischemia in distant brain parenchyma causing cellular dysfunction mediated by cell membrane proton pump failure, intracellular edema, myelin swelling and cessation of energy dependent axoplasmic processes. All these cellular correlates can explain the decreased MD in bilateral PY tracts (50).
DTI application in human subjects with ICH and functional outcome at 1 month
Evidence in the literature suggests the role of DTI in human subjects (46). The goal is to be able to predict the functional outcome in the event of an ICH to enable targeted therapy or to develop a rehabilitation strategy early. A preserved or recovered pyramidal tract is crucial for a good neurological functional outcome following an ICH (55). Several attempts have been made to predict the neurological motor function outcome utilizing various MRI techniques. It has been shown in ischemic stroke, that the functional outcome bears on the extent of wallerian degeneration remote from the lesion as picked up on MRI (56, 57). However, the signal intensity changes appear on MRI approximately 4 weeks from the ictus (58). There has been some evidence that DTI can show changes in signal intensity in the ipsilateral pyramidal tract earlier than the standard MRI sequences. The FA decreases progressively in the ipsilateral pyramidal tract in the subacute and the chronic stages of ischemic stroke, which correlates with motor functional outcome (59–61).
In the clinical scenario of a hemorrhagic stroke a few studies have demonstrated a correlation between decreasing FA on DTI with functional outcome (43). In these studies MRI was performed 3 days following the hematoma or removal of it. There are not many studies that have looked at diffusivity changes in the very early acute phase of the ICH and whether these changes correlate with functional outcome (62). Kusano et al in 18 patients with ICH explored the role of DTI and diffusivity changes in 2 days following the onset of the hematoma and tried to see if the changes correlated with functional outcome (46). They demonstrate that the measured FA in the ipsilateral cerebral peduncle decreased by 11% compared to the contralateral side. The ratio of FA (rFA) between the ipsilateral and contralateral side cerebral peduncle was negatively correlated with the paresis grading at day 0 and day 28 and modified Rankin score (mRS) at 28 days. The rFA showed a significant difference good and poor outcome groups. In addition the authors show that rFA correlated significantly with motor outcome in comparison to other variables used by them in the ICH scales. The mean diffusivity (MD) was unchanged in the affected cerebral peduncle. This is in keeping with prior studies showing unchanged MD on the affected side PY tract in ischemic stroke patients (44, 60, 63). The authors explain this phenomenon by the fact that following an ICH there is loss of axonal structures, membrane disintegration and cellular debris leading to decrease in the first eigenvalue and a relative increase the second and the third diffusion eigenvalues (46). Thus while there is decreased anisotropy the effect on orientationally averaged diffusion index is more challenging to predict and hence MD may remain unchanged in the very early phase i.e. first 2 days of and ICH. Yokoyama et al in another DTI in ICH study in human population have reported that the mean FA ratio decreased by 11% on the ipsilateral pyramidal tract in comparison to the contralateral side in the good recovery group of patients while the reduction in mean FA ratio was 23% in the group of patients with poor recovery (43). In another study Kuzu et al showed a difference of 9% in the absolute FA values between the patients with good or poor recovery (61).
DTI application in humans with ICH and correlation with outcome at 6 months
So we see that there is gathering body of evidence in ICH from animal studies to those in humans that reductions in FA in the acute phase correlate with motor function at 1 month. DaMing Wang et al looked at correlation of DTI with motor function outcome 6 months following an ICH (64). They evaluated DTI performed at 3 days and 2 weeks following ICH in 36 patients. The parameters selected for evaluation were FA, rFA and MD measured at the anterior cerebral peduncle. The results demonstrate that the rFA measured at 2 weeks in the cerebral peduncle correlated significantly with the mRS at 6 months. The authors further opine that their results compare well with those of Kusano et al, Puig et al and Feys et al in so much as non-correlation of the size of the hematoma with motor function outcome (46, 65, 66). As shown in the other studies only the CST DTI showed correlation with motor function outcome. The authors also suggest that DTI measurements at the cerebral peduncle are less prone to miscalculations given the perpendicular arrangement of the fiber tracts with respect to the axial plane of imaging. DaMing Wang et al also recommend further studies to evaluate DTI and diffusion tensor tractography (DTT) correlation with motor function outcome as has been preliminarily demonstrated by Cho et al in a separate study (67). There are other studies that have refined the correlation of DTI parameters with long term motor function (68). Tetsuo Koyama et al in their study demonstrate good correlation of DTI parameters with upper extremity recovery of motor function 6–7 months following and ICH (69).
DTI application in humans with ICH correlating with functional outcome at 1 month and 3 months
As we have seen from the previous studies discussed above they explore the role of DTI of the affected CST in the acute phase of ICH and its correlation with motor function outcome. The aspect of variations in FA and correlation with motor function outcome has not been well addressed in the existing literature. In another study Chi Cheng Ma et al explored the dynamic variations of FA measurements on DTI of the affected CST in patients with ICH over a period of 90 days (70). They performed DTI in 25 ICH patients on day 0, 30 & 90. In this study all patients underwent a motor function score (MFS) assessment before their MRI at day 0, 30 and 90 by experienced neurosurgeons blinded to the DTI results. The MRI sequence utilized was diffusion weighted echo planar imaging with gradients in 6 directions on all the study patients. The authors observed that the day 0 FA correlated significantly with MFS at day 90. In addition patients with an initial FA value above 0.45 could be anticipated to have a good motor function outcome following ICH with conservative management. The authors also demonstrate that progressive reductions of FA values were noted in the ipsilateral CST correlating with poor motor function. On the other hand the FA measurements increased in the good motor function outcome group. In the opinion of the authors of this study the exact modus operandi of the trend of FA values in the opposite direction in its correlation with good outcome is not entirely explicit. They further surmise that wallerian degeneration is induced by direct damage to white matter fibers from the hematoma followed by cytotoxic and vasogenic edema. The cytotoxic edema along the tracts is not readily apparent on conventional MRI which can be detected by DTI. In their study by logistic analysis they showed that the day 0 high FA value was the only one correlating with good motor function outcome. The MFS score at the outset of the ICH did not correlate with day 90 MFS scores to predict a good motor function outcome. This means that some patients with poor motor function in the acute phase would improve in the chronic phase. In addition patients with high MFS of 5 or higher correlates with those patients likely to require surgical intervention to improve functional outcome (71). Thus Ching Ma et al surmise in their article that FA measurements may help to select patients who may benefit from surgical hematoma evacuation in turn enabling physicians with ability to predict motor recovery in advance and also choose effective treatment strategy.
DTI application in humans with ICH to select ones who will benefit from intervention
In another study Guofeng Wan et al explored the role of DTI in evaluation of functional outcome in patients who had treatment of the ICH with a minimally invasive procedure (72). They randomized patients with ICH in the thalamic region into having the minimally invasive procedure or not. DTI was performed on all these patients once at admission and then another at 2 weeks following surgical intervention and also at the same time point in the corresponding medically treated group. Along with the aforementioned imaging motor evoked potentials were also recorded at identical time points. Measurements on DTI were performed on the ipsilateral CST in the internal capsule and contralateral measurements in identical anatomical location. The authors in this study found that DTI imaging demonstrate that the white matter fibers of the CST in the ipsilateral side drop to less than 1/3rd of the fiber density of the contralateral controls. In the group where hematoma was evacuated by minimally invasive technique showed more than 2/3rd of the fibers recovered back to normal while the fiber density measured by tractography was the same in the medically treated group. Both the motor function and the NIHSS scale was significantly more improved in the minimally invasive treated group. This study further proves that DTI technology is robust and fiber tracking can quantify white matter recovery following a spontaneous ICH in the basal ganglia region. There are at least two other studies that have looked at the role of DTI in detecting recovery of the white matter fibers following ICH and treatment with hematoma evacuation (73, 74). Heish et al in a case report demonstrate recovery of white matter fibers in CST damaged by putaminal hematoma correlating with improved motor function post evacuation of hematoma by minimally invasive techniques (75). Similarly Wu et al (ref 2 in Guofeng article) demonstrate similar significant correlation in 27 patients treated with minimally invasive methods to evacuate the hematoma, between improved FA and fiber density in CST and motor function outcome (74).
DTI application in animal IVH models
There are some animal studies that have demonstrated white matter fiber injury in the peri-ventricular location on histopathological examination (76–78). Since its inception only one study has been published showing the value of DTI in IVH (76) in an animal model. In this study Chua et al in a rabbit pup brain demonstrate that there was good correlation with FA and ADC value measurements in the corpus callosum (CC), corona radiate (CR) but not so with the internal capsule (IC). There was significant decrease in FA values at CC and CR correlating with evidence of white matter fiber damage on histology, while the measurements at IC did not correlate. The pathological entities revealed in the rabbit pup brain in the corresponding locations in CC, CR were reduced myelination, gliosis and axonal injury. In the human population relationship of IVH, PVL and hydrocephalus has been shown in the immature brain (79–81). Sang Seok Yeo et al were the first ones to explore the role of DTI in 10 human subjects with a combination of IVH and SAH with IVH (82). They showed that FA value reduction was significant in the fornix, CC, and CR. The ADC values did not show any significant relationship. The authors of this article surmise that this neuronal injury in the periventricular region in IVH could be the reason for poor outcome and high mortality in stroke patients with IVH (83, 84). The authors further suggest that accurate diagnosis of extent of neuronal injury would be crucial in targeting management strategies to improve patient functional outcome.
DTI application in humans with SAH
Amongst the hemorrhagic stroke subtypes the white matter injury is most ill understood in SAH. There are multiple different patho-physiological mechanisms that are at play that inflict neuronal injury in the event of SAH. These include vasospasm, direct injury from hematoma, insufficient blood supply to the paracentral sulci, or injury to the CST from the aneurysm (85, 86). The evidence for reliability of DTI in denoting early injury to the CST in other forms of stroke settings makes it the natural MRI biomarker to study in the clinical scenario of SAH. Sang Seok Yeo et al have explored this aspect in their study of 22 patients with SAH. DTI parameters were measured in the CR, posterior limb of the internal capsule, mid brain, mid pons and medulla in 22 patients and 24 age and sex matched controls at 4 weeks from the ictus (87). They demonstrated significantly low values of FA in ROI within CST at the midbrain in SAH patients compared to the controls. No significant difference was demonstrated in the measurements at other anatomical locations. Moreover, no correlation was observed in the ADC values or between the FA values and the motor function scores. These observations support the hypothesis that CST at the midbrain can be injured during SAH due to early hydrocephalus (76). In addition another hypothesis that the close proximity of blood in the perimesencephalic cistern to the CST in the midbrain makes it a target of damage by SAH is supported by the observations in the aforementioned study (88). It is interesting that motor function outcome did not correlate with the FA values of CST in the midbrain. This only suggests that white matter tract injury is not the only mechanism of injury governing the extent of motor function compromise.
Future directions
Although there is reasonable evidence that has accumulated in the last decade that demonstrates DTI to be sensitive enough to detect white matter tract injury in hemorrhagic stroke a definite correlation with motor function outcome is still preliminary. In parallel our understanding of the pathomechanisms of neuronal damage in hemorrhagic stroke is also increasing. A very good example is the depiction of iron mediated neuronal injury that has come across through several studies on this topic especially in the non-human primate and non-primate animal models (39, 89). The authors of this review firmly believe large human population studies are needed to explore correlation of parenchymal tissue iron levels and damage to white matter tracts detected by DTI and further connection of these biomarkers with motor function outcome. Large prospective studies are needed in the human population to explore this reliably. The next stage will be the evaluation of identified therapeutic targets based on the aforementioned studies to in randomized controlled human population studies to get to meaningful evidence to support therapies to improve functional outcome in patients with hemorrhagic stroke. Furthermore, in future studies, it is important to determine whether DTI can detect white matter edema development and resolution, and white matter recovery.
Conclusion
Hemorrhagic stroke is debilitating and can result in mortality and significant morbidity. Currently no effective therapeutic measure exists that can improve functional outcomes in this clinical scenario. Physiological support is the only management approach currently available with which physicians hope to ride off the insult of hemorrhagic stroke. As we have discussed in the manuscript conventional non-invasive imaging with conventional MRI is not sensitive enough to obtain information about extent of damage to guide management strategies more effectively. DTI technique, as we have discussed, shows preliminary evidence that it may hold promise in predicting patients with poor prognosis early on in hemorrhagic stroke. It may also reveal potential therapeutic targets to ensure good motor function outcomes in these patients. To begin with there is a huge potential for demonstrating DTI as a reliable imaging surrogate of impending neuronal toxicity in patients with hemorrhagic stroke. The next step would be to demonstrate reliable correlation of DTI with functional outcome to be able to stratify risk in patients with hemorrhagic stroke.
Acknowledgments
Supported by grants NS-073595, NS-079157, NS-084049, NS-091545, 973 Program-2014CB541600 and an UMHS-PUHSC Joint Institute grant.
Footnotes
Disclosure: We declare that we have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pendlebury ST. Worldwide under-funding of stroke research. International journal of stroke : official journal of the International Stroke Society. 2007 May;2(2):80–4. doi: 10.1111/j.1747-4949.2007.00126.x. [DOI] [PubMed] [Google Scholar]
- 2.Leak RK, Zheng P, Ji X, Zhang JH, Chen J. From apoplexy to stroke: historical perspectives and new research frontiers. Prog Neurobiol. 2014 Apr;115:1–5. doi: 10.1016/j.pneurobio.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Xi G, Strahle J, Hua Y, Keep RF. Progress in translational research on intracerebral hemorrhage: Is there an end in sight? Prog Neurobiol. 2014 Apr;115C:45–63. doi: 10.1016/j.pneurobio.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S, Feng H, Sherchan P, Klebe D, Zhao G, Sun X, et al. Controversies and evolving new mechanisms in subarachnoid hemorrhage. Prog Neurobiol. 2014 Apr;115:64–91. doi: 10.1016/j.pneurobio.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandey AS, Xi G. Intracerebral hemorrhage: a multimodality approach to improving outcome. Transl Stroke Res. 2014 Jun;5(3):313–5. doi: 10.1007/s12975-014-0344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang JH. Vascular neural network in subarachnoid hemorrhage. Transl Stroke Res. 2014 Aug;5(4):423–8. doi: 10.1007/s12975-014-0355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tso MK, Macdonald RL. Subarachnoid hemorrhage: a review of experimental studies on the microcirculation and the neurovascular unit. Transl Stroke Res. 2014 Apr;5(2):174–89. doi: 10.1007/s12975-014-0323-4. [DOI] [PubMed] [Google Scholar]
- 8.Wagner KR. Modeling intracerebral hemorrhage: glutamate, nuclear factor-kappa B signaling and cytokines. Stroke. 2007;38:753–8. doi: 10.1161/01.STR.0000255033.02904.db. [DOI] [PubMed] [Google Scholar]
- 9.Filler A. Magnetic resonance neurography and diffusion tensor imaging: origins, history, and clinical impact of the first 50,000 cases with an assessment of efficacy and utility in a prospective 5000-patient study group. Neurosurgery. 2009 Oct;65(4 Suppl):A29–43. doi: 10.1227/01.NEU.0000351279.78110.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filler AG. Axonal transport and MR imaging: prospects for contrast agent development. Journal of magnetic resonance imaging : JMRI. 1994 May-Jun;4(3):259–67. doi: 10.1002/jmri.1880040308. [DOI] [PubMed] [Google Scholar]
- 11.Filler AG, Whiteside GT, Bacon M, Frederickson M, Howe FA, Rabinowitz MD, et al. Tri-partite complex for axonal transport drug delivery achieves pharmacological effect. BMC neuroscience. 2010;11:8. doi: 10.1186/1471-2202-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Bihan D. Molecular diffusion nuclear magnetic resonance imaging. Magnetic resonance quarterly. 1991 Jan;7(1):1–30. [PubMed] [Google Scholar]
- 13.Kakuda W, Lansberg MG, Thijs VN, Kemp SM, Bammer R, Wechsler LR, et al. Optimal definition for PWI/DWI mismatch in acute ischemic stroke patients. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2008 May;28(5):887–91. doi: 10.1038/sj.jcbfm.9600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lansberg MG, Thijs VN, O’Brien MW, Ali JO, de Crespigny AJ, Tong DC, et al. Evolution of apparent diffusion coefficient, diffusion-weighted, and T2-weighted signal intensity of acute stroke. AJNR American journal of neuroradiology. 2001 Apr;22(4):637–44. [PMC free article] [PubMed] [Google Scholar]
- 15.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of neurology. 1999 Feb;45(2):265–9. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Jones DK, Pierpaoli C. Confidence mapping in diffusion tensor magnetic resonance imaging tractography using a bootstrap approach. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2005 May;53(5):1143–9. doi: 10.1002/mrm.20466. [DOI] [PubMed] [Google Scholar]
- 17.Lazar M, Alexander AL. An error analysis of white matter tractography methods: synthetic diffusion tensor field simulations. NeuroImage. 2003 Oct;20(2):1140–53. doi: 10.1016/S1053-8119(03)00277-5. [DOI] [PubMed] [Google Scholar]
- 18.Parker GJ, Haroon HA, Wheeler-Kingshott CA. A framework for a streamline-based probabilistic index of connectivity (PICo) using a structural interpretation of MRI diffusion measurements. Journal of magnetic resonance imaging : JMRI. 2003 Aug;18(2):242–54. doi: 10.1002/jmri.10350. [DOI] [PubMed] [Google Scholar]
- 19.Bucci M, Mandelli ML, Berman JI, Amirbekian B, Nguyen C, Berger MS, et al. Quantifying diffusion MRI tractography of the corticospinal tract in brain tumors with deterministic and probabilistic methods. NeuroImage Clinical. 2013;3:361–8. doi: 10.1016/j.nicl.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Presseau C, Jodoin PM, Houde JC, Descoteaux M. A new compression format for fiber tracking datasets. NeuroImage. 2015 Apr 1;109:73–83. doi: 10.1016/j.neuroimage.2014.12.058. [DOI] [PubMed] [Google Scholar]
- 21.Sidaros A, Engberg AW, Sidaros K, Liptrot MG, Herning M, Petersen P, et al. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain : a journal of neurology. 2008 Feb;131(Pt 2):559–72. doi: 10.1093/brain/awm294. [DOI] [PubMed] [Google Scholar]
- 22.Wu JS, Zhou LF, Tang WJ, Mao Y, Hu J, Song YY, et al. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: a prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery. 2007 Nov;61(5):935–48. doi: 10.1227/01.neu.0000303189.80049.ab. discussion 48–9. [DOI] [PubMed] [Google Scholar]
- 23.Yoshioka H, Horikoshi T, Aoki S, Hori M, Ishigame K, Uchida M, et al. Diffusion tensor tractography predicts motor functional outcome in patients with spontaneous intracerebral hemorrhage. Neurosurgery. 2008 Jan;62(1):97–103. doi: 10.1227/01.NEU.0000311066.03121.B8. discussion. [DOI] [PubMed] [Google Scholar]
- 24.Chua TC, Wen W, Slavin MJ, Sachdev PS. Diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease: a review. Current opinion in neurology. 2008 Feb;21(1):83–92. doi: 10.1097/WCO.0b013e3282f4594b. [DOI] [PubMed] [Google Scholar]
- 25.Diehl B, Busch RM, Duncan JS, Piao Z, Tkach J, Luders HO. Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia. 2008 Aug;49(8):1409–18. doi: 10.1111/j.1528-1167.2008.01596.x. [DOI] [PubMed] [Google Scholar]
- 26.Yue JK, Vassar MJ, Lingsma HF, Cooper SR, Okonkwo DO, Valadka AB, et al. Transforming research and clinical knowledge in traumatic brain injury pilot: multicenter implementation of the common data elements for traumatic brain injury. Journal of neurotrauma. 2013 Nov 15;30(22):1831–44. doi: 10.1089/neu.2013.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. AJNR American journal of neuroradiology. 2002 May;23(5):794–802. [PMC free article] [PubMed] [Google Scholar]
- 28.Bazarian JJ, Zhong J, Blyth B, Zhu T, Kavcic V, Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. Journal of neurotrauma. 2007 Sep;24(9):1447–59. doi: 10.1089/neu.2007.0241. [DOI] [PubMed] [Google Scholar]
- 29.Chu Z, Wilde EA, Hunter JV, McCauley SR, Bigler ED, Troyanskaya M, et al. Voxel-based analysis of diffusion tensor imaging in mild traumatic brain injury in adolescents. AJNR American journal of neuroradiology. 2010 Feb;31(2):340–6. doi: 10.3174/ajnr.A1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim N, Branch CA, Kim M, Lipton ML. Whole brain approaches for identification of microstructural abnormalities in individual patients: comparison of techniques applied to mild traumatic brain injury. PloS one. 2013;8(3):e59382. doi: 10.1371/journal.pone.0059382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipton ML, Kim N, Park YK, Hulkower MB, Gardin TM, Shifteh K, et al. Robust detection of traumatic axonal injury in individual mild traumatic brain injury patients: intersubject variation, change over time and bidirectional changes in anisotropy. Brain imaging and behavior. 2012 Jun;6(2):329–42. doi: 10.1007/s11682-012-9175-2. [DOI] [PubMed] [Google Scholar]
- 32.Mayer AR, Ling J, Mannell MV, Gasparovic C, Phillips JP, Doezema D, et al. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology. 2010 Feb 23;74(8):643–50. doi: 10.1212/WNL.0b013e3181d0ccdd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Messe A, Caplain S, Paradot G, Garrigue D, Mineo JF, Soto Ares G, et al. Diffusion tensor imaging and white matter lesions at the subacute stage in mild traumatic brain injury with persistent neurobehavioral impairment. Human brain mapping. 2011 Jun;32(6):999–1011. doi: 10.1002/hbm.21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smits M, Houston GC, Dippel DW, Wielopolski PA, Vernooij MW, Koudstaal PJ, et al. Microstructural brain injury in post-concussion syndrome after minor head injury. Neuroradiology. 2011 Aug;53(8):553–63. doi: 10.1007/s00234-010-0774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilde EA, McCauley SR, Hunter JV, Bigler ED, Chu Z, Wang ZJ, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008 Mar 18;70(12):948–55. doi: 10.1212/01.wnl.0000305961.68029.54. [DOI] [PubMed] [Google Scholar]
- 36.Yuh EL, Cooper SR, Mukherjee P, Yue JK, Lingsma HF, Gordon WA, et al. Diffusion tensor imaging for outcome prediction in mild traumatic brain injury: a TRACK-TBI study. Journal of neurotrauma. 2014 Sep 1;31(17):1457–77. doi: 10.1089/neu.2013.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao X, Sun G, Zhang H, Ting SM, Song S, Gonzales N, et al. Polymorphonuclear neutrophil in brain parenchyma after experimental intracerebral hemorrhage. Transl Stroke Res. 2014 Oct;5(5):554–61. doi: 10.1007/s12975-014-0341-2. [DOI] [PubMed] [Google Scholar]
- 38.Hatakeyama T, Okauchi M, Hua Y, Keep RF, Xi G. Deferoxamine reduces neuronal death and hematoma lysis after intracerebral hemorrhage in aged rats. Transl Stroke Res. 2013 Oct;4(5):546–53. doi: 10.1007/s12975-013-0270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu Y, Hua Y, Keep RF, Morgenstern LB, Xi G. Deferoxamine reduces intracerebral hematoma-induced iron accumulation and neuronal death in piglets. Stroke. 2009 Jun;40(6):2241–3. doi: 10.1161/STROKEAHA.108.539536. Epub 2009/04/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Y, Wang Y, Wang J, Anne Stetler R, Yang QW. Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog Neurobiol. 2014 Apr;115:25–44. doi: 10.1016/j.pneurobio.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. The Lancet Neurology. 2006 Jan;5(1):53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 42.Egashira Y, Hua Y, Keep RF, Xi G. Acute white matter injury after experimental subarachnoid hemorrhage: potential role of lipocalin 2. Stroke. 2014 Jul;45(7):2141–3. doi: 10.1161/STROKEAHA.114.005307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yokoyama K, Matsuki M, Shimano H, Sumioka S, Ikenaga T, Hanabusa K, et al. Diffusion tensor imaging in chronic subdural hematoma: correlation between clinical signs and fractional anisotropy in the pyramidal tract. AJNR American journal of neuroradiology. 2008 Jun;29(6):1159–63. doi: 10.3174/ajnr.A1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomalla G, Glauche V, Koch MA, Beaulieu C, Weiller C, Rother J. Diffusion tensor imaging detects early Wallerian degeneration of the pyramidal tract after ischemic stroke. NeuroImage. 2004 Aug;22(4):1767–74. doi: 10.1016/j.neuroimage.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 45.Domi T, deVeber G, Shroff M, Kouzmitcheva E, MacGregor DL, Kirton A. Corticospinal tract pre-wallerian degeneration: a novel outcome predictor for pediatric stroke on acute MRI. Stroke; a journal of cerebral circulation. 2009 Mar;40(3):780–7. doi: 10.1161/STROKEAHA.108.529958. [DOI] [PubMed] [Google Scholar]
- 46.Kusano Y, Seguchi T, Horiuchi T, Kakizawa Y, Kobayashi T, Tanaka Y, et al. Prediction of functional outcome in acute cerebral hemorrhage using diffusion tensor imaging at 3T: a prospective study. AJNR American journal of neuroradiology. 2009 Sep;30(8):1561–5. doi: 10.3174/ajnr.A1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DeVetten G, Coutts SB, Hill MD, Goyal M, Eesa M, O’Brien B, et al. Acute corticospinal tract Wallerian degeneration is associated with stroke outcome. Stroke; a journal of cerebral circulation. 2010 Apr;41(4):751–6. doi: 10.1161/STROKEAHA.109.573287. [DOI] [PubMed] [Google Scholar]
- 48.Liu X, Tian W, Li L, Kolar B, Qiu X, Chen F, et al. Hyperintensity on diffusion weighted image along ipsilateral cortical spinal tract after cerebral ischemic stroke: a diffusion tensor analysis. European journal of radiology. 2012 Feb;81(2):292–7. doi: 10.1016/j.ejrad.2010.12.053. [DOI] [PubMed] [Google Scholar]
- 49.Kwak SY, Yeo SS, Choi BY, Chang CH, Jang SH. Corticospinal tract change in the unaffected hemisphere at the early stage of intracerebral hemorrhage: a diffusion tensor tractography study. European neurology. 2010;63(3):149–53. doi: 10.1159/000281108. [DOI] [PubMed] [Google Scholar]
- 50.Fan SJ, Lee FY, Cheung MM, Ding AY, Yang J, Ma SJ, et al. Bilateral substantia nigra and pyramidal tract changes following experimental intracerebral hemorrhage: an MR diffusion tensor imaging study. NMR in biomedicine. 2013 Sep;26(9):1089–95. doi: 10.1002/nbm.2922. [DOI] [PubMed] [Google Scholar]
- 51.Qureshi AI. Significance of lesions with decreased diffusion on MRI in patients with intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2012 Jan;43(1):6–7. doi: 10.1161/STROKEAHA.111.639278. [DOI] [PubMed] [Google Scholar]
- 52.Garg RK, Liebling SM, Maas MB, Nemeth AJ, Russell EJ, Naidech AM. Blood pressure reduction, decreased diffusion on MRI, and outcomes after intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2012 Jan;43(1):67–71. doi: 10.1161/STROKEAHA.111.629493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Condette-Auliac S, Bracard S, Anxionnat R, Schmitt E, Lacour JC, Braun M, et al. Vasospasm after subarachnoid hemorrhage: interest in diffusion-weighted MR imaging. Stroke; a journal of cerebral circulation. 2001 Aug;32(8):1818–24. doi: 10.1161/01.str.32.8.1818. [DOI] [PubMed] [Google Scholar]
- 54.Kiphuth IC, Huttner HB, Breuer L, Engelhorn T, Schwab S, Kohrmann M. Vasospasm in intracerebral hemorrhage with ventricular involvement: a prospective pilot transcranial Doppler sonography study. Cerebrovascular diseases. 2011;32(5):420–5. doi: 10.1159/000330652. [DOI] [PubMed] [Google Scholar]
- 55.Sawlani V, Gupta RK, Singh MK, Kohli A. MRI demonstration of Wallerian degeneration in various intracranial lesions and its clinical implications. Journal of the neurological sciences. 1997 Mar 10;146(2):103–8. doi: 10.1016/s0022-510x(96)00299-7. [DOI] [PubMed] [Google Scholar]
- 56.Binkofski F, Seitz RJ, Arnold S, Classen J, Benecke R, Freund HJ. Thalamic metbolism and corticospinal tract integrity determine motor recovery in stroke. Annals of neurology. 1996 Apr;39(4):460–70. doi: 10.1002/ana.410390408. [DOI] [PubMed] [Google Scholar]
- 57.Mukherjee P. Diffusion tensor imaging and fiber tractography in acute stroke. Neuroimaging clinics of North America. 2005 Aug;15(3):655–65. xii. doi: 10.1016/j.nic.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 58.Inoue Y, Matsumura Y, Fukuda T, Nemoto Y, Shirahata N, Suzuki T, et al. MR imaging of Wallerian degeneration in the brainstem: temporal relationships. AJNR American journal of neuroradiology. 1990 Sep-Oct;11(5):897–902. [PMC free article] [PubMed] [Google Scholar]
- 59.Buffon F, Molko N, Herve D, Porcher R, Denghien I, Pappata S, et al. Longitudinal diffusion changes in cerebral hemispheres after MCA infarcts. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2005 May;25(5):641–50. doi: 10.1038/sj.jcbfm.9600054. [DOI] [PubMed] [Google Scholar]
- 60.Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, et al. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. NeuroImage. 2001 Jun;13(6 Pt 1):1174–85. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- 61.Werring DJ, Toosy AT, Clark CA, Parker GJ, Barker GJ, Miller DH, et al. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. Journal of neurology, neurosurgery, and psychiatry. 2000 Aug;69(2):269–72. doi: 10.1136/jnnp.69.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qiu TM, Zhang Y, Wu JS. Preliminary application of pyramidal tractography in evaluating prognosis of patients with hypertensive intracerebral hemorrhage. Acta neurochirurgica Supplement. 2008;105:165–70. doi: 10.1007/978-3-211-09469-3_33. [DOI] [PubMed] [Google Scholar]
- 63.Delsing BJ, Catsman-Berrevoets CE, Appel IM. Early prognostic indicators of outcome in ischemic childhood stroke. Pediatric neurology. 2001 Apr;24(4):283–9. doi: 10.1016/s0887-8994(01)00245-4. [DOI] [PubMed] [Google Scholar]
- 64.Wang DM, Li J, Liu JR, Hu HY. Diffusion tensor imaging predicts long-term motor functional outcome in patients with acute supratentorial intracranial hemorrhage. Cerebrovascular diseases. 2012;34(3):199–205. doi: 10.1159/000341857. [DOI] [PubMed] [Google Scholar]
- 65.Puig J, Pedraza S, Blasco G, Daunis IEJ, Prados F, Remollo S, et al. Acute damage to the posterior limb of the internal capsule on diffusion tensor tractography as an early imaging predictor of motor outcome after stroke. AJNR American journal of neuroradiology. 2011 May;32(5):857–63. doi: 10.3174/ajnr.A2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feys H, Hetebrij J, Wilms G, Dom R, De Weerdt W. Predicting arm recovery following stroke: value of site of lesion. Acta neurologica Scandinavica. 2000 Dec;102(6):371–7. doi: 10.1034/j.1600-0404.2000.102006371.x. [DOI] [PubMed] [Google Scholar]
- 67.Cho SH, Kim SH, Choi BY, Cho SH, Kang JH, Lee CH, et al. Motor outcome according to diffusion tensor tractography findings in the early stage of intracerebral hemorrhage. Neuroscience letters. 2007 Jun 27;421(2):142–6. doi: 10.1016/j.neulet.2007.04.052. [DOI] [PubMed] [Google Scholar]
- 68.Koyama T, Tsuji M, Miyake H, Ohmura T, Domen K. Motor outcome for patients with acute intracerebral hemorrhage predicted using diffusion tensor imaging: an application of ordinal logistic modeling. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2012 Nov;21(8):704–11. doi: 10.1016/j.jstrokecerebrovasdis.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 69.Koyama T, Marumoto K, Miyake H, Ohmura T, Domen K. Relationship between diffusion-tensor fractional anisotropy and long-term outcome in patients with hemiparesis after intracerebral hemorrhage. NeuroRehabilitation. 2013;32(1):87–94. doi: 10.3233/NRE-130825. [DOI] [PubMed] [Google Scholar]
- 70.Ma C, Liu A, Li Z, Zhou X, Zhou S. Longitudinal study of diffusion tensor imaging properties of affected cortical spinal tracts in acute and chronic hemorrhagic stroke. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2014 Aug;21(8):1388–92. doi: 10.1016/j.jocn.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 71.Kuzu Y, Inoue T, Kanbara Y, Nishimoto H, Fujiwara S, Ogasawara K, et al. Prediction of motor function outcome after intracerebral hemorrhage using fractional anisotropy calculated from diffusion tensor imaging. Cerebrovascular diseases. 2012;33(6):566–73. doi: 10.1159/000338904. [DOI] [PubMed] [Google Scholar]
- 72.Wu G, Wang L, Liu J, Mao Y, Qin G. Minimally invasive procedures reduced the damages to motor function in patients with thalamic hematoma: observed by motor evoked potential and diffusion tensor imaging. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2013 Apr;22(3):232–40. doi: 10.1016/j.jstrokecerebrovasdis.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 73.Hsieh CT, Wu CC, Chiang YH, Chang CF. Stereotactic aspiration of enlarged intracerebral hematoma caused by intraprocedural perforation of aneurysm during coil embolization. Surgical neurology. 2008 Jun;69(6):633–5. doi: 10.1016/j.surneu.2007.03.045. discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 74.Wu G, Wang L, Hong Z, Li C, Long X, Sheng F. Effects of minimally invasive procedures for removal of intracranial hematoma on matrix metalloproteinase expression and blood-brain barrier permeability in perihematomal brain tissues. Neurological research. 2011 Apr;33(3):300–6. doi: 10.1179/016164110X12759951866993. [DOI] [PubMed] [Google Scholar]
- 75.Hsieh CT, Chen CY, Chiang YH, Chang CH, Chang CF. Role of diffusion tensor imaging in a patient with spontaneous intracerebral hematoma treated by stereotactic evacuation. Surgical neurology. 2008 Jul;70(1):75–8. doi: 10.1016/j.surneu.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 76.Chua CO, Chahboune H, Braun A, Dummula K, Chua CE, Yu J, et al. Consequences of intraventricular hemorrhage in a rabbit pup model. Stroke; a journal of cerebral circulation. 2009 Oct;40(10):3369–77. doi: 10.1161/STROKEAHA.109.549212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wasserman JK, Schlichter LC. White matter injury in young and aged rats after intracerebral hemorrhage. Experimental neurology. 2008 Dec;214(2):266–75. doi: 10.1016/j.expneurol.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 78.Pang D, Sclabassi RJ, Horton JA. Lysis of intraventricular blood clot with urokinase in a canine model: Part 2. In vivo safety study of intraventricular urokinase. Neurosurgery. 1986 Oct;19(4):547–52. doi: 10.1227/00006123-198610000-00009. [DOI] [PubMed] [Google Scholar]
- 79.de la Monte SM, Hsu FI, Hedley-Whyte ET, Kupsky W. Morphometric analysis of the human infant brain: effects of intraventricular hemorrhage and periventricular leukomalacia. Journal of child neurology. 1990 Apr;5(2):101–10. doi: 10.1177/088307389000500206. [DOI] [PubMed] [Google Scholar]
- 80.Takashima S, Mito T, Houdou S, Ando Y. Relationship between periventricular hemorrhage, leukomalacia and brainstem lesions in prematurely born infants. Brain & development. 1989;11(2):121–4. doi: 10.1016/s0387-7604(89)80080-4. [DOI] [PubMed] [Google Scholar]
- 81.Larroque B, Marret S, Ancel PY, Arnaud C, Marpeau L, Supernant K, et al. White matter damage and intraventricular hemorrhage in very preterm infants: the EPIPAGE study. The Journal of pediatrics. 2003 Oct;143(4):477–83. doi: 10.1067/S0022-3476(03)00417-7. [DOI] [PubMed] [Google Scholar]
- 82.Yeo SS, Choi BY, Chang CH, Jung YJ, Ahn SH, Son SM, et al. Periventricular white matter injury by primary intraventricular hemorrhage: a diffusion tensor imaging study. European neurology. 2011;66(4):235–41. doi: 10.1159/000330942. [DOI] [PubMed] [Google Scholar]
- 83.Arboix A, Rodriguez-Aguilar R, Oliveres M, Comes E, Garcia-Eroles L, Massons J. Thalamic haemorrhage vs internal capsule-basal ganglia haemorrhage: clinical profile and predictors of in-hospital mortality. BMC neurology. 2007;7:32. doi: 10.1186/1471-2377-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arboix A, Comes E, Garcia-Eroles L, Massons J, Oliveres M, Balcells M, et al. Site of bleeding and early outcome in primary intracerebral hemorrhage. Acta neurologica Scandinavica. 2002 Apr;105(4):282–8. doi: 10.1034/j.1600-0404.2002.1o170.x. [DOI] [PubMed] [Google Scholar]
- 85.Ferrante L, Acqui M, Mastronardi L, Celli P, Lunardi P, Fortuna A. Posterior inferior cerebellar artery (PICA) aneurysm presenting with SAH and contralateral crural monoparesis: a case report. Surgical neurology. 1992 Jul;38(1):43–5. doi: 10.1016/0090-3019(92)90210-e. [DOI] [PubMed] [Google Scholar]
- 86.Greene KA, Marciano FF, Dickman CA, Coons SW, Johnson PC, Bailes JE, et al. Anterior communicating artery aneurysm paraparesis syndrome: clinical manifestations and pathologic correlates. Neurology. 1995 Jan;45(1):45–50. doi: 10.1212/wnl.45.1.45. [DOI] [PubMed] [Google Scholar]
- 87.Yeo SS, Choi BY, Chang CH, Kim SH, Jung YJ, Jang SH. Evidence of corticospinal tract injury at midbrain in patients with subarachnoid hemorrhage. Stroke; a journal of cerebral circulation. 2012 Aug;43(8):2239–41. doi: 10.1161/STROKEAHA.112.661116. [DOI] [PubMed] [Google Scholar]
- 88.van Gijn J, van Dongen KJ, Vermeulen M, Hijdra A. Perimesencephalic hemorrhage: a nonaneurysmal and benign form of subarachnoid hemorrhage. Neurology. 1985 Apr;35(4):493–7. doi: 10.1212/wnl.35.4.493. [DOI] [PubMed] [Google Scholar]
- 89.Fujiwara S, Uhrig L, Amadon A, Jarraya B, Le Bihan D. Quantification of iron in the non-human primate brain with diffusion-weighted magnetic resonance imaging. NeuroImage. 2014 Nov 15;102(Pt 2):789–97. doi: 10.1016/j.neuroimage.2014.08.049. [DOI] [PubMed] [Google Scholar]