Abstract
Phthalates impact adipocyte morphology in vitro, but the sex-specific adipogenic signature immediately after perinatal di(2-ethylhexyl) phthalate (DEHP) exposure and adulthood physiology following a high-fat (HF) dietary challenge are unknown. In the current study, pregnant and lactating dams received DEHP (300 mg/kg body weight) or oil. At weaning (postnatal day (PND) 21), adipose tissue was sampled for real-time PCR. The remaining offspring consumed a control or HF diet. DEHP decreased % fat in males at birth from 13.9%±0.2 to 11.8%±0.6 (mean±SEM), representing a 15.1% decrease in fat by DEHP, and these males caught up in adiposity to controls by PND21. Adult DEHP-exposed males had a 27.5% increase in fat (12.5%±0.9% in controls vs. 15.9%±1.5% in the DEHP group); adipocyte perimeter was increased as well, with fewer small/medium-sized adipocytes, and decreased cell number compared to oil controls. HF diet intake in DEHP-exposed males further increased male energy intake and body weight and led to glucose intolerance. In PND21 males, DEHP increased the expression of adipogenic markers (Pparg1, Cebpa, Adipoq, Ppard, Fabp4, Fasn, Igf1), decreased Lep, and decreased markers of mesenchymal stem cell commitment to the adipogenic lineage (Bmp2, Bmp4, Stat1, Stat5a) compared to oil controls. These data suggest that DEHP may decrease the adipocyte pool at birth, which initially increases adaptive adipocyte maturation and lipid accumulation, but leads to adipose tissue dysfunction in adulthood, decreasing the capacity to adapt to a HF diet, and leading to systemic glucose intolerance.
Keywords: adipose, phthalates, diabetes, Pparg, obesity, programming
1. INTRODUCTION
Despite efforts to curb obesity rates, the obesity epidemic continues to contribute to an increase in metabolic syndrome in developed as well as developing countries. Obesity and metabolic syndrome have been associated with various lifestyle and genetic factors. Importantly, although the role of environmental chemicals in the development of metabolic syndrome is not clearly understood, a growing body of evidence is beginning to suggest that exposures to several endocrine disrupting environmental chemicals (EDCs), including phthalates [1], are associated with metabolism-related diseases in humans, including obesity and type 2 diabetes.
Of the EDCs being investigated, the plasticizer di (2-ethylhexyl) phthalate (DEHP) is one of the most ubiquitous environmental contaminants, with measurable concentrations of its metabolites detected in the urine of nearly 100% of the humans sampled [2–4]. While DEHP exposure has been primarily associated with reproductive problems [5, 6], studies in humans have shown that phthalate exposure is also associated with increased waist circumference as well as diabetes [7, 8]. In animals, adulthood exposure to DEHP has been shown to influence insulin sensitivity, liver metabolism, and energy balance [9–12], and studies specifically focusing on prenatal exposure to numerous EDCs, including phthalates, suggest that prenatal exposure to EDCs may disrupt metabolism (reviewed [13]). However, the mechanisms underlying these effects remain to be elucidated, especially in males versus females.
White adipose tissue (WAT) in adult animals is primarily comprised of mature adipocytes that contain a single lipid vacuole and are responsible for both buffering circulating lipids and contributing to systemic glucose homeostasis. The process of adipocyte maturation occurs in several regulated steps. Immature preadipocytes originate from mesenchymal stem cells in the process that regulates adipocyte cell number (hypo- or hyper-plasia), and preadipocytes further mature to adipocytes, which can then adapt in size (hypo-or hyper-trophy) in response to metabolic cues [14]. Due to this multi-step and ontogenic regulation, the process of white adipose tissue expansion is complicated and involves both prenatal and postnatal cues. Studies in animals suggest that increased adipocyte size and number can both be programmed in utero by numerous factors [15–17]. This prenatal shift in adipocyte morphology likely increases an organism’s propensity for increased fat mass accumulation in response to certain postnatal signals, including the intake of a high-fat diet. Perinatal exposure to DEHP can induce obesity and adipose tissue inflammation in adult offspring [18]. Furthermore, while mono-(2-ethylhexyl) phthalate (MEHP), the active metabolite of DEHP, has previously been shown to induce pre-adipocyte [19] and adipocyte [20] differentiation in human cell lines, and DEHP was shown to induce adipogenesis in a murine mesenchymal stem cell line [21], the molecular mechanisms behind the observed consequences of developmental DEHP exposure in vivo are largely unknown.
Due to the critical role of both in utero and postnatal events in adipose tissue metabolism, the proposed role of DEHP in inducing adipogenesis, and the known sex differences in adipose tissue metabolism, the current study investigated the effect of a postnatal high-fat diet on adipose morphology in male and female rats perinatally exposed to DEHP. Furthermore, unlike previous studies investigating the effects of DEHP on adipogenesis in adults, we specifically assessed sex-specific molecular markers in adipose tissue immediately following developmental exposure, and then investigated physiology and adipose tissue morphology in adult animals. We observed that male, but not female offspring perinatally exposed to DEHP had increased adiposity in adulthood. Furthermore, exposure of these males to a post-weaning HF diet increased energy intake and body weight, decreased the number of small and medium-sized adipocytes, and led to glucose intolerance. At birth, DEHP-exposed pups had the same body weight, but lower % body fat than their oil controls, and these males caught up in adiposity to controls by the end of the weaning period (PND21). Adipose tissue of PND21 male, but not female pups had increased expression of markers of adipogenesis and de novo fatty acid synthesis, but decreased expression of markers of mesenchymal stem cell to pre-adipocyte commitment. These data suggest that in the current model, gestational DEHP exposure may decrease the adipocyte pool at birth, which initially increases adaptive catch-up adipocyte maturation and lipid accumulation, but decreases the capacity of adipose tissue to appropriately respond to a HF diet in adulthood, leading to glucose intolerance.
2. METHODS
2.1. DEHP Dosing and Animal Experimental Design during Gestation and Weaning
Timed-pregnant female Sprague-Dawley rats (n=6 per treatment group) were obtained from Charles River Laboratories (Wilmington, MA, USA) on gestational day 2. Pregnant dams were individually housed in ventilated cages in a temperature-controlled environment, fed a modified AIN-93G diet (Table 1), and received DEHP-free (reverse osmosis) water ad libitum. Beginning on gestational day 6 until postnatal day (PND) 21, pregnant and lactating dams were orally dosed with either vehicle control (Oil; tocopherol-stripped corn oil) or DEHP (DEHP; 300 mg/kg BW; Cat# 36735, Sigma-Aldrich, St. Louis, MO) using a 1000 µL Eppendorf pipette and sterilized tips. Pregnant dams were weighed every 3 days, and the dose of DEHP was adjusted based on body weight. To account for the additional calories being consumed from oil by oil vehicle controls, the liquid DEHP solution for the DEHP treatment group was first mixed with the same volume of oil as was given to controls.
TABLE 1.
Diet Composition*
| Control (C) |
High-Fat (HF) |
|||
|---|---|---|---|---|
| % |
Gram |
kcal |
Gram |
kcal |
| Protein | 20 | 20 | 24 | 20 |
| Carbohydrate | 64 | 64 | 41 | 35 |
| Fat | 7 | 16 | 24 | 45 |
| Ingredients | ||||
| Casein | 200 | 800 | 200 | 800 |
| L-Cystine | 3 | 12 | 3 | 12 |
| Corn Starch | 397.5 | 1590 | 105 | 420 |
| Maltodextrin | 132 | 528 | 132 | 528 |
| Sucrose | 100 | 400 | 100 | 400 |
| Cellulose | 50 | 0 | 50 | 0 |
| Soybean Oil | 70 | 630 | 70 | 630 |
| Lard | 0 | 0 | 130 | 1170 |
| Mineral Mix | 35 | 0 | 35 | 0 |
| Vitamin Mix | 10 | 40 | 10 | 40 |
|
Choline Bitartrate |
2.5 | 0 | 2.5 | 0 |
Research Diets, Inc, New Brunswick, NJ
While most humans have regular daily exposure to DEHP, exact exposures have yet to be characterized. Although the U.S. Environmental Protection Agency reference dose for DEHP is 20 µg/kg/day and the lowest-observed-adverse-effect level (LOAEL) of DEHP is 140 mg/kg/day, it is likely that humans are routinely exposed to much higher doses of phthalates because Becker et al. have shown that levels of DEHP in household dust range from 400–700 mg/kg [22]. Multiple doses were not used in the current study because the addition of postnatal diet as a factor limited our ability to include additional dosing groups. However, the dose (300 mg/kg BW) was selected to mimic other rodent studies that have reported adverse reproductive outcomes at this dose, which allowed for the evaluation of metabolic disruptions by DEHP at a dose where male reproductive dysregulations were previously clearly documented [23, 24].
Immediately after birth, litter sex distributions and offspring weights were recorded, and 4 male and 4 female pups were randomly placed back with each dam in respective oil or DEHP-treated groups.
2.2. Animal Experimental Design after Weaning Using a High-fat Dietary Challenge
To determine the effects of a HF dietary challenge in male and female pups developmentally exposed to DEHP, PND21 pups were mixed (within sex and treatment group), 5 male and 5 female removed from each treatment group for sampling, and the remainder of the pups were randomized and placed into new cages, with 2 or 3 offspring per cage, separated by sex and maternal dosing group. At this point, offspring were separated into 4 groups (n=5 males and n=5 females), such that half of the oil-treated offspring consumed a C diet (AIN93G rodent diet for growth Oil-C), while half consumed a high-fat diet (Oil-HF) to model the intake of a western-style diet alone without DEHP exposure, and half of the DEHP-exposed offspring consumed a C diet (DEHP-C), while half were further challenged with a HF diet (DEHP-HF), to model the intake of a HF diet in animals prenatally exposed to DEHP. The HF diet has a higher amount of kcal coming from fat (45%) when compared to the AIN93G diet (16%). Recent NHANES data show that the average intake of fat in the U.S. is 33.7% of total kcals, which is at the higher range of recommended intake for adults of 20–35% kcals from fat [25] and the intake of an energy-dense western-style or high-fat (HF) diet is unquestionably linked to the development of metabolic syndrome [26]. Since many individuals likely consume more fat than the population mean, the HF diet utilized in the current study models a relevant dietary pattern in humans.
At the onset of puberty, all animals were single-housed, but remained on these different diets until the end of the study (PND110) when they were euthanized with CO2. At both collection points (weaning and adulthood), all tissues were either snap-frozen in liquid nitrogen and stored at −80°C or fixed in 10% formalin for future analyses.
2.3. Serum Testosterone Measurement
As DEHP has been suggested to be anti-androgenic in previous rodent models, total testosterone was measured at weaning on PND21 and in adult males on PND110. Serum samples were thawed on ice and analyzed in triplicate per manufacturer’s instruction using the Testosterone enzyme-linked immuno assay (ELISA) kit from DRG (Cat #EIA-1559). The kit is reported to have 100.0 cross-reactivity with testosterone, 3.3 cross reactivity with 11β-Hydroxytestosterone and 19-Nortestosterone, <1.0 with 5α-Dihydrotestosterone and Androstenedione, and <0.01 with other steroid hormones. The intra-assay variation (as the mean of %CV values) was 1.9%.
2.3. Oral Glucose Tolerance Test (OGTT)
Because DEHP exposure has previously been associated with altered glucose homeostasis in humans and animal models, we assessed glucose tolerance on PND22 and PND100, when offspring were fasted overnight, and fasted blood glucose was measured as the baseline the following morning. Immediately following the fasted measurement, animals were orally gavaged with a 2g/kg BW bolus of D-glucose dissolved in water (50% w/v). Plasma glucose measurements were taken after 30, 60, and 120 minutes of the bolus using the Accu-Chek Glucometer and Comfort Curve strips (Roche, Indianapolis, IN) from a tail vein nick.
2.4. Body Composition
To determine whether DEHP exposure affected fat mass throughout the study, and whether a HF diet had an additional impact, body composition was measured on PND1, at weaning on PND21, after the onset of puberty on PND60, and on PND90 using the EchoMRI-700 Body Composition Analyzer (Echo Medical Systems, Houston, TX), which allows for the measurement of fat and lean mass in conscious and unrestrained animals. On PND1 and PND21, male and female pups were scanned as a litter but separated by sex, and on PND60 and PND90, offspring were scanned individually.
2.5. Adipose Histopathology
To assess the effect of DEHP exposure, with and without a HF diet, on adipose tissue morphology in adult animals, gWAT samples were fixed in formalin, embedded in paraffin using a routine protocol and 3 µm thick sections were stained with H&E. Images were taken under 20X magnification and 2 independent fields were chosen for analysis from 5 male or female offspring. Approximately 80–150 independent adipocytes were manually circled and counted per image in order to calculate the perimeter and cell number (ImageJ Software, NIH, Bethesda, MD). Cell number density calculation was performed using a geometric approach as previously reported [27]. Briefly, “adipose tissue cell density” was calculated by first determining cell area and the number of these cells that are expected to fit into each independent window view without overlap. Where cellular borders were not clearly obvious, circularity was predicted by finding the center of cells where at least half of the perimeter was visible, and using the circle feature within the software to predict the full cell perimeter (and area). The actual number of cells within each view was then divided by the calculated expected cell number, such that a lower ratio indicates a decrease in adipose tissue cell density.
2.6. Adipose RNA Isolation and RT-PCR Analysis in PND21 Pups and PND110 Adults
To investigate the adipogenic expression signature in PND21 pups immediately following developmental DEHP exposure, and the mRNA expression of Ar in adult animals, RNA isolation and RT-PCR were performed as previously described [27]. Briefly, frozen WAT (100 mg) from 5 male and 5 female PND21 pups from each treatment group was ground in liquid nitrogen with mortar and pestle. Because pre-pubertal PND21 pups had very little gWAT, adipose sampling included gonadal and renal adipose tissue to assure that sufficient sample was available for analysis. Total RNA isolation, DNase I treatment (to eliminate any DNA contamination), cDNA synthesis and real-time PCR were performed as previously described [28] with an additional spin down step during RNA isolation to remove the lipid layer in adipose. Pparg1 and Pparg2 transcription rate was determined by designing primers that cover both an exon and intron of the gene to measure unspliced pre-mRNA, as previously described [29]. This procedure has been reported to be a valid technique for analyzing transcription rate [30], and was adapted from a method originally described by Lipson & Baserga [31], except that pre-mRNA amounts were quantified by real-time PCR. For all realtime PCR reactions, a serial dilution was used to create a standard curve for quantification and a dissociation curve was analyzed following each reaction. All primers for real-time PCR analysis (Table 2) were designed using the VectorNTI® software (Life Technologies™, Grand Island, NY), analyzed using BLAST, and synthesized by IDT (Coralville, IA). All mRNA data were normalized to the housekeeping gene encoding ribosomal protein L7a (rpl7a).
TABLE 2.
Primer Information
| Gene | Forward Sequence and Location | Reverse Sequence and Location | |
|---|---|---|---|
| Adipoq | TTCAAGAAGGACAAGGCCGT (+540) | CCAGATGGAGGAGCATGGA(+630) | |
| Ar | GAAATGGGACCTTGGATGGAGA (+2508) | TAAAACGTGGTCCCTGGTACTGTC (+2585) | |
| Bmp2 | TGATGCGATGGACAGCACAGG (+688) | ACCTGGCTTCTCCTCTAAGTGGGC (+764) | |
| Bmp4 | CAGCAGCATCCCAGAGAATGAGG (+573) | GTCCACCTGCTCCCGAAATAGC (+639) | |
| Cebpa | AGTCGGTGGATAAGAACAGCAACG (+821) | GCTGTTTGGCTTTATCTCGGCTC(+910) | |
| Cebpb | AGAACGAGCGGCTGCAGAAGA (+1220) | GAACAAGTTCCGCAGCGTGC(+1287) | |
| Fabp4 | AAGAAGTGGGAGTTGGCTTCGC (+106) | ACCAAGTCCCCTTCTACGCTGATG (+190) | |
| Fasn | CTTTGTGAGCCTCACCGCCAT (+1747) | ATGCCATCAGGTTTCAGCCCC (+1811) | |
| Igf1 | GGTGGACGCTCTTCAGTTCGTG (+454) | TCTGTGGTGCCCTCCGAATG (+545) | |
| Irs1 | AGGACTGGGGGAGACTTAGTC (+307) | TCATGCCCAGAGGGAAAG (+384) | |
| Rpl7a | GAGGCCAAAAAGGTGGTCAATCC (+64) | CCTGCCCAATGCCGAAGTTCT(+127) | |
| Lep | CTCACCAGCTTGCCTTCCCA (+112) | AGGTCTCGCAGGTTCTCCAGGT (+176) | |
| Lpl | GAGAACATTCCCTTCACCCTGCC (+1296) | CCGATGTCCACCTCCGTGTAAA (+1375) | |
| Ppara | ATGGCTGAGAAGACGCTTGTGG (+1122) | TCGGACCTCTGCCTCCTTGTT (+1190) | |
| Ppard | GCTCACCGAGTTCGCCAAGAAC (+1024) | CCTCATGCACGCCGTACTTGAG (+1112) | |
| Pparg1 | |||
| mRNA | CGCTGATGCACTGCCTATGAGC (+51) | GGTCCACAGAGCTGATTCCGAAGT (+148) | |
| Transcription Rate | CGCTGATGCACTGCCTATGAGC (+51) | CCCGAAGCATCCCTTACAGCAA (+123) | |
| Pparg2 | |||
| mRNA | TAGCCTGGGCTGCTTTTATA (+15) | GGGAGTTAAGATGAATTTAGCG (+117) | |
| Transcription Rate | ACTCCCATTGAGTAGGTG (+112) | ACCCTCTAACAAAGTGCC (+164) | |
| Stat1 | CAGTGGCTAGAAAAGCAAGACTGGG (+396) | AGAGCAGGTCATGGAAGCGGAT (+474) | |
| Stat5a | GTCTCAGTTCAGCGTTGGCAGC (+1385) | ACAATGACGACCACAGGGAGGG (+1459) | |
| Stat5b | TCAACGCCACCATCACAGACATC (+962) | GGAGGCTGCTTCTCGATGATGAA (+1031) | |
2.7. Statistical Analysis
Maternal weight gain, litter size, sex distribution, maternal %fat mass at delivery and weaning, % fat or lean mass at PND1 and PND21, glucose values and the area under the curve, and all mRNA data were analyzed using one-way ANOVA and body weight from PND1 to PND21 was analyzed using repeated measures one-way ANOVA to determine the overall effect of DEHP. Body weight from PND1 to PND21 was analyzed using repeated measures. All data in adult animals were analyzed in n=5 offspring (except for the OGTT) by first using one-factor ANOVA to confirm the significance of the model, followed by independent comparisons of all groups. gWAT adipocyte perimeter, adipocyte density, and adipocyte perimeter distribution (small+medium adipocytes) at adulthood were calculated in n=5 male or female offspring from each treatment group with 2 fields counted per animal. Values for the OGTT glucose area under the curve (AUC) at adulthood were analyzed in n=4 male or female offspring and on PND22 in n=3 male or female offspring from each treatment group using the following equation: AUC = (0.25*fasted) + (0.5*30min) + (0.75*60min) + (0.5*120min). For all one-way ANOVA, normality was checked using the Shapiro-Wilk test (with all data being normal); for the repeated measures one-way ANOVA, normality was checked using the Kolmogorov-Smirnov test and confirmed using a Q-Q plot of the residuals. All ANOVA and regression analyses (with Pearson’s correlation being shown to demonstrate relationships between variables) were performed in SAS (Chicago, IL) and differences were considered significant at P<0.05.
3. RESULTS
3.1. DEHP exposure had no observable effect on pregnancy outcomes, liver weights and testosterone levels after weaning or in adulthood
DEHP had no effect on litter size or sex distribution (%male). DEHP exposure also had no effect on maternal gestational weight gain, or % fat mass either at delivery or at weaning on PND21 (Table 3).
TABLE 3.
Maternal Characteristics
| Variable | Oil Control | DEHP |
|---|---|---|
| Maternal Weight Gain (g) | 171.4 ± 6.4 | 179.9 ± 11.4 |
| Litter Size | 12.8 ± 0.7 | 12.7 ± 0.4 |
| Sex Distribution (% Male) | 41.5 ± 5.7 | 51.8 ± 4.6 |
| Maternal % Fat Mass at Delivery (PND1) | 14.7 ± 0.4 | 15.9 ± 0.2 |
| Maternal % Fat Mass at Weaning (PND21) | 10.3 ± 0.9 | 11.3 ± 0.3 |
P<0.05 comparing Oil Control vs. DEHP within each gender
PND: Postnatal day
As a marker of toxicity, DEHP had no significant effect on liver weights, either at weaning on PND21 (1.89±0.07 g in the oil group vs. 1.97±0.89 g in DEHP males, and 1.49±0.07 g in the oil group vs. 1.63+0.06 g in DEHP females), or in adults on PND110 (22.28±0.98 g in the oil group vs. 25.91±1.54 g in DEHP males, and 12.08±0.25 g in the oil group vs. 10.85±1.16g in DEHP females).
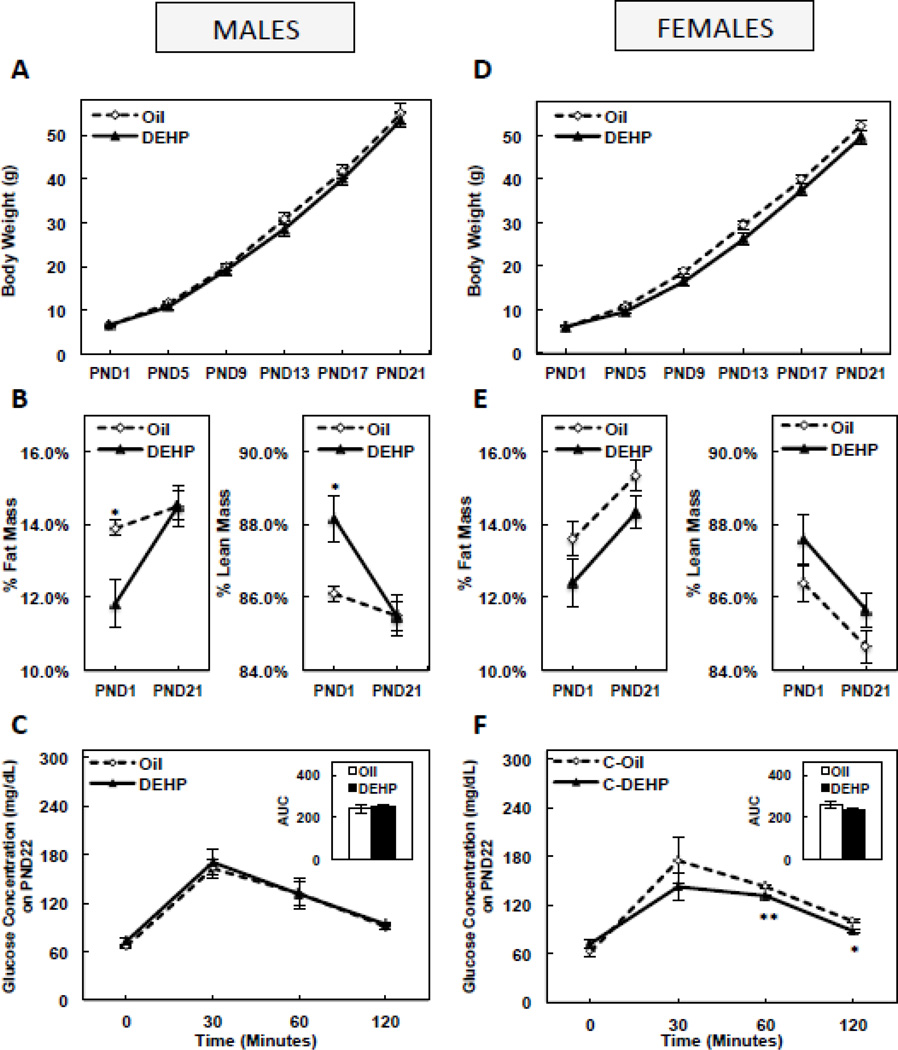
3.2. Perinatal DEHP exposure decreased % fat mass at birth, but induced catch-up adipogenesis during weaning in male, but not female pups, with no effect on circulating testosterone levels
DEHP had no effect on body weight from birth until PND21 in either male (Figure 1A) or female (Figure 1D) pups. However, % fat mass was significantly lower (P<0.05) and lean mass was significantly higher (P<0.05) in males at birth when compared to controls (representing a 15.1% decrease in fat mass or increase in lean mass by DEHP), and adiposity in males caught up to controls by PND 21 (Figure 1B). DEHP trended to decrease adiposity in female pups at birth and on PND21, but this was not significant during either period (Figure 1E). DEHP had no effect on PND21 OGTT glucose levels or the glucose AUC in males (Figure 1C). In females, DEHP decreased OGTT glucose levels at 60 (P<0.005) and 120 (P<0.05) minutes, without a significant effect on the glucose AUC (Figure 1F). DEHP had no significant effect on circulating total testosterone levels in males on PND21 (0.53±0.11 ng/mL in the oil group vs. 0.51±0.12 ng/mL in the DEHP group).
FIGURE 1. Body weight from postnatal day (PND) 1 until PND21 in male (A) and female (D) offspring, fat mass or lean mass on PND1 and PND21 in male (B) and female (E) offspring, and oral glucose tolerance and glucose area under the curve (AUC) on PND 22 in male (C) and female (F) offspring.
Offspring were exposed to maternal DEHP or vehicle (oil). Values are means ± SEM. n=5 male or female offspring. *P<0.05 and **P<0.005.
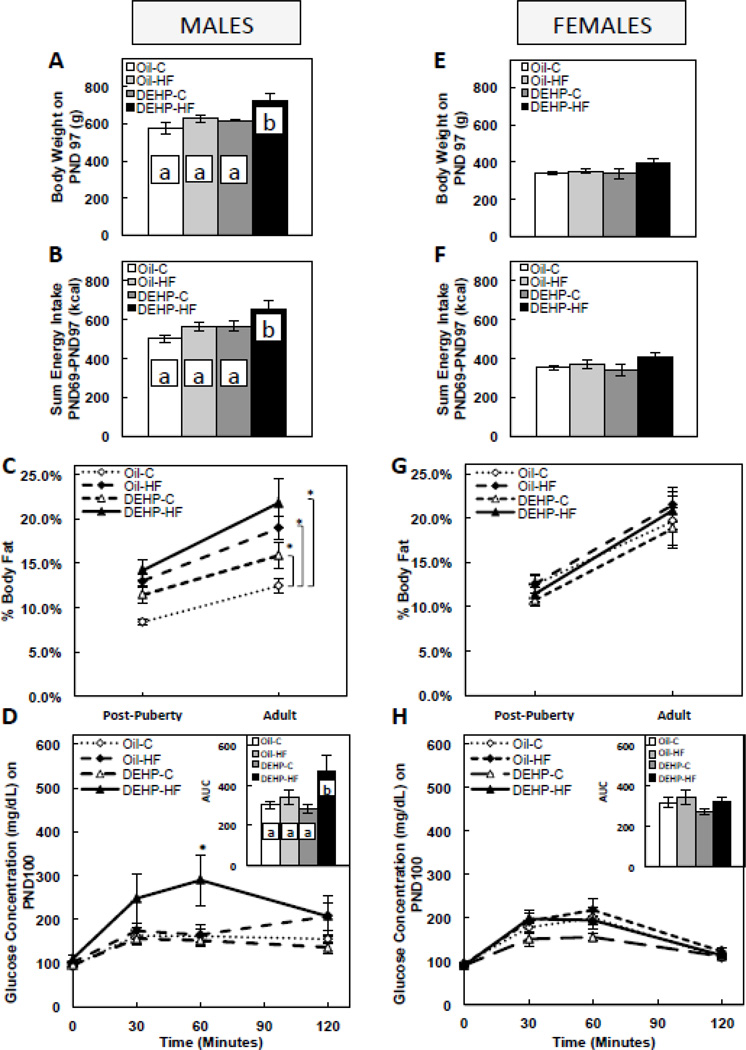
3.3. Perinatal DEHP exposure increased %fat, and a post-weaning HF dietary challenge in DEHP-exposed males increased body weight, energy intake, %fat, and glucose intolerance in adults, with no effect on circulating testosterone levels
In adult males, developmental DEHP exposure alone had no effect on body weight (Figure 2A) or energy intake (Figure 2B), but DEHP-exposed males also consuming a post-weaning HF diet had significantly higher (P<0.05) body weight (Figure 2A) and energy intake (Figure 2B) when compared to controls and either the DEHP or HF groups alone. DEHP-exposed males had increased (P<0.05) % fat mass from the post-puberty until adulthood period (representing a 27.5% increase in fat mass by DEHP), and while the DEHP-HF group appeared to have higher % fat mass when compared to all groups, the difference was only significant (P<0.05) when compared to the control group (Figure 2C). Also in males, developmental DEHP exposure alone had no effect on OGTT glucose levels or the glucose AUC, but DEHP-exposed males also consuming a post-weaning HF diet had significantly higher (P<0.05) glucose levels at 60min, as well as a higher (P<0.05) glucose AUC when compared to controls and either the DEHP or HF groups alone (Figure 2D). In females, neither DEHP exposure alone nor in combination with a post-weaning HF diet affected adulthood body weight (Figure 2E), energy intake (Figure 2F), % fat mass (Figure 2G), or the OGTT glucose levels and the glucose AUC (Figure 2H). DEHP had no significant effect on circulating total testosterone levels in adult males (1.12±0.31 ng/mL in the oil group vs. 1.31±0.21 ng/mL in the DEHP group).
FIGURE 2. Body weight on postnatal day (PND) 97 in male (A) and female (E) offspring, sum of energy intake from PND 69–97 in male (B) and female (F) offspring, % fat mass post-puberty (PND 60) and in adulthood (PND 90) in male (C) and female (G) offspring, and oral glucose tolerance and glucose area under the curve (AUC) in adult male (D) and female (H) offspring (PND 100).
Offspring were exposed to maternal DEHP or vehicle (oil) and then consumed a postnatal control (C) or high-fat (HF) diet. Values are means ± SEM. n=5 male or female offspring for all figures, except n=4 male or female offspring for Figs. D & H. Bars with different letters differ at P<0.05. *P<0.05 when compared to Oil-C (Fig. C) and to Oil-C, Oil-HF, and DEHP-C (Fig. D).
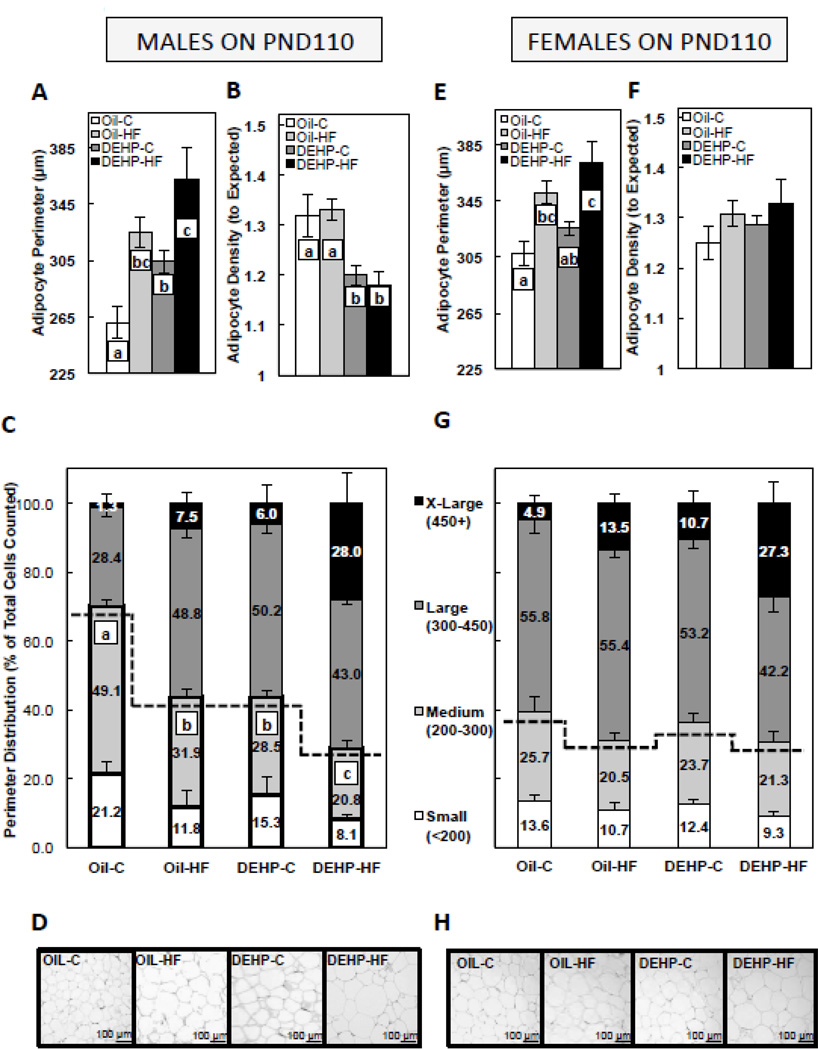
3.4. Perinatal DEHP exposure increased adipocyte perimeter, adipocyte density, and the number of small/medium adipocytes in males, and a post-weaning HF dietary challenge in DEHP-exposed males further decreased the number of small/medium adipocytes
Adipocyte size has been associated with altered insulin sensitivity and glucose uptake [32], and in the current study, glucose AUC was significantly correlated to adipocyte perimeter in males (r = 0.73, P<0.001), where increased adipocyte perimeter was associated with a higher glucose AUC, and no relationship was observed in females. In males, the postnatal HF diet group (Oil-HF) and both DEHP groups (DEHP-C and DEHP-HF) had increased (P<0.05) adipocyte perimeter when compared to the control (Oil-C), and DEHP-HF males also had larger (P<0.05) adipocyte perimeter than DEHP-C males (Figure 3A). Furthermore, male adipocyte density, as a marker of adipocyte number in the sampled depot, was decreased (P<0.05) in both DEHP groups (DEHP-C and DEHP-HF) when compared to unexposed groups (Oil-C and Oil-HF) (Figure 3B). To further characterize the morphology of the adipose tissue, adipocytes were subcategorized by perimeter into 4 size groups: small (<200 µm), medium (200–300 µm), large (300–450 µm), and extra-large (450+ µm). The Oil-HF, DEHP-C, and DEHP-HF had significantly decreased (P<0.05) percentage of small/medium-sized adipocytes in males, and the percentage was lower in DEHP-HF males when compared to DEHP-C and Oil-HF males (Figure 3C, representative images in Figure 3D). In females, the Oil-HF, and DEHP-HF groups had increased (P<0.05) adipocyte perimeter when compared to Oil-C (Figure 3E), whereas there were no group differences in female adipocyte density (Figure 3F) or perimeter distribution (Figure 3G, representative images in Figure 3H).
FIGURE 3. Gonadal white adipose adipocyte perimeter in adult (PND 110) male (A) and female (E) offspring, gonadal white adipose adipocyte density in adult male (B) and female (F) offspring, adipocyte perimeter distribution in male (C) and female (G) offspring, and representative images of gonadal fat depots in male (D) and female (H) offspring.
Offspring were exposed to maternal DEHP or vehicle (oil) and then consumed a postnatal control (C) or high-fat (HF) diet. Values are means ± SEM. n=5 male or female offspring with 2 fields counted per animal. Bars with different letters differ at P<0.05. For perimeter distribution, data are shown as amount of small, medium, large or extra-large adipocytes as percent of total adipocytes counted, and statistical analysis represents group differences of small+medium-sized adipocytes combined. Representative images are 3–4µm hematoxylin & eosin-stained sections under 20X magnification.
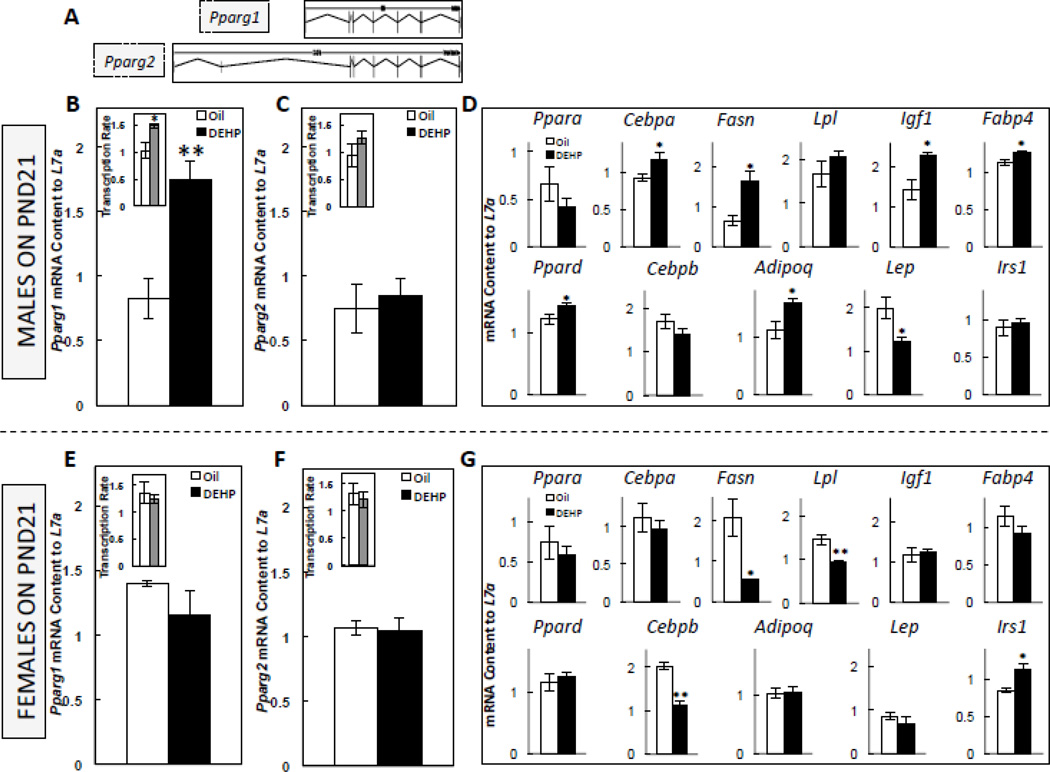
3.5. Perinatal exposure to DEHP increased the PND21 expression of adipogenic genes in the adipose tissue of male offspring
Because DEHP-exposed males had increased adiposity when compared to Oil-C males, and because a post-weaning HF diet increased glucose intolerance in perinatally DEHP-exposed males (DEHP-HF), we assessed the adipose tissue expression of genes associated with the developmental adipogenic cascade immediately after weaning, including the expression of Pparg, the master regulator of adipogenesis (diagram of its 2 major transcripts is shown in Figure 4A). DEHP-exposed male pups had increased expression (P<0.005) and transcription rate (P<0.05) of Pparg1 (Figure 4B), but not Pparg2 (Figure 4C). DEHP-exposed males also had increased (P<0.05) expression of Cebpa, Fasn, Igf1, Adipoq, Ppard, and Fabp4, and decreased (P<0.05) Lep expression, without any change in Cebpb, Irs1, Lpl, or Ppara (Figure 4D). In females, DEHP exposure had no effect on the expression or transcription rate of either Pparg1 (Figure 4E) or Pparg2 (Figure 4F), but DEHP decreased (P<0.05) the expression of Cebpb (P<0.005), Fasn (P<0.05), Lpl (P<0.005), increased Irs1 (P<0.05), and had no effect on Adipoq, Cebpa, Fabp4, Igf1, Lep, Ppara, or Ppard (Figure 4G).
FIGURE 4. Diagram of two major transcripts of the Pparg gene (A), the mRNA expression of Pparg1 in postnatal (PND) 21 male (B) and female offspring (E), the mRNA expression of Pparg2 in PND21 male (C) and female (F) offspring, and the mRNA expression of adipogenic genes in PND 21 male (D) and female (E) offspring.
Offspring were exposed to maternal DEHP or vehicle (oil). Values are means ± SEM. n=5 male or female offspring. *P<0.05 and **P<0.005.
3.6. Perinatal exposure to DEHP decreased the PND21 expression of genes associated with the mesenchymal stem cell commitment to the adipogenic lineage in the adipose tissue of male offspring
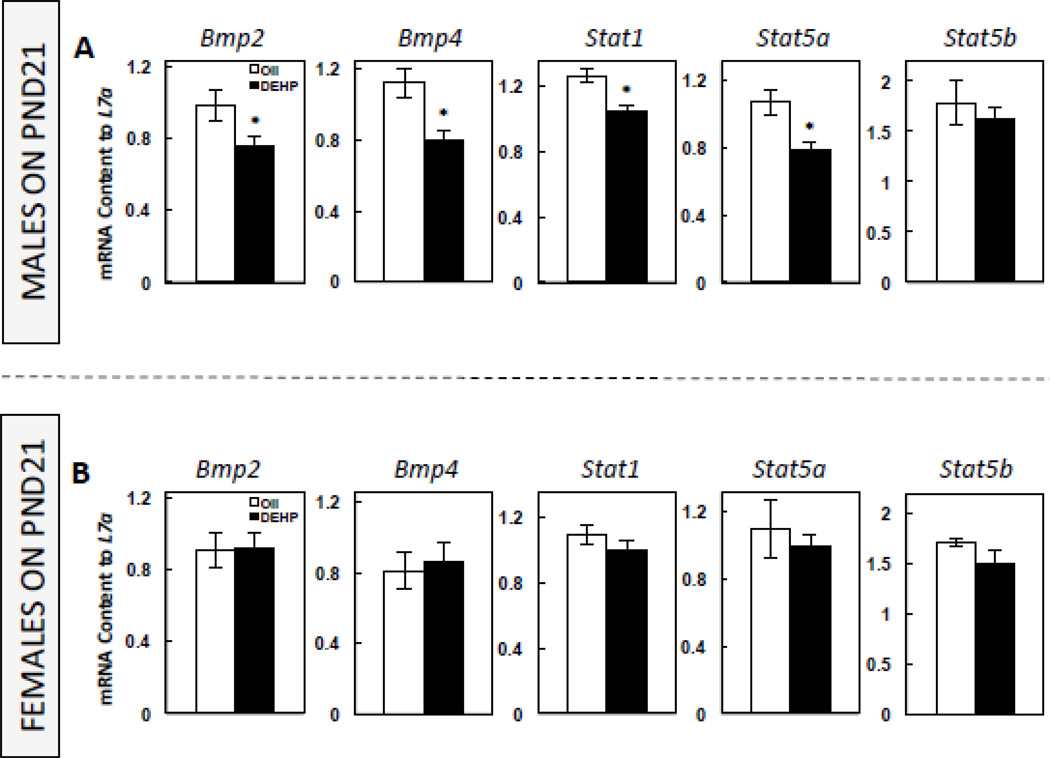
Because adipocyte density, a marker of adipocyte cell number, was decreased in DEHP-exposed males, we also assessed the PND21 expression of genes associated with the mesenchymal stem cell transition to the adipocyte lineage. In males, DEHP significantly decreased (P<0.05) the expression of Bmp2 and Bmp4, as well as of Stat1 and Stat5a, without a significant effect on Stat5b (Figure 5A). DEHP had no effect on the expression of these genes in females (Figure 5B).
FIGURE 5. The mRNA expression of genes associated with the mesenchymal stem cell commitment to the adipogenic lineage in postnatal (PND) 21 male (A) and female offspring (B).
Offspring were exposed to maternal DEHP or vehicle (oil). Values are means ± SEM. n=5 male or female offspring. *P<0.05.
4. DISCUSSION
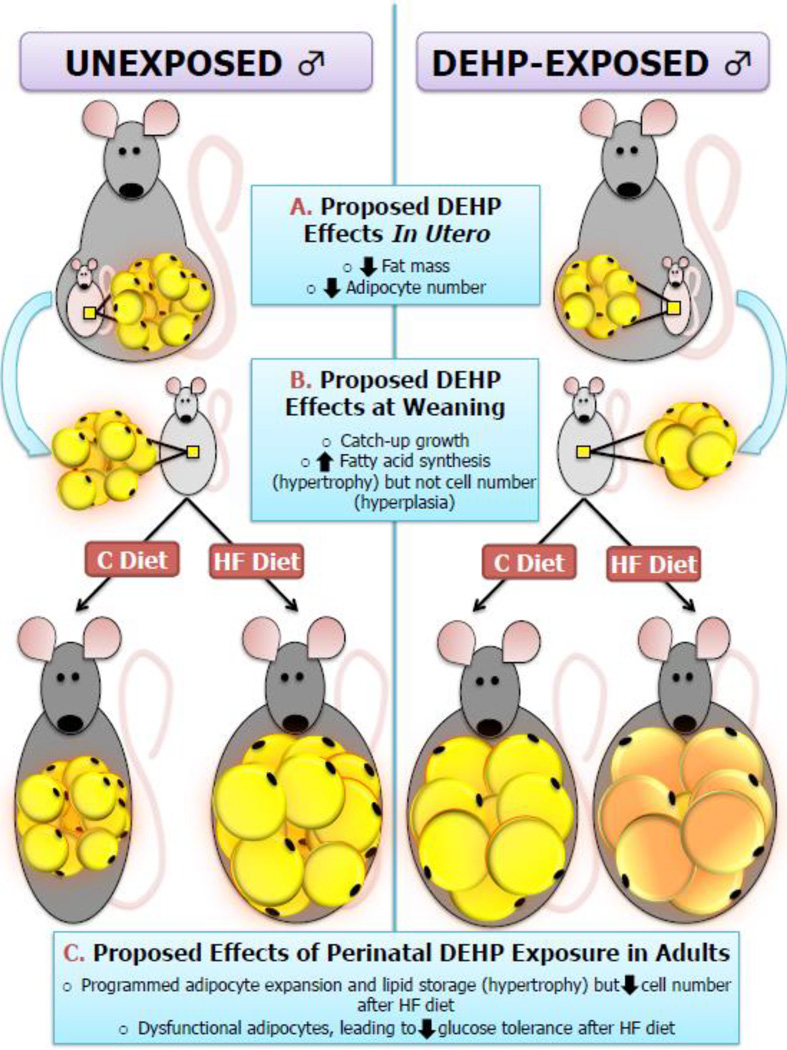
The Developmental Origins of Health and Disease (DOHaD) hypothesis first suggested that maternal nutrition is critical for the fetal programming of adult cardiac and metabolic disorders, and subsequent studies have also suggested that the adulthood adipocyte “set point” can be programmed by numerous in utero factors [33], which may increase the propensity of adipocytes to accumulate lipids and impact the ability of adipose tissue to contribute to systemic glucose handling in response to postnatal cues (including diet). While numerous epidemiological and animal studies have focused on the effects of nutrition and stress during pregnancy on metabolic outcomes, prenatal exposure to environmental endocrine disrupting chemicals has also been proposed to have a role in programming adult-onset metabolic diseases [34]. In the current study, prenatal DEHP exposure resulted in metabolic dysregulations in males, which is consistent with previous studies in rodents [35, 36]. Furthermore, in the current model, glucose intolerance was only observed when developmentally DEHP-exposed males were further challenged with an adulthood HF diet, which could potentially be related to the ability of developmental DEHP exposure to affect adipose tissue structure and function and subsequently alter the response to adulthood fat intake (Figure 6).
FIGURE 6. The proposed action of developmental DEHP exposure on adipose tissue in male offspring in the current model.
Our data suggest that in utero DEHP exposure in males was associated with decreased fat mass, potentially due to a decrease in adipocyte number at birth (A). DEHP-exposed male offspring experienced catch-up adipogenesis at weaning, potentially due to an increase in lipid accumulation and de novo fatty acid synthesis (B). Adult males developmentally exposed to DEHP had increased adiposity and cell perimeter, but fewer small/medium-sized adipocytes and decreased overall cell number. Furthermore, these adult males had glucose intolerance when they also consumed a high-fat diet after weaning.
4.1. Developmental DEHP exposure had sex-specific effects on early-life adipose tissue adipogenic signaling, adulthood morphology, as well as the response to a post-weaning HF diet
In the current study, metabolism-related dysregulations were observed in males, but not females in response to developmental DEHP exposure and a post-weaning HF diet. Adipose metabolism and glucose homeostasis are intrinsically different between males and females, with females being more insulin sensitive, as well as having differently localized adipose depots and increased lipogenesis when compared with postmenopausal females and males [37–39]. A study in mice also showed that in response to a high-fat diet, males and ovariectomized females, but not intact females, were more susceptible to developing obesity and glucose intolerance than controls [40], which agrees with our finding that females did not respond to a HF diet, and that adipocyte size was associated with glucose AUC in males, but not in females. Because animals in the current study were still relatively young adults at the end of the study, it is possible that HF females would have increased adiposity and metabolic disorders with age.
The sex-specific response to DEHP treatment might be due to intrinsic baseline morphological differences between adipose depots, likely driven by the differential expression levels observed in PND21 pups. For example, in the current study, adult females had a higher average % fat mass at baseline compared to males (19.7% and 12.5%, respectively) and also presented with larger adipocytes at baseline than males (320 µm and 262 µm, respectively). These morphological differences were accompanied by obvious sex differences in the baseline expression of several genes at PND21 (Pparg1, Fasn, and Lep), and a drastically different adipogenic signature in response to DEHP. For example, numerous adipogenic genes were affected in males but not females (Pparg1, Cebpa, Igf1, Adipoq, Lep, Ppard, Fabp4), while several other genes affected in females appeared to suggest an anti-adipogenic signature in females in response to DEHP (decreased Cebpb, Fasn, Lpl and increased Irs1). The exact cause for this sex-specific response is unknown, but may be linked to endocrine signals within the adipose tissue itself. DEHP has been proposed to be anti-androgenic, and in men, testosterone has been shown to be inversely correlated with % body fat [41] and to also decrease adipogenesis in vitro [42]. Previous studies in male rodents utilizing doses of DEHP that are similar to the dose used in the current study have shown decreased testosterone levels in adult offspring [23, 24]. While we did not observe differences in circulating total testosterone in adult male offspring in the current study, we assessed the mRNA expression of Androgen Receptor (Ar) in gWAT of adult animals on PND110 (data not shown), and found that in males (but not females), DEHP significantly decreased Ar mRNA expression (1.00±0.04 Oil-C vs. 0.79±0.05 DEHP-C, P<0.05). HF diet also decreased Ar expression (1.00±0.04 Oil-C vs. 0.68±0.60 Oil-HF, P=0.01), and the DEHP-HF group had the lowest Ar expression (0.43±0.03, P<0.0001 to Oil-C; P<0.05 to Oil-HF; and P<0.001 to DEHP-C). Furthermore, there was a negative correlation between adipocyte size in all adult males and Ar expression (r=−0.73, P<0.01). Ar knockout mice have been shown to develop late-onset obesity, but have increased adiponectin release and are not insulin resistant or diabetic [43], which agrees with the observed increased adiposity (but not disrupted glucose homeostasis) in DEHP-exposed males on a control diet in the current study. Based on these observations, as well as the differences in baseline adipocyte measures between the sexes and the effect of DEHP on male adipocyte parameter, it is possible that DEHP directly disrupted hormonal signaling within adipose tissue, and future studies that focus on developmental endocrine disruption within gWAT itself will be needed to determine the precise endocrine mechanisms for the metabolic dysregulation observed in males but not females in response to prenatal DEHP exposure and a postnatal HF diet.
4.2. Developmental DEHP exposure initially decreased adipose tissue expansion, but this was followed by catch-up adipogenesis and increased adulthood adiposity in males
Fetal growth restriction has long been associated with increased adiposity in adulthood, both in humans and animal models [44]. David Barker’s “fetal programming” hypothesis in humans led to numerous studies in animals that utilized developmental nutrition deficits to established intrauterine growth restriction (IUGR) that was followed by an increase in adulthood chronic diseases. Furthermore, the adaptive process of rapid postnatal catch-up growth has been shown to be strongly associated with adulthood chronic diseases, including diabetes and insulin resistance in animal models and human [45, 46]. In the current study, PND1 DEHP-treated males had significantly lower adiposity than oil-treated controls (but not lower body weight), and caught up to the controls by PND21, reflecting a more rapid gain in adiposity during the weaning period. These males also had increased adiposity in adulthood when compared to the oil controls. The mechanisms involved in this adaptive process have not been fully elucidated, but in the case of gestational nutrient restriction, it has been suggested that IUGR establishes a “thrifty phenotype” that is well adapted to its predicted nutrient-deficient postnatal environment. Whether this is also true in the case of environmental chemical exposure is unclear. As previously discussed, DEHP exposure has been associated with metabolic disturbances in humans, and several studies have also shown that DEHP exposure is associated with altered birth outcomes. A population study in China showed that prenatal DEHP exposure was associated with decreased birth length and exposure to another phthalate, dibutyl phthalate, was associated with low birth weight [47]. Furthermore, a study in rats showed that DEHP-exposed rat pups experienced intrauterine growth restriction [48], suggesting that DEHP exposure can interfere with certain aspects of fetal growth, which likely includes metabolic maturation. Additional studies in humans and mechanistic studies in animals are needed to investigate whether growth restriction mediates the proposed relationship between DEHP exposure and increased metabolic syndrome.
4.3. Catch-up adipogenesis in DEHP-exposed males was associated with the increased expression of adipogenic markers but decreased expression of genes associated with the commitment of stem cells to the adipogenic lineage
The commitment of mesenchymal pluripotent stem cells to the adipogenic lineage involves numerous factors. Bmp2 and Bmp4 have been shown to drive stem cell commitment, giving rise to preadipocytes [49], and members of the STAT family of proteins have also been implicated in adipogenic commitment [50, 51]. In the current study, males developmentally exposed to DEHP had decreased Bmp2, Bmp4, Stat1, and Stat5a expression at weaning, which was also associated with a significant decrease in adipocyte density (as a marker of cell number) in adulthood. At birth, these males had decreased adiposity and increased lean mass when compared to controls. These data suggest that decreased adiposity at birth in response to DEHP exposure may be due to a decrease in the number of adipocyte precursors.
We also observed rapid catch-up adipogenesis in these male pups after birth, which was likely due to an increase in adipocyte size and de novo fatty acid synthesis, rather than adipocyte hyperplasia. The differentiation of preadipocytes to mature adipocytes that are capable of storing excess lipids and synthesizing lipids as well as contributing to systemic glucose homeostasis is primarily regulated by peroxisome proliferator-activated receptor gamma (PPARg)-mediated signaling [52], which is accompanied by the increased expression of CCAAT/enhancer binding proteins (Cebpa and Cebpb) [53], as well as other genes associated with lipid accumulation and de novo fatty acid synthesis, including Fasn [54] and Fabp4 [55]. In the current study, PND21 DEHP-exposed males had increased expression of Pparg1, Cebpa, Fasn, Fabp4, Ppard, and Igf1. These mRNA expression data suggest that PND21 male pups had increased adipogenesis at weaning, potentially as an adaptive mechanism in response to restricted adipose tissue development in utero. Interestingly, Adipoq mRNA expression was increased in PND21 DEHP-exposed males. Insulin resistance and obesity are typically associated with a decrease in circulating adiponectin. However, It has been shown that testosterone could actually decrease adiponectin secretion from 3T3-L1 adipocytes and mice (high molecular weight form) [56, 57], and body fat and adiponectin are elevated in androgen receptor knockout mice [58]. As discussed above, we did not observe an overall decrease in circulating testosterone in adult males, but did find that DEHP decreased androgen receptor expression (together with adiponectin expression), which agrees with the data from androgen receptor knockout mice. It is possible that increased Adipoq mRNA expression on PND21 acts as an adaptive mechanism to compensate for the decrease in adipogenic precursors at birth, but additional studies will be needed to determine whether this has a direct impact on the observed consequence of DEHP exposure on adulthood glucose homeostasis in response to a HF diet.
4.4. Developmental DEHP exposure decreased the number of small and medium adipocytes in males, which was associated with glucose intolerance in adults consuming a HF diet
Adipose tissue expansion and large adipocytes, in particular, are thought to contribute to the insulin resistant phenotype [32, 59]. This is due to the fact that in addition to acting as the body’s energy storage depot (in the form of fatty acids and triglycerides), mature adipocytes are also critical for regulating whole-body glucose homeostasis by clearing circulating glucose in response to insulin signals. In the current study, DEHP-exposed males that were further challenged by a post-weaning HF diet had glucose intolerance, which was associated with increased adipocyte perimeter, decreased adipocyte number density, and decreased % of small/medium-sized adipocytes. As previously discussed, PND21 males experienced catch-up adipose tissue expansion, which was potentially associated with an increased rate of adipogenesis but a decrease in the number of adipogenic precursors. Leptin is released from mature adipocytes [60], and PND21 males had decreased adipose Lep expression, which has been shown to positively correlate to preadipocyte number [61], preadipogenic induction [62], and adipogenic differentiation [63]. These data suggest that developmental DEHP exposure and subsequent catch-up adipogenic expansion during weaning increased the tendency for adipocytes from DEHP-exposed males to efficiently store lipids. Increased cell size is known to cause inflammation that leads to insulin resistance [32], so while adipocyte size (and subsequent glucose intolerance) were further increased in DEHP males in response to a HF diet, this potentially occurred due to the apparent decrease in adipocyte number, which may have put additional stress on the adipose tissue “organ” by decreasing individual storage depots (adipocytes).
Interestingly, these physiological adaptations in males in response to DEHP were associated with a substantial increase in the expression and transcription rate of Pparg1 on PND21, without a concurrent increase in Pparg2 expression. The nuclear hormone receptor peroxisome proliferator-activated receptor gamma (PPARg) is a critical transcription factor during adipogenesis. In addition to regulating adipose tissue expansion, PPARg has been shown to directly participate in adipose tissue-related insulin sensitivity. Pparg adipose-specific knockout mice have lipoatrophy and uncontrolled diabetes [64], and individuals with the Pro12Ala polymorphism within the Pparg gene are at an increased risk of having type 2 diabetes [65], highlighting the critical role of this transcription factor in regulating glucose homeostasis. While Pparg has widespread tissue distribution, mature human adipocytes have been shown to have different levels of Pparg1 and Pparg2, which have identical sequences, except for additional nucleotides at the 5-prime end of Pparg2. Mutations in Pparg2, but not Pparg1, have been associated with obesity and diabetes in humans, and Pparg2 has been designated as the “nutrition-responsive” transcript. However, Pparg1 protein appears to be activated earlier during pre-adipocyte differentiation and is expressed at a higher level than that of Pparg2 [66], and both Pparg1 and Pparg2 have been shown to have the ability to independently induce adipogenic differentiation and adipogenesis in 3T3 fibroblasts, but through different transcription factor mechanisms [67]. These observations suggest that the two isoforms have unique upstream regulation as well as differential downstream consequences. Numerous reproductive toxicity and carcinogenicity studies in animals have established that DEHP can act through PPPARg-mediated signaling [68], and cell culture modeling studies suggest that phthalates can efficiently bind to the human Pparg [69], as well as to induce Pparg expression and induce adipogenesis [70]. However, this is the first report, to our knowledge, to suggest that DEHP exposure has transcript-selective effects during development, which appears to be associated with increased lipid accumulation but decreased adipogenic precursor formation. These data suggest that in the current model of developmental DEHP exposure, Pparg1, rather than Pparg2 may have a critical role in driving DEHP-induced adipocyte morphology during development, thus selectively activating signals responsible for lipid accumulation and de novo fatty acid synthesis but decreasing stem cell commitment and decreasing the capacity of adipose tissue to contribute to systemic glucose homeostasis.
In conclusion, the present study is the first to demonstrate that the early-life adipogenic signature in males in response to DEHP-induced growth restriction and catch-up adipogenesis is predictive of adulthood adipocyte morphology and glucose intolerance following a high-fat dietary challenge. Our data suggest that DEHP may decrease the adipocyte pool but hasten early-life adipogenesis, thus disrupting adulthood glucose homeostasis, which is a marker of the metabolic syndrome. The sex-specific deleterious effects of prenatal DEHP exposure and a postnatal HF diet on whole-body energy homeostasis is especially relevant in the current obesogenic environment, and data from the current study characterizing these energy-balance sex differences in vivo contributes to unraveling the roles of EDCs in metabolism-related chronic diseases.
HIGHLIGHTS.
Adult DEHP-exposed males had increased % fat and adipocyte perimeter
HF diet in DEHP-exposed adult males led to glucose intolerance
At birth, DEHP decreased % fat mass in males
At weaning, DEHP increased adipogenic markers in males
At weaning, DEHP decreased markers of commitment to the adipogenic lineage in males
Acknowledgements
The work was funded by an NIEHS training grant (T32ES007326), the College of ACES pilot grant (to Y-X.P.), NIH R01ES019178 (JAF), NIH P01 ES022848 (JAF), and EPA RD-83459301 (JAF). The authors thank Dr. Anna Dilger for the EchoMri use and training, Dr. Hong Chen for her technical expertise, and Dr. Susan Schantz for her careful review of the manuscript.
Glossary
Abbreviations Used
- Adipoq
adiponectin
- AUC
area under the curve
- Bmp2 or 4
bone morphogenic protein 2 or 4
- Cebpa or b
CCAAT/enhancer binding protein alpha or beta
- DEHP
di (2-ethylhexyl) phthalate
- EDC
endocrine disrupting chemical
- Fabp4
fatty acid binding protein 4
- Fasn
fatty acid synthase
- gWAT
gonadal white adipose tissue
- HF
high-fat
- Igf1
insulin-like growth factor 1
- Irs1
insulin receptor substrate 1
- L7a
ribosomal protein L7a
- Lep
leptin
- Lpl
lipoprotein lipase
- MRI
magnetic resonance imaging
- OGTT
oral glucose tolerance test
- Ppara, Ppard, Pparg 1 or 2
peroxisome proliferator-activated receptor alpha, delta, or gamma 1 or 2
- PND
postnatal day
- Stat1 or 5a or 5b
Signal Transducers and Activators of Transcription
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of Ethics: The authors declare no financial conflict of interest. We certify that all applicable institutional and governmental regulations regarding the ethical use of animals were followed during this research (University of Illinois Institutional Animal Care and Use Committee approval #11191).
REFERENCES
- 1.Lind PM, Zethelius B, Lind L. Circulating levels of phthalate metabolites are associated with prevalent diabetes in the elderly. Diabetes Care. 2012;35:1519–1524. doi: 10.2337/dc11-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heudorf U, Mersch-Sundermann V, Angerer J. Phthalates: toxicology and exposure. Int J Hyg Environ Health. 2007;210:623–634. doi: 10.1016/j.ijheh.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Ye X, Pierik FH, Hauser R, Duty S, Angerer J, Park MM, et al. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, the Netherlands: the Generation R study. Environ Res. 2008;108:260–267. doi: 10.1016/j.envres.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adibi JJ, Whyatt RM, Williams PL, Calafat AM, Camann D, Herrick R, et al. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ Health Perspect. 2008;116:467–473. doi: 10.1289/ehp.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christiansen S, Boberg J, Axelstad M, Dalgaard M, Vinggaard AM, Metzdorff SB, et al. Low-dose perinatal exposure to di(2-ethylhexyl) phthalate induces anti-androgenic effects in male rats. Reprod Toxicol. 2010;30:313–321. doi: 10.1016/j.reprotox.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Marsee K, Woodruff TJ, Axelrad DA, Calafat AM, Swan SH. Estimated daily phthalate exposures in a population of mothers of male infants exhibiting reduced anogenital distance. Environ Health Perspect. 2006;114:805–809. doi: 10.1289/ehp.8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James-Todd T, Stahlhut R, Meeker JD, Powell SG, Hauser R, Huang T, et al. Urinary Phthalate Metabolite Concentrations and Diabetes among Women in the National Health and Nutrition Examination Survey (NHANES) 2001–2008. Environ Health Perspect. 2012;120:1307–1313. doi: 10.1289/ehp.1104717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH. Concentrations of urinary phthalate metabolites are associated with increased waist circumference, insulin resistance in adult U.Smales. Environ Health Perspect. 2007;115:876–882. doi: 10.1289/ehp.9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feige JN, Gerber A, Casals-Casas C, Yang Q, Winkler C, Bedu E, et al. The pollutant diethylhexyl phthalate regulates hepatic energy metabolism via species-specific PPARalpha-dependent mechanisms. Environ Health Perspect. 2010;118:234–241. doi: 10.1289/ehp.0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinelli MI, Mocchiutti NO, Bernal CA. Dietary di(2-ethylhexyl)phthalate-impaired glucose metabolism in experimental animals. Hum Exp Toxicol. 2006;25:531–538. doi: 10.1191/0960327106het651oa. [DOI] [PubMed] [Google Scholar]
- 11.Rajesh P, Sathish S, Srinivasan C, Selvaraj J, Balasubramanian K. Exposure to diethyl hexyl phthalate (DEHP) to adult male rat is associated with insulin resistance in adipose tisssue: Protective role of antioxidant vitamins (C & E) J Cell Biochem. 2012 doi: 10.1002/jcb.24399. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt JS, Schaedlich K, Fiandanese N, Pocar P, Fischer B. Effects of Di(2-ethylhexyl) Phthalate (DEHP) on Female Fertility and Adipogenesis in C3H/N Mice. Environ Health Perspect. 2012;120:1123–1129. doi: 10.1289/ehp.1104016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unuvar T, Buyukgebiz A. Fetal and neonatal endocrine disruptors. J Clin Res Pediatr Endocrinol. 2012;4:51–60. doi: 10.4274/Jcrpe.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Billon N, Monteiro MC, Dani C. Developmental origin of adipocytes: new insights into a pending question. Biology of the cell / under the auspices of the European Cell Biology Organization. 2008;100:563–575. doi: 10.1042/BC20080011. [DOI] [PubMed] [Google Scholar]
- 15.Kozak LP, Newman S, Chao PM, Mendoza T, Koza RA. The early nutritional environment of mice determines the capacity for adipose tissue expansion by modulating genes of caveolae structure. PLoS One. 2010;5:e11015. doi: 10.1371/journal.pone.0011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conceicao EP, Trevenzoli IH, Oliveira E, Franco JG, Carlos AS, Nascimento-Saba CC, et al. Higher white adipocyte area and lower leptin production in adult rats overfed during lactation. Horm Metab Res. 2011;43:513–516. doi: 10.1055/s-0031-1275702. [DOI] [PubMed] [Google Scholar]
- 17.Petrighi Polidori G, Lomax MA, Docherty K. Palmitate enhances the differentiation of mouse embryonic stem cells towards white adipocyte lineages. Mol Cell Endocrinol. 2012;361:40–50. doi: 10.1016/j.mce.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Campioli E, Martinez-Arguelles DB, Papadopoulos V. In utero exposure to the endocrine disruptor di-(2-ethylhexyl) phthalate promotes local adipose and systemic inflammation in adult male offspring. Nutrition & diabetes. 2014;4:e115. doi: 10.1038/nutd.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campioli E, Batarseh A, Li J, Papadopoulos V. The endocrine disruptor mono-(2-ethylhexyl) phthalate affects the differentiation of human liposarcoma cells (SW 872) PLoS One. 2011;6:e28750. doi: 10.1371/journal.pone.0028750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feige JN, Gelman L, Rossi D, Zoete V, Metivier R, Tudor C, et al. The endocrine disruptor monoethyl-hexyl-phthalate is a selective peroxisome proliferator-activated receptor gamma modulator that promotes adipogenesis. J Biol Chem. 2007;282:19152–19166. doi: 10.1074/jbc.M702724200. [DOI] [PubMed] [Google Scholar]
- 21.Biemann R, Navarrete Santos A, Navarrete Santos A, Riemann D, Knelangen J, Bluher M, et al. Endocrine disrupting chemicals affect the adipogenic differentiation of mesenchymal stem cells in distinct ontogenetic windows. Biochem Biophys Res Commun. 2012;417:747–752. doi: 10.1016/j.bbrc.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 22.Becker K, Seiwert M, Angerer J, Heger W, Koch HM, Nagorka R, et al. DEHP metabolites in urine of children and DEHP in house dust. International journal of hygiene and environmental health. 2004;207:409–417. doi: 10.1078/1438-4639-00309. [DOI] [PubMed] [Google Scholar]
- 23.Andrade AJ, Grande SW, Talsness CE, Gericke C, Grote K, Golombiewski A, et al. A dose response study following in utero and lactational exposure to di-(2-ethylhexyl) phthalate (DEHP): reproductive effects on adult male offspring rats. Toxicology. 2006;228:85–97. doi: 10.1016/j.tox.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 24.Gray LE, Jr, Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci. 2000;58:350–365. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- 25.Austin GL, Ogden LG, Hill JO. Trends in carbohydrate, fat, and protein intakes and association with energy intake in normal-weight, overweight, and obese individuals: 1971–2006. Am J Clin Nutr. 2011;93:836–843. doi: 10.3945/ajcn.110.000141. [DOI] [PubMed] [Google Scholar]
- 26.Misra A, Singhal N, Khurana L. Obesity, the metabolic syndrome, and type 2 diabetes in developing countries: role of dietary fats and oils. J Am Coll Nutr. 2010;29:289S–301S. doi: 10.1080/07315724.2010.10719844. [DOI] [PubMed] [Google Scholar]
- 27.Strakovsky RS, Lezmi S, Flaws JA, Schantz SL, Pan YX, Helferich WG. Genistein exposure during the early postnatal period favors the development of obesity in female, but not male rats. Toxicol Sci. 2014;138:161–174. doi: 10.1093/toxsci/kft331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strakovsky RS, Pan Y-X. A Decrease in DKK1, a WNT Inhibitor, Contributes to Placental Lipid Accumulation in an Obesity-Prone Rat Model. Biol Reprod. 2011 doi: 10.1095/biolreprod.111.094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strakovsky RS, Zhang X, Zhou D, Pan YX. Gestational High Fat Diet Programs Hepatic Phosphoenolpyruvate Carboxykinase (Pck) Expression and Histone Modification in Neonatal Offspring Rats. J Physiol. 2011 doi: 10.1113/jphysiol.2010.203950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen H, Pan YX, Dudenhausen EE, Kilberg MS. Amino acid deprivation induces the transcription rate of the human asparagine synthetase gene through a timed program of expression and promoter binding of nutrient-responsive basic region/leucine zipper transcription factors as well as localized histone acetylation. J Biol Chem. 2004;279:50829–50839. doi: 10.1074/jbc.M409173200. [DOI] [PubMed] [Google Scholar]
- 31.Lipson KE, Baserga R. Transcriptional activity of the human thymidine kinase gene determined by a method using the polymerase chain reaction and an intron-specific probe. Proc Natl Acad Sci U S A. 1989;86:9774–9777. doi: 10.1073/pnas.86.24.9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammarstedt A, Graham TE, Kahn BB. Adipose tissue dysregulation and reduced insulin sensitivity in non-obese individuals with enlarged abdominal adipose cells. Diabetology & metabolic syndrome. 2012;4:42. doi: 10.1186/1758-5996-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMillen IC, Rattanatray L, Duffield JA, Morrison JL, MacLaughlin SM, Gentili S, et al. The early origins of later obesity: pathways and mechanisms. Adv Exp Med Biol. 2009;646:71–81. doi: 10.1007/978-1-4020-9173-5_8. [DOI] [PubMed] [Google Scholar]
- 34.Casals-Casas C, Desvergne B. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol. 2011;73:135–162. doi: 10.1146/annurev-physiol-012110-142200. [DOI] [PubMed] [Google Scholar]
- 35.Lin Y, Wei J, Li Y, Chen J, Zhou Z, Song L, et al. Developmental exposure to di(2-ethylhexyl) phthalate impairs endocrine pancreas and leads to long-term adverse effects on glucose homeostasis in the rat. Am J Physiol Endocrinol Metab. 2011;301:E527–E538. doi: 10.1152/ajpendo.00233.2011. [DOI] [PubMed] [Google Scholar]
- 36.Maranghi F, Lorenzetti S, Tassinari R, Moracci G, Tassinari V, Marcoccia D, et al. In utero exposure to di-(2-ethylhexyl) phthalate affects liver morphology and metabolism in post-natal CD-1 mice. Reprod Toxicol. 2010;29:427–432. doi: 10.1016/j.reprotox.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Gower BA, Munoz J, Desmond R, Hilario-Hailey T, Jiao X. Changes in intra-abdominal fat in early postmenopausal women: effects of hormone use. Obesity (Silver Spring) 2006;14:1046–1055. doi: 10.1038/oby.2006.120. [DOI] [PubMed] [Google Scholar]
- 38.Kuhl J, Hilding A, Ostenson CG, Grill V, Efendic S, Bavenholm P. Characterisation of subjects with early abnormalities of glucose tolerance in the Stockholm Diabetes Prevention Programme: the impact of sex and type 2 diabetes heredity. Diabetologia. 2005;48:35–40. doi: 10.1007/s00125-004-1614-1. [DOI] [PubMed] [Google Scholar]
- 39.Macotela Y, Boucher J, Tran TT, Kahn CR. Sex and depot differences in adipocyte insulin sensitivity and glucose metabolism. Diabetes. 2009;58:803–812. doi: 10.2337/db08-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stubbins RE, Holcomb VB, Hong J, Nunez NP. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur J Nutr. 2012;51:861–870. doi: 10.1007/s00394-011-0266-4. [DOI] [PubMed] [Google Scholar]
- 41.Allan CA, McLachlan RI. Androgens and obesity. Current opinion in endocrinology, diabetes, and obesity. 2010;17:224–232. doi: 10.1097/MED.0b013e3283398ee2. [DOI] [PubMed] [Google Scholar]
- 42.Singh R, Artaza JN, Taylor WE, Braga M, Yuan X, Gonzalez-Cadavid NF, et al. Testosterone inhibits adipogenic differentiation in 3T3-L1 cells: nuclear translocation of androgen receptor complex with beta-catenin and T-cell factor 4 may bypass canonical Wnt signaling to down-regulate adipogenic transcription factors. Endocrinology. 2006;147:141–154. doi: 10.1210/en.2004-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sato T, Matsumoto T, Yamada T, Watanabe T, Kawano H, Kato S. Late onset of obesity in male androgen receptor-deficient (AR KO) mice. Biochem Biophys Res Commun. 2003;300:167–671. doi: 10.1016/s0006-291x(02)02774-2. [DOI] [PubMed] [Google Scholar]
- 44.Sarr O, Yang K, Regnault TR. In utero programming of later adiposity: the role of fetal growth restriction. Journal of pregnancy. 2012;2012:134758. doi: 10.1155/2012/134758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morrison JL, Duffield JA, Muhlhausler BS, Gentili S, McMillen IC. Fetal growth restriction, catch-up growth and the early origins of insulin resistance and visceral obesity. Pediatr Nephrol. 2010;25:669–677. doi: 10.1007/s00467-009-1407-3. [DOI] [PubMed] [Google Scholar]
- 46.Claris O, Beltrand J, Levy-Marchal C. Consequences of intrauterine growth and early neonatal catch-up growth. Semin Perinatol. 2010;34:207–210. doi: 10.1053/j.semperi.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Lin L, Cao Y, Chen B, Zheng L, Ge RS. Phthalate levels and low birth weight: a nested case-control study of Chinese newborns. J Pediatr. 2009;155:500–504. doi: 10.1016/j.jpeds.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen SQ, Chen JN, Cai XH, Chen GR, Gao Y, Ge RS, et al. Perinatal exposure to di-(2-ethylhexyl) phthalate leads to restricted growth and delayed lung maturation in newborn rats. J Perinat Med. 2010;38:515–521. doi: 10.1515/jpm.2010.083. [DOI] [PubMed] [Google Scholar]
- 49.Huang H, Song TJ, Li X, Hu L, He Q, Liu M, et al. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci U S A. 2009;106:12670–12675. doi: 10.1073/pnas.0906266106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jung HS, Lee YJ, Kim YH, Paik S, Kim JW, Lee JW. Peroxisome proliferator-activated receptor gamma/signal transducers and activators of transcription 5A pathway plays a key factor in adipogenesis of human bone marrow-derived stromal cells and 3T3-L1 preadipocytes. Stem cells and development. 2012;21:465–475. doi: 10.1089/scd.2010.0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stewart WC, Morrison RF, Young SL, Stephens JM. Regulation of signal transducers and activators of transcription (STATs) by effectors of adipogenesis: coordinate regulation of STATs 1, 5A, and 5B with peroxisome proliferator-activated receptor-gamma and C/AAAT enhancer binding protein-alpha. Biochim Biophys Acta. 1999;1452:188–196. doi: 10.1016/s0167-4889(99)00129-9. [DOI] [PubMed] [Google Scholar]
- 52.Tontonoz P, Hu E, Spiegelman BM. Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor gamma. Curr Opin Genet Dev. 1995;5:571–576. doi: 10.1016/0959-437x(95)80025-5. [DOI] [PubMed] [Google Scholar]
- 53.Lane MD, Tang QQ, Jiang MS. Role of the CCAAT enhancer binding proteins (C/EBPs) in adipocyte differentiation. Biochem Biophys Res Commun. 1999;266:677–683. doi: 10.1006/bbrc.1999.1885. [DOI] [PubMed] [Google Scholar]
- 54.Schmid B, Rippmann JF, Tadayyon M, Hamilton BS. Inhibition of fatty acid synthase prevents preadipocyte differentiation. Biochem Biophys Res Commun. 2005;328:1073–1082. doi: 10.1016/j.bbrc.2005.01.067. [DOI] [PubMed] [Google Scholar]
- 55.Samulin J, Berget I, Lien S, Sundvold H. Differential gene expression of fatty acid binding proteins during porcine adipogenesis. Comparative biochemistry and physiology Part B, Biochemistry & molecular biology. 2008;151:147–152. doi: 10.1016/j.cbpb.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 56.Xu A, Chan KW, Hoo RL, Wang Y, Tan KC, Zhang J, et al. Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Biol Chem. 2005;280:18073–18080. doi: 10.1074/jbc.M414231200. [DOI] [PubMed] [Google Scholar]
- 57.Nishizawa H, Shimomura I, Kishida K, Maeda N, Kuriyama H, Nagaretani H, et al. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes. 2002;51:2734–2741. doi: 10.2337/diabetes.51.9.2734. [DOI] [PubMed] [Google Scholar]
- 58.Yanase T, Fan W, Kyoya K, Min L, Takayanagi R, Kato S, et al. Androgens and metabolic syndrome: lessons from androgen receptor knock out (ARKO) mice. J Steroid Biochem Mol Biol. 2008;109:254–257. doi: 10.1016/j.jsbmb.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 59.Monickaraj F, Gokulakrishnan K, Prabu P, Sathishkumar C, Anjana RM, Rajkumar JS, et al. Convergence of adipocyte hypertrophy, telomere shortening and hypoadiponectinemia in obese subjects and in patients with type 2 diabetes. Clin Biochem. 2012 doi: 10.1016/j.clinbiochem.2012.07.097. [DOI] [PubMed] [Google Scholar]
- 60.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 61.Chen XL, Hausman DB, Dean RG, Hausman GJ. Hormonal regulation of leptin mRNA expression and preadipocyte recruitment and differentiation in porcine primary cultures of S-V cells. Obesity research. 1998;6:164–172. doi: 10.1002/j.1550-8528.1998.tb00331.x. [DOI] [PubMed] [Google Scholar]
- 62.Kollmer M, Buhrman JS, Zhang Y, Gemeinhart RA. Markers Are Shared Between Adipogenic and Osteogenic Differentiated Mesenchymal Stem Cells. Journal of developmental biology and tissue engineering. 2013;5:18–25. doi: 10.5897/JDBTE2013.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Melzner I, Scott V, Dorsch K, Fischer P, Wabitsch M, Bruderlein S, et al. Leptin gene expression in human preadipocytes is switched on by maturation-induced demethylation of distinct CpGs in its proximal promoter. J Biol Chem. 2002;277:45420–45427. doi: 10.1074/jbc.M208511200. [DOI] [PubMed] [Google Scholar]
- 64.Wang F, Mullican SE, DiSpirito JR, Peed LC, Lazar MA. Lipoatrophy and severe metabolic disturbance in mice with fat-specific deletion of PPARgamma. Proc Natl Acad Sci U S A. 2013;110:18656–18661. doi: 10.1073/pnas.1314863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tonjes A, Stumvoll M. The role of the Pro12Ala polymorphism in peroxisome proliferator-activated receptor gamma in diabetes risk. Curr Opin Clin Nutr Metab Care. 2007;10:410–414. doi: 10.1097/MCO.0b013e3281e389d9. [DOI] [PubMed] [Google Scholar]
- 66.Morrison RF, Farmer SR. Role of PPARgamma in regulating a cascade expression of cyclin-dependent kinase inhibitors, p18(INK4c) and p21(Waf1/Cip1), during adipogenesis. J Biol Chem. 1999;274:17088–17097. doi: 10.1074/jbc.274.24.17088. [DOI] [PubMed] [Google Scholar]
- 67.Mueller E, Drori S, Aiyer A, Yie J, Sarraf P, Chen H, et al. Genetic analysis of adipogenesis through peroxisome proliferator-activated receptor gamma isoforms. J Biol Chem. 2002;277:41925–41930. doi: 10.1074/jbc.M206950200. [DOI] [PubMed] [Google Scholar]
- 68.Melnick RL. Is peroxisome proliferation an obligatory precursor step in the carcinogenicity of di(2-ethylhexyl)phthalate (DEHP)? Environ Health Perspect. 2001;109:437–442. doi: 10.1289/ehp.01109437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maloney EK, Waxman DJ. trans-Activation of PPARalpha and PPARgamma by structurally diverse environmental chemicals. Toxicol Appl Pharmacol. 1999;161:209–218. doi: 10.1006/taap.1999.8809. [DOI] [PubMed] [Google Scholar]
- 70.Ellero-Simatos S, Claus SP, Benelli C, Forest C, Letourneur F, Cagnard N, et al. Combined transcriptomic-(1)H NMR metabonomic study reveals that monoethylhexyl phthalate stimulates adipogenesis and glyceroneogenesis in human adipocytes. J Proteome Res. 2011;10:5493–5502. doi: 10.1021/pr200765v. [DOI] [PMC free article] [PubMed] [Google Scholar]