Abstract
Background
Few studies examining the genetic architecture of cigarette smoking have focused on adolescents or examined developmental changes in additive genetic, shared environment and unique environmental influences on liability to initiate cigarette smoking and quantity of cigarettes smoked. The aim of this study is to add to the literature on liability to initiate and use cigarettes during adolescence using a nationally representative sample.
Method
Data for this study came from adolescent and young adult twin pairs (ages 14-33) from the National Longitudinal Study of Adolescent to Adult Health. We ran a series of developmental causal-contingent-common pathway models to examine whether additive genetic, shared and unique environmental influences on liability to the initiation of cigarette use are shared with those on smoking quantity, and whether their contributions change across development.
Results
We found evidence for a developmental shift in genetic and shared environmental contributions to cigarette use. Early in adolescence genetic and environmental influences work independently on liability to cigarette smoking initiation and quantity of cigarettes smoked, but liability to these behaviors becomes correlated as individuals age into young adulthood.
Conclusions
These findings provide insight into the causal processes underlying the liability to smoke cigarettes. With age, there is greater overlap in the genetic and environmental factors that influence the initiation of cigarette smoking and quantity of cigarettes smoked.
Keywords: adolescents, development, genetic influences, environmental influences, cigarette smoking
Introduction
Tobacco use during adolescence continues to be a serious public health concern associated with increased mortality and personal and public costs (SAMHSA, 2009). Adolescence is a critical period for the onset of cigarette use, regular cigarette use (SAMHSA, 2007), nicotine dependence (DHHS, 1994), and a risk factor for the development of smoking-related illnesses (Heron, Hickman, Macleod, & Munafò, 2011) and other drug-related problems. Previous reviews of adult twin studies have found that additive genetic, shared and unique environmental influences all play a role in smoking initiation (Li, Cheng, Ma, & Swan, 2003; Sullivan & Kendler, 1999{Li, 2003 #109), but primarily additive and unique environmental influences influence smoking progression (i.e. developing symptoms of nicotine dependence) (Kendler, Neale, et al., 1999; Neale, Harvey, Maes, Sullivan, & Kendler, 2006). When smoking progression is operationalized as number of cigarettes smoked, then a small effect of the shared environment is found for adults (Li et al., 2003). Other work has found that there is overlap in the genetic contribution to smoking initiation, regular tobacco use, and nicotine dependence (Maes et al., 2004; Morley et al., 2007; Öncel, Dick, Maes, & Alıev, 2014)) suggesting that the some of the genetic factors that influence whether individuals initiate smoking also have an impact on how many cigarettes they subsequently smoke. Additionally, studies of adult twins have shown that additive genetic factors influence age at smoking initiation but a different set of genetic factors contribute to the number of cigarettes smoked (Broms, Silventoinen, Madden, Heath, & Kaprio, 2006).
Work with adolescent and young adult twins has suggested that smoking initiation is primarily influenced by shared environmental factors early in adolescence (Dick, Barman, & Pitkänen, 2006; Unger et al., 2011) although some studies do suggest that additive genetic effects play a role in having ever smoked a cigarette (Huizink et al., 2010; Kendler et al., 1993; Maes et al., 1999). As adolescents reach late adolescence and young adulthood additive genetic factors become increasingly more important (Kendler, Schmitt, Aggen, & Prescott, 2008; Koopmans, Doornen, & Boomsma, 1997; Maes & Neale, 2009; Maes et al., 2004).
Due to the conditional nature of drug use (Kendler, Neale, et al., 1999) properly estimating the heritability of smoking quantity requires employing a conditional model that takes into account the heritability of cigarette smoking initiation. The appropriate method to examine additive genetic and shared environmental factors to liability of drug use quantity is a causal-contingent-common (CCC) pathway model (Kendler, Karkowski, Corey, Prescott, & Neale, 1999) which allows for the concurrent estimation of quantity conditional on drug use initiation as well as for estimating the relationship between drug use initiation and drug use quantity (see Figure 1). The CCC model estimates separate liabilities for drug use initiation and for quantity of drug use given initiation. Once adolescents initiate smoking, previous studies have found that heaviness of smoking (light, moderate, or heavy cigarette use) (Huizink et al., 2010; Koopmans, Slutske, Heath, Neale, & Boomsma, 1999) and the presence of nicotine dependence as measured by the Fagerstrom Test for Nicotine Dependence (Haberstick et al., 2007; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991; Vink, Willemsen, & Boomsma, 2005) are influenced by genetic factors but no significant shared environmental effects have been reported. In a study of 11- to 19-year-old twins the same additive genetic and shared environmental factors that influenced initiation of cigarette use were found to contribute to the variance in the number of cigarettes smoked (Fowler et al., 2007). This study extends previous research in this area (Do et al., 2015; Öncel et al., 2014) by characterizing in greater detail the developmental changes in the heritability of quantity of cigarettes smoked per day conditional on cigarette use initiation across different ages spanning adolescence and young adulthood and testing for age-specific sex differences among twins from a nationally representative U. S. sample.
Figure 1.
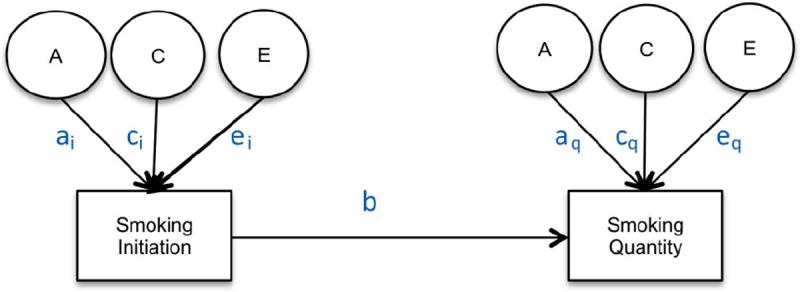
Causal-Contingent-Common Pathway Model
Material and methods
Sample
Data for this study came from twin pairs available in the National Longitudinal Study of Adolescent to Adult Health (Add Health) (Harris, Halpern, Haberstick, & Smolen, 2013; Harris, Halpern, Smolen, & Haberstick, 2006). The Add Health study recruited adolescent participants when they were enrolled in high school and subsequently assessed them through three in-home interviews, for a total of four waves of longitudinal data. In the first wave, participant ages ranged from 12 to 17 and in the last wave participants ranged in age from 28 to 33. At each wave of the 4 wave longitudinal study, participants reported on whether they had ever smoked a cigarette, and if they indicated they had smoked, they were subsequently asked how many cigarettes they had smoked per day in the previous 30 days.
Zygosity of the twin pairs was determined by matching on 12 molecular genetic markers and through self-report questions regarding the twins’ similarity (Harris et al., 2006). For this study, participants were divided into five groups based on sex and zygosity (monozygotic males (MZM), monozygotic females (MZF), dizygotic males (DZM), dizygotic females (DZF) and opposite sex dizygotic twins (DZO) so that we could test for qualitative and quantitative sex differences in the sources of genetic and environmental influences on the phenotypes.
In the Add Health study, data were available for 740 twin pairs from age 12 to age 33. At the younger ages (age 12 and 13), the prevalence of smoking was very low and no participants smoked more than 11 cigarettes per day, so data for this age group was not used. Beginning with age 14 we collapsed across ages resulting in having an age group of adolescent twins who were between 14 and 15 years of age (n=571 individuals), a group of 16- to 17-year-olds (n=776 individuals), a group of 18- to 25-year-olds (n=1,329 individuals), and a group of participants who were 26-33 years of age (n=1,308 individuals). Table I presents demographic information, the prevalence of having ever smoked a cigarette, and the percentage of individuals in each of the three categories of cigarette smoking quantity for each age group. Tetrachoric and polychoric correlations for cigarette smoking initiation and for cigarette smoking quantity are available in Tables II and III, respectively.
Table I.
Demographic characteristics by age group
| Ages
|
|||||
|---|---|---|---|---|---|
| 12-13 (n=100)* | 14-15 (n=571)* | 16-17 (n=776)* | 18-25 (n=1,329)* | 26+ (n=1,308)* | |
| Mean Age (SD) | 13 (0) | 14.7 (0.46) | 16.6 (0.49) | 21.7 (1.76) | 29.0 (1.64) |
| % MZ | 24.0% | 34.3% | 37.5% | 37.7% | 36.7% |
| % Female | 42.0% | 51.6% | 49.9% | 50.9% | 51.5% |
| Ever smoking | |||||
| Total sample | 39.0% | 53.8% | 70.4% | 76.4% | 76.2% |
| Males | 41.4% | 55.4% | 73.8% | 80.4% | 81.1% |
| Females | 35.7% | 52.2% | 66.9% | 72.5% | 71.7% |
| Categorical quantity smoked - None | |||||
| Total sample | 69.2% | 64.2% | 68.0% | 74.1% | 74.4% |
| Males | 70.8% | 58.8% | 62.0% | 71.4% | 72.8% |
| Females | 66.7% | 69.5% | 74.5% | 76.9% | 76.2% |
| Categorical quantity smoked - Light: 1-10 CPD | |||||
| Total sample | 30.8% | 29.6% | 18.0% | 17.0% | 16.9% |
| Males | 29.2% | 31.4% | 18.8% | 16.4% | 16.0% |
| Females | 33.3% | 27.9% | 17.0% | 17.8% | 17.8% |
| Categorical quantity smoked - Heavy: 11+ CPD | |||||
| Total sample | no obs | 6.2% | 14.1% | 8.9% | 8.7% |
| Males | no obs | 9.8% | 19.2% | 12.2% | 11.3% |
| Females | no obs | 2.6% | 8.5% | 5.3% | 6.0% |
Note:
n indicates number of individuals
Table II.
Tetrachoric Correlations for Cigarette Use Initiation (95% CI) by Sex, Zygosity and Age Group
| Females
|
Males
|
DZO
|
|||
|---|---|---|---|---|---|
| MZ
|
DZ
|
MZ
|
DZ
|
||
| 14-15 year-olds | 0.91 | 0.88 | 0.85 | 0.72 | 0.38 |
| (n=287 twin pairs) | (0.85, 0.97) | (0.81, 0.95) | (0.75, 0.94) | (0.59, 0.85) | (0.21, 0.55) |
| 16-17 year-olds | 0.64 | 0.63 | 0.82 | 0.57 | 0.55 |
| (n=389 twin pairs) | (0.50, 0.79) | (0.50, 0.76) | (0.73, 0.91) | (0.40, 0.74) | (0.42, 0.69) |
| 18-25 year-olds | 0.83 | 0.41 | 0.88 | 0.34 | 0.16 |
| (n=709 twin pairs) | (0.76, 0.90) | (0.27, 0.55) | (0.82, 0.94) | (0.17, 0.51) | (0.01, 0.31) |
| 26-33 year-olds | 0.87 | 0.41 | 0.87 | 0.51 | 0.03 |
| (n=723 twin pairs) | (0.81, 0.93) | (0.28, 0.55) | (0.81, 0.94) | (0.36, 0.67) | (-0.15, 0.22) |
Table III.
Polychoric Correlations for Cigarette Quantity (95% CI) by Sex, Zygosity and Age Group
| Females
|
Males
|
DZO
|
|||
|---|---|---|---|---|---|
| MZ
|
DZ
|
MZ
|
DZ
|
||
| 14-15 year-olds | 0.47 | -0.23 | -0.17 | 0.04 | 0.61 |
| (n=287 twin pairs) | (0.23, 0.71) | (-0.54, 0.08) | (-0.50, 0.15) | (-0.21, 0.29) | (0.42, 0.81) |
| 16-17 year-olds | 0.76 | 0.47 | 0.33 | 0.26 | 0.55 |
| (n=389 twin pairs) | (0.63, 0.88) | (0.26, 0.68) | (0.13, 0.54) | (0.07, 0.45) | (0.40, 0.70) |
| 18-25 year-olds | 0.61 | 0.24 | 0.55 | 0.08 | 0.24 |
| (n=709 twin pairs) | (0.49, 0.73) | (0.04, 0.44) | (0.42, 0.68) | (-0.10, 0.25) | (0.07, 0.40) |
| 26-33 year-olds | 0.59 | 0.00 | 0.69 | 0.00 | 0.18 |
| (n=723 twin pairs) | (0.46, 0.72) | (-0.23, 0.23) | (0.57, 0.80) | (-0.18, 0.17) | (0.11, 0.25) |
Note: Non-smokers were not included in the correlations presented here for cigarette quantity. If the liabilities to cigarette quantity are independent of one another then the twin correlations for cigarette quantity will accurately reflect the findings from the causal-contingent-common pathway model, otherwise, if the liabilities are dependent, they will not.
Measures
The aim of this study was to examine developmental changes in the etiology of smoking cigarettes, so cigarette smoking initiation and cigarette smoking quantity variables were created for each available age in the Add Health data. The smoking initiation variable was coded 1 when participants responded “Yes”, or 0 when the answer was “No”, to the survey question “Have you ever smoked a cigarette?”. Subsequently, those participants who answered “Yes” to having initiated smoking were asked how many cigarettes they had smoked in the previous 30 days. The answer to this question was used to create the smoking quantity variable. In Wave 3 of the Add Health data, the options available to participants to respond to the question about how many cigarettes they had smoked in the previous 30 days were ordinal categories rather than open-ended, as in waves 1, 2 and 4. Therefore, the quantity smoked variable used in the present analyses was an ordered categorical variable such that “0” referred to not having smoked any cigarettes in the previous 30 days, “1” referred to smoking between 1 and 11 cigarettes per day, and “2” indicated smoking more than 11 cigarettes per day. Further, those participants who reported not having ever smoked a cigarette (the never smokers) were coded as having missing information for the smoking quantity variable as has been suggested by Neale and colleagues (Neale et al., 2006).
Analysis Plan
Behavioral genetic studies are designed to uncover the sources of individual differences that give rise to variations in a phenotype and take advantage of the differences in the proportion of segregating genes between pairs of monozygotic and dizygotic twins who are reared together. Monozygotic (MZ) twins share 100% of their segregating genes while dizygotic twins (DZ) share on average 50% of their genes. If genetic factors contribute to a phenotype then MZ twins would be expected to be more similar than DZ twins. The variance in a phenotype is partitioned into additive genetic (A), shared environmental (C), or unique environmental (E) sources where additive genetic sources of variance involve the cumulative effects of individual genes on a phenotype. Shared environmental sources are aspects of the environment that both twins experience and increase their similarity. Non-shared environmental sources of variance are aspects of the environment that contribute to making twins in the same family different. Measurement error is also contained in the non-shared environmental component.
The first step in data analysis involved testing the assumptions that the thresholds for cigarette smoking initiation were equal across twin order, sex, and zygosity for each age group. Then, univariate analyses for binary variables decomposed the variance of cigarette smoking initiation into additive genetic (A), shared environmental (C), and unique environmental (E) sources and tested sex differences. Two types of sex differences were tested for; differences in the magnitude of genetic effects between males and females (quantitative sex differences) as well as differences due to a different set of genes influencing the phenotype in males than in females (qualitative sex differences). Quantitative and qualitative sex differences in cigarette smoking initiation were tested by comparing the likelihood statistic (-2LL) produced by reduced models to the likelihood of the full model. The difference in likelihood between the two models is known as a likelihood ratio test and is asymptotically distributed as chi-square.
Causal-Contingent-Common Analyses
Age-specific CCC models (Kendler, Karkowski, et al., 1999; Maes et al., 2004) for ordered categorical variables were run starting with a full, sex-specific model where the three sources (A, C, E) of variance were included for both cigarette smoking initiation and cigarette quantity smoked, and included a common pathway between initiation and quantity (Model 1). First, an overall test for sex differences was performed (Model 2), followed by an overall test for the significance of genetic factors for both smoking initiation (ai in Figure 1) and smoking quantity (aq in Figure 1) at once (Model 3) and shared environmental contributions to smoking initiation and smoking quantity simultaneously (Model 4). The significance of the common pathway between smoking initiation and quantity smoked (b in Figure 1) was tested at each age group (Model 5). A common pathway coefficient of 0 would indicate that smoking initiation and quantity smoked are independent processes for that particular age group while a common pathway coefficient of 1 would indicate that smoking quantity is on the same continuum as smoking initiation. The statistical significance of the variance components for smoking initiation (ai & ci in Figure 1) and for smoking quantity (aq & cq in Figure 1) was tested, separately, by fixing each to zero and examining model fit (Models 6-9). Analyses were done in R (RCore, 2012) using the OpenMx package (Boker et al., 2011).
Results
Prevalence of lifetime cigarette use by age group is presented in Table I. The prevalence rates for this sample are in line with the national estimates available for the years during which the survey was carried out (Johnston, O’Malley, Bachman, & Schulenberg, 2014). Slightly over half of the 14-15, 70% of the 16-17, and over three quarters of both the 18-25 and 26-33 year olds had ever smoked a cigarette. Of the 14-15 year-olds who had ever smoked, 29.6% were light smokers, and 6.2% were heavy smokers. The proportion of heavy smokers was highest in the 16-17 year-olds (14.1%) and lowest in the 26-33 year-old group (8.7%). Table I presents the prevalence of lifetime cigarette use and quantity of cigarettes smoked by sex and zygosity.
Age-Specific Univariate Models of Smoking Initiation
For each age group, we first fitted a saturated model where the thresholds and correlations for cigarette smoking initiation were allowed to vary by twin order, sex and zygosity. Then twin model assumptions were tested by evaluating changes in model fit when the thresholds were constrained across twin order, zygosity, same-sex and opposite-sex twins, and for males and females (results available upon request). Thresholds could be equated across twin order, zygosity (except at young adulthood), same-sex and opposite sex, and sex (except in the oldest age group). Next, whether different genetic factors or environmental influences in males and females contribute to the variance in cigarette smoking initiation (qualitative sex differences) as well as whether males and females differed in the magnitude of additive genetic effects (quantitative sex differences) was tested for at each age group. There was no evidence of qualitative or quantitative sex differences in cigarette smoking initiation at any of the age groups (results available upon request).
Age-Specific Causal -Contingent-Common Pathway Models
For the 14- to 15-year old group, the full causal-contingent-common pathway model allowed the loadings of the variance components for cigarette smoking initiation and quantity of cigarettes smoked to be freely estimated across sexes (Table IV, Model 1). Constraining the parameters to be equal across sex did not cause the fit of the model to deteriorate (Model 2; χ2=7.52, df=9, p=0.583, see Table III). Next, we tested the overall significance of the shared environment or the genetic contribution to liability of smoking initiation and smoking quantity and found that excluding the shared environment significantly worsened the fit of the model (Model 3; χ2=6.87, df=2, p=0.032) but not when dropping the influence of additive genetic variance (Model 4; χ2=4.72, df=2, p=0.094). We then tested the significance of the beta coefficient between smoking initiation and smoking quantity and found that the common pathway in this age group was not significant (Model 5, χ2=2.54, df=1, p=0.111). Subsequent models for this age group did not include the common pathway between smoking initiation and smoking quantity. We then tested the significance of the additive genetic contribution to smoking initiation (Model 6) and smoking quantity (Model 7) and that of the shared environment on smoking initiation (Model 8) and smoking quantity (Model 9). We found that additive genetic and shared environmental influences were significant for smoking initiation (Model 6; χ2=4.58, df=1, p=0.032 & Model 8; χ2=6.15, df=1, p=0.013) but not for smoking quantity (Model 7; χ2=0.0, df=1, p=1.000 & Model 9; χ2=0.34, df=1, p=0.56).
Table IV.
Causal-Contingent-Common Pathway Model Comparison by Age Group
| Age Group | Model | Parameters | - 2LL | df | AIC | Δ LL | Δ df | p |
|---|---|---|---|---|---|---|---|---|
| 14-15 | 1. CCC - ACE | 22 | 1181.22 | 860 | -538.78 | - | - | - |
| 2. CCC - ACE no sex differences | 13 | 1188.75 | 869 | -549.25 | 7.52 | 9 | 0.583 | |
| 3. CCC - AE | 11 | 1195.62 | 871 | -546.38 | 6.87 | 2 | 0.032 | |
| 4. CCC - CE | 11 | 1193.47 | 871 | -548.53 | 4.72 | 2 | 0.094 | |
| 5. Independent | 12 | 1191.29 | 870 | -548.71 | 2.54 | 1 | 0.111 | |
| 6. CCC - Drop initiation A | 11 | 1195.87 | 871 | -546.13 | 4.58 | 1 | 0.032 | |
| 7. CCC - Drop quantity A | 11 | 1191.29 | 871 | -550.71 | 0.00 | 1 | 1.000 | |
| 8. CCC - Drop initiation C | 11 | 1197.44 | 871 | -544.56 | 6.15 | 1 | 0.013 | |
| 9. CCC - Drop quantity C | 11 | 1191.63 | 871 | -550.37 | 0.34 | 1 | 0.560 | |
| 16-17 | 1. CCC - ACE | 22 | 1734.41 | 1304 | -873.59 | - | - | - |
| 2. CCC - ACE no sex differences | 13 | 1741.91 | 1313 | -884.09 | 7.50 | 9 | 0.585 | |
| 3. CCC - AE | 11 | 1749.23 | 1315 | -880.77 | 7.32 | 2 | 0.026 | |
| 4. CCC - CE | 11 | 1747.42 | 1315 | -882.58 | 5.51 | 2 | 0.063 | |
| 5. Independent | 12 | 1761.75 | 1314 | -866.25 | 19.84 | 1 | 0.000 | |
| 6. CCC - Drop initiation A | 12 | 1747.20 | 1314 | -880.80 | 5.29 | 1 | 0.021 | |
| 7. CCC - Drop quantity A | 12 | 1743.15 | 1314 | -884.85 | 1.25 | 1 | 0.264 | |
| 8. CCC - Drop initiation C | 12 | 1748.11 | 1314 | -879.89 | 6.21 | 1 | 0.013 | |
| 9. CCC - Drop quantity C | 12 | 1742.68 | 1314 | -885.32 | 0.78 | 1 | 0.379 | |
| 18-25 | 1. CCC - ACE | 22 | 2803.21 | 2326 | -1848.79 | - | - | - |
| 2. CCC - ACE no sex differences | 13 | 2816.48 | 2335 | -1853.52 | 13.27 | 9 | 0.151 | |
| 3. CCC - AE | 11 | 2816.48 | 2337 | -1857.521 | 0.00 | 2 | 1.000 | |
| 4. CCC - CE | 11 | 2854.90 | 2337 | -1819.101 | 38.42 | 2 | 0.000 | |
| 5. Independent | 12 | 2825.35 | 2336 | -1846.653 | 8.87 | 1 | 0.003 | |
| 6. CCC - Drop initiation A | 12 | 3350.42 | 2336 | -1321.581 | 533.94 | 1 | 0.000 | |
| 7. CCC - Drop quantity A | 12 | 2819.10 | 2336 | -1852.904 | 2.62 | 1 | 0.106 | |
| 8. CCC - Drop initiation C | 12 | 2816.48 | 2336 | -1855.521 | 0.00 | 1 | 1.000 | |
| 9. CCC - Drop quantity C | 12 | 2816.48 | 2336 | -1855.521 | 0.00 | 1 | 1.000 | |
| 26-33 | 1. CCC - ACE | 22 | 2746.08 | 2287 | -1827.92 | - | - | - |
| 2. CCC - ACE no sex differences | 13 | 2757.19 | 2296 | -1834.81 | 11.10 | 9 | 0.269 | |
| 3. CCC - AE | 11 | 2757.19 | 2298 | -1838.81 | 0.00 | 2 | 1.000 | |
| 4. CCC - CE | 11 | 3033.37 | 2298 | -1562.631 | 276.18 | 2 | 0.000 | |
| 5. Independent | 12 | 2767.72 | 2297 | -1826.281 | 10.53 | 1 | 0.001 | |
| 6. CCC - Drop initiation A | 12 | 2788.61 | 2297 | -1805.393 | 31.42 | 1 | 0.000 | |
| 7. CCC - Drop quantity A | 12 | 2760.25 | 2297 | -1833.752 | 3.06 | 1 | 0.080 | |
| 8. CCC - Drop initiation C | 12 | 2757.19 | 2297 | -1836.81 | 0.00 | 1 | 1.000 | |
| 9. CCC - Drop quantity C | 12 | 2757.19 | 2297 | -1836.81 | 0.00 | 1 | 1.000 |
For the 16- to 17-year old group, constraining the variance components and the common pathway between cigarette smoking initiation to quantity of cigarettes smoked to be equal across males and females did not worsen model fit (χ2=7.50, df=9, p=0.585). Removing the effects of the shared environment (Model 3: χ2=7.32, df=2, p=0.026) caused a significant reduction in the fit of the model but not when additive genetic effects were removed (Model 4: χ2=5.51, df=2, p=0.063). The significance of the common pathway between cigarette smoking initiation and quantity of cigarettes smoked was tested by setting it to 0 and resulted in worse model fit (Model 5: χ2=19.84, df=1, p<0.0001), suggesting that for the 16- to 17-year-old group the liabilities to smoking initiation and smoking quantity were correlated. Dropping either additive genetic effects from smoking initiation (Model 6: χ2=5.29, df=1, p=0.021) or shared environmental contributions to smoking initiation (Model 8: χ2=6.21, df=1, p=0.013) worsened the fit of the model but removing the influence of additive genetic effects (Model 7: χ2=1.25, df=1, p=0.264) or shared environmental influences from smoking quantity (Model 9: χ2=0.78, df=1, p=0.379) did not.
For the 18- to 25-year-old, testing a model where the estimates in the parameters were equated across males and females resulted in no significant reduction in model fit (Model 2: χ2=13.27, df=9, p=0.151), so the subsequent models contained equal A, C, E estimates and the common pathway across sex. Next, a model that tested the overall contribution of the shared environment was not significant (Model 3: χ2=0.0, df=2, p=1.0), but that of additive genetic effects was significant (Model 4: χ2=38.42, df=2, p<0.001). Next, whether the liabilities to smoking initiation and smoking quantity were independent of one another was tested by removing the common pathway from the model and resulted in a worse fit (Model 5: χ2=8.87, df=1, p<0.01). In subsequent models for this age group, the common pathway was included. The specific influence of additive genetic effects was found to be important for smoking initiation (Model 6: χ2=534.23, df=1, p<0.001) but not for smoking quantity (Model 7: χ2=2.62, df=1, p=0.106). The influence of the shared environment did not deteriorate model fit on liability to smoking initiation (Model 8: χ2=0.0, df=1, p=1.0), or smoking quantity (Model 9: χ2=0.0, df=1, p=1.0).
Lastly, for the 26-33 age group, equating the estimates across sex did not result in worse model fit (Model 2; χ2=11.1, df=9, p=0.269) suggesting that for this age group there were no significant differences by sex in the estimates for A, C, and E or in the beta coefficient of the common pathway. Removing the overall influence of the shared environment did not cause a reduction in model fit (Model 3; χ2=0.0, df=2, p=1.0) but the removal of additive genetic influences did cause model to fit worse (Model 4; χ2=276.18, df=2, p<0.001). When the common pathway between smoking initiation and smoking quantity was removed in Model 5, the model fit worse (χ2=10.53, df=1, p<0.01) suggesting that the liabilities to smoking initiation and smoking quantity are correlated at this age. Additive genetic influences were important for smoking initiation (Model 6: χ2=460.24, df=1, p<0.001), but not for smoking quantity (Model 7: χ2=3.06, df=1, p=0.08). The influence of shared environment was not significant for smoking initiation or smoking quantity (Model 8: χ2=0.0, df=1, p=1.0; Model 9: χ2=0.0, df=1, p=1.0).
The variance component estimates and associated 95% confidence intervals from the causal-contingent-common pathway model without sex differences are presented in Table V for each age group. The influence of risk factors for quantity of cigarettes smoked that exert their influence through their effect on smoking initiation was found to increase through adolescence and into young adulthood. For the 14-15 year old group, we found that risk factors influencing smoking initiation were independent of those that contributed to smoking quantity. In the three older age groups, the risk factors predisposing to smoking initiation were correlated with those that influence smoking quantity (98%, 62%, and 65%, respectively) suggesting substantial overlap in the factors that influence initiating cigarette use and the number of cigarettes smoked among older adolescents and young adults.
Table V.
Parameter estimates (95% Confidence Interval) from causal-contingent-causal pathway no sex differences model
| Age group | Smoking Initiation
|
|
Smoking Quantity
|
Total
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| a2 | c2 | e2 | Beta | a2s | c2s | e2s | a2t | c2t | e2t | |
| 14-15 year olds | 0.42 | 0.46 | 0.12 | 0.52 | 0.00 | 0.10 | 0.63 | 0.11 | 0.23 | 0.66 |
| (0.04, 0.80) | (0.12, 0.75) | (0.04, 0.26) | (-0.16, 0.82) | (0.00, 0.00) | (0.00, 0.38) | (0.30, 0.98) | ||||
| 16-17 year olds | 0.43 | 0.10 | 0.26 | 0.93 | 0.00 | 0.00 | 0.10 | 0.37 | 0.09 | 0.32 |
| (0.41, 0.76) | (0.05, 0.28) | (0.13, 0.27) | (0.92, 0.95) | (0.00, 0.13) | (0.00, 0.00) | (0.05, 0.28) | ||||
| 18-25 year olds | 0.84 | 0.00 | 0.16 | 0.62 | 0.30 | 0.00 | 0.32 | 0.62 | 0.00 | 0.38 |
| (0.73, 0.92) | (0.00, 0.00) | (0.08, 0.27) | (0.26, 0.78) | (0.02, 0.57) | (0.00, 0.00) | (0.13, 0.53) | ||||
| 26-33 year olds | 0.87 | 0.00 | 0.13 | 0.65 | 0.27 | 0.00 | 0.31 | 0.63 | 0.00 | 0.37 |
| (0.76, 0.93) | (0.00, 0.00) | (0.07, 0.24) | (0.32, 0.80) | (0.01, 0.54) | (0.00, 0.00) | (0.12, 0.52) | ||||
We present estimates of the variance components for the same model across all ages (Table V), even though the causal path was not significant for age 14-15 and some of the genetic and shared environmental contributions were not significant. We estimated that for 14-15 year olds 42% of the variance in liability to smoking initiation was accounted for by additive genetic factors, 46% by shared environmental factors and 12% by non-shared environmental factors. Only non-shared environmental factors accounted for a significant portion of the variance (63%) in quantity of cigarettes smoked. In the 16-17 year olds, 43% of the variance in liability to smoking initiation was accounted for by additive genetic factors, 10% was attributable to the shared environment and 26% by non-shared environment. The additive genetic and shared environmental influences on smoking initiation were found to be almost completely shared with those that influence smoking quantity (causal path estimated at .93) but no additional genetic effects or shared environmental effects were found for this age group to influence quantity of cigarettes smoked. The estimates for the18-25 year olds and the 26-33 year olds indicated that only additive genetic factors accounted for familial resemblance in liability to smoking initiation (84% and 87% respectively) and that these factors influenced smoking quantity through a substantial common path. Additional genetic effects were found to influence the quantity of cigarettes smoked for both young adult age groups (about 30% each).
Discussion
The aim of the present study was to examine developmental changes in the etiology of smoking behavior by modeling the influence of additive genetic and shared environmental influences of quantity of cigarettes smoked conditional on having initiated cigarette smoking. Consistent with previous work, the results of the present study did not find sex differences in the proportion of variance explained by genetic or environmental factors in adolescent smoking initiation or quantity of cigarettes smoked (Boomsma, Koopmans, Doornen, & Orlebeke, 1994; McGue, Elkins, & Iacono, 2000; Rende, Slomkowski, McCaffery, Lloyd-Richardson, & Niaura, 2005).
The results indicate the presence of developmental changes in the degree of overlap between the liability of smoking initiation and smoking quantity from adolescence and into young adulthood. The common pathway between liability to cigarette smoking initiation and cigarette smoking quantity could be dropped from the model in early adolescence suggesting that different risk factors influence whether individuals have initiated cigarette use and how many cigarettes they subsequently smoked. For older adolescents and young adults, removing the common pathway between liability to initiate smoking and quantity smoked worsened model fit suggesting the presence of correlated liabilities between cigarette use initiation and cigarette smoking quantity as has been found in previous adult twin studies (Boomsma et al., 1994; Maes et al., 2004). Thus, with age, the factors that influence the initiation of smoking also play a role in how many cigarettes individuals smoke. At ages 16-17, we found that 93% of the variance in liability to the amount of cigarette smoked was shared with the liability to initiate cigarette smoking. The corresponding proportions of variance in liability of cigarette smoking quantity shared with smoking initiation are 62% for the 18-25 year group, and 65% for the 26-33 year group. Recent reports of adolescent and young adult smoking have shown similar estimates for the proportion of variance in liability shared between smoking initiation and quantity (Do et al., 2015; Öncel et al., 2014).
Further, the findings suggest developmental changes in the factors that contribute to smoking initiation and number of cigarettes smoked when quantity smoked is modeled contingent on initiation. For the youngest age group, additive genetic and shared environmental factors were found to contribute to the liability of smoking initiation but there were no new additive genetic or shared environmental influences specific to the liability of smoking quantity. Previous adolescent and young adult twin studies that have estimated the heritability of cigarettes smoked contingent on cigarette use initiation have included twins of a broader age range (Boomsma et al., 1994; Öncel et al., 2014) during a time when etiology of smoking behavior is changing rapidly. The differences in the results observed in the present study compared to previous findings may be due to our age-specific focus which uncovered a developmental change that may have been previously overlooked. Consistent with our findings, recently, Do and colleagues reported no evidence of new additive genetic effects on quantity of cigarettes smoked in early adolescence (Do et al., 2015). As individuals aged into late adolescence and young adulthood, the influence of additive genetic factors became more important for the initiation of cigarette use, a finding that is consistent with previous research (Do et al., 2015; Kendler et al., 2012; Koopmans et al., 1997). For the two oldest age groups (18-25 and 26-33) shared environmental influences did not contribute significantly to the liability to initiate smoking but additive genetic effects were significant.
We show that there are changes in the overlap of additive genetic and environmental factors that play a role in the initiation of smoking during early adolescence and into young adulthood. This finding adds to the literature on the developmental changes in the contributions of additive genetic and shared environmental factors on smoking initiation (Boomsma et al., 1994; Han, McGue, & Iacono, 1999) and smoking quantity and delineates the developmental shift from shared environmental influences on smoking initiation to additive genetic ones (Do et al., 2015; Koopmans et al., 1997; Öncel et al., 2014).
It has been known that smoking behaviors are complex behavioral traits which are under genetic and environmental influence (Kendler et al., 2012; Munafo, Clark, Johnstone, Murphy, & Walton, 2004). The present study adds to a growing body of literature (Do et al., 2015; Öncel et al., 2014) indicating that the genetic and environmental risk factors for smoking behaviors change over adolescence and into young adulthood. Efforts to find the gene clusters associated with cigarette use have resulted in identifying dopamine receptor genes associated with both cigarette use initiation, progression into regular use, and nicotine dependence (Munafo et al., 2004). Further, once individuals begin using cigarettes and are exposed to nicotine, nicotinic acetylcholine receptors genes have been found to be associated with both the number of cigarettes smoked per day (Liu et al., 2010; Saccone et al., 2010; Tobacco & Consortium, 2010) and nicotine dependence (Chen et al., 2009; Wang et al., 2014; Ware, van den Bree, & Munafò, 2011). However, this work has focused almost exclusively on adult samples. Whether the same genes are important for younger samples has yet to be determined. Our results indicate that during early adolescence the shared environment plays a substantial role in cigarette use, thus, characterizing how different environmental exposures interact with gene clusters in increasing risk for initiating and maintaining cigarette use during this age group will provide a comprehensive understanding of the unfolding of cigarette use. The implications of these findings for future gene finding research suggest the need to search for genetic variants for cigarette use initiation and quantity that are developmentally specific.
Limitations
The findings of the present study should be considered with the following limitations in mind. First, to examine developmental changes, the sample of available twins in the data was divided into groups according to age and this may have reduced the power to detect significant sex effects, especially at the youngest ages where the sample of twin pairs was 287. Second, to have a comparable quantity smoked variable across each assessment we turned a continuous measure of cigarettes smoked per day into an ordinal variable, which may have resulted in loss of information to detect individual differences. Lastly, recent research has indicated important racial/ethnic differences in age of smoking onset (Clark, Doyle, & Clincy, 2013), in quantity of cigarettes smoked (Gutman, Eccles, Peck, & Malanchuk, 2011), and in the development of nicotine dependence (Duncan, Lessov-Schlaggar, Sartor, & Bucholz, 2012). In the present analyses we did not test for the possibility that additive genetic influences on the liability to initiate smoking and in the quantity of cigarette smoked would differ by racial/ethnic background.
Acknowledgments
Financial support:
The research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under award numbers R01DA025109 (H.H.M.) and K01DA036681 (C.B.B.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest:
None.
Ethical standards:
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.
References
- Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick T, Bates T, et al. OpenMx: an open source extended structural equation modeling framework. Psychometrika. 2011;76(2):306–317. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomsma DI, Koopmans JR, Doornen LJ, Orlebeke JF. Genetic and social influences on starting to smoke: a study of Dutch adolescent twins and their parents. Addiction. 1994;89(2):219–226. doi: 10.1111/j.1360-0443.1994.tb00881.x. [DOI] [PubMed] [Google Scholar]
- Broms U, Silventoinen K, Madden PA, Heath AC, Kaprio J. Genetic architecture of smoking behavior: a study of Finnish adult twins. Twin Research and Human Genetics. 2006;9(01):64–72. doi: 10.1375/183242706776403046. [DOI] [PubMed] [Google Scholar]
- Chen X, Chen J, Williamson VS, An SS, Hettema JM, Aggen SH, Kendler KS, et al. Variants in nicotinic acetylcholine receptors α5 and α3 increase risks to nicotine dependence. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2009;150(7):926–933. doi: 10.1002/ajmg.b.30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark TT, Doyle O, Clincy A. Age of first cigarette, alcohol, and marijuana use among US biracial/ethnic youth: A population-based study. Addictive behaviors. 2013;38(9):2450–2454. doi: 10.1016/j.addbeh.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DHHS, U. D. o. H. a. H. S. Preventing tobacco use among young people: A report of the Surgeon General. US Department of Health and Human Services; 1994. [Google Scholar]
- Dick D, Barman S, Pitkänen T. Genetic and environmental influences on the initiation and continuation of smoking and drinking. Socioemotional development and health from adolescence to adulthood. 2006:126–145. [Google Scholar]
- Do EK, Prom-Wormley EC, Eaves LJ, Silberg JL, Miles DR, Maes HH. Genetic and Environmental Influences on Smoking Behavior across Adolescence and Young Adulthood in the Virginia Twin Study of Adolescent Behavioral Development and the Transitions to Substance Abuse Follow-Up. Twin Research and Human Genetics. 2015;18(01):43–51. doi: 10.1017/thg.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AE, Lessov-Schlaggar CN, Sartor CE, Bucholz KK. Differences in time to onset of smoking and nicotine dependence by race/ethnicity in a Midwestern sample of adolescents and young adults from a high risk family study. Drug and alcohol dependence. 2012;125(1):140–145. doi: 10.1016/j.drugalcdep.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler T, Lifford K, Shelton K, Rice F, Thapar A, Neale MC, Van Den Bree M, et al. Exploring the relationship between genetic and environmental influences on initiation and progression of substance use. Addiction. 2007;102(3):413–422. doi: 10.1111/j.1360-0443.2006.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman LM, Eccles JS, Peck S, Malanchuk O. The influence of family relations on trajectories of cigarette and alcohol use from early to late adolescence. Journal of Adolescence. 2011;34(1):119–128. doi: 10.1016/j.adolescence.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Haberstick BC, Timberlake D, Ehringer MA, Lessem JM, Hopfer CJ, Smolen A, Hewitt JK. Genes, time to first cigarette and nicotine dependence in a general population sample of young adults. Addiction. 2007;102(4):655–665. doi: 10.1111/j.1360-0443.2007.01746.x. [DOI] [PubMed] [Google Scholar]
- Han C, McGue MK, Iacono WG. Lifetime tobacco, alcohol and other substance use in adolescent Minnesota twins: univariate and multivariate behavioral genetic analyses. Addiction. 1999;94(7):981–993. doi: 10.1046/j.1360-0443.1999.9479814.x. [DOI] [PubMed] [Google Scholar]
- Harris KM, Halpern CT, Haberstick BC, Smolen A. The National Longitudinal Study of Adolescent Health (Add Health) Sibling Pairs Data. Twin Research and Human Genetics. 2013;16(01):391–398. doi: 10.1017/thg.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Halpern CT, Smolen A, Haberstick BC. The National Longitudinal Study of Adolescent Health (Add Health) Twin Data. Twin Research and Human Genetics. 2006;9(06):988–997. doi: 10.1375/183242706779462787. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K-O. The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. British journal of addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heron J, Hickman M, Macleod J, Munafò MR. Characterizing patterns of smoking initiation in adolescence: comparison of methods for dealing with missing data. Nicotine & Tobacco Research. 2011 doi: 10.1093/ntr/ntr161. ntr161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink AC, Levälahti E, Korhonen T, Dick DM, Pulkkinen L, Rose RJ, Kaprio J. Tobacco, cannabis, and other illicit drug use among Finnish adolescent twins: causal relationship or correlated liabilities? Journal of Studies on Alcohol and Drugs. 2010;715(1) doi: 10.15288/jsad.2010.71.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley P, Bachman J, Schulenberg J. Teen smoking continues to decline in 2013 2014 [Google Scholar]
- Kendler KS, Chen X, Dick D, Maes H, Gillespie N, Neale MC, Riley B. Recent advances in the genetic epidemiology and molecular genetics of substance use disorders. Nature neuroscience. 2012;15(2):181–189. doi: 10.1038/nn.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski L, Corey L, Prescott C, Neale M. Genetic and environmental risk factors in the aetiology of illicit drug initiation and subsequent misuse in women. The British Journal of Psychiatry. 1999;175(4):351–356. doi: 10.1192/bjp.175.4.351. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale M, Sullivan P, Corey L, Gardner C, Prescott C. A population-based twin study in women of smoking initiation and nicotine dependence. Psychological medicine. 1999;29(02):299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, MacLean CJ, Heath AC, Eaves LJ, Kessler RC. Smoking and major depression: a causal analysis. Archives of general psychiatry. 1993;50(1):36–43. doi: 10.1001/archpsyc.1993.01820130038007. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Schmitt E, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Archives of general psychiatry. 2008;65(6):674–682. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans JR, Doornen LJ, Boomsma DI. Association between alcohol use and smoking in adolescent and young adult twins: a bivariate genetic analysis. Alcoholism: Clinical and Experimental Research. 1997;21(3):537–546. [PubMed] [Google Scholar]
- Koopmans JR, Slutske WS, Heath AC, Neale MC, Boomsma DI. The genetics of smoking initiation and quantity smoked in Dutch adolescent and young adult twins. Behavior Genetics. 1999;29(6):383–393. doi: 10.1023/a:1021618719735. [DOI] [PubMed] [Google Scholar]
- Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003;98(1):23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- Liu JZ, Tozzi F, Waterworth DM, Pillai SG, Muglia P, Middleton L, Waeber G, et al. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nature genetics. 2010;42(5):436–440. doi: 10.1038/ng.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes HH, Neale MC. Genetic modeling of tobacco use behavior and trajectories. NCI Tobacco Control Monograph Series. 2009;20 [Google Scholar]
- Maes HH, Sullivan PF, Bulik CM, Neale MC, Prescott CA, Eaves LJ, Kendler KS. A twin study of genetic and environmental influences on tobacco initiation, regular tobacco use and nicotine dependence. Psychological medicine. 2004;34(07):1251–1261. doi: 10.1017/s0033291704002405. [DOI] [PubMed] [Google Scholar]
- Maes HH, Woodard CE, Murrelle L, Meyer JM, Silberg JL, Hewitt JK, Carbonneau R, et al. Tobacco, alcohol and drug use in eight-to sixteen-year-old twins: the Virginia Twin Study of Adolescent Behavioral Development. Journal of Studies on Alcohol and Drugs. 1999;60(3):293. doi: 10.15288/jsa.1999.60.293. [DOI] [PubMed] [Google Scholar]
- McGue M, Elkins I, Iacono WG. Genetic and environmental influences on adolescent substance use and abuse. American journal of medical genetics. 2000;96(5):671–677. doi: 10.1002/1096-8628(20001009)96:5<671::aid-ajmg14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Morley KI, Lynskey MT, Madden PA, Treloar SA, Heath AC, Martin NG. Exploring the inter-relationship of smoking age-at-onset, cigarette consumption and smoking persistence: genes or environment? Psychological medicine. 2007;37(09):1357–1367. doi: 10.1017/S0033291707000748. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Clark TG, Johnstone EC, Murphy MF, Walton RT. The genetic basis for smoking behavior: a systematic review and meta-analysis. Nicotine & Tobacco Research. 2004;6(4):583–597. doi: 10.1080/14622200410001734030. [DOI] [PubMed] [Google Scholar]
- Neale MC, Harvey E, Maes HH, Sullivan PF, Kendler KS. Extensions to the modeling of initiation and progression: applications to substance use and abuse. Behavior Genetics. 2006;36(4):507–524. doi: 10.1007/s10519-006-9063-x. [DOI] [PubMed] [Google Scholar]
- Öncel SY, Dick DM, Maes HH, Alıev F. Risk Factors Influencing Smoking Behavior: A Turkish Twin Study. Twin Research and Human Genetics. 2014;17(06):563–573. doi: 10.1017/thg.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RCore, T. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. URL http://www.R-project.org. [Google Scholar]
- Rende R, Slomkowski C, McCaffery J, Lloyd-Richardson EE, Niaura R. A twin-sibling study of tobacco use in adolescence: Etiology of individual differences and extreme scores. Nicotine & Tobacco Research. 2005;7(3):413–419. doi: 10.1080/14622200500125609. [DOI] [PubMed] [Google Scholar]
- Saccone NL, Culverhouse RC, Schwantes-An T-H, Cannon DS, Chen X, Cichon S, Keskitalo-Vuokko K, et al. Multiple independent loci at chromosome 15q25. 1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS genetics. 2010;6(8):e1001053. doi: 10.1371/journal.pgen.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. Mental Health Services Administration, Office of Applied Studies: The OAS Report: A day in the life of American adolescents: Substance use facts. Rockville, MD: 2007. [Google Scholar]
- SAMHSA. NSDUH Series H-36, HHS Publication No SMA. Vol. 9. Office of Applied Studies; 2009. Mental Health Services Administration (2009) Results from the 2008 national survey on drug use and health: National findings; p. 4434. [Google Scholar]
- Sullivan PF, Kendler KS. The genetic epidemiology of smoking. Nicotine & Tobacco Research. 1999;1(Suppl 2):S51–S57. doi: 10.1080/14622299050011811. [DOI] [PubMed] [Google Scholar]
- Tobacco, & Consortium, G. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nature genetics. 2010;42(5):441–447. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger JB, Lessov-Schlaggar CN, Pang Z, Guo Q, Ning F, Gallaher P, Johnson CA, et al. Heritability of Smoking, Alcohol Use, and Psychological Characteristics Among Adolescent Twins in Qingdao, China. Asia-Pacific Journal of Public Health. 2011;23(4):568–580. doi: 10.1177/1010539509351052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink JM, Willemsen G, Boomsma DI. Heritability of smoking initiation and nicotine dependence. Behavior Genetics. 2005;35(4):397–406. doi: 10.1007/s10519-004-1327-8. [DOI] [PubMed] [Google Scholar]
- Wang S, van der Vaart AD, Xu Q, Seneviratne C, Pomerleau OF, Pomerleau CS, Li MD, et al. Significant associations of CHRNA2 and CHRNA6 with nicotine dependence in European American and African American populations. Human Genetics. 2014;133(5):575–586. doi: 10.1007/s00439-013-1398-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JJ, van den Bree MBM, Munafò MR. Association of the CHRNA5-A3-B4 Gene Cluster With Heaviness of Smoking: A Meta-Analysis. Nicotine & Tobacco Research. 2011;13(12):1167–1175. doi: 10.1093/ntr/ntr118. [DOI] [PMC free article] [PubMed] [Google Scholar]


