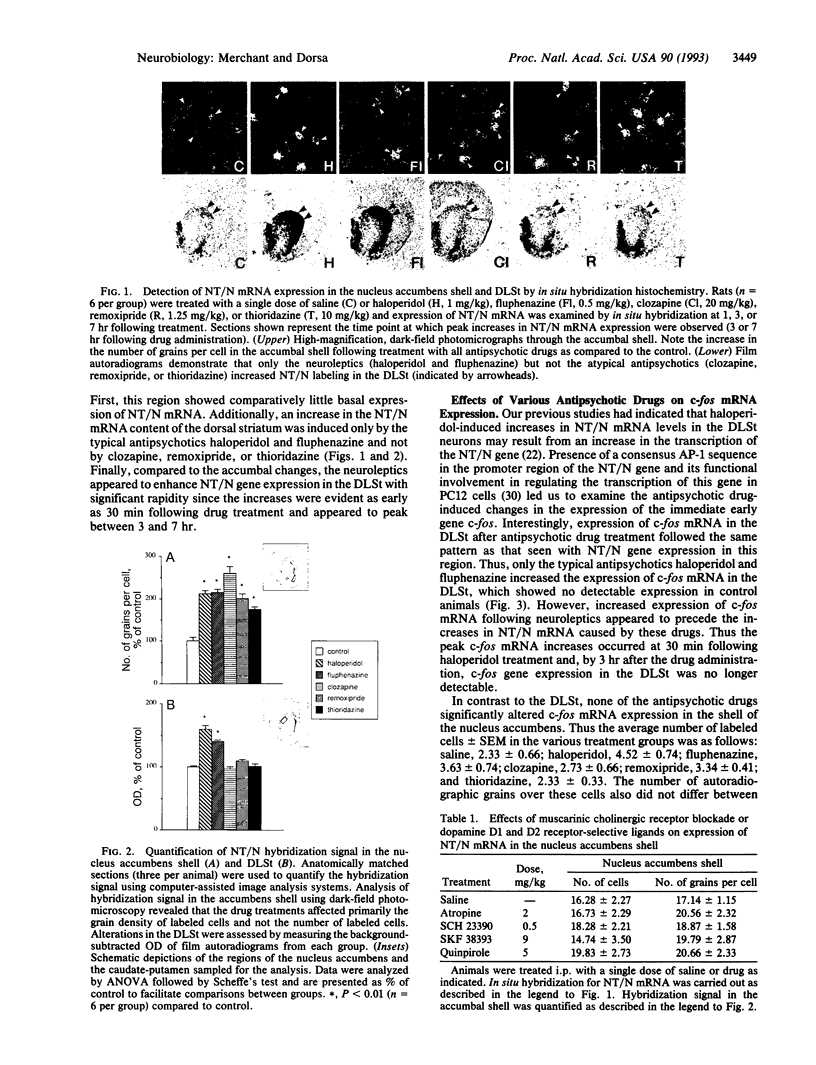
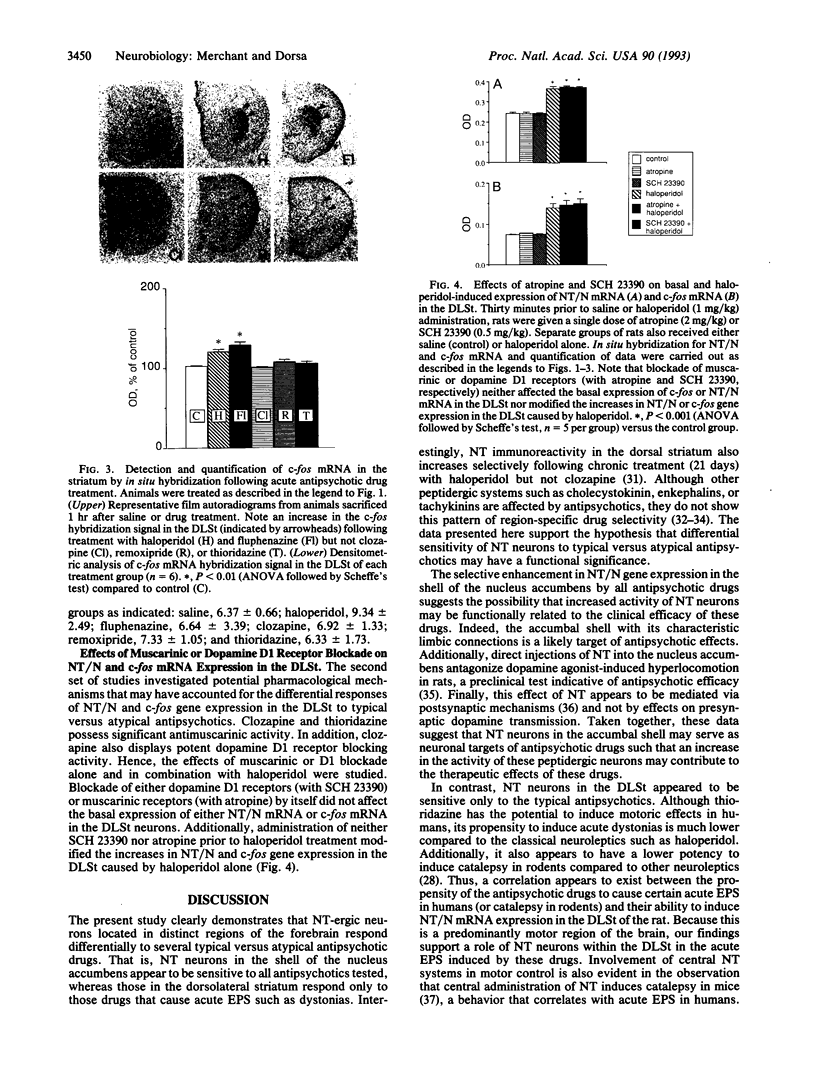
Abstract
Precise neural mechanisms underlying the pathophysiology and pharmacotherapy of psychotic disorders remain largely unknown. Present studies investigated the effects of various antipsychotic drugs on expression of the gene encoding the purported endogenous antipsychotic-like peptide neurotensin (NT) in striatal regions of the rat brain. The results demonstrate that several clinically efficacious antipsychotic drugs selectively and specifically increase expression of NT/neuromedin N (NT/N) mRNA in the shell of the nucleus accumbens, a region of the forebrain associated with limbic systems. On the other hand, only typical antipsychotics that cause a high incidence of acute motor side effects increased the expression of NT/N mRNA in the dorsolateral striatum, an extrapyramidal region primarily involved in motor control. In addition, it appears that distinct mechanisms may be involved in the effects of antipsychotics on NT/N gene expression in the dorsolateral striatum versus the accumbal shell. Thus neuroleptic-induced increases in NT/N mRNA expression in the dorsolateral striatum were preceded by a rapid and transient activation of c-fos mRNA, whereas none of the antipsychotics affected c-fos mRNA expression in the accumbal shell. The anatomical characteristics of NT/N gene expression induced by typical versus atypical antipsychotics raise the possibility that increased activity of specific NT neurons may contribute to the therapeutic effects (NT neurons in the accumbal shell) or motor side effects (NT neurons in the dorsolateral striatum) of these drugs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alheid G. F., Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988 Oct;27(1):1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Angulo J. A., Cadet J. L., Woolley C. S., Suber F., McEwen B. S. Effect of chronic typical and atypical neuroleptic treatment on proenkephalin mRNA levels in the striatum and nucleus accumbens of the rat. J Neurochem. 1990 Jun;54(6):1889–1894. doi: 10.1111/j.1471-4159.1990.tb04887.x. [DOI] [PubMed] [Google Scholar]
- Baldessarini R. J., Frankenburg F. R. Clozapine. A novel antipsychotic agent. N Engl J Med. 1991 Mar 14;324(11):746–754. doi: 10.1056/NEJM199103143241107. [DOI] [PubMed] [Google Scholar]
- Carraway R., Leeman S. E. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem. 1973 Oct 10;248(19):6854–6861. [PubMed] [Google Scholar]
- Creese I., Burt D. R., Snyder S. H. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976 Apr 30;192(4238):481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- Deutch A. Y., Cameron D. S. Pharmacological characterization of dopamine systems in the nucleus accumbens core and shell. Neuroscience. 1992;46(1):49–56. doi: 10.1016/0306-4522(92)90007-o. [DOI] [PubMed] [Google Scholar]
- Deutch A. Y., Moghaddam B., Innis R. B., Krystal J. H., Aghajanian G. K., Bunney B. S., Charney D. S. Mechanisms of action of atypical antipsychotic drugs. Implications for novel therapeutic strategies for schizophrenia. Schizophr Res. 1991 Mar-Apr;4(2):121–156. doi: 10.1016/0920-9964(91)90030-u. [DOI] [PubMed] [Google Scholar]
- Eggerman K. W., Zahm D. S. Numbers of neurotensin-immunoreactive neurons selectively increased in rat ventral striatum following acute haloperidol administration. Neuropeptides. 1988 Apr;11(3):125–132. doi: 10.1016/0143-4179(88)90081-9. [DOI] [PubMed] [Google Scholar]
- Ervin G. N., Birkemo L. S., Nemeroff C. B., Prange A. J., Jr Neurotensin blocks certain amphetamine-induced behaviours. Nature. 1981 May 7;291(5810):73–76. doi: 10.1038/291073a0. [DOI] [PubMed] [Google Scholar]
- Farde L., Hall H., Ehrin E., Sedvall G. Quantitative analysis of D2 dopamine receptor binding in the living human brain by PET. Science. 1986 Jan 17;231(4735):258–261. doi: 10.1126/science.2867601. [DOI] [PubMed] [Google Scholar]
- Frey P. Cholecystokinin octapeptide levels in rat brain are changed after subchronic neuroleptic treatment. Eur J Pharmacol. 1983 Nov 11;95(1-2):87–92. doi: 10.1016/0014-2999(83)90270-4. [DOI] [PubMed] [Google Scholar]
- Govoni S., Hong J. S., Yang H. Y., Costa E. Increase of neurotensin content elicited by neuroleptics in nucleus accumbens. J Pharmacol Exp Ther. 1980 Nov;215(2):413–417. [PubMed] [Google Scholar]
- Heimer L., Zahm D. S., Churchill L., Kalivas P. W., Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41(1):89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- Idänpän-Heikkilä J., Alhava E., Olkinuora M., Palva I. P. Agranulocytosis during treatment with chlozapine. Eur J Clin Pharmacol. 1977 Mar 11;11(3):193–198. doi: 10.1007/BF00606409. [DOI] [PubMed] [Google Scholar]
- Jolicoeur F. B., Rivest R., St-Pierre S., Gagné M. A., Dumais M. The effects of neurotensin and [D-Tyr11]-NT on the hyperactivity induced by intra-accumbens administration of a potent dopamine receptor agonist. Neuropeptides. 1985 Apr;6(2):143–156. doi: 10.1016/0143-4179(85)90105-2. [DOI] [PubMed] [Google Scholar]
- Kilts C. D., Anderson C. M., Bissette G., Ely T. D., Nemeroff C. B. Differential effects of antipsychotic drugs on the neurotensin concentration of discrete rat brain nuclei. Biochem Pharmacol. 1988 Apr 15;37(8):1547–1554. doi: 10.1016/0006-2952(88)90017-2. [DOI] [PubMed] [Google Scholar]
- Kislauskis E., Dobner P. R. Mutually dependent response elements in the cis-regulatory region of the neurotensin/neuromedin N gene integrate environmental stimuli in PC12 cells. Neuron. 1990 May;4(5):783–795. doi: 10.1016/0896-6273(90)90205-t. [DOI] [PubMed] [Google Scholar]
- Letter A. A., Merchant K., Gibb J. W., Hanson G. R. Effect of methamphetamine on neurotensin concentrations in rat brain regions. J Pharmacol Exp Ther. 1987 May;241(2):443–447. [PubMed] [Google Scholar]
- Merchant K. M., Bush L. G., Gibb J. W., Hanson G. R. Dopamine D2 receptors exert tonic regulation over discrete neurotensin systems of the rat brain. Brain Res. 1989 Oct 23;500(1-2):21–29. doi: 10.1016/0006-8993(89)90295-3. [DOI] [PubMed] [Google Scholar]
- Merchant K. M., Dobner P. R., Dorsa D. M. Differential effects of haloperidol and clozapine on neurotensin gene transcription in rat neostriatum. J Neurosci. 1992 Feb;12(2):652–663. doi: 10.1523/JNEUROSCI.12-02-00652.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant K. M., Gibb J. W., Hanson G. R. Role of dopamine D-1 and D-2 receptors in the regulation of neurotensin systems of the neostriatum and the nucleus accumbens. Eur J Pharmacol. 1989 Feb 7;160(3):409–412. doi: 10.1016/0014-2999(89)90098-8. [DOI] [PubMed] [Google Scholar]
- Merchant K. M., Miller M. A., Ashleigh E. A., Dorsa D. M. Haloperidol rapidly increases the number of neurotensin mRNA-expressing neurons in neostriatum of the rat brain. Brain Res. 1991 Feb 1;540(1-2):311–314. doi: 10.1016/0006-8993(91)90526-2. [DOI] [PubMed] [Google Scholar]
- Miller J. C. Induction of c-fos mRNA expression in rat striatum by neuroleptic drugs. J Neurochem. 1990 Apr;54(4):1453–1455. doi: 10.1111/j.1471-4159.1990.tb01983.x. [DOI] [PubMed] [Google Scholar]
- Nemeroff C. B., Luttinger D., Hernandez D. E., Mailman R. B., Mason G. A., Davis S. D., Widerlöv E., Frye G. D., Kilts C. A., Beaumont K. Interactions of neurotensin with brain dopamine systems: biochemical and behavioral studies. J Pharmacol Exp Ther. 1983 May;225(2):337–345. [PubMed] [Google Scholar]
- Nemeroff C. B. Neurotensin: perchance an endogenous neuroleptic? Biol Psychiatry. 1980 Apr;15(2):283–302. [PubMed] [Google Scholar]
- Ogren S. O., Hall H., Köhler C., Magnusson O., Lindbom L. O., Angeby K., Florvall L. Remoxipride, a new potential antipsychotic compound with selective antidopaminergic actions in the rat brain. Eur J Pharmacol. 1984 Jul 20;102(3-4):459–474. doi: 10.1016/0014-2999(84)90567-3. [DOI] [PubMed] [Google Scholar]
- Quirion R. Interactions between neurotensin and dopamine in the brain: an overview. Peptides. 1983 Sep-Oct;4(5):609–615. doi: 10.1016/0196-9781(83)90005-0. [DOI] [PubMed] [Google Scholar]
- Robertson G. S., Fibiger H. C. Neuroleptics increase c-fos expression in the forebrain: contrasting effects of haloperidol and clozapine. Neuroscience. 1992;46(2):315–328. doi: 10.1016/0306-4522(92)90054-6. [DOI] [PubMed] [Google Scholar]
- Seeman P., Lee T., Chau-Wong M., Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976 Jun 24;261(5562):717–719. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- Shibata K., Haverstick D. M., Bannon M. J. Tachykinin gene expression in rat limbic nuclei: modulation by dopamine antagonists. J Pharmacol Exp Ther. 1990 Oct;255(1):388–392. [PubMed] [Google Scholar]
- Shibata K., Yamada K., Furukawa T. Possible neuronal mechanisms involved in neurotensin-induced catalepsy in mice. Psychopharmacology (Berl) 1987;91(3):288–292. doi: 10.1007/BF00518179. [DOI] [PubMed] [Google Scholar]
- Wadworth A. N., Heel R. C. Remoxipride. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in schizophrenia. Drugs. 1990 Dec;40(6):863–879. doi: 10.2165/00003495-199040060-00008. [DOI] [PubMed] [Google Scholar]
- Widerlöv E., Lindström L. H., Besev G., Manberg P. J., Nemeroff C. B., Breese G. R., Kizer J. S., Prange A. J., Jr Subnormal CSF levels of neurotensin in a subgroup of schizophrenic patients: normalization after neuroleptic treatment. Am J Psychiatry. 1982 Sep;139(9):1122–1126. doi: 10.1176/ajp.139.9.1122. [DOI] [PubMed] [Google Scholar]
- Wong D. F., Wagner H. N., Jr, Tune L. E., Dannals R. F., Pearlson G. D., Links J. M., Tamminga C. A., Broussolle E. P., Ravert H. T., Wilson A. A. Positron emission tomography reveals elevated D2 dopamine receptors in drug-naive schizophrenics. Science. 1986 Dec 19;234(4783):1558–1563. doi: 10.1126/science.2878495. [DOI] [PubMed] [Google Scholar]
- Zahm D. S. Subsets of neurotensin-immunoreactive neurons revealed following antagonism of the dopamine-mediated suppression of neurotensin immunoreactivity in the rat striatum. Neuroscience. 1992;46(2):335–350. doi: 10.1016/0306-4522(92)90056-8. [DOI] [PubMed] [Google Scholar]