Abstract
Planar cell polarity (PCP) is a ubiquitous property of animal tissues and is essential for morphogenesis and homeostasis. In most cases, this fundamental property is governed by a deeply conserved set of ‘core PCP’ proteins, which includes the transmembrane proteins Van Gogh-like (Vangl) and Frizzled (Fzd), as well as the cytoplasmic effectors Prickle (Pk) and Dishevelled (Dvl). Asymmetric localization of these proteins is thought to be central to their function, and understanding the dynamics of these proteins is an important challenge in developmental biology. Among the processes that are organized by the core PCP proteins is the directional beating of cilia, such as those in the vertebrate node, airway and brain. Here, we exploit the live imaging capabilities of Xenopus to chart the progressive asymmetric localization of fluorescent reporters of Dvl1, Pk2 and Vangl1 in a planar polarized ciliated epithelium. Using this system, we also characterize the influence of Pk2 on the asymmetric dynamics of Vangl1 at the cell cortex, and we define regions of Pk2 that control its own localization and those impacting Vangl1. Finally, our data reveal a striking uncoupling of Vangl1 and Dvl1 asymmetry. This study advances our understanding of conserved PCP protein functions and also establishes a rapid, tractable platform to facilitate future in vivo studies of vertebrate PCP protein dynamics.
KEY WORDS: Planar cell polarity, Core PCP, Prickle, Pk2, Dishevelled, Dvl1, Van Gogh-like, Vangl1, Cilia, Multiciliated cell, Xenopus
Summary: Live imaging in Xenopus reveals that Pk2, a cytoplasmic effector of the PCP pathway, controls the directional ciliary beating in multiciliated cells by regulating Vangl1 dynamics at the cell cortex.
INTRODUCTION
The polarization of cellular shape, structure and behavior across epithelial sheets is established by the action of the planar cell polarity (PCP) pathway. Pioneering work in Drosophila revealed that the coordinated orientation of bristles and hairs throughout the body is governed by ‘core PCP’ genes (Adler, 1992; Gubb, 1993; Gubb and Garcia-Bellido, 1982), and this genetic module has since been associated with a diverse set of developmental processes spanning invertebrates to mammals. Some examples in vertebrates include polarized cell shape changes and rearrangements during gastrulation, axis elongation and neural tube closure, the polarized orientation of nodal cilia required during the establishment of left-right asymmetry, polarized beating of cilia on multiciliated cells (MCCs), and the polarization of cochlear mechanosensory stereocilia (Goodrich and Strutt, 2011; Gray et al., 2011; Wallingford, 2012).
A hallmark of planar polarized epithelia is the asymmetric localization of core PCP proteins in each cell. The core PCP genes include those encoding the transmembrane proteins Frizzled (Fz in Drosophila, Fzd in vertebrates), Van Gogh [Vang; or Strabismus (Stbm) in invertebrates, Vangl in vertebrates] and Flamingo [Fmi; or Starry night (Stan) in invertebrates, Celsr in vertebrates], as well as the intracellular proteins Dishevelled (Dsh; Dvl in vertebrates), Diego (Dgo; inversin in vertebrates) and Prickle (Pk) (Strutt, 2008). These proteins form complementary domains of enrichment at the level of apical junctions within the cells of planar polarized epithelia. In the Drosophila wing, for example, the localization of Fz and Dsh becomes restricted to the distal edge of the apical cell membrane, and these PCP complexes are mirrored at the proximal edge by accumulations of Vang and Pk (Axelrod, 2001; Bastock et al., 2003; Strutt, 2001; Strutt and Strutt, 2002). Both proximal and distal complexes associate with Fmi and, together, they pattern the orientation of wing hairs from high Fz levels in one cell towards low levels of Fz across shared junctions in the neighboring cells (Adler et al., 1997; Chen et al., 2008; Strutt and Strutt, 2008). The transmembrane proteins are sufficient to propagate PCP patterning signals between cells in a non-autonomous fashion, coordinating alignment, while the cytoplasmic members amplify intracellular asymmetry (Adler et al., 2000; Das et al., 2004; Jenny et al., 2003, 2005; Strutt and Strutt, 2007, 2008; Tree et al., 2002; Vinson and Adler, 2002; Wu and Mlodzik, 2008).
In the fly wing, core PCP proteins are initially trafficked apically and are symmetrically distributed at the level of cellular junctions. Transmembrane Fmi is thought to be subject to endocytic flux, and stability at the membrane is gradually increased over time in a Fz- and Vang-dependent manner, presumably as these core PCP complexes become associated with Fmi-Vang or Fmi-Fz pairs of a neighboring cell (Strutt et al., 2011). The preferential stabilization of Fmi-Vangl pairs at proximal junctions and Fmi-Fz pairs at distal junctions has been suggested to involve an initial upstream bias that is then amplified via both positive- and negative-feedback loops (Amonlirdviman et al., 2005; Tree et al., 2002). These proposed feedback loops that amplify intracellular PCP asymmetry have been shown in Drosophila to involve a network of positive and negative interactions centered on the cytoplasmic core PCP members (Cho et al., 2015; Strutt and Strutt, 2007). In brief, Pk binds and inhibits the membrane localization of Dsh, but Dgo competes with Pk for Dsh binding, promoting the association of Dsh with Fz (Jenny et al., 2005; Tree et al., 2002). Pk also physically interacts with Vang, and this interaction leads to the clustering of Vang on the opposite side of the cell (Bastock et al., 2003; Jenny et al., 2003; Strutt et al., 2011). In addition, Pk mediates the internalization of Vang or Fmi-Vang molecules that are not associated with stable, patterned complexes (Cho et al., 2015). Because of the delicate balance required for asymmetry between these proteins, an intricate system of ubiquitylation and proteosomal degradation is in place to control the protein levels of core PCP components (Cho et al., 2015; Narimatsu et al., 2009; Strutt et al., 2013a,b), and overexpression or loss of function of one core PCP protein in a given cell is generally sufficient to disrupt core PCP asymmetry and result in a loss or reversal of structural polarity (Adler et al., 2000; Bastock et al., 2003; Strutt and Strutt, 2007; Tree et al., 2002).
Consistent with data from Drosophila, asymmetry has also been observed for various core PCP proteins in MCCs of the mouse airway and brain ventricles (Guirao et al., 2010; Vladar et al., 2012), where these cells drive directional fluid flow that is essential for proper development and homeostasis (Brooks and Wallingford, 2014). Here, PCP proteins control the coordinated orientation of ciliary structures that interface with the apical cytoskeleton within each cell, thus aligning the cilia within these cells to establish polarized, synchronous beating; the term rotational polarity refers to these intracellular orientations of ciliary basal bodies (Wallingford, 2010). Furthermore, the asymmetric patterning of core PCP proteins allows for the alignment of cellular polarity between cells across entire tissues, and this intercellular tissue-wide polarity promotes strong, effective flow (Wallingford, 2010).
In vertebrates, core PCP proteins are encoded by families of related genes, including two Vangl, three Dvl and at least three Pk genes. Several examples of cell- and tissue-specific differences in protein localization and function have been observed for different vertebrate PCP family members in the context of these multiciliated epithelia. For example, Dvl2 is present at the basal bodies of MCCs but does not display cortical asymmetry, whereas Dvl1 and Dvl3 are not at basal bodies but rather are present at planar polarized cortical crescents (Park et al., 2008; Vladar et al., 2012). Additional examples of differences in the activity of related core PCP homologs are provided by the analysis of ciliary and cytoskeletal patterning throughout the multiciliated ependymal cells of various PCP mutant mice. The ciliary rotational polarity within cells appears to be disrupted in Celsr2 and Celsr3 mutants, while the tissue-wide coordination of mean directionality remains intact (Boutin et al., 2014). In stark contrast, Celsr1 mutant mice exhibit the seemingly opposite characteristics, with properly organized ciliary patches within cells that fail to oriented in a coordinated fashion across cells throughout the tissue (Boutin et al., 2014). The increased complexity seen in vertebrate PCP warrants further study of individual core PCP family members and challenging any assumed redundancy in their activity.
Interestingly, MCCs are also present in the epidermis of developing amphibian embryos, and the relative ease of observing these epidermal MCCs has contributed to their application in the analysis of planar polarization for decades (König and Hausen, 1993; Twitty, 1928). Early studies of the role for PCP signaling in MCC directional beating were performed in these cells, prefiguring the work in mammals (Mitchell et al., 2009; Park et al., 2008). In this context, disruption of core PCP components can lead to defects in either rotational polarity within the cells or tissue-wide polarity across the cells in the Xenopus epidermis (Mitchell et al., 2009; Park et al., 2008). For example, the transplantation of epidermal tissue overexpressing Vangl2 onto an otherwise normal embryo reverses the orientation of basal bodies in cells situated anterior to the transplant, demonstrating the tissue-level, non-autonomous effects of transmembrane core PCP components (Mitchell et al., 2009). By contrast, the expression of a PCP-specific, dominant-negative form of Dvl that lacks a large C-terminal portion of the PDZ domain [Dvl2-ΔPDZpartial – previously referred to as Xdd1 (Sokol, 1996)] completely randomizes the initial rotational polarity within MCCs, and tissue grafting experiments revealed that this Dvl2-ΔPDZpartial dominant negative acts in a strictly cell-autonomous manner (Mitchell et al., 2009). In addition to the basal body localization that was also later reported in the mouse ependyma and medullary thymic epithelial cells, Dvl2 immunostaining shows symmetrical distributions at the apical junctions of cells regardless of its polarizing effects (Park et al., 2008). In fact, despite the promising capability of protein dynamics studies in this in vivo vertebrate system, there is as yet no report of planar polarized, apicolateral localization of core PCP proteins in the Xenopus epidermis. Here, we report that planar polarity of the Xenopus multiciliated epithelium is progressively patterned by the asymmetric localization of a subset of core PCP family members, and we go on to leverage the strengths of this Xenopus platform by performing quantitative analyses of subcellular dynamics that serve to dissect the role of Pk2 in the establishment of these asymmetric patterns.
RESULTS
Asymmetric patterning of specific core PCP proteins in Xenopus MCCs
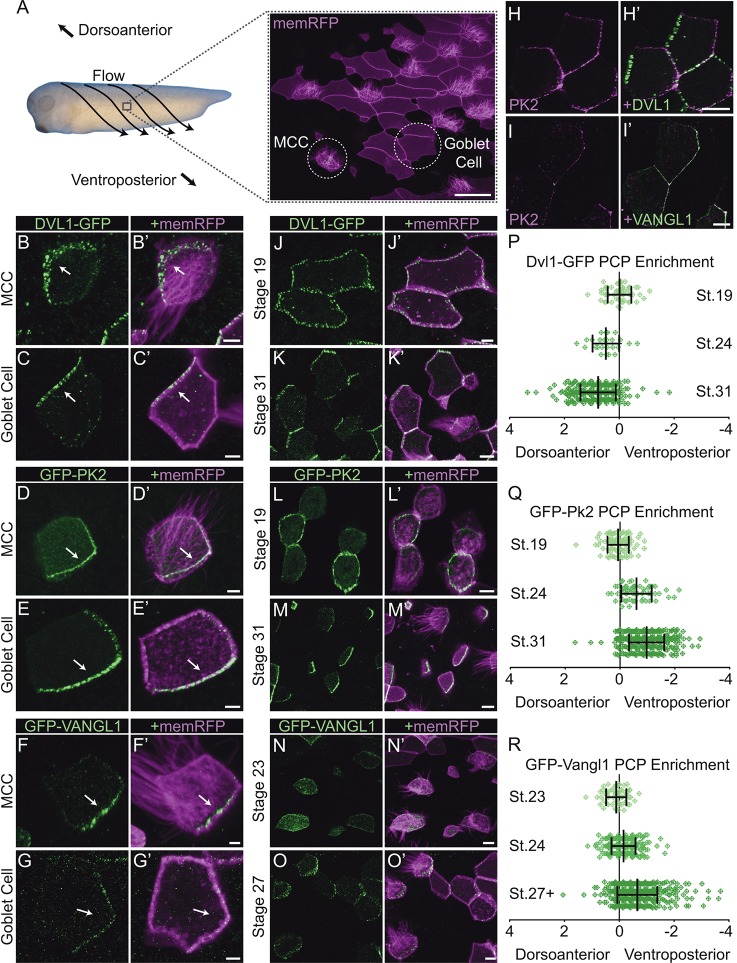
A role for PCP proteins in the orientation of ciliary beating was first described in Xenopus epidermal MCCs (Mitchell et al., 2009; Park et al., 2008). Surprisingly, there are as yet no reports of asymmetric localization of PCP proteins at the cell cortex in these cells, although such localization has been reported for both airway and ependymal MCCs in mouse (Guirao et al., 2010; Vladar et al., 2012). Because vertebrate PCP proteins are encoded by multi-gene families, we surveyed the localization of GFP fusions to a wide range of Xenopus PCP proteins, using mosaic expression for accurate assessment of asymmetric localization. Whereas many previously studied Xenopus core PCP proteins localized symmetrically around the cell cortex, including Dvl2, Dvl3, Fzd7, Fzd8 and Vangl2, we found that Dvl1, Pk2, Vangl1 and Fzd6 displayed striking asymmetric localizations (Fig. 1; supplementary material Fig. S1). Transcripts for eight of these nine family members were identified in RNA-seq analyses of organotypic cultures of the Xenopus mucociliary epidermis, with only fzd7 being absent (Chung et al., 2014).
Fig. 1.
Core PCP proteins pattern the Xenopus epidermis during the refinement of basal body orientation. (A) Xenopus laevis embryo with flow and anatomical directionality indicated along with a confocal slice showing the different cell types visualized via memRFP in a mosaically labeled epidermis. MCC, multiciliated cell. (B-G′) MCCs (B,D,F) and goblet cells (C,E,G) in a St.31 embryo surrounded by unlabeled neighbors display asymmetric core PCP protein localization in the direction indicated by the arrows. (H-I′) Groups of cells labeled with RFP-Pk2 and either mutually exclusive Dvl1-GFP (H) or colocalizing GFP-Vangl1 (I). (J-M′) Patches of cells mosaically labeled with either Dvl1-GFP (J,K) or GFP-Pk2 (L,M) at stages prior to ciliogenesis (St.19) and after basal body refinements (St.31). (N-O′) Patches of cells mosaically labeled with GFP-Vangl1 at stages during ciliogenesis (St.23) and during basal body refinement (St.27). (P-R) Quantifications of PCP enrichment at different developmental stages show that increasing asymmetry develops during basal body refinement stages. Each mark represents the enrichment value for a single cell. All comparisons within graphs are highly significant (P<0.0001) except for a modest increase between Dvl1 St.24 and St.31 (P=0.0366). In P: St.19, n=58 cells; St.24, n=25 cells; St.31, n=321 cells. In Q: St.19, n=106 cells; St.24, n=61 cells; St.31, n=344 cells. In R: St.23, n=61 cells; St.24, n=268 cells; St.27+, n=465 cells. Error bars indicate s.e.m. Scale bars: 50 μm in A; 5 μm in B-G′; 10 μm in H-O′.
In Xenopus embryos, fluid flow is directed across the epidermis from the dorsoanterior to ventroposterior direction (Fig. 1A). Accordingly, we observed punctate accumulations of Dvl1-GFP that were restricted to the dorsoanterior apical cell cortex of MCCs found here, corresponding to asymmetry in the direction upstream of flow (Fig. 1B). Mucociliary epithelia are composed of two principal cell types, namely MCCs and mucus-secreting goblet cells, and because the MCCs are separated from one another by intervening goblet cells, PCP signaling must be transmitted evenly across both cell types. It is notable, then, that we also observed asymmetric accumulations of Dvl1 in goblet cells (Fig. 1C). Comparable asymmetry appeared to occur in other intercalating cell types, such as ionocytes (Dubaissi and Papalopulu, 2011; Quigley et al., 2011) and small secretory cells (Dubaissi et al., 2014; Walentek et al., 2014), which were identifiable by their significantly smaller surface area and lack of cilia in regions where only intercalating cells had been labeled.
Typically, asymmetric Dvl co-accumulates with asymmetric Fzd at the cell cortex (Seifert and Mlodzik, 2007) and, indeed, Fzd6-GFP also localized to the dorsoanterior cell cortex (supplementary material Fig. S1A). In most planar polarized tissues, domains enriched for Dvl and Fzd are mirrored by complementary accumulations of Pk and Vangl (Seifert and Mlodzik, 2007), and we observed asymmetric accumulations of GFP-Pk2 (Fig. 1D,E) and GFP-Vangl1 (Fig. 1F,G) at the ventroposterior cell cortex in both MCCs and goblet cells. Moreover, co-expression of Dvl1-GFP and RFP-Pk2 revealed mutually exclusive domains of enrichment (Fig. 1H), and clear colocalization was observed for RFP-Pk2 and GFP-Vangl1 proteins (Fig. 1I). These observations of an Fzd family member on the anterior cell face and Vangl at the posterior are consistent with previous functional studies of the domineering non-autonomy of PCP signaling in this tissue (Mitchell et al., 2009), although, on the other hand, it was surprising that their orientation relative to the direction of ciliary beating is reversed compared with that of MCCs in the mouse trachea (Vladar et al., 2012).
Dynamics of the asymmetric localization of Pk2, Vangl1 and Dvl1
Having identified useful reporters for PCP patterning in the Xenopus embryonic epidermis, we next sought to characterize the developmental dynamics of core PCP protein localization. MCCs are derived from a basal layer of progenitor cells and insert into the mucociliary epithelium at the early tailbud stage [around stage (St.) 22], after which cilia are assembled and polarization of ciliary beating is established progressively over the next several hours (until roughly St.30) (Billett and Gould, 1971; Drysdale and Elinson, 1992; König and Hausen, 1993). Prior to the insertion of MCCs, Dvl1-GFP decorated the apicolateral regions of goblet cells symmetrically and in a punctate fashion (Fig. 1J). During tailbud stages, as ciliogenesis is completed and the refinement of ciliary orientation begins, Dvl1-GFP asymmetry is present, although the degree and coordination of asymmetry is variable at this time. Dvl1-GFP asymmetry finally reaches a maximal level in both goblet cells and MCCs across the tissue around St.30 (Fig. 1K), a time at which ciliary basal body orientations are locked in place and flow has strengthened across the epithelium (König and Hausen, 1993; Mitchell et al., 2007; Werner et al., 2007).
GFP-Pk2 displayed similar dynamics to Dvl1-GFP, with asymmetric accumulations also apparent by St.24-25 that reach a maximum around St.30 (Fig. 1L,M), but with some notable differences. First, although GFP-Pk2 also localizes symmetrically to apical accumulations just prior to ciliogenesis, the punctate pattern observed for Dvl1-GFP was less prominent for GFP-Pk2 (Fig. 1L). Another interesting dissimilarity was the localization of GFP-Pk2 near ciliary basal bodies labeled with Centrin-RFP at later stages (supplementary material Fig. S1G). For the transmembrane protein Vangl1, localization of GFP fusions at early stages consisted primarily of cytoplasmic puncta, and labeling at the cell cortex was weak and diffuse at these stages (Fig. 1N). This pattern might reflect the vesicular transport proteins known to be important for the processing and trafficking of Vangl proteins (Guo et al., 2013; Merte et al., 2009; Yin et al., 2012). Both the cortical localization and asymmetry of these accumulations increased as development proceeded, with a timeframe similar to that for Dvl1 and Pk2 (Fig. 1O).
We quantified these dynamic localization patterns using the relative level of reporter fluorescence intensity at dorsoanterior and ventroposterior cell cortices, and mosaic labeling allowed us to score cells abutting unlabeled neighbors (‘PCP enrichment’; see supplementary material Fig. S5A and Materials and Methods). This metric demonstrates the degree to which asymmetry increased over time during PCP patterning for all three reporters examined (Fig. 1P-R). Interestingly, PCP enrichment increased along with increased coordination of fluid flow (König and Hausen, 1993), and asymmetries were not easily detectable at times corresponding to stages of initial MCC intercalations. Once MCCs have intercalated and expanded their apical surface, basal bodies are docked and serve as the foundations for cilia formation. Once these cilia are visibly projecting from the apical surface, the surrounding goblet cells can be seen exhibiting asymmetric patterns that are readily detectible by eye, although no such patterns are easily discernible in the MCCs. This result suggests that the initial rotational polarity of basal bodies that is in place at the onset of ciliogenesis, which clearly depends upon intact Dvl2 function (Mitchell et al., 2007), might not be reliant upon intracellular PCP asymmetry, but rather that directional information is imparted by the surrounding tissue. However, it has also been reported that polarity information can be relayed in the absence of detectible asymmetry in the fly wing (Strutt and Strutt, 2007), so perhaps asymmetric stability or activity in the absence of clear asymmetric localization might be present in these early, maturing MCCs.
Interplay of cortical asymmetric localization of Pk2 and Dvl1
We next explored the interplay between Pk2 and Dvl1 during asymmetric localization, as antagonistic interactions between homologs of these proteins are fundamental to amplifying visually detectable intracellular asymmetry in other contexts (Strutt and Strutt, 2007), yet have never been examined for these particular family members or in this tissue. We first determined the effect of the well-characterized and PCP-specific dominant-negative Dvl2-ΔPDZpartial (Xdd1) (Sokol, 1996), shown to negatively affect PCP signaling in a variety of contexts, including in MCCs (Park et al., 2008; Wallingford et al., 2000). Expression of Dvl2-ΔPDZpartial significantly disrupted the normal ventroposterior restriction of Pk2-GFP (Fig. 2A-C), demonstrating that asymmetric Pk2 localization is dependent upon the ability of the cell to adopt a planar polarized state. We then performed the complementary experiment by reducing Pk2 levels using an antisense morpholino (Pk2-MO#1) that disrupts splicing of pk2 (supplementary material Fig. S2A). Whereas discrete crescents of Dvl1-GFP accumulation were normally oriented in the dorsoanterior direction in controls (Fig. 2D), Pk2 knockdown significantly reduced this asymmetric enrichment (Fig. 2E,F), and a second morpholino targeting an alternate splicing sequence provided similar results (supplementary material Fig. S2A-C). Together with the observed progressive asymmetric localization (Fig. 1), these data demonstrate the efficacy and veracity of our GFP reporters for core PCP protein localization in this tissue. We then used these novel reporters to address outstanding questions in vertebrate PCP signaling.
Fig. 2.
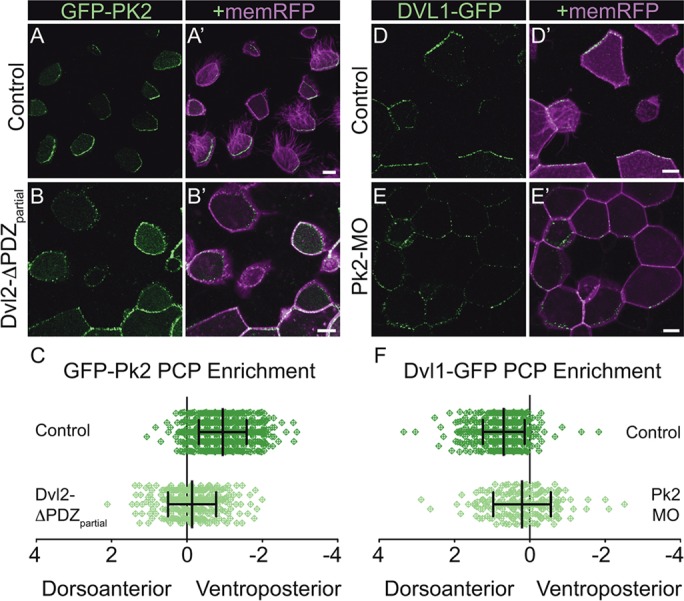
Intact PCP signaling is required for the formation of asymmetric core PCP complexes. (A-B′) Mosaically labeled epidermal cells in St.31 X. laevis embryos have GFP-Pk2 localized asymmetrically in the control situation (A) and symmetrically upon overexpression of Dvl2-ΔPDZpartial (B). (C) Quantification of PCP enrichment shows a significant shift upon Dvl2-ΔPDZpartial expression (P<0.0001; Control, n=584 cells; Dvl2-ΔPDZpartial, n=326 cells). (D-E′) Mosaically labeled epidermal cells in St.31 X. laevis embryos have GFP-Pk2 localized asymmetrically in the control situation (D) and symmetrically upon Pk2-MO knockdown (E). (F) Quantification of PCP enrichment shows a significant shift upon Pk2-MO knockdown (P<0.0001; Control, n=508 cells; Pk2-MO, n=210 cells). Error bars indicate s.e.m. Scale bars: 10 μm.
Role of the PCP effectors Intu and Wdpcp in Pk2 protein localization
First, we assessed the role of the ‘PCP effector’ proteins in the patterning of polarity complexes, as the role of these proteins in PCP signaling remain contentious. First identified as planar polarity proteins by genetic screens in Drosophila (Collier et al., 2005; Gubb and Garcia-Bellido, 1982), Inturned and Fritz were placed genetically downstream of core PCP protein function (Collier et al., 2005; Lee and Adler, 2002; Wong and Adler, 1993), although a recent paper suggests that Fritz overexpression can influence core PCP protein localization in Drosophila (Wang et al., 2014). Curiously, the vertebrate orthologs (Intu and Wdpcp/Fritz) were found to control ciliogenesis, first in the Xenopus epidermis (Kim et al., 2010; Park et al., 2006) and later in mice (Cui et al., 2013; Zeng et al., 2010). Intu apparently plays only a modest role in PCP-mediated processes such as convergent extension (Park et al., 2006), whereas Wdpcp is essential for both convergent extension in Xenopus (Kim et al., 2010) and planar polarization of cochlear hair cells in mouse (Cui et al., 2013). We performed knockdown of Wdpcp and Intu using morpholinos that have been validated by genetic studies in mice (Cui et al., 2013; Kim et al., 2010; Park et al., 2006; Zeng et al., 2010). Wdpcp knockdown strongly disrupted the planar polarized localization of Pk2, whereas Intu knockdown had a far more modest, although still significant, effect (Fig. 3A-C). These data reveal an important role for Wdpcp in the control of core PCP protein asymmetry in vertebrates, the mechanism of which will be important to determine.
Fig. 3.
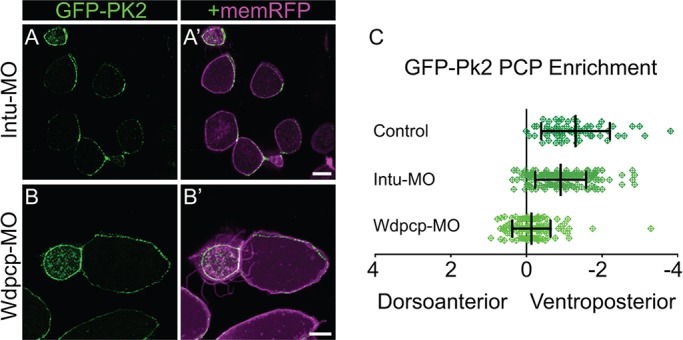
Wdpcp knockdown disrupts core PCP patterning. (A,B) Mosaically labeled epidermal cells in St.31 X. laevis embryos have GFP-Pk2 localized asymmetrically upon Intu-MO knockdown (A) and symmetrically upon Wdpcp-MO knockdown (B). (C) Quantification of PCP enrichment shows a significant shift upon Intu-MO knockdown (P=0.0031, n=187 cells) and a more significant shift upon Wdpcp-MO knockdown (P<0.0001, n=137 cells) in comparison to controls (n=64 cells). Error bars indicate s.e.m. Scale bars: 10 μm.
Role of Pk2 in the control of asymmetric cortical Vangl1 dynamics
The next question we chose to address concerns the interplay between Pk2 and Vangl1. In Drosophila, Pk physically interacts with and clusters Vang at the apicolateral membrane, and this behavior promotes Vang accumulation on the proximal side of cells in the wing epithelium (Bastock et al., 2003; Jenny et al., 2003). Although both are implicated in vertebrate PCP (Liu et al., 2014; Song et al., 2010; Takeuchi et al., 2003; Torban et al., 2008; Vladar et al., 2012), Vangl1 and Pk2 remain relatively seldom studied, and it is unknown in vertebrates if either Pk1 or Pk2 is required for the normal localization of Vangl1 or Vangl2 protein.
We found that Pk2 knockdown eliminated the asymmetric accumulation of GFP-Vangl1 at the ventroposterior cell cortex (Fig. 4A,B), whereas, conversely, Pk2 overexpression increased Vangl1 apicolateral enrichment to such a degree that accumulations were no longer restricted to ventroposterior regions but were enriched in other areas around the cell periphery (Fig. 4A,C). Additional analysis confirmed that Pk2 knockdown disrupts asymmetry by suppressing the enrichment of Vangl1 at the cortical regions of the cell (supplementary material Fig. S3B) and, together, these observations are consistent with a previously defined role for Pk in the clustering of Vang (Bastock et al., 2003; Cho et al., 2015; Strutt and Strutt, 2007). We conclude that Pk2 promotes cortical Vangl1 concentration, and that an excess of Pk2 promotes further GFP-Vangl1 enrichment that becomes increasingly unrestricted from the ventroposterior cell face. In the reciprocal experiment, overexpression of Vangl1 showed a clear reduction in the planar polarized, cortical accumulation of GFP-Pk2 (supplementary material Fig. S4A-C), while enrichments of Dvl1-GFP were still present but not asymmetrically polarized (supplementary material Fig. S4A-C). In light of previous evidence that Vang participates in the control of Pk protein levels (Strutt et al., 2013b), our results suggest that Vangl1 overexpression might be leading to increased levels of Pk2 degradation, which in turn leads to a reduction in Pk2 and to associated Dvl1 patterning defects.
Fig. 4.
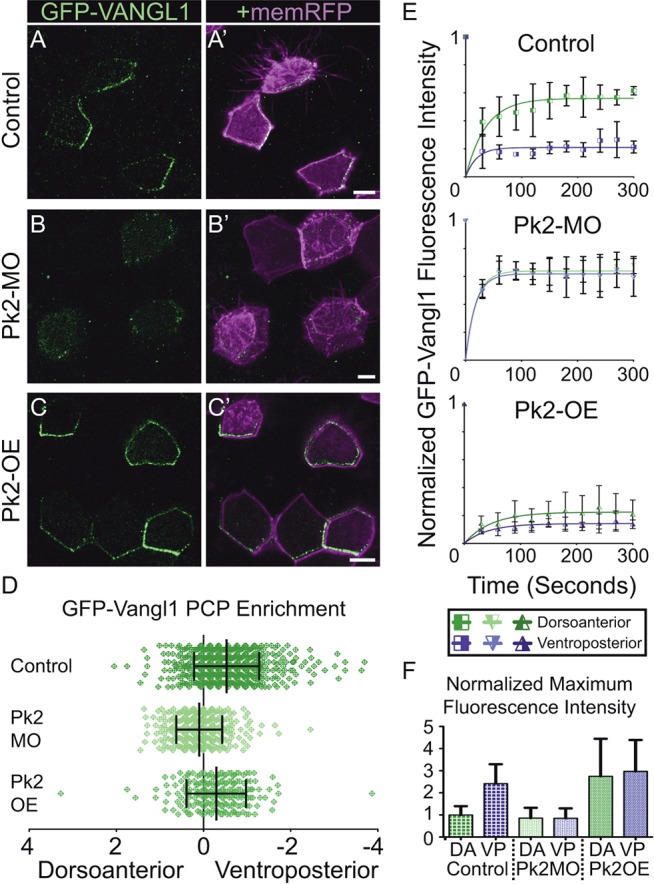
Pk2 expression levels influence the dynamic localization of apicolateral Vangl1 accumulations. (A-C′) Mosaically labeled epidermal cells in St.31 X. laevis embryos have GFP-Vangl1 localized asymmetrically in the control situation (A), whereas it is absent from apicolateral enrichments upon Pk2-MO knockdown (B) and localized more symmetrically with Pk2 overexpression (C). (D) Quantification of PCP enrichment shows a significant shift upon Pk2-MO knockdown (P<0.0001, n=355 cells) and Pk2 overexpression (P=0.0002, n=217 cells) in comparison to controls (n=519 cells). (E) FRAP recovery trends of GFP-Vangl1 fluorescence intensity following simultaneous bleaching at discrete dorsoanterior (green lines) and ventroposterior (purple lines) cortical regions in St.25 embryos. Error bars represent s.e.m. from three separately bleached cells in two or three different embryos. (F) Comparison of the maximum fluorescence intensities at regions measured in E prior to bleaching, which are normalized to dorsoanterior intensity in the control situation. DA, dorsoanterior; VP, ventroposterior. Error bars in D and F indicate s.e.m. Scale bars: 10 μm.
Given the demonstrated effects of Pk2 on the asymmetric enrichment of Vangl1, we next asked if Pk2 influences the dynamics of Vangl1 turnover at the cell cortex. Indeed, asymmetric enrichment of Fzd is driven in part by differences in Fzd turnover at distinct locations along the cell cortex (Strutt et al., 2011), and we wanted to test whether a similar mechanism might act on Vangl. We measured fluorescence recovery after photobleaching (FRAP) of GFP-Vangl1. In control embryos, enriched regions at the ventroposterior cortex contained a significantly larger stable fraction of GFP-Vangl1 when compared with the less enriched dorsoanterior cortex (Fig. 4E; supplementary material Fig. S5C,D). This result was evident in the ∼30% increase in normalized fluorescence recovery at the latter (Fig. 4E). This difference was potently extinguished by Pk2 knockdown, in which case both dorsoanterior and ventroposterior cortical regions contained similarly unstable fractions of GFP-Vangl1 (Fig. 4E). In the complementary experiment, overexpression of Pk2 had the opposite effect, eliciting increased stability of GFP-Vangl1 on both sides of the cell (Fig. 4E). Comparison of these measurements with the maximum intensities prior to bleaching (Fig. 4F) reveals that areas more highly enriched for GFP-Vangl1 were indeed less dynamic. These data complement the previous studies of Dsh-dependent Fz stabilization in Drosophila (Strutt et al., 2011), not only by extending the work to a vertebrate epithelium, but also by demonstrating that asymmetric protein turnover is an attribute of core PCP protein localization on the Vangl/Pk side of the cell.
Structure/function analysis of Pk2
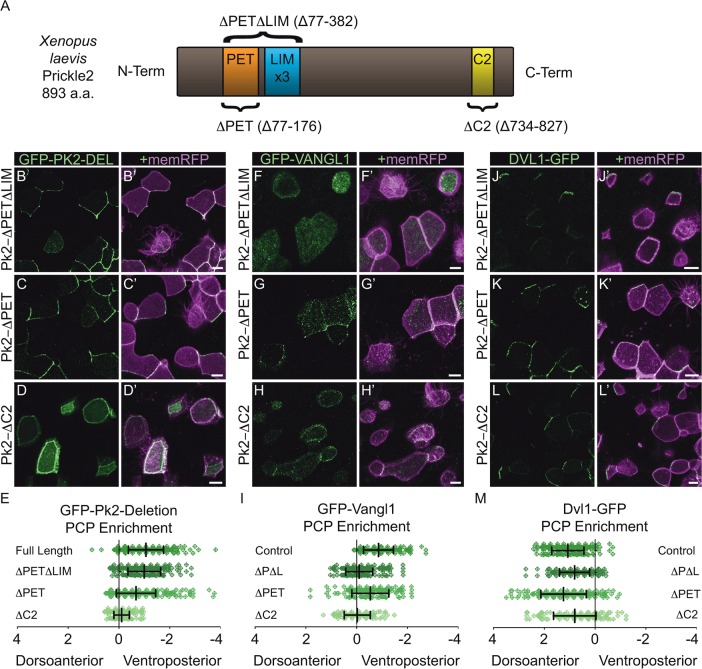
As the role for Pk2 in the control of core PCP dynamics became clear, we sought to determine the structural components of the protein necessary for eliciting the observed polarized behaviors. Pk2 contains three domains that are conserved in vertebrate Pk protein family members and in Pk proteins across species (Fig. 5A). These include the PET and LIM domains, as well as the C2 domain, which is populated by many serines and basic amino acids near the C-terminus of the protein (Jenny et al., 2003; Tree et al., 2002). The roles for various domains of vertebrate Pk proteins in PCP-dependent processes have been assessed (Daulat et al., 2011; Jenny et al., 2003; Lin and Gubb, 2009; Takeuchi et al., 2003), but how these roles relate to PCP protein localization remains unknown in vertebrates. We therefore constructed three Pk2 deletion constructs corresponding to previously described versions of Xenopus Pk1 (Takeuchi et al., 2003) and Drosophila Pk (Jenny et al., 2003); we then assessed both their localizations and their effects on the localization of Vangl1 and Dvl1.
Fig. 5.
The Pk2 C2 domain is required for Pk2 asymmetry, while both the LIM and C2 domains promote the asymmetric enrichment of Vangl1. (A) Schematic of X. laevis Pk2 showing the location of conserved domains removed from Pk2 deletion constructs. (B-D′) Cellular localizations of GFP-Pk2 with sequence deletions that correspond to the PET and LIM domains (B), PET domain alone (C), and C-terminal region C2 (D). (E) Graph depicting changes in the localization of Pk2 caused by conserved domain deletions, with the normal localization of GFP-Pk2-ΔPETΔLIM (P=0.4037, n=312 cells), still asymmetric yet significantly misaligned GFP-Pk2-ΔPET (P<0.0001, n=379 cells), and symmetric GFP-Pk2-ΔC2 (P<0.0001, n=302 cells), compared to full-length (n=374 cells). (F-H′) Cellular localizations of GFP-Vangl1 upon overexpression of Pk2-ΔPETΔLIM (F), Pk2-ΔPET (G) and Pk2-ΔC2 (H). (I) Graph depicting changes in localization of Vangl1 caused by overexpression of Pk2 deletions, with a loss of asymmetry upon Pk2-ΔPETΔLIM and Pk2-ΔC2 overexpression (P<0.0001, ΔPETΔLIM, n=310 cells; ΔC2, n=125 cells) and significant reduction upon Pk2-ΔPET overexpression (P=0.0001, n=203 cells), compared to full-length (n=215 cells). (J-L′) Cellular localizations of Dvl1-GFP upon overexpression of Pk2-ΔPETΔLIM (J), Pk2-ΔPET (K) and Pk2-ΔC2 (L). (M) Graph depicting changes in localization of Dvl1 caused by overexpression of Pk2 deletions, with a significant shift in, but not loss of, asymmetric enrichment upon Pk2-ΔPETΔLIM (P<0.0001, n=206 cells), Pk2-ΔPET (P=0.0181, n=212 cells) and Pk2-ΔC2 (P=0.0002, n=163 cells) overexpression, compared to full-length (n=243 cells). Error bars indicate s.e.m. Scale bars: 10 μm.
We first examined GFP fusions to a deletion of the PET domain and a combined deletion lacking both the PET and LIM domains. Strikingly, both GFP-Pk2-ΔPET and Pk2-ΔPETΔLIM were enriched ventroposteriorly (Fig. 5B,C). Although this polarization for GFP-Pk2-ΔPET was not as robust as that of full-length GFP-Pk2 (Fig. 5E), it was remarkably similar and suggests that neither the PET nor LIM domain is strictly required for proper Pk2 asymmetric localization. Despite their similar localizations, however, these two constructs had divergent effects on Vangl1 when expressed at high levels. Pk2-ΔPETΔLIM severely perturbed not only the asymmetry of Vangl1, but also its association with the cell cortex (Fig. 5F), comparable to the effects of Pk2 knockdown (supplementary material Fig. S3C). By contrast, both cortical recruitment and asymmetry of Vangl1 were intact after overexpression of Pk2-ΔPET (Fig. 5G), despite a significant reduction in the overall enrichment measure. Together, these findings are consistent with the opposing effects of ΔPETΔLIM and ΔPET deletions of Pk1 on PCP-dependent convergent extension (Takeuchi et al., 2003).
We next assessed a deletion of the C2 domain, which in Drosophila allows for binding to Vang, to other Pk molecules, and to Dgo (Jenny et al., 2003). Unlike the other deletions, GFP-Pk2-ΔC2 failed to adopt a polarized localization and instead remained symmetrical around the cell cortex (Fig. 5D,E). As such, this domain appears to be essential for the normal asymmetric localization of Pk2. Increased expression of Pk2-ΔC2 also severely disrupted the asymmetry of cortical Vangl1, although Vangl1 was present around the cell cortex (Fig. 5H). These data suggest that the C2 domain is required for the regulation of Pk2 localization and that the mislocalization that results from the C2 domain deletion promotes the co-mislocalization of associated Vangl1 (Fig. 5I), although we cannot rule out the possibility of an indirect or alternative mechanism of Vangl1 mislocalization around the cortex.
Lastly, we determined the effects of our Pk2 deletions on Dvl1 localization. In Drosophila, overexpression of solely the PET and LIM domains together has been suggested to inhibit the membrane association of Dsh (Tree et al., 2002), and the C2 domain that binds Dgo is likely to be important in the competition between Dgo and Pk for Dsh binding (Jenny et al., 2005). Strikingly, we found that Dvl1 remained polarized in the presence of any of the three Pk2 deletion constructs (Fig. 5J-M), although the degree of polarization was modestly reduced in some cases.
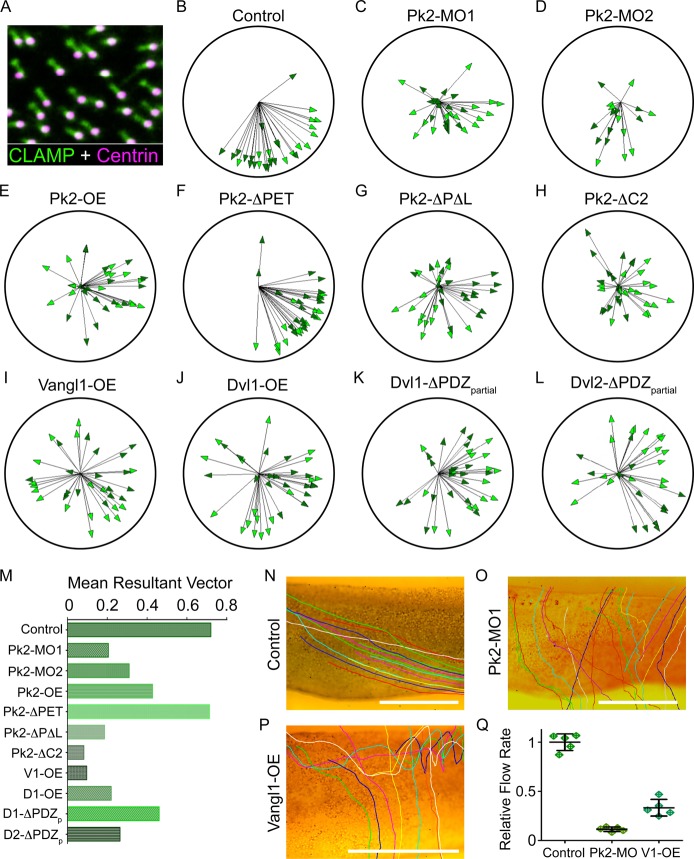
Effects of PCP manipulation on ciliary polarity
To determine how the various PCP patterning defects we observed affect structural and thus functional polarity in this epithelium, we assessed ciliary orientation using a CLAMP-GFP (CLAMP is also known as spef1 – Xenbase) ciliary rootlet marker and Centrin 4-RFP basal body marker (Park et al., 2008). Under control conditions, CLAMP-GFP localized to the dorsoanterior side of basal bodies with little variance, as expected because striated rootlets are oriented oppositely to the direction of the effective stroke (Fig. 6A). As previously used to provide ciliary orientation data (Mitchell et al., 2009), we present the state of basal body polarity using a single arrow for each individual cell in a circular diagram; arrows point in the mean direction of the effective ciliary strokes, and the length of the arrow represents the mean resultant vector, a measure of variation from the mean orientation within that cell. The majority of cells under control conditions were oriented in the ventroposterior direction, with little variation in the individual orientations observed (Fig. 6B). With an obvious exception for Pk2-ΔPET overexpression, all manipulations that were used to assess the molecular interactions affecting PCP protein enrichment resulted in clear defects in ciliary orientation (Fig. 6C-J). The exception for Pk2-ΔPET overexpression is significant because this reagent also did not disrupt the asymmetry of other core PCP proteins (Fig. 5), although this result is dissimilar to that of a previous report of Pk2-ΔPET overexpression disrupting fly wing hair polarity (Lin and Gubb, 2009). In addition, because Dvl1 and Dvl2 displayed divergent localizations at the cell cortex and basal bodies, respectively, we tested the effects of overexpressing Dvl1-ΔPDZpartial, a Dvl1 construct analogous to the Dvl2-ΔPDZpartial deletion of Dvl2 (Mitchell et al., 2009; Park et al., 2008; Sokol, 1996). Similarly to Dvl2-ΔPDZpartial, this resulted in a strong perturbation of ciliary polarity (Fig. 6K,L).
Fig. 6.
Disrupting PCP patterning leads to structural defects in basal body polarity. (A) Apical intercellular region of a single MCC labeled with CLAMP-GFP and Centrin-RFP, which when combined display the orientation of cilia with respect to the direction of ciliary beating (CLAMP points in the opposite direction). (B-L) Plots displaying the mean basal body orientation and associated mean vector length of MCCs under various experimental conditions. Each arrow represents a measure of the orientations of a single MCC, with increased length correlated with less variation between basal bodies within each MCC. Dark and light arrowheads represent data from separate experimental repeats, with each experiment involving measures from at least five cells from at least three embryos each. The combined data are displayed for control (B, n=2113 orientations, 31 cells), Pk2-MO#1 knockdown (C, n=1556 orientations, 32 cells), Pk2-MO#2 knockdown (D, n=1057 orientations, 18 cells), Pk2 overexpression (E, n=2426 orientations, 34 cells), Pk2-ΔPET overexpression (F, n=3122 orientations, 35 cells), Pk2-ΔPETΔLIM overexpression (G, n=1941 orientations, 34 cells), Pk2-ΔC2 overexpression (H, n=2275 orientations, 33 cells), Vangl1 overexpression (I, n=2843 orientations, 35 cells), Dvl1 overexpression (J, n=2086 orientations, 37 cells), Dvl1-ΔPDZpartial overexpression (K, n=2797 orientations, 36 cells) and Dvl2-ΔPDZpartial overexpression (L, n=2177 orientations, 32 cells) conditions. (M) The mean resultant vector length from the combined measurements for all MCCs across all embryos from both experimental treatments, serving as an overall metric for PCP disruption. (N-P) Stills from the end of movies showing the traces of beads carried by flow across the epidermis of St.31 X. laevis embryos that were uninjected (N) or were injected with either Pk2-MO (O) or a high dose of vangl1 mRNA (P). (Q) Quantification of the flow rate from five beads traced in the movies of which the final frame is shown in N-P. Error bars indicate s.e.m. Scale bars: 1 mm.
Comparing the mean vector length of individual cells between Pk2 misexpression conditions and Vangl1 overexpression reveals a difference in the type of polarity defects observed, with seemingly more defective rotational polarity for the former and tissue-wide polarity for the latter. This interpretation is further supported not only by previous characterization of Vangl2 in this respect (Mitchell et al., 2009), but also by a closer examination of Vangl1 overexpression at the edge of clones, where basal bodies tend to orient their striated rootlets towards higher Vangl1 levels irrespective of their orientation relative to the body axis (supplementary material Fig. S4B). As would be expected from the observed defects in ciliary orientation upon disrupting core PCP patterning, the resulting strength and directionality of flow at the surface of the embryo were severely impaired upon Pk2 morpholino knockdown and Vangl1 overexpression (Fig. 6N-Q; supplementary material Movies 1-3).
DISCUSSION
Understanding the dynamics of PCP patterning events in vivo provides potential insights into the function and interactions of the molecular players involved, yet studies in vertebrates have been limited in this respect. Here, we describe the action of useful reporters for in vivo imaging of the dynamic localization of vertebrate core PCP proteins in Xenopus, further supplementing this established rapid and tractable vertebrate PCP model. The observed behavior of symmetrically distributed PCP complexes later resolving into asymmetric accumulations is reminiscent of early PCP patterning events in the fly wing (Seifert and Mlodzik, 2007; Strutt and Strutt, 2009; Strutt et al., 2011). In addition, there appears to be a common theme of increasing asymmetry coinciding with increasing polarized cellular behaviors. Here, core PCP protein asymmetry reflects oriented cilia beating, and in Drosophila wings the degree of aligned asymmetry of core PCP complexes increases following hinge contraction and reaches a maximum level just prior to trichome formation (Aigouy et al., 2010; Wong and Adler, 1993). Despite this and other behavioral similarities, these reporters also highlight important differences between planar polarized tissues and proteins encoded by distinct members of PCP gene families. For example, we previously reported that Dvl2 localizes to basal bodies in MCCs (Park et al., 2008), and here we find that Dvl1 localizes to asymmetric cortical crescents, a situation similar to that reported in the mouse airway (Vladar et al., 2012). Similarly, we find that although Vangl2 is cortically localized in this tissue it remains symmetrical, whereas Vangl1 becomes progressively planar polarized.
A second notable finding in this respect concerns the relationship between PCP protein asymmetry and the direction of ciliary beating. We find that Pk2-Vangl1 complexes are enriched on the ‘downstream’ side of the cell and are thus aligned in the direction of the effective stroke, which is consistent with previous functional studies suggesting that Fzd points ‘upstream’ of flow (Mitchell et al., 2009). This situation is exactly the opposite of that found in MCCs of the mammalian airway and ependyma, where Pk and/or Vangl mark the ‘upstream’ face of the cell (Guirao et al., 2010; Vladar et al., 2012). This argues against there being an intrinsic link between cortical enrichment of core PCP protein complexes and basal body orientation. This notion is consistent with the decoupling of Pk2 localization and kinocilia positioning in the mammalian inner ear (Deans et al., 2007), but it is nonetheless curious in light of the known localization of core PCP proteins to basal bodies (Park et al., 2008; Vladar et al., 2012) and the requirement of intact PCP signaling for asymmetry of the MCC microtubule cytoskeleton (Vladar et al., 2012).
Another related observation of note is the basal body localization of Pk2 that we found, which is not shown in the murine airway (Vladar et al., 2012). The potential limitations of using the synthetic GFP protein fusions necessary for live imaging and the chance of mislocalization should not be ignored, although our particular finding that GFP-Pk2 localizes to basal bodies several stages after ciliogenesis is supported by Pk3 being identified as asymmetrically associating with the more ‘mature’ mother centrioles (but not daughter centrioles) during cell divisions in U-2 OS cells, as well as with the axoneme-associated mother centriole in ciliated hTERT-RPE1 cells (Jakobsen et al., 2011). Determining if Pk2 localization near basal bodies marks or mediates a state in which ciliary orientations are ‘locked’ in place could provide additional insight into the function of this protein in planar polarization.
How the core planar polarity module interfaces with proteins such as Intu and Wdpcp in vertebrates remains an important question. These proteins have been described as ‘PCP effectors’ in the fly wing, yet have a wide range of PCP phenotypes, from strong to mild to no phenotype, in vertebrates depending on the protein in question and the developmental context (Cui et al., 2013; Gray et al., 2009; Kim et al., 2010; Park et al., 2006; Zeng et al., 2010). Further complicating matters is a recent report that both fritz overexpression and fuzzy/inturned co-overexpression can effect the patterning of core PCP patterns (Wang et al., 2014), as these genes have been considered to be downstream based on epistasis experiments (Wong and Adler, 1993) and other genetic studies (Collier et al., 2005; Lee and Adler, 2002). However, Inturned overexpression alone did not produce a significant phenotype in that study (Wang et al., 2014), consistent with previous data showing normal Fz localization in inturned mutants (Strutt, 2001), a mild convergent extension phenotype upon morpholino knockdown in Xenopus gastrulating mesoderm (Park et al., 2006), and only a modest effect of Intu-MO treatment on PCP pattering as shown here. By contrast, Wdpcp knockdown severely inhibits PCP-dependent convergent extension in Xenopus (Kim, 2010; Shindo, 2014), consistent with the robust effect on the polarized localization of Pk2 that we observed. These data argue that Wdpcp might function independently of Intu in the control of PCP. Intriguingly, loss of Wdpcp in mice is associated with loss of cortical Vangl2 (Cui et al., 2013).
Additionally, our data shed new light on how mechanisms and relationships identified in Drosophila translate to vertebrate PCP signaling, specifically in a multiciliated epithelia consisting of multilayered, heterogeneous populations of cells shaped by families of PCP proteins acting at multiple levels. In particular, we expand upon relationships established between Vang and Pk (Bastock et al., 2003) and localization dynamics of Fz dependent upon Dsh (Strutt et al., 2011) to verify that Pk has an analogous effect on Vangl localization dynamics. Beyond this, we demonstrate that the activity of the Pk2 LIM domain is likely to be important for this Vangl-enriching behavior, and we also identify the C2 region as crucial for the asymmetric cortical localization of Pk2 itself. The fact that Dvl1 remained asymmetric under conditions when Vangl1 asymmetry was lost is of particular interest because scenarios in which one core PCP protein is noticeably asymmetric while others are not are extremely rare, a finding that could potentially be attributed to the increased complexity of vertebrate PCP and lack of similar analysis in vertebrate tissues in general. However, data from Drosophila cells argue that polarization can occur through Fmi/Celsr and Fz even in the absence of Vang and obvious asymmetric enrichments (Struhl et al., 2012; Strutt and Strutt, 2007). The normal localization of Dvl1 in the face of mislocalized Vangl1 and/or Pk2 could in part be explained by communication from neighboring cells not expressing Pk2 deletions in our mosaic embryos, which would provide further support for Fz/Dsh being the more ‘dominant’ module in comparison to Vangl/Pk (Struhl et al., 2012; Strutt and Strutt, 2007). In addition to providing localization-altering behaviors in the context of previously reported dominant-negative activity (Takeuchi et al., 2003; Lin and Gubb, 2009), our results provide a cell biological context for previous biochemical data indicating that PET domain function is regulated via intramolecular physical interactions with the LIM domains (Sweede et al., 2008).
Ultimately, this work provides a foundation for future studies into the role of vertebrate Pk proteins, and thus might help to elucidate the etiology of Pk-associated human pathologies, such as epilepsy, congenital birth defects and, possibly, autism (Okumura et al., 2014; Sowers et al., 2013; Tao et al., 2011; Wen et al., 2010).
MATERIALS AND METHODS
Plasmid and morpholino design
We designed the splice-blocking Pk2 morpholino oligonucleotide based on the longest mRNA sequence obtained from the UTexas Oktoberfest gene models available from Xenbase.org. The chosen splice-blocking Pk2-MO#1 (GATTGGACAAAGGATTCTCACCTCA) is complementary to the region spanning the 3′ end of exon 4 and the 5′ end of intron 4 of sequence JGIv6.000014371_26644_262472 and was prepared by GeneTools. The second splicing morpholino Pk2-MO#2 (GAACCCAAACAAACACTTACCTGTT) is complementary to the region spanning the 3′ end of exon 3 and 5′ end of intron 3.
The primers used for cloning the pk2 coding sequence into Pk2 CS107-GFP using StuI and NotI are listed in supplementary material Table S1. The remaining gene sequences were cloned into Gateway entry vectors using the Invitrogen pENTR/D-TOPO cloning system for recombination of coding sequences into pCSDest GFP or RFP vectors; the pk2, dvl1, dvl3, fzd6 and vangl1 sequence sources and primers are listed in supplementary material Table S1.
Pk2 deletions were amplified by fusing two PCR products generated with the above as template and primers ΔPET-1/2, ΔPETΔLIM-1/2 and ΔC2-1/2 as listed in supplementary material Table S1. These fusions resulted in the removal of amino acids 77-176 for Pk2-ΔPET, 77-382 for Pk2-ΔPETΔLIM and 734-827 for Pk2-ΔC2.
Dvl1-ΔPDZpartial was amplified by fusing two PCR products generated with the above as template and the primers listed in supplementary material Table S1. This fusion resulted in the removal of amino acids 300-380.
Xenopus manipulations
Embryos were obtained and externally fertilized according to standard protocols (Sive et al., 2000). Developmental stages were determined according to Nieuwkoop and Faber (1967). For Pk2 morpholino treatments, 20-25 ng was injected into one cell at the 16-cell stage for mosaic analysis or 30-35 ng at the 4-cell stage, depending on the experiment, and Pk2 was overexpressed by injecting either 600-700 pg or up to 1 ng mRNA similarly. About 22 ng was injected for both Wdpcp and Intu morpholinos. Core PCP fluorescent protein fusion mRNA was injected into one of 16 or 32 cells along with ∼50 pg memRFP mRNA, with ∼20-30 pg for Dvl1-GFP, Dvl2-GFP and Dvl3-GFP, ∼60-80 pg for Fzd6-GFP, Fzd7-GFP and Fzd8-GFP, ∼150 pg for GFP/RFP-Pk2, and ∼50-60 pg for GFP-Vangl1 and GFP-Vangl2. Pk2 deletion constructs were injected at 125-150 pg for localization of GFP-fused constructs and 700-800 pg for overexpression of non-GFP-fused constructs at the 16-cell stage. This amount was increased to 1 ng at the 4-cell stage for the determination of effects on ciliary orientation. For Vangl1 and Dvl-ΔPDZpartial constructs injected at the 4-cell stage, 880 pg was introduced. Centrin-RFP, CLAMP-GFP and GFP-Pk2 were co-injected into one of four cells at 60 pg, 60 pg and 150 pg, respectively.
Live imaging and confocal image quantification
All imaging was performed using live embryos gently sandwiched between coverglasses using silicon grease as an adhesive spacer and carried out with a Zeiss LSM700 confocal microscope. Images were processed with the Fiji distribution of ImageJ (NIH), Imaris (Bitplane) and Photoshop (Adobe) software suites, and figures were assembled in Illustrator (Adobe).
For PCP enrichment measures, freehand lines (3-5 pixels wide, depending on image zoom) were drawn over dorsoanterior and ventroposterior memRFP-labeled cell membranes with unlabeled neighbors on images in the Fiji distribution of ImageJ. Intensity measurements were then taken from these areas as well as from the GFP-PCP protein channel at the same area drawn. PCP enrichment was calculated by taking the natural log of the final value obtained after dividing the dorsoanterior measurements by the ventroposterior measurements, both of which were normalized values of PCP-GFP average intensity divided by the memRFP intensity at the same region. Statistical analyses were carried out using GraphPad Prism software with Mann-Whitney tests for significance.
For FRAP analysis, time-lapse movies were acquired after simultaneously photobleaching anterior and posterior membrane domains of core PCP-GFP fusions under control conditions and after the above described Pk2 overexpression and morpholino knockdown manipulations. Intensity measurements were performed in Fiji, similarly to those for PCP enrichment, with recordings for each time point taken individually from each frame captured at bleached regions and normalized as detailed by Goldman et al. (2005). Recovery trendlines were calculated and plotted in GraphPad Prism software with one phase exponential association curve fitting.
Ciliary orientations were determined manually in Fiji, and mean polarity vectors and mean vector lengths were calculated with the CircStat Matlab circular statistics toolbox (Berens, 2009) before values were plotted with Oriana software (Kovach Computing Services).
For the assessment of flow across the epidermis, embryos were placed on modeling clay submerged in 1/3× Modified Marc's Ringer Solution (Sive et al., 2000), and latex beads were loaded into the medium. Time-lapse imaging was performed using a Zeiss Axio Zoom.V16 stereomicroscope and associated Zen software. Movies were processed in Photoshop (Adobe), and bead traces were performed manually with Fiji.
Acknowledgements
We thank A. Shindo, J. Tabler and our reviewers for critical reading of the manuscript, Peter Klein for Fzd8-GFP and Herbert Steinbeisser for Fzd7-GFP plasmids.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
M.T.B. and J.B.W. designed experiments, interpreted results, and wrote the paper. M.T.B. performed all experiments.
Funding
This work was supported by grants from the National Institute of General Medical Sciences (NIGMS) and National Heart, Lung, and Blood Institute (NHLBI) to J.B.W. J.B.W. is an Early Career Scientist of the Howard Hughes Medical Institute (HHMI). Deposited in PMC for release after 6 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.121384/-/DC1
References
- Adler P. N. (1992). The genetic control of tissue polarity in Drosophila. Bioessays 14, 735-741. 10.1002/bies.950141103 [DOI] [PubMed] [Google Scholar]
- Adler P. N., Krasnow R. E. and Liu J. (1997). Tissue polarity points from cells that have higher Frizzled levels towards cells that have lower Frizzled levels. Curr. Biol. 7, 940-949. 10.1016/S0960-9822(06)00413-1 [DOI] [PubMed] [Google Scholar]
- Adler P. N., Taylor J. and Charlton J. (2000). The domineering non-autonomy of frizzled and van Gogh clones in the Drosophila wing is a consequence of a disruption in local signaling. Mech. Dev. 96, 197-207. 10.1016/S0925-4773(00)00392-0 [DOI] [PubMed] [Google Scholar]
- Aigouy B., Farhadifar R., Staple D. B., Sagner A., Röper J.-C., Jülicher F. and Eaton S. (2010). Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell 142, 773-786. 10.1016/j.cell.2010.07.042 [DOI] [PubMed] [Google Scholar]
- Amonlirdviman K., Khare N. A., Tree D. R. P., Chen W.-S., Axelrod J. D. and Tomlin C. J. (2005). Mathematical modeling of planar cell polarity to understand domineering nonautonomy. Science 307, 423-426. 10.1126/science.1105471 [DOI] [PubMed] [Google Scholar]
- Axelrod J. D. (2001). Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes Dev. 15, 1182-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastock R., Strutt H. and Strutt D. (2003). Strabismus is asymmetrically localised and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development 130, 3007-3014. 10.1242/dev.00526 [DOI] [PubMed] [Google Scholar]
- Berens P. (2009). CircStat: A MATLAB toolbox for circular statistics. J. Stat. Soft. 31, 1-21. [Google Scholar]
- Billett F. S. and Gould R. P. (1971). Fine structural changes in the differentiating epidermis of Xenopus laevis embryos. J. Anat. 108, 465-480. [PMC free article] [PubMed] [Google Scholar]
- Boutin C., Labedan P., Dimidschstein J., Richard F., Cremer H., André P., Yang Y., Montcouquiol M., Goffinet A. M. and Tissir F. (2014). A dual role for planar cell polarity genes in ciliated cells. Proc. Natl. Acad. Sci. USA 111, E3129-E3138. 10.1073/pnas.1404988111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks E. R. and Wallingford J. B. (2014). Multiciliated cells. Curr. Biol. 24, R973-R982. 10.1016/j.cub.2014.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.-S., Antic D., Matis M., Logan C. Y., Povelones M., Anderson G. A., Nusse R. and Axelrod J. D. (2008). Asymmetric homotypic interactions of the atypical cadherin flamingo mediate intercellular polarity signaling. Cell 133, 1093-1105. 10.1016/j.cell.2008.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho B., Pierre-Louis G., Sagner A., Eaton S. and Axelrod J. D. (2015). Clustering and negative feedback by endocytosis in planar cell polarity signaling is modulated by ubiquitinylation of prickle. PLoS Genet. 11, e1005259 10.1371/journal.pgen.1005259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung M.-I., Kwon T., Tu F., Brooks E. R., Gupta R., Meyer M., Baker J. C., Marcotte E. M. and Wallingford J. B. (2014). Coordinated genomic control of ciliogenesis and cell movement by RFX2. eLife 3, e01439 10.7554/eLife.01439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier S., Lee H., Burgess R. and Adler P. (2005). The WD40 repeat protein fritz links cytoskeletal planar polarity to frizzled subcellular localization in the Drosophila epidermis. Genetics 169, 2035-2045. 10.1534/genetics.104.033381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C., Chatterjee B., Lozito T. P., Zhang Z., Francis R. J., Yagi H., Swanhart L. M., Sanker S., Francis D., Yu Q. et al. (2013). Wdpcp, a PCP protein required for ciliogenesis, regulates directional cell migration and cell polarity by direct modulation of the actin cytoskeleton. PLoS Biol. 11, e1001720 10.1371/journal.pbio.1001720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das G., Jenny A., Klein T. J., Eaton S. and Mlodzik M. (2004). Diego interacts with Prickle and Strabismus/Van Gogh to localize planar cell polarity complexes. Development 131, 4467-4476. 10.1242/dev.01317 [DOI] [PubMed] [Google Scholar]
- Daulat A. M., Luu O., Sing A., Zhang L., Wrana J. L., McNeill H., Winklbauer R. and Angers S. (2011). Mink1 regulates beta-catenin-independent Wnt signaling via Prickle phosphorylation. Mol. Cell. Biol. 32, 173-185. 10.1128/MCB.06320-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans M. R., Antic D., Suyama K., Scott M. P., Axelrod J. D. and Goodrich L. V. (2007). Asymmetric distribution of prickle-like 2 reveals an early underlying polarization of vestibular sensory epithelia in the inner ear. J. Neurosci. 27, 3139-3147. 10.1523/JNEUROSCI.5151-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale T. A. and Elinson R. P. (1992). Cell migration and induction in the development of the surface ectodermal pattern of the Xenopus laevis tadpole. Dev. Growth Differ. 34, 51-59. 10.1111/j.1440-169X.1992.00051.x [DOI] [PubMed] [Google Scholar]
- Dubaissi E. and Papalopulu N. (2011). Embryonic frog epidermis: a model for the study of cell-cell interactions in the development of mucociliary disease. Dis. Model. Mech. 4, 179-192. 10.1242/dmm.006494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubaissi E., Rousseau K., Lea R., Soto X., Nardeosingh S., Schweickert A., Amaya E., Thornton D. J. and Papalopulu N. (2014). A secretory cell type develops alongside multiciliated cells, ionocytes and goblet cells, and provides a protective, anti-infective function in the frog embryonic mucociliary epidermis. Development 141, 1514-1525. 10.1242/dev.102426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. D., Spector D. L. and Swedlund A. C. (2005). Live Cell Imaging: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Goodrich L. V. and Strutt D. (2011). Principles of planar polarity in animal development. Development 138, 1877-1892. 10.1242/dev.054080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R. S., Abitua P. B., Wlodarczyk B. J., Szabo-Rogers H. L., Blanchard O., Lee I., Weiss G. S., Liu K. J., Marcotte E. M., Wallingford J. B. et al. (2009). The planar cell polarity effector Fuz is essential for targeted membrane trafficking, ciliogenesis and mouse embryonic development. Nat. Cell Biol. 11, 1225-1232. 10.1038/ncb1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R. S., Roszko I. and Solnica-Krezel L. (2011). Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity. Dev. Cell 21, 120-133. 10.1016/j.devcel.2011.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubb D. (1993). Genes controlling cellular polarity in Drosophila. Dev. Suppl. 269-277. [PubMed] [Google Scholar]
- Gubb D. and Garcia-Bellido A. (1982). A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J. Embryol. Exp. Morphol. 68, 37-57. [PubMed] [Google Scholar]
- Guirao B., Meunier A., Mortaud S., Aguilar A., Corsi J.-M., Strehl L., Hirota Y., Desoeuvre A., Boutin C., Han Y.-G. et al. (2010). Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat. Cell Biol. 12, 341-350. 10.1038/ncb2040 [DOI] [PubMed] [Google Scholar]
- Guo Y., Zanetti G. and Schekman R. (2013). A novel GTP-binding protein-adaptor protein complex responsible for export of Vangl2 from the trans Golgi network. eLife 2, e00160 10.7554/eLife.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen L., Vanselow K., Skogs M., Toyoda Y., Lundberg E., Poser I., Falkenby L. G., Bennetzen M., Westendorf J., Nigg E. A. et al. (2011). Novel asymmetrically localizing components of human centrosomes identified by complementary proteomics methods. EMBO J. 30, 1520-1535. 10.1038/emboj.2011.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny A., Darken R. S., Wilson P. A. and Mlodzik M. (2003). Prickle and Strabismus form a functional complex to generate a correct axis during planar cell polarity signaling. EMBO J. 22, 4409-4420. 10.1093/emboj/cdg424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny A., Reynolds-Kenneally J., Das G., Burnett M. and Mlodzik M. (2005). Diego and Prickle regulate Frizzled planar cell polarity signalling by competing for Dishevelled binding. Nat. Cell Biol. 7, 691-697. 10.1038/ncb1271 [DOI] [PubMed] [Google Scholar]
- Kim S. K., Shindo A., Park T. J., Oh E. C., Ghosh S., Gray R. S., Lewis R. A., Johnson C. A., Attie-Bittach T., Katsanis N. et al. (2010). Planar cell polarity acts through septins to control collective cell movement and ciliogenesis. Science 329, 1337-1340. 10.1126/science.1191184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- König G. and Hausen P. (1993). Planar polarity in the ciliated epidermis of Xenopus Embryos. Dev. Biol. 160, 355-368. 10.1006/dbio.1993.1312 [DOI] [PubMed] [Google Scholar]
- Lee H. and Adler P. N. (2002). The function of the frizzled pathway in the Drosophila wing is dependent on inturned and fuzzy. Genetics 160, 1535-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.-Y. and Gubb D. (2009). Molecular dissection of Drosophila Prickle isoforms distinguishes their essential and overlapping roles in planar cell polarity. Dev. Biol. 325, 386-399. 10.1016/j.ydbio.2008.10.042 [DOI] [PubMed] [Google Scholar]
- Liu C., Lin C., Gao C., May-Simera H., Swaroop A. and Li T. (2014). Null and hypomorph Prickle1 alleles in mice phenocopy human Robinow syndrome and disrupt signaling downstream of Wnt5a. Biol. Open 3, 861-870. 10.1242/bio.20148375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merte J., Jensen D., Wright K., Sarsfield S., Wang Y., Schekman R. and Ginty D. D. (2009). Sec24b selectively sorts Vangl2 to regulate planar cell polarity during neural tube closure. Nat. Cell Biol. 12, 41-46. 10.1038/ncb2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell B., Jacobs R., Li J., Chien S. and Kintner C. (2007). A positive feedback mechanism governs the polarity and motion of motile cilia. Nature 447, 97-101. 10.1038/nature05771 [DOI] [PubMed] [Google Scholar]
- Mitchell B., Stubbs J. L., Huisman F., Taborek P., Yu C. and Kintner C. (2009). The PCP pathway instructs the planar orientation of ciliated cells in the xenopus larval skin. Curr. Biol. 19, 924-929. 10.1016/j.cub.2009.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narimatsu M., Bose R., Pye M., Zhang L., Miller B., Ching P., Sakuma R., Luga V., Roncari L., Attisano L. et al. (2009). Regulation of planar cell polarity by smurf ubiquitin ligases. Cell 137, 295-307. 10.1016/j.cell.2009.02.025 [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P. D. and Faber J. (1967). Normal Tables of Xenopus Laevis:(Daudin) a Systematical and Chronological Survey of the Development from the Fertilized Egg Till the End of the Metamorphosis. Netherlands: Hubrecht-Laboratorium (Embryologisch Instituut). [Google Scholar]
- Okumura A., Yamamoto T., Miyajima M., Shimojima K., Kondo S., Abe S., Ikeno M. and Shimizu T. (2014). 3p interstitial deletion including PRICKLE2 in identical twins with autistic features. Pediatr. Neurol. 51, 730-733. 10.1016/j.pediatrneurol.2014.07.025 [DOI] [PubMed] [Google Scholar]
- Park T. J., Haigo S. L. and Wallingford J. B. (2006). Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat. Genet. 38, 303-311. 10.1038/ng1753 [DOI] [PubMed] [Google Scholar]
- Park T. J., Mitchell B. J., Abitua P. B., Kintner C. and Wallingford J. B. (2008). Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat. Genet. 40, 871-879. 10.1038/ng.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley I. K., Stubbs J. L. and Kintner C. (2011). Specification of ion transport cells in the Xenopus larval skin. Development 138, 705-714. 10.1242/dev.055699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert J. R. K. and Mlodzik M. (2007). Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat. Rev. Genet. 8, 126-138. 10.1038/nrg2042 [DOI] [PubMed] [Google Scholar]
- Shindo A. and Wallingford J. B. (2014). PCP and septins compartmentalize cortical actomyosin to direct collective cell movement. Science 343, 649-652. 10.1126/science.1243126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sive H. L., Grainger R. M. and Harland R. M. (2000). Early development of Xenopus laevis: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Sokol S. Y. (1996). Analysis of Dishevelled signalling pathways during Xenopus development. Curr. Biol. 6, 1456-1467. 10.1016/S0960-9822(96)00750-6 [DOI] [PubMed] [Google Scholar]
- Song H., Hu J., Chen W., Elliott G., Andre P., Gao B. and Yang Y. (2010). Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature 466, 378-382. 10.1038/nature09129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers L. P., Loo L., Wu Y., Campbell E., Ulrich J. D., Wu S., Paemka L., Wassink T., Meyer K., Bing X. et al. (2013). Disruption of the non-canonical Wnt gene PRICKLE2 leads to autism-like behaviors with evidence for hippocampal synaptic dysfunction. Mol. Psychiatry 18, 1077-1089. 10.1038/mp.2013.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G., Casal J. and Lawrence P. A. (2012). Dissecting the molecular bridges that mediate the function of Frizzled in planar cell polarity. Development 139, 3665-3674. 10.1242/dev.083550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt D. I. (2001). Asymmetric localization of frizzled and the establishment of cell polarity in the Drosophila wing. Mol. Cell 7, 367-375. 10.1016/S1097-2765(01)00184-8 [DOI] [PubMed] [Google Scholar]
- Strutt D. (2008). The planar polarity pathway. Curr. Biol. 18, R898-R902. 10.1016/j.cub.2008.07.055 [DOI] [PubMed] [Google Scholar]
- Strutt H. and Strutt D. (2002). Nonautonomous planar polarity patterning in Drosophila: dishevelled-independent functions of frizzled. Dev. Cell 3, 851-863. 10.1016/S1534-5807(02)00363-5 [DOI] [PubMed] [Google Scholar]
- Strutt D. and Strutt H. (2007). Differential activities of the core planar polarity proteins during Drosophila wing patterning. Dev. Biol. 302, 181-194. 10.1016/j.ydbio.2006.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt H. and Strutt D. (2008). Differential stability of flamingo protein complexes underlies the establishment of planar polarity. Curr. Biol. 18, 1555-1564. 10.1016/j.cub.2008.08.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt H. and Strutt D. (2009). Asymmetric localisation of planar polarity proteins: Mechanisms and consequences. Semin. Cell Dev. Biol. 20, 957-963. 10.1016/j.semcdb.2009.03.006 [DOI] [PubMed] [Google Scholar]
- Strutt H., Warrington S. J. and Strutt D. (2011). Dynamics of core planar polarity protein turnover and stable assembly into discrete membrane subdomains. Dev. Cell 20, 511-525. 10.1016/j.devcel.2011.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt H., Searle E., Thomas-MacArthur V., Brookfield R. and Strutt D. (2013a). A Cul-3-BTB ubiquitylation pathway regulates junctional levels and asymmetry of core planar polarity proteins. Development 140, 1693-1702. 10.1242/dev.089656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt H., Thomas-MacArthur V. and Strutt D. (2013b). Strabismus promotes recruitment and degradation of farnesylated prickle in Drosophila melanogaster planar polarity specification. PLoS Genet. 9, e1003654 10.1371/journal.pgen.1003654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweede M., Ankem G., Chutvirasakul B., Azurmendi H. F., Chbeir S., Watkins J., Helm R. F., Finkielstein C. V. and Capelluto D. G. S. (2008). Structural and membrane binding properties of the prickle PET domain. Biochemistry 47, 13524-13536. 10.1021/bi801037h [DOI] [PubMed] [Google Scholar]
- Takeuchi M., Nakabayashi J., Sakaguchi T., Yamamoto T. S., Takahashi H., Takeda H. and Ueno N. (2003). The prickle-related gene in vertebrates is essential for gastrulation cell movements. Curr. Biol. 13, 674-679. 10.1016/S0960-9822(03)00245-8 [DOI] [PubMed] [Google Scholar]
- Tao H., Manak J. R., Sowers L., Mei X., Kiyonari H., Abe T., Dahdaleh N. S., Yang T., Wu S., Chen S. et al. (2011). Mutations in prickle orthologs cause seizures in flies, mice, and humans. Am. J. Hum. Genet. 88, 138-149. 10.1016/j.ajhg.2010.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torban E., Patenaude A.-M., Leclerc S., Rakowiecki S., Gauthier S., Andelfinger G., Epstein D. J. and Gros P. (2008). Genetic interaction between members of the Vangl family causes neural tube defects in mice. Proc. Natl. Acad. Sci. USA 105, 3449-3454. 10.1073/pnas.0712126105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tree D. R. P., Shulman J. M., Rousset R., Scott M. P., Gubb D. and Axelrod J. D. (2002). Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell 109, 371-381. 10.1016/S0092-8674(02)00715-8 [DOI] [PubMed] [Google Scholar]
- Twitty V. C. (1928). Experimental studies on the ciliary action of amphibian embryos. J. Exp. Zool. 50, 319-344. 10.1002/jez.1400500302 [DOI] [Google Scholar]
- Vinson C. R. and Adler P. N. (2002). Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature 329, 549-551. 10.1038/329549a0 [DOI] [PubMed] [Google Scholar]
- Vladar E. K., Bayly R. D., Sangoram A. M., Scott M. P. and Axelrod J. D. (2012). Microtubules enable the planar cell polarity of airway cilia. Curr. Biol. 22, 2203-2212. 10.1016/j.cub.2012.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walentek P., Bogusch S., Thumberger T., Vick P., Dubaissi E., Beyer T., Blum M. and Schweickert A. (2014). A novel serotonin-secreting cell type regulates ciliary motility in the mucociliary epidermis of Xenopus tadpoles. Development 141, 1526-1533. 10.1242/dev.102343 [DOI] [PubMed] [Google Scholar]
- Wallingford J. B. (2010). Planar cell polarity signaling, cilia and polarized ciliary beating. Curr. Opin. Cell Biol. 22, 597-604. 10.1016/j.ceb.2010.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford J. B. (2012). Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Annu. Rev. Cell Dev. Biol. 28, 627-653. 10.1146/annurev-cellbio-092910-154208 [DOI] [PubMed] [Google Scholar]
- Wallingford J. B., Rowning B. A., Vogeli K. M., Rothbacher U., Fraser S. E. and Harland R. M. (2000). Dishevelled controls cell polarity during Xenopus gastrulation. Nature 405, 81-85. 10.1038/35011077 [DOI] [PubMed] [Google Scholar]
- Wang Y., Yan J., Lee H., Lu Q. and Adler P. N. (2014). The proteins encoded by the Drosophila planar polarity effector genes inturned, fuzzy and fritz interact physically and can re-pattern the accumulation of “upstream” planar cell polarity proteins. Dev. Biol. 394, 156-169. 10.1016/j.ydbio.2014.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen S., Zhu H., Lu W., Mitchell L. E., Shaw G. M., Lammer E. J. and Finnell R. H. (2010). Planar cell polarity pathway genes and risk for spina bifida. Am. J. Med. Genet. 152A, 299-304. 10.1002/ajmg.a.33230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner M. E., Hwang P., Huisman F., Taborek P., Yu C. C. and Mitchell B. J. (2007). Actin and microtubules drive differential aspects of planar cell polarity in multiciliated cells. Annu. Rev. Physiol. 195, 423-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong L. L. and Adler P. N. (1993). Tissue polarity genes of Drosophila regulate the subcellular location for prehair initiation in pupal wing cells. J. Cell Biol. 123, 209-221. 10.1083/jcb.123.1.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. and Mlodzik M. (2008). The frizzled extracellular domain is a ligand for Van Gogh/Stbm during nonautonomous planar cell polarity signaling. Dev. Cell 15, 462-469. 10.1016/j.devcel.2008.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H., Copley C. O., Goodrich L. V. and Deans M. R. (2012). Comparison of phenotypes between different vangl2 mutants demonstrates dominant effects of the looptail mutation during hair cell development. PLoS ONE 7, e31988 10.1371/journal.pone.0031988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H., Hoover A. N. and Liu A. (2010). PCP effector gene Inturned is an important regulator of cilia formation and embryonic development in mammals. Dev. Biol. 339, 418-428. 10.1016/j.ydbio.2010.01.003 [DOI] [PubMed] [Google Scholar]