Abstract
V1 interneurons are inhibitory neurons that play an essential role in vertebrate locomotion. The molecular mechanisms underlying their genesis remain, however, largely undefined. Here, we show that the transcription factor Prdm12 is selectively expressed in p1 progenitors of the hindbrain and spinal cord in the frog embryo, and that a similar restricted expression profile is observed in the nerve cord of other vertebrates as well as of the cephalochordate amphioxus. Using frog, chick and mice, we analyzed the regulation of Prdm12 and found that its expression in the caudal neural tube is dependent on retinoic acid and Pax6, and that it is restricted to p1 progenitors, due to the repressive action of Dbx1 and Nkx6-1/2 expressed in the adjacent p0 and p2 domains. Functional studies in the frog, including genome-wide identification of its targets by RNA-seq and ChIP-Seq, reveal that vertebrate Prdm12 proteins act as a general determinant of V1 cell fate, at least in part, by directly repressing Dbx1 and Nkx6 genes. This probably occurs by recruiting the methyltransferase G9a, an activity that is not displayed by the amphioxus Prdm12 protein. Together, these findings indicate that Prdm12 promotes V1 interneurons through cross-repressive interactions with Dbx1 and Nkx6 genes, and suggest that this function might have only been acquired after the split of the vertebrate and cephalochordate lineages.
KEY WORDS: Prdm, Interneuron, Neurogenesis, Spinal cord, Transcription factor
Summary: In vertebrates, V1 interneuron specification requires Prdm12, whose expression depends on Pax6 and retinoic acid and is restricted to the p1 domain by Dbx1 and Nkx6.1/2, themselves repressed by Prdm12.
INTRODUCTION
In the ventral neural tube, distinct classes of neurons (V0, V1, V2 and V3 interneurons, INs) and motor neurons are generated at specific dorsoventral levels in response to a gradient of sonic hedgehog (Shh) activity. Shh functions by regulating the expression of genes encoding homeodomain (HD) (i.e. Pax6, Irx3 and Dbx1/2 are repressed and Nkx6 and Nkx2 are induced by Shh) and basic helix-loop-helix (bHLH) transcription factors (TFs). These TFs produce a combinatorial transcriptional code that delineates spatially the distinct ventral progenitor domains (Briscoe and Novitch, 2008; Dessaud et al., 2008). Cross-repressive interactions between complementary pairs of these TFs further refine and sharpen the boundaries of the progenitor domains. Perturbation of this code of HD and bHLH TFs leads to aberrant dorsoventral patterning and cell type mis-specification (Briscoe and Novitch, 2008; Dessaud et al., 2008).
In the adult mammalian spinal cord, probably as the result of evolutionary pressures toward functional specialization during transition from swimming to terrestrial locomotion, there is a large diversity of spinal INs modulating motor output that arise from this limited number of progenitor domains (Goulding, 2009; Grillner and Jessell, 2009). As an example, one can cite the V1 class of inhibitory pre-motor INs that, at the onset of post-mitotic differentiation, is characterized in all vertebrates studied so far by the expression of Engrailed-1 (En1) (Saueressig et al., 1999; Wenner et al., 2000; Li et al., 2004; Higashijima et al., 2004; Alvarez et al., 2005). In aquatic vertebrates, this class of INs comprises only one type of multifunctional INs, known as circumferential ipsilateral ascending neurons (CiA) in fish and ascending interneurons (aINs) in frogs. In mammals, the V1 class of INs that plays a crucial role in regulating the rhythm of locomotor outputs and flexor-extensor inhibition (Gosgnach et al., 2006; Zhang et al., 2014) comprises a large variety of derivatives, including Renshaw cells (RCs) and putative reciprocal Ia inhibitory INs (Ia INs) (Alvarez et al., 2005; Siembab et al., 2010; Benito-Gonzalez and Alvarez, 2012; Francius et al., 2013). In the chick, the population of En1-expressing neurons in the embryonic spinal cord has equally been shown to be heterogeneous (Wenner et al., 2000). However, how these V1 INs and their subtypes are generated remains largely unknown.
Prdm proteins are a family of epigenetic zinc-finger transcriptional regulators with important roles in cell differentiation and diseases (Fog et al., 2012; Hohenauer and Moore, 2012). In mouse and zebrafish embryos, Prdm12 is expressed in specific regions of the developing brain, in spinal cord p1 progenitors and in dorsal root and trigeminal ganglia (Kinameri et al., 2008). In P19 embryonic carcinoma cells, Prdm12 is induced by retinoic acid (RA) and inhibits cell proliferation (Yang and Shinkai, 2013). Recently, it has been shown that Prdm12 is required for V1 interneurons in zebrafish (Zannino et al., 2014). However, the mechanisms that are responsible for its selective expression in p1 progenitors and its mode of action remain elusive. Here, we approached these mechanisms and dissected its mode of action in Xenopus V1 cell fate specification.
RESULTS
Cloning and expression of Xenopus, chicken and amphioxus Prdm12 during development
Xenopus Prdm12 encodes a nuclear protein with an N-terminal PR domain followed by three zinc-finger motifs (Hanotel et al., 2014). As previously reported for the mouse protein (Yang and Shinkai, 2013), Xenopus Prdm12 exhibits histone methyltransferase activity. Prdm12 is highly conserved during evolution (Nagy et al., 2015), and we identified orthologous sequences in the chicken and the cephalochordate amphioxus (Branchiostoma lanceolatum), a basal chordate (supplementary material Fig. S1).
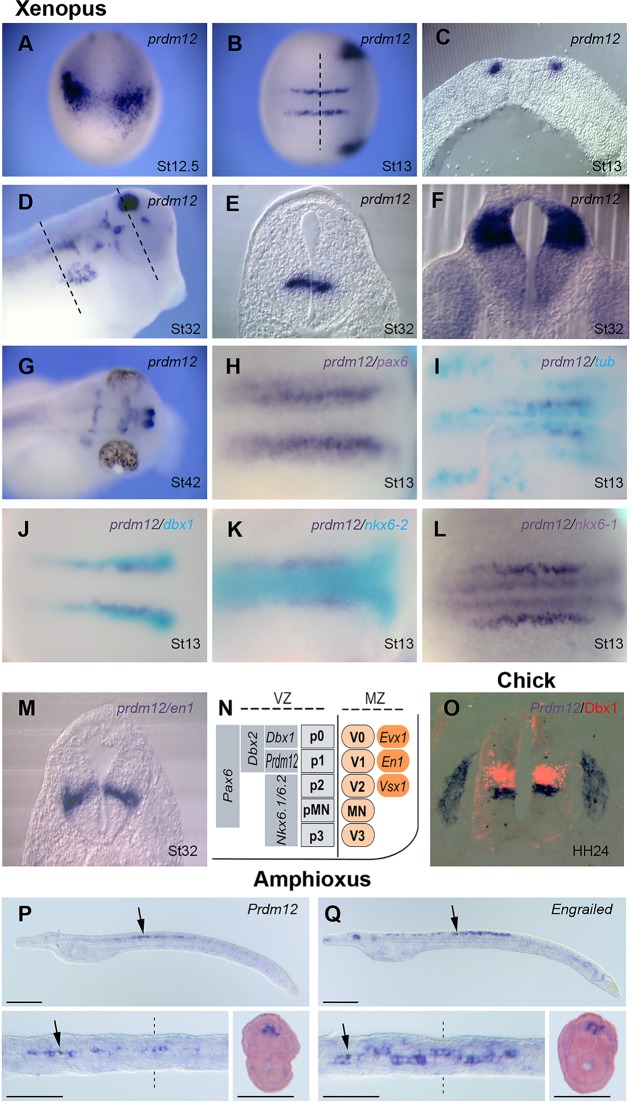
Prdm12b is selectively expressed in p1 progenitors of the zebrafish and mouse neural tube (Kinameri et al., 2008; Zannino et al., 2014; and supplementary material Fig. S2). To determine prdm12 expression during Xenopus neural development, we used ISH. prdm12 is first detected at stage 12.5-13 in two bilateral longitudinal stripes of cells running along the posterior neural plate as well as in cranial placodes (Fig. 1A-C). At this stage, these stripes have an anterior limit in rhombomere 7. After neural tube closure, prdm12 is also detected in specific brain regions, and its expression in neural tube ventral progenitors has a rostral limit corresponding to the anterior hindbrain (Fig. 1D,F; supplementary material Fig. S3). Within the neural plate, prdm12-expressing cells are included in the Pax6 domain. They are located between the medial and intermediate stripes of the primary neuronal marker N-tubulin (tubb2b – Xenbase), at the medial boundary of dbx1 that in the mouse spinal cord marks p0 progenitors (Fig. 1H-J) and at the lateral boundary of nkx6-1 and nkx6-2 (Fig. 1K,L). At this stage, the medial limit of prdm12 appears to correspond to the lateral limit of nkx6-2, while nkx6-1 is separated from prdm12 by a gap. At later tailbud and tadpole stages, the dorsal limits of nkx6-1 and nkx6-2 appear to be similar and correspond to the ventral border of prdm12. Thus, in contrast to the mouse (Vallstedt et al., 2001), nkx6-1 and nkx6-2 are broadly co-expressed in Xenopus, from neurula to tadpole stages, and appear excluded from p1 progenitors. dbx2 is very weak at neurula stage. In tadpoles, it is more broadly expressed than dbx1, suggesting that, like in the mouse (Pierani et al., 2001), dbx2 extends ventrally into the p1 progenitor territory (supplementary material Figs S4 and S5). In tadpoles, prdm12 staining in the ventricular zone of the neural tube appears in the marginal zone at the level of en1, a selective marker of Xenopus aINs and mouse V1 INs (Matise and Joyner, 1997; Li et al., 2004; and Fig. 1M). Thus, as in zebrafish and mouse, prdm12 is selectively expressed in the spinal cord in p1 progenitors (Fig. 1N). A similar restricted expression was also observed in the neural tube of chick embryos (Fig. 1O) and in the ventral nerve cord of the larvae of the basal chordate amphioxus (Fig. 1P,Q).
Fig. 1.
Prdm12 expression in Xenopus, chicken and amphioxus embryos. (A-G) Whole-mount ISH analysis of prdm12 expression in Xenopus. In C,E,F, transverse sections at the levels indicated in B,D are shown. (H-M) Double ISH comparing prdm12 with the indicated genes. In panels H,L and M, both Prdm12 and the second probe are revealed in dark blue. In panels I-K, prdm12 is in dark blue and the second probe is in light blue. (N) Diagram summarizing the expression domains of Prdm12 and of HD TFs in the ventricular zone of the Xenopus ventral caudal neural tube. (O) A transverse section of the neural tube of a chick embryo hybridized for Prdm12 and immunostained for Dbx1 (red). (P,Q) Prdm12 and Engrailed are co-expressed in restricted cells of the ventral nerve cord (arrows) of amphioxus larvae (48 hpf). Lateral and dorsal views as well as a transverse section are shown.
Xenopus prdm12 expression in the spinal cord is dependent on RA signaling and Pax6
RA plays a role in spinal cord neurogenesis and is known to posteriorize induced neural tissue (Briscoe and Novitch, 2008; Durston et al., 1989). To examine whether RA regulates prdm12 during Xenopus neural development, we analyzed its expression in animal cap ectodermal explants. These explants are specified to become epidermal tissue, but can be converted to derivatives of all three germ layers. Embryos were injected with noggin mRNA to neuralize the cells, and dissected animal caps were cultured to the equivalent of stage 28 in the presence or absence of RA. Total RNA was isolated and prdm12 expression was analyzed by RT-qPCR. We also monitored en1 and, as controls, the epidermal marker epidermal keratin (epK), the anterior neural marker otx2, the hindbrain markers egr2 (krox20) and mafb, and the posterior marker hoxb9 (Hb9). As expected, in neuralized caps, epidermal keratin is inhibited and otx2 is strongly expressed. egr2, mafB and hoxb9 are only weakly upregulated, demonstrating that the explants have adopted an anterior neural fate. In neuralized caps treated with RA, otx2, egr2 and mafB were downregulated and hoxB9 was induced, consistent with their differentiation into spinal cord. prdm12 and en1 were not efficiently induced in neuralized caps, but were strongly upregulated in neuralized explants treated with RA (Fig. 2A). The requirement of RA for prdm12 expression during spinal cord development was next evaluated in both frog and chick embryos. In Xenopus, RA signaling was blocked by overexpressing the RA-catabolizing enzyme Cyp26. In the chick, we interfered with RA signaling using a dominant negative form of the human RA receptor α (dnRAR403). As shown in supplementary material Fig. S6, Prdm12 is reduced in both Xenopus and chick embryos in response to the inhibition of RA. Thus, RA appears important for Prdm12 expression in the ventral spinal cord. However, we cannot exclude that up- and downregulation of Prdm12 observed in our manipulations of RA is indirect, related to the change of A/P identity of the cells.
Fig. 2.
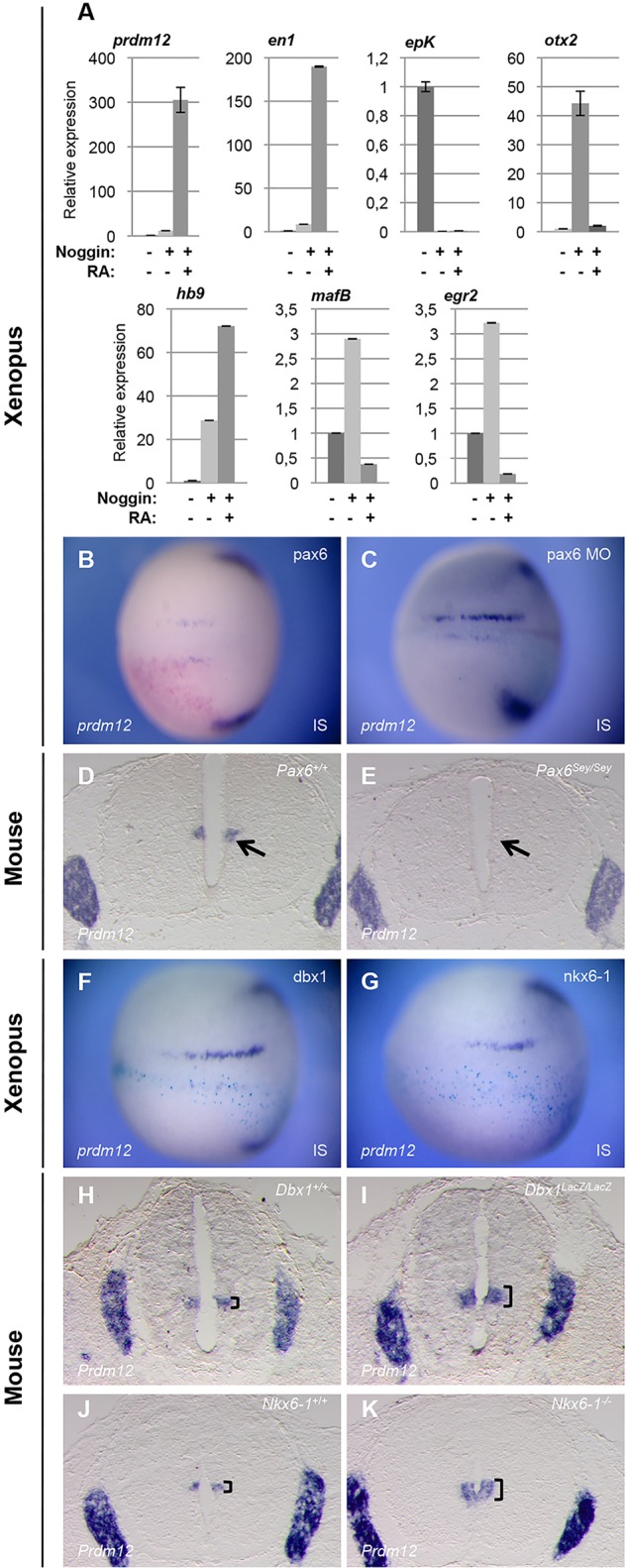
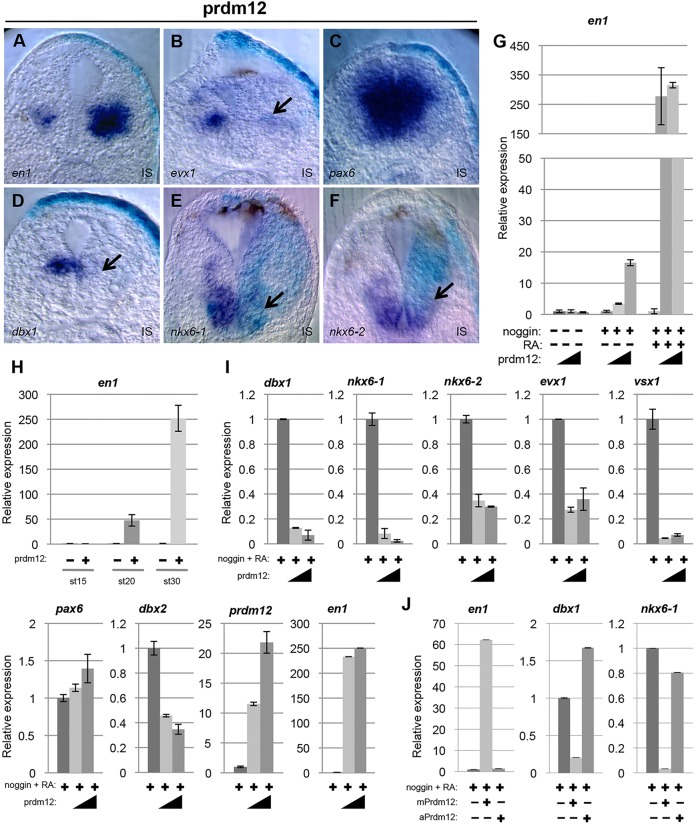
prdm12 is dependent on RA signaling and Pax6, and is repressed by Dbx1 and Nkx6. (A) RT-PCR analysis of the expression of the indicated genes in animal caps isolated from wild-type or noggin-injected (100 pg/blastomere) embryos, cultivated in the presence or absence of RA. Expression levels were compared with the expression level of uninjected caps, which was defined as 1. (B,C,F,G) prdm12 expression in Xenopus neurula-stage embryos injected unilaterally at the 2-cell stage with the indicated mRNA (500 pg/blastomere). (D,E,H,K). prdm12 is downregulated in the spinal cord of E12.5 pax6Sey/Sey mutants and is expanded in dbx1lacZ/lacZ and nkx6-1−/− embryos (brackets) (n≥2 for all mutant lines). IS, injected side.
Downstream of RA signaling, Pax6 constitutes one of the major stimuli for IN development in the intermediate spinal cord (Ericson et al., 1997; Pierani et al., 1999). Therefore, we investigated the regulation of Prdm12 by Pax6. As a first step, we assessed the effects of Pax6 overexpression on Prdm12 expression in the frog and chick embryos. Fig. 2B shows that pax6 overexpression in Xenopus neurula-stage embryos is insufficient to upregulate prdm12 (71% unaffected, 29% decreased, n=24), and similar results were obtained in the chick (supplementary material Fig. S7). We next assessed in both frog and mice whether the loss of Pax6 affects Prdm12 expression. To block pax6 activity in Xenopus, embryos were injected with a Pax6 morpholino oligonucleotide (MO). Fig. 2C shows that injection of the Pax6 MO inhibits prdm12. The penetrance of this phenotype, however, was weak (58% inhibited, 42% unaffected, n=45), which might be due to incomplete depletion of the protein (Rungger et al., 2010). We therefore examined prdm12 expression in pax6Sey/Sey mutants. In pax6Sey/Sey embryos, in which en1 is lost (Burrill et al., 1997), prdm12 was strongly reduced (Fig. 2D,E).
In silico, we identified several potential Pax6 binding sites in a conserved 600-bp sequence stretch located within the first intron of the Prdm12 locus with enhancer activity in the neural tube of E11.5 mouse embryos (Visel et al., 2007). To determine whether Pax6 binds this enhancer, ChIP-qPCR was performed on caudal neural tube tissues from E11.5 embryos using an anti-Pax6 antibody (Coutinho et al., 2011) and primers that span the identified potential Pax6 binding sites. This region was found to be significantly enriched compared with a negative control region (Kirrel2) (Borromeo et al., 2014) (supplementary material Fig. S8). Thus, Prdm12 is activated, perhaps directly, by Pax6. However, this activation could be also indirect as the absence of Pax6 results in the dysregulation of other TFs expressed in ventral progenitors (Takahashi and Osumi, 2002).
Prdm12 is confined to p1 progenitors due to the repressive action of Dbx1 and Nkx6
Progenitor identity in the spinal cord often relies on cross-repressive interactions between specific TFs (Briscoe and Novitch, 2008; Dessaud et al., 2008). We therefore tested whether Prdm12 is regulated by Dbx1, Nkx6-1 and Nkx6-2. In the frog, we found that their overexpression inhibits Prdm12 in neurula-stage embryos (71% reduced, n=28 for Dbx1; 78% reduced, n=18 for nkx6-1; 71% reduced, n=17 for nkx6-2) (Fig. 2F,G; and data not shown). As in the frog embryo, mis-expression of Dbx1 or Nkx6-1 in the chick neural tube resulted in a decrease of Prdm12 (supplementary material Fig. S9). The relationship between Prdm12 and these genes was further analyzed in the mouse by examining Prdm12 expression in DbxllacZ/lacZ and Nkx6-1−/− knockout mice (Pierani et al., 2001; Sander et al., 2000; Vallstedt et al., 2001). Compared with control mice, Prdm12 is expanded in Dbx1lacZ/lacZ mutants (Fig. 2H,I). Comparing Prdm12 and Prdm13 expressed in dp2-dp6 (Chang et al., 2013), this expansion appears limited to the p0 domain, which is defective in Dbx1lacZ/lacZ embryos (supplementary material Fig. S10). In Nkx6-1−/− knockout mice, the Prdm12 domain was also expanded (Fig. 2J,K). This expansion, however, is also limited, probably due to a switch of identity of some more ventral progenitors to p1 (Sander et al., 2000). Together, these results reveal that prdm12 expression in the Xenopus ventral spinal cord is excluded from the p0 and p2 domains due to the repressive action of Dbx1 and Nkx6-1/2, and suggest that other TFs also participate in its repression. In the mouse, Nkx6-1 alone is probably responsible for the ventral restriction of Prdm12, as Nkx6-2 is expressed in p1 and no change in Prdm12 could be detected in Nkx6-2Cre/Cre null mice (data not shown).
Xenopus and mouse, but not amphioxus, Prdm12 induces a V1 cell fate
Loss of Prdm12 in zebrafish inhibits the V1-specific marker En1 and affects swimming movements (Zannino et al., 2014). To determine whether Prdm12 has a similar function in Xenopus, we designed a prdm12 translation-blocking MO and inject it bilaterally at the 2-cell stage. As in zebrafish, Prdm12 morphants show impaired swimming movements and reduced en1 levels (99% reduced, n=78) (Fig. 3A; supplementary material Fig. S11 and Movies 1 and 2). Similar results were obtained with a second independent MO (data not shown). Injection of a Prdm12 mismatch MO had no effect on en1 (none affected, n=21), and the phenotype could be rescued by co-injection of mRNA encoding mouse Flag-Prdm12 (150 pg mPrdm12/blastomere), which is insensitive to the Prdm12 MO (94% increased, n=33) (Fig. 3B,C).
Fig. 3.
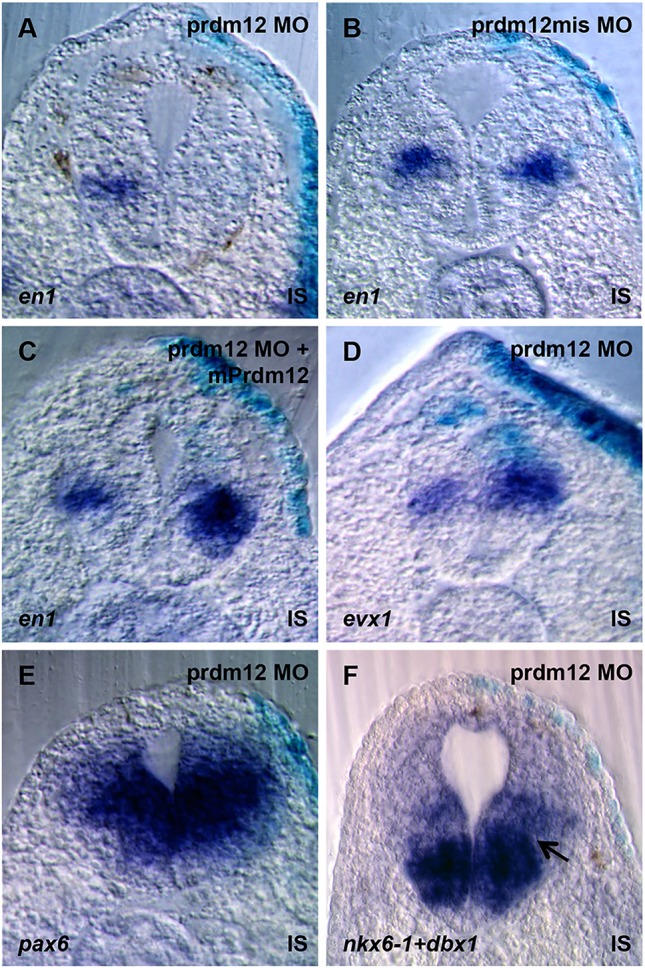
prdm12 knockdown in Xenopus dramatically reduces the V1 marker en1 and affects gene expression in ventral spinal cord progenitors. (A-F) Transverse sections at the neural tube of stage 32 embryos unilaterally injected with 20 ng of Prdm12 MO and hybridized as indicated. The disappearance of the gap between nkx6-1 and dbx1 expression in Prdm12 morphants is indicated by an arrow. IS, injected side.
We next determined whether the postmitotic V0 and V2 markers evx1 and vsx1 are affected in Prdm12 morphants. While vsx1 is unaffected in most injected embryos (87% unaffected, n=47) (data not shown), evx1 is increased (92% upregulated, n=78) (Fig. 3D).
Zannino et al. reported that the dorsal limit of Nkx6-1 is expanded in ventral neural tube progenitor cells of zebrafish prdm12b morphants (Zannino et al., 2014). We thus analyzed nkx6-1 as well as nkx6-2, pax6 and dbx1. In most Prdm12 MO-injected embryos, pax6 and dbx1 were unaffected (pax6, none affected, n=7; dbx1, 89% unaffected and 11% increased, n=28). In the case of nkx6-1, a slight dorsal expansion was detectable in 66% of the injected embryos (n=44). This expansion was most noticeable in embryos stained for both nkx6-1 and dbx1, as the region of expansion corresponds to a gap normally present between the expression domains of the two markers (Fig. 3E,F). The nkx6-2 signal also appears to be extended dorsally in some Prdm12 MO-injected embryos (37% expanded, n=8) (data not shown). Thus, as in zebrafish, prdm12 is required for the specification of V1 INs, and these neurons are important for swimming movements.
To characterize the function of Prdm12 further, we next performed gain-of-function assays in the frog and chick embryos. While frog embryos overexpressing a Flag version of mouse or Xenopus prdm12 appear morphologically normal at the early tadpole stage, they did not respond to touch (supplementary material Fig. S11 and Movies 1 and 3), suggesting that prdm12 affects neuronal specification. In these prdm12 mRNA-injected embryos, we first examined en1 expression. In embryos overexpressing Xenopus or mouse Prdm12, en1 was induced (all induced, n=35, for Xenopus prdm12; n=23 for mouse Prdm12). The induction observed was, however, stronger with mouse Prdm12 (Fig. 4A, and data not shown). We therefore performed all our subsequent Prdm12 overexpression experiments using the Flag version of mouse Prdm12. We next looked at the effects of Prdm12 overexpression on the postmitotic V0 and V2 markers evx1 and vsx1. While vsx1 is mainly unaffected (92% unchanged, n=75) (data not shown), evx1 was often reduced (89% decreased, n=90) (Fig. 4B). We next investigated the effects of Prdm12 overexpression on pax6 and irx3, co-expressed with Prdm12 in p1 progenitors, dbx1, nkx6-1 and nkx6-2 expressed in adjacent progenitor domains and on pax3, pax7 and ptf1a expressed in neural progenitors of the dorsal neural tube. prdm12 overexpression had no effect on pax6 (89% unaffected, n=19), irx3 (100%, unaffected, n=14), pax3, pax7 and ptf1a (supplementary material Fig. S12). By contrast, dbx1, nkx6-1 and nkx6-2 were decreased (97% decreased, n=33 for dbx1; 71% decreased, n=42, for nkx6-1; 76% decreased, n=42, for nkx6-2) (Fig. 4C-F; supplementary material Fig. S12). Thus, in Xenopus, Prdm12 activity appears mainly limited to the repression of neighboring genes.
Fig. 4.
Xenopus and mouse Prdm12, but not amphioxus Prdm12, downregulate dbx1, nkx6-1 and nkx6-2, and induces en1 in Xenopus. (A-F) Transverse sections at the neural tube of stage 32 embryos injected unilaterally with mPrdm12 mRNA (200 pg/blastomere) and hybridized with indicated probes. The decrease of dbx1, nkx6-1, nkx6-2 and evx1 is indicated by arrows. IS, injected side. (G-J) RT-PCR analysis of the expression of indicated genes in RA-treated neuralized animal caps isolated from embryos injected with mouse Prdm12 mRNA (50 or 250 pg/blastomere), amphioxus Prdm12 and chimeric constructs mRNA (250 pg/blastomere) as indicated. In G and H, expression levels were compared with the expression level of uninjected caps, defined as 1.
The effects of Prdm12 overexpression were further examined in animal caps. We found that Prdm12 induces en1 in neuralized explants but not in naïve caps and that Prdm12 is much more active in RA-treated neuralized caps than in untreated ones (Fig. 4G). We also observed that in RA-treated neuralized caps, Prdm12 overexpression efficiently increases en1 level only starting from tailbud stages (st. 20), thus at around its normal onset of expression during development (Fig. 4H). Next, we further evaluated Prdm12 activity by analyzing the expression of pax6, dbx1/2, Nkx6-1/2 (and prdm12 itself), as well as of the postmitotic markers evx1 and vsx1. Fig. 4I shows that, as observed in embryos, dbx1, nkx6-1, nkx6-2 that are excluded from p1 progenitors and evx1 were all downregulated by prdm12 overexpression. vsx1, which appears unaffected in embryos, was also downregulated. By contrast, pax6 was not affected and dbx2, which is co-expressed with prdm12 in p1 progenitors, was only slightly reduced. Interestingly, prdm12 was upregulated by its own overexpression. Finally, we also tested the activity of the amphioxus Prdm12 protein. Fig. 4J shows that in contrast to the mouse protein, amphioxus Prdm12 is unable both to increase en1 and to efficiently downregulate dbx1 and nkx6-1. Thus, amphioxus and vertebrate Prdm12 proteins appear to differ functionally.
In the chick neural tube, Prdm12 increases En1 and induces markers of the different subtypes of V1 INs (supplementary material Fig. S13). Together, these results indicate that Prdm12 is involved in patterning ventral neuronal progenitors and acts as a general determinant of V1 cell fate, a property that appears specific to the vertebrate lineage.
Prdm12 requires both its PR and zinc-finger domains and acts as a repressor
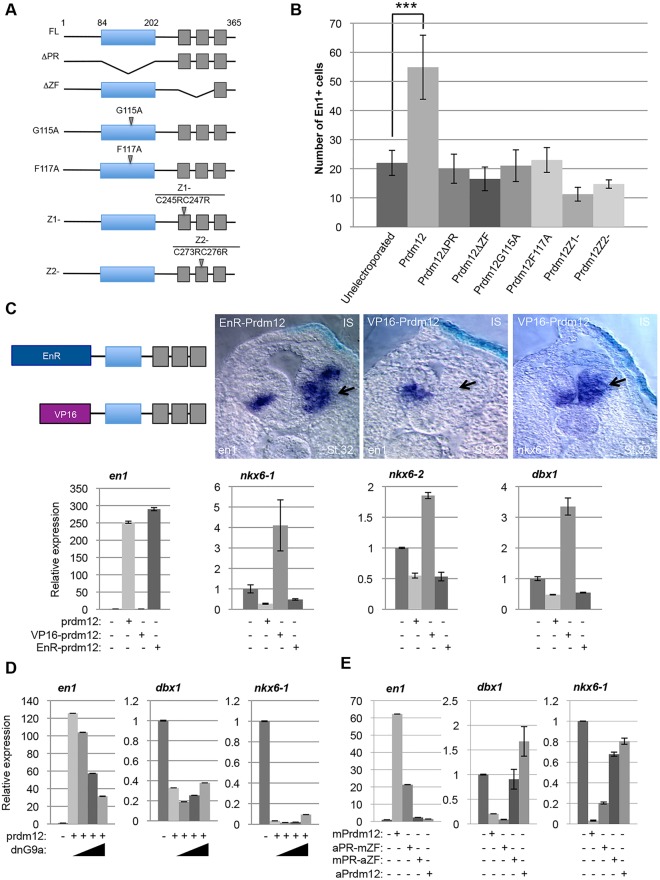
Prdm family members contain two major motifs, the PR domain similar to the SET domain found in histone methyltransferases, and zinc-fingers. To determine their functional contribution, we tested the activity of a series of mutants (Yang and Shinkai, 2013) in the chick neural tube (Fig. 5A). Fig. 5B shows that mutations that disrupt either the PR or the zinc-fingers impair its ability to induce En1.
Fig. 5.
Prdm12 function requires both the PR and zinc-finger domains and acts as a G9a-dependent repressor to induce en1. (A) Diagram of the Flag-mPrdm12 wild-type and mutants. (B) Average number of En1+ cells detected on the electroporated side of embryos overexpressing the indicated constructs. *, P<0.05; **, P<0.01; ***, P<0.001; n≥3. (C) Top panels: scheme of the VP16-Prdm12 and EnR-Prdm12 constructs and transverse sections of the neural tube of embryos overexpressing the indicated constructs (250 pg/blastomere for EnR-Prdm12 and 50 pg/blastomere for VP16-Prdm12) hybridized with the indicated probes. The upregulation of en1 by EnR-Prdm12, the downregulation of en1 and the upregulation of nkx6-1 by VP16-Prdm12 are indicated (arrows). Bottom panels: RT-PCR analysis of the expression of the indicated genes in RA-treated neuralized animal caps overexpressing mouse Prdm12 (250 pg/blastomere), EnR-Prdm12 (250 pg/blastomere) or VP16-Prdm12 (50 pg/blastomere) mRNA as indicated. (D) A dominant negative form of G9a (25, 500 and 100 pg mRNA injected) reduces the ability of Prdm12 to induce en1 and slightly attenuates the repression of dbx1 and nkx6-1 in RA-treated neuralized animal caps. (E) The mouse-amphioxus Prdm12 chimeric protein aPR-mZF, but not the complementary chimeric protein mPR-aZF, induces en1 and represses dbx1 and nkx6-1 in RA-treated neuralized animal caps.
To determine whether the activity of Prdm12 to induce V1 INs reflects a transcriptional activator or repressor function, we created fusion constructs of Xenopus Prdm12 with the engrailed repressor (EnR) (Smith and Jaynes, 1996) or with the VP16 activator domain (Triezenberg et al., 1988) and tested the en1-inducing activity of the chimeric proteins in the frog embryo. We found that EnR-Prdm12 functions as the wild-type protein in that it induces en1 (97%, n=78). By contrast, VP16-Prdm12 functions as an antimorphic mutant, as its overexpression inhibits en1 (98%, n=125). Interestingly, nkx6-1 that is downregulated by Prdm12 is upregulated by VP16-Prdm12 (64%, n=22). In animal caps, like in the embryos, EnR-Prdm12 induces en1. It also downregulates nkx6-1, nkx6-2 and dbx1, whereas VP16-Prdm12 upregulates their expression (Fig. 5C).
Unlike other Prdm proteins, Prdm12 lacks direct histone methyltransferase activity and repress target gene expression by recruiting the methyltransferase G9a to dimethylate histone H3 at lysine 9 (H3K9me2) (Yang and Shinkai, 2013). Therefore, we asked whether G9a is necessary for Prdm12 function in the spinal cord. We found that a dominant negative G9a overexpressed together with Prdm12 in RA-treated neuralized caps diminishes its ability to induce en1 and, at high dose, slightly attenuates nkx6-1 and dbx1 repression (Fig. 5D).
The amphioxus Prdm12 protein appears functionally distinct from the vertebrate Prdm12 proteins. To identify the regions responsible for this differential activity, we generated two mouse-amphioxus Prdm12 chimeric proteins, substituting their PR or zinc-finger domains, and tested their activity using our animal cap assay. The chimeric protein retaining the mouse zinc-finger domain (aPR-mZF) was still able to induce en1 and to repress dbx1 and nkx6-1. By contrast, the other chimeric protein with the amphioxus zinc-finger domain (mPR-aZF) was inactive (Fig. 5E). Together, these data indicate that Prdm12 acts in V1 cell specification as a G9a-dependent transcriptional repressor, the activity of which requires both the PR and zinc-finger domains. They also suggest that differences in the C-terminal part of the protein, including the zinc-fingers, are responsible for the differential functions of the amphioxus and vertebrate Prdm12 proteins.
dbx1 and nkx6 gene overexpression blocks Prdm12 activity, and their knockdown upregulates en1
The above results suggest that Prdm12 induces V1 expressing cells by repressing dbx1 and nkx6 genes. To test this idea, we co-expressed them with prdm12 in RA-treated neuralized caps. Fig. 6A shows that the induction of en1 by Prdm12 is abrogated by the overexpression of dbx1, nkx6-1 or nkx6-2. Co-expression of dbx2 or lacZ had no such repressive effect. We next tested whether the knockdown of dbx1 and/or nkx6-1 and nkx6-2 in RA-treated neuralized caps could be sufficient to increase en1 expression. Fig. 6B shows that en1 is upregulated when dbx1, nkx6-1 or nkx6-2 were knocked down, and that en1 levels were increased even further when combinations of these MOs were used. As expected, prdm12 was also upregulated when dbx1, nkx6-1 or nkx6-2 were knocked down, Thus, Prdm12 mainly promotes V1 cell fate in Xenopus by repressing dbx1 and nkx6 genes.
Fig. 6.
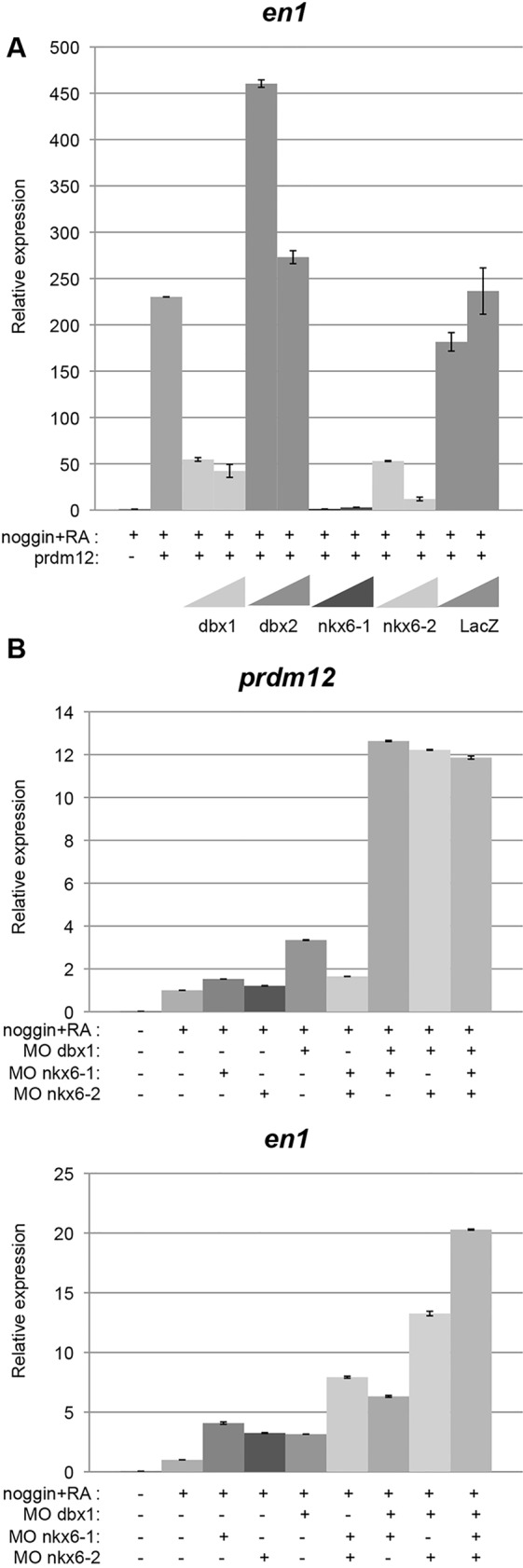
dbx1 and nkx6 overexpression blocks Prdm12 activity, and their knockdown upregulates En1. (A) RT-PCR analysis of en1 in neuralized animal caps overexpressing mouse Prdm12 mRNA (250 pg/blastomere), dbx1, dbx2, nkx6-1 or nkx6-2 mRNA (50 and 250 pg/blastomere) and treated with RA as indicated. (B) RT-PCR analysis of en1 and prdm12 in neuralized animal caps derived from embryos injected with MO against dbx1, nkx6-1 and nkx6-2 as indicated.
RNA-Seq and ChIP-Seq analyses support a role for Prdm12 in promoting V1 cell fate through direct repression of dbx1 and nkx6 genes
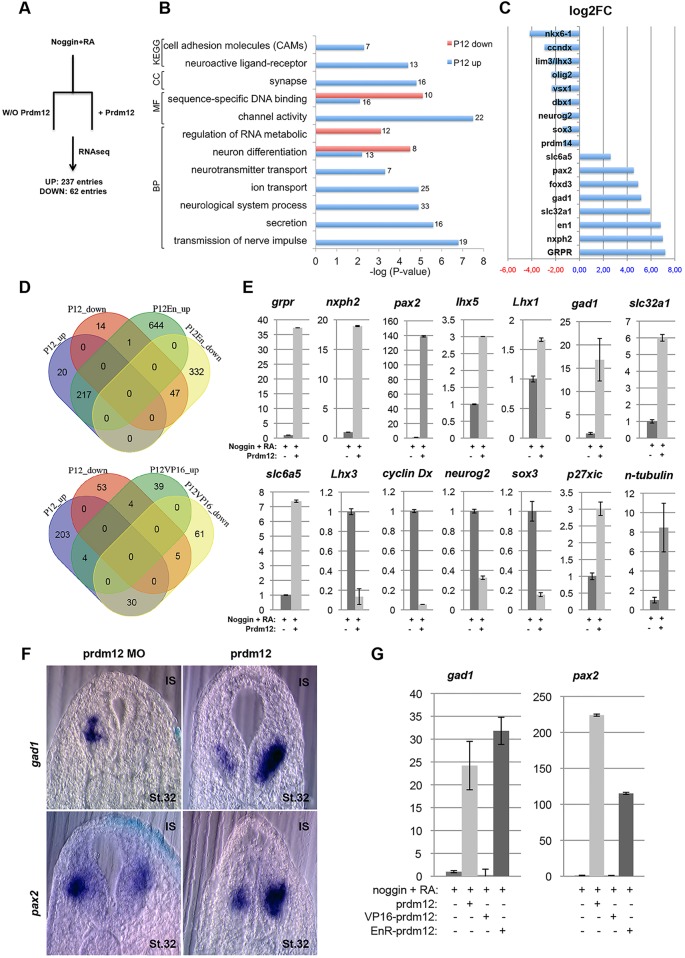
To gain further insights into the transcription network regulated by Prdm12, RNAseq analyses were carried out on RA-treated neuralized caps derived from wild-type or prdm12-overexpressing embryos (Fig. 7A). Applying a minimal cutoff of twofold change and P<0.05, a total of 299 differentially expressed genes were identified. Of these, 62 were downregulated and 237 were upregulated by Prdm12 (supplementary material Table S2). Gene ontology (GO) analyses revealed that genes positively regulated by Prdm12 are enriched in GO categories relevant to neuronal function such as transmission of nerve impulses, neurotransmitter transport, secretion, synapse and channel activity (Fig. 7B). Enriched in the both up- and downregulated categories were genes involved in sequence-specific DNA binding and neuron differentiation. Fig. 7C shows a curated list of the most highly upregulated and downregulated genes (together with their fold change). The list of upregulated genes contains, as expected, many genes know to be expressed in the postmitotic V1 domain, including TFs en1, pax2 and foxd3. Consistent with the inhibitory neurotransmitter phenotype of V1 neurons, markers such as gad1 and slc32a1 were also found upregulated. In the list of downregulated genes were TFs expressed in both the progenitor and postmitotic cells of the adjacent domains, including olig2, nkx6-1, lhx3, dbx1 and vsx1/chx10.
Fig. 7.
RNA-Seq identification of Prdm12-regulated genes. (A) Experimental setup for the RNA-Seq analysis. (B) Selected ontological classification of genes regulated by Prdm12. (C) Examples of identified differentially expressed genes. (D) Venn diagram showing the overlap of differentially regulated genes between Prdm12, Prdm12-EnR and Prdm12-VP16. (E) Validation of some of the identified genes as prdm12 target genes by RT-PCR in RA-treated neuralized animal caps. (F) pax2 and gad1 on sections of embryos injected with either mouse Prdm12 mRNA (200 pg/blastomere) or with a Prdm12 MO. IS, injected side. (G) RT-qPCR analysis of pax2 and gad1 in EnR-Prdm12- and VP16-Prdm12-overexpressing RA-treated neuralized caps.
To obtain additional evidence that those genes are indeed regulated by Prdm12, the transcriptome of RA-treated neuralized caps overexpressing the EnR-Prdm12 or VP16-Prdm12 constructs was determined. Consistent with our results suggesting that Prdm12 functions as a repressor, 76% of the genes downregulated by Prdm12 were present in the list of the 379 genes downregulated by EnR-Prdm12, and 92% of the genes upregulated by Prdm12 were present in the 862 genes induced by EnR-Prdm12. The overlap of differentially expressed genes between Prdm12 and Prdm12-VP16 was limited, with the highest overlap represented by the 30 genes upregulated by Prdm12 and downregulated by VP16-Prdm12 (Fig. 7D; supplementary material Tables S2, S3).
For some of these genes, we have confirmed their disregulation by Prdm12 using RT-qPCR. Fig. 7E shows that two of the most upregulated genes in our RNAseq list, the gastrin-releasing peptide receptor (grpr) that is involved in neurogenesis (Walton et al., 2014), and neurexophilin2 (nxph2), a member of a family of secreted glycoprotein that function as neuropeptides (Missler and Südhof, 1998), are indeed strongly increased by Prdm12. The upregulation of pax2, lhx5 (induced more than twofold by EnR-Prdm12 but not by Prdm12) and lhx1 (induced by both Prdm12 and EnR-Prdm12, but less than twofold), which are expressed in spinal cord V1 INs, as well as of the inhibitory markers gad1, slc32a1 and slc6a5, was also validated. We were further able to verify that lhx3 (lim3) and cyclin Dx (ccndx), which are expressed more ventrally, as well as neurog2 and sox3, which are expressed in undifferentiated neural cells, are indeed downregulated by Prdm12. Finally, we also tested p27Xic1 (cdknx), encoding a cyclin-dependent kinase inhibitory protein and the pan-neuronal differentiation marker N-tubulin as those genes were found upregulated by Prdm12 in P19 cells (Yang and Shinkai, 2013). Both of them were also upregulated by Prdm12 in our neuralized RA-treated caps. The deregulation of two of these targets, pax2 and gad1, has further been validated by ISH in prdm12-overexpressing and morphant embryos (Fig. 7F). Their up- and downregulation by Prdm12-EnR and Prdm12-VP16 has been equally confirmed by RT-qPCR (Fig. 7G).
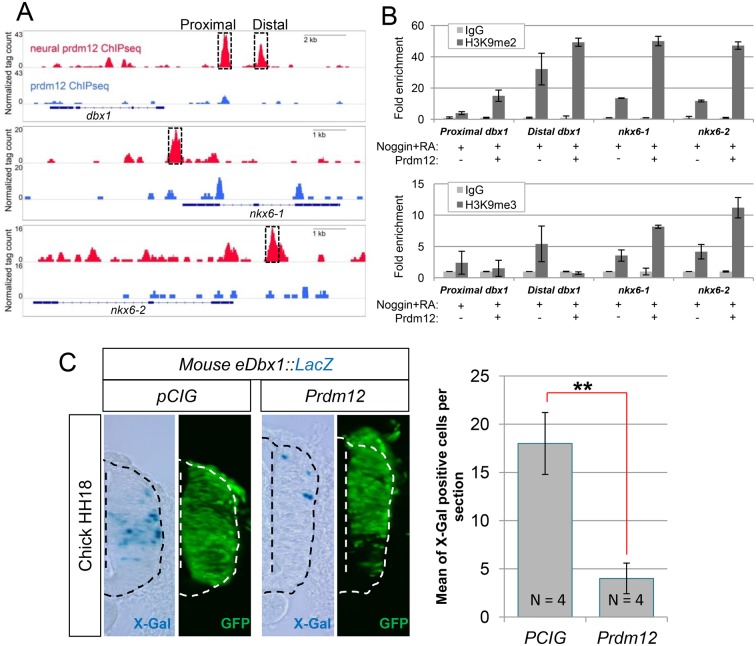
To identify direct downstream targets of Prdm12, chromatin immunoprecipitation-sequencing (ChIP-Seq) experiments were performed using neuralized RA-treated animal caps overexpressing mouse Flag-Prdm12 and anti-Flag antibodies. Naïve animal caps overexpressing the mouse Flag-Prdm12 construct were used as a control, as Prdm12 is unable to promote en1 in epidermal cells (Fig. 4G). In these conditions, Prdm12 binds to several hundreds of sites within the genome (supplementary material Table S4).
Only sites differentially bound at fourfold were retained, which numbered ∼20,000. To determine where Prdm12 binds relative to other genomic features, we annotated its positions and found that Prdm12 strongly prefers enhancers to promoters (data not shown). We then tested how many Prdm12 peaks were flanking genes regulated by Prdm12, as shown by our RNA-seq. We found Prdm12 peaks flanking many of these targets, such as dbx1, nkx6-1, nkx6-2, vsx1, lhx3, prdm14, sox3 and neurog2 (Fig. 8A). In some cases, genes were surrounded by a large number of Prdm12 peaks, hinting at complex regulation. For example, dbx1 has some 18 Prdm12 peaks flanking its promoter at distances ranging from 2 to 70 kb. Among the genes upregulated by Prdm12, en1 and other genes, such as slc32a1, slc6a5 and nxph2 had no or very low peaks, which is consistent with the idea that they are indirectly regulated by Prdm12.
Fig. 8.
Prdm12 binds dbx1 and nkx6 promoter regions, promotes their methylation and affects the activity of a dbx1 promoter-lacZ reporter construct. (A) IGV browser screenshots showing differential binding of Flag-mPrdm12 in RA-treated neuralized caps versus naïve caps in the promoters of dbx1, nxk6-1 and nkx6-2. Two major peaks in the dbx1 locus (at −3 kb and −5 kb), one in the nkx6-1 locus (at −0.5 kb), and another in the nkx6-2 locus (at −1.0 kb) are boxed. (B) ChIP-qPCR analysis of the dbx1, nkx6-1 and nkx6-2 promoter regions (with primers designed at the dashed boxes highlighted in A) in RA-treated neuralized caps overexpressing or not mPrdm12 with α-H3K9me2 (upper histogram) or α-H3K9me3 (lower histogram) antibodies. Fold enrichment is shown. (C) X-gal staining of a transverse section of the neural tube of a chick embryo electroporated with a 5.7 kb mouse eDbx1::lacZ reporter, plus or minus a pCIG-Prdm12 or an empty pCIG vector, and immunostained for GFP to show electroporation efficiency. Quantification of the number of X-gal+ cells observed in the electroporated side is shown (n≥4 embryos). Error bars denote s.e.m. **P<0.01.
Overexpression of Prdm12 increases H3K9me2 and H3K9me3 levels in Xenopus embryos (Chen et al., 2015; Matsukawa et al., 2015). To investigate whether Prdm12 methylates histone H3 at lysine 9 at the dbx1, nkx6-1 and nkx6-2 promoters, ChIP-qPCR experiments were performed with anti-H3K9me2 and H3K9me3 antibodies on RA-treated neuralized animal caps that were either overexpressing or not Flag-mPrdm12. Fig. 8B shows an enrichment of H3K9me2 on the dbx1, nkx6-1 and nkx6-2 promoter regions bound by Prdm12 in caps overexpressing prdm12 compared with controls. An enrichment of H3K9me3 marks was also observed, but only in the nkx6-1 and nkx6-2 upstream sequences.
A 5.7 kb DNA fragment of the mouse Dbx1 promoter is sufficient to direct the expression of a lacZ transgene in the intermediate region of the developing spinal cord where Dbx1 is expressed (Lu et al., 1996). This fragment includes a conserved non-coding sequence (CNS), present in human, chicken, Xenopus tropicalis and zebrafish, that corresponds to a distal Prdm12 peak identified by ChIP-Seq (data not shown).
As previously reported, when a construct containing this mouse 5.7 kb Dbx1 promoter fragment cloned upstream of a lacZ reporter was electroporated into the chick neural tube, staining was mainly restricted to the intermediate spinal cord where Dbx1 is expressed (Fig. 8C). Upon co-electroporation with Prdm12, β-Gal staining was strongly reduced. Thus, the Prdm12-bound region in the Dbx1 promoter appears functional. Together, these data suggest that Prdm12 promotes V1 cell fate through, at least in part, direct repression of Dbx1 and Nkx6 genes.
DISCUSSION
Prdm12 function in vertebrate V1 IN specification
In the mouse, V1 INs play an important role in locomotion (Gosgnach et al., 2006; Zhang et al., 2014). Our Prdm12 knockdown experiments indicate that the aINs, the homologues of V1 INs, are required for swimming movement in Xenopus, as observed for CiA INs in zebrafish (Zannino et al., 2014). Thus, V1 INs have a conserved role in controlling locomotor outputs in vertebrates. Whether changes in these V1 INs occur in the metamorphing frog during the transition from swimming to walking behavior remains to be determined.
As in zebrafish (Zannino et al., 2014), loss of Prdm12 in Xenopus affects en1-expressing V1 INs. En1 is also lost in the spinal cord of Prdm12 mutant mice (unpublished data), indicating that Prdm12 has an evolutionarily conserved role in V1 IN specification in vertebrates. In zebrafish, Prdm12 overexpression does not lead to ectopic V1 neurons (Zannino et al., 2014). By contrast, our data indicate that Xenopus or mouse Prdm12 promotes En1+ V1 INs when overexpressed in the frog or chick embryo. Human PRDM12 also induces en1 when overexpressed in the frog (Nagy et al., 2015). The reasons for this functional difference between zebrafish and tetrapode vertebrate Prdm12 are unclear and remain to be determined. We also observed that the amphioxus Prdm12 ortholog, when overexpressed in the frog, does not induce En1. This suggests that Prdm12 function in V1 IN specification has specifically evolved in the vertebrate lineage after the split of the amphioxus and vertebrate lineages. This function of Prdm12 might have, however, already been present in the last common ancestor of all chordates and then specifically been lost in the lineage leading to extant cephalochordates. We favor the first scenario, given that vertebrate diversification was marked by a significant increase of the diversity of neuronal cell types, which required the elaboration of novel regulatory circuits, mediated, for example, by novel protein functions, as we report here for Prdm12 and as has previously been reported for other Prdm proteins (Vincent et al., 2012).
Prdm12 mechanism of action in V1 INs
Our results suggest that Prdm12 acts in p1 progenitors as a repressor by suppressing dbx1, nkx6-1 and nkx6-2. Whether Prdm12 can, in addition, also act as an activator is unclear. Prdm12 ChIP-Seq peaks around genes such as pax2, foxd3, lhx5 and lhx1, all expressed in postmitotic V1 INs, have been observed. As (1) prdm12 is not maintained in postmitotic cells, and (2) these ChIP-Seq data have been obtained in conditions of prdm12 overexpression, it is questionable and remains to be investigated whether they correspond to true binding sites. It is not clear why dbx1, which is increased by VP16-Prdm12 in noggin-injected RA-treated caps, is not upregulated in most Prdm12 morphants and in VP16-prdm12 overexpressing embryos (data not shown). Dbx1 is also unaffected by the loss of Prdm12 in zebrafish embryos (Zannino et al., 2014). It is possible that dbx1 is not upregulated in the Prdm12 morphants and in VP16-prdm12-overexpressing embryos because other TFs cooperate with Prdm12 to exclude dbx1 from p1 progenitors. The fact that Vsx1 appears unaffected in embryos but is, however, downregulated in the noggin mRNA-injected RA-treated explants is also puzzling.
Our results also indicate that the proneural gene neurog2 is downregulated by Prdm12, whereas the negative regulators of the cell cycle p27Xic and the pan-neuronal differentiated marker N-tubulin are upregulated. These findings, which are consistent with the observation that Prdm12 has anti-proliferative activity in P19 cells (Yang and Shinkai, 2013), suggest that Prdm12 plays a role in the coordination of the proliferation and differentiation of p1 progenitors.
Our data indicate that Prdm12 acts as a G9a-dependent transcriptional repressor in V1 cell specification, as in P19 cells (Yang and Shinkai, 2013). Prdm12 recruits G9a via its second zinc-finger (Yang and Shinkai, 2013). While confirming the importance of the zinc-fingers for Prdm12 activity, our results suggest a specific role for the PR domain. We speculate that Prdm12 might recruit, via its PR domain, other chromatin-modifying proteins to create a repressive state. This has previously been shown for Prdm6 that associates with HDAC1-3 via its PR domain (Davis et al., 2006).
The key differences responsible for the differential function of the amphioxus and vertebrate Prdm12 proteins appear to be located in the C-terminal part of the protein, including the zinc-fingers. In a recent study, different homozygous mutations have been identified throughout Prdm12 that all abrogates its histone-modifying activity (Chen et al., 2015). Some of these mutated forms of Prdm12, when overexpressed in Xenopus, exhibit an impaired activity to induce en1 (Nagy et al., 2015). It remains to be tested whether the amphioxus Prdm12 is inactive because of its impaired histone-modifying potential.
Our results indicate that Prdm12 occupies regions in the dbx1, nkx6-1 and nkx6-2 loci, increases in these regions H3K9 methylation and represses the transcriptional activity of a dbx1 promoter construct. These observations strongly suggest that prdm12 acts as a patterning gene in V1 cell fate determination by directly repressing dbx1 and nkx6. The high number of postmitotic genes that are upregulated upon prdm12 overexpression is probably an indirect consequence of its role in progenitors in the induction of V1 and sensory cell lineages. Whether Prdm12 binds directly to DNA or indirectly, via other TFs, is unknown. De novo motif analysis of the Prdm12 peaks revealed a strong enrichment for Sox and homeobox binding motifs (data not shown). Whether Prdm12 binds DNA via these TFs or whether it cooperates with them at these sites remains to be tested.
MATERIALS AND METHODS
Prdm12 cDNAs and expression constructs
Xenopus laevis (EST BM179581), zebrafish (Danio rerio) (EST BC085382) and chicken (Gallus gallus) (EST BU233582) Prdm12 cDNA clones were identified by BLAST searches of EST databases (NCBI), using the mouse Prdm12 cDNA sequence as bait. An amphioxus (B. lanceolatum) Prdm12 cDNA was amplified by PCR from cDNA generated from total RNA from adults, using primers forward 5′-ATGAAGCCGACCCTGTTTGATC-3′ and reverse 5′-ACATGGCGACCGAACGAACGTC-3′ (Oulion et al., 2012).
Prdm12 and previously described expression vectors used in frog microinjections and in ovo electroporations are described in further detail in the supplementary material methods.
Xenopus embryo micro-injections and animal caps
Synthetic mRNAs were prepared using the SP6 mMessage mMachine kit (Ambion). The pCS2-Flag-dnG9a construct was obtained by PCR from pCDNA3-human dnG9a (Győry et al., 2004) using primers 5′-GACGATGACAAGAATTCAAGTGATGATGTCCACTCACTGGGAAAG-3′ and reverse 5′-GTTCTAGAGGCTCGAGTCATGTGTTGACAGGGGGCAGGGA-3′, and cloned into the EcoRI and XhoI sites of pCS2-FLAG. The Prdm12 antisense MOs (5′-CCGGCAGCACCGAGCCCATCATTAA-3′ and 5′-CATTAATTCTGCCTGCGAGTCTGAC-3′) and the control Prdm12 antisense mismatch MO (5′-CCCGCACCACGGAGCGCATGATTAA-3′) were injected at 20 ng/blastomere. Pax6, Dbx1, Nkx6-1 and Nkx6-2 MOs were as described (Dichmann and Harland, 2011; Ma et al., 2013, 2011; Rungger-Brändle et al., 2010). For in situ hybridization (ISH) analyses, embryos were injected in one cell of two- to four-cell-stage embryos. In all experiments, embryos were co-injected with lacZ mRNA (50 pg/blastomere), and β-Gal activity was revealed by X-gal staining (in light blue or red). For animal cap assays, mRNA was microinjected into the animal region of each blastomere of four-cell stage embryos. Dissected animal caps were cultured in 1× Steinberg medium, 0.1% BSA with or without RA (10 µM) until sibling embryos reach stage 28, unless indicated. All microinjections were repeated independently at least twice.
In situ hybridization and immunohistochemistry
ISH and immunohistochemistry (IHC) procedures used in amphioxus, frog, chick, zebrafish and mouse embryos are described in further detail in the supplementary material methods.
Real-time RT-PCR
Real-time RT-PCR was performed as reported (Hanotel et al., 2014). Gene expression levels were normalized with GAPDH and, unless indicated, compared with the level observed in naïve RA-treated neuralized caps (defined as 1). Primers are listed in supplementary material Table S1. All assays were carried out in duplicates or triplicates. Error bars represent s.d. The values indicated in the figures are derived from one representative experiment.
Chick embryo in ovo electroporation
Hamburger–Hamilton (HH) stage 12-14 embryos were electroporated in the neural tube as described (Hanotel et al., 2014). 48 h (HH24-25) or 72 h (HH27-28) after electroporation, embryos were harvested, fixed and processed for IHC or ISH. Embryos electroporated with the 5.7 kb mouse Dbx1 promoter fragment were collected 24 h later (HH18) and processed for β-gal activity as described (Lu et al., 1996).
Mouse embryo preparation
Pax6Sey/Sey, Dbx1lacZ/lacZ, Nkx6-1−/− and Nkx6-2Cre/Cre mutant mice were as described (Baudet et al., 2008; Ericson et al., 1997; Pierani et al., 2001; Sander et al., 2000).
Histone methyltransferase activity
The HMTase assays were conducted as described (Hanotel et al., 2014).
RNA sequencing and chromatin immunoprecipitation
RNAseq from Xenopus animal caps was performed essentially as described (Ma et al., 2014). ChIP was essentially conducted in mouse (Borromeo et al., 2014) and in Xenopus as described (Ma et al., 2014; Colin et al., 2011) (see supplementary material methods). RNAseq and ChIP data have been deposited at GEO under accession GSE64551.
Acknowledgements
The authors acknowledge M. Andreazzoli, F. Clotman, J. Ericson, M. Götz, R. Harland, C. Logan, B. Mao, A. Moore, A. H. Monsoro-Burq, T. Müller, B. Novitch, Y. Shinkai, E. Winterbottom and K. Wright for reagents, N. Bessodes for experimental help, L. Delhaye for technical assistance, and G. Salinas-Riester and T. Linger for the RNAseq analysis.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
A.T., S.D., E.B. and C.V.C. designed the experiments and analyzed the data, with contributions from J.H., I.Q., A.R., M.D.B., B.V.D., C.R.K., T.P. and K.A.H. A.T., S.D., J.H., S.K., E.J.B. and C.V.C. carried out the experiments, with contributions from I.Q., A.R., M.D.B., F.L., J.C., B.V.D., G.C.-M., J.O.F. and B.B. S.D., E.J.B. and C.V.C. prepared the manuscript, incorporating comments from K.L., M.Sa., A.P., C.V.L., M.Sc., J.E.J. and K.A.H.
Funding
This work was supported by grants from the Belgian Fonds de la Recherche Scientifique (FNRS) [FRC 3.4635.06] (to E.J.B. and C.V.L.); from the French Agence Nationale de la Recherche (ANR) [11-JSV2-002-01 to M.S.]; from the Medical Research Council (MRC) [G0600877 and G0801283] to K.E.L.; and from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (NIH/NIDDK) to M.S. A.T. and C.V.C. are FNRS postdoctoral fellows. S.D. and J.H. are Research Foundation for Industry and Agriculture (FRIA) doctoral fellows. Deposited in PMC for release after 6 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.121871/-/DC1
References
- Alvarez F. J., Jonas P. C., Sapir T., Hartley R., Berrocal M. C., Geiman E. J., Todd A. J. and Goulding M. (2005). Postnatal phenotype and localization of spinal cord V1 derived interneurons. J. Comp. Neurol. 493, 177-192. 10.1002/cne.20711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudet C., Pozas E., Adameyko I., Andersson E., Ericson J. and Ernfors P. (2008). Retrograde signaling onto Ret during motor nerve terminal maturation. J. Neurosci. 28, 963-975. 10.1523/JNEUROSCI.4489-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito-Gonzalez A. and Alvarez F. J. (2012). Renshaw cells and Ia inhibitory interneurons are generated at different times from p1 progenitors and differentiate shortly after exiting the cell cycle. J. Neurosci. 32, 1156-1170. 10.1523/JNEUROSCI.3630-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borromeo M. D., Meredith D. M., Castro D. S., Chang J. C., Tung K.-C., Guillemot F. and Johnson J. E. (2014). A transcription factor network specifying inhibitory versus excitatory neurons in the dorsal spinal cord. Development 141, 2803-2812. 10.1242/dev.105866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J. and Novitch B. G. (2008). Regulatory pathways linking progenitor patterning, cell fates and neurogenesis in the ventral neural tube. Philos. Trans. R. Soc. B Biol. Sci. 363, 57-70. 10.1098/rstb.2006.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrill J. D., Moran L., Goulding M. D. and Saueressig H. (1997). PAX2 is expressed in multiple spinal cord interneurons, including a population of EN1+ interneurons that require PAX6 for their development. Development 124, 4493-4503. [DOI] [PubMed] [Google Scholar]
- Chang J. C., Meredith D. M., Mayer P. R., Borromeo M. D., Lai H. C., Ou Y.-H. and Johnson J. E. (2013). Prdm13 mediates the balance of inhibitory and excitatory neurons in somatosensory circuits. Dev. Cell 25, 182-195. 10.1016/j.devcel.2013.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.-C., Auer-Grumbach M., Matsukawa S., Zitzelsberger M., Themistocleous A. C., Strom T. M., Samara C., Moore A. W., Cho L. T.-Y., Young G. T. et al. (2015). Transcriptional regulator PRDM12 is essential for human pain perception. Nat. Genet. 47, 803-808. 10.1038/ng.3308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin L., Dekoninck A., Reichert M., Calao M., Merimi M., Van den Broeke A., Vierendeel V., Cleuter Y., Burny A., Rohr O. et al. (2011). Chromatin disruption in the promoter of Bovine Leukemia Virus during transcriptional activation. Nucleic Acids Res. 39, 9559-9573. 10.1093/nar/gkr671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho P., Pavlou S., Bhatia S., Chalmers K. J., Kleinjan D. A. and van Heyningen V. (2011). Discovery and assessment of conserved Pax6 target genes and enhancers. Genome Res. 21, 1349-1359. 10.1101/gr.124115.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. A., Haberland M., Arnold M. A., Sutherland L. B., McDonald O. G., Richardson J. A., Childs G., Harris S., Owens G. K. and Olson E. N. (2006). PRISM/PRDM6, a transcriptional repressor that promotes the proliferative gene program in smooth muscle cells. Mol. Cell. Biol. 26, 2626-2636. 10.1128/MCB.26.7.2626-2636.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessaud E., McMahon A. P. and Briscoe J. (2008). Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development 135, 2489-2503. 10.1242/dev.009324 [DOI] [PubMed] [Google Scholar]
- Dichmann D. S. and Harland R. M. (2011). Nkx6 genes pattern the frog neural plate and Nkx6.1 is necessary for motoneuron axon projection. Dev. Biol. 349, 378-386. 10.1016/j.ydbio.2010.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston A. J., Timmermans J. P. M., Hage W. J., Hendriks H. F. J., de Vries N. J., Heideveld M. and Nieuwkoop P. D. (1989). Retinoic acid causes an anteroposterior transformation in the developing central nervous system. Nature 340, 140-144. 10.1038/340140a0 [DOI] [PubMed] [Google Scholar]
- Ericson J., Rashbass P., Schedl A., Brenner-Morton S., Kawakami A., van Heyningen V., Jessell T. M. and Briscoe J. (1997). Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell 90, 169-180. 10.1016/S0092-8674(00)80323-2 [DOI] [PubMed] [Google Scholar]
- Fog C. K., Galli G. G. and Lund A. H. (2012). PRDM proteins: important players in differentiation and disease. Bioessays 34, 50-60. 10.1002/bies.201100107 [DOI] [PubMed] [Google Scholar]
- Francius C., Harris A., Rucchin V., Hendricks T. J., Stam F. J., Barber M., Kurek D., Grosveld F. G., Pierani A., Goulding M. et al. (2013). Identification of multiple subsets of ventral interneurons and differential distribution along the rostrocaudal axis of the developing spinal cord. PLoS ONE 8, e70325 10.1371/journal.pone.0070325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosgnach S., Lanuza G. M., Butt S. J. B., Saueressig H., Zhang Y., Velasquez T., Riethmacher D., Callaway E. M., Kiehn O. and Goulding M. (2006). V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature 440, 215-219. 10.1038/nature04545 [DOI] [PubMed] [Google Scholar]
- Goulding M. (2009). Circuits controlling vertebrate locomotion: moving in a new direction. Nat. Rev. Neurosci. 10, 507-518. 10.1038/nrn2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. and Jessell T. M. (2009). Measured motion: searching for simplicity in spinal locomotor networks. Curr. Opin. Neurobiol. 19, 572-586. 10.1016/j.conb.2009.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Győry I., Wu J., Fejér G., Seto E. and Wright K. L. (2004). PRDI-BF1 recruits the histone H3 methyltransferase G9a in transcriptional silencing. Nat. Immunol. 5, 299-308. 10.1038/ni1046 [DOI] [PubMed] [Google Scholar]
- Hanotel J., Bessodes N., Thélie A., Hedderich M., Parain K., Driessche B. V., Brandão K. D. O., Kricha S., Jorgensen M. C., Grapin-Botton A. et al. (2014). The Prdm13 histone methyltransferase encoding gene is a Ptf1a–Rbpj downstream target that suppresses glutamatergic and promotes GABAergic neuronal fate in the dorsal neural tube. Dev. Biol. 386, 340-357. 10.1016/j.ydbio.2013.12.024 [DOI] [PubMed] [Google Scholar]
- Higashijima S.-i., Masino M. A., Mandel G. and Fetcho J. R. (2004). Engrailed-1 expression marks a primitive class of inhibitory spinal interneuron. J. Neurosci. 24, 5827-5839. 10.1523/JNEUROSCI.5342-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenauer T. and Moore A. W. (2012). The Prdm family: expanding roles in stem cells and development. Development 139, 2267-2282. 10.1242/dev.070110 [DOI] [PubMed] [Google Scholar]
- Kinameri E., Inoue T., Aruga J., Imayoshi I., Kageyama R., Shimogori T. and Moore A. W. (2008). Prdm proto-oncogene transcription factor family expression and interaction with the Notch-Hes pathway in mouse neurogenesis. PLoS ONE 3, e3859 10.1371/journal.pone.0003859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.-C., Higashijima S.-i., Parry D. M., Roberts A. and Soffe S. R. (2004). Primitive roles for inhibitory interneurons in developing frog spinal cord. J. Neurosci. 24, 5840-5848. 10.1523/JNEUROSCI.1633-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S., Shashikant C. S. and Ruddle F. H. (1996). Separate cis-acting elements determine the expression of mouse DBX gene in multiple spatial domains of the central nervous system. Mech. Dev. 58, 193-202. 10.1016/S0925-4773(96)00576-X [DOI] [PubMed] [Google Scholar]
- Ma P., Zhao S., Zeng W., Yang Q., Li C., Lv X., Zhou Q. and Mao B. (2011). Xenopus Dbx2 is involved in primary neurogenesis and early neural plate patterning. Biochem. Biophys. Res. Commun. 412, 170-174. 10.1016/j.bbrc.2011.07.068 [DOI] [PubMed] [Google Scholar]
- Ma P., Xia Y., Ma L., Zhao S. and Mao B. (2013). Xenopus Nkx6.1 and Nkx6.2 are required for mid–hindbrain boundary development. Dev. Genes Evol. 223, 253-259. 10.1007/s00427-013-0437-9 [DOI] [PubMed] [Google Scholar]
- Ma L., Quigley I., Omran H. and Kintner C. (2014). Multicilin drives centriole biogenesis via E2f proteins. Genes Dev. 28, 1461-1471. 10.1101/gad.243832.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matise M. P. and Joyner A. L. (1997). Expression patterns of developmental control genes in normal and Engrailed-1 mutant mouse spinal cord reveal early diversity in developing interneurons. J. Neurosci. 17, 7805-7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa S., Miwata K., Asashima M. and Michiue T. (2015). The requirement of histone modification by PRDM12 and Kdm4a for the development of pre-placodal ectoderm and neural crest in Xenopus. Dev. Biol. 399, 164-176. 10.1016/j.ydbio.2014.12.028 [DOI] [PubMed] [Google Scholar]
- Missler M. and Südhof T. C. (1998). Neurexophilins form a conserved family of neuropeptide-like glycoproteins. J. Neurosci. 18, 3630-3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy V., Cole T., Van Campenhout C., Khoung T. M., Leung C., Vermeiren S., Novatchkova M., Wenzel D., Cikes D., Polyansky A. A. et al. (2015). The evolutionarily conserved transcription factor PRDM12 controls sensory neuron development and pain perception. Cell Cycle 14, 1799-1808. 10.1080/15384101.2015.1036209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulion S., Bertrand S., Belgacem M. R., Le Petillon Y. and Escriva H. (2012). Sequencing and analysis of the Mediterranean amphioxus (Branchiostoma lanceolatum) transcriptome. PLoS ONE 7, e36554 10.1371/journal.pone.0036554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierani A., Brenner-Morton S., Chiang C. and Jessell T. M. (1999). A sonic hedgehog–independent, retinoid-activated pathway of neurogenesis in the ventral spinal cord. Cell 97, 903-915. 10.1016/S0092-8674(00)80802-8 [DOI] [PubMed] [Google Scholar]
- Pierani A., Moran-Rivard L., Sunshine M. J., Littman D. R., Goulding M. and Jessell T. M. (2001). Control of interneuron fate in the developing spinal cord by the progenitor homeodomain protein Dbx1. Neuron 29, 367-384. 10.1016/S0896-6273(01)00212-4 [DOI] [PubMed] [Google Scholar]
- Rungger-Brändle E., Ripperger J. A., Steiner K., Conti A., Stieger A., Soltanieh S. and Rungger D. (2010). Retinal patterning by Pax6-dependent cell adhesion molecules. Dev. Neurobiol. 70, 764-780. 10.1002/dneu.20816 [DOI] [PubMed] [Google Scholar]
- Sander M., Paydar S., Ericson J., Briscoe J., Berber E., German M., Jessell T. M. and Rubenstein J. L. (2000). Ventral neural patterning by Nkx homeobox genes: Nkx6.1 controls somatic motor neuron and ventral interneuron fates. Genes Dev. 14, 2134-2139. 10.1101/gad.820400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saueressig H., Burrill J. and Goulding M. (1999). Engrailed-1 and netrin-1 regulate axon pathfinding by association interneurons that project to motor neurons. Development 126, 4201-4212. [DOI] [PubMed] [Google Scholar]
- Siembab V. C., Smith C. A., Zagoraiou L., Berrocal M. C., Mentis G. Z. and Alvarez F. J. (2010). Target selection of proprioceptive and motor axon synapses on neonatal V1-derived Ia inhibitory interneurons and Renshaw cells. J. Comp. Neurol. 518, 4675-4701. 10.1002/cne.22441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. T. and Jaynes J. B. (1996). A conserved region of engrailed, shared among all en-, gsc-, Nk1-, Nk2- and msh-class homeoproteins, mediates active transcriptional repression in vivo. Development 122, 3141-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triezenberg S. J., Kingsbury R. C. and McKnight S. L. (1988). Functional dissection of VP16, the trans-activator of herpes simplex virus immediate early gene expression. Genes Dev. 2, 718-729. 10.1101/gad.2.6.718 [DOI] [PubMed] [Google Scholar]
- Vallstedt A., Muhr J., Pattyn A., Pierani A., Mendelsohn M., Sander M., Jessell T. M. and Ericson J. (2001). Different levels of repressor activity assign redundant and specific roles to Nkx6 genes in motor neuron and interneuron specification. Neuron 31, 743-755. 10.1016/S0896-6273(01)00412-3 [DOI] [PubMed] [Google Scholar]
- Vincent S. D., Mayeuf A., Niro C., Saitou M. and Buckingham M. (2012). Non conservation of function for the evolutionarily conserved prdm1 protein in the control of the slow twitch myogenic program in the mouse embryo. Mol. Biol. Evol. 29, 3181-3191. 10.1093/molbev/mss125 [DOI] [PubMed] [Google Scholar]
- Visel A., Minovitsky S., Dubchak I. and Pennacchio L. A. (2007). VISTA Enhancer Browser--a database of tissue-specific human enhancers. Nucleic Acids Res. 35, D88-D92. 10.1093/nar/gkl822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton N. M., de Koning A., Xie X., Shin R., Chen Q., Miyake S., Tajinda K., Gross A. K., Kogan J. H., Heusner C. L. et al. (2014). Gastrin-releasing peptide contributes to the regulation of adult hippocampal neurogenesis and neuronal development. Stem Cells 32, 2454-2466. 10.1002/stem.1740 [DOI] [PubMed] [Google Scholar]
- Wenner P., O'Donovan M. J. and Matise M. P. (2000). Topographical and physiological characterization of interneurons that express engrailed-1 in the embryonic chick spinal cord. J. Neurophysiol. 84, 2651-2657. [DOI] [PubMed] [Google Scholar]
- Yang C. M. and Shinkai Y. (2013). Prdm12 is induced by retinoic acid and exhibits anti-proliferative properties through the cell cycle modulation of P19 embryonic carcinoma cells. Cell Struct. Funct. 38, 195-204. 10.1247/csf.13010 [DOI] [PubMed] [Google Scholar]
- Zannino D. A., Downes G. B. and Sagerström C. G. (2014). prdm12b specifies the p1 progenitor domain and reveals a role for V1 interneurons in swim movements. Dev. Biol. 390, 247-260. 10.1016/j.ydbio.2014.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Lanuza G. M., Britz O., Wang Z., Siembab V. C., Zhang Y., Velasquez T., Alvarez F. J., Frank E. and Goulding M. (2014). V1 and v2b interneurons secure the alternating flexor-extensor motor activity mice require for limbed locomotion. Neuron 82, 138-150. 10.1016/j.neuron.2014.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]