Abstract
A leading cause of human birth defects is the incomplete fusion of tissues, often manifested in the palate, heart or neural tube. To investigate the molecular control of tissue fusion, embryonic dorsal closure and pupal thorax closure in Drosophila are useful experimental models. We find that Pvr mutants have defects in dorsal midline closure with incomplete amnioserosa internalization and epidermal zippering, as well as cardia bifida. These defects are relatively mild in comparison to those seen with other signaling mutants, such as in the JNK pathway, and we demonstrate that JNK signaling is not perturbed by altering Pvr receptor tyrosine kinase activity. Rather, modulation of Pvr levels in the ectoderm has an impact on PIP3 membrane accumulation, consistent with a link to PI3K signal transduction. Polarized PI3K activity influences protrusive activity from the epidermal leading edge and the protrusion area changes in accord with Pvr signaling intensity, providing a possible mechanism to explain Pvr mutant phenotypes. Tissue-specific rescue experiments indicate a partial requirement in epithelial tissue, but confirm the essential role of Pvr in hemocytes for embryonic survival. Taken together, we argue that inefficient removal of the internalizing amnioserosa tissue by mutant hemocytes coupled with impaired midline zippering of mutant epithelium creates a situation in some embryos whereby dorsal midline closure is incomplete. Based on these observations, we suggest that efferocytosis (corpse clearance) could contribute to proper tissue closure and thus might underlie some congenital birth defects.
KEY WORDS: Cell removal, Dorsal closure, Drosophila, Jun kinase, PIP3, Pvr, Receptor tyrosine kinase, Morphometric analysis, Thorax closure
Summary: During tissue closure in Drosophila, Pvr is required for efficient corpse clearance by hemocytes and for protrusive activity in the epidermis.
INTRODUCTION
Tissue closure is an essential developmental process to seal a hole or generate a contiguous tissue layer. Its common occurrence during embryogenesis underscores the prevalence of birth defects in the human population, such as cleft palate, spina bifida, ventral body wall and cardiac septum defects, involving incomplete or improper fusion (Brewer and Williams, 2004; Ray and Niswander, 2012). Injury-induced tissue closure, or wound healing, is also essential over a lifetime to repair damage and help repel infections (Martin and Parkhurst, 2004).
Two genetically tractable models of tissue closure that have contributed to our current understanding are dorsal closure and thorax closure in Drosophila. Dorsal closure of the embryo proceeds by the coordinated activities of a central squamous epithelium, the amnioserosa (AS), and surrounding dorsal epidermal epithelium (Kiehart et al., 2000). Cell contraction and limited apoptosis of the AS, along with epidermal cell stretching tightened by an actomyosin pursestring, together drive the dorsalward movement of the epidermal leading edges (LEs) (Blanchard et al., 2010; Fernández et al., 2007; Gorfinkiel et al., 2009; Solon et al., 2009; Toyama et al., 2008). Dynamic LE cell protrusions then facilitate zippering of the epithelium across the midline concomitant with AS internalization and degeneration (Jacinto et al., 2000, 2002; Kaltschmidt et al., 2002; Millard and Martin, 2008). Although dorsal closure is robust to mechanical perturbations due to distributed tension-based forces (Kiehart et al., 2000; Rodriguez-Diaz et al., 2008), mutations in many signaling, adhesion, cytoskeleton and trafficking proteins impact the speed and efficiency of the process (Harden, 2002; Heisenberg, 2009).
Thorax closure occurs shortly after the Drosophila larva pupariates to commence metamorphosis of the adult. A pair of imaginal discs, which are epithelial precursors to the adult wing and dorsal thorax (or notum), evert over the larval epidermis, displacing it as they spread from lateral positions toward the midline (Pastor-Pareja et al., 2004; Usui and Simpson, 2000). LE cells take on a mesenchymal character and exhibit extensive actin-based protrusions (Martin-Blanco et al., 2000). Within 8 h, midline fusion bridges the contralateral cell sheets into a continuous epithelium (Martin-Blanco et al., 2000; Usui and Simpson, 2000; Zeitlinger and Bohmann, 1999), and further remodeling occurs to form a mature epidermal barrier (Pastor-Pareja et al., 2004). Such dynamic reorganization of epithelial margin cells to promote or curtail motility highlights a similarity of Drosophila and vertebrate tissue closure, in which mesenchyme function and signaling with neighboring epithelia are major contributing factors (Heller et al., 2014; Smith and Tallquist, 2010). One key difference is that in Drosophila closure does not depend heavily on cell proliferation to fuel tissue outgrowth across large distances (Bush and Jiang, 2012). Despite this, the molecular pathways that orchestrate such large-scale tissue fusions are generally well conserved, reflecting fundamental strategies for animal morphogenesis.
Evidence from vertebrate studies indicates that Platelet-derived growth factor receptor (Pdgfr) signaling organizes cell behaviors that drive developmental closure processes in various organ systems and organisms (Bush and Jiang, 2012). For instance, zebrafish pdgfra directs morphogenesis of the craniofacial skeleton, and its activity is sensitive to genetic and environmental perturbations suspected in the etiology of mammalian clefting disorders (Eberhart et al., 2008; McCarthy et al., 2013). Similarly, mutations in murine Pdgfc ligand and Pdgfra receptor result in facial and palate clefts due to both reduced cell proliferation and inadequate migration of neural crest derivatives (Ding et al., 2004; He and Soriano, 2013; Smith and Tallquist, 2010). A human PDGFC noncoding polymorphism has also been associated with cleft lip/palate (Choi et al., 2009), signifying the importance of these growth factors and cognate receptors for proper tissue closure. Although Pdgf receptor tyrosine kinases signal through multiple molecular pathways, namely the Mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K) pathways (Heldin and Westermark, 1999; Hoch and Soriano, 2003; Tallquist and Kazlauskas, 2004), it is the loss of PI3K-mediated signaling downstream of Pdgfrα that primarily accounts for such phenotypes in the mouse and zebrafish mutants (Klinghoffer et al., 2002; McCarthy et al., 2013; Pickett et al., 2008).
In Drosophila, receptor tyrosine kinase isoforms resembling vertebrate Pdgfr, displaying extracellular Ig domains and a split tyrosine kinase domain intracellularly, are encoded by the Pvr (PDGF- and VEGF-receptor related) gene (Cho et al., 2002; Duchek et al., 2001; Heino et al., 2001). Pvr knockdown has been shown to impair thorax closure, suggesting an evolutionary conservation of function in tissue closure; in this context, Jun kinase (JNK) signaling is thought to mediate this effect (Ishimaru et al., 2004). Numerous studies have shown that JNK signaling is a key modulator of embryonic dorsal closure (Glise et al., 1995; Riesgo-Escovar et al., 1996; Sluss et al., 1996), but curiously the trigger for its activation in the embryo has been elusive. Together, these observations spurred us to test directly the requirement for Pvr and its three ligands, Pvf1-3 (Duchek et al., 2001), in dorsal closure as upstream activators of JNK signaling. Here, we describe a role for Pvr in embryonic dorsal closure and investigate the cause of closure defects, the consequence for JNK signaling, and the cellular effects on thorax closure. Moreover, we implicate Pvf1 as a relevant ligand in these processes.
RESULTS
Pvr is required for the completion of dorsal closure
Pvr signaling is implicated in the process of thorax closure during metamorphosis (Ishimaru et al., 2004). We hypothesized that Pvr could play a role in embryonic dorsal closure as well. To test this, the phenotypic consequences of embryonic lethal Pvr null mutations were determined, paying specific attention to the dorsal aspect of the secreted cuticle, revealing the fidelity of the closure process. Cuticle preparations from Pvr zygotic or germline clone (GLC) mutant embryos displayed mild dorsal midline defects centered around the last segment to close (Fig. 1A-F). Phenotypes varied from wild type, midline pinching and scabs, to dorsal holes with no more than four segments unfused (Fig. 1G). Head involution defects were rare. Although the cuticle phenotypes of Pvr mutant embryos were relatively mild, they indicate an undescribed role for Pvr during embryogenesis.
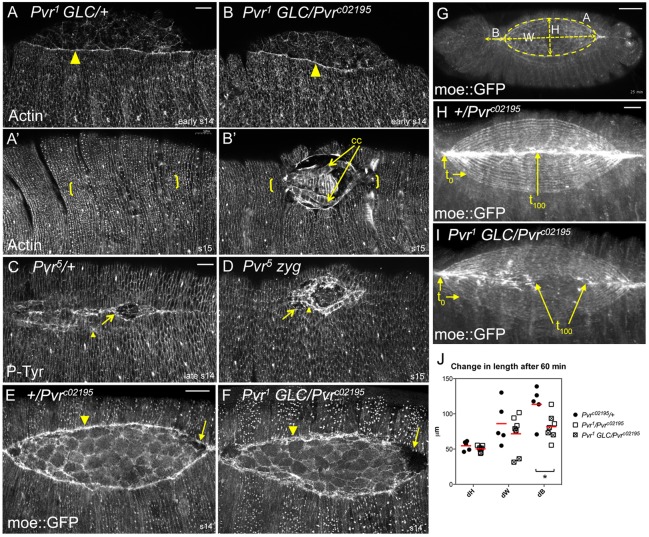
Fig. 1.
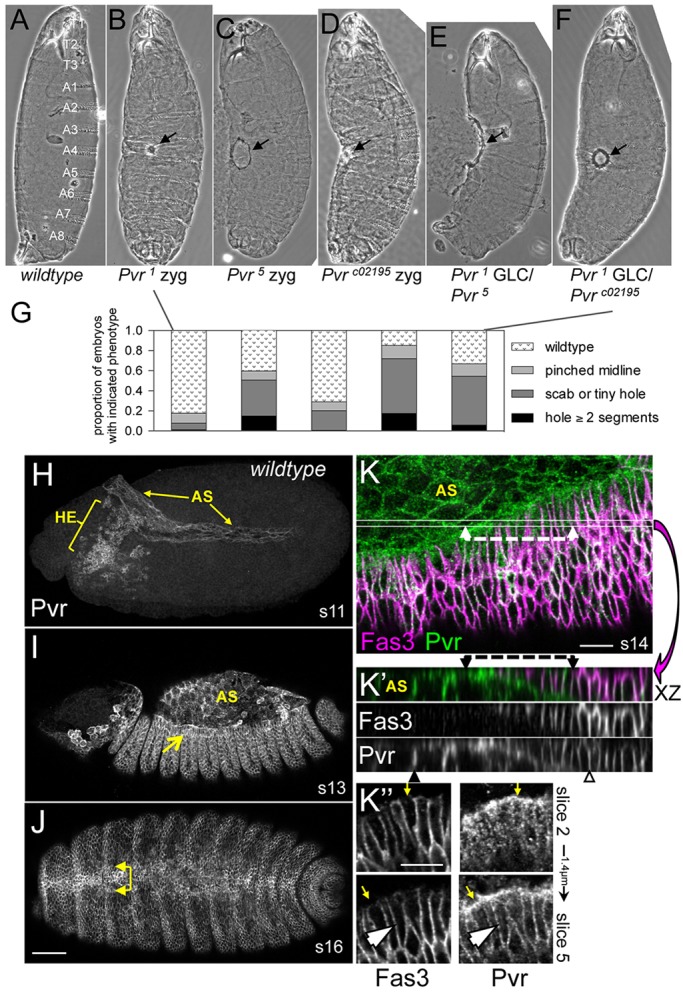
Embryonic closure phenotypes and protein expression of Pvr. (A-F) Larval cuticles. Wild type, with the segments indicated (A), is compared with Pvr zygotic (B-D) or germline clone (GLC) (E,F) mutants displaying midline scabs or holes (arrows). (G) Quantification of phenotypes in B-F (n=124-310). (H-K″) Pvr immunostaining in wild-type embryos, without (H-J) or with (K-K″) Fas3 colocalization. Germband extended (H), early dorsal closure (I) and post-closure (J) embryos depict Pvr expression in hemocytes (HE), amnioserosa (AS) and leading edge (LE) of the dorsal ectoderm (arrows, I,J). (K) Confocal z-projection of dorsal quadrant from mid-closure embryo. Thin lines demarcate the position of the x/z projection beneath (K′); single channels are also shown. Dashed lines highlight Pvr localization at the interface between LE and underlying AS. Note Pvr at lateral membranes of AS (black arrowhead). Ectoderm has extensive colocalization of markers (open arrowhead). Planar polarized enrichment of Pvr is seen in LE cells compared with Fas3 (K″, arrows). Lateral membrane colocalization is seen in the deeper section (arrowhead). Scale bars: 50 μm in H-J; 10 μm in K-K″.
Pvr protein expression in the AS and ectoderm
The expression and requirement of Pvr in embryonic hemocytes have been described (Brückner et al., 2004; Cho et al., 2002), but, in addition, we detected Pvr protein in the AS and ectoderm, two tissues orchestrating the closure process (Fig. 1H-K). Staining in the AS developed during stage 10/11 when this tissue is flattened between the extended germband and Pvr-expressing hemocytes are migrating out of the head (Fig. 1H). During stage 12, Pvr was detected in the ectoderm. Strong expression continued in these tissues throughout the rest of embryogenesis (Fig. 1I,J). Upon closure, dorsal midline cells remained enriched for Pvr (Fig. 1J).
Surface (Fig. 1K,K″) and transverse (Fig. 1K′) views highlighted Pvr along the lateral membranes of ectodermal epithelial cells, similar to Fasciclin 3 (Fas3). Laterally localized Pvr was also prominent in AS cells lacking Fas3. z-projection also revealed Pvr enrichment at the adhesive interface between the ectodermal LE and underlying peripheral AS cells (Fig. 1K,K′) (Wada et al., 2007). Notably, single confocal sections of the LE showed marked planar polarization of Pvr at the ‘free’ edge excluding Fas3 (Fig. 1K″), where LE cells take on a mesenchymal-like phenotype with extensive cellular projections (Bahri et al., 2010). In apical surface sections, Pvr had a punctate appearance suggesting receptor trafficking in endo/exocytic vesicles (Fig. 1K″). Antibody specificity was confirmed by comparisons among wild-type, heterozygous and homozygous Pvr mutant embryos in which the fluorescence intensity correlated directly with wild-type gene copy number (supplementary material Fig. S1).
Late defects at the completion of dorsal closure in Pvr mutant embryos
The cuticle defects in Pvr mutants suggested a requirement at late stages; however, this could be secondary to delayed or inefficient progression of closure. To determine the cause of the midline defects, we evaluated molecular markers and dynamic parameters in fixed and live embryos. Phalloidin staining for filamentous actin (F-actin) or phosphotyrosine immunostaining in fixed embryos showed no difference in the ability of Pvr mutants to fortify a prominent actin pursestring at the LE or to assemble and maintain cellular adherens junctions throughout the ectoderm and AS (Fig. 2A-D). Time-lapse imaging of live embryos expressing a green fluorescent protein (GFP)-tagged moesin actin-binding domain, moe::GFP (Kiehart et al., 2000), revealed similar deployment of the actomyosin cytoskeleton and dynamic AS behavior (Fig. 2E,F; supplementary material Movie 1). In fact, measurements of the width and height of the eye-shaped opening and the area of the exposed AS in projected 2D images over time demonstrated that closure of Pvr mutant embryos was nearly indistinguishable from that of control heterozygotes (Fig. 2G,J; supplementary material Fig. S2), arguing against inefficient closure as the basis for the late defects. The velocity of the LEs, defined as dH/dt averaged over the duration of closure, was 14±4 and 13±4 nm/s for Pvr heterozygous and homozygous mutant embryos, respectively, similar to published velocities (Hutson et al., 2003; Wells et al., 2014). Time-lapse imaging did however suggest faltering of zipping in some embryos, which was appreciated by projecting time series data as 2D images (Fig. 2H,I). Consistent with this, quantification of the change in length over time between the anterior canthus and cephalic dorsal ridge (dB) demonstrated shorter mean lengths in the mutants (Fig. 2I,J). We also noted that the plane of the zipping canthi was typically deeper than the rest of the LE (Fig. 2E,F), a pattern observed in other mutants (Nowotarski et al., 2014) but of unknown cause. Finally, cell adhesion between the AS and epidermis was typically maintained as closure proceeded, although in some specimens the LE pursestring was less uniform and tissues detached as final segments approached the midline, presumably yielding those cuticles with a hole or scab.
Fig. 2.
Dorsal closure progression in Pvr mutants. (A-D) F-actin or phosphotyrosine distribution in mid- and late-stage heterozygous (A,C) or homozygous (B,D) Pvr mutant embryos. LE actin (arrowhead) accumulates normally in the mutant (B). At late stages, note the midline (bracketed) defects and unapposed cardiac cells (cc) in mutants (B′,D) compared with controls (A′,C). Cardiac cells (arrowhead), which normally trail the LE (arrow, C), were exposed in unclosed Pvr5 mutants (D). (E-I) moe::GFP localization in live embryos. LE pursestring (arrowhead, E,F) is less uniform and zipping canthi are in different focal planes in Pvr mutants (arrows, E,F). (G) Single image from time-lapse movie of dorsal closure illustrating the values quantified in J (see Materials and methods). (H,I) Cumulative projections of 20 frames from time-lapse movies of Pvr heterozygote (H) or GLC mutant (I). Contours show 5-min intervals. Control embryo has closed by t100, but the mutant has stalled. (J) Scatter plot comparing change in length of the indicated parameters after 60 min; red bars show mean value. t0 was set when AS height (H) equaled 85±4 μm. dB was significantly different between heterozygous control and Pvr mutants. *P<0.05 (unpaired t-test). n=5 control, n=7 mutant embryos. Scale bars: 20 μm, except 50 μm in G.
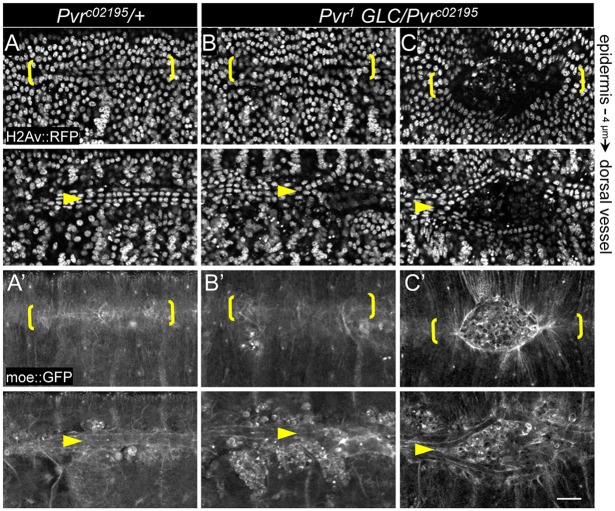
Another terminal phenotype in Pvr mutants was a split dorsal vessel, or cardia bifida (Chartier et al., 2002), observed in 6% of heterozygotes (n=16) versus 65% of homozygous mutants (n=37). Single rows of cardiac cells remained adherent with their lateral neighbors, but failed to coalesce across the midline to form the heart tube (Fig. 2B′,D and Fig. 3). The position and extent of the unfused dorsal vessel corresponded precisely with the overlying epidermal hole, but, even in embryos that appeared closed, incomplete vessel apposition was sometimes evident in deeper focal planes (Fig. 3B). Incidentally, we have encountered dorsal open embryos of unrelated genotypes without cardia bifida (supplementary material Fig. S3A), implying that closure of the different tissues is not absolutely interdependent, a conclusion corroborated by a recent study (Haack et al., 2014). Thus, the cardia bifida phenotype described here might reflect a direct requirement for Pvr and is likely to underlie a reported malfunction in heart pumping mechanics in late-stage Pvr mutant embryos (Wu and Sato, 2008).
Fig. 3.
Cardia bifida in Pvr mutants. (A-C) Single confocal sections of epidermis or dorsal vessel showing the indicated fluorescent markers in live embryos. Late-stage heterozygotes (A,A′) have closed (midline bracketed) and cardiac cells have coalesced underneath in an aligned double row (arrowheads). Pvr mutants have mild (dorsal vessel only, B,B′) or moderate (C,C′) ectodermal hole and split vessel phenotypes. Scale bar: 20 μm.
Often, Pvr mutants displayed cells and/or debris within the unclosed hole (Fig. 3C) or outside the epidermis (supplementary material Fig. S3B). In wild-type embryos, concurrent with dorsal vessel and epidermal closure, the AS becomes internalized, degenerates, and disappears rapidly through hemocyte phagocytosis (Reed et al., 2004; Rugendorff et al., 1994). Presumably, the AS has to get out of the way before converging tissues meet at the midline, but inefficient hemocyte phagocytosis might result in corpses or debris obstructing the fusing tissues. Hemocytes also secrete extracellular matrix proteins required for embryonic morphogenesis (Bunt et al., 2010; Olofsson and Page, 2005). Together, impaired hemocyte survival and migration, two characteristic phenotypes of Pvr mutants (Brückner et al., 2004; Cho et al., 2002), could contribute to both decreased removal of cell corpses and compromised integrity of tissues fusing at the midline.
Pvr function in multiple tissues accounts for embryonic viability and completion of closure
To determine the tissue requirements for Pvr in proper dorsal closure, tissue-specific rescue experiments were conducted using various Gal4 drivers to induce wild-type receptor in a Pvr mutant background. Rescue was assessed by quantifying the lethal fraction of embryos and the penetrance of cuticle phenotypes compared with controls without either the rescue transgene or the driver. Both control crosses resulted in an embryonic lethal fraction of one-quarter, the expected Mendelian frequency for homozygous recessive Pvr mutants (Fig. 4A). Expression of Pvr in epithelial tissues, such as the ectoderm, AS and endoderm, with 69B-G4 significantly reduced embryonic lethality. By contrast, restricted expression of Pvr in the AS using LP1-G4 provided no rescue, and the majority of cuticles had obvious dorsal defects (Fig. 4A). These results suggest that embryonic survival requires Pvr expression in both dorsal ectoderm and AS, or in the ectoderm alone. We confirmed transgene expression and the effects on different tissues by immunostaining for Pvr and Fas3, which showed that transgenic Pvr protein exceeded the levels of endogenous protein (Fig. 4B-E). Interestingly, we found that high-level expression of Pvr in the AS (with LP1-G4 or 69B-G4) led to abnormal upregulation of Fas3 (Fig. 4C; supplementary material Fig. S4). Coincidentally, remnants of AS tissue were left behind at the midline after closure (supplementary material Fig. S4), suggesting that Pvr could modulate the cell adhesion machinery.
Fig. 4.
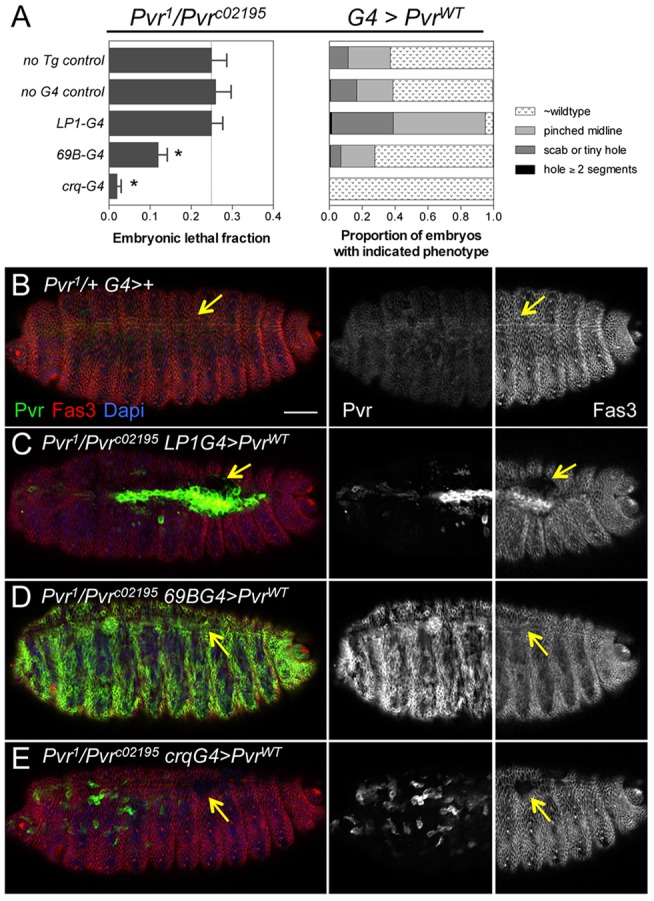
Complementation of Pvr in embryonic hemocytes rescues lethality. (A) Quantification of embryonic lethal fraction (left) and cuticle phenotypes (right) from tissue-specific rescue crosses. The lethal fraction was significantly reduced by expression of Pvr in ectodermal derivatives (69B-G4) or hemocytes (crq-G4). Bars show mean±s.d. *P<0.0001 (Chi-square test). n=269-394. Line indicates the expected fraction (0.25) for homozygous recessive embryonic lethal mutation. (B-E) Pvr immunostaining detects endogenous and overexpressed protein in merged and single-channel images (embryo anterior). Fas3 is shown in merged and righthand panels (embryo posterior). Arrows point to the dorsal midline, which is closed in similarly staged control (B) and 69B-G4 (D) embryos, but open in LP1-G4 (C) and crq-G4 (E) embryos. Scale bar: 20 μm.
With the crq-G4 driver directing Pvr expression in the hemocytes as early as stage 11, the fraction of lethal embryos was dramatically reduced and the hemocyte survival and migration phenotypes were rescued (Fig. 4A,E; supplementary material Fig. S5). Despite this, Fas3 staining revealed remaining dorsal midline defects, including lack of epidermal fusion and cardia bifida, as compared with similarly staged heterozygous embryos (Fig. 4E). Therefore, the embryonic lethality of Pvr mutants is largely attributable to impaired hemocyte functions; however, complementation of Pvr in epithelial derivatives excluding the hemocytes (using 69B-G4) promoted survival to a small degree, with demonstrable rescue of the midline closure defects. Altogether, we conclude that there is a dual tissue requirement for endogenous Pvr in the hemocytes and ectoderm to allow embryonic survival and proper tissue closure.
Pvr signals independently of the JNK pathway during dorsal closure
Constitutively active Pvr is reportedly sufficient to induce JNK target gene expression in the embryo (Ishimaru et al., 2004), predicting that Pvr mutations might diminish JNK signaling, causing dorsal closure defects. Yet our observations revealed that the phenotype of Pvr mutants was comparatively mild. Thus, we tested directly whether Pvr activity is necessary or sufficient for induction of JNK signaling by systematically evaluating a transcriptional reporter for JNK pathway activity in Pvr mutant and overexpression contexts. In control embryos, the puc-lacZ reporter was prominently expressed in the most dorsal epidermal cells surrounding the AS tissue (Fig. 5A). As positive controls, dorsal overexpression of wild-type or kinase-dead versions of the JNK kinase kinase Slpr led to ectopic induction or inhibition of puc-lacZ, respectively (Fig. 5B,C,H). By contrast, puc-lacZ expression was unaltered in embryos similarly expressing wild-type or constitutively active Pvr (λPvr) (Fig. 5D,E,H), and in embryos with Pvr expression or activity depleted by RNA interference (RNAi), dominant-negative inhibition, or GLC mutation (Fig. 5F-H; supplementary material Fig. S6). Collectively, these results demonstrate that Pvr signaling does not directly regulate JNK signaling in the embryonic dorsal ectoderm.
Fig. 5.
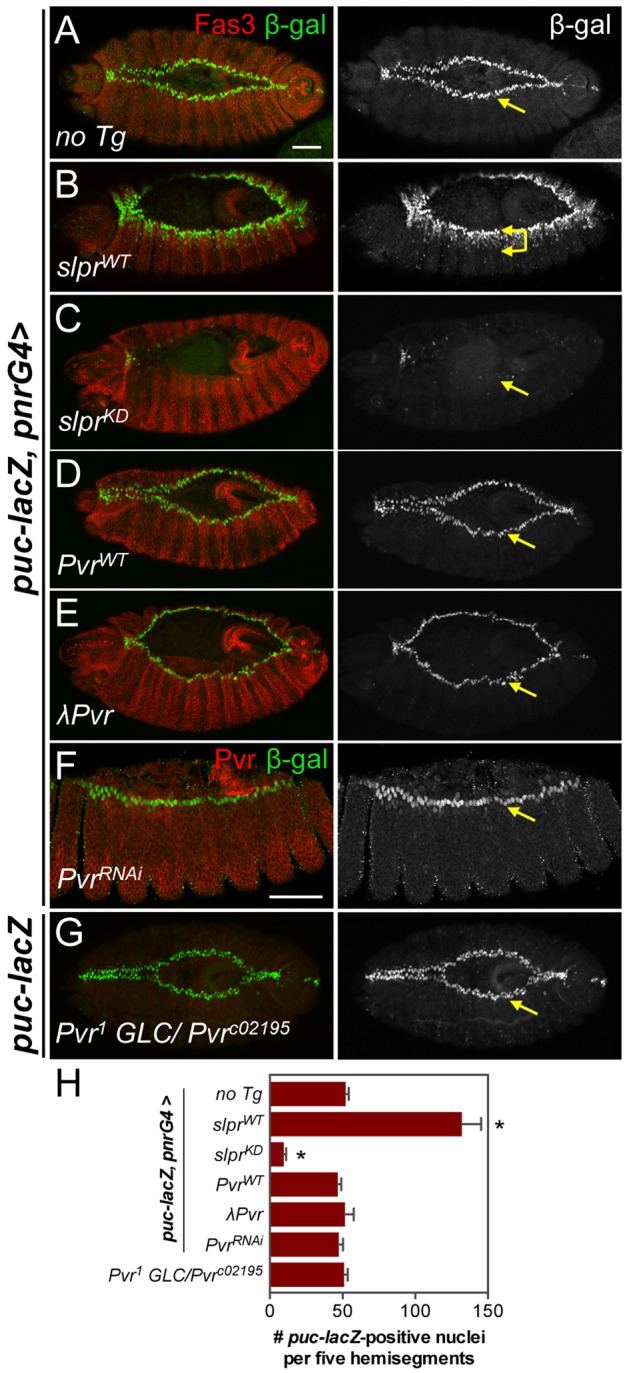
JNK signaling is unaffected by changes in Pvr activity. (A-G) Immunostaining of mid-stage dorsal closure embryos for Fas3 (or Pvr) and β-gal protein from the JNK transcriptional reporter puc-lacZ at the LE (arrows). pnr-G4 directs transgene expression in the dorsal ectoderm. Slpr overexpression induced ectopic puc-lacZ (B), whereas kinase-dead Slpr blocked it (C). Overexpression of wild-type (D) or constitutively active (E) Pvr had no effect on puc-lacZ. Similarly, reporter expression was normal upon depletion of Pvr by RNAi (F) or in GLC mutants (G), as confirmed by anti-Pvr co-immunostaining. Scale bars: 50 μm. (H) Quantification of β-gal-positive nuclei, n≥4 embryos. Bars show mean±s.d. *P<0.05 (one-way ANOVA, with Bonferroni post-hoc test) for comparison with ‘no Tg’ control.
To reconcile these results with previous studies, we considered the effects of excessive Pvr activation over a prolonged period of λPvr overexpression. In late-stage embryos specifically, we counted the same or slightly increased numbers of puc-lacZ-positive cells, but they were disorganized, reflecting a striking alteration of cell and embryo morphology including induction of Matrix metalloprotease 1 (Mmp1) expression (supplementary material Fig. S7). Under these circumstances, the apparent increase in JNK target gene expression is likely to have resulted from effects secondary to epithelial disorganization, as described previously for imaginal epithelial tissues (Rosin et al., 2004).
Pvr is necessary and sufficient for maximal PI3K pathway signaling
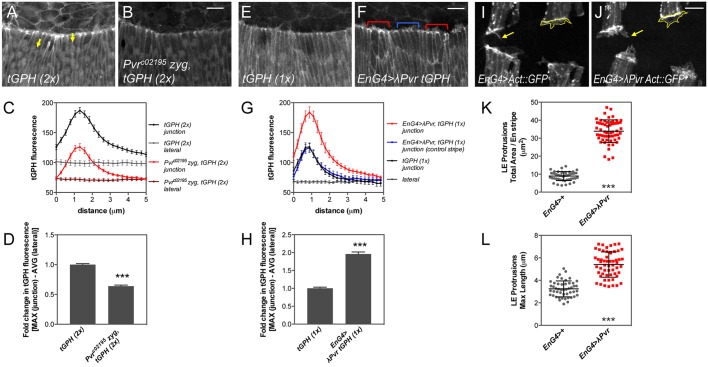
Given the distinct embryonic phenotypes and lack of impact on JNK signaling during dorsal closure, we considered whether Pvr, like its mammalian counterparts, could regulate PI3K signaling, using a fluorescent reporter to monitor production of phosphatidylinositol (3,4,5)-trisphosphate (PIP3). tGPH encodes GFP fused to a PIP3-binding PH domain, which relocates to the plasma membrane in response to PIP3 production, reflecting a bias in the opposing enzymatic activities of PI3K and the phosphatase Pten (Britton et al., 2002). During dorsal closure, PIP3 distribution is polarized at the LE interface, driving actin-based protrusive activity to facilitate midline zippering (Pickering et al., 2013). We confirmed this localization and quantified tGPH fluorescence at the LE junctions versus lateral membranes in live wild-type or Pvr mutant embryos. Mutants had significantly reduced (by 36%) fluorescence intensity relative to the control, although the overall profile of junctional enrichment was preserved (Fig. 6A-D).
Fig. 6.
Pvr is required for maximal PIP3 accumulation at the LE. (A,B,E,F) Confocal z-projections of the LE of mid-dorsal closure stage live embryos expressing the PIP3 reporter tGPH. Controls with two (A) or one (E) copy of tGPH compared with Pvr mutant (B) or constitutively active Pvr expression in segmental stripes with en-G4 (red bracket) versus control stripe (blue bracket). (C,G) Mean tGPH fluorescence along 5 μm spanning the LE cell junction enriched for PIP3 or lateral membrane control (yellow arrows, A). Error bars represent s.e.m. n=24-28 junctions, 6-7 embryos (C); n=60 junctions, 15 embryos (G). Line color (G) matches bracketed stripes in F. (D,H) Fold change in maximum tGPH fluorescence between control and experimental genotypes, normalized to corresponding averaged lateral membrane values. Error bars show s.e.m. (I,J) en-G4 driving GFP-labeled Actin to reveal LE protrusions (outlines, arrows), without (I) or with (J) λPvr co-expression. (K,L) Quantification of LE protrusion area and length, respectively. Mean±s.d. n=4 stripes, two time points, 6-7 embryos. ***P<0.0001 (unpaired t-test). Scale bars: 10 μm.
Next, we expressed λPvr in segmental stripes of cells using the en-Gal4 driver and measured tGPH fluorescence. In these embryos, the reporter was strongly recruited to the membranes of cells in the expressing stripes, while the adjacent non-expressing stripes showed a profile equivalent to that of control embryos (Fig. 6E-G; supplementary material Fig. S8). Quantification revealed a two-fold increase in fluorescence at the LE junctions upon λPvr expression (Fig. 6H). In keeping with the role of elevated PIP3 in regulating dynamic protrusive activity, large protrusions were observed to emanate from LE and AS cells with λPvr overexpression (Fig. 6I,J; supplementary material Fig. S8A-D). The total area and maximum length of LE projections from cells co-expressing λPvr and GFP-labeled actin were significantly enhanced relative to wild type (Fig. 6K,L). Collectively, these data suggest that Pvr function is necessary for maximal PIP3 enrichment at the membrane and sufficient for protrusions, but is not necessary for the polarized accumulation of PIP3 at the LE junctions.
These results point to a signaling mechanism linking Pvr to PI3K in the epidermis. To test this model further, we expressed wild-type PI3K in Pvr mutant embryos using the 69B-Gal4 driver. Although there was no effect on embryonic lethality, boosting PI3K levels increased the fraction of wild-type cuticles by 10% relative to the control, comparable to expression of PvrWT (supplementary material Fig. S8E). Moreover, adult progeny from these crosses revealed dominant genetic suppression of a PI3K-induced phenotype by heterozygosity of Pvr (supplementary material Fig. S8F). Thus, mechanistically, the influence of Pvr on the PI3K pathway could account in part for the ectodermal requirement during closure.
Synthetic lethal interaction between the JNK pathway and Pvf1
Although our observations demonstrate that Pvr does not directly signal through JNK in embryonic dorsal closure, published studies do provide evidence that a Pvr ligand, Pvf1, and JNK signaling participate in some of the same developmental processes. For example, Pvf1 null mutants are adult viable, but mutant males show morphological defects of the external genitalia (Macias et al., 2004), a phenotype seen in slprBS06 zygotic mutant males, in which JNK signaling is impaired (Polaski et al., 2006). Pvf1/Pvr and JNK signaling are also required for wound healing, regulating distinct aspects of the process (Brock et al., 2012; Wu et al., 2009).
To test whether Pvf1 might also participate in embryonic dorsal closure along with Pvr and JNK, we generated slpr, Pvf1 double mutants and discovered a synthetic lethal genetic interaction (Table 1) resulting in fully penetrant embryonic lethality and failure of dorsal closure, comparable to slpr GLC mutants (Fig. 7). The lethal phase and phenotype of a maternal effect viable JNKK [hemipterous (hep) – FlyBase] allele, hep1, alone or with Pvf1, were also compared, revealing similar synthetic lethality (Table 1, Fig. 7A). Given possible redundancy among Pvf ligands, the slprBS06 mutation was combined with alleles of Pvf2 and Pvf3. Although the lethality associated with Pvf2 was partially suppressed by simultaneous loss of slpr in the double mutants, neither of the ligands enhanced the lethality or cuticle phenotype of the slpr mutant, in contrast to Pvf1 (Fig. 7A). These data thereby support the idea that Pvf1 is a candidate for the activation of embryonic Pvr and that this activity, in connection with JNK signaling, is required for proper dorsal closure.
Table 1.
Synthetic lethal genetic interaction between Pvf1 and JNK pathway mutants
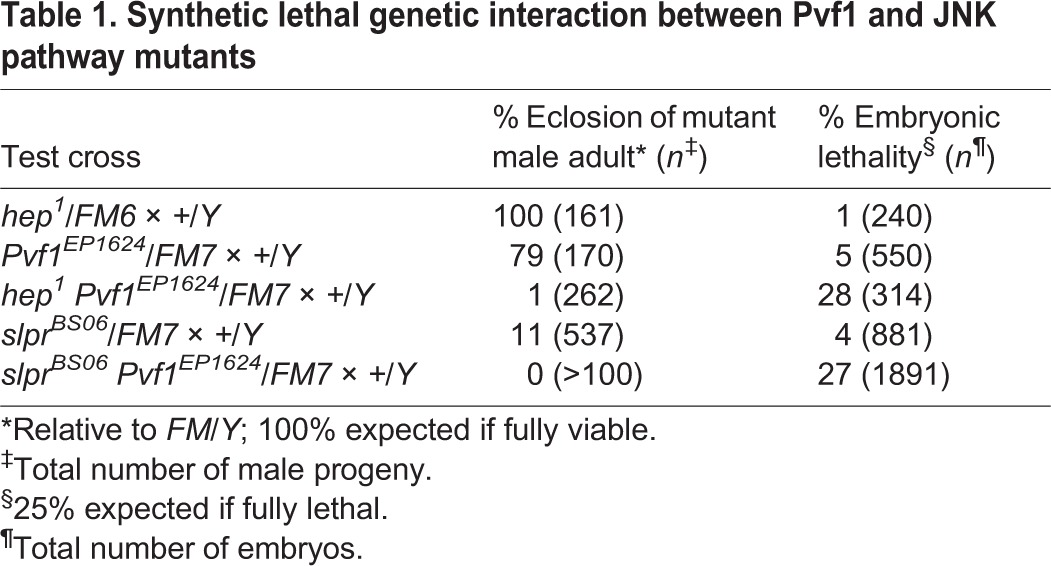
Fig. 7.
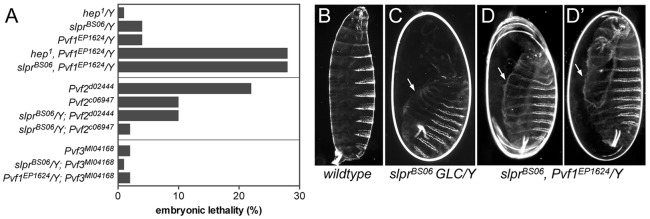
Specificity of synthetic lethal interaction between Pvf1 and the JNK pathway. (A) Quantification of percent embryonic lethality of single versus double mutants. (B-D′) Darkfield images of cuticles from control (B) and mutant embryos with large dorsal holes (arrows, C-D′). D and D′ show two examples of the same genotype.
Notum morphology reveals opposing effects of Pvr and JNK signaling on thorax closure
We showed previously that modulating the levels of JNK signaling during thorax closure leads to concomitant changes in midline notum morphology in the adult (Garlena et al., 2010). Underlying these morphological changes is the participation of JNK in regulating cell adhesion, shape, polarity and motility during spreading of the imaginal cell sheets toward the midline (Martin-Blanco et al., 2000; Pastor-Pareja et al., 2004; Zeitlinger and Bohmann, 1999). Ishimaru et al. (2004) described thorax closure defects arising from knockdown of Pvr and, based on genetic interactions and reduced puc-lacZ expression, proposed that the phenotypes were due to loss of JNK signaling. Since we found no direct dependence of JNK signaling on Pvr in the embryo, we re-evaluated their relationship in thorax closure using a geometric morphometric strategy with adult phenotypes (Fig. 8) and cell biological observations of pupal development (Figs 9 and 10).
Fig. 8.
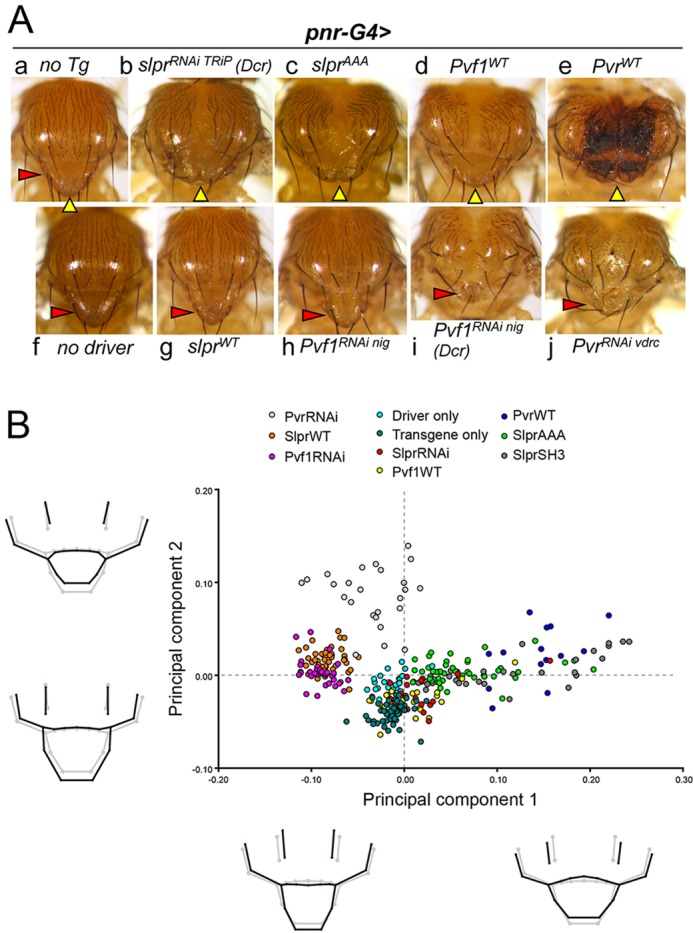
JNK and Pvr signaling during thorax closure cause reciprocal changes in adult notum morphology. (A) Examples of notum morphology of control groups (a,f) or adults with pnr-G4-directed transgene expression (b-e,g-j). Yellow arrowheads indicate the dorsal midline, red arrowheads point to the scutellum. (B) Principal components analysis (PCA) of group shapes from expression of the indicated transgenes driven by pnr-G4. Individuals are represented graphically as points. Group differences are depicted as deformations of landmark positions in a wireframe diagram (black) corresponding to position along the adjacent PC axis, as compared with the mean shape from all groups (gray).
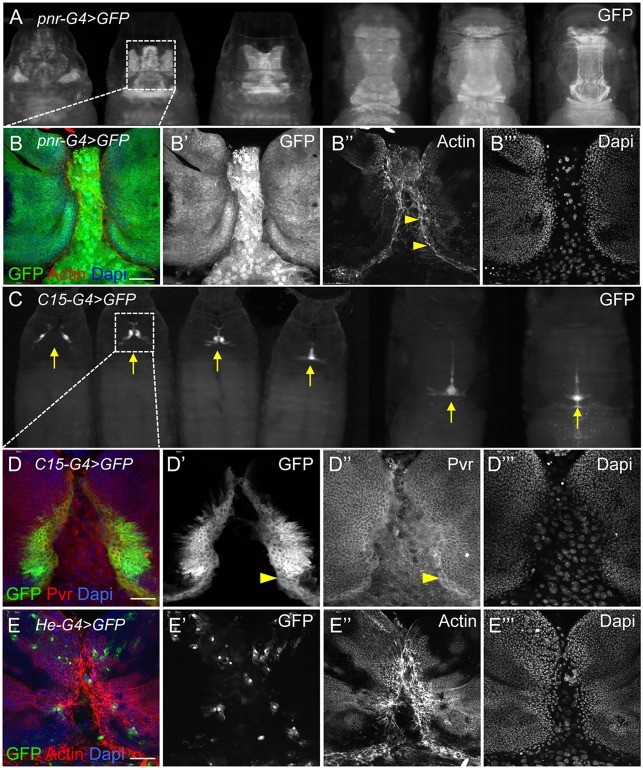
Fig. 9.
Pvr expression at the LE of spreading imaginal discs during thorax closure. (A,C) GFP expression with pnr-G4 (A) or C15-G4 (C) drivers in progressively older live pupae reveals apposition and fusion of notum tissue at the midline (arrows) during thorax closure. Boxes indicate regions shown as dissected, fixed tissues in panels beneath. (B-B‴) pnr drives expression in discs (small packed nuclei) in a broad proximal domain and in the intervening larval epidermal tissue (large nuclei). DAPI staining shows nuclei (blue). F-actin is enriched at the LE margins (arrowheads). (D-D‴) GFP expression in the C15 domain is restricted to a narrow region of the disc margins. Endogenous Pvr protein is enriched in mesenchymal-like marginal cells (arrowheads). (E-E‴) For comparison, the He-G4 driver directs GFP expression in hemocytes infiltrating the tissues. Actin protrusions are prominent, knitting together disc margins. Scale bars: 50 μm.
Fig. 10.
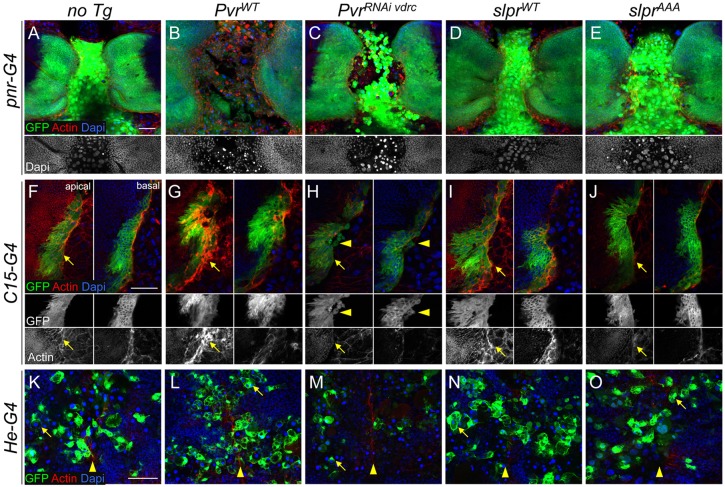
Differential effects of Pvr on tissues during thorax closure. (A-O) Confocal images of fixed pupal tissues during thorax closure with the indicated markers. (A-E) pnr-G4-directed GFP expression without (A) or with (B-E) transgene co-expression. Altering Pvr levels has a dramatic effect on centrally positioned larval cells. The grayscale DAPI channel (beneath) highlights pyknotic nuclei (B,C) versus the control (A) or slpr-expressing (D,E) samples. (F-J) Single apical and basal sections of pupal wing discs expressing GFP and transgenes with C15-G4. The midline is toward the right in merged and single-channel images (beneath). F-actin at the leading front (arrows) is highly enriched upon Pvr overexpression (G), and blunted by Pvr knockdown (H) or Slpr inhibition (J). Slpr overexpression has a mild enhancing effect on actin levels (I). Arrowheads identify round stray cells depleted for Pvr and leaving the leading edge (H), whereas cells with increased Pvr are flat and spreading (G). (K-O) Confocal projection of circulating hemocytes labeled by He-G4-directed GFP expression 12 μm below the notum epithelium, which has closed at the midline (arrowheads). Internalized larval cells with pyknotic nuclei are seen engulfed inside hemocytes (arrows). PvrRNAi depletes hemocyte numbers. Scale bars: 50 μm.
Geometric morphometrics is well suited to account for shape variation among individuals in a population, separating or clustering groups with statistical power. To investigate how notum shape correlates with signaling intensity, we systematically perturbed slpr-dependent JNK signaling, or Pvr signaling through knockdown or overexpression constructs within the pnr-Gal4 domain, encompassing a broad region spanning the dorsal midline. Altered signaling of these two pathways resulted in reproducible phenotypes with some superficial similarities (Fig. 8A, compare b-e with g-j). After digitizing landmarks on images of specimens of different genotypes (supplementary material Fig. S9A, Table S1), the resulting coordinates were characterized using principal components analysis (PCA) to quantify the major modes of shape variation. The two pathways were first analyzed individually (supplementary material Fig. S9B,C).
Analysis of all genotypes together revealed an overlap of group clusters, with the notable outcome that increased signaling in one pathway phenocopied reduced signaling in the other (Fig. 8B), suggesting a reciprocal relationship in thorax closure. For instance, SlprWT and Pvf1RNAi individuals grouped together on the negative side of the PC1 axis, reflecting shape variation characterized by longer, narrower notum morphology, as depicted beneath in the wireframe diagram (Fig. 8B). PvrRNAi also distributed on this side but separated from the former groups and controls by differences in PC2, where lateral landmarks were wider and the scutellum was notably reduced. Substantial overlap of groups along the positive PC1 axis correlated with widening of all landmarks and a slight anterior shift in the scutellum. These shapes arose from reduced JNK activity using dominant interfering proteins (SlprAAA, SlprSH3) or from elevated Pvr signaling (Fig. 8B). Together, PC1 and PC2 described nearly 71% of the total shape variation.
In light of these results, we next asked which cell behaviors induced during the pupal stages might account for the morphological phenotypes in adults. Several drivers were used to express GFP and experimental constructs in particular tissue regions. The pnr-Gal4 pattern evolved from expression in the proximal part of the larval wing discs that prefigure the adult notum and in the larval dorsal epidermis upon which the disc cells migrate toward the midline, to a broad dorsal stripe overlapping the midline of the scutum and entire scutellum (Fig. 9A,B). The domain of a second driver, C15-Gal4, was restricted more proximally in the wing discs and delimited the margins of the fusing tissues in pupae, making it an excellent marker of thorax closure (Fig. 9C). Confocal imaging further showed GFP expression in ∼2-8 cells at the leading front of the pupal disc during closure, but not in the underlying larval epithelium, in contrast to pnr-Gal4 (Fig. 9, compare B with D). LE cells, distinguished by slightly larger nuclei, were strikingly enriched for Pvr protein, above the uniform levels present in the rest of the disc epithelium and larval epidermis (Fig. 9D,D″). Although the narrower expression domain of C15-Gal4 facilitated the best view of the leading front during closure, the ultimate effects on adult morphology were mild and limited to the scutellum (not shown). A third reagent, He-Gal4, was used to drive expression of constructs in larval hemocytes that pervade the tissues during closure (Fig. 9E), analogous to our use of crq-Gal4 in the embryo.
The most striking tissue-level phenotypes during thorax closure were the effects of Pvr signaling on the integrity of the larval epidermis specific to pnr-Gal4 (Fig. 10B,C) and the accumulation of F-actin and changes in cell morphology at the tissue margins visualized clearly with C15-Gal4 (Fig. 10G,H). Altering Pvr activity in the pnr domain led to dissolution of the larval epithelium and fragility of the specimens during dissection (Fig. 10B,C), phenotypes rarely encountered with perturbation of JNK signaling (Fig. 10D,E). With Pvr knockdown, larval cells rounded up and nuclei condensed, reminiscent of apoptosis (Fig. 10C). These cells persisted at the midline without being engulfed by hemocytes, which are normally present during closure as seen with He-Gal4>GFP (Fig. 10K-O). As such, the adult notum possessed occasional midline scabs (Fig. 8Aj), recalling the phenotype of Pvr mutant embryos. In the C15 domain, Pvr-depleted margin cells also rounded up, lost contact and dispersed, consistent with blunted actin accumulation and protrusions (Fig. 10H). Although some pyknotic nuclei and dissolution of the larval epidermis also resulted from elevated Pvr in the pnr domain, the phenotype was distinct in that the bright round larval cells seen in Pvr-depleted pupae were not evident (Fig. 10B,C). Some larval cells had rounded up but the central tissue appeared highly disorganized, less adherent and more mesenchymal, with cells of different shapes, possibly contributing to impaired disc migration due to the compromised larval epithelial substrate. C15-Gal4 expression of PvrWT revealed intense F-actin staining in the imaginal cells, which was especially dramatic at the apical surface compared with the control (Fig. 10G), and the disc margin appeared broader and less tightly constrained compared with the other genotypes (Fig. 10G). Hemocytes expressing Pvr were clearly present and capable of corpse engulfment, but were sparse with PvrRNAi (Fig. 10L,M).
The effects of altering JNK signaling with all three drivers were subtle in comparison to Pvr (Fig. 10D,E,I,J,N,O). Slpr overexpression moderately enhanced F-actin accumulation, whereas Slpr inhibition reduced it (Fig. 10I,J), but JNK signaling appeared to have much less impact on the larval epithelium. Thus, the thorax morphologies induced by reciprocal changes in both pathways cannot be explained simply by their effects on F-actin alone, or the LE in isolation, but must also take into account effects on the intervening larval tissue, the interface between them, and possibly the phagocytic hemocytes, which are also thought to be part of the pnr expression domain (Minakhina et al., 2011).
DISCUSSION
Here, we show evidence of a role for Pvf1/Pvr in tissue closure, acting independently of JNK signaling, but with an influence on the PI3K pathway. Foremost, in the embryo, Pvr is required in both hemocytes and ectoderm to support embryo viability and proper midline zippering. We envision that impaired hemocyte migration and function in Pvr mutants (Brückner et al., 2004) slows AS internalization and removal. This occurs to a varying extent, likely contributing to the low penetrance and variable expressivity of the Pvr midline phenotypes. Indeed, Brückner et al. (2004) predicted that many phenotypes found in Pvr mutants might be indirect consequences of hemocyte dysfunction. This has certainly been borne out in independent studies of central nervous system development (Olofsson and Page, 2005; Sears et al., 2003), malpighian tubule morphogenesis (Bunt et al., 2010), and now dorsal closure. Yet, sole rescue of hemocyte function did not eliminate midline phenotypes compared with similarly staged control embryos, and a significant degree of rescue was achieved by Pvr expression in ectodermal derivatives. In fact, PIP3 accumulation at the ectodermal LE is altered in Pvr loss- and gain-of-function contexts, denoting input to the PI3K pathway. In this light, the planar polarized distribution of Pvr at the LE is noteworthy and could differentially impact PI3K signaling components. The effects of Pvr on the magnitude of membrane-associated PIP3 appear distinct, however, from that of Bazooka and Pten phosphatase, which are required to polarize PIP3 distribution in LE cells (Pickering et al., 2013). Altogether, the cumulative defects in hemocyte function, slowing removal of the AS in Pvr mutants, coupled with submaximal PIP3-dependent protrusive activity of the epidermal LE, result in compromised midline zippering, leaving debris-filled dorsal holes and causing cardia bifida. This multi-tissue requirement for Pvr in facilitating dorsal closure recalls the process of wound healing in which contraction of wound bed mesenchyme cooperates with wound edge filopodial extensions to facilitate re-epithelialization of embryonic wounds (Redd et al., 2004).
Re-evaluation of JNK and Pvr signaling in thorax closure provided several new insights. First, the application of geometric morphometrics provided a powerful method to objectively quantify morphological features that can often be subtle or vary among individuals. Our analyses revealed evidence for an inverse relationship between pathway activity and notum shape. Upregulation of JNK signaling phenocopied downregulation of Pvf1/Pvr activity and vice versa, which is incompatible with a direct role for Pvr in JNK activation as proposed (Ishimaru et al., 2004). Instead, the implication is that the pathways act in opposition or in a balanced manner to regulate cellular machinery driving the closure process. Second, our evidence supports the notion that Pvr regulates PI3K signaling at this later stage. We found that Pvr dominantly suppressed a PI3K overexpression phenotype consistent with positive regulation of signaling by Pvr. Also, the induction of cuticle melanization by Pvr overexpression and reduced pigmentation with RNAi might connect to PI3K. Cuticle pigmentation relies on catecholamine derivatives, the enzymatic production of which is sensitive to nutrient status through the Insulin receptor/PI3K/Akt signaling axis (Shakhmantsir et al., 2014; Zitserman et al., 2012). The strength of Akt activity towards its targets, Tor and FoxO, correlates directly with the intensity of pigmentation, and our data suggest that this cascade is also responsive to Pvr activity. Pvr activation of PI3K signaling could therefore explain the inverse phenotypic effects with JNK described here, as antagonistic crosstalk between Akt/Tor and JNK signaling is believed to regulate inverse cell fates, such as survival or apoptosis (Eijkelenboom and Burgering, 2013; Puig and Mattila, 2011). It is likely, though, that the function of Pvr in thorax closure is multifaceted, providing trophic signals for the larval epidermis and hemocytes, and morphogenetic signals in the imaginal epithelium and LE, similar to its roles in the embryo. Indeed, there is a striking parallel between disruption of tissue in the pupae upon Pvr knockdown – as characterized by diminished actin, loss of structured cell shape, tissue break up, limited corpse clearance and defective midline fusion – and the embryonic tissue and cuticle phenotypes.
While it is commonly appreciated that the failure to remove apoptotic cells during tissue repair or homeostasis can result in pathology, as debris and leaky corpses trigger the immune response and inflammation (Franc, 2002; Hochreiter-Hufford and Ravichandran, 2013), the consequences for proper developmental tissue closure might be underappreciated as a possible contribution to embryonic clefting defects. Intriguingly, murine JNK pathway mutants with increased apoptosis and neural tube closure defects have been described (Chi et al., 2005; Sabapathy et al., 1999), but to what extent the removal of dead cells contributes to the phenotype, if at all, is not clear. Moreover, programmed cell death is a normal part of other tissue closure events in craniofacial development and palatogenesis (Ray and Niswander, 2012). Thus, it will be important to ascertain the consequences not only of regulated apoptosis, but also of the fate of dying cells in cleft mutants. The characterization of Pvr mutants, impaired in hemocyte and ectodermal function, provides an opportunity to explore this phenomenon further. It will be interesting to determine whether the prolonged presence of debris or corpses simply poses a physical barrier to tissue closure, triggers secondary signals detrimental to further development, or whether there is a temporal window in which a series of developmental events must proceed without disruption to subsequent steps. Closely coupling programmed cell death and phagocytosis appears to be a conserved mechanism to ensure expeditious corpse removal while maintaining immune tolerance during tissue homeostasis and infection (Stefater et al., 2011). Likewise, efficient coupling during organogenesis provides added value especially for organisms with rapid embryonic development, just like maintaining a streamlined factory assembly line.
MATERIALS AND METHODS
Fly stocks
UAS-Pvf1 (McDonald et al., 2003); UAS-PvrWT, UAS-λPvr, P{EP}Pvf1EP1624 (Duchek et al., 2001); UAS-PvrΔC (UAS-PvrDN), Pvr1, Pvr5 (Brückner et al., 2004); UAS-slprWT, UAS-slprKD, UAS-slprAAA, UAS-slprSH3 (Garlena et al., 2010); slprBS06 (Polaski et al., 2006); hep1 (Glise et al., 1995); slprHMS00742 (TRiP) (Ni et al., 2008); PvrKK101575 (VDRC) (Dietzl et al., 2007); PvrNIG.8222R, Pvf1NIG.7103R (NIG-Fly); UAS-mCherry.CAAX, UAS-srcEGFP, UAS-Act5C::GFP, PBac{PB}Pvrc02195, P{XP}Pvf2d02444, PBac{PB}Pvf2c06947, Mi{MIC}Pvf3MI04168, En-Gal4e16E, UAS-PI3K92E.Exel (BDSC); pucE69 (also known as puc-lacZ) (Ring and Martinez Arias, 1993); pnr-Gal4MD237 (Calleja et al., 1996); C15-Gal4 (also known as P{GMR40B01-Gal4}attP2) (Pfeiffer et al., 2008); crq-Gal4 (Olofsson and Page, 2005); 69B-Gal4 (Brand and Perrimon, 1993); LP1-Gal4 (also known as cald-Gal4; LP1 is also known as Orct2 – FlyBase) (Herranz et al., 2006); He-Gal4, UAS-GFP.nls (Zettervall et al., 2004); P{His2Av-mRFP}, P{sGMCA} (also known as H2Av::RFP, moe::GFP) (Kiehart et al., 2000); tGPH (Britton et al., 2002). Pvf1 recombinants with hep and slpr were verified by complementation and PCR to detect the P{EP} insertion. w1118 was our experimental control.
Clonal analysis
hs-Flp; ovoD1, FRT40 males were mated with heterozygous Pvr1, FRT40 females. Larvae were heat shocked for 2 h at 37°C on two consecutive days to produce female GLCs. Paternal rescue was 50%.
Tissue staining
Embryo immunostaining used standard methods (Rothwell and Sullivan, 2000). F-actin staining was similar but excludes methanol in favor of hand devitellinization. Staining reagents were: rat anti-Pvr 1:500 (Sears et al., 2003), rat anti-Pvr 1:500 (Rosin et al., 2004), mouse anti-Fas3 1:40 (7G10, DSHB), presorbed rabbit anti-β-gal 1:1500 (Cappel, 55976), mouse anti-phosphotyrosine 1:1000 (4G10, EMD Millipore, 05-321), Texas Red-phalloidin 1:500 (Life Technologies), and Vectashield with DAPI mounting media (Vector Labs). Balancer chromosomes carrying lacZ, GFP or RFP markers were used to distinguish genotypes in embryo populations. Preparation of larval cuticles has been described (Baril et al., 2009).
Pupal dissections
GFP-expressing pupae, aged 4.5-6 h after puparium formation, were poked with fine-tipped forceps in the posterior pupal case, releasing pressure. The posterior third of the case was peeled away and gut and internal tissues were removed. Remaining tissue was fixed overnight at 4°C in 4% formaldehyde diluted in PBT (1× PBS pH 7, 0.1% Triton X-100), followed by three 10 min washes in PBS. Samples were dissected further under GFP fluorescence by pushing tissue away from the casing. Samples were then prepared for phalloidin or immunostaining and imaging.
Geometric morphometric analysis
Adult flies were glued on a slide, ventral side down. Digital images of the notum were taken and the program tpsDIG2 (http://life.bio.sunysb.edu/morph/) was used to collect 14 landmarks and five semi-landmarks covering features of the scutum, scutellum and scuto-scutellar suture (supplementary material Fig. S9A). The resulting x,y coordinate positions were aligned using tpsRelw v1.49 (http://life.bio.sunysb.edu/morph/), in which the raw landmarks are translated, rotated and scaled to remove any variation not due to shape. Semi-landmarks were slid along a path tangent to the curve at that semi-landmark to minimize the Procrustes distance between each target and reference specimen. The resulting shape coordinates were imported into MorphoJ v1.05f (Klingenberg, 2011) for statistical analysis using PCA to quantify the major modes of shape variation and to test for differences among the groups. Shape variation and group differences were then visualized graphically and as deformations in wireframe diagrams.
Microscopy
Images of adult flies and pupae were obtained with NIS-Elements software using a DS-Fi1 digital camera and SMZ1500 stereomicroscope (all Nikon). Embryonic cuticles were imaged with a Leica DMI4000B inverted microscope and DFC480 camera. Stained embryos and pupal tissues were imaged using an Olympus FV1000 Fluoview confocal system on an IX81 compound inverted microscope and processed with Adobe Photoshop. Preparation and time-lapse imaging of embryos was as previously described (Evans et al., 2010) with the following details. moe::GFP embryos were imaged with a 20× objective for up to 2 h. Every 5 min, z-series of four to eight slices (1.5 μm step size) were captured. A 40× objective was used for 5-min movies with 30-s intervals to visualize cell protrusions and contractility. Files were imported into ImageJ (NIH), assembled into movies, cropped, and adjusted for brightness and contrast. Height (h, H) and width (W) were measured as straight-line lengths (Fig. 2). AS area (A) was defined using the Freehand selection tool followed by a Fit ellipse function. An arbitrary value (B) was defined as the midline distance between the anterior canthus and cephalic dorsal ridge. Live tGPH-expressing stage 14 embryos were imaged at 40× using identical settings for all genotypes. Projected z-stacks of four slices were quantified in ImageJ with a Profile plot of gray values (Pickering et al., 2013). Fold change was calculated by dividing maximum mean pixel intensity for control and experimental genotypes by mean lateral membrane intensity to control for different background intensities. puc-lacZ quantification in the embryo has been described (Stronach et al., 2014). Graphing and statistical tests were performed with Prism GraphPad. Details are provided in the figure legends.
Acknowledgements
We are grateful to members of the fly community, D. Montell, K. Bruckner, P. Rorth, P. Garrity, B. Shilo, G. Campbell and B. McCartney, and the Vienna Drosophila Resource Center (VDRC), Bloomington Drosophila Stock Center (BDSC) and National Institute of Genetics (NIG-Fly) for stocks and reagents, to Developmental Studies Hybridoma Bank (DSHB) for antibodies, and to F. Homa and G. Campbell for use of their microscopes.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
B.E.S. conceived the experiments. R.A.G., A.L.L. and B.E.S. performed the experiments and analyzed the results. L.R.B. obtained images and performed landmarking and data conversion for the morphometric analysis. T.E.P. and S.M.W. analyzed the morphometric data and contributed to presentation and description of the data. B.E.S. wrote the manuscript.
Funding
This work was supported by funding from the National Institutes of Health/Institute for Child Health and Human Development (NIH/NICHD) [grant number HD045836] to B.E.S., along with start-up funding from the University of Pittsburgh School of Medicine. L.R.B. was supported by a Brackenridge Research Fellowship from the University of Pittsburgh Honors College. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.122226/-/DC1
References
- Bahri S., Wang S., Conder R., Choy J., Vlachos S., Dong K., Merino C., Sigrist S., Molnar C., Yang X. et al. (2010). The leading edge during dorsal closure as a model for epithelial plasticity: Pak is required for recruitment of the Scribble complex and septate junction formation. Development 137, 2023-2032. 10.1242/dev.045088 [DOI] [PubMed] [Google Scholar]
- Baril C., Sahmi M., Ashton-Beaucage D., Stronach B. and Therrien M. (2009). The PP2C Alphabet is a negative regulator of stress-activated protein kinase signaling in Drosophila. Genetics 181, 567-579. 10.1534/genetics.108.096461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard G. B., Murugesu S., Adams R. J., Martinez-Arias A. and Gorfinkiel N. (2010). Cytoskeletal dynamics and supracellular organisation of cell shape fluctuations during dorsal closure. Development 137, 2743-2752. 10.1242/dev.045872 [DOI] [PubMed] [Google Scholar]
- Brand A. H. and Perrimon N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401-415. [DOI] [PubMed] [Google Scholar]
- Brewer S. and Williams T. (2004). Finally, a sense of closure? Animal models of human ventral body wall defects. Bioessays 26, 1307-1321. 10.1002/bies.20137 [DOI] [PubMed] [Google Scholar]
- Britton J. S., Lockwood W. K., Li L., Cohen S. M. and Edgar B. A. (2002). Drosophila's insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev. Cell 2, 239-249. 10.1016/S1534-5807(02)00117-X [DOI] [PubMed] [Google Scholar]
- Brock A. R., Wang Y., Berger S., Renkawitz-Pohl R., Han V. C., Wu Y. and Galko M. J. (2012). Transcriptional regulation of Profilin during wound closure in Drosophila larvae. J. Cell Sci. 125, 5667-5676. 10.1242/jcs.107490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brückner K., Kockel L., Duchek P., Luque C. M., Rørth P. and Perrimon N. (2004). The PDGF/VEGF receptor controls blood cell survival in Drosophila. Dev. Cell 7, 73-84. 10.1016/j.devcel.2004.06.007 [DOI] [PubMed] [Google Scholar]
- Bunt S., Hooley C., Hu N., Scahill C., Weavers H. and Skaer H. (2010). Hemocyte-secreted type IV collagen enhances BMP signaling to guide renal tubule morphogenesis in Drosophila. Dev. Cell 19, 296-306. 10.1016/j.devcel.2010.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush J. O. and Jiang R. (2012). Palatogenesis: morphogenetic and molecular mechanisms of secondary palate development. Development 139, 231-243. 10.1242/dev.067082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calleja M., Moreno E., Pelaz S. and Morata G. (1996). Visualization of gene expression in living adult Drosophila. Science 274, 252-255. 10.1126/science.274.5285.252 [DOI] [PubMed] [Google Scholar]
- Chartier A., Zaffran S., Astier M., Semeriva M. and Gratecos D. (2002). Pericardin, a Drosophila type IV collagen-like protein is involved in the morphogenesis and maintenance of the heart epithelium during dorsal ectoderm closure. Development 129, 3241-3253. [DOI] [PubMed] [Google Scholar]
- Chi H., Sarkisian M. R., Rakic P. and Flavell R. A. (2005). Loss of mitogen-activated protein kinase kinase kinase 4 (MEKK4) results in enhanced apoptosis and defective neural tube development. Proc. Natl. Acad. Sci. USA 102, 3846-3851. 10.1073/pnas.0500026102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho N. K., Keyes L., Johnson E., Heller J., Ryner L., Karim F. and Krasnow M. A. (2002). Developmental control of blood cell migration by the Drosophila VEGF pathway. Cell 108, 865-876. 10.1016/S0092-8674(02)00676-1 [DOI] [PubMed] [Google Scholar]
- Choi S. J., Marazita M. L., Hart P. S., Sulima P. P., Field L. L., McHenry T. G., Govil M., Cooper M. E., Letra A., Menezes R. et al. (2009). The PDGF-C regulatory region SNP rs28999109 decreases promoter transcriptional activity and is associated with CL/P. Eur. J. Hum. Genet. 17, 774-784. 10.1038/ejhg.2008.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G., Chen D., Schnorrer F., Su K.-C., Barinova Y., Fellner M., Gasser B., Kinsey K., Oppel S., Scheiblauer S. et al. (2007). A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151-156. 10.1038/nature05954 [DOI] [PubMed] [Google Scholar]
- Ding H., Wu X., Boström H., Kim I., Wong N., Tsoi B., O'Rourke M., Koh G. Y., Soriano P., Betsholtz C. et al. (2004). A specific requirement for PDGF-C in palate formation and PDGFR-alpha signaling. Nat. Genet. 36, 1111-1116. 10.1038/ng1415 [DOI] [PubMed] [Google Scholar]
- Duchek P., Somogyi K., Jékely G., Beccari S. and Rørth P. (2001). Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell 107, 17-26. 10.1016/S0092-8674(01)00502-5 [DOI] [PubMed] [Google Scholar]
- Eberhart J. K., He X., Swartz M. E., Yan Y.-L., Song H., Boling T. C., Kunerth A. K., Walker M. B., Kimmel C. B. and Postlethwait J. H. (2008). MicroRNA Mirn140 modulates Pdgf signaling during palatogenesis. Nat. Genet. 40, 290-298. 10.1038/ng.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkelenboom A. and Burgering B. M. T. (2013). FOXOs: signalling integrators for homeostasis maintenance. Nat. Rev. Mol. Cell Biol. 14, 83-97. 10.1038/nrm3507 [DOI] [PubMed] [Google Scholar]
- Evans I. R., Zanet J., Wood W. and Stramer B. M. (2010). Live imaging of Drosophila melanogaster embryonic hemocyte migrations. J. Vis. Exp. 36, e1696 10.3791/1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández B. G., Arias A. M. and Jacinto A. (2007). Dpp signalling orchestrates dorsal closure by regulating cell shape changes both in the amnioserosa and in the epidermis. Mech. Dev. 124, 884-897. 10.1016/j.mod.2007.09.002 [DOI] [PubMed] [Google Scholar]
- Franc N. C. (2002). Phagocytosis of apoptotic cells in mammals, caenorhabditis elegans and Drosophila melanogaster: molecular mechanisms and physiological consequences. Front. Biosci. 7, d1298-d1313. 10.2741/franc [DOI] [PubMed] [Google Scholar]
- Garlena R. A., Gonda R. L., Green A. B., Pileggi R. M. and Stronach B. (2010). Regulation of mixed-lineage kinase activation in JNK-dependent morphogenesis. J. Cell Sci. 123, 3177-3188. 10.1242/jcs.063313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glise B., Bourbon H. and Noselli S. (1995). hemipterous encodes a novel Drosophila MAP kinase kinase, required for epithelial cell sheet movement. Cell 83, 451-461. 10.1016/0092-8674(95)90123-X [DOI] [PubMed] [Google Scholar]
- Gorfinkiel N., Blanchard G. B., Adams R. J. and Martinez Arias A. (2009). Mechanical control of global cell behaviour during dorsal closure in Drosophila. Development 136, 1889-1898. 10.1242/dev.030866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack T., Schneider M., Schwendele B. and Renault A. D. (2014). Drosophila heart cell movement to the midline occurs through both cell autonomous migration and dorsal closure. Dev. Biol. 396, 69-182. 10.1016/j.ydbio.2014.08.033 [DOI] [PubMed] [Google Scholar]
- Harden N. (2002). Signaling pathways directing the movement and fusion of epithelial sheets: lessons from dorsal closure in Drosophila. Differentiation 70, 181-203. 10.1046/j.1432-0436.2002.700408.x [DOI] [PubMed] [Google Scholar]
- He F. and Soriano P. (2013). A critical role for PDGFRalpha signaling in medial nasal process development. PLoS Genet. 9, e1003851 10.1371/journal.pgen.1003851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heino T. I., Kärpänen T., Wahlström G., Pulkkinen M., Eriksson U., Alitalo K. and Roos C. (2001). The Drosophila VEGF receptor homolog is expressed in hemocytes. Mech. Dev. 109, 69-77. 10.1016/S0925-4773(01)00510-X [DOI] [PubMed] [Google Scholar]
- Heisenberg C.-P. (2009). Dorsal closure in Drosophila: cells cannot get out of the tight spot. Bioessays 31, 1284-1287. 10.1002/bies.200900109 [DOI] [PubMed] [Google Scholar]
- Heldin C. H. and Westermark B. (1999). Mechanism of action and in vivo role of platelet-derived growth factor. Physiol. Rev. 79, 1283-1316. [DOI] [PubMed] [Google Scholar]
- Heller E., Kumar K. V., Grill S. W. and Fuchs E. (2014). Forces generated by cell intercalation tow epidermal sheets in mammalian tissue morphogenesis. Dev. Cell 28, 617-632. 10.1016/j.devcel.2014.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz H., Morata G. and Milán M. (2006). calderon encodes an organic cation transporter of the major facilitator superfamily required for cell growth and proliferation of Drosophila tissues. Development 133, 2617-2625. 10.1242/dev.02436 [DOI] [PubMed] [Google Scholar]
- Hoch R. V. and Soriano P. (2003). Roles of PDGF in animal development. Development 130, 4769-4784. 10.1242/dev.00721 [DOI] [PubMed] [Google Scholar]
- Hochreiter-Hufford A. and Ravichandran K. S. (2013). Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb. Perspect. Biol. 5, a008748 10.1101/cshperspect.a008748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson M. S., Tokutake Y., Chang M.-S., Bloor J. W., Venakides S., Kiehart D. P. and Edwards G. S. (2003). Forces for morphogenesis investigated with laser microsurgery and quantitative modeling. Science 300, 145-149. 10.1126/science.1079552 [DOI] [PubMed] [Google Scholar]
- Ishimaru S., Ueda R., Hinohara Y., Ohtani M. and Hanafusa H. (2004). PVR plays a critical role via JNK activation in thorax closure during Drosophila metamorphosis. EMBO J. 23, 3984-3994. 10.1038/sj.emboj.7600417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto A., Wood W., Balayo T., Turmaine M., Martinez-Arias A. and Martin P. (2000). Dynamic actin-based epithelial adhesion and cell matching during Drosophila dorsal closure. Curr. Biol. 10, 1420-1426. 10.1016/S0960-9822(00)00796-X [DOI] [PubMed] [Google Scholar]
- Jacinto A., Wood W., Woolner S., Hiley C., Turner L., Wilson C., Martinez-Arias A. and Martin P. (2002). Dynamic analysis of actin cable function during Drosophila dorsal closure. Curr. Biol. 12, 1245-1250. 10.1016/S0960-9822(02)00955-7 [DOI] [PubMed] [Google Scholar]
- Kaltschmidt J. A., Lawrence N., Morel V., Balayo T., Fernández B. G., Pelissier A., Jacinto A. and Martinez Arias A. (2002). Planar polarity and actin dynamics in the epidermis of Drosophila. Nat. Cell Biol. 4, 937-944. 10.1038/ncb882 [DOI] [PubMed] [Google Scholar]
- Kiehart D. P., Galbraith C. G., Edwards K. A., Rickoll W. L. and Montague R. A. (2000). Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J. Cell Biol. 149, 471-490. 10.1083/jcb.149.2.471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg C. P. (2011). MorphoJ: an integrated software package for geometric morphometrics. Mol. Ecol. Resour. 11, 353-357. 10.1111/j.1755-0998.2010.02924.x [DOI] [PubMed] [Google Scholar]
- Klinghoffer R. A., Hamilton T. G., Hoch R. and Soriano P. (2002). An allelic series at the PDGFalphaR locus indicates unequal contributions of distinct signaling pathways during development. Dev. Cell 2, 103-113. 10.1016/S1534-5807(01)00103-4 [DOI] [PubMed] [Google Scholar]
- Macias A., Romero N. M., Martin F., Suarez L., Rosa A. L. and Morata G. (2004). PVF1/PVR signaling and apoptosis promotes the rotation and dorsal closure of the Drosophila male terminalia. Int. J. Dev. Biol. 48, 1087-1094. 10.1387/ijdb.041859am [DOI] [PubMed] [Google Scholar]
- Martin P. and Parkhurst S. M. (2004). Parallels between tissue repair and embryo morphogenesis. Development 131, 3021-3034. 10.1242/dev.01253 [DOI] [PubMed] [Google Scholar]
- Martin-Blanco E., Pastor-Pareja J. C. and Garcia-Bellido A. (2000). JNK and decapentaplegic signaling control adhesiveness and cytoskeleton dynamics during thorax closure in Drosophila. Proc. Natl. Acad. Sci. USA 97, 7888-7893. 10.1073/pnas.97.14.7888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy N., Wetherill L., Lovely C. B., Swartz M. E., Foroud T. M. and Eberhart J. K. (2013). Pdgfra protects against ethanol-induced craniofacial defects in a zebrafish model of FASD. Development 140, 3254-3265. 10.1242/dev.094938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. A., Pinheiro E. M. and Montell D. J. (2003). PVF1, a PDGF/VEGF homolog, is sufficient to guide border cells and interacts genetically with Taiman. Development 130, 3469-3478. 10.1242/dev.00574 [DOI] [PubMed] [Google Scholar]
- Millard T. H. and Martin P. (2008). Dynamic analysis of filopodial interactions during the zippering phase of Drosophila dorsal closure. Development 135, 621-626. 10.1242/dev.014001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakhina S., Tan W. and Steward R. (2011). JAK/STAT and the GATA factor Pannier control hemocyte maturation and differentiation in Drosophila. Dev. Biol. 352, 308-316. 10.1016/j.ydbio.2011.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J.-Q., Markstein M., Binari R., Pfeiffer B., Liu L.-P., Villalta C., Booker M., Perkins L. and Perrimon N. (2008). Vector and parameters for targeted transgenic RNA interference in Drosophila melanogaster. Nat. Methods 5, 49-51. 10.1038/nmeth1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotarski S. H., McKeon N., Moser R. J. and Peifer M. (2014). The actin regulators Enabled and Diaphanous direct distinct protrusive behaviors in different tissues during Drosophila development. Mol. Biol. Cell 25, 3147-3165. 10.1091/mbc.E14-05-0951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson B. and Page D. T. (2005). Condensation of the central nervous system in embryonic Drosophila is inhibited by blocking hemocyte migration or neural activity. Dev. Biol. 279, 233-243. 10.1016/j.ydbio.2004.12.020 [DOI] [PubMed] [Google Scholar]
- Pastor-Pareja J. C., Grawe F., Martı´n-Blanco E. and Garcı´a-Bellido A. (2004). Invasive cell behavior during Drosophila imaginal disc eversion is mediated by the JNK signaling cascade. Dev. Cell 7, 387-399. 10.1016/j.devcel.2004.07.022 [DOI] [PubMed] [Google Scholar]
- Pfeiffer B. D., Jenett A., Hammonds A. S., Ngo T.-T. B., Misra S., Murphy C., Scully A., Carlson J. W., Wan K. H., Laverty T. R. et al. (2008). Tools for neuroanatomy and neurogenetics in Drosophila. Proc. Natl. Acad. Sci. USA 105, 9715-9720. 10.1073/pnas.0803697105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering K., Alves-Silva J., Goberdhan D. and Millard T. H. (2013). Par3/Bazooka and phosphoinositides regulate actin protrusion formation during Drosophila dorsal closure and wound healing. Development 140, 800-809. 10.1242/dev.089557 [DOI] [PubMed] [Google Scholar]
- Pickett E. A., Olsen G. S. and Tallquist M. D. (2008). Disruption of PDGFRalpha-initiated PI3K activation and migration of somite derivatives leads to spina bifida. Development 135, 589-598. 10.1242/dev.013763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polaski S., Whitney L., Barker B. W. and Stronach B. (2006). Genetic analysis of slipper/mixed lineage kinase reveals requirements in multiple Jun-N-terminal kinase-dependent morphogenetic events during Drosophila development. Genetics 174, 719-733. 10.1534/genetics.106.056564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig O. and Mattila J. (2011). Understanding Forkhead box class O function: lessons from Drosophila melanogaster. Antioxid. Redox Signal. 14, 635-647. 10.1089/ars.2010.3407 [DOI] [PubMed] [Google Scholar]
- Ray H. J. and Niswander L. (2012). Mechanisms of tissue fusion during development. Development 139, 1701-1711. 10.1242/dev.068338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redd M. J., Cooper L., Wood W., Stramer B. and Martin P. (2004). Wound healing and inflammation: embryos reveal the way to perfect repair. Philos. Trans. R. Soc. Lond. B Biol. Sci. 359, 777-784. 10.1098/rstb.2004.1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed B. H., Wilk R., Schöck F. and Lipshitz H. D. (2004). Integrin-dependent apposition of Drosophila extraembryonic membranes promotes morphogenesis and prevents anoikis. Curr. Biol. 14, 372-380. 10.1016/j.cub.2004.02.029 [DOI] [PubMed] [Google Scholar]
- Riesgo-Escovar J. R., Jenni M., Fritz A. and Hafen E. (1996). The Drosophila Jun-N-terminal kinase is required for cell morphogenesis but not for DJun-dependent cell fate specification in the eye. Genes Dev. 10, 2759-2768. 10.1101/gad.10.21.2759 [DOI] [PubMed] [Google Scholar]
- Ring J. M. and Martinez Arias A. (1993). puckered, a gene involved in position-specific cell differentiation in the dorsal epidermis of the Drosophila larva. Dev. Suppl., 251-259. [PubMed] [Google Scholar]
- Rodriguez-Diaz A., Toyama Y., Abravanel D. L., Wiemann J. M., Wells A. R., Tulu U. S., Edwards G. S. and Kiehart D. P. (2008). Actomyosin purse strings: renewable resources that make morphogenesis robust and resilient. HFSP J. 2, 220-237. 10.2976/1.2955565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin D., Schejter E., Volk T. and Shilo B.-Z. (2004). Apical accumulation of the Drosophila PDGF/VEGF receptor ligands provides a mechanism for triggering localized actin polymerization. Development 131, 1939-1948. 10.1242/dev.01101 [DOI] [PubMed] [Google Scholar]
- Rothwell W. and Sullivan W. (2000). Fluorescent analysis of Drosophila embryos. In Drosophila Protocols (ed. Sullivan W., Ashburner M. and Hawley R. S.), pp. 141-157. New York: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Rugendorff A., Younossi-Hartenstein A. and Hartenstein V. (1994). Embryonic origin and differentiation of the Drosophila heart. Roux's Arch. Dev. Biol. 203, 266-280. 10.1007/BF00360522 [DOI] [PubMed] [Google Scholar]
- Sabapathy K., Jochum W., Hochedlinger K., Chang L., Karin M. and Wagner E. F. (1999). Defective neural tube morphogenesis and altered apoptosis in the absence of both JNK1 and JNK2. Mech. Dev. 89, 115-124. 10.1016/S0925-4773(99)00213-0 [DOI] [PubMed] [Google Scholar]
- Sears H. C., Kennedy C. J. and Garrity P. A. (2003). Macrophage-mediated corpse engulfment is required for normal Drosophila CNS morphogenesis. Development 130, 3557-3565. 10.1242/dev.00586 [DOI] [PubMed] [Google Scholar]
- Shakhmantsir I., Massad N. L. and Kennell J. A. (2014). Regulation of cuticle pigmentation in drosophila by the nutrient sensing insulin and TOR signaling pathways. Dev. Dyn. 243, 393-401. 10.1002/dvdy.24080 [DOI] [PubMed] [Google Scholar]
- Sluss H. K., Han Z., Barrett T., Davis R. J. and Ip Y. T. (1996). A JNK signal transduction pathway that mediates morphogenesis and an immune response in Drosophila. Genes Dev. 10, 2745-2758. 10.1101/gad.10.21.2745 [DOI] [PubMed] [Google Scholar]
- Smith C. L. and Tallquist M. D. (2010). PDGF function in diverse neural crest cell populations. Cell Adh. Migr. 4, 561-566. 10.4161/cam.4.4.12829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon J., Kaya-Çopur A., Colombelli J. and Brunner D. (2009). Pulsed forces timed by a ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell 137, 1331-1342. 10.1016/j.cell.2009.03.050 [DOI] [PubMed] [Google Scholar]
- Stefater J. A. III, Ren S., Lang R. A. and Duffield J. S. (2011). Metchnikoff's policemen: macrophages in development, homeostasis and regeneration. Trends Mol. Med. 17, 743-752. 10.1016/j.molmed.2011.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stronach B., Lennox A. L. and Garlena R. A. (2014). Domain specificity of MAP3K family members, MLK and Tak1, for JNK signaling in Drosophila. Genetics 197, 497-513. 10.1534/genetics.113.160937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallquist M. and Kazlauskas A. (2004). PDGF signaling in cells and mice. Cytokine Growth Factor Rev. 15, 205-213. 10.1016/j.cytogfr.2004.03.003 [DOI] [PubMed] [Google Scholar]
- Toyama Y., Peralta X. G., Wells A. R., Kiehart D. P. and Edwards G. S. (2008). Apoptotic force and tissue dynamics during Drosophila embryogenesis. Science 321, 1683-1686. 10.1126/science.1157052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui K. and Simpson P. (2000). Cellular basis of the dynamic behavior of the imaginal thoracic discs during Drosophila metamorphosis. Dev. Biol. 225, 13-25. 10.1006/dbio.2000.9766 [DOI] [PubMed] [Google Scholar]
- Wada A., Kato K., Uwo M. F., Yonemura S. and Hayashi S. (2007). Specialized extraembryonic cells connect embryonic and extraembryonic epidermis in response to Dpp during dorsal closure in Drosophila. Dev. Biol. 301, 340-349. 10.1016/j.ydbio.2006.09.020 [DOI] [PubMed] [Google Scholar]
- Wells A. R., Zou R. S., Tulu U. S., Sokolow A. C., Crawford J. M., Edwards G. S. and Kiehart D. P. (2014). Complete canthi removal reveals that forces from the amnioserosa alone are sufficient to drive dorsal closure in Drosophila. Mol. Biol. Cell 25, 3552-3568. 10.1091/mbc.E14-07-1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. and Sato T. N. (2008). On the mechanics of cardiac function of Drosophila embryo. PLoS ONE 3, e4045 10.1371/journal.pone.0004045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Brock A. R., Wang Y., Fujitani K., Ueda R. and Galko M. J. (2009). A blood-borne PDGF/VEGF-like ligand initiates wound-induced epidermal cell migration in Drosophila larvae. Curr. Biol. 19, 1473-1477. 10.1016/j.cub.2009.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlinger J. and Bohmann D. (1999). Thorax closure in Drosophila: involvement of Fos and the JNK pathway. Development 126, 3947-3956. [DOI] [PubMed] [Google Scholar]
- Zettervall C.-J., Anderl I., Williams M. J., Palmer R., Kurucz E., Ando I. and Hultmark D. (2004). A directed screen for genes involved in Drosophila blood cell activation. Proc. Natl. Acad. Sci. USA 101, 14192-14197. 10.1073/pnas.0403789101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitserman D., Gupta S., Kruger W. D., Karbowniczek M. and Roegiers F. (2012). The TSC1/2 complex controls Drosophila pigmentation through TORC1-dependent regulation of catecholamine biosynthesis. PLoS ONE 7, e48720 10.1371/journal.pone.0048720 [DOI] [PMC free article] [PubMed] [Google Scholar]