Abstract
Aims/hypothesis
Statins and niacin (nicotinic acid) reduce circulating LDL-cholesterol (LDLC) levels by different mechanisms. Yet, both increase the risk of diabetes mellitus. Our objective was to relate blood LDL-C concentrations and a genetic risk score (GRS) for LDLC to the risk of incident diabetes in individuals not treated with lipid-modifying therapy.
Methods
We evaluated participants of the Framingham Heart Study who attended any of Offspring cohort examination cycles 3–8 and Third Generation cohort examination cycle 1 (N =14,120 person-observations, 6,011 unique individuals; mean age 50 ± 11 years, 56% women), who were not treated with lipid-modifying or antihypertensive medications and who were free from cardiovascular disease at baseline. Incident diabetes was assessed at the next examination.
Results
The GRS was significantly associated with LDL-C concentrations (sex- and age-adjusted estimated influence 0.24, p < 0.0001). On follow-up (mean 4.5 ± 1.5 years), 312 individuals (2.2%) developed new-onset diabetes. In multivariable models, a higher LDL-C concentration was associated with lower risk of diabetes (OR per SD increment 0.81, 95% CI 0.70, 0.93, p = 0.004). The GRS was associated with incident diabetes in similar direction and of comparable magnitude (OR per SD increment 0.85, 95% CI 0.76, 0.96, p = 0.009).
Conclusions/interpretation
Among individuals not treated with lipid-modifying therapy low LDL-C concentrations were associated with increased diabetes risk. These observations may contribute to our understanding of why lipid-lowering treatment may cause diabetes in some individuals. Additional studies are warranted to elucidate the molecular mechanisms underlying our observations.
Keywords: Low-density lipoprotein concentrations, Diabetes risk, Mendelian randomisation, Untreated individuals
Introduction
Statins and niacin (nicotinic acid) reduce circulating LDL-cholesterol (LDL-C) levels by different mechanisms. Yet, it is notable that both drugs have been associated with an increased risk of diabetes mellitus in randomised controlled trials [1, 2]. These observations could suggest that low LDL-C concentrations per se (independent of off-target effects of lipid-lowering medications) may be associated with an increased diabetes risk. A Mendelian randomisation analysis (i.e. an analysis linking genetic variations in single-nucleotide polymorphisms [SNPs] to diabetes risk) recently suggested that the risk of diabetes observed with statin treatment could be attributable to ‘on-target’ effects of the drug, possibly supporting the idea that blood cholesterol concentrations may influence diabetes risk directly [3]. Consistent with this line of enquiry, another recent study reported that several lipid-related SNPs were oppositely associated with LDL-C concentrations and blood glucose levels [4] and a very recent cross-sectional study suggested that a genetic risk score (GRS) for LDL-C was associated with diabetes risk [5]. Additional supportive evidence linking low LDL-C concentrations to increased diabetes risk was suggested by a recent large cross-sectional study of individuals screened for familial hypercholesterolaemia [6]. The authors of that study reported a lower prevalence of diabetes in individuals with familial hypercholesterolaemia, compared with individuals without familial hypercholesterolaemia (with a graded association dependent on the mutation subtype) [6]. At least three smaller cross-sectional observational studies have similarly reported lower circulating LDL-C concentrations among people with insulin resistance and diabetes, compared with people without insulin resistance and diabetes [7–9]. Studies investigating the longitudinal relationship between blood LDL-C concentrations and incident diabetes are, however, lacking. Accordingly, we hypothesised that lower levels of LDL-C may be associated with greater risk of diabetes prospectively in individuals who are not on any lipid-lowering medications. We tested this hypothesis in the community-based Framingham Offspring and Third Generation cohorts. We also related a previously used GRS for LDL-C to the risk of developing diabetes [10] to assess whether individuals genetically predisposed to higher levels of LDL-C have a lower incidence of diabetes, thereby evaluating concordance between the two sets of epidemiological observations.
Methods
Participants
We included participants who attended any of Framingham Offspring cohort examination cycles 3 through 8, or Framingham Third Generation cohort examination cycle 1 (combined N = 29,346 person-observations) provided they were free from diabetes (n with diabetes =1,644), not using lipid-modifying therapy (n using lipid therapy = 2,718) and attended the next examination cycle (where incident diabetes was determined, n attendees = 21,117). We chose a priori to exclude people with prevalent cardiovascular disease and those using any antihypertensive medications (combined n =3,788 person-observations) because these conditions or the medications used to treat them have been associated with the risk of future diabetes in previous studies.[11–15] Additionally, we excluded people with missing data on key covariates (age, sex, blood pressure, antihypertensive medications, cholesterol treatment, lipid values and/or blood glucose concentrations; n = 3,209).
At each examination cycle, blood was drawn from participants after an overnight fast (typically 10–12 h). Triacylglycerol, total cholesterol and HDL-cholesterol (HDL-C) concentrations were measured directly using standardised assays and LDL-C concentrations were calculated using the Friedewald formula [16]. We excluded people with blood triacylglycerol concentrations > 10.34 mmol/l (> 400 mg/dl) (n = 415) because LDL-C concentrations cannot be calculated reliably above this value using the Friedewald formula. As a sensitivity analysis, we used directly measured total cholesterol concentrations to further ensure that any observed associations were not due to inaccurate calculations of LDL-C concentrations by the Friedewald formula. Diabetes was defined as a fasting plasma glucose concentration > 3.23 mmol/l (> 125 mg/dl) or the use of glucose-lowering medications. All participants provided written informed consent and the study protocol was approved by the institutional review board of the Boston University Medical Center.
Genotyping
Participants from the Offspring and Third Generation cohorts had blood drawn for DNA analyses during their sixth and first examination cycles, respectively. DNA variants were genotyped on the Affymetrix 500K (Affymetrix, Santa Clara, CA, USA) and MIPS 50K platforms and imputations were based on MACH version 1.0 (http://csg.sph.umich.edu//abecasis/MACH/). The LDL-C GRS was calculated based on 37 SNPs (in accordance with previous work [10]) using a weighted summation of genotypes (coded additively for the risk allele; β estimates were derived from the genome-wide association study by Teslovich et al [17]). Since assembly of that risk score, more SNPs have been identified in larger meta-analyses but we used the ‘older’ 37 SNP score in order to maximise our sample size. Typically, the effect sizes of the newly discovered SNPs are small or have a low prevalence and, therefore, the contributions of the newly discovered SNPs to circulating LDL-C concentrations in community-based settings is modest. We used a corresponding weighted risk score of total serum cholesterol concentrations based on 52 SNPs from the study by Teslovich et al [17].
Statistical analyses
Data from the different examination cycles were pooled to increase sample size (pooled repeated observations) [18]. Characteristics, including blood lipid concentrations, were updated at each examination cycle. All participants were followed until the next examination cycle for incident diabetes. Because we had no diabetes events registered in between examination cycles (ascertainment was made at the examinations), we used multivariable logistic regression analyses with generalised estimating equations (accounting for repeated observations within participants) to calculate the ORs for development of diabetes in relation to blood LDL-C and the GRS. The interval between Framingham examinations was included as a covariate in the models (this method yields essentially similar results to time-dependent Cox models) [19]. We adjusted all statistical models for the following covariates (all covariates were included in the same model): examination cycle (as a categorical variable, which captured both calendar time period, and the cohort status), age, sex, blood HDL-C and log(triacylglycerol) concentrations, BMI, systolic and diastolic blood pressure and fasting glucose level. Previous work based on the Framingham Offspring Study has demonstrated that these variables are important predictors of the risk of developing diabetes [20]. To facilitate comparison in OR estimates between LDL-C concentrations and GRS, we standardised these two variables prior to analyses (mean = 0 and SD = 1). We tested for effect modification by sex, age, BMI, fasting glucose concentrations, HDL-C and triacylglycerol concentrations (all but triacylglycerol concentrations were tested as continuous measures; the latter was tested as quintiles because of its wide distribution) by incorporating the interaction terms into multivariable models. Cubic regression splines were used to assess and graphically visualise the linearity/nonlinearity of associations of LDL-C or GRS with incident diabetes. We performed an instrumental variable (IV) analysis (i.e. Mendelian randomisation analysis) for the binary outcome incident diabetes (Y) using the ratio method [21]. First, we regressed LDL-C (X, standardised) on GRS (the IV variable, standardised) to estimate β(GX). Next, we ran logistic regression of diabetes incidence on GRS to estimate β(GY). We included age and sex in each model. We estimated β(IV) using the ratio β(IV) = β(GY) / β(GX) and we estimated variance, β(IV), as in Burgess [21]. As sensitivity analyses, all models were repeated using directly measured total cholesterol concentrations instead of estimated LDL-C. As another sensitivity analysis we also investigated the associations between GRS for HDL-C and for triacylglycerols and incident diabetes. A two-sided p value < 0.05 was considered statistically significant for all analyses.
Results
A total of 14,120 person-observations were included (age [mean ± SD] 50 ± 11 years, 56% women; 6,011 unique individuals) (Table 1). The mean concentration of LDL-C was 3.23 ± 0.89 mmol/l (limits 0.57–8.52 mmol/l; corresponding to 125 ± 34 mg/dl [limits 22– 330 mg/dl]). The GRS varied from 47 to 110, with a mean of 78 ± 8. The GRS was positively associated with LDL-C concentrations (age- and sex-adjusted estimated influence 0.24, p < 0.0001). The GRS was also positively associated with circulating levels of triacylglycerols (age- and sex-adjusted estimated influence 0.08, p < 0.0001) and negatively associated with circulating HDL-C concentrations (age- and sex-adjusted estimated influence −0.04, p < 0.0001).
Table 1.
Study sample characteristics
| Characteristic | Offspring sample | Third Generation sample |
|---|---|---|
| N | 11,364 | 2,756 |
| Age, years | 53±10 | 39±8 |
| Female sex, n (%) | 6,398 (56) | 1,524 (55) |
| LDL-C concentration range, mmol/l | 0.79–8.52 | 0.57–7.27 |
| LDL-C concentration, mmol/l | 3.32±0.89 | 2.90±0.81 |
| HDL-C concentration, mmol/l | 1.39±0.41 | 1.44±0.42 |
| Triacylglycerol concentration, mmol/l | 2.89±1.60 | 2.61±1.44 |
| GRS 2010, sum mmol/l | 2.02±0.20 | 2.03±0.20 |
| GRS 2013, sum mmol/l | 4.22±0.27 | 4.24±0.27 |
| BMI, kg/m2 | 26.4±4.7 | 26.1±5.1 |
| Systolic blood pressure, mmHg | 122±17 | 115±13 |
| Diastolic blood pressure, mmHg | 76±10 | 74±9 |
| Fasting blood glucose concentration, mmol/l | 2.41±0.24 | 2.39±0.21 |
| Incident diabetes during follow-up, n (%) | 257 (2.3) | 55 (2.0) |
Continuous variables are presented as mean ± SD Offspring sample comprises pooled observations from different examinations
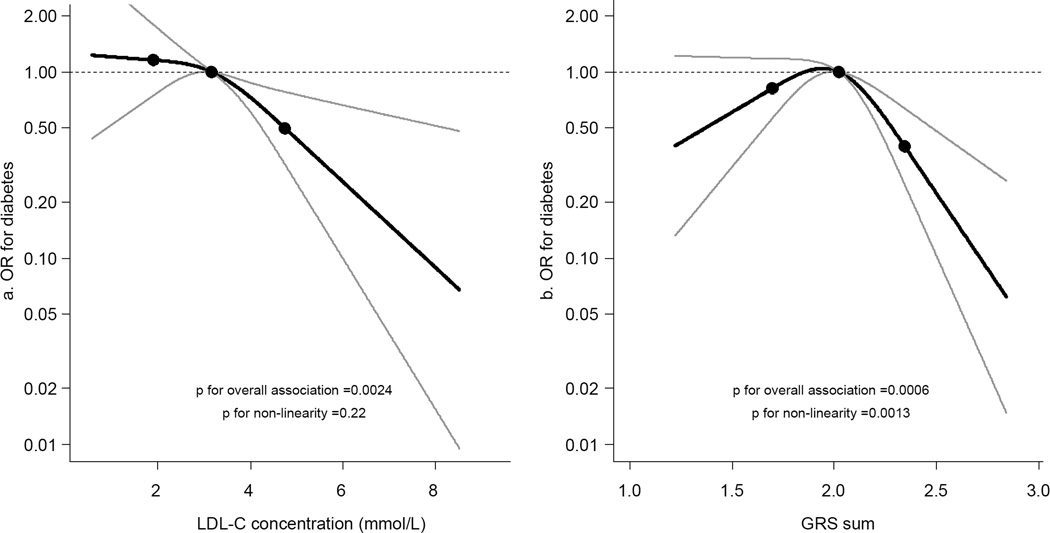
On follow-up (mean 4.5 ± 1.5 years), 312 individuals (2.2%) developed diabetes. The number of diabetes events and total observational time by examination are presented in electronic supplementary material (ESM) Table 1. In multivariable-adjusted models, a higher LDL-C concentration was associated with a lower risk of diabetes (OR per SD increment 0.81, 95% CI 0.70, 0.93, p = 0.004) (Fig. 1a). Models adjusted for fewer variables are available in ESM Table 2. The GRS was also associated inversely with incident diabetes (OR per SD increment of 0.85, 95% CI 0.76, 0.96, p = 0.009). The relationship was, however, not linear for GRS (p for non-linearity = 0.001) (Fig. 1b). Therefore, we performed additional analyses using tertiles of both GRS and LDL-C concentrations (Table 2). Compared with the lowest tertile, adjusted ORs were lower for the second and third tertiles without a clear dose– response relationship for either GRS or LDL-C. Formal Mendelian randomisation analysis supported a causal relationship between LDL-C concentrations and incident diabetes: the estimated OR of diabetes per 1 SD higher LDL-C concentration was 0.81 (95% CI 0.70, 0.93, p = 0.004) for directly measured LDL-C levels and 0.49 (95% CI 0.29, 0.83, p = 0.008) for LDL-C levels estimated from the GRS-based instrument variable.
Fig. 1.
Splines illustrating the OR of developing diabetes for LDL-C concentration (a) and the GRS underlying LDL-C concentration (b). Knots are placed at the 5th, 50th and 95th percentiles. Both models were adjusted for time between examinations, examination, HDL-C and triacylglycerol concentrations, sex, age, BMI, systolic and diastolic blood pressures and fasting blood glucose levels. p for overall association = 0.0024 (a) and 0.0006 (b); p for nonlinearity = 0.22 (a) and 0.0013 (b). The y-axes are on a log scale
Table 2.
Adjusted ORs of new-onset diabetes by tertiles of cholesterol concentration and GRS
| Concentration/GRS | Adjusted ORa | p value |
|---|---|---|
| LDL-C concentration | ||
| Tertile 1 | 1.42 (1.01, 2.00) | 0.044 |
| Tertile 2 | 1.48 (1.09, 2.00) | 0.012 |
| Tertile 3 | 1.00 (reference) | |
| GRS for LDL-C | ||
| Tertile 1 | 1.56 (1.12, 2.24) | 0.012 |
| Tertile 2 | 1.77 (1.25, 2.49) | 0.001 |
| Tertile 3 | 1.00 (reference) | |
| Total cholesterol concentration | ||
| Tertile 1 | 1.43 (0.98, 2.08) | 0.064 |
| Tertile 2 | 1.52 (1.12, 2.06) | 0.007 |
| Tertile 3 | 1.00 (reference) | |
| GRS for total cholesterol | ||
| Tertile 1 | 1.52 (1.06, 2.18) | 0.023 |
| Tertile 2 | 1.76 (1.25, 2.46) | 0.001 |
| Tertile 3 | 1.00 (reference) |
Estimates were adjusted for time between examinations, examination, HDL-C and log(triacylglycerol) concentrations, sex, age, BMI, systolic and diastolic blood pressure and fasting blood glucose level
Compared with the third tertile
Associations between LDL-C and diabetes risk were generally similar across age, for the two sexes, and for different BMIs (p for all interactions > 0.2). Also, the association of LDL-C and incident diabetes was consistent across levels of HDL-C concentration (p for interaction = 0.1) and fasting plasma glucose concentration (p for interaction > 0.9) and did not differ significantly across quintiles of triacylglycerol concentration (p for interaction = 0.4). There were also no effect modifications by age, sex, BMI, fasting glucose or HDL-C or triacylglycerol concentrations for the association between GRS and incident diabetes risk (p for all interactions > 0.4).
Sensitivity analyses
The association of a GRS underlying total cholesterol and circulating total cholesterol levels was of similar magnitude to that of LDL-C (sex- and age-adjusted estimated influence 0.24, p < 0.0001). The GRS for total cholesterol was modestly associated with log-triacylglycerol concentration (sex- and age-adjusted estimated influence 0.07, p < 0.0001) and very modestly associated with HDL-C concentration (sex- and age-adjusted estimated influence −0.04, p < 0.0001). Analyses based on direct measurement of blood total cholesterol concentration yielded results consistent with those observed for LDL-C concentration,(Table 2 and ESM Fig. 1).
The correlation coefficients between the GRS for HDL-C and triacylglycerols with circulating concentrations of the corresponding lipid subtype were of similar magnitude to that of LDL-C (ESM Tables 3, 4). Neither the GRS for HDL-C nor the GRS for triacylglycerol was associated with incident diabetes (multivariable-adjusted OR for 1 SD increment in GRS 0.89 [95% CI 0.79, 1.02], p = 0.11 and 0.88 [95% CI 0.77, 1.01], p = 0.064 for HDL-C and triacylglycerol, respectively). When all three GRSs for LDL-C, HDL-C and triacylglycerol were included in the same model, the GRS for LDL-C remained borderline statistically significant (multivariable adjusted OR for 1 SD increment in GRS 0.89 [95% CI 0.79, 1.00], p = 0.060), whereas neither the GRS for HDL-C nor the GRS for triacylglycerol was significantly associated with incident diabetes (OR 0.97 [95% CI 0.85, 1.11], p = 0.64 and 0.94 [95% CI 0.81, 1.09], p = 0.38, respectively).
When using a more extensive GRS based on the 2013 genome-wide association study (157 SNPs) [22] the associations with incident diabetes were similar (multivariable adjusted OR 0.86 [95% 0.75, 0.98], p = 0.020 per 1 SD greater GRS).
Discussion
In the present investigation, we evaluated the association of blood LDL-C concentration with the risk of incident diabetes during a mean follow-up of 4.5 years in a large community-based sample of individuals who were not using antihypertensive or lipid-lowering medications. Our study supports recent evidence that low LDL-C concentrations might be associated with increased diabetes risk both in the presence and absence of cholesterol-lowering drug treatment [6]. Although our observational study precludes any causal inference, the temporal relation of the association (blood LDL-C assessment pre-dated diabetes incidence), the dose–response observed across the range of values (splines, Fig. 1), the consistency of results in multiple analyses and the observation of a similar relationship between the GRS and incident diabetes all strengthen the likelihood that the observed association is causal [23]. To the best of our knowledge, previous studies that have investigated the association between blood LDL-C concentration and diabetes have been cross-sectional in design [3, 4, 6–9] Therefore, we believe our prospective analyses are important and should encourage future studies to elucidate the biological underpinnings of the observed association, which may be important for future efforts to minimise the risk of diabetes with cholesterol-lowering treatment.
It is very likely that the observed inverse association of LDL-C with diabetes risk is more complex than our study was able to illuminate. For instance, diabetes risk may vary according to endogenous and exogenous cholesterol metabolic pathways, which may or may not have contributed to our observation of a non-linear relationship between GRS and diabetes risk (this possibly being due to the rather small sample size). Within this context, several cross-sectional studies have noted a direct association between insulin resistance and high endogenous cholesterol synthesis coupled with low intestinal cholesterol absorption [7– 9]. Yet, statin treatment inhibits endogenous cholesterol synthesis but increases the risk of future diabetes [3]. Hepatic endogenous cholesterol synthesis is linked to glucose metabolism via common metabolic intermediates including acetyl coenzyme A. It could be hypothesised that one way to adapt to subtle impairments in glucose metabolism is by means of increasing the production of endogenous cholesterol and triacylglycerol. A genetically reduced capacity to produce endogenous cholesterol (marked by lower LDL-C levels) would, in such case, perhaps be associated with increased diabetes risk in predisposed individuals, as suggested by previous case reports describing clustering of diabetes in individuals with familial hypobetalipoproteinaemia [24]. Further, in a recent study examining SNP variants relating to different lipid-associated genes, most SNPs showed opposite directionality of relations between LDL-C levels and fasting glucose / HbA1c / HOMA-IR [4]. Also, interestingly, because the rate of intestinal cholesterol absorption is reciprocally related to the rate of endogenous cholesterol synthesis, genetic variation related to higher intestinal cholesterol absorption pathways may perhaps lead to lower endogenous cholesterol production and a greater risk of diabetes. Consistent with this premise, an SNP variant within the Niemann– Pick C1-like 1 gene (NPC1L1) was shown to be associated with LDL-C concentration and insulin resistance in similar directions (i.e. low LDL-C and low HOMA-IR) [4]. This gene codes for a transmembrane protein located in the apical part of the intestinal enterocytes and canicular membrane of hepatocytes [25].
The net effect of blocking this channel is lower intestinal cholesterol uptake and perhaps to some extent higher endogenous cholesterol production (the channel is the target of the lipid-lowering drug ezetimibe) [25]. Another proposed mechanism is that pancreatic cholesterol uptake may be of importance for beta cell function and insulin secretion. Higher intracellular cholesterol concentrations have been suggested to be detrimental for pancreatic beta cell function [6, 26]. Such a hypothesis is consistent with the study linking familial hypercholesterolaemia (with defective LDL-receptors) to a lower prevalence of diabetes. Clearly, more studies are needed to delineate the relative importance of different cholesterol metabolism pathways and determine their impact on the risk of diabetes.
Limitations
Our study sample was modest in size and thereby precluded exploratory pathway-based genetic analyses or analyses aiming to address the relationship between individual SNPs in lipid genes and incident diabetes. We linked an aggregated risk score including SNPs that have previously been associated with LDL-C and total cholesterol concentrations to the risk of diabetes, but acknowledge that this may have been a simplification of the true relationship between lipids and diabetes risk [17]. Because the LDL-C GRS was also weakly associated with concentrations of triacylglycerols (positively) and HDL-C cholesterol (negatively) it cannot be excluded that our observations might have been biased towards the null (because high triacylglycerol and low HDL-C concentrations are markers of diabetes risk). However, consistent with prior analyses, we did not observe any evidence of causal influence of HDL-C or triacylglycerols on diabetes risk (i.e. the GRSs for these two lipid measures were not associated with diabetes) [5]. Further, the magnitude of the association between GRS and LDL-C concentrations was rather weak (age- and sex-adjusted estimated influence was 0.24). The GRS (which was based on rather fewer selected SNPs), therefore, left a proportion of the circulating LDL-C concentration unexplained. Finally, our sample mainly comprised white people of European ancestry and the generalisability to other races is unknown.
Clinical implications and future directions
As noted above, a recent community-based study showed that people with familial hypercholesterolaemia had a substantially lower prevalence of diabetes than people without familial hypercholesterolaemia [6]. This finding is interesting and is in agreement with our observations, where especially individuals with LDL-C concentrations or GRS above the median values had a lower risk of diabetes (estimated from the splines). Further, based on our observations it may be hypothesised that people with relatively low baseline cholesterol levels have the greatest risk of developing diabetes with statin treatment; however, this premise warrants further studies. Despite a plausible association of low LDL-C concentrations with diabetes risk, it is important to emphasise that the net effect of lowering cholesterol is cardioprotective among high-risk individuals. Thus, cholesterol-lowering treatment should not be withheld in individuals with prevalent, or at high risk of, cardiovascular disease. More research is warranted to confirm our observations and to elucidate the molecular mechanisms underlying the observed associations. Such studies may also help to guide future efforts to lower cholesterol levels without increasing diabetes risk.
Supplementary Material
Acknowledgments
Funding
The study was supported by NIH Heart, Lung and Blood Institute (contract no. N01-HC-25195). CA was supported by an independent research grant from the Danish agency for science, technology and innovation (grant no. FSS-11-120873). The sponsors had no influence on the design or conduct of the study, collection, management, analysis or interpretation of the data, preparation, review or approval of the manuscript or decision to submit the manuscript for publication.
Abbreviations
- GRS
Genetic risk score
- IV
Instrument variable
- HDL-C
HDL-cholesterol
- LDL-C
LDL-cholesterol
- SNP
Single-nucleotide polymorphism
Footnotes
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
All authors contributed to the conception and design of the study and acquisition of data. AL, CA and MGL performed the analyses. CA wrote the initial draft of the manuscript. All authors interpreted the data and contributed to the revision process with important intellectual content. All authors gave their final approval to the version to be published. RSV and CA are guarantors of this work.
References
- 1.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 2.Landray MJ, Haynes R, Hopewell JC, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 3.Swerdlow DI, Preiss D, Kuchenbaecker KB, et al. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet. 2015;385:351–361. doi: 10.1016/S0140-6736(14)61183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li N, van der Sijde MR, Bakker SJ, et al. Pleiotropic effects of lipid genes on plasma glucose, HbA1c, and HOMA-IR levels. Diabetes. 2014;63:3149–3158. doi: 10.2337/db13-1800. [DOI] [PubMed] [Google Scholar]
- 5.Fall T, Xie W, Poon W, et al. Using genetic variants to assess the relationship between circulating lipids and type 2 diabetes. Diabetes. 2015;64:2676–2684. doi: 10.2337/db14-1710. [DOI] [PubMed] [Google Scholar]
- 6.Besseling J, Kastelein JJ, Defesche JC, Hutten BA, Hovingh GK. Association between familial hypercholesterolemia and prevalence of type 2 diabetes mellitus. Jama. 2015;313:1029–1036. doi: 10.1001/jama.2015.1206. [DOI] [PubMed] [Google Scholar]
- 7.Pihlajamaki J, Gylling H, Miettinen TA, Laakso M. Insulin resistance is associated with increased cholesterol synthesis and decreased cholesterol absorption in normoglycemic men. J Lipid Res. 2004;45:507–512. doi: 10.1194/jlr.M300368-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Hoenig MR, Sellke FW. Insulin resistance is associated with increased cholesterol synthesis, decreased cholesterol absorption and enhanced lipid response to statin therapy. Atherosclerosis. 2010;211:260–265. doi: 10.1016/j.atherosclerosis.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 9.Simonen PP, Gylling HK, Miettinen TA. Diabetes contributes to cholesterol metabolism regardless of obesity. Diabetes Care. 2002;25:1511–1515. doi: 10.2337/diacare.25.9.1511. [DOI] [PubMed] [Google Scholar]
- 10.Tsao CW, Preis SR, Peloso GM, et al. Relations of long-term and contemporary lipid levels and lipid genetic risk scores with coronary artery calcium in the framingham heart study. J Am Coll Cardiol. 2012;60:2364–2371. doi: 10.1016/j.jacc.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demant MN, Gislason GH, Kober L, Vaag A, Torp-Pedersen C, Andersson C. Association of heart failure severity with risk of diabetes: a Danish nationwide cohort study. Diabetologia. 2014;57:1595–1600. doi: 10.1007/s00125-014-3259-z. [DOI] [PubMed] [Google Scholar]
- 12.Andersson C, Norgaard ML, Hansen PR, et al. Heart failure severity, as determined by loop diuretic dosages, predicts the risk of developing diabetes after myocardial infarction: a nationwide cohort study. Eur J Heart Fail. 2010;12:1333–1338. doi: 10.1093/eurjhf/hfq160. [DOI] [PubMed] [Google Scholar]
- 13.Zillich AJ, Garg J, Basu S, Bakris GL, Carter BL. Thiazide diuretics, potassium, and the development of diabetes: a quantitative review. Hypertension. 2006;48:219–224. doi: 10.1161/01.HYP.0000231552.10054.aa. [DOI] [PubMed] [Google Scholar]
- 14.Niklason A, Hedner T, Niskanen L, Lanke J. Development of diabetes is retarded by ACE inhibition in hypertensive patients--a subanalysis of the Captopril Prevention Project (CAPPP) J Hypertens. 2004;22:645–652. doi: 10.1097/00004872-200403000-00029. [DOI] [PubMed] [Google Scholar]
- 15.Shen L, Shah BR, Reyes EM, et al. Role of diuretics, beta blockers, and statins in increasing the risk of diabetes in patients with impaired glucose tolerance: reanalysis of data from the NAVIGATOR study. Bmj. 2013;347:f6745. doi: 10.1136/bmj.f6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 17.Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cupples LA, D’Agostino RB, Anderson K, Kannel WB. Comparison of baseline and repeated measure covariate techniques in the Framingham Heart Study. Stat Med. 1988;7:205–222. doi: 10.1002/sim.4780070122. [DOI] [PubMed] [Google Scholar]
- 19.D’Agostino RB, Lee ML, Belanger AJ, Cupples LA, Anderson K, Kannel WB. Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study. Stat Med. 1990;9:1501–1515. doi: 10.1002/sim.4780091214. [DOI] [PubMed] [Google Scholar]
- 20.Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM, D’Agostino RB., Sr Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med. 2007;167:1068–1074. doi: 10.1001/archinte.167.10.1068. [DOI] [PubMed] [Google Scholar]
- 21.Burgess S. Sample size and power calculations in Mendelian randomization with a single instrumental variable and a binary outcome. Int J Epidemiol. 2014;43:922–929. doi: 10.1093/ije/dyu005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deloukas P, Kanoni S, Willenborg C, et al. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pulai JI, Latour MA, Kwok PY, Schonfeld G. Diabetes mellitus in a new kindred with familial hypobetalipoproteinemia and an apolipoprotein B truncation (apoB-55) Atherosclerosis. 1998;136:289–295. doi: 10.1016/s0021-9150(97)00222-0. [DOI] [PubMed] [Google Scholar]
- 25.Jia L, Betters JL, Yu L. Niemann-pick C1-like 1 (NPC1L1) protein in intestinal and hepatic cholesterol transport. Annu Rev Physiol. 2011;73:239–259. doi: 10.1146/annurev-physiol-012110-142233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Preiss D, Sattar N. Does the LDL receptor play a role in the risk of developing type 2 diabetes? Jama. 2015;313:1016–1017. doi: 10.1001/jama.2015.1275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.