Abstract
Goblet cells and their main secretory product, mucus, have long been poorly appreciated; however, recent discoveries have changed this and placed these cells at the center stage of our understanding of mucosal biology and the immunology of the intestinal tract. The mucus system differs substantially between the small and large intestine, although it is built around MUC2 mucin polymers in both cases. Furthermore, that goblet cells and the regulation of their secretion also differ between these two parts of the intestine is of fundamental importance for a better understanding of mucosal immunology. There are several types of goblet cell which can be delineated based on their location and function. The surface colonic goblet cells secrete continuously to maintain the inner mucus layer, whereas goblet cells of the colonic and small intestinal crypts secrete upon stimulation, for example after endocytosis or in response to acetyl choline. However, despite much progress in recent years our understanding of goblet cell function and regulation is still in its infancy.
Keywords: Mucin, mucus, goblet cell, intestine, colon, colitis
The intestine
The gastrointestinal tract is a remarkable organ. Not only can it digest most of our food into small components, but it is it also filled with kilograms of microbes that live in stable equilibrium with us and our immune system. A digestive system can be traced back to early metazoans and Cambrian evolution (1), long before an adaptive immune system developed. The first appearance of molecules with protein domains typical to polymeric mucins was among metazoans as was the appearance of glycosyltransferases necessary for decorating the mucin protein core (2;3). Together this allowed the biosynthesis of molecules that are virtually indigestible by the host’s digestive enzymes. Avoiding self-digestion is a primary prerequisite of a digestive system, but handling of the luminal content without triggering an overt immune reaction while generating tolerance requires an intricate balance that is still poorly understood. A better understanding of epithelial cells and their function as part of the innate immune system, as well as their communication with lamina propria and intraepithelial adaptive immune cells, are a likely source of further understanding (4).
The single layer of epithelial cells along the gastrointestinal tract is very active and takes part in different absorption and secretion processes. To be able to withstand the physical and mechanical stress placed on these cells there is a continuous system of mucus covering the epithelium. There is a two-layered mucus system in the stomach and colon and a single layered mucus in the small intestine (5). The mucus layers in these three regions perform their protective function using different mechanisms and must be considered separately in order to properly comprehend the overall mucus system, a consideration that is often overlooked in the immunological literature.
The principle of separation
For quite some time the importance of the mucus covering the epithelium was overlooked and mucosal immunologists instead attempted to understand how the immune system could tolerate luminal bacteria which were assumed to be in direct contact with epithelial cells. However, this assumption was changed by the observation of an inner colonic mucus layer separating the bacteria from the host epithelium (6-8). Further studies of the gastrointestinal mucus systems have shown that all mucus functions to separate the luminal content, especially bacteria, from direct contact with the epithelial cells. The epithelial cells and especially the enterocytes are providing the best separation of luminal material from the lamina propria. Of special importance is the enterocyte apical glycocalyx that is built by transmembrane mucins and the tight junctions that firmly anchors the cells to each other (9;10). These systems are of course not perfect and some bacteria can come in contact with the epithelium even in the healthy intestine However, the mucosal immune system is programmed to tolerate low numbers of bacteria as exemplified by macrophage populations in the colon that can engulf bacteria, but still do not induce inflammatory responses or activate the adaptive immune system (11;12).
The net-like mucins forming the intestinal mucus have different properties in the small and large intestine. In the small intestine the pore sizes are large allowing bacteria and bacteria-sized particles to penetrate the mucus (5). The mucus fills the space between the villi and covers the villi tips, but bacteria are typically not found in contact with the epithelium except at the villus tip. The carbohydrate-rich polymeric mucin binds water that limits and slows down diffusion. The antibacterial peptides and proteins secreted from crypt Paneth cells and enterocytes into the mucus are of major importance for keeping bacteria at a distance (13-15). However, without the mucus, these antibacterial components would quickly be diluted into the intestinal lumen. The mucus concentrates them near to the epithelium and thus generates an antibacterial gradient from the epithelial cells out towards the lumen. The mucus also slows down bacterial penetration and is continuously renewed by the goblet cells, a process that pushes bacteria out towards the lumen. Together this explains the lack of bacteria in contact with the epithelial cells in the small intestine and may also suggest why most pathogenic bacteria are motile, i.e. in order to swim against the mucus flow and overcome the diffusion barrier. This penetrability of the small intestinal mucus may be the reason why pathogenic bacteria mostly infect this region of the gut using different mechanisms as discussed elsewhere (16).
The small intestinal mucus is normally easily aspirated and non-attached. However, this is not the case in germ-free animals where the MUC2 mucin, and thus the mucus, remains anchored to the goblet cells. The reason for this is that a proteolytic enzyme, meprin β, is required for cleaving MUC2 and releasing it from the goblet cell attachment (17). Meprin β is anchored in the enterocyte apical membrane and cannot access the mucin goblet cell anchor from this position. To contact MUC2 it needs to first be released by extracellular cleavage allowing it to diffuse into the mucus. It is the release of anchored meprin β that is controlled and triggered by exposure to bacteria. This regulation of the mechanism for releasing anchored mucus suggests that it has an important role in the small intestine’s handling of bacteria. A hint of this function can be observed in the small intestine lacking a functional CFTR chloride and bicarbonate channel as in the disease cystic fibrosis (18). In this case the mucins also remain anchored as low levels of bicarbonate do not sufficiently well remove calcium ions to allow for unfolding the packed MUC2, necessary for exposing the meprin β cleavage sites (17). In mice and humans a non-functional CFTR channel causes bacterial overgrowth, distal intestinal obstruction syndrome (DIOS) and sometimes obstructive ileus. This shows how important mucus is for trapping and moving bacteria away from the host surface.
In the colon there is a two-layered mucus system where the inner mucus layer is stratified and organized as a filter that physically separates the bacteria from the epithelial cells (6). This layer is anchored to the goblet cells. At about 50 µm (mouse) or 200 µm (human) distance from the epithelium endogenous protease activities convert the inner mucus layer into the outer non-attached mucus that is slowly expanded 2-3 fold in volume. This expansion increases the pore sizes allowing bacteria to penetrate. The pore sizes of the inner mucus layer are sufficiently small to hinder penetration of bacteria or beads down to 0.5 µm diameter. Interestingly, this penetrability is regulated. Germ-free animals have a penetrable mucus as is also the case for animals lacking the cytokine IL10 or the NHE3 ion transporter (19). The composition of the bacterial flora can also influence the mucus penetrability as observed in genetically identical animals (20). The molecular mechanisms underlying this modulation of inner mucus penetrability are not yet understood.
In the absence of MUC2 there is no mucus and bacteria are in direct contact with the epithelium which triggers inflammation similar to that found in ulcerative colitis and, in the long-term, can lead to cancer. Furthermore, animals with a penetrable inner mucus layer develop spontaneous colitis. Patients with active ulcerative colitis have a fully penetrable mucus, whereas patients in remission have a more variable profile with some looking perfectly normal (19). Together this demonstrates the importance of separating the majority of the luminal contents from the epithelial cells, a function that is dependent on the mucus layer.
The mucins are the structural building block in mucus
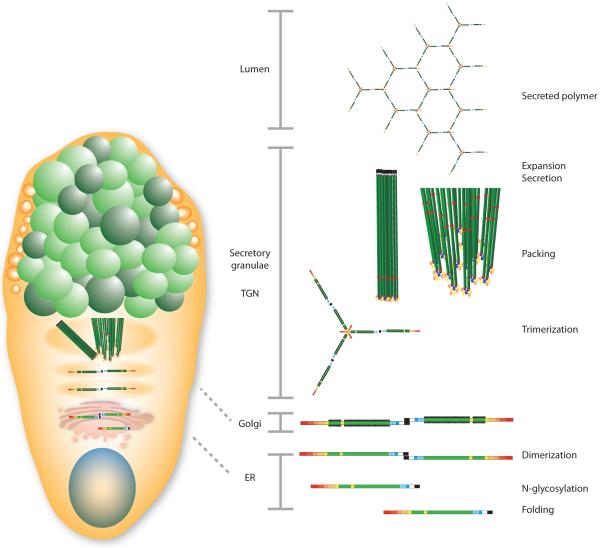
The intestinal mucus proteome is around 50 proteins with a few that are very abundant. The major component is the MUC2 mucin, the function of which has been determined in recent years. However, the functions of most of the other more abundant proteins, such as FCGBP, CLCA1, AGR2, and ZG16 remain mostly unknown (6). MUC2 comprises approximately 5,100 amino acids, with the uncertainty originating from the central region that is still not fully sequenced (21). MUC2 contains two long central regions called PTS domains that become rigid, outstretched rods after O-glycosylation and are referred to as mucin domains (Fig. 1). In MUC2 these regions are about 0.5 µm long. The N- and C-terminal regions are about 1,300 and 1,000 amino acids, respectively, and are tightly folded and stabilized by numerous disulfide bonds. The MUC2 monomeric building block has a mass of about 2.5 MDa when fully glycosylated and is polymerized by C-terminal dimerization and N-terminal trimerization, resulting in the formation of enormous net-like polymeric sheets (22).
Figure 1.
Assembly of the MUC2 mucin in the goblet cell.
Goblet cells
Goblet cells are specialized for the synthesis and secretion of mucus. They acquired their name for their typical goblet, cup-like, appearance formed by the mucin granulae which fill up the cytoplasm (Fig. 1). In addition to MUC2 the mucin granulae are filled with the other typical mucus components FCGBP, CLCA1, ZG16, and AGR2. Mice lacking Muc2 have the same number of goblet cells but these do not have the typical goblet cell shape.
The intestinal surface epithelium, including goblet cells, is continuously renewed from the stem cells at the crypt base with normal cell turnover between 3-7 days (23). The stem cells give rise to all epithelial cells including the enterocytes, goblet cells, Paneth cells, enteroendocrine cells, and Tuft cells. The enterocyte lineage is the primary cell lineage and its differentiation pathway is controlled by Notch signaling. When Notch signaling is blocked by γ-secretase inhibition, the Notch cytoplasmic component no longer enters the nucleus, inhibiting transcriptional signaling and shifting differentiation into the secretory pathway (24). Without further changes in signaling, goblet cells are the default result of this shift. Furthermore, the transcription factor Spdef is important for full maturation into goblet cells (25).
Although there is still limited knowledge of the cell biology of goblet cells, it has become obvious that there are several types that function in different ways. In the small intestine the goblet cell adjacent enterocytes supply the required bicarbonate for proper mucin unfolding (18). This is not the case in the large intestine where the goblet cell might supply bicarbonate via its own bestrophin-2 bicarbonate transporter (26). Other examples are the inter-crypt colonic surface goblet cells that continuously secrete mucus and by this build the inner mucus layer contrast with the goblet cells in the upper part of the colonic crypts that can secrete by rapid compound exocytosis (27;28). In order to better understand goblet cell secretion and function we first need to realize that these cells are more diverse and capable than has been previously anticipated.
Goblet cell exocytosis
MUC2 polymers are densely packed in the regulated secretory vesicles of the goblet cell due to the low pH and high calcium in these compartments. In this milieu the N-terminal trimers spontaneously form concatenated rings with the long extended mucin domains standing perpendicular to this structure and joined to other MUC2 molecules end-to-end by their C-termini (Fig. 1 (22). More recent structural studies of the MUC2 N-terminal D3 domain suggested that every second MUC2 mucin layer is rotated 180° and the N-terminal ring structures meet (29). Upon secretion, the densely packed mucin expands >1,000-fold into large nets (Fig. 1). This process requires an increase in pH to and the removal of N-terminally bound single calcium ion (30). Bicarbonate is the ideal and physiological solution for precipitating calcium and raising the pH (18). Previous models of mucin packing in the goblet cell have suggested that this is less organized and that the calcium only acts as a shield preventing electrostatic repulsion by negatively charged glycans; however, this model does not allow for the rapid and well organized mucin expansion observed (31).
Secretion of mucins can take place in at least two ways; regulated vesicle secretion and compound exocytosis. Regulated vesicle secretion has been well studied in airway cells (32). This process includes the fusion of single vesicles with the plasma membrane mediated by typical vesicle exocytosis components like Syntaxins, Munc18, VAMP, and SNAP proteins. Secretion from surface goblet cells of both the small and large intestine, although not studied, probably follows similar mechanisms. Compound exocytosis is a more dramatic event where most of the vesicles that make up the goblet cell theca fuse and empty their content. There are no molecular studies on this type of secretion, but there are very clear morphological studies which show that the whole cell essentially explodes during this secretory process (33).
Renewal of the colonic inner mucus layer by mucus secretion is important for its protective function. Mucus renewal is much faster than epithelial cell turnover and is most likely tightly coupled to propulsion of the luminal material. Turnover studies have been conducted by in vivo glycoprotein labeling using an azide modified N-Acetylgalactosamine, GalNAz, which is incorporated into glycans during protein biosynthesis (27). Mucins, with their numerous O-glycans, are very effectively labeled by this method. The goblet cells on the colon surface epithelium showed rapid mucin synthesis and secrete the mucus without extensive storage. This was in contrast to the goblet cells in the upper crypts, where the mucus accumulated in granulae over time and secretion was slower. In the small intestine, with its larger epithelial surface, cell renewal takes between 3-5 days (23). Investigation of this region using the same method demonstrated that crypt goblet cells were labeled very slowly, but massive secretion was observed at the crypt opening a few hours after GalNAz injection, suggesting an enforced protection of the vulnerable crypts (27). Conversely, the goblet cells on the villi have faster mucin biosynthesis and secretion.
Goblet cell endocytosis
Recent research has indicated that optimal intestinal goblet cell mucus exocytosis is dependent on several intersecting cellular processes which together appear to modulate mucin granule accumulation and secretion. These include endocytosis, autophagy, ROS generation and inflammasome assembly and activation.
Endocytosis of luminal material by colonic goblet cells was described in the 1980’s by showing the uptake of cationic ferritin using electron microscopy (34;35). These studies observed that in some goblet cells intracellular cationic ferritin was localized to endosomes located at the periphery of the theca and that these vesicles eventually fused with lysosomes. Although the extent of endocytosis was relatively limited in adult tissues, it was substantially elevated in fetal tissue indicating a developmental aspect to this process (35). At the time it was believed that this was a mechanism for recycling the plasma membrane which accumulated apically during exocytosis (36); however, the endocytotic pathway has recently been shown to have a function beyond membrane salvage.
Using a novel method for culturing differentiated intestinal cells from mouse stem cells, Patel et al. demonstrated that inhibition of clathrin-mediated endocytosis resulted in the accumulation of goblet cell mucin granulae (37). Importantly, a similar phenotype was observed in cells lacking proteins involved in conventional autophagy including Atg5, Atg14 and FIP200. Mucin accumulation occurred independently of mucin expression, indicating that the effect was likely secondary to defects in mucin secretion. Electron microscopy showed that LC3β, a marker for autophagosomes, could be localized to multivesicular vacuoles that were also positive for the endosomal marker EEA1. This association was abrogated in endocytosis-inhibited cells, thus potentially linking the autophagy and endocytotic machinery to mucin secretion in goblet cells.
Autophagy is a ubiquitous cytoplasmic process in which a double membrane autophagosome is formed and subsequently fused with lysosomes for degradation of enclosed components, a process linked to immunity and inflammation (38). An autophagosome can also fuse with endosomes to form intermediate structures called amphisomes which closely resemble the LC3β/EEA1-positive vacuoles observed by Patel et al. (37). This study also provided evidence that these amphisome-like structures possess NADPH oxidases and that their loss, due to inhibition of endocytosis or knock-out of autophagy genes, results in decreased cellular ROS production. Crucially, application of exogenous ROS rescued the mucin accumulation phenotype, indicating that ROS may be involved in regulating mucin secretion.
Another potential actor in the regulation of goblet cell secretion is the inflammasome. These are multi-protein complexes that are typically comprised of one of the NOD-like receptor (NLR) family members linked to an inflammatory caspase, such as Caspase 1, via homotypic interactions between PYD and CARD domains present on the adapter protein ASC (39). NLRPs are thought to function as sensors for various motifs in pathogen-associated molecules and for endogenous damage or danger signals. Upon activation, NLRPs bind ASC which in turn recruits Procaspase 1. NLRPs oligomerize via their NACHT domains, resulting in a large ring-shaped inflammasome complex with 7- or 8-fold symmetry. Inflammasome assembly permits proximity-induced autoactivation of inflammatory caspases which classically proceed to cleave the proforms of IL1α, IL1β and IL18 cytokines into their mature active forms (40). Inflammasomes are abundantly expressed among immune cells and most current knowledge is derived from studies in these cell types; however, some NLRPs are also found in mucosal epithelial cells and are suggested to have roles in intestinal homeostasis (41-44).
NLRP6 is expressed by colonic goblet cells and was recently suggested to have a functional role in mucin exocytosis as mice lacking NLRP6 displayed a similar mucin granule accumulation as reported for Atg5−/− intestinal cells (37;44). Lack of NLRP6 was previously shown to have functional consequences in vivo as knockout mice were more susceptible to both DSS and Citrobacter rodentium-induced colitis (41;44). Similar outcomes were observed in both Caspase 1/11−/− and ASC−/− animals, implying that activated and fully assembled inflammasomes are required for functional mucus secretion. In addition, mice lacking NLRP6 had increased numbers of damaged mitochondria and alterations in autophagic flux, both of which are symptomatic of defective autophagy.
Current data suggests that there are strong links between endocytosis, autophagy, inflammasome activity, ROS generation, and mucin secretion. Understanding how these systems are coupled together and the molecular basis for their regulatory effect on goblet cell exocytosis will be interesting avenues of future research. However, it is already clear that mucus secretion is an end point of the interaction of all of these innate immune system molecules, once more pointing to the central role of mucus secretion and the generation of a functional mucus layer in protecting the host epithelium.
Induced goblet cell mucus secretion
Exocytosis of mucin granulae can also be regulated by intracellular Ca2+ levels and Ca2+ mobilizing agents such as acetylcholine and histamine are potent inducers of intestinal mucus secretion. Acetylcholine is by far the most studied mucus secretagogue and has been shown to induce mucus secretion in both the small and large intestine of mouse, rat and rabbit, and in the human colon (5;33;45-47). Stimulation with acetylcholine or other cholinergic agonists such as carbachol results in a rapid transient increase in mucus secretion rates that reverts to baseline values within 30 min (47). Acetylcholine primarily targets the goblet cells in the small intestinal crypts, resulting in more or less complete emptying of the stored mucin granule pool. The villus goblet cells in the small intestine and the surface goblet cells in the colon do not respond to acetylcholine or carbachol with exocytosis. Although cholinergic agonists are inducers of mucus secretion in both the small and large intestine, the small intestine is by far the most sensitive to carbachol (5;47). Conversely, the opposite pattern is instead true for histamine which induces mucus secretion in the colon, but not in the small intestine (45;48). The immune modulator prostaglandin E2 (PGE2) has been shown to induce mucus secretion in rat colon and mouse small intestine, but only induces fluid secretion in the human colon (48;49). Species and segmental differences in the responsiveness to PGE2 may be explained by PGE2 being able to signal via both cAMP and Ca2+ dependent pathways depending on which of its receptors is present.
The triggers for compound exocytosis from the colonic crypts have not yet been clearly identified. However, a coordinated process of mucus secretion, ion and fluid secretion, and motility is an efficient way to dispose harmful agents. The importance of this secretory process in maintaining the intestinal barrier was recently described by Grootjans et al. who showed that ischemia induced tissue damage was prevented by compound exocytosis of mucin vesicles in the colonic crypt goblet cells that cleared the crypts of bacteria that had managed to penetrate the crypts during the ischemic period (50).
Goblet cells as antigen importers
Although production and secretion of mucus are the main functions of the intestinal goblet cells, recent studies have shown that the small intestinal population is also able to acquire luminal antigens and present these to CD103+ dendritic cells in the lamina propria (51;52). Spontaneous antigen uptake was observed in the small intestine of conventionally raised mice, but not in the colon. However, in mice raised under germ free conditions and in conventionally raised mice following oral treatment with antibiotics spontaneous antigen uptake was also observed in the colon, suggesting that the uptake process is inhibited by the colonic microbiota (51;53). Similarly to regulation of mucus secretion, antigen uptake by the small intestinal goblet cells is augmented by cholinergic agonists and both baseline and agonist induced antigen uptake is mediated via muscarinic receptor 4 (53). The ability of the intestinal goblet cells to acquire luminal antigens correlates with the expression of the m4 receptor i.e. higher expression in the small intestine compared to the colon and increased expression following antibiotics treatment (53). The observed differences in carbachol induced antigen uptake in the small and large intestine correlate with the observed differences in the sensitivity to carbachol induced mucus secretion, suggesting that the two processes are regulated by similar mechanisms.
Immune regulation of goblet cell function
That the intestinal goblet cells are under direct regulation by the immune system is best exemplified by the striking goblet cell hyperplasia and mucus hypersecretion associated with parasitic helminth infections (54;55). These infections elicit a T helper type 2 (Th2) response with increased levels of cytokines such as interleukin IL-4, IL-5, IL-9 and IL-13, where IL-13 is considered the major effector cytokine. Intestinal epithelial cells have been shown to express the IL-4Rα and IL13Rα1 subunits, making it likely that IL-13 acts directly on the epithelium to induce goblet cell hyperplasia via STAT6 signaling (56). Further support in favor of IL-13 being an important regulator of goblet cell hyperplasia is found in studies showing that IL-13 overexpressing mice develop intestinal goblet cell hyperplasia and that exogenous IL-25 or IL-9 overexpression induce goblet cell hyperplasia and increased mucin expression via IL-13 dependent pathways (57). Th2 responses, including goblet cell hyperplasia, are also fundamental to allergic asthma. Interestingly, recent observations suggest that the Spdef transcription factor is required for the normal pulmonary Th2 inflammatory response and goblet cell hyperplasia (58). Spdef regulates goblet cell maturation and differentiation in both the lung and intestine (25;58;59), further suggesting that the goblet cell is involved in the regulation of the immune system (60).
In addition to the importance of Th2 cytokines in regulation of goblet cell function, recent findings also implicate the Th17 associated cytokine IL-22 in regulation of goblet cell differentiation and mucin expression as IL-22 deficient mice fail to increase Muc2 expression and have reduced levels of goblet cell hyperplasia in response to N. brasiliensis and T. muris infections compared to wild-type animals (61). Interestingly, these effects were observed in the presence of increased levels of IL-4 and IL-13, suggesting overlapping pathways for induction of mucin expression and goblet cell differentiation. Less is understood on the role of Th1 cytokines such as interferon-γ (IFNγ), TNFα, and Th17 cytokines such as IL-23 and IL-17 in regulation of goblet cell function.
Regulation of goblet cells by immunomodulatory cytokines
The MUC2 mucin is not only large, but also has termini that are stabilized by disulfide bonding between the cysteine residues that comprise more than 10% of the amino acids present in these regions. Correct folding of the terminal regions requires the careful joining of each disulfide bond making it one of the more difficult proteins for the ER to handle. This is reflected in the need for MUC2 to have a special chaperone, ERN2 (also called IRE1β), that seems to be utilized exclusively for mucin folding (62;63). Misfolded proteins trigger an unfolded protein response (UPR) that is in turn coupled to inflammation (64). Single mutations in mouse Muc2 can cause ER accumulation that triggers a UPR response and increased levels of inflammatory cytokines (65;66). Higher quantitative demands for MUC2 synthesis, such as an increased bacterial load in contact with the epithelium, will further challenge the ER folding system and trigger UPR responses and inflammation. However, there seem to be at least two immunological systems that function to limit such deleterious developments. These are based on the protective cytokines IL22 and IL10.
IL22, which is primarily produced by NK cells, has protective effects on intestinal epithelial cells (67;68). The mechanism for this is not yet known, but recent observations on the effects of IL22 on insulin producing cells suggests that IL22 can reduce and alleviate inflammation in conjunction with the UPR (69).
IL10 has an immune regulatory function and is critical for intestinal homeostasis as IL10 and IL10 receptor deficient mice develop colitis that is dependent on bacteria (70-72). Genome-wide association studies (GWAS) in IBD patients have associated single nucleotide polymorphisms (SNPs) in the IL10 loci with IBD (73;74) and mutations in the IL10 receptors are also linked to IBD (75). IL10 is produced by several cells of the immune system but has also been shown to be produced by the epithelium (76;77). The effect of this cytokine has mainly been studied in th econtext of its effect on immune cells, and its effects on epithelial cells are less explored. Interestingly, bacteria manipulated to express IL10 have been shown to reduce experimental colitis (78). IL10 has a direct effect on goblet cells by modulating their ability to manage mucin biosynthesis and misfolding (79) and decreased MUC2 production has been demonstrated in IL10 deficient mice (80). In young IL10 deficient mice, prior to the development of inflammation, the inner colonic mucus layer has a normal thickness, but is fully penetrable to bacteria and beads the size of bacteria (19). The bacteria in contact with the epithelium likely trigger inflammatory responses leading to colitis as seen in mice lacking the Muc2 mucin (6). Together it can be suggested that IL10 and IL22 have important positive effects on the goblet cell function, again suggesting important connections between the immune system and goblet cells.
Conclusions
Goblet cells form the major line of defense at the intestinal mucosa, based primarily on secretion of the MUC2 mucin. Although all goblet cells have a common secretory role, evidence suggests that cells in different segments of the intestine, and different segmental sub-regions, are responsive to variable stimuli and have distinct roles to play in maintaining intestinal homeostasis. Although the existence of different goblet cell populations is only now becoming clear, it should not be considered surprising given the diversity of other cell types of the innate immune system. Indeed, the findings summarized in this review demonstrate that immune system associated intracellular processes and intercellular signaling have a significant role to play in regulating goblet cell activity, strongly indicating that these cells have a central role to play as the gatekeepers of the mucosal immune system.
Acknowledgements
Without the hard work of all the members of the Mucin Biology Groups this contribution would not be possible. This work was supported by the Swedish Research Council, The Swedish Cancer Foundation, The Knut and Alice Wallenberg Foundation, IngaBritt and Arne Lundberg Foundation, Sahlgren's University Hospital (LUA-ALF), Wilhelm and Martina Lundgren’s Foundation, Torsten och Ragnar Söderbergs Stiftelser, The Sahlgrenska Academy, National Institute of Allergy and Infectious Diseases (U01AI095473, the content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH), The Swedish Foundation for Strategic Research - The Mucus-Bacteria-Colitis Center (MBC) of the Innate Immunity Program, The Swedish CF Foundation, Erica Lederhausen’s Foundation, and Lederhausen’s Center for CF Research at Univ. Gothenburg. We also acknowledge the Mammalian Protein Expression Core Facility and the Centre for Cellular Imaging at the University of Gothenburg.
Footnotes
No conflict of interest
References
- 1.Erwin DH, Valentine JW. The Cambrian explosion: The construction of animal diversity. Roberts and Companry Publishers; Greenwood Village, CO: 2013. [Google Scholar]
- 2.Lang T, Hansson GC, Samuelsson T. Gel-forming mucins appeared early in metazoan evolution. Proc. Natl. Acad. Sci. USA. 2007;104:16209–14. doi: 10.1073/pnas.0705984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett EP, Mandel U, Clausen H, Gerken TA, Fritz TA, Tabak LA. Control of mucin-type O-glycosylation: A classification of the polypeptide GalNAc-transferase gene family. Glycobiology. 2012;22:736–56. doi: 10.1093/glycob/cwr182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swamy M, Jamora C, Havran W, Hayday A. Epithelial decision makers: in search of the 'epimmunome'. Nat Immunol. 2010;11:656–65. doi: 10.1038/ni.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ermund A, Schutte A, Johansson MEV, Gustafsson JK, Hansson GC. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer's patches. Am. J. Physiol. Gastroint. Liver Physiol. 2013;305:G341–G347. doi: 10.1152/ajpgi.00046.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansson MEV, Phillipson M, Petersson J, Holm L, Velcich A, Hansson GC. The inner of the two Muc2 mucin dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA. 2008;105:15064–9. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swidsinski A, Sydora B, Doerffel Y, Loening-Baucke V, Vaneechoutte M, Lupicki M, et al. Viscosity gradient within the mucus layer determines the mucosal barrier function and the spatial organization of the intestinal microbiota. Inflam. Bowel Dis. 2007;13:693–70. doi: 10.1002/ibd.20163. [DOI] [PubMed] [Google Scholar]
- 8.van der Waaij LA, Harmsen HJ, Madjipour M, Kroese FG, Zwiers M, van Dullemen HM, et al. Bacterial population analysis of human colon and terminal ileum biopsies with 16S rRNA-based fluorescent probes: commensal bacteria live in suspension and have no direct contact with epithelial cells. Inflamm. Bowel Dis. 2005;11:865–71. doi: 10.1097/01.mib.0000179212.80778.d3. [DOI] [PubMed] [Google Scholar]
- 9.Sheng YH, Triyana S, Wang R, Das I, Gerloff K, Florin TH, et al. MUC1 and MUC13 differentially regulate epithelial inflammation in response to inflammatory and infectious stimuli. Mucosal Immunol. 2013;6:557–68. doi: 10.1038/mi.2012.98. [DOI] [PubMed] [Google Scholar]
- 10.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 11.Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013;6:498–510. doi: 10.1038/mi.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grainger JR, Wohlfert EA, Fuss IJ, Bouladoux N, Askenase MH, Legrand F, et al. Inflammatory monocytes regulate pathologic responses to commensals during acute gastrointestinal infection. Nat Med. 2013;19:713–21. doi: 10.1038/nm.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, et al. The Antibacterial Lectin RegIIIg Promotes the Spatial Segregation of Microbiota and Host in the Intestine. Science. 2011;33:255–8. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansson MEV, Hansson GC. Keeping Bacteria at a Distance. Science. 2011;334:182–3. doi: 10.1126/science.1213909. [DOI] [PubMed] [Google Scholar]
- 15.Clevers HC, Bevins CL. Paneth cells: maestros of the small intestinal crypts. Annu. Rev Physiol. 2013;75:289–311. doi: 10.1146/annurev-physiol-030212-183744. [DOI] [PubMed] [Google Scholar]
- 16.McGuckin MA, Linden SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat Rev Micro. 2011;9:265–78. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- 17.Schütte A, Ermund A, Becker-Pauly C, Johansson MEV, Rodriguez-Pineiro AM, Bäckhed F, et al. Microbial Induced Meprin â Cleavage in MUC2 Mucin and Functional CFTR Channel are Required to Release Anchored Small Intestinal Mucus. Proc. Natl. Acad. Sci. USA. 2014;111:12396–401. doi: 10.1073/pnas.1407597111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gustafsson JK, Ermund A, Ambort D, Johansson MEV, Nilsson HE, Thorell K, et al. Bicarbonate and functional CFTR channel is required for proper mucin secretion and link Cystic Fibrosis with its mucus phenotype. J. Exp. Med. 2012;209:1263–72. doi: 10.1084/jem.20120562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johansson MEV, Gustafsson JK, Holmen-Larsson J, Jabbar KS, Xia L, Xu H, et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and in patients with ulcerative colitis. Gut. 2014;213:281–91. doi: 10.1136/gutjnl-2012-303207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jakobsson HE, Rodriguez-Pineiro AM, Schütte A, Ermund A, Boysen P, Sommer F, et al. The gut microbiota composition impairs the colon inner mucus layer barrier. EMBO Reports. 2014 doi: 10.15252/embr.201439263. on line. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gum JR, Hicks JW, Toribara NW, Siddiki B, Kim YS. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J. Biol. Chem. 1994;269:2440–6. [PubMed] [Google Scholar]
- 22.Ambort D, Johansson MEV, Gustafsson JK, Nilsson H, Ermund A, Johansson BR, et al. Calcium and pH-dependent Packing and Release of the Gel-forming MUC2 Mucin. Proc. Natl. Acad. Sci. U. S. A. 2012;109:5645–50. doi: 10.1073/pnas.1120269109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 24.Clevers H. The Intestinal Crypt, A Prototype Stem Cell Compartment. Cell. 2013;154:274–84. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Gregorieff A, Strange DE, Kujala P, Begthel H, van den Born M., Korving J, et al. The Ets-Domain Transcription Factor Spdef Promotes Maturation of Goblet and Paneth Cells in the Intestinal Epithelium. Gastroenterology. 2009;137:1333–45. doi: 10.1053/j.gastro.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 26.Yu K, Lujan R, Marmorstein A, Gabriel S, Hartzell HC. Bestrophin-2 mediates bicarbonate transport by goblet cells in mouse colon. J Clin Invest. 2010 doi: 10.1172/JCI41129. 0, 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johansson MEV. Fast Renewal of the Distal Colonic Mucus Layers by the Surface Goblet Cells as Measured by In Vivo Labeling of Mucin Glycoproteins. PLoS ONE. 2012;7:e41009. doi: 10.1371/journal.pone.0041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Specian RD, Neutra MR. Regulation of intestinal goblet cell secretion. I. Role of parasympathetic stimulation. Am J Physiol. 1982;242:G370–G379. doi: 10.1152/ajpgi.1982.242.4.G370. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson HE, Ambort D, Backstrom M, Thomsson E, Koeck PJ, Hansson GC, et al. Intestinal MUC2 mucin supramolecular topology by packing and release resting on D3 domain assembly. J. Mol. Biol. 2014;426:2567–79. doi: 10.1016/j.jmb.2014.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ridley C, Kouvatsos N, Raynal BD, Howard M, Collins RF, Desseyn JL, et al. Assembly of the Respiratory Mucin MUC5B: A NEW MODEL FOR A GEL-FORMING MUCIN. J. Biol. Chem. 2014;289:16409–20. doi: 10.1074/jbc.M114.566679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verdugo P. Mucin Exocytosis. Am. Rev. Resp. Dis. 1991;144:S33–S37. doi: 10.1164/ajrccm/144.3_pt_2.S33. [DOI] [PubMed] [Google Scholar]
- 32.Adler KB, Tuvim MJ, Dickey BF. Regulated Mucin Secretion from Airway Epithelial Cells. Frontiers in Endocrinology. 2013;4 doi: 10.3389/fendo.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Specian D, Neutra MR. Mechanism of rapid mucus secretion in goblet cells stimulated by acetylcholine. J. Cell Biol. 1980;85:626–40. doi: 10.1083/jcb.85.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbour WM, Hopwood D. Uptake of cationized ferritin by colonic epithelium. J Pathol. 1983;139:167–78. doi: 10.1002/path.1711390208. [DOI] [PubMed] [Google Scholar]
- 35.Colony PC, Specian RD. Endocytosis and vesicular traffic in fetal and adult colonic goblet cells. Anat. Rec. 1987;218:365–72. doi: 10.1002/ar.1092180403. [DOI] [PubMed] [Google Scholar]
- 36.Herzog V. Pathways of endocytosis in secretory cells. Trends Biochem. Sci. 1981;6:319–22. [Google Scholar]
- 37.Patel KK, Miyoshi H, Beatty WL, Head RD, Malvin NP, Cadwell K, et al. Autophagy proteins control goblet cell function by potentiating reactive oxygen species production. Embo J. 2013;32:3130–44. doi: 10.1038/emboj.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–35. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–22. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–26. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 41.Chen GY, Liu M, Wang F, Bertin J, Nunez G. A Functional Role for Nlrp6 in Intestinal Inflammation and Tumorigenesis. J Immunol. 2011;186:7187–94. doi: 10.4049/jimmunol.1100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elinav E, Strowig T, Kau A, Henao-Mejia J, Thaiss C, Booth C, et al. NLRP6 Inflammasome Regulates Colonic Microbial Ecology and Risk for Colitis. Cell. 2011;145:745–57. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sellin M, M++ller A, Felmy B, Dolowschiak T, Diard M+, Tardivel A, et al. Epithelium-Intrinsic NAIP/NLRC4 Inflammasome Drives Infected Enterocyte Expulsion to Restrict Salmonella Replication in the Intestinal Mucosa. Cell Host & Microbe. 2014;16:237–48. doi: 10.1016/j.chom.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Wlodarska M, Thaiss CA, Nowarski R, Henao-Mejia J, Zhang JP, Brown EM, et al. NLRP6 inflammasome orchestrates the colonic host-microbial interpace by regulating goblet cell mucus secretion. Cell. 2014 doi: 10.1016/j.cell.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neutra MR, O'Malley LJ, Specian RD. Regulation of intestinal goblet cell secretion. II. A survey of potentid secretugogues. Am. J. Physiol. Gastroint. Liver Physiol. 1982;242:G380–G387. doi: 10.1152/ajpgi.1982.242.4.G380. [DOI] [PubMed] [Google Scholar]
- 46.Halm DR, Halm ST. Secretagogue response of goblet cells and columnar cells in human colonic crypts. Am J Physiol Cell Physiol. 2000;278:C212–C233. doi: 10.1152/ajpcell.2000.278.1.C212. [DOI] [PubMed] [Google Scholar]
- 47.Gustafsson JK, Ermund A, Johansson MEV, Schutte A, Hansson GC, Sjovall H. An ex vivo method for studying mucus formation, properties and thickness in human colonic biopsies and mouse small and large intestinal explants. Am. J. Physiol. Gastrointest. Liver. Physiol. 2012;302:G430–G438. doi: 10.1152/ajpgi.00405.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Halm DR, Halm ST. Secretagogue response of goblet cells and columnar cells in human colonic crypts. Am. J. Physiol. - Cell Physiol. 2000;278:C212–C233. doi: 10.1152/ajpcell.2000.278.1.C212. [DOI] [PubMed] [Google Scholar]
- 49.Garcia MA, Yang N, Quinton PM. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator−dependent bicarbonate secretion. J. Clin. Invest. 2009;119:2613–22. doi: 10.1172/JCI38662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grootjans J, Hundscheild IH, Lenaerts K, Boonen B, Renes IB, Verheyen FK, et al. Ischemia-induced mucus barrier loss and bacterial penetration are rapidly counteracted by increased goblet cell secretory activity in human and rat colon. Gut. 2013;62:250–8. doi: 10.1136/gutjnl-2011-301956. [DOI] [PubMed] [Google Scholar]
- 51.McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483:345–9. doi: 10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Howe SE, Lickteig DJ, Plunkett KN, Ryerse JS, Konjufca V. The Uptake of Soluble and Particulate Antigens by Epithelial Cells in the Mouse Small Intestine. PLoS ONE. 2014;9:e86656. doi: 10.1371/journal.pone.0086656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knoop KA, McDonald KG, McCrate S, McDole JR, Newberry RD. Microbial sensing by goblet cells controls immune surveillance of luminal antigens in the colon. Mucosal Immunol. 2014 doi: 10.1038/mi.2014.58. on line, doi:10.1038/mi.2014.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marillier RG, Michels C, Smith EM, Fick LC, Leeto M, Dewals B, et al. IL-4/IL-13 independent goblet cell hyperplasia in experimental helminth infections. BMC Immunol. 2008;9:11, 11–9. doi: 10.1186/1471-2172-9-11. doi: 10.1186/1471-2172-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oeser K, Schwartz C, Voehringer D. Conditional IL-4/IL-13-deficient mice reveal a critical role of innate immune cells for protective immunity against gastrointestinal helminths. Mucosal Immunol. 2014;10 doi: 10.1038/mi.2014.101. [DOI] [PubMed] [Google Scholar]
- 56.Finkelman FD, Shea-Donohue T, Morris SC, Gildea L, Strait R, Madden KB, et al. Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol Rev. 2004;201:139–55. doi: 10.1111/j.0105-2896.2004.00192.x. 139-55. [DOI] [PubMed] [Google Scholar]
- 57.Steenwinckel V. r., Louahed J, Lemaire MM, Sommereyns C, Warnier G, McKenzie A, et al. IL-9 Promotes IL-13-Dependent Paneth Cell Hyperplasia and Up-Regulation of Innate Immunity Mediators in Intestinal Mucosa. J Immunol. 2009;182:4737–43. doi: 10.4049/jimmunol.0801941. [DOI] [PubMed] [Google Scholar]
- 58.Rajavelu P, Cehn G, Xu Y, Kitzmiller JA, Korfhagen TR, Whitsett JA. Airway Epithelial SPDEF Integrates Goblet Cell Differentiation and Pulmonary Th2 Inflammation. J. Clin. Invest. 2015 doi: 10.1172/JCI79422. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, Maeda Y, et al. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest. 2009 doi: 10.1172/JCI39731. 0, 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johansson MEV, Hansson GC. Is the intestinal goblet cell a major immune cell? Cell Host & Microbe. 2014;15:251–2. doi: 10.1016/j.chom.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turner JE, Stockinger B, Helmby H. IL-22 mediates goblet cell hyperplasia and worm expulsion in intestinal helminth infection. PLoS Pathog. 2013;9:e1003698. doi: 10.1371/journal.ppat.1003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martino MB, Jones L, Brighton B, Ehre C, Abdulah L, Davis CW, et al. The ER stress transducer IRE1beta is required for airway epithelial mucin production. Mucosal Immunol. 2012;6:639–54. doi: 10.1038/mi.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsuru A, Fujimoto N, Takahashi S, Saito M, Nakamura D, Iwano M, et al. Negative feedback by IRE1beta optimizes mucin production in goblet cells. Proc. Natl. Acad. Sci. USA. 2013;110:2864–9. doi: 10.1073/pnas.1212484110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 65.Heazlewood CK, Cook MC, Eri R, Price GR, Tauro SB, Taupin D, et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5:e54. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eri RD, Adams RJ, Tran TV, Tong H, Das I, Roche DK, et al. An intestinal epithelial defect conferring ER stress results in inflammation involving both innate and adaptive immunity. Mucosal Immunol. 2011;4:354–64. doi: 10.1038/mi.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 2009;31:15–23. doi: 10.1016/j.immuni.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 68.Wang X, Ota N, Manzanillo P, Kates L, Zavala-Solorio J, Eidenschenk C, et al. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature. 2014;514:237–41. doi: 10.1038/nature13564. [DOI] [PubMed] [Google Scholar]
- 69.Hasnain SZ, Borg DJ, Harcourt BE, Tong H, Sheng YH, Ng CP, et al. Glycemic control in diabetes is restored by therapeutic manipulation of cytokines that regulate beta cell stress. Nat Med. 2014;20:1417–26. doi: 10.1038/nm.3705. [DOI] [PubMed] [Google Scholar]
- 70.Kuhn R, Löhler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 71.Spencer SD, Di MF, Hooley J, Pitts-Meek S, Bauer M, Ryan AM, et al. The orphan receptor CRF2-4 is an essential subunit of the interleukin 10 receptor. J Exp Med. 1998;187:571–8. doi: 10.1084/jem.187.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect. Immun. 1998;66:5224–31. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Franke A, Balschun T, Karlsen TH, Hedderich J, May S, Lu T, et al. Replication of signals from recent studies of Crohn's disease identifies previously unknown disease loci for ulcerative colitis. Nat Genet. 2008;40:713–5. doi: 10.1038/ng.148. [DOI] [PubMed] [Google Scholar]
- 74.Franke A, Balschun T, Karlsen TH, Sventoraityte J, Nikolaus S, Mayr G, et al. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat Genet. 2008;40:1319–23. doi: 10.1038/ng.221. [DOI] [PubMed] [Google Scholar]
- 75.Begue B, Verdier J, Rieux-Laucat F, Goulet O, Morali A, Canioni D, et al. Defective IL10 Signaling Defining a Subgroup of Patients With Inflammatory Bowel Disease. Am J Gastroenterol. 2011;106:1544–55. doi: 10.1038/ajg.2011.112. [DOI] [PubMed] [Google Scholar]
- 76.Autschbach F, Braunstein J, Helmke B, Zuna I, Schurmann G, Niemir ZI, et al. In situ expression of interleukin-10 in noninflamed human gut and in inflammatory bowel disease. Am J Pathol. 1998;153:121–30. doi: 10.1016/S0002-9440(10)65552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jarry A, Bossard C, Bou-Hanna C, Masson D, Espaze E, Denis MG, et al. Mucosal IL-10 and TGF-beta play crucial roles in preventing LPS-driven, IFN-gamma-mediated epithelial damage in human colon explants. J Clin Invest. 2008;118:1132–42. doi: 10.1172/JCI32140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, et al. Treatment of murine colitis by lactococcus lactis secreting interleukin-10. Science. 2000;289:1352–5. doi: 10.1126/science.289.5483.1352. [DOI] [PubMed] [Google Scholar]
- 79.Hasnain SZ, Tauro S, Das I, Tong H, Chen AC, Jeffery PL, et al. IL-10 promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells. Gastroenterology. 2013;144:357–68. doi: 10.1053/j.gastro.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 80.van der Sluis M, Bouma J, Vincent A, Velcich A, Carraway KL, Buller HA, et al. Combined defects in epithelial and immunoregulatory factors exacerbate the pathogenesis of inflammation: mucin 2-interleukin 10-deficient mice. Lab Invest. 2008;88:634–42. doi: 10.1038/labinvest.2008.28. [DOI] [PubMed] [Google Scholar]