Abstract
Acute respiratory distress syndrome (ARDS) represents a serious problem in critically ill patients and is associated with in-hospital mortality rates of 33%-52%. Recruitment maneuvers (RMs) are a simple, low-cost, feasible intervention that can be performed at the bedside in patients with ARDS. RMs are characterized by the application of airway pressure to increase transpulmonary pressure transiently. Once non-aerated lung units are reopened, improvements are observed in respiratory system mechanics, alveolar reaeration on computed tomography, and improvements in gas exchange (functional recruitment). However, the reopening process could lead to vascular compression, which can be associated with overinflation, and gas exchange may not improve as expected (anatomical recruitment). The purpose of this review was to discuss the effects of different RM strategies - sustained inflation, intermittent sighs, and stepwise increases of positive end-expiratory pressure (PEEP) and/or airway inspiratory pressure - on the following parameters: hemodynamics, oxygenation, barotrauma episodes, and lung recruitability through physiological variables and imaging techniques. RMs and PEEP titration are interdependent events for the success of ventilatory management. PEEP should be adjusted on the basis of respiratory system mechanics and oxygenation. Recent systematic reviews and meta-analyses suggest that RMs are associated with lower mortality in patients with ARDS. However, the optimal RM method (i.e., that providing the best balance of benefit and harm) and the effects of RMs on clinical outcome are still under discussion, and further evidence is needed.
Keywords: Recruitment maneuvers, Acute respiratory distress syndrome, Positive end-expiratory pressure, Transpulmonary pressure, Lung ultrasonography
Core tip: Experimental and clinical studies show that stepwise recruitment maneuvers (RMs) improve oxygenation and lung aeration and are associated with less hemodynamic instability and inflammatory impact on lung tissue compared to traditional abrupt maneuvers. Patients with severe acute respiratory distress syndrome, characterized by increased edema and atelectasis, are good candidates for RMs. Patients whose oxygenation improves with increased pressure are at lower risk of death. Post-recruitment positive end-expiratory pressure (PEEP) titration is critical to maintaining stabilization of alveolar units and avoiding derecruitment. The use of individualized PEEP based on lung compliance might move clinical management forward.
INTRODUCTION
The acute respiratory distress syndrome (ARDS) is clinically characterized by severe hypoxemia, reduced lung compliance, and bilateral radiographic infiltrates[1]. Protective mechanical ventilation strategies, which are characterized by protective tidal volumes [VT = 6 mL/kg, predicted body weight (PBW)] and end-inspiratory (plateau) airway pressures lower than 28 cm H2O, have been associated with improved survival in randomized clinical trials[2,3]. However, the use of protective VT alone seems to be not enough to maintain homogeneous distribution of ventilation across different alveolar units[4]. In this line, VT titrated to 6 mL/kg (PBW) may result in repetitive opening and closing of such units, which may result in atelectrauma unless sufficient positive end-expiratory pressure (PEEP) is applied. On the other hand, overdistension and disruption of alveolar units may develop if high PEEP values are used[5].
General anesthesia and neuromuscular blockade may potentiate the generation of atelectatic areas[6]. In a normal homeostatic condition, the sigh reflex maintains lung compliance and decreases atelectasis[7]. However, during mechanical ventilation, there is no sigh reflex. One possibly way to maintain oxygenation, functional residual capacity, and respiratory system elastance is the application of recruitment maneuvers (RMs), which have become a component of lung-protective ventilation strategies[8,9]. A recent systematic review suggested that, when included in ventilatory strategies, RMs reduced mortality by 6% in patients with moderate to severe ARDS[10]. Since this is only a slight improvement in mortality and no major differences in length of intensive care unit or hospital stay were observed, subsequent studies raised concerns regarding the beneficial effects and the safety of RMs.
This review sought to discuss: (1) the physiologic effects of RMs; (2) describe different types of RMs and their safety; (3) techniques of positive end-expiratory pressure titration; and (4) the future perspectives of RMs in the presence of protective ventilation strategies.
PHYSIOLOGICAL EFFECTS OF RMS
A RM is a dynamic, transient increase in transpulmonary pressure (difference between airway pressure and pleural pressure) which is directly proportional to the reopening of lung units. Its success and/or adverse events can be predicted by the magnitude of transpulmonary pressure, balancing the increase in aerated lung areas and the reduction of mechanical stress between the edge of collapsed and aerated areas[11]. Traditionally, RMs usually improve lung mechanics and oxygenation, but whether these are the only positives consequences of RM use remains unknown. Thus far, no randomized clinical trial has aimed to show whether the presence or absence of RM among the constituent elements of a protective ventilator strategy bundle makes a difference. A randomized clinical trial designed to answer this question with sufficient statistical power, the alveolar recruitment for ARDS trial, is ongoing. Nevertheless, important, physiologically based studies have attempted to answer key questions. In a prospective study of 16 mechanically ventilated patients with ARDS by Di Marco et al[12] divided participants into responders and non-responders based on an increase in diffusing capacity for carbon monoxide associated with a higher PEEP. Increasing PEEP from 5 to 15 cm H2O has been demonstrated to yield increased lung volume (anatomical recruitment) in half of patients, while in other patients, higher PEEP results in improvement of lung volume and perfusion (functional recruitment). In other words, opening of alveolar units does not necessarily entail restoration of lung perfusion in that specific region. In cases of functional recruitment, an increment in PaO2/FiO2 can be expected (Figure 1).
Figure 1.
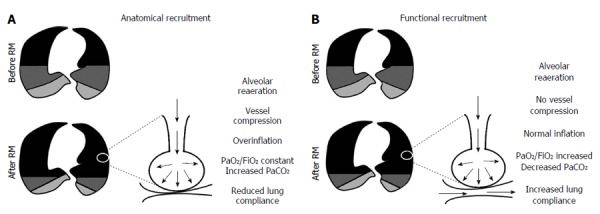
Schematic representation of lung morphology before and after application of recruitment maneuvers. A: Anatomical recruitment. Alveolar reopening is not accompanied by reperfusion and PaO2/FiO2 remains unchanged; B: Functional recruitment. Reperfusion is a landmark of functional recruitment and, after application of a recruitment maneuver, an increment in PaO2/FiO2 ratio is expected. RM: Recruitment maneuver.
The viscoelastance and time-dependent force required to open collapsed areas is a function of both transpulmonary pressure and time[13], known as the pressure-time product. In an attempt to evaluate optimal RM duration and hemodynamic changes, Arnal et al[14] conducted a prospective clinical trial of 12 recruited patients with ARDS. The authors found that most recruitment occurs in the first few seconds of a sustained inflation, suggesting that time is less important as a determinant of RM success. Instead, time plays a critical role in hemodynamic alterations, which generally occur with a longer duration of inflation.
RMs are largely related to reversal of atelectasis in the context of ARDS. Moreover, their beneficial effects have also been described in patients under general anesthesia, during postoperative ventilation, and in other conditions related to hypoxemia, including heart failure[7,15].
TYPES OF RMS
Tables 1 and 2 summarize clinical and experimental studies comparing different RM methods according to Population, Intervention, Comparison, Outcome criteria. Sigh was the first reported RM, applied interposed with monotonous ventilation to mimic physiological breathing as it occurs in healthy subjects[16]. This RM consists of high VT in controlled mode or high PEEP up to a specific plateau pressure level, for a selected number of cycles. In this line, Pelosi et al[17], in an observational study, ventilated 10 ARDS patients for 1 h with a lung-protective strategy consisting of three consecutive sighs/minute at 45 cm H2O plateau pressure. These patients exhibited improvement in oxygenation, lung elastance, and functional residual capacity compared to patients who did not receive sighs. Despite the beneficial effects of this maneuver, high sigh frequency (up to 180/h) was associated with hyperinflation and expression of type III procollagen mRNA in lung tissue in experimental models[18]. Lower sigh frequency can protect the lung[18], mainly when combined with pressure-support ventilation[19]. The most widely described RM is sustained inflation, in which airway pressure is abruptly raised for a given time interval. A common sustained inflation is 40 cm H2O for 40 s[20-22]. More recently, RMs with a stepwise increase in airway pressure and/or PEEP (stepwise RM) have been proposed to provide slowly increasing transpulmonary pressure instead of the rapid increase used in sustained inflation, in experimental[8,23,24] and clinical studies[25-27]. Both sustained inflation (fast RM) and stepwise RM (slow RM) have been reported to improve oxygenation and lung function and minimize atelectasis in experimental[8,24,28] and clinical scenarios[20,25]. Since stepwise RMs recruit lung units as effectively as sustained inflation with a lower mean airway pressure, they may lead to less hemodynamic compromise and hyperinflation. In this context, sustained inflation has also been associated with risk of hypotension[29], barotrauma[29], and has even been reported to be ineffective in improving oxygenation and reducing intrapulmonary shunting[30]. In an observational study and randomized controlled trial, respectively, stepwise RM improved lung compliance, shunt fraction, and oxygen saturation[25] and was associated with less release of inflammatory mediators[24] compared to a ventilator strategy that did not incorporate RMs. However, stepwise RMs may have a heterogeneous impact on respiratory mechanics and cause adverse hemodynamic effects in an observational clinical study[26]. In experimental endotoxin-induced mild ARDS, stepwise RM, compared to sustained inflation, was associated with reduced type II epithelial cell damage and decreased expression of markers associated with fibrosis and endothelial cell damage, depending on ARDS etiology[8].
Table 1.
Recruitment maneuver methods and outcomes reported in the literature about clinical studies
| Ref. | Population | Design | Interventions | Comparison | Outcome |
| Pelosi et al[17] | Patients with pulmonary and extrapulmonary ARDS | Observational study | 3 sighs/min at Pplat 45 cm H2O, VT to maintain Pplat ≤ 35 cm H2O. PEEP level to keep the lung open | (1) 1 h of ventilator strategy; (2) 2 h of ventilator strategy; and (3) 1 h of ventilator strategy with three consecutive sighs/min at Plat 45 cm H2O | Sigh during protective ventilation improved lung recruitment |
| Borges et al[44] | Patients with early ARDS | Observational study | Stepwise maximum-recruitment strategy with sequential increments in Paw, in 5-cm H2O steps, until the detection of PaO2 + PaCO2 = 400 mmHg | No comparisons | Stepwise maximum recruitment reverted hypoxemia and fully recruited the lungs |
| Meade et al[29] | Patients with ARDS (PaO2/FiO2 ≤ 250 mmHg) | Randomized controlled trial | Low VT, Pplat ≤ 30 cm H2O or ≥ 40 cm H2O, and lower or higher PEEP levels according to PEEP/FiO2 table | (1) Ventilator strategy with Pplat ≤ 30 cm H2O, and conventional PEEP levels; (2) “open lung” approach with Pplat ≤ 40 cm H2O, RM, and higher PEEP levels | “Open-lung” approach improved oxygenation associated with lower use of rescue therapies |
| Hodgson et al[25] | Patients with early ARDS | Observational study | Staircase RM, Paw set to 15 cm H2O above the PEEP, which was increased in a stepwise manner to 20, 30 and then 40 cm H2O every 2 min, followed by PEEP titration | No comparisons | 80% of early ARDS patients responded to staircase RM |
| Hodgson et al[27] | Patients with ARDS | Randomized controlled trial | Control ventilation strategy compared to staircase recruitment maneuver | (1) Control group: PCV, Pplat < 30 cm H2O, VT < 6 mL/kg. FiO2 adjusted to SaO2: 90% to 92%; and (2) Staircase RM: Paw adjusted to 15 cm H2O above PEEP level, which was increased in a stepwise manner to 20, 30 and 40 cm H2O every 2 min, and then reduced in steps of 2.5 from 25 to 15 cm H2O every 3 min until a decrease in SaO2 ≥ 1% | Staircase RM improved plasma cytokines, oxygenation and lung function over 7 d |
| Morán et al[26] | Patients with early ARDS | Observational study | Stepwise RM started from plateau pressure/PEEP of 40/25 cm H2O, 5 cm H2O of PEEP was sequentially increased until PaO2/FiO2 of 350 mmHg or plateau pressure/PEEP of 60/40 cm H2O | No comparisons | Stepwise RM improved oxygenation but caused hemodynamic instability and transient hypoxemia |
Summary of the results of clinical and experimental studies comparing different recruitment maneuver (RM) methods, according to population, intervention, comparison, outcome criteria. ARDS: Acute respiratory distress syndrome; FiO2: Inspiratory oxygen fraction; PaO2: Arterial oxygen partial pressure; PaCO2: Arterial carbon dioxide partial pressure; PCV: Pressure-controlled ventilation; PEEP: Positive end-expiratory pressure; Pplat: Plateau pressure; SaO2: Arterial oxygen saturation; VT: Tidal volume.
Table 2.
Recruitment maneuver methods and outcomes reported in the literature about experimental studies
| Ref. | Population | Design | Interventions | Comparison | Outcome |
| Rzezinski et al[23] | Animals with mild extrapulmonary lung injury | Randomized experimental study | Prolonged RM stepwise increase in PIP of 15-20-25 cm H2O above a PEEP of 15 cm H2O (maximal PIP = 40 cm H2O) | (1) Animals ventilated with VT = 6 mL/kg and PEEP = 5 cm H2O with no RMs; (2) Sustained inflation (40 cm H2O for 40 s); or (3) Stepwise increase in Paw of 15, 20, 25 cm H2O above a PEEP of 15 cm H2O (maximal PIP = 40 cm H2O), with interposed periods of Paw = 10 cm H2O above a PEEP = 15 cm H2O | Prolonged RM improved lung function, with less damage to alveolar epithelium, resulting in reduced pulmonary injury |
| Steimback et al[18] | Animals with extrapulmonary lung injury | Randomized experimental study | Sigh with different PIP and frequencies | (1) Animals ventilated with VT = 6 mL/kg and PEEP = 5 cm H2O with no RMs; (2) Sustained inflation (40 cm H2O for 40 s); (3) RM (180 sighs/h) and PIP (40 cm H2O) (S180/40); (4) RM (10 sighs/h) and PIP (40 cm H2O) (S10/40); and (5) RM (10 sighs/h) and PIP (20 cm H2O) (S10/20) | The reduction in sigh frequency led to a protective effect on the lung and distal organs |
| Silva et al[8] | Animals with pulmonary and extrapulmonary lung injury | Randomized experimental study | Stepwise RM (5 cm H2O/step, 8.5 s at each step during 51 s); Stepwise RM (5 cm H2O/step, 5 s at each step during 30 s) | (1) Sustained inflation (30 cm H2O for 30 s; (2) Stepwise PIP increase 30 cm H2O over 51 s (STEP-51); and (3) Stepwise PIP increase over 30 s with maximum PIP sustained for a further 30 s (STEP-30/30) | Stepwise RM prevented fibrogenesis and endothelial cell damage |
Summary of the results of clinical and experimental studies comparing different recruitment maneuver (RM) methods, according to population, intervention, comparison, outcome criteria. PEEP: Positive end-expiratory pressure; PIP: Peak inspiratory pressure; VT: Tidal volume.
Despite extensive research into the applications of RMs, definitive guidelines for these maneuvers have not been established. As a step toward standardization, a trial with high methodological quality is being conducted to assess the 28-d survival of ARDS patients subjected to maximum stepwise alveolar recruitment followed by ventilation with PEEP titrated according to best compliance[31]. This multicenter study may represent a valuable contribution to the treatment of patients with ARDS[31].
Assisted ventilation may be associated with homogeneous lung recruitment. In the presence of lung recruitment, end-expiratory lung volume increases, thus reducing strain, while lung elastance decreases, resulting in lower inspiratory transpulmonary pressure and stress[32]. However, in the absence of lung recruitment, transpulmonary pressure might be higher than during controlled mechanical ventilation and thus, assisted ventilation may lead to deleterious effects[33,34]. Additionally, spontaneous breathing during assisted mechanical ventilation may exacerbate lung injury by increasing patient-ventilator asynchrony and rapid shallow breathing[35]. Furthermore, negative pleural pressures may increase intrathoracic blood volume, worsening pulmonary edema and lung damage[36]. In short, we suggest that assisted mechanical ventilation can be applied for mild and moderate ARDS.
It is well established that prone positioning improves oxygenation in patients who require mechanical ventilatory support for management of ARDS[37]. Guérin et al[38] recently showed that early application of prolonged prone positioning significantly reduces mortality in patients with severe ARDS. Pronation acts as a RM, increasing transpulmonary pressure in dorsal regions and reducing alveolar instability and hyperinflation. In this line, Galiatsou et al[39] assessed lung computed tomography findings in ARDS patients in the supine and prone positions after RM application. The authors found that prone position had an additive effect on oxygenation and recruitment of dependent lung regions, and was associated with a reduction in ventral overinflation areas. These findings were confirmed by Cornejo et al[40] who evaluated the interaction of lung recruitability, high PEEP values, and prone positioning. Reductions in atelectasis and/or overdistension were observed in patients in both categories (low and high recruitability) at both low and high PEEP in the prone position. Furthermore, in a subgroup of patients with high recruitability, prone positioning added to the effect of high PEEP on atelectrauma, and prevented its effects on tidal overinflation.
SAFETY OF RMS
RMs are being increasingly used in clinical practice, and even if full re-expansion is expected, negative effects can occur, especially on hemodynamics. The type of RM seems to be a crucial predictor of hemodynamic adverse effects. In a prospective clinical trial, Iannuzzi et al[41] evaluated hemodynamic changes in 40 patients with ARDS randomized to receive RMs with sustained inflation or pressure-controlled ventilation (PCV) adjusted to generate the same pressure-time product. PCV-RM, compared to sustained inflation, resulted in greater oxygenation and less hemodynamic impairment as reflected by lower central venous and pulmonary artery pressures, lower right ventricle workload, and higher cardiac output. In addition, the post-RM level of PEEP and lung recruitability should be taken into account to avoid complications related to high intrathoracic pressure during RMs[42,43].
Desaturation and barotrauma are less common complications of RMs. Hodgson et al[25], demonstrated that although 8 of 20 patients desaturated and exhibited transient circulatory depression during application of RMs, they had improved shunt fraction, oxygenation, and respiratory system compliance 60 min after maneuver application followed by PEEP titration. In a randomized controlled trial by Meade et al[29], five patients with ARDS developed ventilator asynchrony, three experienced discomfort during the RM, two had hypotension, and four developed barotrauma. However, some issues should be taken into account, such as the sedation protocol allowing spontaneous cycles during the application of a sustained maneuver for 40 s. In addition, the level of PEEP was returned to the same value as before RM application. On the other hand, in a previous observational study, Borges et al[44] demonstrated that two of 26 patients developed barotrauma; one case occurred 24 h and the other 12 h after application of the RM. Despite the preceding reports, recent data confirm that RMs are not associated with an increased risk of barotrauma[10,45].
Lung recruitability could provide valuable information before RM application to prevent possible deleterious effects. Oxygenation and respiratory system elastance are often used to evaluate response to RMs. Gattinoni et al[42] aimed to establish an estimation of lung recruitability in patients with ARDS based on three physiological variables: Oxygenation, respiratory system compliance, and alveolar dead space in patients exposed to a progressive increase in PEEP. However, these variables had low sensitivity and specificity to predict higher lung recruitability. Static lung compliance (the difference between respiratory system compliance and chest wall compliance) reflects transpulmonary pressure as well as lung recruitment, and could be used instead of respiratory system compliance to measure lung recruitability[46]. Esophageal pressure monitoring permits measurement of lung compliance, but its implementation in the intensive care unit setting is still a challenge. In research settings, computed tomography can be used to assess recruitment, as well as to individualize ventilation strategies in order to keep the lungs open[45,47,48]. Additionally, the use of lung ultrasonography (LUS) can be a useful imaging tool to assess lung aeration in critically ill patients[49,50]. In this context, studies have shown the utility of LUS in the detection and quantification of lung recruitment via a transesophageal approach[51] and via a transthoracic approach[50]. Electrical impedance tomography (EIT) can provide a good estimate of the amount of tidal recruitment and may be useful to individualize ventilatory settings[52,53]. Even though LUS and EIT offer, at the bedside, an easy, alternative way to evaluate lung recruitment, both are inappropriate to detect hyperinflation.
Response to RMs and/or lung recruitability cannot be predicted a priori, and require individualized assessment. Recently, Cressoni et al[47] showed that extent of lung inhomogeneities increases as poorly aerated tissue increases from mild to severe ARDS (from 14% to 23%). In this study, high lung recruitability was considered in patients in whom the poorly aerated tissue area decreased with increasing PEEP, unlike in patients in whom poorly aerated tissue increased with increasing pressure[47]. Additionally, poorly aerated tissue areas, i.e., areas of tidal recruitment/derecruitment, are the primary targets of the inflammatory process in ventilator-induced lung injury[54]. In this context, severe ARDS is more recruitable than mild or moderate disease[42,47], and extrapulmonary ARDS is more recruitable than cases of pulmonary etiology. Several studies[55-57] have demonstrated that focal lung injury (pulmonary etiology) is associated with lower recruitability and alveolar overinflation in response to increased PEEP levels. In contrast, within the group of ARDS responders, in those with diffuse loss of aeration (extrapulmonary etiology), alveolar recruitment resulting from PEEP is not accompanied by lung overinflation[42,55].
Recently, Caironi et al[58] retrospectively analyzed a large cohort of patients with ARDS, aiming to describe lung edema and recruitability according to the Berlin definition and elucidate whether assessment of PaO2/FiO2 at standardized PEEP (5 or 15 cm H2O) allows a more accurate description of ARDS severity as compared to its clinical assessment. They reported that the clinical PEEP applied when assessing PaO2/FiO2 may mask the underlying ARDS severity, and that application of the Berlin definition at 5 cm H2O PEEP more accurately matches ARDS lung injury severity and recruitability, providing important information to guide ventilator strategies and to assess mortality risk.
TITRATION OF POSITIVE END-EXPIRATION PRESSURE
PEEP is required to recruit or maintain recruitment in the heterogeneous ARDS lung. The most common method for PEEP level selection is the use of PEEP/FiO2 tables, introduced by the ARDS Network[3] and the LOVS study[29]. Although high PEEP values improve oxygenation and decrease alveolar stress[44], they can sometimes result in lung overdistension and hemodynamic instability[59]. An explanation for this discrepancy may be found in the heterogeneity of ARDS: A subpopulation of non-responders (patients with low recruitability) experience no change in arterial oxygenation with higher PEEP[60], and may be at greater risk of ventilator-induced lung injury from overdistention[61]. On the other hand, patients with predominantly recruitable lung (severe ARDS; PaO2/FiO2 < 150 mmHg) exhibit an association of oxygenation response and PEEP adjustment, as well as lower risk of death[62]. Recently, a Cochrane review of seven trials concluded that high PEEP levels are unrelated to hospital outcome as compared with low levels[63]. A relationship between higher PEEP and low mortality could be achieved in patients with more severe ARDS, in whom lung recruitability is higher[59]. In the era of identification of PEEP responders and/or high recruitability, attention to prevention of intratidal collapse and decollapse (“open the lung and keep it open!”)[64] and lung function seems to be more relevant than oxygenation.
In a study of 57 patients with ARDS, Huh et al[65] compared daily decremental PEEP titration according to the best dynamic compliance performed after an RM vs PEEP selection as suggested by ARDSnet[3], based on a PEEP/FiO2 table. In this protocol, an initial improvement in oxygenation occurred in patients who received decremental PEEP titration after RM compared to those in whom the PEEP/FiO2 table method was used. This earlier improvement in oxygenation was not related to any advantage in respiratory mechanics within 1 wk, nor with 28-d intensive care unit mortality.
Cressoni et al[66] reported that, in mechanically ventilated patients in the supine position, collapse occurs first in the most dependent areas and overinflation in the less dependent regions, as observed on computed tomography analysis. This finding calls into question the use of a single pressure parameter to reflect the entire lung structure. Pintado et al[67], in a randomized controlled pilot study, suggested that PEEP application according to the highest compliance was associated with more organ dysfunction-free days and a trend toward lower mortality at 28 d as compared with FiO2-guided PEEP selection, with no differences in oxygenation ratio or PEEP level among groups.
The new concept of transpulmonary pressure to titrate PEEP during the decremental method has emerged as a measurement of alveolar stability and alveolar stress. Rodriguez et al[68] showed that high and low transpulmonary pressure values were associated with lung overdistension and with reductions in oxygenation and collapse, respectively. In addition, a positive correlation has been observed between transpulmonary and airway pressures. Transpulmonary pressure reflects pleural pressure surrounding dependent lung regions at a given point, while airway pressure only reflects opened alveolar units. In this context, transpulmonary pressure could be a more representative measure to guide PEEP selection and prevent alveolar unit instability.
“Open-lung PEEP”, first described more than 2 decades ago by Lachmann et al[64], represents the level of PEEP that combines the minimal tidal recruitment/derecruitment, overinflation, and dead space with optimal oxygenation and lung compliance. Open-lung PEEP should be achieved after an application of RM[44], which may open collapsed alveolar units, and should then be titrated gradually toward the minimum value that can stabilize the previously recruited lung[67]. RMs and PEEP titration are interdependent events for the success of ventilatory management.
CONCLUSION
RMs are a simple, low-cost, feasible intervention that can be performed at bedside in intensive care units. A wealth of experimental and clinical data has demonstrated improvements in oxygenation, lung mechanics, and lung re-aeration after application of RMs. Recent systematic reviews and meta-analyses suggest that RMs are associated with lower mortality in patients with ARDS. However, the optimal RM method (i.e., that with the best balance of benefit and harm) and the effects of RMs on clinical outcome are still under discussion, and further evidence is needed.
ACKNOWLEDGMENTS
We express our gratitude to Mrs. Moira Elizabeth Schottler and Mr. Filippe Vasconcellos for their assistance in editing the manuscript.
Footnotes
P- Reviewer: Inchauspe A S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
Supported by Brazilian Council for Scientific and Technological Development (CNPq), Carlos Chagas Filho Rio de Janeiro State Research Foundation (FAPERJ), Department of Science and Technology (DECIT)/Brazilian Ministry of Health; and Coordination for the Improvement of Higher Level Personnel (CAPES).
Conflict-of-interest statement: Authors have no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 30, 2015
First decision: August 14, 2015
Article in press: October 27, 2015
References
- 1.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 2.Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, Slutsky AS, Pullenayegum E, Zhou Q, Cook D, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303:865–873. doi: 10.1001/jama.2010.218. [DOI] [PubMed] [Google Scholar]
- 3.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 4.Treschan TA, Beiderlinden M. Role of recruitment maneuvers for lung-protective ventilation in the operating room remains unclear. Anesthesiology. 2015;122:472–473. doi: 10.1097/ALN.0000000000000549. [DOI] [PubMed] [Google Scholar]
- 5.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 6.Spieth PM, Güldner A, Uhlig C, Bluth T, Kiss T, Schultz MJ, Pelosi P, Koch T, Gama de Abreu M. Variable versus conventional lung protective mechanical ventilation during open abdominal surgery: study protocol for a randomized controlled trial. Trials. 2014;15:155. doi: 10.1186/1745-6215-15-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartland BL, Newell TJ, Damico N. Alveolar recruitment maneuvers under general anesthesia: a systematic review of the literature. Respir Care. 2015;60:609–620. doi: 10.4187/respcare.03488. [DOI] [PubMed] [Google Scholar]
- 8.Silva PL, Moraes L, Santos RS, Samary C, Ramos MB, Santos CL, Morales MM, Capelozzi VL, Garcia CS, de Abreu MG, et al. Recruitment maneuvers modulate epithelial and endothelial cell response according to acute lung injury etiology. Crit Care Med. 2013;41:e256–e265. doi: 10.1097/CCM.0b013e31828a3c13. [DOI] [PubMed] [Google Scholar]
- 9.Keenan JC, Formenti P, Marini JJ. Lung recruitment in acute respiratory distress syndrome: what is the best strategy? Curr Opin Crit Care. 2014;20:63–68. doi: 10.1097/MCC.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 10.Suzumura EA, Figueiró M, Normilio-Silva K, Laranjeira L, Oliveira C, Buehler AM, Bugano D, Passos Amato MB, Ribeiro Carvalho CR, Berwanger O, et al. Effects of alveolar recruitment maneuvers on clinical outcomes in patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Intensive Care Med. 2014;40:1227–1240. doi: 10.1007/s00134-014-3413-6. [DOI] [PubMed] [Google Scholar]
- 11.Brunner JX, Wysocki M. Is there an optimal breath pattern to minimize stress and strain during mechanical ventilation? Intensive Care Med. 2009;35:1479–1483. doi: 10.1007/s00134-009-1510-8. [DOI] [PubMed] [Google Scholar]
- 12.Di Marco F, Devaquet J, Lyazidi A, Galia F, da Costa NP, Fumagalli R, Brochard L. Positive end-expiratory pressure-induced functional recruitment in patients with acute respiratory distress syndrome. Crit Care Med. 2010;38:127–132. doi: 10.1097/CCM.0b013e3181b4a7e7. [DOI] [PubMed] [Google Scholar]
- 13.Marini JJ, Gattinoni L. Propagation prevention: a complementary mechanism for “lung protective” ventilation in acute respiratory distress syndrome. Crit Care Med. 2008;36:3252–3258. doi: 10.1097/CCM.0b013e31818f0e68. [DOI] [PubMed] [Google Scholar]
- 14.Arnal JM, Paquet J, Wysocki M, Demory D, Donati S, Granier I, Corno G, Durand-Gasselin J. Optimal duration of a sustained inflation recruitment maneuver in ARDS patients. Intensive Care Med. 2011;37:1588–1594. doi: 10.1007/s00134-011-2323-0. [DOI] [PubMed] [Google Scholar]
- 15.Constantin JM, Futier E, Cherprenet AL, Chanques G, Guerin R, Cayot-Constantin S, Jabaudon M, Perbet S, Chartier C, Jung B, et al. A recruitment maneuver increases oxygenation after intubation of hypoxemic intensive care unit patients: a randomized controlled study. Crit Care. 2010;14:R76. doi: 10.1186/cc8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levine M, Gilbert R, Auchincloss JH. A comparison of the effects of sighs, large tidal volumes, and positive end expiratory pressure in assisted ventilation. Scand J Respir Dis. 1972;53:101–108. [PubMed] [Google Scholar]
- 17.Pelosi P, Cadringher P, Bottino N, Panigada M, Carrieri F, Riva E, Lissoni A, Gattinoni L. Sigh in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;159:872–880. doi: 10.1164/ajrccm.159.3.9802090. [DOI] [PubMed] [Google Scholar]
- 18.Steimback PW, Oliveira GP, Rzezinski AF, Silva PL, Garcia CS, Rangel G, Morales MM, Lapa E Silva JR, Capelozzi VL, Pelosi P, et al. Effects of frequency and inspiratory plateau pressure during recruitment manoeuvres on lung and distal organs in acute lung injury. Intensive Care Med. 2009;35:1120–1128. doi: 10.1007/s00134-009-1439-y. [DOI] [PubMed] [Google Scholar]
- 19.Moraes L, Santos CL, Santos RS, Cruz FF, Saddy F, Morales MM, Capelozzi VL, Silva PL, de Abreu MG, Garcia CS, et al. Effects of sigh during pressure control and pressure support ventilation in pulmonary and extrapulmonary mild acute lung injury. Crit Care. 2014;18:474. doi: 10.1186/s13054-014-0474-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oczenski W, Hörmann C, Keller C, Lorenzl N, Kepka A, Schwarz S, Fitzgerald RD. Recruitment maneuvers after a positive end-expiratory pressure trial do not induce sustained effects in early adult respiratory distress syndrome. Anesthesiology. 2004;101:620–625. doi: 10.1097/00000542-200409000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Oczenski W, Hörmann C, Keller C, Lorenzl N, Kepka A, Schwarz S, Fitzgerald RD. Recruitment maneuvers during prone positioning in patients with acute respiratory distress syndrome. Crit Care Med. 2005;33:54–61; quiz 62. doi: 10.1097/01.ccm.0000149853.47651.f0. [DOI] [PubMed] [Google Scholar]
- 22.Grasso S, Terragni P, Mascia L, Fanelli V, Quintel M, Herrmann P, Hedenstierna G, Slutsky AS, Ranieri VM. Airway pressure-time curve profile (stress index) detects tidal recruitment/hyperinflation in experimental acute lung injury. Crit Care Med. 2004;32:1018–1027. doi: 10.1097/01.ccm.0000120059.94009.ad. [DOI] [PubMed] [Google Scholar]
- 23.Rzezinski AF, Oliveira GP, Santiago VR, Santos RS, Ornellas DS, Morales MM, Capelozzi VL, Amato MB, Conde MB, Pelosi P, et al. Prolonged recruitment manoeuvre improves lung function with less ultrastructural damage in experimental mild acute lung injury. Respir Physiol Neurobiol. 2009;169:271–281. doi: 10.1016/j.resp.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Silva PL, Moraes L, Santos RS, Samary C, Ornellas DS, Maron-Gutierrez T, Morales MM, Saddy F, Capelozzi VL, Pelosi P, et al. Impact of pressure profile and duration of recruitment maneuvers on morphofunctional and biochemical variables in experimental lung injury. Crit Care Med. 2011;39:1074–1081. doi: 10.1097/CCM.0b013e318206d69a. [DOI] [PubMed] [Google Scholar]
- 25.Hodgson CL, Tuxen DV, Bailey MJ, Holland AE, Keating JL, Pilcher D, Thomson KR, Varma D. A positive response to a recruitment maneuver with PEEP titration in patients with ARDS, regardless of transient oxygen desaturation during the maneuver. J Intensive Care Med. 2011;26:41–49. doi: 10.1177/0885066610383953. [DOI] [PubMed] [Google Scholar]
- 26.Morán I, Blanch L, Fernández R, Fernández-Mondéjar E, Zavala E, Mancebo J. Acute physiologic effects of a stepwise recruitment maneuver in acute respiratory distress syndrome. Minerva Anestesiol. 2011;77:1167–1175. [PubMed] [Google Scholar]
- 27.Hodgson CL, Tuxen DV, Davies AR, Bailey MJ, Higgins AM, Holland AE, Keating JL, Pilcher DV, Westbrook AJ, Cooper DJ, et al. A randomised controlled trial of an open lung strategy with staircase recruitment, titrated PEEP and targeted low airway pressures in patients with acute respiratory distress syndrome. Crit Care. 2011;15:R133. doi: 10.1186/cc10249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riva DR, Oliveira MB, Rzezinski AF, Rangel G, Capelozzi VL, Zin WA, Morales MM, Pelosi P, Rocco PR. Recruitment maneuver in pulmonary and extrapulmonary experimental acute lung injury. Crit Care Med. 2008;36:1900–1908. doi: 10.1097/CCM.0b013e3181760e5d. [DOI] [PubMed] [Google Scholar]
- 29.Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, Davies AR, Hand LE, Zhou Q, Thabane L, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:637–645. doi: 10.1001/jama.299.6.637. [DOI] [PubMed] [Google Scholar]
- 30.Villagrá A, Ochagavía A, Vatua S, Murias G, Del Mar Fernández M, Lopez Aguilar J, Fernández R, Blanch L. Recruitment maneuvers during lung protective ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002;165:165–170. doi: 10.1164/ajrccm.165.2.2104092. [DOI] [PubMed] [Google Scholar]
- 31.ART Investigators. Rationale, study design, and analysis plan of the Alveolar Recruitment for ARDS Trial (ART): study protocol for a randomized controlled trial. Trials. 2012;13:153. doi: 10.1186/1745-6215-13-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saddy F, Sutherasan Y, Rocco PR, Pelosi P. Ventilator-associated lung injury during assisted mechanical ventilation. Semin Respir Crit Care Med. 2014;35:409–417. doi: 10.1055/s-0034-1382153. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida T, Torsani V, Gomes S, De Santis RR, Beraldo MA, Costa EL, Tucci MR, Zin WA, Kavanagh BP, Amato MB. Spontaneous effort causes occult pendelluft during mechanical ventilation. Am J Respir Crit Care Med. 2013;188:1420–1427. doi: 10.1164/rccm.201303-0539OC. [DOI] [PubMed] [Google Scholar]
- 34.Güldner A, Kiss T, Bluth T, Uhlig C, Braune A, Carvalho N, Quast T, Rentzsch I, Huhle R, Spieth P, et al. Effects of ultraprotective ventilation, extracorporeal carbon dioxide removal, and spontaneous breathing on lung morphofunction and inflammation in experimental severe acute respiratory distress syndrome. Anesthesiology. 2015;122:631–646. doi: 10.1097/ALN.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 35.Thille AW, Rodriguez P, Cabello B, Lellouche F, Brochard L. Patient-ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med. 2006;32:1515–1522. doi: 10.1007/s00134-006-0301-8. [DOI] [PubMed] [Google Scholar]
- 36.Kallet RH, Alonso JA, Luce JM, Matthay MA. Exacerbation of acute pulmonary edema during assisted mechanical ventilation using a low-tidal volume, lung-protective ventilator strategy. Chest. 1999;116:1826–1832. doi: 10.1378/chest.116.6.1826. [DOI] [PubMed] [Google Scholar]
- 37.Guérin C. Prone ventilation in acute respiratory distress syndrome. Eur Respir Rev. 2014;23:249–257. doi: 10.1183/09059180.00001114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guérin C, Reignier J, Richard JC. Prone positioning in the acute respiratory distress syndrome. N Engl J Med. 2013;369:980–981. doi: 10.1056/NEJMc1308895. [DOI] [PubMed] [Google Scholar]
- 39.Galiatsou E, Kostanti E, Svarna E, Kitsakos A, Koulouras V, Efremidis SC, Nakos G. Prone position augments recruitment and prevents alveolar overinflation in acute lung injury. Am J Respir Crit Care Med. 2006;174:187–197. doi: 10.1164/rccm.200506-899OC. [DOI] [PubMed] [Google Scholar]
- 40.Cornejo RA, Díaz JC, Tobar EA, Bruhn AR, Ramos CA, González RA, Repetto CA, Romero CM, Gálvez LR, Llanos O, et al. Effects of prone positioning on lung protection in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2013;188:440–448. doi: 10.1164/rccm.201207-1279OC. [DOI] [PubMed] [Google Scholar]
- 41.Iannuzzi M, De Sio A, De Robertis E, Piazza O, Servillo G, Tufano R. Different patterns of lung recruitment maneuvers in primary acute respiratory distress syndrome: effects on oxygenation and central hemodynamics. Minerva Anestesiol. 2010;76:692–698. [PubMed] [Google Scholar]
- 42.Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354:1775–1786. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- 43.Lim CM, Jung H, Koh Y, Lee JS, Shim TS, Lee SD, Kim WS, Kim DS, Kim WD. Effect of alveolar recruitment maneuver in early acute respiratory distress syndrome according to antiderecruitment strategy, etiological category of diffuse lung injury, and body position of the patient. Crit Care Med. 2003;31:411–418. doi: 10.1097/01.CCM.0000048631.88155.39. [DOI] [PubMed] [Google Scholar]
- 44.Borges JB, Okamoto VN, Matos GF, Caramez MP, Arantes PR, Barros F, Souza CE, Victorino JA, Kacmarek RM, Barbas CS, et al. Reversibility of lung collapse and hypoxemia in early acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;174:268–278. doi: 10.1164/rccm.200506-976OC. [DOI] [PubMed] [Google Scholar]
- 45.de Matos GF, Stanzani F, Passos RH, Fontana MF, Albaladejo R, Caserta RE, Santos DC, Borges JB, Amato MB, Barbas CS. How large is the lung recruitability in early acute respiratory distress syndrome: a prospective case series of patients monitored by computed tomography. Crit Care. 2012;16:R4. doi: 10.1186/cc10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akoumianaki E, Maggiore SM, Valenza F, Bellani G, Jubran A, Loring SH, Pelosi P, Talmor D, Grasso S, Chiumello D, et al. The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med. 2014;189:520–531. doi: 10.1164/rccm.201312-2193CI. [DOI] [PubMed] [Google Scholar]
- 47.Cressoni M, Cadringher P, Chiurazzi C, Amini M, Gallazzi E, Marino A, Brioni M, Carlesso E, Chiumello D, Quintel M, et al. Lung inhomogeneity in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2014;189:149–158. doi: 10.1164/rccm.201308-1567OC. [DOI] [PubMed] [Google Scholar]
- 48.Rocco PR, Pelosi P, de Abreu MG. Pros and cons of recruitment maneuvers in acute lung injury and acute respiratory distress syndrome. Expert Rev Respir Med. 2010;4:479–489. doi: 10.1586/ers.10.43. [DOI] [PubMed] [Google Scholar]
- 49.Stefanidis K, Dimopoulos S, Tripodaki ES, Vitzilaios K, Politis P, Piperopoulos P, Nanas S. Lung sonography and recruitment in patients with early acute respiratory distress syndrome: a pilot study. Crit Care. 2011;15:R185. doi: 10.1186/cc10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bouhemad B, Brisson H, Le-Guen M, Arbelot C, Lu Q, Rouby JJ. Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment. Am J Respir Crit Care Med. 2011;183:341–347. doi: 10.1164/rccm.201003-0369OC. [DOI] [PubMed] [Google Scholar]
- 51.Tsubo T, Yatsu Y, Tanabe T, Okawa H, Ishihara H, Matsuki A. Evaluation of density area in dorsal lung region during prone position using transesophageal echocardiography. Crit Care Med. 2004;32:83–87. doi: 10.1097/01.CCM.0000104944.18636.B2. [DOI] [PubMed] [Google Scholar]
- 52.Muders T, Luepschen H, Zinserling J, Greschus S, Fimmers R, Guenther U, Buchwald M, Grigutsch D, Leonhardt S, Putensen C, et al. Tidal recruitment assessed by electrical impedance tomography and computed tomography in a porcine model of lung injury*. Crit Care Med. 2012;40:903–911. doi: 10.1097/CCM.0b013e318236f452. [DOI] [PubMed] [Google Scholar]
- 53.Bikker IG, Leonhardt S, Reis Miranda D, Bakker J, Gommers D. Bedside measurement of changes in lung impedance to monitor alveolar ventilation in dependent and non-dependent parts by electrical impedance tomography during a positive end-expiratory pressure trial in mechanically ventilated intensive care unit patients. Crit Care. 2010;14:R100. doi: 10.1186/cc9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borges JB, Costa EL, Suarez-Sipmann F, Widström C, Larsson A, Amato M, Hedenstierna G. Early inflammation mainly affects normally and poorly aerated lung in experimental ventilator-induced lung injury*. Crit Care Med. 2014;42:e279–e287. doi: 10.1097/CCM.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 55.Rouby JJ, Puybasset L, Nieszkowska A, Lu Q. Acute respiratory distress syndrome: lessons from computed tomography of the whole lung. Crit Care Med. 2003;31:S285–S295. doi: 10.1097/01.CCM.0000057905.74813.BC. [DOI] [PubMed] [Google Scholar]
- 56.Constantin JM, Jaber S, Futier E, Cayot-Constantin S, Verny-Pic M, Jung B, Bailly A, Guerin R, Bazin JE. Respiratory effects of different recruitment maneuvers in acute respiratory distress syndrome. Crit Care. 2008;12:R50. doi: 10.1186/cc6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grasso S, Stripoli T, De Michele M, Bruno F, Moschetta M, Angelelli G, Munno I, Ruggiero V, Anaclerio R, Cafarelli A, et al. ARDSnet ventilatory protocol and alveolar hyperinflation: role of positive end-expiratory pressure. Am J Respir Crit Care Med. 2007;176:761–767. doi: 10.1164/rccm.200702-193OC. [DOI] [PubMed] [Google Scholar]
- 58.Caironi P, Carlesso E, Cressoni M, Chiumello D, Moerer O, Chiurazzi C, Brioni M, Bottino N, Lazzerini M, Bugedo G, et al. Lung recruitability is better estimated according to the Berlin definition of acute respiratory distress syndrome at standard 5 cm H2O rather than higher positive end-expiratory pressure: a retrospective cohort study. Crit Care Med. 2015;43:781–790. doi: 10.1097/CCM.0000000000000770. [DOI] [PubMed] [Google Scholar]
- 59.Dasenbrook EC, Needham DM, Brower RG, Fan E. Higher PEEP in patients with acute lung injury: a systematic review and meta-analysis. Respir Care. 2011;56:568–575. doi: 10.4187/respcare.01011. [DOI] [PubMed] [Google Scholar]
- 60.Grasso S, Fanelli V, Cafarelli A, Anaclerio R, Amabile M, Ancona G, Fiore T. Effects of high versus low positive end-expiratory pressures in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171:1002–1008. doi: 10.1164/rccm.200407-940OC. [DOI] [PubMed] [Google Scholar]
- 61.Slutsky AS, Hudson LD. PEEP or no PEEP--lung recruitment may be the solution. N Engl J Med. 2006;354:1839–1841. doi: 10.1056/NEJMe068045. [DOI] [PubMed] [Google Scholar]
- 62.Goligher EC, Villar J, Slutsky AS. Positive end-expiratory pressure in acute respiratory distress syndrome: when should we turn up the pressure? Crit Care Med. 2014;42:448–450. doi: 10.1097/01.ccm.0000435685.00716.48. [DOI] [PubMed] [Google Scholar]
- 63.Santa Cruz R, Rojas JI, Nervi R, Heredia R, Ciapponi A. High versus low positive end-expiratory pressure (PEEP) levels for mechanically ventilated adult patients with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev. 2013;6:CD009098. doi: 10.1002/14651858.CD009098.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lachmann B. Open up the lung and keep the lung open. Intensive Care Med. 1992;18:319–321. doi: 10.1007/BF01694358. [DOI] [PubMed] [Google Scholar]
- 65.Huh JW, Jung H, Choi HS, Hong SB, Lim CM, Koh Y. Efficacy of positive end-expiratory pressure titration after the alveolar recruitment manoeuvre in patients with acute respiratory distress syndrome. Crit Care. 2009;13:R22. doi: 10.1186/cc7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cressoni M, Chiumello D, Carlesso E, Chiurazzi C, Amini M, Brioni M, Cadringher P, Quintel M, Gattinoni L. Compressive forces and computed tomography-derived positive end-expiratory pressure in acute respiratory distress syndrome. Anesthesiology. 2014;121:572–581. doi: 10.1097/ALN.0000000000000373. [DOI] [PubMed] [Google Scholar]
- 67.Pintado MC, de Pablo R, Trascasa M, Milicua JM, Rogero S, Daguerre M, Cambronero JA, Arribas I, Sánchez-García M. Individualized PEEP setting in subjects with ARDS: a randomized controlled pilot study. Respir Care. 2013;58:1416–1423. doi: 10.4187/respcare.02068. [DOI] [PubMed] [Google Scholar]
- 68.Rodriguez PO, Esperanza JA, Valentini R. Transpulmonary pressure in acute respiratory distress syndrome. Crit Care Med. 2013;41:e9–10. doi: 10.1097/CCM.0b013e318270e569. [DOI] [PubMed] [Google Scholar]


