Abstract
Beyond canonical signaling via Gαs and cAMP, the concept of functional selectivity at β2-adrenoceptors (β2ARs) describes the ability of adrenergic drugs to stabilize ligand-specific receptor conformations to initiate further signaling cascades comprising additional G-protein classes or β-arrestins (βarr). A set of 65 adrenergic ligands including 40 agonists and 25 antagonists in either racemic or enantiopure forms was used for βarr recruitment experiments based on a split-luciferase assay in a cellular system expressing β2AR. Many agonists showed only (weak) partial agonism regarding βarr recruitment. Potencies and/or efficacies increased depending on the number of chirality centers in (R) configuration; no (S)-configured distomer was more effective at inducing βarr recruitment other than the eutomer. βarr2 was recruited more effectively than βarr1. The analysis of antagonists revealed no significant effects on βarr recruitment. Several agonists showed preference for activation of Gαs GTPase relative to βarr recruitment, and no βarr-biased ligand was identified. In conclusion: 1) agonists show strong bias for Gαs activation relative to βarr recruitment; 2) agonists recruit βarr1 and βarr2 with subtle differences; and 3) there is no evidence for βarr recruitment by antagonists.
Introduction
According to the concept of canonical signaling, β2-adrenoceptors (β2ARs) induce bronchodilatory effects by activation of Gαs and an increase in intracellular cAMP (Samama et al., 1993; Johnson, 1998). β2-Sympathomimetics are agonists at the β2ARs derived from the endogenous agonist epinephrine (EPI) (Supplemental Fig. 1) and constitute essential drugs in the treatment of bronchial asthma and chronic obstructive pulmonary disease. Representatives are the rapid-acting β2AR agonists fenoterol (FEN) and albuterol (ALB) used to counter immediate asthmatic attacks, or the long-acting β2AR agonists formoterol (FOR) and salmeterol (SAL) used for prolonged respiratory control (Hochhaus and Möllmann, 1992; Delmotte and Sanderson, 2010).
The concept of functional selectivity describes the ability of agonists to stabilize ligand-specific receptor conformations triggering the activation of multiple signaling cascades as well as the bias of ligands to preferentially activate certain cascades (Evans et al., 2010; Reiner et al., 2010; Seifert, 2013; van der Westhuizen et al., 2014). With regard to racemic sympathomimetic drug formulations consisting of (R)- and (S)-isoproterenol (ISOs), stereochemistry-related problems have been reported, including paradoxical proinflammatory effects caused by the inactive (S)-distomer (Mazzoni et al., 1994; Mitra et al., 1998; Nelson et al., 1998; Templeton et al., 1998; Zhang et al., 1998; Gawchik et al., 1999; Handley et al., 2000, 2002; Baramki et al., 2002; Volcheck et al., 2005; Patel and Thomson, 2012). These side effects may be the consequence of yet unknown distomer-triggered activation of noncanonical signaling pathways. In addition to canonical signaling by Gαs, the β2AR couples to Gαi proteins (Wenzel-Seifert and Seifert, 2000; Seifert et al., 2002; Birnbaumer, 2007; Magocsi et al., 2007) and β-arrestins (βarrs) (Oakley et al., 2000; Shenoy et al., 2006; Audet et al., 2010; Shukla et al., 2011; Reiter et al., 2012). Arrestins are responsible for desensitization of G-protein-coupled receptors upon prolonged stimulation and noncanonical signaling via mitogen-activated protein kinases (Freedman and Lefkowitz, 1996; Baillie et al., 2003; Beaulieu et al., 2005; Shenoy et al., 2006; Luttrell and Gesty-Palmer, 2010; Shukla et al., 2011). There are four known arrestin isoforms with arrestin1 and arrestin4 being restricted to visual sensory tissue and arrestin2 and arrestin3 [also referred to as β-arrestin1 (βarr1) and β-arrestin2 (βarr2), respectively] being ubiquitously expressed (Ferguson, 2001). In recent studies, we have shown that FEN stereoisomers stabilize ligand-specific β2AR conformations and exhibit strong bias for Gαs activation relative to Gαi activation and βarr1 and βarr2 recruitment (Reinartz et al., 2015a,b).
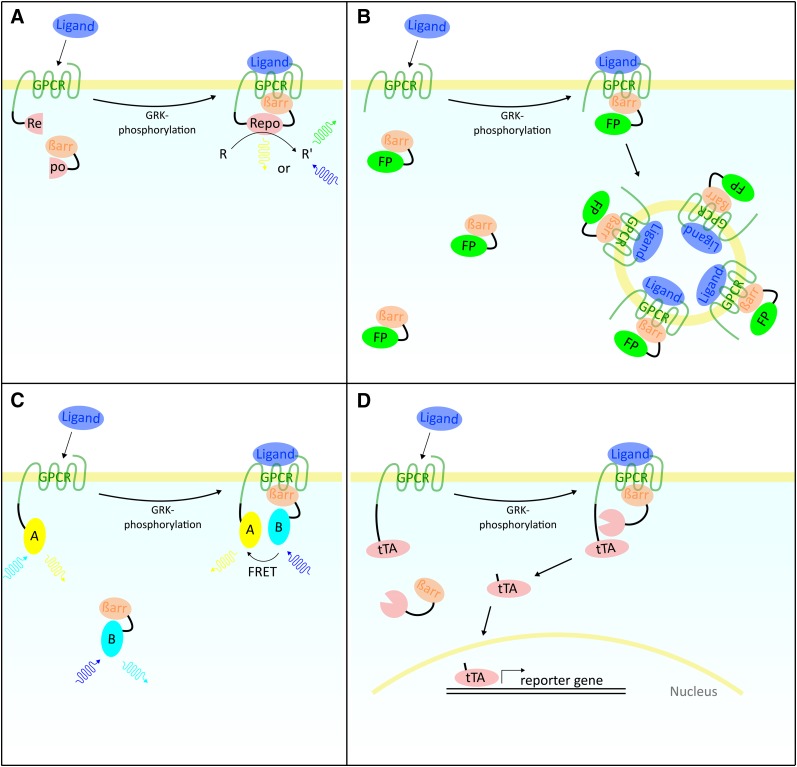
Several experimental setups are available for the investigation of receptor-arrestin interactions. In the complementation assay, a split reporter protein such as luciferase becomes functional upon recruitment of βarr to the G-protein-coupled receptor (Fig. 1A). One example is the split-luciferase assay system used in this study, which is based on cells expressing two fusion proteins: The first protein consists of the receptor and the N-terminal fragment of a luciferase from the click beetle Photinus pyralis and the second protein consists of the βarr fused to the C-terminal fragment of the luciferase. Upon recruitment of βarr to the β2AR, the two fragments of the luciferase complement each other and light at λ = 558 nm is emitted (Takakura et al., 2012). In this study, a cell line expressing the β2AR-luciferase protein and the βarr1-luciferase protein (β2AR-βarr1) as well as a cell line expressing the β2AR-luciferase protein and the βarr2-luciferase protein (β2AR-βarr2) were analyzed. In the DiscoveRx (Fremont, CA) PathHunter βarr assay, a β-galactosidase enzyme is complemented upon βarr recruitment (Yin et al., 2009). Alternative experimental approaches are the GFP (YFP)-distribution assays (Fig. 1B), the CFP/YFP Förster resonance energy transfer assay technique (Fig. 1C), and the Tango assay format (Fig. 1D).
Fig. 1.
Assay setups for the investigation of arrestin recruitment. (A) Complementation assay. Fusion proteins of G-protein-coupled receptor (GPCR) and βarr are each used with one part of a trenched reporter protein (Repo). Upon recruitment of βarr to the GPCR the two parts of the reporter protein complement each other and it becomes functional. Reporter proteins are luciferases, β-lactamase, or β-galactosidase that process specific substrates (R). Their activity can be detected, either by measuring luminescence (luciferase, β-galactosidase), or fluorescence (β-lactamase). (B) GFP (YFP)-distribution assays. The distribution of fluorescent proteins (FPs) (e.g., GFP, YFP) is analyzed. Without the influence of ligands the FP-βarr fusion proteins are uniformly distributed in the cell. Upon recruitment of the FP-βarr fusion proteins to the GPCR, internalization of the receptors in vesicles is mediated. GPCRs and bound FP-βarr are colocalizing, leading to the formation of grains within the cells, which is quantified using fluorescence microscopy and specific software. (C) CFP/YFP Förster resonance energy transfer (FRET) assay. These methods use FRET for quantification. Two fusion proteins of the GPCR and βarr are used. Each protein is fused to a fluorescent protein of which the emission spectrum of the first overlaps the excitation spectrum of the second (e.g., YFP and CFP). When both proteins come in close proximity to each other FRET occurs and is measured. Another possibility is to use a luciferase as a FRET donor instead of a fluorescent protein. (D) Tango assay. The βarr is fused to a tobacco etch virus (TEV) protease. The GPCR is fused to a linker region containing a cleavage site for the TEV protease and the tTA transcription factor. Upon recruitment of βarr to the GPCR, the protease fusion protein is close enough to the GPCR fusion protein to cut off the tTA, which is now able to translocate to the nucleus where it induces transcription of a reporter gene (e.g., luciferase).
To this end, the effects of β2AR ligands on βarr1 and βarr2 recruitment have not yet been systematically analyzed. Therefore, the aim of this study was to fill this gap in our knowledge and to investigate the recruitment of βarr1 and βarr2 to the β2AR using 65 adrenergic ligands either as racemic forms or, if available, as pure enantiomers. Ligands were chosen from different structural classes (Supplemental Figs. 1 and 2) and included antagonists because several studies have shown that βarr recruitment can be induced by antagonists as well (Wisler et al., 2007; Erickson et al., 2013).
Materials and Methods
Materials.
The following ligands and reagents were obtained from Sigma (Steinheim, Germany): alprenolol [racemic, (R)-, 1-(2-allylphenoxy)-3-(isopropylamino)propan-2-ol]; atenolol (racemic, 2-{4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl}acetamide); CGP 20712A (2-hydroxy-5-[2-[[2-hydroxy-3-[4-[1-methyl-4-(trifluoromethyl)-1H-imidazol-2-yl]phenoxy]propyl]amino]ethoxy]-benzamide methanesulfonate salt); ephedrine (enantiopure, (1R,2S)-2-(methylamino)-1-phenylpropan-1-ol); EPI (enantiopure, (R)-, [4-(1-hydroxy-2-(methylamino)ethyl)benzene-1,2-diol]; FEN (racemic, enantiopure, (R,R′)-, (R,S′)-, (S,R′)-, (S,S′)-, [5-(1-hydroxy-2-{[2-(4-hydroxyphenyl)-1-methylethyl]amino}ethyl)benzene-1,3-diol]; isoproterenol (enantiopure, (R)-, (S)-, 4-[1-hydroxy-2-(isopropylamino)ethyl]benzene-1,2-diol); labetalol (racemic, 2-hydroxy-5-{1-hydroxy-2-[(1-methyl-3-phenylpropyl)amino]ethyl}benzamide); metoprolol (racemic, 1-(isopropylamino)-3-[4-(2-methoxyethyl)phenoxy]propan-2-ol); nadolol (racemic, (2R*,3S*)-5-{[(2R*)-3-(tert-butylamino)-2-hydroxypropyl]oxy}-1,2,3,4-tetrahydronaphthalene-2,3-diol); norepinephrine (enantiopure, (R)-, (S)-, 4-[(1R)-2-amino-1-hydroxyethyl]benzene-1,2-diol); propranolol (enantiopure, (R)-, (S)-, 1-(1-methylethylamino)-3-(1-naphthyloxy)propan-2-ol); salbutamol (racemic, 4-[2-(tert-butylamino)-1-hydroxyethyl]-2-(hydroxymethyl)phenol); sotalol (racemic, N-{4-[1-hydroxy-2-(propan-2-ylamino)ethyl]phenyl}methanesulfonamide); timolol [(S)-1-(tert-butylamino)-3-[(4-morpholin-4-yl-1,2,5-thiadiazol-3-yl)oxy]propan-2-ol]; Dulbecco’s modified Eagle’s medium; and fetal bovine serum. Dulbecco’s modified Eagle’s medium without phenol red was obtained from GE Healthcare (Pasching, Austria). The following ligands were obtained from Tocris (Avonmouth, United Kingdom): betaxolol (racemic, 1-{4-[2-(cyclopropylmethoxy)ethyl]-phenoxy}-3-(isopropylamino)propan-2-ol); bisoprolol (racemic, 1-{4-[(2-isopropoxyethoxy)methyl]phenoxy}-3-(isopropylamino)propan-2-ol); BRL 37344 [(R*,R*)-(±)-4-[2-[(2-(3-chlorophenyl)-2-hydroxyethyl)amino]propyl]phenoxyacetic acid, sodium salt]; CGP 12177 (4-[3-[(1,1-dimethylethyl)amino]2-hydroxypropoxy]-1,3-dihydro-2H-benzimidazol-2-one hydrochloride); CGP 20712 (1-[2-((3-carbamoyl-4-hydroxy)phenoxy)ethylamino]-3-[4-(1-methyl-4-trifluoromethyl-2-imidazolyl)phenoxy]-2-propanol dihydrochloride); ICI 118,551 (racemic, [erythro-(S*,S*)-1-[2,3-(dihydro-7-methyl-1H-inden-4-yl)oxy]-3-[(1-methylethyl)amino]-2-butanol hydrochloride]); ICI 215,001 [(S)-4-[2-hydroxy-3-phenoxypropylaminoethoxy]phenoxyacetic acid hydrochloride]; pindolol (racemic, [1-(1H-indol-4-yloxy)-3-[(1-methylethyl)amino]-2-propanol]); nebivolol (racemic, (±)-[2R*(1S*5S*(S*))]-α,α′-[Iminobis(methylene)bis(6-fluoro-3,4-dihydro-2H-1-benzopyran-2-methanol)); practolol (racemic, N-{4-[2-hydroxy-3-(isopropylamino)propoxy]phenyl}acetamide); xamoterol (racemic, 1-(4-hydroxyphenoxy)-3-[2-(4-morpholinocarboxamido)ethylamino]-2-propanol hemifumarate); and zinterol (racemic, N-[5-[2-[(1,1-dimethyl-2-phenylethyl)amino]-1-hydroxyethyl]-2-hydroxyphenyl]methanesulphonamide hydrochloride). The following ligands were obtained from Boehringer-Ingelheim (Biberach, Germany): carvedilol (CAR) (racemic, [3-(9H-carbazol-4-yloxy)-2-hydroxypropyl][2-(2-methoxyphenoxy)ethyl]amine); FOR (racemic, (R,R)-, (S,S)-, N-[2-hydroxy-5-[1-hydroxy-2-[1-(4-methoxyphenyl)propan-2-ylamino]ethyl]phenyl]formamide); olodaterol (enantiopure, (R)-, (S)-, 6-hydroxy-8-{(1R)-1-hydroxy-2-{[1-(4-methoxyphenyl)-2-methylpropan-2-yl]amino}ethyl}-4H-1,4-benzoxazin-3-one); salbutamol (racemic, enantiopure, (R)-, (S)-, 4-[2-(tert-butylamino)-1-hydroxyethyl]-2-(hydroxymethyl)phenol); and salmeterol (racemic, enantiopure, (R)-, (S)-, 2-(hydroxymethyl)-4-{1-hydroxy-2-[6-(4-phenylbutoxy)hexylamino]ethyl}phenol). CAR (enantiopure, (R)-, (S)-, [3-(9H-carbazol-4-yloxy)-2-hydroxypropyl][2-(2-methoxyphenoxy)ethyl]amine) was obtained from Dr. Peter Gmeiner (University of Erlangen, Erlangen, Germany). Cyanopindolol (racemic, 4-[3-(tert-Butylamino)-2-hydroxypropoxy]-1H-indole-2-carbonitrile) was obtained from Biotrend (Cologne, Germany). Racemic FEN [5-(1-hydroxy-2-{[2-(4-hydroxyphenyl)-1-methylethyl]amino}ethyl)benzene-1,3-diol)] and derivatives [enantiopure, (R,R′)-, (R,S′)-, (S,R′)-, (S,S′)-] were obtained from SRI (Menlo Park, CA). The Bright-Glo luciferase reagent was obtained from Promega (Mannheim, Germany).
Cells and Cultures.
Cells were maintained and used in the experiments as described previously (Takakura et al., 2012). HEK293 cells transfected with the β2AR fused to one fragment of split luciferase derived from the click beetle P. pyralis and either βarr1 or βarr2 linked to a second fragment of split luciferase were grown in Dulbecco’s modified Eagle’s medium (high glucose, free of phenol red) containing 10% (v/v) fetal bovine serum, 1% (v/v) penicillin/streptomycin, 1% (v/v) L-glutamine, 0.8 mg/ml G418, and 0.04 mg/ml zeocin at 37°C in the presence of 5% (v/v) CO2.
Arrestin Recruitment Experiments.
Experiments for the investigation of βarr1 or βarr2 recruitment to the β2AR were conducted as described previously (Takakura et al., 2012). In brief, 24 hours prior to the experiments, 100,000 cells in 90 µl of growth medium were seeded in 96-well microtiter plates suitable for cell culture and luminescence detection (Corning, Kaiserslautern, Germany). Cells were incubated in triplicate with 10 µl of ligand for 10 minutes at 37°C in a 96-well microtiter plate reader (Synergy 4, BioTek, Winooski, VT). When antagonism was analyzed, another 10 minute incubation period with 10 µl of antagonist solution was conducted prior to agonist incubation. Next, 50 µl/well of medium was removed and 50 µl/well of Bright-Glo luciferase reagent (Promega) was added. After shaking for 2 minutes, luminescence was measured at λ = 558 nm for 2 seconds/well. Raw data were normalized to a solvent control (baseline signal) as well as to data obtained from incubation with 10 µM (R)-ISO (maximal response of the system, 100%). The time courses of βarr1 or βarr2 recruitment for various selected ligands were similar (Supplemental Figs. 3 and 4). Hence, in the following experiments, to ensure high signal intensities and for practical reasons, all experiments were conducted for 10 minutes. Data were fitted, plotted, and statistically analyzed with Graph Pad Prism 5 (Graph Pad Software, La Jolla, CA). To further validate the results obtained using the previously described method, some β2AR-βarr2 recruitment experiments were replicated with the commercially available PathHunter system, PathHunter eXpress (DiscoveRx, Fremont, CA) according to the manufacturer’s instructions. Confluent Chinese hamster ovary cell layers in 96-well plates were inoculated with different concentrations of the ligands in duplicate for 90 minutes at 37°C. Reactions were stopped by the addition of the detection reagent. Luminescence was measured after 60 minute incubation at room temperature. Readings were normalized between the basal signal without ligand and maximal stimulation by 10 µM (R)-ISO.
Bias Quantification.
Bias quantification was performed according to van der Westhuizen et al. (2014). In brief, the transduction coefficients [log(τ/KA)] were obtained by fitting the concentration-response data to the operational model for agonism by Black and Leff (1983). For each ligand the transduction coefficients were compared by subtraction to the reference ligand (R)-EPI to correct for system bias [Δlog(τ/KA)]. The comparison between two pathways was performed by subtracting the Δlog(τ/KA) values for each individual ligand for one pathway by those from another one. This subtraction yielded the ΔΔlog(τ/KA) values, which represent the bias of a ligand toward a certain pathway. A bias was defined as significant if the 95% interval of confidence of the investigated ligand did not overlap that of the reference ligand (R)-EPI. Several ligands were defined as extremely biased because they were either giving no signal in one of the two compared pathways, or the signal in one of the two pathways was too low to get a robust fit with the operational model.
Results
Endogenous Catecholamines and Isoproterenol.
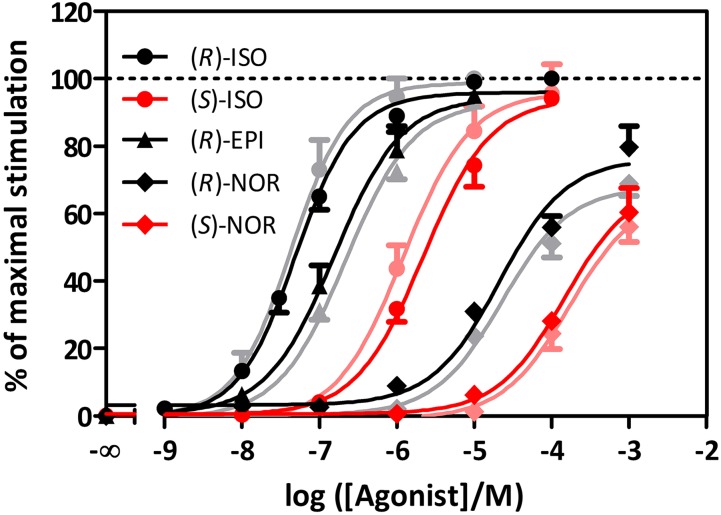
Representative concentration-response curves for βarr1 or βarr2 recruitment by agonists are shown in Fig. 2. (R)-Stereoisomers generally showed higher potencies compared with (S)-ISOs. Moreover, efficacies for (R)-stereoisomers were higher compared with (S)-isomers for most agonists (Table 1). (S)-ISO and (R)-ISO exhibited similar efficacies. With respect to biased recruitment of βarr1 or βarr2 subtypes, neither ISO nor endogenous catecholamines showed significant selectivity with respect to potency and efficacy. Comparison of the split-luciferase assay and the DiscoveRx PathHunter assay revealed similar pharmacological properties of (R)-ISO and (R)-EPI in both assay systems (Supplemental Fig. 5). In contrast to the endogenous catecholamines, a series of antagonists showed no or only minimal stimulatory effects on βarr1 or βarr2 recruitment (Supplemental Figs. 4, 6, and 7), pointing to the specificity of the assay for agonists.
Fig. 2.
Concentration-response curves for β2AR agonists in relation to βarr recruitment. Concentration-response curves for pairs of enantiomers of ISO and norepinephrine (NOR), and (R)-EPI. Cells were treated as described in Materials and Methods. Curves and symbols for βarr1 are given in lighter colors than those for βarr2. (N ≥ 3; data ± S.D.).
TABLE 1.
Potencies and efficacies of agonists at inducing βarr1 or βarr2 recruitment
When concentration-response curves did not reach saturation, the activation at the highest measured concentration is given as Emax. To analyze the data for preferred activation of βarr1 versus βarr2, two-way analysis of variance and the Bonferroni post-test were performed. *P < 0.05; **P < 0.01; ***P < 0.001; data ± S.D.; N ≥ 4.
| Ligand |
Stereo-Configuration |
pEC50
βarr1 |
pEC50
βarr2 |
Statistical Significance |
Emax
βarr1 |
Emax
βarr2 |
Statistical Significance |
|---|---|---|---|---|---|---|---|
| % | % | ||||||
| ISO | (R) | 7.38 ± 0.05 | 7.24 ± 0.03 | ns | 100 | 100 | ns |
| (S) | 5.92 ± 0.07 | 5.66 ± 0.05 | * | 96.07 ± 2.54 | 94.22 ± 1.96 | ns | |
| EPI | (R) | 6.66 ± 0.05 | 6.81 ± 0.05 | ns | 93.10 ± 1.78 | 94.42 ± 1.82 | ns |
| NOR | (R) | 4.64 ± 0.08 | 4.68 ± 0.10 | ns | 67.79 ± 2.08 | 76.20 ± 3.13 | *** |
| (S) | No saturation | No saturation | 56.01 ± 4.35 | 60.36 ± 7.21 | * | ||
| DCI | Racemic | No signal | No signal | No signal | No signal | ||
| ALB | Racemic | 6.32 ± 0.05 | 6.71 ± 0.07 | *** | 16.58 ± 0.42 | 19.79 ± 0.51 | ns |
| (R) | 6.55 ± 0.05 | 6.82 ± 0.09 | *** | 19.59 ± 3.32 | 19.82 ± 1.92 | ns | |
| (S) | 4.77 ± 0.34 | 4.65 ± 0.10 | ns | 0.36 ± 0.05 | 3.45 ± 0.13 | ns | |
| FEN | Racemic | 7.02 ± 0.06 | 7.04 ± 0.06 | ns | 63.94 ± 1.45 | 61.48 ± 1.51 | ns |
| (R,R′) | 7.28 ± 0.07 | 7.20 ± 0.06 | ns | 70.56 ± 1.63 | 64.69 ± 1.48 | *** | |
| (R,S′) | 5.78 ± 0.09 | 5.85 ± 0.05 | ns | 31.69 ± 1.17 | 29.27 ± 0.61 | ns | |
| (S,R′) | 4.48 ± 0.07 | 4.76 ± 0.07 | *** | 11.20 ± 0.34 | 17.32 ± 0.48 | *** | |
| (S,S′) | 4.49 ± 0.09 | 4.62 ± 0.06 | ns | 25.69 ± 1.02 | 28.71 ± 0.79 | ns | |
| SAL | Racemic | 7.76 ± 0.09 | 7.83 ± 0.08 | ns | 10.35 ± 0.39 | 14.69 ± 0.46 | ** |
| (R) | 7.44 ± 0.07 | 7.42 ± 0.11 | ns | 10.90 ± 0.36 | 14.26 ± 0.69 | ns | |
| (S) | 6.79 ± 0.14 | 6.99 ± 0.11 | *** | 3.90 ± 1.24 | 7.39 ± 1.88 | *** | |
| FOR | Racemic | 7.69 ± 0.06 | 7.63 ± 0.05 | ns | 76.80 ± 1.77 | 84.86 ± 1.78 | *** |
| (R,R′) | 7.95 ± 0.03 | 8.03 ± 0.08 | ns | 73.29 ± 0.96 | 80.33 ± 2.45 | *** | |
| (S,S′) | 5.91 ± 0.07 | 5.82 ± 0.07 | ns | 65.13 ± 1.79 | 73.12 ± 2.03 | *** | |
| OLO | (R) | 8.11 ± 0.04 | 8.11 ± 0.08 | ns | 34.43 ± 0.58 | 34.49 ± 1.11 | ns |
| (S) | 6.06 ± 0.07 | 6.00 ± 0.08 | ns | 31.15 ± 0.81 | 32.11 ± 1.08 | ns | |
| MDF | (R) | 5.47 ± 0.08 | 5.89 ± 0.10 | *** | 26.51 ± 0.94 | 26.35 ± 1.02 | ns |
| (S) | No saturation | No saturation | 7.02 ± 0.23 | 14.52 ± 2.00 | *** | ||
| MEF | (R,R′) | 7.00 ± 0.05 | 7.10 ± 0.05 | ns | 63.14 ± 1.31 | 61.18 ± 1.23 | ns |
| (R,S′) | 6.03 ± 0.07 | 5.91 ± 0.06 | ns | 39.45 ± 1.02 | 42.48 ± 0.97 | ns | |
| (S,R′) | No saturation | 4.92 ± 0.07 | 3.17 ± 0.53 | 10.09 ± 0.32 | *** | ||
| (S,S′) | No saturation | 4.76 ± 0.08 | 3.64 ± 1.25 | 9.43 ± 0.39 | *** | ||
| MEtF | (R,R′) | 6.36 ± 0.08 | 6.47 ± 0.05 | ns | 47.24 ± 1.67 | 40.37 ± 0.83 | *** |
| (R,S′) | No saturation | No saturation | −0.04 ± 0.14 | 2.69 ± 0.21 | ns | ||
| MnF | (R,R′) | No saturation | No saturation | 5.75 ± 1.34 | 12.83 ± 0.98 | *** | |
| (R,S′) | No saturation | No saturation | −0.14 ± 0.13 | 3.15 ± 0.36 | ns | ||
| MiF | (R,R′) | No signal | No signal | No signal | No signal | ||
| (R,S′) | No saturation | No saturation | −0.01 ± 0.19 | 3.61 ± 0.26 | ns | ||
| MNF | (R,R′) | 6.6 ± 0.12 | 6.84 ± 0.06 | ** | 34.04 ± 1.67 | 35.96 ± 0.88 | ns |
| (R,S′) | No saturation | 6.39 ± 0.11 | 0.07 ± 0.17 | 2.17 ± 0.11 | ns | ||
| (S,R′) | No saturation | 5.71 ± 0.07 | 9.42 ± 1.47 | 17.15 ± 0.47 | *** | ||
| (S,S′) | No saturation | 6.02 ± 0.39 | −0.06 ± 0.11 | 0.75 ± 0.16 | ns | ||
| ZIN | Racemic | 7.62 ± 0.10 | 7.65 ± 0.07 | ns | 26.38 ± 1.07 | 28.22 ± 0.77 | ns |
| BRL | Racemic | No saturation | 6.26 ± 0.19 | 0.02 ± 0.23 | 1.52 ± 0.13 | ns |
BRL, BRL 37344; DCI, sodium dichloroisoproterenol; MDF, 4′-Methoxy-desmethylfenoterol; MEF, 4′-methoxyfenoterol; MEtF, 4′-methoxy-ethylfenoterol; MiF, 4′-methoxy-i-propylfenoterol; MnF, 4′-methoxy-n-propylfenoterol; NOR, norepinephrine; ns, not significant; OLO, olodaterol; ZIN, zinterol.
Rapid-Acting β2AR Agonists.
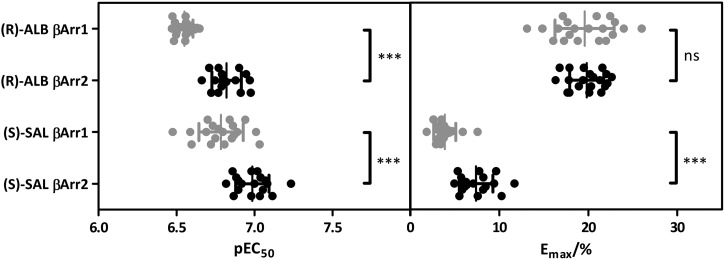
FEN possesses two stereocenters yielding four stereoisomers (Supplemental Fig. 1). The most potent and efficacious FEN isomer was (R,R′), followed by (R,S′), (S,S′), and (S,R′) (Supplemental Figs. 8 and 9; Table 1). The racemic mixture exhibited similar pharmacological properties as the (R,R′)-isomer. (S,R′)-FEN recruited βarr2 with higher potency and efficacy compared with βarr1, and (R,R′)-FEN induced βarr2 recruitment with higher efficacy. (R)-ALB showed higher potency and efficacy than the racemic mixture and (S)-ALB. In-depth analysis of (R)-ALB confirmed the small bias toward βarr2 recruitment since the differences in potencies of βarr subtype recruitment were significant (Fig. 3).
Fig. 3.
Scatter-plot of pEC50 and Emax values for (R)-ALB and (S)-SAL. Cells were treated as described in Materials and Methods. While (S)-SAL exhibited significantly different pEC50 and Emax values comparing βarr1 (gray) and βarr2 (black), (R)-ALB only showed significant differences with regard to potency. Detailed values can be found in Table 1. (N ≥ 18; data ± S.D.; unpaired t test with two-tailed P values; ns, not significant; ***P < 0.001).
Long-Acting β2AR Agonists.
Racemic SAL and (R)-SAL showed similar potencies and efficacies of about 15% for βarr2 and 10% for βarr1 normalized to (R)-ISO. Again, the (S)-isomer showed reduced potency and efficacy. In-depth analysis of (S)-SAL yielded significantly increased potency and efficacy for βarr2 compared with βarr1 recruitment (Fig. 3; Table 1). The racemic mixture of FOR and (R,R)-FOR exhibited similar pharmacological properties, whereas the concentration-response curve of (S,S)-FOR showed a right shift as well as a slight reduction in maximal response. All three substances showed higher efficacies for βarr2 recruitment. Olodaterol isomers showed no bias between βarr1 and βarr2.
Derivatives of FEN.
4′-Methoxy-desmethylfenoterol showed a similar pattern as the SAL and FOR isomers. (S)-isomer exhibited lower potency and efficacy than the (R)-isomer. Moreover, (R)-4′-methoxy-desmethylfenoterol showed higher potency at inducing recruitment of βarr2 and (S)-4′-methoxy-desmethylfenoterol showed higher efficacy at inducing recruitment of βarr2 compared with βarr1. In the case of 4′-methoxyfenoterol, potencies and efficacies increased depending on the number of chirality centers in the (R) configuration, attributing major importance the center closest to the catechol moiety. (S,R′)-Methoxyfenoterol and (S,S′)-4′-methoxyfenoterol were more effective in inducing βarr2 than βarr1 recruitment. Regarding 4′-methoxy-1-naphthylfenoterol (MNF), (S,R′)-MNF was the second efficacious isomer after (R,R′)-MNF. The lowest efficacy was again found for (S,S′)-ISO. (R,S′)-MNF, (S,R′)-MNF, and (S,S′)-MNF preferred βarr2 over βarr1 recruitment. (R,R′)-MNF preferred βarr2 concerning potency. Interestingly, (R,S′)-MNF and (S,S′)-MNF showed slight inverse agonism concerning βarr1 recruitment. The (R,R′) configuration of 4′-methoxy-ethylfenoterol showed considerably higher potency and efficacy relative to (R,S′)-4′-methoxy-ethylfenoterol regardless of the βarr subtype. (R,S′)-4′-Methoxy-ethylfenoterol effected weak βarr2 recruitment and was incapable of recruiting βarr1. In the case of 4′-methoxy-n-propylfenoterol, the (R,R′) and (R,S′) configurations both revealed a bias toward βarr2. No activity was detected for the induction of βarr1 recruitment by (R,S′)-4′-methoxy-n-propylfenoterol. In contrast to all other FEN derivatives yielding higher potencies and efficacies for the (R,R′) configuration, (R,R′)-4′-methoxy-i-propylfenoterol did not induce recruitment of either of the analyzed βarr subtypes. For this compound the only effect that could be detected was βarr2 recruitment by (R,S′)-4′-methoxy-i-propylfenoterol.
Other Ligands.
While racemic zinterol showed no bias toward a given arrestin isoform, racemic BRL 37344 was only capable of inducing βarr2 recruitment.
Antagonist Effects.
As exemplarily shown in Supplemental Fig. 10 for (R)-CAR and (S)-CAR, β-adrenergic antagonists inhibited signals induced by (R)-ISO. However, when β-adrenergic antagonists and partial agonists were examined in the absence of ISO at a fixed concentration of 10 µM, only one ligand was found to significantly influence βarr recruitment. Specifically, the β3AR agonist ICI 215,001 showed very weak but significant inverse agonism concerning βarr1 recruitment (Fig. 4; Table 2). Even when tested at different time points and at a broad range of concentrations, antagonists were virtually devoid of stimulatory effects on βarr recruitment (Supplemental Figs. 4, 6, and 7).
Fig. 4.
Effect of antagonists (10 µM each) on the recruitment of βarr. Cells were treated as described in Materials and Methods. Each analyzed ligand was examined for the induction of βarr1 (gray) and βarr2 (black) recruitment. Unless otherwise noted, racemic mixtures were analyzed. (A) Ligands are presented with an uninterrupted y-axis to show relative differences from the reference ligand (R)-ISO. (B) The y-axis is interrupted to show ligand effects in detail. Statistical analysis of antagonist effects compared with solvent control yielded no significance except for the β3AR agonist ICI 215,001, which is a very week inverse agonist concerning βarr2 recruitment. (N = 4; data ± S.D.; two-way analysis of variance with Bonferroni post-test; *P < 0.05).
TABLE 2.
Stimulation of βarr1 or βarr2 recruitment by antagonists and partial agonists analyzed in the split-luciferase assay
Ligand activities were normalized to (R)-ISO (100%), and statistical significance was determined compared with values from the solvent control (two-way analysis of variance with the Bonferroni post-test).
| Ligand |
Stimulation βarr1 |
Stimulation βarr2 |
Significance βarr1 |
Significance βarr2 |
|---|---|---|---|---|
| % | % | |||
| (R)-ISO | 100.0 | 100.0 | P < 0.001 | P < 0.001 |
| Timolol | 0.22 | 0.17 | P > 0.05 | P > 0.05 |
| Nadolol | 0.10 | −0.02 | P > 0.05 | P > 0.05 |
| Labetalol | 0.16 | 0.10 | P > 0.05 | P > 0.05 |
| Metoprolol | −0.33 | −0.10 | P > 0.05 | P > 0.05 |
| Nebivolol | 0.09 | 0.06 | P > 0.05 | P > 0.05 |
| Alprenolol | 0.09 | −0.04 | P > 0.05 | P > 0.05 |
| Atenolol | −0.43 | −0.30 | P > 0.05 | P > 0.05 |
| Sotalol | −0.34 | −0.26 | P > 0.05 | P > 0.05 |
| Practolol | −0.25 | −0.01 | P > 0.05 | P > 0.05 |
| Bisoprolol | −0.07 | −0.14 | P > 0.05 | P > 0.05 |
| Xamoterol | −0.25 | −0.16 | P > 0.05 | P > 0.05 |
| Betaxolol | −0.19 | −0.21 | P > 0.05 | P > 0.05 |
| (R)-Alprenolol | 0.14 | 0.03 | P > 0.05 | P > 0.05 |
| (R)-Propranolol | −0.06 | −0.11 | P > 0.05 | P > 0.05 |
| (S)-Propranolol | −0.02 | −0.05 | P > 0.05 | P > 0.05 |
| CGP 20712A | −0.37 | −0.25 | P > 0.05 | P > 0.05 |
| CGP 20712 | −0.22 | −0.06 | P > 0.05 | P > 0.05 |
| Pindolol | 0.40 | 0.16 | P > 0.05 | P > 0.05 |
| Cyanopindolol | 0.15 | 0.08 | P > 0.05 | P > 0.05 |
| Rac-CAR | −0.22 | −0.31 | P > 0.05 | P > 0.05 |
| (R)-CAR | −0.12 | −0.18 | P > 0.05 | P > 0.05 |
| (S)-CAR | −0.001 | −0.22 | P > 0.05 | P > 0.05 |
| ICI 118,551 | −0.30 | −0.24 | P > 0.05 | P > 0.05 |
| CGP 12177 | −0.23 | 0.06 | P > 0.05 | P > 0.05 |
| ICI 215,001 | −0.63 | −0.12 | P < 0.05 | P > 0.05 |
GTPase Data.
For comparison with βarr recruitment, we also examined activation of Gαs by selected ligands. These data are listed in Supplemental Tables 1 and 2. In general, agonists were more potent and efficacious at stimulating Gαs than at recruiting βarr. The corresponding bias analyses are shown in Supplemental Tables 3 and 4 and Fig. 5.
Fig. 5.
The ΔΔlog(τ/KA) values for three different pathways. The ΔΔlog(τ/KA) values were obtained by subtracting the system bias-corrected Δlog(τ/KA) values for each ligand for one pathway from those of another pathway. Shown are the values with their corresponding 95% confidence interval (CI). The 95% CI of the reference ligand (R)-EPI is shown as a red bar. A ligand is defined as biased if its 95% CI does not overlap with the one from the reference.
Discussion
βarr Recruitment by β2AR ligands: Comparison with Literature Data.
Potency and efficacy data differ depending on the experimental readout system as well as the cellular system used (Supplemental Table 5). Strikingly, values determined in the Tango assay systemically showed higher potencies but lower efficacies, which may be associated with the normalization of ligand parameters to those of the partial agonist FOR instead of the endogenous ligand EPI or its substitute ISO (Rajagopal et al., 2011). Considering results from fluorescent protein-distribution assays, differences in comparison with results from this study were rather small and not as systemic (Oakley et al., 2002; Reiner et al., 2010). These differences may result from the rather challenging quantification of changes in fluorescence distribution within cells. Data from β-galactosidase complementation assays yielded different ligand parameters depending on the cell type used. The results obtained by Kopra et al. (2013) using Chinese hamster ovary cells are in good agreement with the results from this study, whereas Yaffe and Saxel (1977) and Carter and Hill (2005) obtained higher efficacies on myocytic, polynuclear C2C12 cells. It is difficult to provide a definitive explanation for these differences.
Partial Agonism.
Many agonists analyzed in this study are full agonists with respect to G-protein activation and cAMP production (Toll et al., 2011; Brunskole Hummel et al., 2013); however, they turned out to be only (weak) partial agonists regarding βarr recruitment, indicating a strong bias toward Gαs-mediated cAMP formation. Compounds showing low efficacies for βarr recruitment were ALB, FOR, SAL, zinterol, and in particular the FEN derivatives (Baker, 2010; Brunskole Hummel et al., 2013; Plazinska et al., 2014). Reduced efficacies may result from modification of the two hydroxyl groups in the m and p positions of the catechol moiety of the endogenous agonists and ISO. The replacement of these hydroxyl groups by chlorine moieties in sodium dichloroisocyanurate and BRL 37344 is associated with the complete loss of βarr recruitment. In addition, secondary amines appeared to effect full activation of the system, while the primary amine norepinephrine only exhibits an Emax value of about 80%.
Signaling Bias.
βarr1 and βarr2 function in similar ways within the cell, for example, in the case of knockout of one subtype, the other βarr isoform can rescue the phenotype nearly completely (Conner et al., 1997; Bohn et al., 2002). Moreover, β2AR exhibits higher affinities for βarr2 than for βarr1 (Oakley et al., 2000). Few ligands exhibited biased βarr signaling (Fig. 5), and if so, βarr2 was recruited more effectively and potently than βarr1 (Table 1).
Stereochemistry.
(R)-Configured agonists activated βarr more effectively than (S)-stereoisomers. Presumably, the stereocenter next to the catechol moiety significantly contributes to receptor activation by the formation of specific hydrogen bonds. MNF is an exception to the rule because only a very small signal could be detected for the (R,S′)-enantiomer, which may be a result from the size of the naphthyl moiety and the resulting steric hindrance. No distomer was more potent and effective at inducing βarr recruitment than its eutomer, which could have explained some of the reported paradoxical proinflammatory adverse effects of clinically administered racemic drugs (Templeton et al., 1998; Volcheck et al., 2005; Patel and Thomson, 2012). Further research that focuses on downstream signaling is required to determine whether the two different subtypes of βarr differ in their ability to activate signaling pathways within cells apart from receptor internalization.
Antagonists.
Several groups have reported βarr recruitment as a result from antagonist binding to G-protein-coupled receptors. Antagonists induced receptor internalization and G-protein-independent (but βarr-dependent) activation of the mitogen-activated protein kinase ERK1/2 (Wisler et al., 2007; Erickson et al., 2013). Normalized to the effect of 10 µM (R)-ISO, CAR induced about 14% of receptor internalization and generated about 40% of ERK1/2 activation, which was reduced to about 15% by knocking down βarr2 with siRNA (Wisler et al., 2007). Similar characteristics have also been described for the βAR antagonist nebivolol (Erickson et al., 2013). In contrast, the analysis of antagonists with the split-luciferase assay revealed no significant effects on the recruitment of βarr, although each ligand was used at a concentration of 10 µM (Fig. 4). Several explanations are possible. First, experiments by Wisler et al. (2007) were performed with β2AR/vasopressin V2 receptor chimeras, which may have altered receptor characteristics. Second, CAR effects may also result from antagonizing α1ARs and not only βARs (Pedersen and Cockcroft, 2007). Third, the high concentrations of antagonists used may have caused off-target effects (Hagelüken et al., 1994). In conclusion, βAR agonists show strong bias for Gαs activation relative to βarr activation, agonists activate βarr1 and βarr2 signaling with subtle differences, and there is no evidence for βarr recruitment by βAR antagonists.
Supplementary Material
Acknowledgments
The authors thank Dr. Peter Gmeiner and Markus Stanek from Friedrich-Alexander-University Erlangen-Nürnberg, Erlangen, Germany, for providing pure carvedilol stereoisomers, as well as Dr. Andreas Schnapp and Dr. Michael Pieper from Boehringer-Ingelheim, Biberach, Germany, for generously providing several adrenergic ligands. Thanks are also due to the reviewers for the helpful critique of this paper.
Abbreviations
- ALB
albuterol
- β2AR
β2-adrenoceptor
- βarr
β-arrestin
- CAR
carvedilol
- EPI
epinephrine
- FEN
fenoterol
- FOR
formoterol
- ISO
isomer
- MNF
4′-methoxy-1-naphthylfenoterol
- SAL
salmeterol
Authorship Contributions
Participated in research design: Littmann, Göttle, Seifert.
Conducted experiments: Littmann, Göttle, Reinartz, Kälble.
Contributed new reagents or analytic tools: Wainer, Ozawa.
Performed data analysis: Littmann, Göttle, Reinartz, Seifert.
Wrote or contributed the writing of the manuscript: Göttle, Littmann, Seifert.
Footnotes
This work was supported by internal funds of the Hannover Medical School.
The authors declare no conflict of interest.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Audet M, Lagacé M, Silversides DW, Bouvier M. (2010) Protein-protein interactions monitored in cells from transgenic mice using bioluminescence resonance energy transfer. FASEB J 24:2829–2838. [DOI] [PubMed] [Google Scholar]
- Baillie GS, Sood A, McPhee I, Gall I, Perry SJ, Lefkowitz RJ, Houslay MD. (2003) β-Arrestin-mediated PDE4 cAMP phosphodiesterase recruitment regulates β-adrenoceptor switching from Gs to Gi. Proc Natl Acad Sci USA 100:940–945. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Baker JG. (2010) The selectivity of β-adrenoceptor agonists at human β1-, β2- and β3-adrenoceptors. Br J Pharmacol 160:1048–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baramki D, Koester J, Anderson AJ, Borish L. (2002) Modulation of T-cell function by (R)- and (S)-isomers of albuterol: anti-inflammatory influences of (R)-isomers are negated in the presence of the (S)-isomer. J Allergy Clin Immunol 109:449–454. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. (2005) An Akt/β-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 122:261–273. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L. (2007) Expansion of signal transduction by G proteins. The second 15 years or so: from 3 to 16 α subunits plus βγ dimers. Biochim Biophys Acta 1768:772–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JW, Leff P. (1983) Operational models of pharmacological agonism. Proc R Soc Lond B Biol Sci 220:141–162. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Caron MG. (2002) Differential mechanisms of morphine antinociceptive tolerance revealed in βarrestin-2 knock-out mice. J Neurosci 22:10494–10500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunskole Hummel I, Reinartz MT, Kälble S, Burhenne H, Schwede F, Buschauer A, Seifert R. (2013) Dissociations in the effects of β2-adrenergic receptor agonists on cAMP formation and superoxide production in human neutrophils: support for the concept of functional selectivity. PLoS One 8:e64556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AA, Hill SJ. (2005) Characterization of isoprenaline- and salmeterol-stimulated interactions between β2-adrenoceptors and β-arrestin 2 using β-galactosidase complementation in C2C12 cells. J Pharmacol Exp Ther 315:839–848. [DOI] [PubMed] [Google Scholar]
- Conner DA, Mathier MA, Mortensen RM, Christe M, Vatner SF, Seidman CE, Seidman JG. (1997) β-Arrestin1 knockout mice appear normal but demonstrate altered cardiac responses to β-adrenergic stimulation. Circ Res 81:1021–1026. [DOI] [PubMed] [Google Scholar]
- Delmotte P, Sanderson MJ. (2010) Effects of formoterol on contraction and Ca2+ signaling of mouse airway smooth muscle cells. Am J Respir Cell Mol Biol 42:373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson CE, Gul R, Blessing CP, Nguyen J, Liu T, Pulakat L, Bastepe M, Jackson EK, Andresen BT. (2013) The β-blocker nebivolol is a GRK/β-arrestin biased agonist. PLoS One 8:e71980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans BA, Sato M, Sarwar M, Hutchinson DS, Summers RJ. (2010) Ligand-directed signalling at β-adrenoceptors. Br J Pharmacol 159:1022–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SS. (2001) Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev 53:1–24. [PubMed] [Google Scholar]
- Freedman NJ, Lefkowitz RJ. (1996) Desensitization of G protein-coupled receptors. Recent Prog Horm Res 51:319–351 (discussion 352–353). [PubMed] [Google Scholar]
- Gawchik SM, Saccar CL, Noonan M, Reasner DS, DeGraw SS. (1999) The safety and efficacy of nebulized levalbuterol compared with racemic albuterol and placebo in the treatment of asthma in pediatric patients. J Allergy Clin Immunol 103:615–621. [DOI] [PubMed] [Google Scholar]
- Hagelüken A, Grünbaum L, Nürnberg B, Harhammer R, Schunack W, Seifert R. (1994) Lipophilic β-adrenoceptor antagonists and local anesthetics are effective direct activators of G-proteins. Biochem Pharmacol 47:1789–1795. [DOI] [PubMed] [Google Scholar]
- Handley DA, Anderson AJ, Koester J, Snider ME. (2000) New millennium bronchodilators for asthma: single-isomer β agonists. Curr Opin Pulm Med 6:43–49. [DOI] [PubMed] [Google Scholar]
- Handley DA, Senanayake CH, Dutczak W, Benovic JL, Walle T, Penn RB, Wilkinson HS, Tanoury GJ, Andersson RG, Johansson F, et al. (2002) Biological actions of formoterol isomers. Pulm Pharmacol Ther 15:135–145. [DOI] [PubMed] [Google Scholar]
- Hochhaus G, Möllmann H. (1992) Pharmacokinetic/pharmacodynamic characteristics of the β-2-agonists terbutaline, salbutamol and fenoterol. Int J Clin Pharmacol Ther Toxicol 30:342–362. [PubMed] [Google Scholar]
- Johnson M. (1998) The β-adrenoceptor. Am J Respir Crit Care Med 158:S146–S153. [DOI] [PubMed] [Google Scholar]
- Kopra K, Kainulainen M, Mikkonen P, Rozwandowicz-Jansen A, Hänninen P, Härmä H. (2013) Multiparametric homogeneous method for identification of ligand binding to G protein-coupled receptors: receptor-ligand binding and β-arrestin assay. Anal Chem 85:2276–2281. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Gesty-Palmer D. (2010) Beyond desensitization: physiological relevance of arrestin-dependent signaling. Pharmacol Rev 62:305–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magocsi M, Vizi ES, Selmeczy Z, Brózik A, Szelenyi J. (2007) Multiple G-protein-coupling specificity of β-adrenoceptor in macrophages. Immunology 122:503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni L, Naef R, Chapman ID, Morley J. (1994) Hyperresponsiveness of the airways following exposure of guinea-pigs to racemic mixtures and distomers of β2-selective sympathomimetics. Pulm Pharmacol 7:367–376. [DOI] [PubMed] [Google Scholar]
- Mitra S, Ugur M, Ugur O, Goodman HM, McCullough JR, Yamaguchi H. (1998) (S)-Albuterol increases intracellular free calcium by muscarinic receptor activation and a phospholipase C-dependent mechanism in airway smooth muscle. Mol Pharmacol 53:347–354. [DOI] [PubMed] [Google Scholar]
- Nelson HS, Bensch G, Pleskow WW, DiSantostefano R, DeGraw S, Reasner DS, Rollins TE, Rubin PD. (1998) Improved bronchodilation with levalbuterol compared with racemic albuterol in patients with asthma. J Allergy Clin Immunol 102:943–952. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Hudson CC, Cruickshank RD, Meyers DM, Payne RE, Jr, Rhem SM, Loomis CR. (2002) The cellular distribution of fluorescently labeled arrestins provides a robust, sensitive, and universal assay for screening G protein-coupled receptors. Assay Drug Dev Technol 1:21–30. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. (2000) Differential affinities of visual arrestin, βarrestin1, and βarrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem 275:17201–17210. [DOI] [PubMed] [Google Scholar]
- Patel M, Thomson NC. (2012) Levosalbutamol for chronic obstructive pulmonary disease: a treatment evaluation. Expert Opin Pharmacother 13:1069–1075. [DOI] [PubMed] [Google Scholar]
- Pedersen ME, Cockcroft JR. (2007) The vasodilatory β-blockers. Curr Hypertens Rep 9:269–277. [DOI] [PubMed] [Google Scholar]
- Plazinska A, Pajak K, Rutkowska E, Jimenez L, Kozocas J, Koolpe G, Tanga M, Toll L, Wainer IW, Jozwiak K. (2014) Comparative molecular field analysis of fenoterol derivatives interacting with an agonist-stabilized form of the β₂-adrenergic receptor. Bioorg Med Chem 22:234–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal S, Ahn S, Rominger DH, Gowen-MacDonald W, Lam CM, Dewire SM, Violin JD, Lefkowitz RJ. (2011) Quantifying ligand bias at seven-transmembrane receptors. Mol Pharmacol 80:367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinartz MT, Kälble S, Littmann T, Ozawa T, Dove S, Kaever V, Wainer IW, Seifert R. (2015a) Structure-bias relationships for fenoterol stereoisomers in six molecular and cellular assays at the β2-adrenoceptor. Naunyn Schmiedebergs Arch Pharmacol 388:51–65. [DOI] [PubMed] [Google Scholar]
- Reinartz MT, Kälble S, Wainer IW, Seifert R. (2015b) Interaction of fenoterol stereoisomers with β2-adrenoceptor-Gsα fusion proteins: antagonist and agonist competition binding. Naunyn Schmiedebergs Arch Pharmacol 388:517–524. [DOI] [PubMed] [Google Scholar]
- Reiner S, Ambrosio M, Hoffmann C, Lohse MJ. (2010) Differential signaling of the endogenous agonists at the β2-adrenergic receptor. J Biol Chem 285:36188–36198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter E, Ahn S, Shukla AK, Lefkowitz RJ. (2012) Molecular mechanism of β-arrestin-biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol Toxicol 52:179–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samama P, Cotecchia S, Costa T, Lefkowitz RJ. (1993) A mutation-induced activated state of the β2-adrenergic receptor. Extending the ternary complex model. J Biol Chem 268:4625–4636. [PubMed] [Google Scholar]
- Seifert R. (2013) Functional selectivity of G-protein-coupled receptors: from recombinant systems to native human cells. Biochem Pharmacol 86:853–861. [DOI] [PubMed] [Google Scholar]
- Seifert R, Wenzel-Seifert K, Arthur JM, Jose PO, Kobilka BK. (2002) Efficient adenylyl cyclase activation by a β2-adrenoceptor-Giα2 fusion protein. Biochem Biophys Res Commun 298:824–828. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. (2006) β-arrestin-dependent, G protein-independent ERK1/2 activation by the β2 adrenergic receptor. J Biol Chem 281:1261–1273. [DOI] [PubMed] [Google Scholar]
- Shukla AK, Xiao K, Lefkowitz RJ. (2011) Emerging paradigms of β-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem Sci 36:457–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura H, Hattori M, Takeuchi M, Ozawa T. (2012) Visualization and quantitative analysis of G protein-coupled receptor-β-arrestin interaction in single cells and specific organs of living mice using split luciferase complementation. ACS Chem Biol 7:901–910. [DOI] [PubMed] [Google Scholar]
- Templeton AG, Chapman ID, Chilvers ER, Morley J, Handley DA. (1998) Effects of S-salbutamol on human isolated bronchus. Pulm Pharmacol Ther 11:1–6. [DOI] [PubMed] [Google Scholar]
- Toll L, Jimenez L, Waleh N, Jozwiak K, Woo AY, Xiao RP, Bernier M, Wainer IW. (2011) β2-adrenergic receptor agonists inhibit the proliferation of 1321N1 astrocytoma cells. J Pharmacol Exp Ther 336:524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Westhuizen ET, Breton B, Christopoulos A, Bouvier M. (2014) Quantification of ligand bias for clinically relevant β2-adrenergic receptor ligands: implications for drug taxonomy. Mol Pharmacol 85:492–509. [DOI] [PubMed] [Google Scholar]
- Volcheck GW, Kelkar P, Bartemes KR, Gleich GJ, Kita H. (2005) Effects of (R)- and (S)-isomers of β-adrenergic agonists on eosinophil response to interleukin-5. Clin Exp Allergy 35:1341–1346. [DOI] [PubMed] [Google Scholar]
- Wenzel-Seifert K, Seifert R. (2000) Molecular analysis of β2-adrenoceptor coupling to Gs-, Gi-, and Gq-proteins. Mol Pharmacol 58:954–966. [DOI] [PubMed] [Google Scholar]
- Wisler JW, DeWire SM, Whalen EJ, Violin JD, Drake MT, Ahn S, Shenoy SK, Lefkowitz RJ. (2007) A unique mechanism of β-blocker action: carvedilol stimulates β-arrestin signaling. Proc Natl Acad Sci USA 104:16657–16662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D, Saxel O. (1977) Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270:725–727. [DOI] [PubMed] [Google Scholar]
- Yin H, Chu A, Li W, Wang B, Shelton F, Otero F, Nguyen DG, Caldwell JS, Chen YA. (2009) Lipid G protein-coupled receptor ligand identification using β-arrestin PathHunter assay. J Biol Chem 284:12328–12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Zhu FX, Olszewski MA, Robinson NE. (1998) Effects of enantiomers of β2-agonists on ACh release and smooth muscle contraction in the trachea. Am J Physiol 274:L32–L38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.