Abstract
Acetaminophen (APAP) is the most commonly used over-the-counter analgesic. However, hepatotoxicity induced by APAP is a major clinical issue, and the factors that define sensitivity to APAP remain unclear. We have previously demonstrated that mice nulled for glutathione S-transferase Pi (GSTP) are resistant to APAP-induced hepatotoxicity. This study aims to exploit this difference to delineate pathways of importance in APAP toxicity. We used mice nulled for GSTP and heme oxygenase-1 oxidative stress reporter mice, together with a novel nanoflow liquid chromatography–tandem mass spectrometry methodology to investigate the role of oxidative stress, cell signaling, and protein S-glutathionylation in APAP hepatotoxicity. We provide evidence that the sensitivity difference between wild-type and Gstp1/2−/− mice is unrelated to the ability of APAP to induce oxidative stress, despite observing significant increases in c-Jun N-terminal kinase and extracellular signal-regulated kinase phosphorylation in wild-type mice. The major difference in response to APAP was in the levels of protein S-glutathionylation: Gstp1/2−/− mice exhibited a significant increase in the number of S-glutathionylated proteins compared with wild-type animals. Remarkably, these S-glutathionylated proteins are involved in oxidative phosphorylation, respiratory complexes, drug metabolism, and mitochondrial apoptosis. Furthermore, we found that S-glutathionylation of the rate-limiting glutathione-synthesizing enzyme, glutamate cysteine ligase, was markedly increased in Gstp1/2−/− mice in response to APAP. The data demonstrate that S-glutathionylation provides an adaptive response to APAP and, as a consequence, suggest that this is an important determinant in APAP hepatotoxicity. This work identifies potential novel avenues associated with cell survival for the treatment of chemical-induced hepatotoxicity.
Introduction
Acetaminophen (APAP) is an analgesic and antipyretic compound which causes hepatic necrosis and acute liver failure at high doses (Hinson et al., 2010). Drug-induced liver injury as a result of APAP overdose is particularly problematic in the United States and UK, where it is the most common cause of acute liver failure (Ferner et al., 2011; Tujios and Fontana, 2011). Metabolism of APAP is well established, with the majority of the parent compound typically conjugated with sulfate or glucuronide (Jollow et al., 1974) and around 5% of APAP oxidized by cytochrome P450 to form the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI) (Laine et al., 2009). Whereas at nontoxic doses NAPQI is conjugated to the tripeptide glutathione, at toxic doses glucuronidation and sulfation pathways become saturated, leading to increased NAPQI production and depletion of hepatic glutathione levels (McGill and Jaeschke, 2013).
Glutathione S-transferase (GST) isoenzymes form a vital function in chemical detoxification and cytoprotection through conjugation of glutathione with reactive electrophiles and endogenous substrates (Hayes et al., 2005). Of the cytosolic classes, GST Pi (GSTP) has attracted much attention because of its significant role in cellular homeostasis. GSTP has been found to regulate the activities of a number of biologic pathways, such as c-Jun N-terminal kinase (JNK) and tumor necrosis factor receptor–associated factor 2 (Adler et al., 1999; Wu et al., 2006). Furthermore, evidence suggests that GSTP may catalyze the binding of glutathione to cellular proteins (S-glutathionylation) (Townsend et al., 2009) and facilitate the transport of nitric oxide via dinitrosyl-dithiol-iron complexes (Lok et al., 2012).
To study the function of GSTP in vivo, we have generated mice that are nulled at the Gstp gene locus (Henderson et al., 1998). Gstp1/2−/− mice are resistant to hepatotoxic doses of APAP (Henderson et al., 2000); the mechanism(s) behind this effect remains unclear. There is no difference in the metabolism of APAP between wild-type and Gstp1/2−/− mice (Henderson et al., 2000), suggesting that the difference in toxicity is downstream of APAP metabolite formation. In mice and humans, it is clear that a number of intracellular signaling pathways are affected in the development of APAP-induced hepatotoxicity, such as oxidative stress (McGill et al., 2012), mitogen-activated protein (MAP) kinase phosphorylation (Gunawan et al., 2006), mitochondrial injury (Kon et al., 2004), and glutathione depletion (Henderson et al., 2000). Importantly, GSTP has been found to play a significant role in the regulation of a number of these signaling events (Henderson et al., 2000; Schroer et al., 2011; Castro-Caldas et al., 2012), and therefore it remains unclear why GSTP deletion confers resistance to APAP injury.
In the present study, we examined the downstream consequence of APAP overdose in Gstp1/2−/− mice to understand the mechanisms associated with hepatotoxic resistance. These data demonstrate that protein S-glutathionylation may provide a means of protection against APAP toxicity and electrophilic stress in general in vivo.
Materials and Methods
Animals.
All experiments were undertaken in accordance with the Animals (Scientific Procedures) Act 1986 and approved by the Welfare and Ethical Use of Animals Committee of the University of Dundee (Dundee, UK). Gstp1WT and Gstp1/2−/− mouse lines were generated as previously described and backcrossed onto a C57/BL6J background for at least eight generations. Mice had ad libitum access to standard rodent diet prior to conducting experiments (RM1; Special Diet Services, Essex, UK) and were kept in a 12-hour light/12-hour dark cycle.
Administration of APAP and Buthionine Sulfoximine.
APAP was prepared as a 15-mg/ml suspension in phosphate-buffered saline (PBS) and administered by gavage at 300 mg/kg (20 ml/kg). Mice were starved for 16 hours prior to APAP administration. After APAP treatment, mice were harvested at various time points, as described in the Results, by CO2 inhalation. Blood was collected by cardiac puncture into heparinized tubes before analysis for serum transaminase levels.
Buthionine sulfoximine (BSO) was prepared at 50 mg/ml in water and administered by i.p. injection at 0.9 g/kg (18 ml/kg). After BSO treatment, mice were harvested at various time points, as described in the Results, by CO2 inhalation.
Liver Histopathology.
Mice livers were removed, washed in PBS, and fixed in 10% formalin before being embedded in paraffin wax. Sections (5 µm) were prepared and stained using hematoxylin and eosin to determine structural damage, as previously described (Henderson et al., 2014).
Immunoblotting.
Liver samples were lysed in radioimmunoprecipitation assay lysis buffer [50 mM Tris (pH 7.4), 150 mM NaCl, 1% Igepal-630, 5 mM EDTA, 0.1% SDS, and 0.5% sodium deoxycholate] containing protease and phosphatase inhibitors (Roche, Basel, Switzerland). Samples were denatured in lithium dodecyl sulphate loading buffer containing 10 mM dithiothreitol before being resolved on 10 or 12% polyacrylamide gels, as previously described (McGarry et al., 2015). Membranes were incubated with antibodies against glyceraldehyde-3-phosphate dehydrogenase (1:10,000; Sigma-Aldrich, St. Louis, MO), GSTP1 (1:2000), extracellular signal-regulated kinase (ERK), phospho-ERK, JNK, phospho-JNK, phospho-p38, p38 (1:1000; Cell Signaling Technology, Danvers, MA), or heme oxygenase-1 (1:1000; Abcam, Cambridge, MA) overnight at 4°C. Membranes were washed and incubated with a polyclonal goat anti-rabbit immunoglobulin–horseradish peroxidase secondary antibody (Dako, High Wycombe, UK) for 1 hour at room temperature at a dilution of 1:5000. Signals were visualized on X-ray film using a Xograph (Gloucestershire, UK) Compact ×4 film processor.
Immunohistochemistry.
Paraffin tissue sections (5 µm) were deparaffinized and rehydrated using gradient alcohols. Sections underwent microwave antigen retrieval for 25 minutes in 0.01 M sodium citrate buffer (pH 6.5) and allowed to cool to room temperature. Immunohistochemistry was performed using the Dako Cytomation EnVision Dual Link System-HRP (DAB+) kit according to the manufacturer’s protocols. Sections were blocked using 2% goat serum prepared in 1% bovine serum albumin, 0.1% Triton X-100, and 0.05% Tween in PBS before incubation with either an anti– heme oxygenase-1 antibody (1:200 dilution; Abcam) or an anti–phospho-ERK antibody (1:100Cell Signaling Technology) for 1 hour at room temperature.
To determine lacZ expression in liver samples, livers were fixed in 1% PFA for 4 hours at 4°C and then incubated overnight in 30% sucrose solution before being frozen in Shandon M-1 Embedding Matrix (Thermo Fisher Scientific, Waltham, MA) using a dry ice–isopentane bath. Cryosections (15 µm) were prepared using a Bright Instruments cryostat (Bright Instruments, Huntingdon, Cambridgeshire, UK), and sections were stained for β-galactosidase activity, as previously described (Henderson et al., 2014).
Glutathione Levels.
Levels of total, oxidized, and protein-bound glutathione were determined spectrophotometrically using the sulfhydryl reagent 5,5′-dithio-bis (2-nitrobenzoic acid) as described previously (Rahman et al., 2006). In brief, tissues were homogenized in 0.25 M sucrose before being extracted in 0.6% salicylic acid, 5% metaphosphoric acid, 0.1% Triton X-100, and 0.1% NP40. Homogenates were centrifuged at 3000 × g for 4 minutes at 4°C, and 500 µl of the aqueous upper layer was neutralized with 1 ml of 1 M Tris (pH 7.5) and used to determine total and oxidized glutathione levels. The remaining pellet was resuspended in 1 ml of 1% sodium borohydride and neutralized with 0.4 ml of 30% metaphosphoric acid. The suspension was centrifuged at 1000 × g for 15 minutes and the supernatant used to determine the amount of protein-bound glutathione.
Identification of S-Glutathionylated Proteins.
S-glutathionylated proteins were isolated as previously described (McGarry et al., 2015). In brief, tissues were homogenized in radioimmunoprecipitation assay lysis buffer supplemented with 1% methyl methanethiosulfonate. Lysates were incubated in a buffer containing 100 mM HEPES (pH 7.5), 1 mM EDTA, 2.5% SDS, and 1% methyl methanethiosulfonate for 20 minutes at 50°C to block all free thiol groups. Proteins were precipitated in ice-cold acetone and washed in 70% acetone. Pellets were resuspended in 400 µl of binding buffer [10 mM HEPES (pH 8.0), 0.1 mM EDTA, 0.1% SDS] before S-glutathionylated proteins were reduced with 1 mM NADPH, 35 µg/ml glutathione reductase, 0.2 mM reduced glutathione, and 25 µg/ml of human glutaredoxin (Sigma-Aldrich, St. Louis, MO) in a final volume of 500 µl. Lysates were mixed carefully before being incubated at 37°C for 5 minutes. Lysates were incubated with 50 µl of thiopropyl Sepharose 6B beads (GE Healthcare, Little Chalfont, UK) for 1 hour at room temperature and then washed in 100 mM HEPES (pH 8.0), 1 mM EDTA, 1% SDS, and 300 mM NaCl. Proteins were eluted from beads by incubation in 1× lithium dodecyl sulphate sample buffer (Invitrogen, Carlsbad, CA) supplemented with 10 mM Dithiothreitol.
Mass Spectrometry Analysis.
Isolated S-glutathionylated proteins were analyzed by nLC–tandem mass spectrometry (MS/MS) using an LTQ Orbitrap (FingerPrints Proteomics, Dougie Lamont, University of Dundee, UK). All MS/MS samples were analyzed using Mascot (version 2.3.02; Matrix Science, London, UK). Scaffold (version Scaffold_4.0.5; Proteome Software Inc., Portland, OR) was used to validate MS/MS-based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 95.0% probability using the Peptide Prophet algorithm (Keller et al., 2002) with Scaffold delta-mass correction. Protein identifications were accepted at greater than 99.9% probability and containing at least two identified peptides.
Bioinformatics Analysis.
Bioinformatic analysis of S-glutathionylated proteins was performed as previously described (McGarry et al., 2015). Pathway and process enrichment was determined using MetaCore from Thompson Reuters (http://thomsonreuters.com/metacore/). The mouse proteome was used as the background. The enrichment analysis used hypergeometric testing, and the resulting P value was adjusted for multiple testing corrections using the Benjamini-Hochberg correction method (Benjamini and Hochberg, 1995). An adjusted P value of less than 0.05 was considered to be statistically significant.
Results
Liver Pathology and Oxidative Stress in Acetaminophen-Treated Gstp1/2−/− Mice.
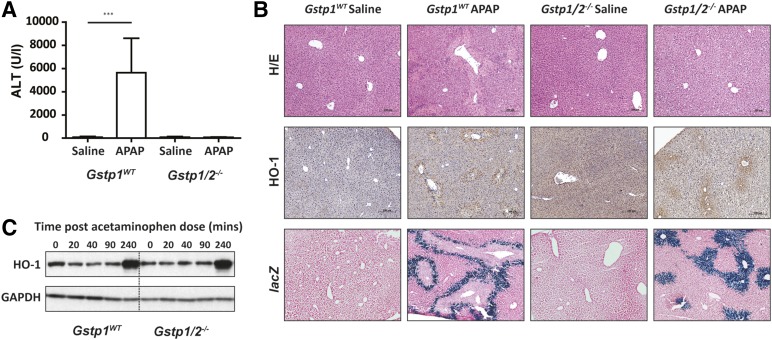
Following APAP treatment (300 mg/kg), Gstp1WT mice had significantly elevated serum alanine aminotransferase (ALT) levels and exhibited areas of centrilobular necrosis 24 hours after treatment (Fig. 1, A and B). In line with previous data (Henderson et al., 2000), ALT levels remained low in Gstp1/2−/− mice, and no evidence of hepatotoxicity could be observed by histochemical examination of the liver sections. Differences in the level of oxidative stress between wild-type and Gstp1/2−/− mice could be the reason for the sensitivity difference to APAP (Cover et al., 2006) and may activate a number of stress response genes, including heme oxygenase-1 (Young et al., 2010). Initially, we examined the expression of heme oxygenase-1 as a marker of oxidative stress in Gstp1WT and Gstp1/2−/− mouse liver 24 hours after a single oral dose of APAP (300 mg/kg). Immunohistochemical analysis showed heme oxygenase-1 expression around the outer centrilobular necrotic areas in the livers of Gstp1WT mice 24 hours after APAP treatment (Fig. 1B). Interestingly, Gstp1/2−/− mice also exhibited increased heme oxygenase-1 expression around hepatic centrilobular regions in response to APAP, despite no signs of hepatic damage. This observation is further supported through examination of mice carrying an heme oxygenase-1 reporter transgene, in which the lacZ reporter is fused to the C terminus of the Hmox1 gene (Young et al., 2010) and crossed onto Gstp1/2−/− mice. β-Galactosidase staining of liver sections from these mice correlated strongly with heme oxygenase-1 immunohistochemical staining, demonstrating reproducibility between the transgenic models used. Analysis of heme oxygenase-1 induction over time by western blotting and densitometry showed that there was little difference in heme oxygenase-1 induction between Gstp1WT and Gstp1/2−/− mice (Fig. 1C; Supplemental Fig. 1). Therefore, the generation of oxidative stress does not appear to underlie the difference in severity of the toxicity between these mice.
Fig. 1.
HO-1 stress response in Gstp1WT and Gstp1/2−/− mice after acetaminophen administration. (A) Plasma ALT levels in Gstp1WT and Gstp1/2−/− male mice 24 hours after a single oral dose of acetaminophen (300 mg/kg, n = 9–12; ***P < 0.001). Error bars show the mean ± S.D. (B) Liver histopathology determined in Gstp1WT and Gstp1/2−/− male mice 24 hours after a single oral dose of APAP (300 mg/kg, n = 9–12). At 24 hours, livers were removed, fixed, sectioned (5 µm for hematoxylin and eosin (H/E) and HO-1 and 15 µm for lacZ), and processed using hematoxylin and eosin, β-galactosidase (for lacZ), or antibody against HO-1. Representative sections are shown. (C) Western blot analysis of HO-1 in pooled whole-cell liver lysates (10 µg) from Gstp1WT and Gstp1/2−/− male mice after a single oral dose of APAP (300 mg/kg, n = 8) and harvested at the time points shown. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Phosphorylation of JNK, ERK, and p38 MAP Kinases in Gstp1/2−/− Mice after Acetaminophen Treatment.
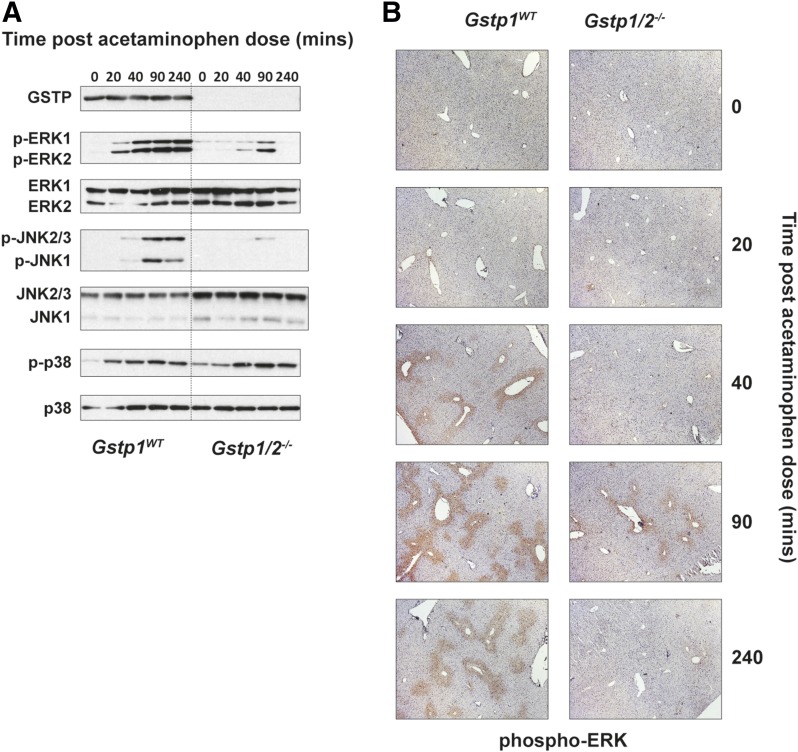
It has been reported that GSTP is a modulator of cell signaling through inhibition of JNK phosphorylation. JNK has been reported to potentiate APAP toxicity in mice (Gunawan et al., 2006); therefore, we studied whether JNK phosphorylation in response to APAP was different between the mouse lines after a single oral dose of APAP (300 mg/kg). Western blot analysis showed a time-dependent increase in phosphorylation of both JNK1 and JNK2/3 isoforms in Gstp1WT post APAP dosing (Fig. 2A). In addition to changes in JNK phosphorylation, activation of ERK1/2 and p38 phosphorylation was also observed. The p38 MAP kinase pathway can be induced in response to a number of stressors, but critically also by oxidative stress (Ito et al., 2006). This is supported in our data, where similar levels of heme oxygenase-1, glutathione disulfide (GSSG), and p38 MAP kinase phosphorylation were observed in Gstp1WT and Gstp1/2−/− mice in response to APAP. However, the levels of phosphorylated JNK and phosphorylated ERK were greatly attenuated in Gstp1/2−/− mice in response to APAP, and therefore their regulation and activation appear independent from that of p38 MAP kinase in this study. This suggests that, in this case, GSTP does not inhibit JNK phosphorylation, as has been previously described. Immunohistochemical analysis demonstrated that expression of phosphorylated ERK is predominantly centrilobular (Fig. 2B).
Fig. 2.
Attenuation of the JNK/ERK pathway in Gstp1/2−/− mice in response to acetaminophen. (A) Western blot analysis of the MAP kinase pathway in pooled whole-cell liver lysates (10 µg) from Gstp1WT and Gstp1/2−/− male mice after a single oral dose of acetaminophen (300 mg/kg, n = 8) and harvested at the time points shown. Blots show a reduction in JNK/ERK phosphorylation in Gstp1/2−/− mice in response to APAP. (B) Immunohistochemical staining of phospho-ERK in liver sections of Gstp1WT and Gstp1/2−/− male mice after a single oral dose of APAP (300 mg/kg, n = 8). Increased phospho-ERK staining can be found around centrilobular regions in livers of Gstp1WT mice over a 4-hour period, whereas Gstp1/2−/− mice demonstrate little phospho-ERK staining at these time points. Representative sections are shown. p-ERK, phospho-ERK; p-JNK, phospho-JNK; p-p38, phospho-p38.
Following this observation, we examined whether the expression of GSTP was necessary for the phosphorylation of JNK/ERK. Despite differences in JNK/ERK phosphorylation in response to APAP, we could not find any difference in JNK phosphorylation between Gstp1WT and Gstp1/2−/− primary mouse embryonic fibroblasts in response to anisomycin (Supplemental Fig. 2), suggesting that the activation of JNK in this study reflects the condition of the liver in response to APAP and not an intrinsic mechanism by which GSTP functions.
Glutathione Levels and Protein S-Glutathionylation following Acetaminophen Treatment.
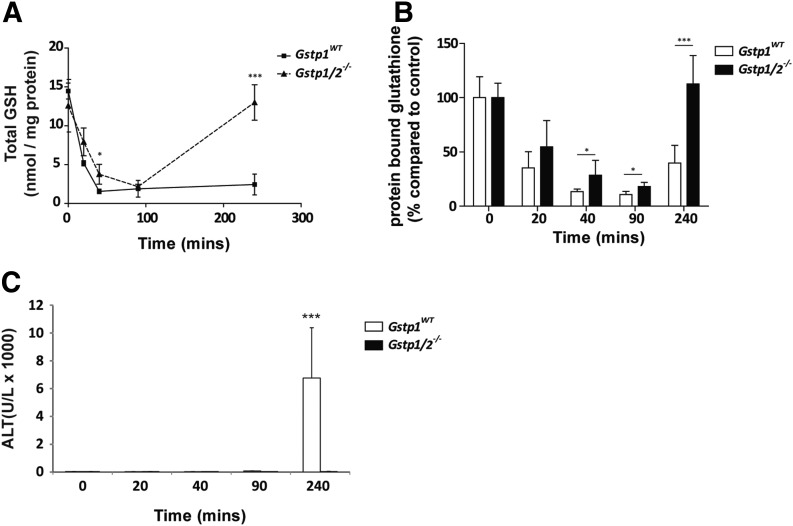
At high doses, NAPQI accumulates in the liver, leading to depletion of hepatic glutathione and subsequent hepatotoxicity. In our study, we observed no difference in resting glutathione levels, whereas rapid depletion of hepatic glutathione was found in both Gstp1WT and Gstp1/2−/− mice after APAP administration (Fig. 3A). The rate of glutathione depletion was similar in both genotypes, although there was a slight trend showing a faster rate of depletion in Gstp1WT mice. Nonetheless, glutathione levels were depleted to the same level in both mouse lines. Significantly, hepatic glutathione levels remained low in Gstp1WT mice but were fully recovered in Gstp1/2−/− mice 4 hours after APAP treatment. Interestingly, there was an inverse correlation between the level of glutathione depletion and increase in JNK/ERK phosphorylation, an induction that was no longer observed in Gstp1/2−/− mice after glutathione levels were fully recovered (Fig. 2A). Measurement of GSSG, indicative of cellular oxidation, increased significantly after APAP treatment in both genotypes, but no difference in the level of GSSG was observed between Gstp1WT and Gstp1/2−/− mice (Supplemental Fig. 3), providing further evidence that the difference in APAP sensitivity is not due to differences in oxidative stress.
Fig. 3.
Glutathione (GSH) levels and protein S-glutathionylation in Gstp1WT and Gstp1/2−/− mice in response to acetaminophen. Male Gstp1WT and Gstp1/2−/− mice were administered a single oral dose of APAP (300 mg/kg) and harvested at the time points indicated. Livers were removed, washed in PBS, and analyzed for total glutathione levels (A) and total levels of protein S-glutathionylation (B) as detailed in Materials and Methods (n = 8; *P < 0.05; ***P < 0.001). Error bars show the mean ±S.D. (C) Male Gstp1WT and Gstp1/2−/− mice were administered a single oral dose of APAP (300 mg/kg), and cardiac punctures were taken at the time points indicated. Blood samples were collected into heparin tubes and centrifuged at 13,000 rpm for 10 minutes at room temperature, and the plasma was analyzed for ALT content (n = 3, ***P < 0.001). Error bars show the mean ±S.D.
We examined the relationship between changes in protein S-glutathionylation and the levels of hepatic glutathione. Protein S-glutathionylation is a reversible post-translational modification in which glutathione binds cellular protein thiols, resulting in protection against irreversible oxidative damage (Grek et al., 2013). At resting levels, we could not detect any significant difference in the level of S-glutathionylation between Gstp1WT and Gstp1/2−/− mice (Fig. 3B), in agreement with our previous findings (McGarry et al., 2015). In response to APAP, a significant decrease in the level of protein S-glutathionylation was evident in both Gstp1WT and Gstp1/2−/− mice soon after treatment. However, Gstp1/2−/− mice retained a significantly higher proportion of protein-bound glutathione than did Gstp1WT mice prior to the onset of hepatotoxicity. After 4 hours, levels of protein S-glutathionylation were fully restored in Gstp1/2−/− mice, whereas they remained reduced in Gstp1WT mice. We next determined if these changes correlated with increased plasma ALT levels, indicative of hepatotoxicity. As shown in Fig. 3C, APAP induces ALT levels in Gstp1WT mice 4 hours after treatment, whereas ALT levels remain low in Gstp1/2−/− mice.
To assess whether these observations are dependent on glutathione depletion alone, Gstp1WT and Gstp1/2−/− mice were administered a separate glutathione-depleting agent, l-BSO, and hepatic glutathione and protein S-glutathionylation levels were determined. Although BSO reduced hepatic glutathione and protein S-glutathionylation levels, no significant difference between Gstp1WT and Gstp1/2−/− mice was observed (Supplemental Fig. 4).
Identification of S-Glutathionylated Proteins in Response to Acetaminophen.
To identify the proteins S-glutathionylated in response to APAP, we analyzed samples 40 minutes after a single oral dose of APAP (300 mg/kg), i.e., prior to the point at which hepatic glutathione becomes critically depleted and before the onset of hepatotoxicity as determined by ALT levels.
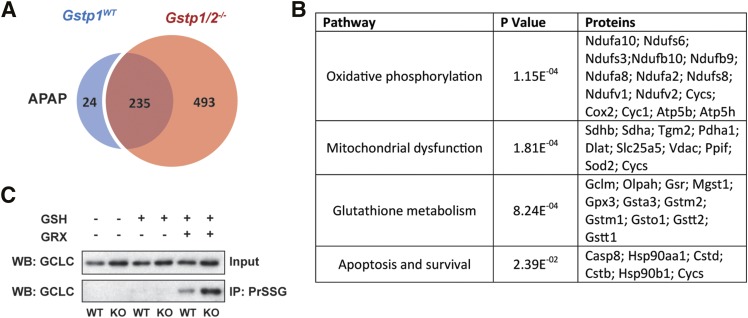
S-glutathionylation profiles between Gstp1WT and Gstp1/2−/− mice were very similar (McGarry et al., 2015). However, APAP treatment markedly changed the proportion of proteins S-glutathionylated between Gstp1WT and Gstp1/2−/− mice (Fig. 4A). nLC-MS/MS analysis of isolated S-glutathionylated proteins 40 minutes after APAP treatment identified 235 proteins that were common to both Gstp1WT and Gstp1/2−/− mice, 24 proteins that were unique to Gstp1WT mice, and, remarkably, 493 proteins that were unique to Gstp1/2−/− mice (Fig. 4A; Supplemental Table 1). We only included proteins that met strict minimum criteria as detailed in the Experimental section. Of particular note was that peroxiredoxin 1, succinate dehydrogenase, NADH dehydrogenase, cytochrome P450 2E1, heat shock protein 90, caspase-8, cytochrome c oxidase, and cytochrome c (somatic) were specifically S-glutathionylated in Gstp1/2−/− mice in response to APAP.
Fig. 4.
Changes in hepatic protein S-glutathionylation after acetaminophen treatment in Gstp1WT and Gstp1/2−/− mice. (A) Number of hepatic proteins identified as S-glutathionylated after APAP treatment (300 mg/kg, 40 minutes). (B) Pathway analysis of proteins specifically S-glutathionylated in Gstp1/2−/− mice after APAP treatment (300 mg/kg, 40 minutes). The P value represents the significance of each pathway enriched as described in the Experimental section. See Supplemental Table 2 for a full list of pathways. (C) S-glutathionylated proteins were isolated from wild-type (WT) and Gstp1/2−/− mice [knockout (KO)] 40 minutes after an oral dose of APAP (300 mg/kg) and analyzed for glutamate cysteine ligase catalytic subunit (GCLC) expression using western blotting. S-glutathionylated proteins were isolated as described in the methodology. To demonstrate the specificity of the reaction, only proteins reduced with the deglutathionylating enzyme, glutaredoxin (GRX), are isolated from protein lysates. GSH, glutathione; IP, immunoprecipitate; PrSSG, glutathionylated protein; WB, Western blot.
We performed unbiased bioinformatic analysis on the 493 glutathionylated proteins using MetaCore software to determine which biologic pathways and processes were enriched after APAP treatment. In Gstp1/2−/− mice, the S-glutathionylation of proteins of a number of key cellular pathways such as oxidative phosphorylation, glutathione metabolism, and mitochondrial apoptosis was maintained in response to APAP (Fig. 4B; Supplemental Table 2). Furthermore, western blot analysis of extracts isolated from mouse liver confirmed that glutamate cysteine ligase catalytic subunit, the rate-limiting enzyme in glutathione synthesis, remained heavily S-glutathionylated in Gstp1/2−/− mouse liver 40 minutes after APAP administration (Fig. 4C). These data suggest that, in the initial phase of APAP toxicity, Gstp1/2−/− mice are better suited to protect critical pathways and proteins involved in cell survival and glutathione regeneration through protein S-glutathionylation.
Discussion
We have previously shown that Gstp1/2−/− mice demonstrate increased susceptibility to chemically induced skin (Henderson et al., 1998) and lung tumors (Ritchie et al., 2007) and develop a higher incidence of tumors when crossed onto genetically initiated mouse models of colon (Apcmin mice) (Ritchie et al., 2009) and skin (TgAc mice) (Henderson et al., 2011) cancers. Paradoxically, Gstp1/2−/− mice are resistant to APAP-induced hepatotoxicity; in this study, we investigated a number of pathways that could account for this intriguing observation.
Our previous studies demonstrated that the absence of GSTP does not alter the pathways of APAP metabolism (Henderson et al., 2000). Here, we have demonstrated that the capacity of APAP to induce hepatic oxidative stress, which is critical for the development of APAP toxicity, is also similar between the Gstp1WT and Gstp1/2−/− mice. The mechanism of protection in the Gstp1/2−/− model must therefore be downstream of the initial toxic insult. This conclusion is consistent with previous studies that demonstrate N-acetylcysteine treatment of hepatocytes blocks mitochondrial permeability transition activation and downstream activity, despite APAP-induced oxidative stress (Reid et al., 2005). In this manuscript, we provide strong evidence that the lack of GSTP relates to cell survival pathways in response to APAP.
Our data provide further evidence for the involvement of JNK/ERK phosphorylation in the development of hepatic damage. In Gstp1WT mice, increased JNK phosphorylation confirms previous findings that JNK activation is associated with sensitivity to APAP (Gunawan et al., 2006). ERK phosphorylation has similarly been found to sensitize to APAP hepatotoxicity (Stamper et al., 2010; Wakabayashi et al., 2012). Although initial phosphorylation signals precede increased ALT levels, it is not yet clear from our data whether phosphorylation of JNK/ERK contributes to hepatotoxicity in Gstp1WT mice or is reflective of the condition of the liver in response to APAP. Defining the role of JNK in APAP toxicity is complex, as deletion of individual JNK isoforms in mice has demonstrated inconsistent changes in APAP sensitivity (Gunawan et al., 2006; Bourdi et al., 2008). A number of studies have reported that GSTP can modulate the activity of JNK by binding through its C terminus (Wang et al., 2001), preventing phosphorylation of its downstream targets in response to stress (Castro-Caldas et al., 2012). However, we could not find any direct evidence for negative regulation of JNK phosphorylation by GSTP in our studies; under basal (untreated) conditions, there was no difference in JNK phosphorylation between Gstp1WT and Gstp1/2−/− mice (Fig. 3A), consistent with a number of studies in a range of other tissues or cells on different genetic backgrounds (Gate et al., 2004; Castro-Caldas et al., 2012; Bartolini et al., 2015; Conklin et al., 2015). JNK phosphorylates downstream transcription factors in response to a wide variety of stressors to induce apoptosis, cell proliferation, or differentiation. Therefore, due to the diverse nature of JNK in cell regulation, the effect of APAP on JNK activity may be stress-specific and unrelated to its interaction with GSTP. Furthermore, there is growing evidence to suggest that the dysregulation of protein kinase phosphatases may influence APAP toxicity (Wancket et al., 2012). Protein tyrosine phosphatase 1B null mice are more resistant to APAP than wild-type mice and have reduced levels of phosphorylated JNK in response to APAP (Mobasher et al., 2013). Interestingly, previous reports have demonstrated that protein tyrosine phosphatase 1B S-glutathionylation results in enzyme inactivation (Barrett et al., 1999). In our study, we identified that protein tyrosine phosphatase 1B is specifically S-glutathionylated in Gstp1/2−/− mice in response to APAP (Supplemental Table 1) and could potentially result in enzyme inactivation, leading to APAP resistance. Therefore, it cannot be ruled out that GSTP-mediated effects on protein phosphorylation are involved in the resistance mechanism.
Proteins S-glutathionylated under basal conditions have roles in key biologic pathways such as energy metabolism, cytoskeleton remodeling, oxidative stress, and proteasomal degradation (McGarry et al., 2015). In this manuscript, we demonstrate an adaptive response in protein S-glutathionylation, and subsequent hepatic glutathione regeneration, in response to electrophilic stress in vivo. Our data are in line with previous reports that demonstrate decreased protein S-glutathionylation in response to APAP in wild-type mice (Yang et al., 2012). Significantly, Yang et al. suggested that a subset of cells lining the centrilobular region exhibited increased protein S-glutathionylation and showed less damage than those cells with depleted levels of S-glutathionylation. In our study, the observation that Gstp1/2−/− mice show increased S-glutathionylation despite no increase in ALT levels up to 4 hours after treatment correlates with these findings and provides compelling evidence for the role of protein S-glutathionylation in the protection against APAP hepatotoxicity. Furthermore, we are able to provide a deeper understanding of the individual proteins susceptible to S-glutathionylation and suggest that they are likely involved in the protection against APAP hepatotoxicity.
Several proteins S-glutathionylated in Gstp1/2−/− mice, such as succinate dehydrogenase, cytochrome c, and NADH dehydrogenase, form critical components of pathways important in the development of toxicity. NAPQI induces mitochondrial permeability transition, resulting in depolarization of the mitochondrial membrane, uncoupling of oxidative phosphorylation, and activation of proapoptotic proteins, including the release of cytochrome c (Masubuchi et al., 2005; Lee et al., 2015). The ability of cytochrome c to activate the caspase pathway is dependent on its redox state, where oxidized cytochrome c stimulates apoptotic activation, whereas reduction of cytochrome c prevents activation (Borutaite and Brown, 2007; Barros et al., 2013). As the majority of S-glutathionylation reactions are believed to result in enzyme inactivation, it can be hypothesized that S-glutathionylation prevents transition to cellular necrosis by inhibiting this enzyme activity. In support of this, we identified S-glutathionylation of caspase 8, whose activation initiates apoptosis (Li et al., 1998), and also demonstrated the S-glutathionylation of the catalytic subunit of glutamate cysteine ligase (GCL) was increased in Gstp1/2−/− mice in response to APAP. Mice nulled for Gclm exhibit extensive APAP-induced hepatotoxicity compared with their wild-type counterparts (McConnachie et al., 2007), whereas severe steatosis in Gclc hepatic-specific knockout mice can be rescued through N-acetylcysteine supplementation (Chen et al., 2010). Increased S-glutathionylation of GCL may protect GCL integrity, allowing for further glutathione synthesis after APAP administration. Furthermore, NAPQI has been found to target and inactivate respiratory complex II of mitochondria (Lee et al., 2015). Protection of sulfhydryl/thiol-NAPQI adducts through protein S-glutathionylation may present one mechanism to prevent irreversible inactivation.
A lack of GSTP contributes to the protection of hepatic proteins in response to APAP, but the precise mechanism remains unclear. Previous work has demonstrated that there is little/no difference in the S-glutathionylation profile of Gstp1WT and Gstp1/2−/− mice under basal conditions (McGarry et al., 2015), and therefore, any direct effect on protein S-glutathionylation may be stress-dependent. It has been suggested that the omega class of GSTs (GSTO1) can act as a deglutathionylating enzyme (Menon and Board, 2013), and that this activity contributes to inflammation and reactive oxygen species production in response to lipopolysaccharide (Menon et al., 2014). Unpublished data suggest that this glutaredoxin-like activity may be reserved specifically for the omega class of GSTs (Board et al., 2000). However, there is evidence to suggest that, under certain stresses, redox enzymes may have multiple roles in cycling S-glutathionylation (Starke et al., 2003), and it is therefore conceivable that GSTP may have multiple functions depending on the source and nature of the stress. A possible explanation could be that GSTP similarly acts as a deglutathionylating enzyme in response to APAP, resulting in the reduction of S-glutathionylated proteins and increased sensitivity to hepatotoxicity.
Our original observations of APAP resistance in Gstp1/2−/− mice were made on a mixed 129xMF1 background. In this study, mice were bred onto a C57/BL6J background (10 generations), demonstrating that the difference in response to APAP treatment is conserved across these sets of mouse strains. In contrast to mice, GSTP expression is restricted to the biliary epithelium in normal human liver. A number of transcription binding sites, such as AP-1 (Moffat et al., 1997), p53 (Lo et al., 2008), retinoic acid, and nuclear factor κB (Xia et al., 1993), have been identified within the human GSTP gene. Recently, we have made further efforts to characterize the regulation of human GSTP1 in an animal model and demonstrated upregulation of human hepatic GSTP expression in response to the chemopreventive agents ethoxyquin and butylated hydroxyanisole (Henderson et al., 2014). Therefore, hepatic GSTP induction through chemical toxicity or hepatic disease may contribute to the known increase in the risk of drug-induced liver injury associated with APAP in these instances.
We have shown that the modulation of the function of a single protein can profoundly alter the hepatotoxicity induced by APAP. The cytoprotective effect does not appear to be related to the initiation of the pathway of toxicity, i.e., the induction of oxidative stress, but appears to be related to the activation of cell survival signals and changes in protein S-glutathionylation. Understanding the mechanism of these effects will provide important insights into the factors which define cell survival resulting from toxic insult and raises the possibility that their activation, such as by pharmacological means, may provide new avenues for therapeutic intervention and the prevention of serious hepatotoxicity.
Supplementary Material
Acknowledgments
The authors thank Catherine Meakin and Julia Carr for invaluable technical assistance; Dr. Linda Rushworth for JNK, p38, and ERK antibodies; and members of the C.R.W. laboratory for valuable discussion.
Abbreviations
- ALT
alanine aminotransferase
- APAP
acetaminophen
- BSO
buthionine sulfoximine
- ERK
extracellular signal-regulated kinase
- GCL
glutamate cysteine ligase
- GSSG
glutathione disulfide
- GST
glutathione S-transferase
- GSTP
glutathione S-transferase Pi
- JNK
c-Jun N-terminal kinase
- MAP
mitogen-activated protein
- MS/MS
tandem mass spectrometry
- NAPQI
N-acetyl-p-benzoquinone imine
- PBS
phosphate-buffered saline
Authorship Contributions
Participated in research design: McGarry, Wolf, Henderson.
Conducted experiments: McGarry.
Performed data analysis: Chakravarty.
Wrote or contributed to the writing of the manuscript: McGarry, Wolf, Henderson.
Footnotes
This work was funded by a Cancer Research UK program grant awarded to C.R.W. [Grant C4639/A10822] and the Medical Research Council Integrative Toxicology Training Partnership (C.J.H.). The authors declare no conflict of interest.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Adler V, Yin Z, Fuchs SY, Benezra M, Rosario L, Tew KD, Pincus MR, Sardana M, Henderson CJ, Wolf CR, et al. (1999) Regulation of JNK signaling by GSTp. EMBO J 18:1321–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett WC, DeGnore JP, Keng YF, Zhang ZY, Yim MB, Chock PB. (1999) Roles of superoxide radical anion in signal transduction mediated by reversible regulation of protein-tyrosine phosphatase 1B. J Biol Chem 274:34543–34546. [DOI] [PubMed] [Google Scholar]
- Barros S, Mencia N, Rodríguez L, Oleaga C, Santos C, Noé V, Ciudad CJ. (2013) The redox state of cytochrome c modulates resistance to methotrexate in human MCF7 breast cancer cells. PLoS One 8:e63276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolini D, Commodi J, Piroddi M, Incipini L, Sancineto L, Santi C, Galli F. (2015) Glutathione S-transferase Pi expression regulates the Nrf2-dependent response to hormetic diselenides. Free Radic Biol Med DOI: 10.1016/j.freeradbiomed.2015.06.039 [published ahead of print]. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc, B 57:289–300. [Google Scholar]
- Board PG, Coggan M, Chelvanayagam G, Easteal S, Jermiin LS, Schulte GK, Danley DE, Hoth LR, Griffor MC, Kamath AV, et al. (2000) Identification, characterization, and crystal structure of the Omega class glutathione transferases. J Biol Chem 275:24798–24806. [DOI] [PubMed] [Google Scholar]
- Borutaite V, Brown GC. (2007) Mitochondrial regulation of caspase activation by cytochrome oxidase and tetramethylphenylenediamine via cytosolic cytochrome c redox state. J Biol Chem 282:31124–31130. [DOI] [PubMed] [Google Scholar]
- Bourdi M, Korrapati MC, Chakraborty M, Yee SB, Pohl LR. (2008) Protective role of c-Jun N-terminal kinase 2 in acetaminophen-induced liver injury. Biochem Biophys Res Commun 374:6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Caldas M, Carvalho AN, Rodrigues E, Henderson C, Wolf CR, Gama MJ. (2012) Glutathione S-transferase pi mediates MPTP-induced c-Jun N-terminal kinase activation in the nigrostriatal pathway. Mol Neurobiol 45:466–477. [DOI] [PubMed] [Google Scholar]
- Chen Y, Johansson E, Yang Y, Miller ML, Shen D, Orlicky DJ, Shertzer HG, Vasiliou V, Nebert DW, Dalton TP. (2010) Oral N-acetylcysteine rescues lethality of hepatocyte-specific Gclc-knockout mice, providing a model for hepatic cirrhosis. J Hepatol 53:1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin DJ, Haberzettl P, Jagatheesan G, Baba S, Merchant ML, Prough RA, Williams JD, Prabhu SD, Bhatnagar A. (2015) Glutathione S-transferase P protects against cyclophosphamide-induced cardiotoxicity in mice. Toxicol Appl Pharmacol 285:136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover C, Liu J, Farhood A, Malle E, Waalkes MP, Bajt ML, Jaeschke H. (2006) Pathophysiological role of the acute inflammatory response during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol 216:98–107. [DOI] [PubMed] [Google Scholar]
- Ferner RE, Dear JW, Bateman DN. (2011) Management of paracetamol poisoning. BMJ 342:d2218. [DOI] [PubMed] [Google Scholar]
- Gate L, Majumdar RS, Lunk A, Tew KD. (2004) Increased myeloproliferation in glutathione S-transferase pi-deficient mice is associated with a deregulation of JNK and Janus kinase/STAT pathways. J Biol Chem 279:8608–8616. [DOI] [PubMed] [Google Scholar]
- Grek CL, Zhang J, Manevich Y, Townsend DM, Tew KD. (2013) Causes and consequences of cysteine S-glutathionylation. J Biol Chem 288:26497–26504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. (2006) c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology 131:165–178. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Flanagan JU, Jowsey IR. (2005) Glutathione transferases. Annu Rev Pharmacol Toxicol 45:51–88. [DOI] [PubMed] [Google Scholar]
- Henderson CJ, McLaren AW, Wolf CR. (2014) In vivo regulation of human glutathione transferase GSTP by chemopreventive agents. Cancer Res 74:4378–4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson CJ, Ritchie KJ, McLaren A, Chakravarty P, Wolf CR. (2011) Increased skin papilloma formation in mice lacking glutathione transferase GSTP. Cancer Res 71:7048–7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson CJ, Smith AG, Ure J, Brown K, Bacon EJ, Wolf CR. (1998) Increased skin tumorigenesis in mice lacking pi class glutathione S-transferases. Proc Natl Acad Sci USA 95:5275–5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson CJ, Wolf CR, Kitteringham N, Powell H, Otto D, Park BK. (2000) Increased resistance to acetaminophen hepatotoxicity in mice lacking glutathione S-transferase Pi. Proc Natl Acad Sci USA 97:12741–12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JA, Roberts DW, James LP. (2010) Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol (196):369–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, et al. (2006) Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med 12:446–451. [DOI] [PubMed] [Google Scholar]
- Jollow DJ, Thorgeirsson SS, Potter WZ, Hashimoto M, Mitchell JR. (1974) Acetaminophen-induced hepatic necrosis. VI. Metabolic disposition of toxic and nontoxic doses of acetaminophen. Pharmacology 12:251–271. [DOI] [PubMed] [Google Scholar]
- Keller A, Nesvizhskii AI, Kolker E, Aebersold R. (2002) Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem 74:5383–5392. [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ. (2004) Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology 40:1170–1179. [DOI] [PubMed] [Google Scholar]
- Laine JE, Auriola S, Pasanen M, Juvonen RO. (2009) Acetaminophen bioactivation by human cytochrome P450 enzymes and animal microsomes. Xenobiotica 39:11–21. [DOI] [PubMed] [Google Scholar]
- Lee KK, Imaizumi N, Chamberland SR, Alder NN, Boelsterli UA. (2015) Targeting mitochondria with methylene blue protects mice against acetaminophen-induced liver injury. Hepatology 61:326–336. [DOI] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491–501. [DOI] [PubMed] [Google Scholar]
- Lo H-W, Stephenson L, Cao X, Milas M, Pollock R, Ali-Osman F. (2008) Identification and functional characterization of the human glutathione S-transferase P1 gene as a novel transcriptional target of the p53 tumor suppressor gene. Mol Cancer Res 6:843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok HC, Suryo Rahmanto Y, Hawkins CL, Kalinowski DS, Morrow CS, Townsend AJ, Ponka P, Richardson DR. (2012) Nitric oxide storage and transport in cells are mediated by glutathione S-transferase P1-1 and multidrug resistance protein 1 via dinitrosyl iron complexes. J Biol Chem 287:607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masubuchi Y, Suda C, Horie T. (2005) Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J Hepatol 42:110–116. [DOI] [PubMed] [Google Scholar]
- McConnachie LA, Mohar I, Hudson FN, Ware CB, Ladiges WC, Fernandez C, Chatterton-Kirchmeier S, White CC, Pierce RH, Kavanagh TJ. (2007) Glutamate cysteine ligase modifier subunit deficiency and gender as determinants of acetaminophen-induced hepatotoxicity in mice. Toxicol Sci 99:628–636. [DOI] [PubMed] [Google Scholar]
- McGarry DJ, Chen W, Chakravarty P, Lamont DJ, Wolf CR, Henderson CJ. (2015) Proteome-wide identification and quantification of S-glutathionylation targets in mouse liver. Biochem J 469:25–32. [DOI] [PubMed] [Google Scholar]
- McGill MR, Jaeschke H. (2013) Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res 30:2174–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Williams CD, Xie Y, Ramachandran A, Jaeschke H. (2012) Acetaminophen-induced liver injury in rats and mice: comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol Appl Pharmacol 264:387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon D, Board PG. (2013) A role for glutathione transferase Omega 1 (GSTO1-1) in the glutathionylation cycle. J Biol Chem 288:25769–25779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon D, Coll R, O’Neill LA, Board PG. (2014) Glutathione transferase omega 1 is required for the lipopolysaccharide-stimulated induction of NADPH oxidase 1 and the production of reactive oxygen species in macrophages. Free Radic Biol Med 73:318–327. [DOI] [PubMed] [Google Scholar]
- Mobasher MA, González-Rodriguez A, Santamaría B, Ramos S, Martín MA, Goya L, Rada P, Letzig L, James LP, Cuadrado A, et al. (2013) Protein tyrosine phosphatase 1B modulates GSK3β/Nrf2 and IGFIR signaling pathways in acetaminophen-induced hepatotoxicity. Cell Death Dis 4:e626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat GJ, McLaren AW, Wolf CR. (1997) Transcriptional and post-transcriptional mechanisms can regulate cell-specific expression of the human Pi-class glutathione S-transferase gene. Biochem J 324:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman I, Kode A, Biswas SK. (2006) Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 1:3159–3165. [DOI] [PubMed] [Google Scholar]
- Reid AB, Kurten RC, McCullough SS, Brock RW, Hinson JA. (2005) Mechanisms of acetaminophen-induced hepatotoxicity: role of oxidative stress and mitochondrial permeability transition in freshly isolated mouse hepatocytes. J Pharmacol Exp Ther 312:509–516. [DOI] [PubMed] [Google Scholar]
- Ritchie KJ, Henderson CJ, Wang XJ, Vassieva O, Carrie D, Farmer PB, Gaskell M, Park K, Wolf CR. (2007) Glutathione transferase pi plays a critical role in the development of lung carcinogenesis following exposure to tobacco-related carcinogens and urethane. Cancer Res 67:9248–9257. [DOI] [PubMed] [Google Scholar]
- Ritchie KJ, Walsh S, Sansom OJ, Henderson CJ, Wolf CR. (2009) Markedly enhanced colon tumorigenesis in Apc(Min) mice lacking glutathione S-transferase Pi. Proc Natl Acad Sci USA 106:20859–20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer KT, Gibson AM, Sivaprasad U, Bass SA, Ericksen MB, Wills-Karp M, Lecras T, Fitzpatrick AM, Brown LAS, Stringer KF, et al. (2011) Downregulation of glutathione S-transferase pi in asthma contributes to enhanced oxidative stress. J Allergy Clin Immunol 128:539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamper BD, Bammler TK, Beyer RP, Farin FM, Nelson SD. (2010) Differential regulation of mitogen-activated protein kinase pathways by acetaminophen and its nonhepatotoxic regioisomer 3′-hydroxyacetanilide in TAMH cells. Toxicol Sci 116:164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke DW, Chock PB, Mieyal JJ. (2003) Glutathione-thiyl radical scavenging and transferase properties of human glutaredoxin (thioltransferase). Potential role in redox signal transduction. J Biol Chem 278:14607–14613. [DOI] [PubMed] [Google Scholar]
- Townsend DM, Manevich Y, He L, Hutchens S, Pazoles CJ, Tew KD. (2009) Novel role for glutathione S-transferase pi. Regulator of protein S-Glutathionylation following oxidative and nitrosative stress. J Biol Chem 284:436–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tujios S, Fontana RJ. (2011) Mechanisms of drug-induced liver injury: from bedside to bench. Nat Rev Gastroenterol Hepatol 8:202–211. [DOI] [PubMed] [Google Scholar]
- Wakabayashi H, Ito T, Fushimi S, Nakashima Y, Itakura J, Qiuying L, Win MM, Cuiming S, Chen C, Sato M, et al. (2012) Spred-2 deficiency exacerbates acetaminophen-induced hepatotoxicity in mice. Clin Immunol 144:272–282. [DOI] [PubMed] [Google Scholar]
- Wancket LM, Meng X, Rogers LK, Liu Y. (2012) Mitogen-activated protein kinase phosphatase (Mkp)-1 protects mice against acetaminophen-induced hepatic injury. E 40:1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Arifoglu P, Ronai Z, Tew KD. (2001) Glutathione S-transferase P1-1 (GSTP1-1) inhibits c-Jun N-terminal kinase (JNK1) signaling through interaction with the C terminus. J Biol Chem 276:20999–21003. [DOI] [PubMed] [Google Scholar]
- Wu Y, Fan Y, Xue B, Luo L, Shen J, Zhang S, Jiang Y, Yin Z. (2006) Human glutathione S-transferase P1-1 interacts with TRAF2 and regulates TRAF2-ASK1 signals. Oncogene 25:5787–5800. [DOI] [PubMed] [Google Scholar]
- Xia C, Taylor JB, Spencer SR, Ketterer B. (1993) The human glutathione S-transferase P1-1 gene: modulation of expression by retinoic acid and insulin. Biochem J 292:845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Greenhaw J, Ali A, Shi Q, Roberts DW, Hinson JA, Muskhelishvili L, Beger R, Pence LM, Ando Y, et al. (2012) Changes in mouse liver protein glutathionylation after acetaminophen exposure. J Pharmacol Exp Ther 340:360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R, Wolf CR, Brown K, Hayes JD, Whitelaw CB. (2010) Spatial monitoring of toxicity in HMOX-LacZ transgenic mice. Transgenic Res 19:897–902. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.