Abstract
The use of norfloxacin either as primary or secondary prophylaxis of bacterial infections in advanced cirrhosis has improved patient’s survival. This may be explained not only due to a significant decrease in the number of infections, but also because of a direct immunomodulatory effect. Selective intestinal decontamination with norfloxacin reduces translocation of either viable bacteria or bacteria-driven products from the intestinal lumen. In addition, norfloxacin directly modulates the systemic inflammatory response. The pro-inflammatory cytokine profile secreted by neutrophils from these patients shows a close, significant, and inverse correlation with serum norfloxacin levels. Similar effects have been described with other quinolones in different clinical conditions. Although the underlying mechanisms are not well defined for most of the antibiotics, the pathways triggered for norfloxacin to induce such immunomodulatory effects involve the down-regulation of pro-inflammatory inducible nitric oxide synthase, cyclooxygenase-2, and NF-κB and the up-regulation of heme-oxygenase 1 and IL-10 expression. The knowledge of these immunomodulatory effects, additional to their bactericidal role, improves our comprehension of the interaction between antibiotics and the cellular host response and offer new possibilities for the development of new therapeutic strategies to manage and prevent bacterial infections in cirrhosis.
Keywords: Cirrhosis, Prophylaxis, Cytokines, Bacterial DNA, Norfloxacin
Core tip: The use of antibiotic therapy to either treat or prevent frequent bacteria-derived complications arising in patients with cirrhosis is well established. The knowledge of antibiotic immunomodulatory mechanism, additional to their bactericidal role, improves our comprehension of the interaction between these molecules and the cellular machinery, and provides insight on the development of alternative strategies in the management and prevention of bacterial infections in cirrhosis.
BACTERIAL INFECTIONS IN CIRRHOSIS
Bacterial infections are among the most frequent complications arising in patients with cirrhosis and are associated with clinical consequences such as failure to control bleeding from oesophageal varices[1] and reduced survival[2]. Up to 35% of patients with cirrhosis develop nosocomial infections whereas this percentage is significantly lower (5%) in the general population[3].
Bacterial infection episodes are mainly caused by Gram-negative bacilli of enteric origin. Bacterial species such as Escherichia coli (E. coli) are responsible for spontaneous bacterial peritonitis (SBP) episodes, urinary tract infections or pneumonia in cirrhosis. The accepted pathogenic mechanism to explain the passage of bacteria or their products from the intestinal lumen through the intestinal wall and to mesenteric lymph nodes (MLNs) is defined as bacterial translocation (BT)[4,5]. Spontaneous BT, evaluated either by culture-positive MLNs or the presence of surrogate markers in blood such as lipopolysaccharide (LPS)-binding protein (LBP) or bacterial DNA, has been reported to occur in 30%-35% of patients with cirrhosis[6-8].
Intestinal bacterial overgrowth (IBO), increased intestinal permeability and immune alterations are considered as the main mechanisms involved in the pathogenesis of BT in cirrhosis (Figure 1). Experimental studies have shown a higher rate of IBO in cirrhotic rats with ascites and BT compared with those without BT[9,10]. However, these studies also report a significant percentage of animals showing IBO without BT, suggesting the implication of other factors in the pathogenesis of BT.
Figure 1.
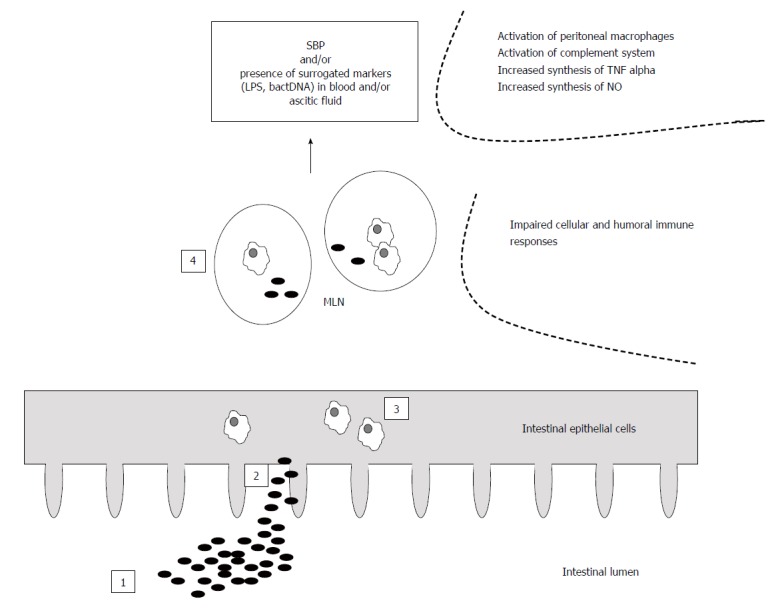
Bacterial translocation and spontaneous bacterial peritonitis - pathogenic mechanisms. Intestinal bacterial overgrowth (1), increased intestinal permeability (2) and immune alterations (3 and 4) are considered as the main mechanisms involved in the pathogenesis of bacterial translocation (BT) defined as the passage of bacteria from the intestinal lumen through the intestinal wall and to mesenteric lymph nodes (MLNs) in cirrhosis. SBP: Spontaneous bacterial peritonitis.
The gut barrier integrity is also considered as an important issue in preventing BT. Recent studies have shown the relevance of maintaining gut barrier integrity as a potential target to modify/reduce BT episodes in experimental cirrhosis. Increased intestinal permeability has been associated with increased serum endotoxin levels in different experimental models of liver damage[11] and the gut barrier protective role of certain probiotics have succeed in decreasing induced BT in CCl4-cirrhotic mice[12] and rats[13]. However, therapy against IBO without interfering intestinal permeability has been shown to decrease the rate of BT in cirrhotic rats[14], suggesting that the increase of intestinal permeability may not be sufficient for explaining BT pathogenesis
Finally, translocating bacterial products into MLNs or to portal blood are usually phagocyted and neutralized, but alterations in local and systemic immunity may favour the development of BT episodes. For example, SBP has been correlated with nucleotide-binding oligomerization domain containing protein 2 (NOD2) gene variants that fail in the recognition of bacterial peptidoglycans therefore affecting local immunity[15].
BACTERIAL TRANSLOCATION AND INFLAMMATION
Decompensated cirrhosis is associated with impaired cellular and humoral immune responses[16,17]. For instance, decreased serum levels of Complement subunit C3 predispose to develop SBP[18]. Cirrhosis and the development of SBP has also been correlated with a decreased phagocytic activity of the reticuloendothelial system[19]. In addition, the translocation of bacteria or their products induces a chronic inflammatory response that may aggravate the haemodynamic disturbances observed in cirrhosis[20].
Our group has extensively worked on the systemic inflammatory mechanisms and consequences of the passage of bacterial products into the blood of patients with decompensated cirrhosis. The cell mediated immune response and nitric oxide production is activated in peritoneal macrophages from patients with cirrhosis and ascites who show translocation of bacterial DNA into blood and ascitic fluid (AF)[21], and the relevance of the inflammatory response is species-specific in serum and AF of these patients[22]. Also, the translocation of bacterial DNA induces the activation of the complement system in patients with advanced cirrhosis[23], probably justifying its consumption when repeated episodes occur[24]. We have reported as well an ERK-related signalling pathway activated in peritoneal macrophages in response to bacterial DNA[25], the similarity between the inflammatory reaction induced by bacterial DNA translocation and that observed in overt infections such as SBP[26] and the detection of bacterial-synthesized peptides in the ascitic fluid of patients with decompensated cirrhosis associated with an increased pro-inflammatory cytokine cascade[27]. In addition, other bacterial products such as LPS have widely shown their immunogenic effect on patients with cirrhosis[28]. All this evidence clearly state not only the translocation of viable bacteria but also the translocation of bacterial products as an immunogenic mechanism directly implicated in the engagement of systemic inflammation in cirrhosis.
Other studies focused on mucosal inflammation have demonstrated that the synthesis of TNF-α and nitric oxide exacerbates oxidative damage in the intestinal mucosa[29], which in turn increases intestinal permeability probably favouring BT and creating a feedback that perpetuates inflammation. In fact, our group has demonstrated that the administration of anti-TNF-α monoclonal antibodies to cirrhotic rats with ascites is associated with a significant decrease in the rate of BT[30].
SELECTIVE INTESTINAL DECONTAMINATION
Selective intestinal decontamination (SID) consists of the aerobic Gram-negative bacteria clearance from intestinal content with oral non-absorbable or poorly absorbable antibiotics. Norfloxacin is the most frequently used antibiotic for long-term SID. Norfloxacin has been proved as poorly absorbable and specifically active against Gram-negative bacteria. In addition, the incidence of adverse effects associated with its long-term administration is low[31].
Norfloxacin is a synthetic 6-fluoro 4-quinolone molecule that antagonizes enzymatic activity of DNA gyrase of bacteria[32]. Norfloxacin is well tolerated according to randomized controlled trials performed[33], it is excreted through the kidneys and the usual pharmacological parameters, such as serum half-life or peak concentration, are not altered in patients with moderate liver damage compared with healthy controls[34].
In the setting of cirrhosis, oral norfloxacin is administered, either as primary prophylaxis (400 mg twice a day for 1 wk) to patients with variceal bleeding, severely decompensated cirrhosis and those with ascites protein concentration of < 15 mg/L or as secondary prophylaxis (indefinitely, 400 mg daily) to those who have survived a previous episode of SBP[35]. In this last condition, norfloxacin administration significantly reduces the incidence of bacterial infections[31,36] and, used as primary prophylaxis, also reduces noninfectious related clinical complications, such as hepatorenal syndrome, thus improving survival[35,37]. Oral ciprofloxacin 500 mg/d is an alternative option to norfloxacin[38]. On the other hand, although norfloxacin decreases certain virulence factors even in quinolone-resitant E. coli, long-term norfloxacin prophylaxis increases 2.7 fold the risk of developing multiresistant bacterial infections and almost 4 fold the risk of infections caused by extended-spectrum β -lactamase-producing Enterobacteriacea[39,40].
Recently, rifaximin, an antibiotic with broad-spectrum antimicrobial activity that eliminates intestinal flora non-selectively has been suggested as an alternative in the prophylaxis of bacterial infections in cirrhosis. This antibiotic reaches high fecal concentrations but is virtually non-absorbed (bioavailability in blood after oral administration < 0.4%), reduces the expression of bacterial virulence factors and compromises plasmid transfer and, despite high gut concentrations and its broad spectrum of activity, produces minimal alterations in the intestinal microflora[35]. Nevertheless, there are no studies comparing rifaximin vs norfloxacin in the prevention of SBP.
Efficacy of norfloxacin in preventing recurrent SBP therefore relies on the removal of enteric Gram-negative bacteria, which are mainly responsable for SBP episodes occuring in patients with cirrhosis (Figure 2). Although norfloxacin is poorly absorbable, its long-term administration upholds serum levels higher than 90% of minimal inhibitory concentration for most aerobic Gram-negative[41].
Figure 2.
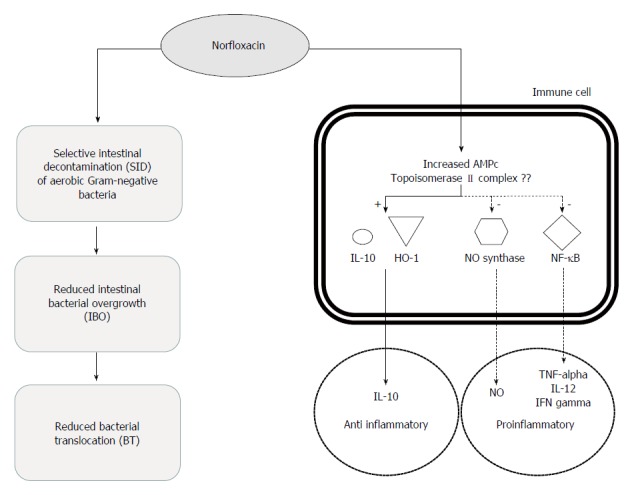
Immunomodulatory and bactericidal effects of norfloxacin in patients with cirrhosis.
ANTIBIOTICS AND INFLAMMATORY DOWNREGULATION
Most antibiotic classes exert effects on the immune system and complement activation. Sulphonamides, tetracyclines, beta-lactams, aminoglycosides, lincosamides, chloramphenicol, rifampin, benzylpyrimidinones, fusidic acid, fosfomycin or gyrase B-inhibitors, and, in particular, macrolides and fluoroquinolones either increase or decrease phagocyte functions. For most of this antibiotics the underlying mechanisms of immunomodulation are not well defined[42].
Fluorquinolones are able to concentrate intracellularly in human phagocytic cells on average 3 to 12-fold depending on the particular cell type and fluoroquinolone studied[43]. This intracellular accumulation of fluoroquinolones is modulated in macrophages by the activity of drug efflux pumps. Thus, gemfibrozil and probenecid, inhibitors of multidrug resistance proteins (MRP) and other transporters of organic anions, increase the intracellular accumulation of ciprofloxacin[44]. Numerous studies have shown that intracellular fluorquinolones remain active against facultative and obligate intracellular pathogens such as mycobacteria, Legionella spp, Streptococcus pneumoniae, Listeria monocytogenes and Staphylococcus aureus despite the acidic intracellular pH. However, little is known about how and to what extent this intracellular accumulation of antibiotics directly and specifically relates to changes in phagocytic and immunomodulatory activity of macrophages[45].
Ciprofloxacin improved survival in mice exposed to sublethal LPS challenge changing the pattern of early cytokine response. In the absence of ciprofloxacin LPS administration resulted in peaks of serum TNF-α and IL-10 concentrations at 1 h that disappeared by 6 h while serum IL12 concentrations peaked at 3 h and decreased by 6 h. Pre-treatment with a dose of ciprofloxacin prevented death in these mice, and consistently and significantly attenuated the TNF-α burst, decreased IL-12 concentrations and increased the IL-10 response. Similarly, trovafloxacin reduced TNF-α levels and decreased mortality in a experimental model of intra-abdominal abscesses in rats despite subtherapeutic doses of the drug were used or animals were challenged with heat-killed bacteria[46]. These results demonstrate that fluoroquinolones affect cytokine responses to bacterial products, an effect that may be particularly important when considering the consequences of early inflammatory responses to infection when cytokines influence the functional differentiation of T lymphocytes to initiate the acquired immune response[47].
Several clinical and experimental studies indicate that the fluoroquinolones exert immunomodulatory activities in latent or chronic infections affecting the synthesis of cytokines. Chronic infections such as those caused by Chlamydia pneumoniae are characterised by a marked inflammatory response, mediated by NF-κB and other transcription factors. Fluoroquinolones show a direct antichlamydial activity and simultaneously produce a reduction in secretion of proinflammatory cytokines via NF-κB necessary to achieve efficacy in this setting[45]. On the other hand, the participation of activated macrophages in the resolution of Listeria monocytogenes infections is determined by interferon, which acts synergistically with sparfloxacin and moxifloxacin[48]. Staphylococcus aureus excretes a wide array of toxins including superantigens, which cause T-cell proliferation, the release of cytokines, and promote apoptosis increasing TNF-RI and Fas receptor expression and Fas ligand production. Moxifloxacin inhibits apoptosis and downregulates the staphylococcal superantigen induced mRNA expression of Fas, FasL, and TNF-RI[45]. In general, these studies indicate that the fluoroquinolones exert immunomodulatory activities besides their own bactericidal properties supporting further their efficacy in latent or chronic infections.
In cirrhotic patients, SID with norfloxacin reduces translocation of either viable bacteria or bacteria-driven products from the intestinal lumen and then modulates the immune system activity but also could modulates patients’ proinflammatory reaction by acting directly on phagocyte response as described in other settings (Figure 2). In fact, we have shown that norfloxacin but not trimethoprim/sulfamethoxazole modulates inflammatory response and directly affects neutrophils activity in patients with cirrhosis. Indeed, serum levels of proinflammatory cytokines TNF-α and IL12 and interferon-γ showed a close, significant, and inverse correlation to serum norfloxacin levels, both at the peak time and at trough stages of the drug concentrations[41]. During the first 4 h after drug administration, when maximum serum and intracellular norfloxacin levels are reached, the transcription factor responsible of transcription of proinflammatory genes and cytokine synthesis as TNF-α and interleukin 8 (NF-κB) is down-regulated. These evidences are in agreement with the low cytokine levels frequently observed in patients with cirrhosis and ascites undergoing SID with norfloxacin[41]. Neutrophils from patients with cirrhosis cultured with LPS showed an up-regulation in IL-10 levels and heme oxygenase-1 expression in patients receiving norfloxacin. IL-10 levels, HO-1 expression and norfloxacin concentrations were directly correlated, whereas proinflammatory inducible nitric oxide synthase, cyclooxygenase-2, and NF-κB were inversely correlated[49]. All these data are consistent with the effects of other quinolones in different clinical conditions and experimental models supporting the hypothesis of the existence of an immunomodulatory effect of norfloxacin in cirrhotic patients that acts synergistically with direct antibiotic effect on the intestinal bacterial flora.
Liver transplantation represents a particular situation characterized by immunosuppression and a high risk of infections associated to an increase in mortality, morbidity, and hospital stay. As a consequence, many patients are subjected to antibiotic prophylaxis. No significant differences in the number of infections between intervention and control groups were found in all seven low-quality trials published up to now[50]. The effect of antibiotics on post-transplantation immunosuppression status is unknown.
It has been proposed that increased levels of cytokines, such as TNF-α, IL12, IL-1 and IL-6, and IFN-γ play a role in the hyperdynamic circulatory syndrome of cirrhosis. In mice the hypotension elicited by TNF-α is reversed after inhibition of NO synthesis, suggesting that l-arginine-derived NO is a principal mediator of TNF-α-induced hypotension[51]. It has been described in experimental cirrhosis that TNF-α induces NO synthesis increasing the activity of the inducible and endothelial forms of NO synthase (iNOS and eNOS, respectively). Several studies in patients with cirrhosis have showed a positive effect of norfloxacin on vascular resistance and pulmonary shunting, reversing the hyperdynamic circulation, increasing mean arterial blood pressure and systemic vascular resistance[52]. In patients with cirrhosis treated with norfloxacin for SID and in parallel to proinflammatory cytokines concentrations, serum NO shows a close, significant, and inverse correlation to serum norfloxacin levels suggesting an effect of norfloxacin regulating cytokine response and then NO production and associated haemodynamic changes[41].
MECHANISM/S
Norfloxacin is a synthetic 6-fluoro-4-quinolone which primary bacterial target is bacterial DNA gyrase or topoisomerase II. Bacterias require DNA gyrase for DNA replication, transcription of certain genes and aspects of DNA repair and recombination. All these activities are antagonized by fluoroquinolones[53]. However, the basic mechanisms underlying their immunomodulatory activity have not been elucidated.
It has been postulated that TNF-α inhibition could be related to changes in intracellular levels of cyclic AMP (cAMP) (Figure 2). In human monocytes with or without the addition of lipopolysaccharide the presence of ciprofloxacin was associated to a significant accumulation of cAMP[54]. Similarly, 2-phenyl-4-quinolone (YT-1), a quinolone compound that did not activate adenylate cyclase but inhibited the cytosolic phosphodiesterase activity, increased the cellular cAMP levels and the cAMP-protein kinase A (PKA) activity in rat neutrophils stimulated with N-formyl-methionyl-leucyl-phenylalanine (fMLP)[55]. These studies suggest evidence that fluoroquinolones can directly inhibits phosphodiesterase leading to accumulation of cAMP and enhanced PKA activity. Activation of PKA is associated with intracellular protein phosphorylations and transcription factors activation, and suppression of TNF-α expression. Another direct effect on cytosolic systems of fluoroquinolones has been hypothesized after findings suggesting that ciprofloxacin induced the production of PGE2 in monocytes in a concentration-dependent manner enhancing the expression of cyclooxygenase (COX-2) protein and the elevation of intracellular cAMP in monocytes[56] and after data showing a parallel increase in IL-10 levels and heme oxygenase-1 expression in patients receiving norfloxacin[49], suggesting the involvement of the pathway 1L-10/heme oxygenase 1, a pathway profoundly effective in dampening the enhanced activation of innate immune responses, in the fluoroquinolone effect (Figure 2).
Most experimental models studying the relationship between cytokine expression and quinolone exposition show that alteration in cytokine production correlated with parallel changes in the concentration of mRNA of the specific cytokine. These mRNA alterations were seen only when cells were stimulated by stimuli such as phytohaemagglutinin, lipopolysaccharide or phorbol-myristate acetate (PMA) before quinolone exposure[47].
NF-κB is the crucial factor in mRNA transcription of many cytokines and chemokines including TNF-α and interleukin 8 molecules. It also has an important role in other central cellular events such as superoxide production and apoptosis. In resting cells, NF-κB is bound to an inhibitory protein, inhibitory kappa B (IκB), and retained in the cytoplasm. Cell stimulation with several agonists triggers signal transduction pathways that ultimately result in activation of IκB kinases (IKK). Phosphorylation of IκB by IKK is followed by a rapid degradation of the IκB proteins thereby freeing NF-κB, which then enters the nucleus, binds to DNA, and activates transcription[45].
In a model of reactivation of latent HIV-1 expression in chronically infected promonocytic U1 cells, ciprofloxacin (at concentrations between 1.5 and 10 mg/L) inhibited HIV-1 expression in PMA-stimulated U1 cells. This effect was associated with a significant decrease in TNF-α production and with significant inhibition of NF-κB[57]. Moxifloxacin-treated mice showed no bronchopneumonia after cyclophosphamide injection and intra-tracheal inoculation of C albicans. This protective effect was associated with a significant inhibition of TNF-α and interleukin 8 secretion compared with controls. In lung tissue analysed by immunohistochemistry, moxifloxacin-treated animals showed a marked and significant decrease of NF-κB staining in epithelial cells and macrophages. When human monocyte cell lines were stimulated by lipopolysaccharide a significant increase in interleukin 8 and a significant degradation of IκB were observed. Both effects were abolished when cells were exposed to moxifloxacin[58]. These results indicate that moxifloxacin inhibits NF-κB activation, probably through its inhibitory effects on IκB degradation. According with these studies we have observed in cirrhotic patients treated with norfloxacin a NF-κB down-regulation in parallel to increases in quinolone levels and to decreases in TNF-α concentrations (Figure 2). Then norfloxacin shows in this setting a similar immunomodulatory effect to that described for other fluoroquinolones[41].
In eukaryotic cells quinolones target the topoisomerase II complex in a similar manner to prokaryotic cells but their inhibitory effects is generally attained at concentrations that are two to three orders of magnitude higher[59]. As in prokaryotic cells, quinolones were shown to result in an increased formation of topoisomerase II-cleaved DNA complexes. If intra-nuclear processes relating to quinolone-topoisomerase II interaction may have an effect on cytosolic activation or inhibition of NF-κB requires further research (Figure 2).
FUTURE DIRECTIONS
The use of norfloxacin either as primary or secondary prophylaxis of bacterial infections in advanced cirrhosis has improved patient’s survival, not only due to a significant decrease in the number of infections, but also in the incidence of other deadly complications such as hepatorenal syndrome. Quinolones not only exert their beneficial effect through bactericidal mechanisms, as shown above, but also by an immunomodulatory effect. The extent to which this immunomodulatory effect actually contributes to norfloxacin efficacy in cirrhotic patients requires further research.
On the other hand, long-term norfloxacin prophylaxis has been related with an increased risk of developing multiresistant bacterial infections being this effect the main factor that can limit the use of this prophylactic strategy in the near future. New studies are needed to assess the possible involvement of the changes on the immune system exerted by fluoroquinolones in the development of these resistances. Related to this, it is necessary to study whether different strategies to modulate the activity of the immune system as well as new drugs with different effects not only on bacteria but also on immune cells can prevent and/or treat the development of multiresistant nosocomial bacterial infections in cirrhotic patients. In fact this situation is considered so relevant that recently a consortium comprised by more than 30 European companies and universities have joined efforts to develop new antibiotics against gram-negative bacteria, a European funding project from the Innovative Medicines Initiative.
Until new drugs are available however, the intimate knowledge of immunomodulatory activities exerted by antibiotics at intracellular levels may improve survival in patients with cirrhosis and open interesting possibilities for future researches.
Footnotes
Supported by Grants PI13/1443 and PI14/01090 from Instituto de Salud Carlos III, Madrid, Spain, and FEDER funds, EU.
Conflict-of-interest statement: All authors declare that they have no competing interests with any financial organization regarding the material discussed in the manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 11, 2015
First decision: May 18, 2015
Article in press: August 31, 2015
P- Reviewer: Ali AEM, Toshikuni N S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
References
- 1.Goulis J, Armonis A, Patch D, Sabin C, Greenslade L, Burroughs AK. Bacterial infection is independently associated with failure to control bleeding in cirrhotic patients with gastrointestinal hemorrhage. Hepatology. 1998;27:1207–1212. doi: 10.1002/hep.510270504. [DOI] [PubMed] [Google Scholar]
- 2.Arvaniti V, D’Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–156, 1246-156. doi: 10.1053/j.gastro.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 3.Fernández J, Navasa M, Gómez J, Colmenero J, Vila J, Arroyo V, Rodés J. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140–148. doi: 10.1053/jhep.2002.30082. [DOI] [PubMed] [Google Scholar]
- 4.Berg RD, Garlington AW. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun. 1979;23:403–411. doi: 10.1128/iai.23.2.403-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422–433. doi: 10.1002/hep.20632. [DOI] [PubMed] [Google Scholar]
- 6.Cirera I, Bauer TM, Navasa M, Vila J, Grande L, Taurá P, Fuster J, García-Valdecasas JC, Lacy A, Suárez MJ, et al. Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatol. 2001;34:32–37. doi: 10.1016/s0168-8278(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 7.Albillos A, de la Hera A, González M, Moya JL, Calleja JL, Monserrat J, Ruiz-del-Arbol L, Alvarez-Mon M. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology. 2003;37:208–217. doi: 10.1053/jhep.2003.50038. [DOI] [PubMed] [Google Scholar]
- 8.Such J, Francés R, Muñoz C, Zapater P, Casellas JA, Cifuentes A, Rodríguez-Valera F, Pascual S, Sola-Vera J, Carnicer F, et al. Detection and identification of bacterial DNA in patients with cirrhosis and culture-negative, nonneutrocytic ascites. Hepatology. 2002;36:135–141. doi: 10.1053/jhep.2002.33715. [DOI] [PubMed] [Google Scholar]
- 9.Pérez-Paramo M, Muñoz J, Albillos A, Freile I, Portero F, Santos M, Ortiz-Berrocal J. Effect of propranolol on the factors promoting bacterial translocation in cirrhotic rats with ascites. Hepatology. 2000;31:43–48. doi: 10.1002/hep.510310109. [DOI] [PubMed] [Google Scholar]
- 10.Guarner C, Runyon BA, Young S, Heck M, Sheikh MY. Intestinal bacterial overgrowth and bacterial translocation in cirrhotic rats with ascites. J Hepatol. 1997;26:1372–1378. doi: 10.1016/s0168-8278(97)80474-6. [DOI] [PubMed] [Google Scholar]
- 11.Hartmann P, Haimerl M, Mazagova M, Brenner DA, Schnabl B. Toll-like receptor 2-mediated intestinal injury and enteric tumor necrosis factor receptor I contribute to liver fibrosis in mice. Gastroenterology. 2012;143:1330–40.e1. doi: 10.1053/j.gastro.2012.07.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moratalla A, Gómez-Hurtado I, Santacruz A, Moya Á, Peiró G, Zapater P, González-Navajas JM, Giménez P, Such J, Sanz Y, et al. Protective effect of Bifidobacterium pseudocatenulatum CECT7765 against induced bacterial antigen translocation in experimental cirrhosis. Liver Int. 2014;34:850–858. doi: 10.1111/liv.12380. [DOI] [PubMed] [Google Scholar]
- 13.Sánchez E, Nieto JC, Boullosa A, Vidal S, Sancho FJ, Rossi G, Sancho-Bru P, Oms R, Mirelis B, Juárez C, et al. VSL#3 probiotic treatment decreases bacterial translocation in rats with carbon tetrachloride-induced cirrhosis. Liver Int. 2015;35:735–745. doi: 10.1111/liv.12566. [DOI] [PubMed] [Google Scholar]
- 14.Llovet JM, Bartolí R, Planas R, Viñado B, Pérez J, Cabré E, Arnal J, Ojanguren I, Ausina V, Gassull MA. Selective intestinal decontamination with norfloxacin reduces bacterial translocation in ascitic cirrhotic rats exposed to hemorrhagic shock. Hepatology. 1996;23:781–787. doi: 10.1002/hep.510230419. [DOI] [PubMed] [Google Scholar]
- 15.Appenrodt B, Grünhage F, Gentemann MG, Thyssen L, Sauerbruch T, Lammert F. Nucleotide-binding oligomerization domain containing 2 (NOD2) variants are genetic risk factors for death and spontaneous bacterial peritonitis in liver cirrhosis. Hepatology. 2010;51:1327–1333. doi: 10.1002/hep.23440. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-González M, Boixeda D, Herrero D, Burgaleta C. Effect of granulocyte-macrophage colony-stimulating factor on leukocyte function in cirrhosis. Gastroenterology. 1993;105:527–531. doi: 10.1016/0016-5085(93)90730-z. [DOI] [PubMed] [Google Scholar]
- 17.Rajkovic IA, Williams R. Abnormalities of neutrophil phagocytosis, intracellular killing and metabolic activity in alcoholic cirrhosis and hepatitis. Hepatology. 1986;6:252–262. doi: 10.1002/hep.1840060217. [DOI] [PubMed] [Google Scholar]
- 18.Such J, Guarner C, Enriquez J, Rodriguez JL, Seres I, Vilardell F. Low C3 in cirrhotic ascites predisposes to spontaneous bacterial peritonitis. J Hepatol. 1988;6:80–84. doi: 10.1016/s0168-8278(88)80465-3. [DOI] [PubMed] [Google Scholar]
- 19.Rimola A, Soto R, Bory F, Arroyo V, Piera C, Rodes J. Reticuloendothelial system phagocytic activity in cirrhosis and its relation to bacterial infections and prognosis. Hepatology. 1984;4:53–58. doi: 10.1002/hep.1840040109. [DOI] [PubMed] [Google Scholar]
- 20.Bellot P, García-Pagán JC, Francés R, Abraldes JG, Navasa M, Pérez-Mateo M, Such J, Bosch J. Bacterial DNA translocation is associated with systemic circulatory abnormalities and intrahepatic endothelial dysfunction in patients with cirrhosis. Hepatology. 2010;52:2044–2052. doi: 10.1002/hep.23918. [DOI] [PubMed] [Google Scholar]
- 21.Francés R, Muñoz C, Zapater P, Uceda F, Gascón I, Pascual S, Pérez-Mateo M, Such J. Bacterial DNA activates cell mediated immune response and nitric oxide overproduction in peritoneal macrophages from patients with cirrhosis and ascites. Gut. 2004;53:860–864. doi: 10.1136/gut.2003.027425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francés R, González-Navajas JM, Zapater P, Muñoz C, Caño R, Pascual S, Santana F, Márquez D, Pérez-Mateo M, Such J. Translocation of bacterial DNA from Gram-positive microorganisms is associated with a species-specific inflammatory response in serum and ascitic fluid of patients with cirrhosis. Clin Exp Immunol. 2007;150:230–237. doi: 10.1111/j.1365-2249.2007.03494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francés R, González-Navajas JM, Zapater P, Muñoz C, Caño R, Pascual S, Márquez D, Santana F, Pérez-Mateo M, Such J. Bacterial DNA induces the complement system activation in serum and ascitic fluid from patients with advanced cirrhosis. J Clin Immunol. 2007;27:438–444. doi: 10.1007/s10875-007-9090-2. [DOI] [PubMed] [Google Scholar]
- 24.Such J, Guarner C, Soriano G, Teixidó M, Barrios J, Tena F, Méndez C, Enríquez J, Rodríguez JL, Vilardell F. Selective intestinal decontamination increases serum and ascitic fluid C3 levels in cirrhosis. Hepatology. 1990;12:1175–1178. doi: 10.1002/hep.1840120516. [DOI] [PubMed] [Google Scholar]
- 25.Ruiz-Alcaraz AJ, Martínez-Esparza M, Caño R, Hernández-Caselles T, Recarti C, Llanos L, Zapater P, Tapia-Abellán A, Martín-Orozco E, Pérez-Mateo M, et al. Peritoneal macrophage priming in cirrhosis is related to ERK phosphorylation and IL-6 secretion. Eur J Clin Invest. 2011;41:8–15. doi: 10.1111/j.1365-2362.2010.02368.x. [DOI] [PubMed] [Google Scholar]
- 26.Francés R, Zapater P, González-Navajas JM, Muñoz C, Caño R, Moreu R, Pascual S, Bellot P, Pérez-Mateo M, Such J. Bacterial DNA in patients with cirrhosis and noninfected ascites mimics the soluble immune response established in patients with spontaneous bacterial peritonitis. Hepatology. 2008;47:978–985. doi: 10.1002/hep.22083. [DOI] [PubMed] [Google Scholar]
- 27.Caño R, Llanos L, Zapater P, Pascual S, Bellot P, Barquero C, Pérez-Mateo M, Such J, Francés R. Proteomic evidence of bacterial peptide translocation in afebrile patients with cirrhosis and ascites. J Mol Med (Berl) 2010;88:487–495. doi: 10.1007/s00109-009-0582-9. [DOI] [PubMed] [Google Scholar]
- 28.Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197–209. doi: 10.1016/j.jhep.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 29.Unno N, Wang H, Menconi MJ, Tytgat SH, Larkin V, Smith M, Morin MJ, Chavez A, Hodin RA, Fink MP. Inhibition of inducible nitric oxide synthase ameliorates endotoxin-induced gut mucosal barrier dysfunction in rats. Gastroenterology. 1997;113:1246–1257. doi: 10.1053/gast.1997.v113.pm9322519. [DOI] [PubMed] [Google Scholar]
- 30.Francés R, Chiva M, Sánchez E, González-Navajas JM, Llovet T, Zapater P, Soriano G, Muñoz C, Balanzó J, Pérez-Mateo M, et al. Bacterial translocation is downregulated by anti-TNF-alpha monoclonal antibody administration in rats with cirrhosis and ascites. J Hepatol. 2007;46:797–803. doi: 10.1016/j.jhep.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 31.Ginés P, Rimola A, Planas R, Vargas V, Marco F, Almela M, Forné M, Miranda ML, Llach J, Salmerón JM. Norfloxacin prevents spontaneous bacterial peritonitis recurrence in cirrhosis: results of a double-blind, placebo-controlled trial. Hepatology. 1990;12:716–724. doi: 10.1002/hep.1840120416. [DOI] [PubMed] [Google Scholar]
- 32.Lee C, Ronald AR. Norfloxacin: its potential in clinical practice. Am J Med. 1987;82:27–34. doi: 10.1016/0002-9343(87)90615-2. [DOI] [PubMed] [Google Scholar]
- 33.Fish DN. Fluoroquinolone adverse effects and drug interactions. Pharmacotherapy. 2001;21:253S–272S. doi: 10.1592/phco.21.16.253s.33993. [DOI] [PubMed] [Google Scholar]
- 34.Wolfson JS, Hooper DC. Norfloxacin: a new targeted fluoroquinolone antimicrobial agent. Ann Intern Med. 1988;108:238–251. doi: 10.7326/0003-4819-108-2-238. [DOI] [PubMed] [Google Scholar]
- 35.Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, Stadlbauer V, Gustot T, Bernardi M, Canton R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310–1324. doi: 10.1016/j.jhep.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 36.Soriano G, Guarner C, Tomás A, Villanueva C, Torras X, González D, Sainz S, Anguera A, Cussó X, Balanzó J. Norfloxacin prevents bacterial infection in cirrhotics with gastrointestinal hemorrhage. Gastroenterology. 1992;103:1267–1272. doi: 10.1016/0016-5085(92)91514-5. [DOI] [PubMed] [Google Scholar]
- 37.Fernández J, Navasa M, Planas R, Montoliu S, Monfort D, Soriano G, Vila C, Pardo A, Quintero E, Vargas V, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818–824. doi: 10.1053/j.gastro.2007.06.065. [DOI] [PubMed] [Google Scholar]
- 38.Fernández J, Gustot T. Management of bacterial infections in cirrhosis. J Hepatol. 2012;56 Suppl 1:S1–12. doi: 10.1016/S0168-8278(12)60002-6. [DOI] [PubMed] [Google Scholar]
- 39.Fernández J, Acevedo J, Castro M, Garcia O, de Lope CR, Roca D, Pavesi M, Sola E, Moreira L, Silva A, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology. 2012;55:1551–1561. doi: 10.1002/hep.25532. [DOI] [PubMed] [Google Scholar]
- 40.Tandon P, Delisle A, Topal JE, Garcia-Tsao G. High prevalence of antibiotic-resistant bacterial infections among patients with cirrhosis at a US liver center. Clin Gastroenterol Hepatol. 2012;10:1291–1298. doi: 10.1016/j.cgh.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zapater P, Caño R, Llanos L, Ruiz-Alcaraz AJ, Pascual S, Barquero C, Moreu R, Bellot P, Horga JF, Muñoz C, et al. Norfloxacin modulates the inflammatory response and directly affects neutrophils in patients with decompensated cirrhosis. Gastroenterology. 2009;137:1669–79.e1. doi: 10.1053/j.gastro.2009.07.058. [DOI] [PubMed] [Google Scholar]
- 42.Labro MT. Interference of antibacterial agents with phagocyte functions: immunomodulation or “immuno-fairy tales”? Clin Microbiol Rev. 2000;13:615–650. doi: 10.1128/cmr.13.4.615-650.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mandell GL, Coleman E. Uptake, transport, and delivery of antimicrobial agents by human polymorphonuclear neutrophils. Antimicrob Agents Chemother. 2001;45:1794–1798. doi: 10.1128/AAC.45.6.1794-1798.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seral C, Carryn S, Tulkens PM, Van Bambeke F. Influence of P-glycoprotein and MRP efflux pump inhibitors on the intracellular activity of azithromycin and ciprofloxacin in macrophages infected by Listeria monocytogenes or Staphylococcus aureus. J Antimicrob Chemother. 2003;51:1167–1173. doi: 10.1093/jac/dkg223. [DOI] [PubMed] [Google Scholar]
- 45.Dalhoff A, Shalit I. Immunomodulatory effects of quinolones. Lancet Infect Dis. 2003;3:359–371. doi: 10.1016/s1473-3099(03)00658-3. [DOI] [PubMed] [Google Scholar]
- 46.Thadepalli H, Reddy U, Chuah SK, Thadepalli F, Malilay C, Polzer RJ, Hanna N, Esfandiari A, Brown P, Gollapudi S. In vivo efficacy of trovafloxacin (CP-99,217), a new quinolone, in experimental intra-abdominal abscesses caused by Bacteroides fragilis and Escherichia coli. Antimicrob Agents Chemother. 1997;41:583–586. doi: 10.1128/aac.41.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Purswani M, Eckert S, Arora H, Johann-Liang R, Noel GJ. The effect of three broad-spectrum antimicrobials on mononuclear cell responses to encapsulated bacteria: evidence for down-regulation of cytokine mRNA transcription by trovafloxacin. J Antimicrob Chemother. 2000;46:921–929. doi: 10.1093/jac/46.6.921. [DOI] [PubMed] [Google Scholar]
- 48.Carryn S, Van Bambeke F, Mingeot-Leclercq MP, Tulkens PM. Comparative intracellular (THP-1 macrophage) and extracellular activities of beta-lactams, azithromycin, gentamicin, and fluoroquinolones against Listeria monocytogenes at clinically relevant concentrations. Antimicrob Agents Chemother. 2002;46:2095–2103. doi: 10.1128/AAC.46.7.2095-2103.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gómez-Hurtado I, Zapater P, Bellot P, Pascual S, Pérez-Mateo M, Such J, Francés R. Interleukin-10-mediated heme oxygenase 1-induced underlying mechanism in inflammatory down-regulation by norfloxacin in cirrhosis. Hepatology. 2011;53:935–944. doi: 10.1002/hep.24102. [DOI] [PubMed] [Google Scholar]
- 50.Gurusamy KS, Nagendran M, Davidson BR. Methods of preventing bacterial sepsis and wound complications after liver transplantation. Cochrane Database Syst Rev. 2014;3:CD006660. doi: 10.1002/14651858.CD006660.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cauwels A, Van Molle W, Janssen B, Everaerdt B, Huang P, Fiers W, Brouckaert P. Protection against TNF-induced lethal shock by soluble guanylate cyclase inhibition requires functional inducible nitric oxide synthase. Immunity. 2000;13:223–231. doi: 10.1016/s1074-7613(00)00022-4. [DOI] [PubMed] [Google Scholar]
- 52.Rasaratnam B, Kaye D, Jennings G, Dudley F, Chin-Dusting J. The effect of selective intestinal decontamination on the hyperdynamic circulatory state in cirrhosis. A randomized trial. Ann Intern Med. 2003;139:186–193. doi: 10.7326/0003-4819-139-3-200308050-00008. [DOI] [PubMed] [Google Scholar]
- 53.Rasaratnam B, Connelly N, Chin-Dusting J. Nitric oxide and the hyperdynamic circulation in cirrhosis: is there a role for selective intestinal decontamination? Clin Sci (Lond) 2004;107:425–434. doi: 10.1042/CS20040157. [DOI] [PubMed] [Google Scholar]
- 54.Bailly S, Fay M, Roche Y, Gougerot-Pocidalo MA. Effects of quinolones on tumor necrosis factor production by human monocytes. Int J Immunopharmacol. 1990;12:31–36. doi: 10.1016/0192-0561(90)90065-u. [DOI] [PubMed] [Google Scholar]
- 55.Wang JP, Chang LC, Raung SL, Hsu MF, Huang LJ, Kuo SC. Inhibition of superoxide anion generation by YC-1 in rat neutrophils through cyclic GMP-dependent and -independent mechanisms. Biochem Pharmacol. 2002;63:577–585. doi: 10.1016/s0006-2952(01)00882-6. [DOI] [PubMed] [Google Scholar]
- 56.Mori S, Takahashi HK, Liu K, Wake H, Zhang J, Liu R, Yoshino T, Nishibori M. Ciprofloxacin inhibits advanced glycation end products-induced adhesion molecule expression on human monocytes. Br J Pharmacol. 2010;161:229–240. doi: 10.1111/j.1476-5381.2010.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gollapudi S, Kim CH, Roshanravan B, Gupta S. Ciprofloxacin inhibits activation of latent human immunodeficiency virus type 1 in chronically infected promonocytic U1 cells. AIDS Res Hum Retroviruses. 1998;14:499–504. doi: 10.1089/aid.1998.14.499. [DOI] [PubMed] [Google Scholar]
- 58.Shalit I, Horev-Azaria L, Fabian I, Blau H, Kariv N, Shechtman I, Alteraz H, Kletter Y. Immunomodulatory and protective effects of moxifloxacin against Candida albicans-induced bronchopneumonia in mice injected with cyclophosphamide. Antimicrob Agents Chemother. 2002;46:2442–2449. doi: 10.1128/AAC.46.8.2442-2449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riesbeck K. Immunomodulating activity of quinolones: review. J Chemother. 2002;14:3–12. doi: 10.1179/joc.2002.14.1.3. [DOI] [PubMed] [Google Scholar]


