Abstract
Hyponatremia is a frequent complication of advanced cirrhosis with ascites associated with increased morbidity and mortality. It is caused by an impairment in the renal capacity to eliminate solute-free water and is considered to be related to persistent secretion of vasopressin despite low serum osmolality. This nonosmotic release of vasopressin is mediated by the autonomic nervous system, which senses the underfilling of arterial vascular component. This reduction of effective arterial blood volume is closely related to the development of ascites. Although the short-time effects of vasopressin V2 receptor antagonists (vaptans) on hyponatremia and ascites have been repeatedly reported, their effects on the long-term management of cirrhotic ascites have not been established yet. Considering that their effects on water diuresis and their safety are limited by severe underfilling state of patients, cautious approaches with adequate monitoring are needed to advanced cirrhosis. Proper indication, adequate doses and new possibility of combination therapy should be explored in the future controlled study. As hyponatremia is frequent obstacle to ascites management, judicious combination with low-dose diuretics may decrease the incidence of refractory ascites. Although vaptans show much promise in the treatment of advanced cirrhosis, the problem of high cost should be solved for the future.
Keywords: Liver cirrhosis, Ascites, Hyponatremia, Pathophysiology, V2 receptor antagonist
Core tip: Dilutional hyponatremia is a frequent complication with high morbidity and mortality in advanced liver cirrhosis with ascites. It is attributable to disturbed water excretion related to enhanced vasopressin activity on the background of underfilling state in the splanchnic arterial circulation. V2 receptor antagonist is theoretically promising for the future treatment of hyponatremia and ascites. The present review aimed to summarize the pathophysiological backgrounds of ascites and hyponatremia and to introduce major results of all controlled trials. Although there existed several unsolved problems and controversies on the topic, discussions from the basic standpoints may light up the dark road.
INTRODUCTION
Hyponatremia is a frequent complication of advanced cirrhosis related to an impairment in the renal capacity to eliminate solute-free water[1]. It becomes an important clinical problem for decompensated cirrhosis associated with increased morbidity and mortality[1]. The failure to excrete solute-free water attributable to persistent secretion of vasopressin despite low serum osmolality has been considered to underlie the development of this hyponatremia[2]. Since vasopressin V2 receptor antagonists or vaptans have selective aquaretic effect, they may open new possibilities for management of hyponatremia in advanced liver cirrhosis[3].
A basic understanding of the pathophysiologic mechanisms leading to water retention and dilutional hyponatremia is required for rational treatment of decompensated cirrhosis[4]. This review introduces the basic mechanisms of ascites and water retention and the major trials of vaptans in patients with liver cirrhosis. It also discusses the areas of uncertainty for the future clinical use of vaptans.
PATHOGENESIS OF ASCITES IN CIRRHOSIS
The pathogenetic events leading to ascites formation in patients with liver cirrhosis are extremely complex, being associated with multiple factors[5,6]. Pathophysiologic backgrounds and main theories of ascites formation in liver cirrhosis are shown in Figure 1. Cirrhotic patients exhibit the hyperdynamic circulation characterized by arterial hypotension, increased cardiac output, and reduced systemic vascular resistance[7]. These changes come from peripheral vasodilation and arteriovenous shunt. The contraction of the effective plasma volume associated with peripheral vasodilation may induce compensatory responses such as mobilization of vasoactive hormones and activation of the sympathetic nervous system[8].
Figure 1.
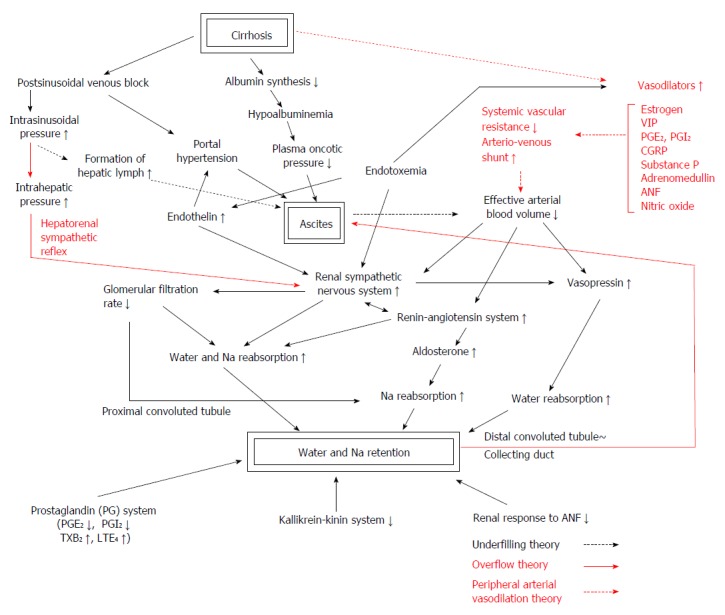
Phatophysiologic backgrounds and main theories of ascites formation in liver cirrhosis. The pathogenetic events leading to ascites formation in patients with liver cirrhosis are multifactorial. They include hepatic venous outflow block, portal hypertension and hypoalbuminemia as hepatic factors, hyperdynamic circulation, peripheral arterial vasodilation, decreased effective circulating blood volume and altered neurohumoral systems as systemic circulatory factors, and enhanced salt and water reabsorption in proximal and distal nephron and convoluted duct related to intrarenal haemodynamic derangement as renal factors. They are closely interrelated and enhance salt and water reabsorption in the kidney. The activities of the two major vasoconstrictor and antinatriuretic systems, the renin-angiotensin-aldosteron system and the sympathetic nervous system, are increased in most cirrhotics with tense ascites. Increased plasma level of arginine vasopressin enhances water reabsorption in the collecting duct and contributes to water retention. Endothelin, an endothelial-derived peptide with marked vasoconstrictor activity, is also increased in advanced cirrhosis[86,87]. Increased endothelin levels were proven to be related to creatinine clearance, effective renal plasma flow[88], serum creatinine and blood pressure[89], and may also contribute to renal dysfunction in patients with cirrhosis[88]. Three main hypotheses, the underfilling theory, the overflow theory and the peripheral arterial vasodilation theory, are considered to explain variable pathophysiological changes occurred in a patient with advanced liver cirrhosis. VIP: Vasoactive intestinal peptide; CGRP: Calcitonin gene-related peptide; PG: Prostaglandin; TX; Thromboxane; LT: Leukotriene.
Renal vasoconstriction is a common finding in patients with cirrhosis and ascites and more intense in the renal cortex, which may lead to a reduction of renal blood flow and glomerular filtration rate (GFR)[9]. Free water clearance and urinary sodium (Na) excretion are markedly decreased in those with tense ascites. Water and Na retention in advanced cirrhosis is due to increased tubular reabsorption. Increased reabsorption in the proximal convoluted tubule leads to a reduced delivery of filtrate. An increased reabsorption in the distal convoluted tubule may further enhance water and Na retention.
Three main hypotheses, the underfilling theory, the overflow theory and the peripheral arterial vasodilation theory, are considered to explain variable pathophysiological changes in a patient with advanced liver cirrhosis. In the underfilling theory, excess lymph accumulation in the peritoneal space attributable to imbalance of Starling forces in the hepatic sinusoids and splanchnic capillaries is considered to bring contraction of circulating plasma volume, which is thought to constitute an afferent signal to the renal tubule to augment salt and water reabsorption[6]. The postsinusoidal venous block and the decreased albumin synthesis in the background of cirrhosis are important initial events. In the overflow theory, renal Na retention and plasma volume expansion are considered to precede rather than follow the formation of ascites[10]. The investigators who supported this hypothesis[5,11,12] speculated that an increase in sinusoidal hydrostatic pressure directly stimulates renal sympathetic nervous system through the hepatorenal reflex[13,14], which initiates primary renal Na retention. Schrier et al[15] proposed the peripheral arterial vasodilation theory as a revised underfilling theory. In this theory, peripheral arterial vasodilation is considered to cause imbalance of capacitance and volume, which leads to decreased effective intravascular volume.
A wide variety of neurohumoral derangements may influence renal handling of salt and water[8]. The activities of the two major vasoconstrictor and antinatriuretic systems, the renin-angiotensin-aldosterone (RAA) system and the sympathetic nervous system, are enhanced in most cirrhotics with tense ascites[9]. Increased plasma level of arginine vasopressin (AVP) also known as antidiuretic hormone (ADH) increases water reabsorption in the collecting duct and contributes to water retention[9].
The mechanism of splanchnic arterial vasodilation in cirrhosis is complex and still undetermined[16]. For many years, arterial vasodilation in cirrhosis has been attributable to increased circulating vasodilators such as estrogen, VIP, prostaglandins[6], CGRP[17], substance P[18], adrenomedullin[19], atrial natriuretic factor[20,21] and nitric oxide[16,22]. As the most important site of vasodilation is the splanchnic circulation, increased production of nitric oxide, that acts in a paracrine manner, has recently been considered essential[16]. Bacterial endotoxin closely related to bacterial translocation is known to stimulate these vasodilators especially nitric oxide[23,24].
BACKGROUNDS OF REFRACTORY ASCITES
Refractory ascites was internationally defined as ascites that does not recede despite Na restriction and maximal diuretic therapy (furosemide 160 mg/d and spironolactone 400 mg/d) or that recurs shortly after therapeutic paracentesis[25]. However, most Japanese cirrhotics with ascites develop electrolyte disturbance or azotemia by this high-dose diuretic treatment. In order to avoid diuretic-induced side effects, we designed a stepped care protocol with a combination of low-dose aldosterone antagonists (400 mg/d of potassium canrenoate, iv) and loop diuretics (40-80 mg/d of furosemide, iv) and studied the pathophysiological backgrounds of these patients[26,27]. In the early step responders, basal renal function, serum Na concentration, plasma renin activity (PRA), plasma levels of aldosterone, norepinephrine (NE), and AVP were within normal limits and basal plasma α-human atrial natriuretic peptide (α-hANP) was elevated[26]. On the contrary, in the late step responders and nonresponders, basal PRA and plasma NE and AVP were progressively elevated with the concomitant decreases in basal creatinine clearance (Ccr), urine volume, urinary Na excretion (UNaV), serum Na levels and the increases in basal blood urea nitrogen (BUN) and serum creatinine levels[26,27]. These results indicate that the early responders were basically in the state of vascular overflow, while the late responders and nonresponders were relatively in the state of vascular underfilling. The pathophysiological backgrounds of ascites are considered to be a continuum involving both overflow (early stage) and underfilling states (late stage). Interestingly enough, in the patients who responded diuretics, PRA, plasma aldosterone and NE were elevated and α-hANP was lowered by the treatment, suggesting that the diuretics themselves may cause relative vascular underfilling[26].
In the advanced stages of decompensated cirrhosis, patients develop low arterial pressure attributable to further reduction of the peripheral vascular resistances[28]. An increase in the cardiac output to refill the expanded intravascular bed and the release of vasoconstrictors (RAA system, sympathetic nervous system and AVP) to raise peripheral vascular resistance are two compensatory mechanisms to overcome a further reduction of the peripheral vascular resistances and to maintain the hemodynamic stability[28]. Reduced renal perfusion and further Na and water retention with dilutional hyponatremia are the natural consequences of this physiological response[28].
WATER RETENTION IN LIVER CIRRHOSIS
An impaired renal water handling, leading to inability to excrete a water load and hyponatremia, represents a common finding in advanced liver cirrhosis[29]. In refractory ascites, hyponatraemia often develops, which indicates more intense water retention[30]. Free water clearance, an index of water excretion, has been reported to be markedly decreased in patients with cirrhosis and ascites.
On the other hand, the results reported in patients with compensated cirrhosis are still conflicting. By an intravenous water overload of 20 mL/kg body weight, we noted that free water clearance was decreased with the progression of liver cirrhosis[18]. Several other authors[29,31,32] showed a blunt diuretic and natriuretic response to water administration in non-ascitic cirrhotic patients. Conversely, Krag et al[30] reported that the Child B cirrhotic patients had increased free water clearance and distal fractional water excretion during a 400 mL/h oral water load.
Pathogenesis of water retention is not fully established, but two major mechanisms have been considered: (1) increased nonosmotic release and decreased clearance of AVP; and (2) decreased GFR and excessive proximal tubular Na reabsorption resulting in impaired free water excretion. For many years, the role of enhanced AVP activity in the cause of subnormal dilution capacity and water retention has received strong support[4]. Taking these results, the current pharmacological therapy has been focused on the release or action of AVP[33]. On the other hand, Gatta et al[29] found that free water clearance corrected for distal Na delivery were normal both in non-ascitic patients and in the majority (75%) of decompensated cirrhotics. They thought that excessive proximal Na reabsorption and reduced distal delivery of fluid may play a primary role in the pathogenesis of the impaired water excretion of these patients[29]. Krag et al[30] noted that Child C cirrhotics with ascites and mild hyponatremia showed a low GFR, a low distal tubular flow and an inability to increase free water clearance during water loading, while plasma AVP levels remained low. In general, the above two mechanisms are hardly evaluated separately, for the maneuvers leading to expansion of extracellular fluid volume not only enhance the distal delivery of filtrate but may also suppress AVP release by attenuating underfilling of arterial vascular compartment[4].
PLASMA LEVELS OF AVP
After the development of sensitive radioimmunoassay, most investigators reported elevated plasma AVP levels in liver cirrhosis[4]. However, its relation to clinical findings including water excretion was variable. Pérez-Ayuso et al[34] reported that plasma AVP levels in cirrhotics were higher than those in normal subjects and plasma AVP levels in cirrhotics with negative free water clearance were even higher than those in cirrhotics with positive free water clearance.
Castellano et al[35] found that basal AVP levels were elevated only in decompensated cirrhotics with hyponatremia, although water diuresis and fractional proximal Na excretion were significantly decreased in patients both with and without hyponatremia. They concluded that impaired water excretion in those without hyponatremia cannot be ascribed to enhanced AVP activity but may be related to reduced delivery of filtrate to the distal segment of the nephron[35].
Although basal plasma AVP levels were not different between well-compensated cirrhosis and healthy controls[32,36], suppression of plasma AVP after water load (20 mL/kg body weight) noted in healthy controls was not observed in cirrhotics[36]. Nicholls et al[37] evaluated diuretic responses to head-out water immersion, another maneuver to increase central blood volume, and reported that the cirrhotic patients with impaired water excretion revealed higher plasma AVP levels than those with moderate water excretion during water immersion. Bichet et al[38] reported that redistribution of central blood volume by water loading in addition to water immersion to the neck resulted in suppression of AVP levels and improvement in water excretion in decompensated cirrhotics. Epstein et al[39], however, failed to demonstrate plasma AVP suppression with their water immersion despite increased diuresis in most decompensated cirrhotics. They concluded that the diuresis in some patients without concomitant suppression of plasma AVP suggests that AVP may constitute a permissive rather than pivotal factor in the impaired water excretion in patients with advanced liver disease[39]. The discrepancies among these results on plasma AVP levels may be partly explained by the differences in the basal and post-immersion central blood volumes. The patients in Bichet’s study[38], who received a stronger central hydration (immersion plus intravenous water load) are considered to reveal more effective suppression of AVP[4].
We designed a “body compression” apparatus as a means to restore effective blood volume in cirrhotics with ascites[40]. All four limbs and the lower abdomen were compressed with constant pressure for 3 h, using stroke rehabilitation splints, while patients lay supine[40]. Repeated body compression alleviated ascites in those with well-preserved renal function, but was ineffective in those with markedly impaired renal function[40]. In the responders, plasma AVP levels were within normal limits during the study, whereas in the nonresponders, markedly elevated AVP was not depressed by the body compression[40].
NONOSMOTIC RELEASE OF AVP
The possible mechanism for increased AVP activity in patients with liver cirrhosis may include enhanced nonosmotic release of AVP, decreased hepatic metabolism and enhanced tubular sensitivity to AVP[4]. Among them, enhanced nonosmotic release of AVP is considered to play most pivotal role. In physiological state, mechanisms of osmoregulation and volume regulation help to maintain water balance and tonicity in the body[41]. AVP is synthesized by two hypothalamic nuclei (supraoptic nuclei and paraventricular nuclei) and secreted by the posterior pituitary in response to an increase in plasma tonicity or decrease in plasma volume. Effective circulating arterial volume acts as a nonosmotic factor which regulates the secretion of AVP[41].
The major nonosmotic pathway for AVP release involves the autonomic nervous system, which is mediated via the baroreceptors located mainly in the left ventricle and carotid sinus[42]. These baroreceptors communicate to the hypothalamus via parasympathetic pathways and cause a release of AVP in response to hypovolemia[33]. Small changes of < 10% in blood pressure or blood volume have no effect on AVP levels. However, once decreases in volume or pressure exceed this value, baroreceptor-mediated signals provide persistent stimuli for AVP secretion[43]. In cirrhotic patients with ascites, the nonosmotic release of AVP from the posterior pituitary becomes the dominant force and the end result is impaired free water excretion and subsequent dilutional hyponatraemia[44].
The peripheral arterial vasodilation of splanchnic vascular bed attributable to vasodilators such as nitric oxide, is considered to cause typical circulatory derangement in liver cirrhosis, i.e., hyperdynamic circulation. Although total plasma volume may be increased in these circumstances, splanchnic arteriolar vasodilation leads to underfilling of arterial vascular component. The reduction of “effective plasma volume” is sensed by the high-pressure osomoreceptors and stimulates RAA system and sympathetic nervous systems, resulting an increase in AVP release[4].
ROLE OF AQUAPORIN-2
AVP plays an important role in water and Na homeostasis. It acts via three receptor subtypes-V1a, V1b, and V2-distributed widely throughout the body[41]. V1a receptors are present on vascular smooth muscle cells, myocardium, platelets, and hepatocytes, and mediate vasoconstriction, platelet aggregation, and glycogenolysis[41]. V1b is expressed in the anterior pituitary gland where it mediates adrenocorticotropin release[45]. V2 receptors are located in principal cells of the renal collecting duct system and mediate water reabsorption. AVP acts on V2 receptors, which activate the cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) signaling pathway and translocate intracellular vesicles of water channel aquaporin-2 (AQP-2) to the apical plasma membrane of collecting duct cells. This AQP-2 in the cell surface increases the reabsorption of free water from the tubular fluid back into the circulation[1,41,46]. AVP further regulates the long-term water permeability of the collecting ducts by increasing AQP-2 gene expression[46] and AQP-2 protein synthesis[47]. AVP thus plays a pivotal role in the on-off regulation of the cellular trafficking of AQP-2 and the synthesis of AQP-2 in collecting duct cells[47]. Nonsuppressible release of AVP is profoundly involved in abnormal antidiuresis in pathological states of impaired water excretion[47]. Such a chronic AVP excess is closely associated with abundance of AQP-2 protein in collecting duct cells despite hypoosmolar condition[47].
In experimental studies, increased expression of AQP-2 mRNA and protein were also found and closely related to volume of ascites in CCl4-induced cirrhotic rats[48]. Jonassen et al[49] conversely showed that the renal expression of the AQP-2 was down-regulated in bile duct-ligated cirrhotic rats with Na retention but without ascites. They further demonstrated that renal AQP-2 expression in membrane fractions of both whole kidney and inner medulla from CCl4-induced cirrhotic rats with hyponatremia and ascites was unchanged[49].
In clinical investigations, Ivarsen et al[50] showed that urinary AQP-2 excretion was increased in parallel with impairment of free water clearance with the progression of liver cirrhosis, although it was not related to plasma AVP levels. They concluded that there is a functional association between increased AQP-2 excretion and increased renal reabsorption of water in cirrhosis. Chung et al[51] also reported that urinary AQP-2 secretion was increased in patients with liver cirrhosis, especially in those with ascites. In contrast to these results, Esteva-Font et al[52] found a progressive decrease in urinary AQP-2 excretion as the progression of liver cirrhosis, from compensated cirrhosis to cirrhosis with ascites and hepatorenal syndrome. Plasma AVP level did not correlate with urinary AQP-2 excretion and markedly increased in those with hepatorenal syndrome[52]. Krag et al[30] reported that plasma AVP level was suppressed but AQP-2 excretion was unchanged and urine volume, free water clearance and distal fractional water excretion were not increased after water load in Child C patients. These discrepancies between plasma AVP level and urinary AQP-2 excretion support an uncoupling of AVP/AQP-2 system attributable to a AVP-independent production of AQP-2 in decompensated liver cirrhosis[30]. The regulation of AQP-2 may be modified by a number of hormones and bioactive substances, such as angiotensin II, aldosterone, dopamine, atrial natriuretic peptide, PGE2 and adrenergic hormones[30]. The lack of responsiveness of the collecting ducts to changes in the plasma AVP could explain why a number of patients respond poorly to vaptans[30].
TREATMENT BY VASOPRESSIN RECEPTOR ANTAGONIST
Given the central role of AVP in limiting renal water excretion, AVP receptor antagonists represent a physiologic and rational method to increase renal water excretion[43]. Several vasopressin receptor antagonists have been evaluated in treating hyponatremia in patients with cirrhosis/end-stage liver disease[53]. These include the intravenous dual V1A/V2 -receptor antagonist conivaptan, and the oral V2 -receptor antagonists lixivaptan, RWJ-351647, satavaptan, and tolvaptan[53]. Main characteristics of randomized double-blind placebo-controlled trials on vaptans for patients with liver cirrhosis are listed in Table 1.
Table 1.
Main characteristics of randomized double-blind, placebo-controlled trials on vaptans for patients with liver cirrhosis
| Ref. | V2RA | Dose | Additional diuretics | Max treatment duration | No of patients vaptan(control) | Efficacy outcome | Main results | |
| Guyader et al[54] | Lixivaptan | 25, 50, 100, 200, 300 mg/d | None (withheld for 48 h) | 24 h | 22 (5) | Daily urine output, urine osmolality, serum osmolality, serum Na | A significant dose-related increase in daily urine output and a dose-related decrease in urine osmolality together with significant increases in serum osmolality, Na, and vasopressin levels | |
| Gerbes et al[55] | Lixivaptan | 100, 200 mg/d | Yes? (no detailed information available) | 7 d | 40 (20) | Serum sodium concentration, urine osmolality, body weight | Normalization of serum Na 27% (100 mg/d group) 50% (200 mg/d group); a significant reduction in urine osmolality and body weight | Fluid intake was restricted to 1000 mL/d |
| Wong et al[56] | Lixivaptan | 50, 250, 500 mg/d | Yes | 8 d | 25 (8) | Free water clearance, serum sodium | Significant dose related increases in free water clearance and serum Na without changes in orthostatic blood pressure and serum creatinine levels | Fluid intake was restricted to 1500 mL/d |
| Schrier et al[63] | Tolvaptan | 15, 30, 60 mg/d | Yes | 30 d | 63 (57) Salt 1 and Salt 2 study including other causes of hyponatremia | Serum Na | Effective in increasing serum Na concentrations at day 4 and day 30 | |
| Thuluvath et al[58] | RWJ-351647 | 1, 2, 5 mg (single oral doses) | Spironolactone 100 mg/d + furosemide 40 mg/d | 24 h | 18 (6) | Urine volume, free water excretion, urine osmolality | Increases in cumulative urine volume and free water excretion, and a decrease in urine osmolality were noted in a dose-dependent manner reaching the statistical significance at the 5-mg dose | No changes in either serum chemistry or plasma AVP and renin levels |
| Ginès et al[59] | Satavaptan | 5, 12.5, 25 mg/d | Spironolactone 100 mg/d | 14 d | 82 (28) | Body weight, abdominal girth, serum Na | Reduction in body weight and abdominal girth with improvements in serum Na | |
| Ginès et al[60] | Satavaptan | 5, 12.5, 25 mg/d | Spironolactone 100 mg/d + furosemide 20-25 mg/d | 14 d | 113 (35) | Change in body weight The percentage of patients with a weight loss > 2 kg | Significant reduction in body weight; percentage of patients with a weight loss > 2 kg was greater | |
| Wong et al[61] | Satavaptan | 5, 12.5, 25 mg/d | Spironolactone 100 mg/d | 12 wk | 115 (36) | Prevention of recurrent ascites after LVP (1) median time to first paracentesis (2) frequency of paracenteses (3) mean increase in ascites | Significant decrease in the frequency of paracenteses; No significant difference of mean increase in ascites | |
| Cárdenas et al[64] | Tolvaptan | 15, 30, 60 mg/d | Spironolactone < 200 mg/d + furosemide < 80 mg/d | 30 d | 63 (57) | Serum Na (average daily area under the curve for serum Na); mental component summary scores of the SF-12 health survey | Improvement in serum Na levels and patient-reported health status without severe adverse effects | Sub-analysis of the SALT-1 and SALT-2 trials |
| Wong et al[62] | Satavaptan | 5, 10 mg/d | Study 1 (None); Study 2 (one or more diuretics); Study 3 (withheld during the first 12 wk) | 52 wk | 720 (478) | Prevention of recurrent ascites after LVP (1) cumulative number of LVPs during the first 12 wk (2) reccurrence of ascites, defined as LVP and/or weight increase of > 4 kg (3) cumulative increase in ascites estimated | Not more effective than placebo in the control of ascites in any of the populations studied as estimated by the primary efficacy endpoints; slight advantages noted in delaying ascites formation and improvement of serum Na concentration in patients with hyponatremia | (Study 3) a higher rate of all-cause mortality, mostly associated with complications of cirrhosis in combination with diuretics |
| Okita et al[67] | Tolvaptan | 7.5, 15 or 30 mg/d | Furosemide ≥ 40 mg/d + spironolactone ≥ 25 mg/d or furosemide ≥ 20 mg/d + spironolactone ≥ 50 mg/d | 7 d | 77 (27) | Body weight, abdominal circumference | 7.5-30 mg/d reduced body weight and abdominal circumference 7.5 mg/d showed the maximum changes together with preferable tolerability | |
| Sakaida et al[68] | Tolvaptan | 7.5 mg/d | furosemide ≥ 40 mg/d + spironolactone ≥ 25 mg/d or furosemide ≥ 20 mg/d + spironolactone ≥ 50 mg/d | 7 d | 84 (80) | Change in body weight from baseline; changes in abdominal circumference and ascites volume: improvement rates of lower limb edema and ascites-related clinical symptoms | change in body weight; -0.44 kg in the placebo group vs -1.95 kg in the tolvaptan group; higher improvement rates of limb edema and ascites-related clinical symptoms | Improve hyponatremia and derease body weight regardless of serum albumin level |
All major studies are summarized in this table. In general, short-term effects of vaptans on hyponatremia and ascites are evident. Long-term effects are still controversial.
Lixivaptan
Guyader et al[54] first reported the randomized double-blind, placebo-controlled trial on the effect of V2-receptor antagonist lixivaptan (VPA-985; ascending single doses of 25, 50, 100, 200, and 300 mg) in patients with liver cirrhosis and ascites and found a dose-related increase in daily urine output and a dose-related decrease in urine osmolality together with increases in serum osmolality and Na concentration for 24 h[54]. Gerbes et al[55] studied the effects of lixivaptan (100 or 200 mg/d) or placebo in cirrhotics with ascites and hyponatremia (serum Na 115-132 mmol/L) in a double-blind controlled trial. The authors found that normalization of serum Na concentration was achieved in 27% and 50% of patients in the lixivaptan 100 mg/d and 200 mg/d groups, respectively, but in none of the patients in the placebo group. Although the effect of lixivaptan on ascites was not described in the report, a significant decrease of body weight was recorded in subjects receiving lixivaptan 200 mg/d[55]. Wong et al[56] investigated the add-on effects of lixivaptan (doses of 25, 125, and 250 mg twice daily) or placebo to diuretics (mostly furosemide and spironolactone) on cirrhotics with ascites and hyponatremia (< 130 mmol/L) and found that lixivaptan produced a significant overall aquaretic response with significant dose related increases in free water clearance and serum Na.
Lixivaptan was also shown to be effective and safe for long-term management of hyponatremia after outpatient initiation[2,57]. Normalization of serum Na levels was maintained during the 24-wk study period. It was titrated safely in the outpatient setting without over-rapid serum Na level correction[2,57].
RWJ-351647
Thuluvath et al[58] evaluated the effect of another selective V2-receptor antagonist RWJ-351647 in cirrhotics with ascites, who failed to lose ≥ 2 kg weight in the week by the diuretic regimen of furosemide (40 mg/d) and spironolactone (100 mg/d), as a randomized double-blind, placebo-controlled trial. They found that this vaptan is an effective aquaretic causing dose-dependent increases in urine output and free water clearance, when co-administered with conventional diuretics[58]. There was no influence on the pharmacokinetics of concomitant furosemide and spironolactone in their study[58].
Satavaptan
Gines et al[59,60] investigated the effects of satavaptan on ascites management and serum Na in decompensated cirrhotic patients with and without hyponatremia. They first compared the effect of three fixed doses of satavaptan (5, 12.5 or 25 mg once daily) versus placebo for 14 d in 110 cirrhotic patients with ascites and hyponatremia (serum Na ≤ 130 mmol/L), who continuously received spironolactone at 100 mg/d[59]. The authors reported that satavaptan was effective for control of ascites (indicated by a reduction in body weight and abdominal girth), which was associated with improvements in serum Na and concluded that this vaptan improves the control of ascites and hyponatremia in patients with cirrhosis under diuretic treatment[59]. They next evaluated the effect of satavaptan on ascites in 148 cirrhotics without hyponatremia as the similar double-blind randomized-controlled trial[60]. Duration of treatment was also 14 d but all patients received spironolactone 100 mg/d plus furosemide 20-25 mg/d. As a result, the administration of satavaptan was associated with reduction of ascites in cirrhotics without hyponatraemia[60].
On the other side, Wong et al[61] evaluated the long-term effect of satavaptan on the recurrence of ascites and survival. They first performed a double-blind placebo-controlled trial investigating the effects of the addition of satavaptan (5, 12.5 or 25 mg) or placebo to 100 mg spironolactone for 12 wk on ascites recurrence after a large volume paracentesis in 151 patients with liver cirrhosis irrespective of the presence of hyponatraemia[61]. Although the frequency of paracentesis was decreased significantly in all satavaptan groups versus placebo, no significant difference of mean increase in ascites was found among the groups[61]. They further evaluated the efficacy and safety of satavaptan in three different populations of patients with cirrhosis and ascites by large-scale randomized, placebo-controlled trials[62]. They included 1200 patients in three studies comparing satavaptan with placebo in uncomplicated ascites (study 1) and difficult-to-treat ascites, with and without concomitant diuretic treatment (studies 2 and 3). They reported that satavaptan was not more effective than placebo in the control of ascites in any of the populations studied as estimated by the primary efficacy endpoints: worsening of ascites (study 1) and the cumulative number of large-volume paracentesis during 12 wk (studies 2 and 3)[62]. However, as for secondary efficacy endpoints, slight advantages of satavaptan over placebo were noted in delaying ascites formation and improving serum Na concentration in patients with hyponatremia[61]. They finally concluded that satavaptan, alone or in combination with diuretics, is not clinically beneficial in the long-term management of ascites in cirrhosis[62]. Moreover, when satavaptan was administered in combination with diuretics to prevent ascites recurrence after large-volume paracentesis (study 3), a higher rate of all-cause mortality, mostly associated with known complications of cirrhosis, was recorded during the 52 wk of follow-up[62]. These limited efficacy and safety concerns resulted in withdrawal of the drug by the pharmaceutical company[33,53].
Tolvaptan
Shrier et al[63] reported the effect of another V2-receptor antagonist tolvaptan for 448 patients with hyponatremia of variable etiology as two double-blind placebo-controlled studies (Study of Ascending Levels of Tolvaptan in Hyponatremia1 and 2 [SALT-1 and SALT-2]). The subjects included 138 patients with congestive heart failure, 190 patients with SIADH and 120 patients with liver cirrhosis[63]. The authors did not evaluate the results separately based on etiology, but simply showed that 15 mg of oral tolvaptan (increased to 30 or 60 mg if needed) was effective in increasing serum Na concentrations in these patients with euvolemic or hypervolemic hyponatremia[63]. Cárdenas et al[64] then performed sub-analysis of these SALT-1 and SALT-2 trials evaluating the efficacy and safety of tolvaptan in patients with cirrhosis and hyponatremia and found improvement in serum Na levels and patient-reported health status without severe adverse effects[64]. Additionally, hyponatremia recurred in tolvaptan-treated patients after discontinuation of tolvaptan[64].
The SALTWATER trial, an open-label extension of SALT 1 and SALT 2, enrolled 111 individuals including 20 cirrhotics to evaluate whether tolvaptan remained safe and effective during long-term use[2,65]. Initial dose was 15 mg, which was increased to attain a normal serum Na level[2,65]. At 50 wk, the serum Na concentration normalized in approximately 60% of patients[41,65]. During the more than 4-year study period, only one required withdrawal from the study. As in the short-term trials, there were no reported adverse effects concerning for osmotic demyelination[2,65].
In a Japanese pilot study[66], tolvaptan was administrated at titrated doses of 15, 30, and 60 mg once daily for 3 d at each dose to 18 cirrhotic patients with persistent ascites and/or lower limb edema despite receiving oral furosemide at 40 mg/d or higher. In this study, tolvaptan was proved to decrease body weight and abdominal circumference dose-dependently and to improve ascites and edema beginning from 15 mg[66]. Japanese study group then designed a 7-d multicenter double-blind trial of tolvaptan to determine the optimal dose for hepatic edema[67]. One hundred four participants were stratified randomly to four groups receiving tolvaptan at 7.5, 15 or 30 mg/d, or placebo as an add-on to conventional diuretics for 7 d. The subjects selected were poor responders to the standard daily dose of concomitant diuretics: (1) a loop diuretic at a daily dose equivalent to furosemide 40 mg/d or higher and spironolactone at 25 mg/d or higher; or (2) a loop diuretic at a daily dose equivalent to furosemide 20 mg/d or higher and spironolactone at 50 mg/d or higher[67]. Although tolvaptan at 7.5-30 mg/d reduced body weight and abdominal circumference compared with placebo, dose of 7.5 mg/d showed the maximum changes together with preferable tolerability[67]. From these results, 7.5 mg/d was considered the optimal dose in patients with liver cirrhosis and ascites, who showed inadequate response to conventional diuretics in Japan[67]. This conclusion was verified by another multicenter double-blind placebo-controlled trial by Sakaida et al[68], by which tolvaptan is proved to improve hyponatremia and to exert its effect on body weight and initial urine volume of the patients regardless of serum albumin level[68,69]. They also doubled the duration of tolvaptan treatment and showed that it’s effect on body weight persisted for 14 d[70]. In order to avoid adverse effects of tolvaptan in cirrhotics, Sakaida et al[71] further tried to decrease the dosage of tolvaptan and compared 3.75 mg/d and 7.5 mg/d schedules by a double-blind, parallel-group study. Although tolvaptan resulted in dose-dependent decreases in body weight and ascites volume and increases in urine output, 3.75 mg/d tolvaptan exerted significant effects as well[71].
Conivaptan
In patients with Child-Pugh class A-C cirrhosis, conivaptan is administered via an intravenous loading dose of 10 mg followed by continuous infusion of 10 mg over 24 h for 2 to 4 d with titration up to 20 mg over 24 h if serum Na is not rising at the desired rate[53].
A retrospective review of 24 cirrhotic patients with hyponatremia showed that it raised serum Na in patients with and without diuretics[53,72]. Because conivaptan is available only as a parenteral formulation, chronic use in cirrhotic patients with hyponatremia may be limited. Theoretically, it should be cautious to use conivaptan in patients with cirrhosis because it blocks both V2 and V1 A receptors[53,73]. There is a possibility that V1A inhibition may result in splanchnic vasodilation, which leads to further reduction in blood pressure and increased risk of variceal bleeding[33]. Dilatation of the splanchnic bed and interference with platelet aggregation could exacerbate complications of variceal bleeding[41].
AREAS OF UNCERTAINTY
It is not determined if V2-receptor antagonists are helpful for asymptomatic hyponatremic patients[43]. It is also undetermined if early use of vaptans prevent diuretic-induced hyponatremia in cirrhotics. Although it remains unclear whether correction of the hyponatremia per se will improve patient outcomes, V2-receptor antagonist offer an opportunity to test this uncertainty in patients with euvolemic and hypervolemic hyponatremia[43].
So far no study has revealed that the effects of vaptans are related to plasma AVP levels in patients with liver cirrhosis. As plasma AVP levels are considered to be normal in cirrhotics without hyponatremia, the reported effects of tolvaptan in these patients may be related to hypersensitivity of V2-receptor to AVP as noted in congestive heart failure[74]. In fact, the effect of spironolactone for cirrhotics with normal plasma aldosterone levels have been explained by the renal tubular hypersensitivity to aldosterone[75].
Proper indication of vaptans
Hospitalized patients with mild to moderate symptoms of hyponatremia can be considered ideal candidates for the use of vaptans. Although restriction of fluid intake has been recommended in patients with severe hyponatremia, it has very limited efficacy for cirrhotic patients[1]. While it remains speculative as to whether correction of the hyponatremia per se will improve patient outcomes, the vaptans offer an opportunity to test this uncertainty in patients with euvolemic and hypervolemic hyponatremia[43]. One remaining question is if they are helpful in hyponatremic patients who are asymptomatic or mildly symptomatic[43]. Hyponatraemia with serum Na ≤ 130 mEq/L is one of several predictive factors for the development of overt hepatic encephalopathy[44,76]. Investigators have hypothesized that low-grade cerebral edema associated with hyponatremia may predispose cirrhotics to encephalopathy[53,77]. Treating hyponatremia is, therefore, considered to be a prudent step to reduce the frequency and severity of encephalopathy in cirrhosis[41]. Hyponatraemia in cirrhosis is associated with impaired cognition and poor health-related quality of life (HRQOL), which were recently proved to be improved by hyponatraemia correction by short-term tolvaptan therapy[78].
Dahl et al[79] made meta-analyses to evaluate the effects of vaptans (tolvaptan, satavaptan and lixivaptan) on patients with cirrhosis and hyponatraemia or ascites. As a result, they did not find clear differences between the vaptan groups and the control groups regarding mortality, variceal bleeding, hepatic encephalopathy, spontaneous bacterial peritonitis, hepatorenal syndrome, or renal failure, although vaptans increased serum Na levels and led to reductions in weight and the time to the first paracentesis. They admitted that vaptans have a small beneficial effect on hyponatremia and ascites but concluded that these data do not support the routine use of vaptans in cirrhosis[79]. Further studies about using different end-points such as hospitalizations for hyponatremia, need for more or less diuretics to control the ascites, and need for paracentesis, would better define how to use this new class of drugs in the patient with cirrhosis and ascites[80].
A recent FDA drug safety communication recommended that tolvaptan should not be used for longer than 30 d and should not be used in patients with underlying liver disease due to a risk of liver injury leading to liver transplant or death[81]. This arose from surveillance in a 3-year placebo-controlled study of 1400 patients with autosomal dominant polycystic kidney disease. Among them 3 patients treated with tolvaptan at a dose of 120 mg/d developed significant increases in serum ALT and total serum bilirubin levels[81]. No vasopressin receptor antagonist is currently approved by the FDA for treatment of hyponatremia in patients with liver disease or cirrhosis[33].
Proper doses of vaptans
The effect of vaptans may be less robust in hyponatremia of cirrhosis compared with other causes[56,63,65]. A subgroup analysis of cirrhotic patients in the SALT trials also supported this observation[2]. Reasons for limited response to vaptans in some hyponatremic patients with advanced cirrhosis may be that avid proximal reabsorption of solute leads to decreased distal delivery of the glomerular filtrate, although this possibility has not been studied. Another possibility is that V2 receptor-independent pathways play a role in APQ-2 regulation in cirrhosis[2,30]. The difference in the doses of tolvaptan between international trials and Japanese trials are noticeable. Of course, it should be kept in mind that the primary efficacy outcomes in the Japanese studies were improvement of ascites and edema of the extremities and not that of severe hyponatremia, hence refractory ascites in end-stage cirrhotics was excluded from these studies. International tolvaptan trials used 15 to 60 mg/d in cirrhotics for decompensated cirrhotics with hyponatremia, whereas the doses of Japanese trials first settled between 7.5 to 30 mg/d[66] finally decreased to 3.75 and 7.5 mg for decompensated cirrhotics without hyponatremia[71]. This study, however, showed that tolvaptan induced significant increases in urine volume and significant decreases in body weight from basal levels for 4 d even in the 3.75 mg/d group[71]. Significant increase in serum Na concentrations within normal limits were noted in the 7.5 mg/d group[71]. Although the approved dose of tolvaptan for cirrhotics is 7.5 mg/d, intial dose of 3.75 mg/d is recommended for safety in advanced cirrhotics in Japan. Although the reason why some Japanese cirrhotic patients responded to such a low dose of tolvaptan as 3.75 to 7.5 mg/d is not clear, it is now advisable to use low-dose tolvaptan for cirrhotic ascites to prevent the risky liver injury warned by FDA[33].
Effect for refractory ascites
Zhang et al[82] recently reported that the combination of 15 mg/d tolvaptan with diuretics effectively increased the urine output in 89.7% of patients with refractory ascites. They selected the patients who were not satisfactorily controlled after either 1 wk of Na intake restrictions, albumin infusion and high doses of diuretics (more than 160 mg/d of furosemide and 200 mg/d of spironolactone) or 2 wk of large volume therapeutic paracentesis.
Patients with Child-Pugh scores of greater than 10 have been excluded in most tolvaptan trials except for this study[82]. The more severe forms of hyponatremia (Na < 125 mmol/L) are seen in patients with more advanced liver disease, and hence the safety and efficacy of vaptans in this group of patients with cirrhosis should be carefully examined[80]. It is conceivable that combining vaptans with diuretics could be beneficial in patients with refractory ascites reducing the frequency of large-volume paracentesis[28]. This hypothesis, however, was not validated with large-scale randomized controlled studies. We cannot generally recommend vaptans for all refractory ascites, until they are proved to be effective in the same kind of study that include only patients with refractory ascites.
A low serum Na level is a strong predictor of pre-transplant mortality, independent of the Model for End-stage Liver Disease score (MELD)[83]. Vaptans may have some merit in the management of patients before liver transplantation. This should be also evaluated in an adequate control study.
FUTURE POSSIBILITY OF ASCITES TREATMENT BY DIURETICS AND VAPTANS
The doses of diuretics in combination with vaptans have been reported to be variable; i.e., furosemide 20-80 mg/d and spironolactone 25-200 mg/d.
Large-doses of diuretics frequently lead difficult-to-treat hyponatremia, which disturbs further use of diuretics. Although the level of hyponatremia at which diuretics should be abandoned is contentious, most experts agree that they should temporarily stop diuretics in patients whose serum Na decreases to less than 120-125 mmol/L[25]. By an addition of vaptans we may continue diuretics to these severe hyponatremic patients with ascites, or we may prevent the development of severe hyponatremia itself in the diuretic treatment. It is advisable to evaluate in a prospective controlled study if we can decrease the incidence of diuretic-intractable ascites by an early combination of diuretics and vaptans. The fact that disturbed water excretion already exists in the early cirrhosis without hyponatremia may become the theoretical basis for this strategy.
Considering the mechanism of water excretion, markedly decreased GFR and distal delivery of fluid may cancel the effect of vaptans. Although we have no definite data yet, the effect of tolvaptan seems weak in patients with renal dysfunction related to the underfilling state. Except for Zhang et al[82]’s report, we have no evidence to say that vaptans benefit these advanced cases. More clinical data are needed to know if tolvaptan is really effective for difficult-to-treat ascites in patients with marked underfilling state. Although vaptans show much promise, further study is needed to clarify whether we can establish this drug in the outpatient setting as a combination with diuretics[80] and whether we can improve the morbidity and cost burden[2].
CONCLUSION
Although there is no evidence that correcting the serum Na influences the patient’s prognosis, it is clear that severe hyponatremia leads to hospitalization, discontinuation of diuretics and fluid restriction, all of which are undesirable outcomes[80]. Even if hyponatremia is not the direct cause of symptoms, it may lower the threshold for changes in mental status resulting from poor cerebral perfusion[43]. Therefore, meticulous use of vaptans may become a choice in the management of ascites and hyponatremia before considering large-volume paracentesis or TIPS. Although tolvaptan has a possibility to make a breakthrough in the treatment of difficult-to-treat ascites, its high price is a major barrier to go beyond for the future[2].
Considering that the use of vaptans is only a symptomatic therapy, we should make every effort to improve the backgrounds of water retention. There is now considerable evidence to suggest that long-term anti-HBV treatment can improve liver fibrosis in patients with advanced hepatitis B virus-related cirrhosis[84]. As endotoxemia and resultant increase of nitric oxide are important precipitating factors for the underfilling state of cirrhotics, adequate management of the gut-liver axis may merit the patients[85].
Footnotes
Conflict-of-interest statement: The author has received fees for serving as a speaker and a moderator of several meetings from Otsuka Pharmaceutical Co., Ltd.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 11, 2015
First decision: July 13, 2015
Article in press: September 15, 2015
P- Reviewer: Joh JW S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
References
- 1.Ginès P, Guevara M. Hyponatremia in cirrhosis: pathogenesis, clinical significance, and management. Hepatology. 2008;48:1002–1010. doi: 10.1002/hep.22418. [DOI] [PubMed] [Google Scholar]
- 2.Lehrich RW, Ortiz-Melo DI, Patel MB, Greenberg A. Role of vaptans in the management of hyponatremia. Am J Kidney Dis. 2013;62:364–376. doi: 10.1053/j.ajkd.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 3.Yi JH, Shin HJ, Kim HJ. V2 receptor antagonist; tolvaptan. Electrolyte Blood Press. 2011;9:50–54. doi: 10.5049/EBP.2011.9.2.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaamonde C. Renal water handling in liver disease. In: Epstein M, editor. The Kidney in Liver Disease. 4th ed. Philadelphia: Hanley and Belfus Inc; 1996. pp. 33–74. [Google Scholar]
- 5.Levy M. Pathophysiology of ascites formation. In: Epstein M, editor. The Kidney in Liver Disease. 3rd ed. Baltimore: Williams and Wilkins; 1988. pp. 209–243. [Google Scholar]
- 6.Epstein M. Renal sodium handling in liver disease. In: Epstein M, editor. The Kidney in Liver Disease. 4th ed. Philadelphia: Hanley and Belfus Inc; 1996. pp. 1–31. [Google Scholar]
- 7.Henriksen JH, Moller S. Hemodynamics, distribution of blood volume, and kinetics of vasoactive substances in cirrhosis. In: Epstein M, editor. The Kidney in Liver Disease. 4th ed. Philadelphia: Hanley and Belfus Inc; 1996. pp. 241–258. [Google Scholar]
- 8.Levy M. Pathophysiology of ascites formation. In: Epstein M, editor. The Kidney in Liver Disease. 4th ed. Philadelphia: Hanley and Belfus lnc; 1996. pp. 179–220. [Google Scholar]
- 9.Ginès P, Fernández-Esparrach G, Arroyo V, Rodés J. Pathogenesis of ascites in cirrhosis. Semin Liver Dis. 1997;17:175–189. doi: 10.1055/s-2007-1007196. [DOI] [PubMed] [Google Scholar]
- 10.Lieberman FL, Reynolds TB. Plasma volume in cirrhosis of the liver: its relation of portal hypertension, ascites, and renal failure. J Clin Invest. 1967;46:1297–1308. doi: 10.1172/JCI105622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong F, Girgrah N, Blendis L. Review: the controversy over the pathophysiology of ascites formation in cirrhosis. J Gastroenterol Hepatol. 1997;12:437–444. doi: 10.1111/j.1440-1746.1997.tb00463.x. [DOI] [PubMed] [Google Scholar]
- 12.Gentilini P, Laffi G, La Villa G, Raggi VC. Pathogenetic factors and clinical elements in ascites and hepatorenal syndrome during liver cirrhosis. Ann Ital Med Int. 1999;14:264–284. [PubMed] [Google Scholar]
- 13.Kostreva DR, Castaner A, Kampine JP. Reflex effects of hepatic baroreceptors on renal and cardiac sympathetic nerve activity. Am J Physiol. 1980;238:R390–R394. doi: 10.1152/ajpregu.1980.238.5.R390. [DOI] [PubMed] [Google Scholar]
- 14.Morita H, Matsuda T, Furuya F, Khanchowdhury MR, Hosomi H. Hepatorenal reflex plays an important role in natriuresis after high-NaCl food intake in conscious dogs. Circ Res. 1993;72:552–559. doi: 10.1161/01.res.72.3.552. [DOI] [PubMed] [Google Scholar]
- 15.Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodés J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151–1157. doi: 10.1002/hep.1840080532. [DOI] [PubMed] [Google Scholar]
- 16.Arroyo V, Fernández J. Renal dysfunction and pathogenesis and treatment of ascites and hepatorenal syndrome in cirrhosis. In: Chen CW, Cheng J, Gines P, Ouyang Q, Scholmerich J, et al., editors. Gut and Liver: Falk Symposium 174. Basel: Karger; 2011. pp. 102–112. [Google Scholar]
- 17.Gupta S, Morgan TR, Gordan GS. Calcitonin gene-related peptide in hepatorenal syndrome. A possible mediator of peripheral vasodilation? J Clin Gastroenterol. 1992;14:122–126. doi: 10.1097/00004836-199203000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Uemura M, Tsujii T, Kikuchi E, Fukui H, Tsukamoto N, Matsumura M, Fujimoto M, Koizumi M, Takaya A, Kojima H, et al. Increased plasma levels of substance P and disturbed water excretion in patients with liver cirrhosis. Scand J Gastroenterol. 1998;33:860–866. doi: 10.1080/00365529850171530. [DOI] [PubMed] [Google Scholar]
- 19.Kojima H, Tsujimoto T, Uemura M, Takaya A, Okamoto S, Ueda S, Nishio K, Miyamoto S, Kubo A, Minamino N, et al. Significance of increased plasma adrenomedullin concentration in patients with cirrhosis. J Hepatol. 1998;28:840–846. doi: 10.1016/s0168-8278(98)80235-3. [DOI] [PubMed] [Google Scholar]
- 20.Gerbes AL, Arendt RM, Paumgartner G. Atrial natriuretic factor. Possible implications in liver disease. J Hepatol. 1987;5:123–132. doi: 10.1016/s0168-8278(87)80070-3. [DOI] [PubMed] [Google Scholar]
- 21.Fukui H, Tsujii T, Matsumura M, Okamoto S, Morita T, Koizumi M, Morimura M, Kojima H, Hokaze Y, Fujimoto M. Plasma levels of atrial natriuretic peptide in patients with liver cirrhosis and its relation to ascites and renal function. Gastroenterol Jpn. 1989;24:149–155. doi: 10.1007/BF02774189. [DOI] [PubMed] [Google Scholar]
- 22.Angeli P, Fernández-Varo G, Dalla Libera V, Fasolato S, Galioto A, Arroyo V, Sticca A, Guarda S, Gatta A, Jiménez W. The role of nitric oxide in the pathogenesis of systemic and splanchnic vasodilation in cirrhotic rats before and after the onset of ascites. Liver Int. 2005;25:429–437. doi: 10.1111/j.1478-3231.2005.01092.x. [DOI] [PubMed] [Google Scholar]
- 23.Shoji H, Minamino N, Kangawa K, Matsuo H. Endotoxin markedly elevates plasma concentration and gene transcription of adrenomedullin in rat. Biochem Biophys Res Commun. 1995;215:531–537. doi: 10.1006/bbrc.1995.2497. [DOI] [PubMed] [Google Scholar]
- 24.Chu CJ, Lee FY, Wang SS, Lu RH, Tsai YT, Lin HC, Hou MC, Chan CC, Lee SD. Hyperdynamic circulation of cirrhotic rats with ascites: role of endotoxin, tumour necrosis factor-alpha and nitric oxide. Clin Sci (Lond) 1997;93:219–225. doi: 10.1042/cs0930219. [DOI] [PubMed] [Google Scholar]
- 25.European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417. doi: 10.1016/j.jhep.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Takaya A, Fukui H, Matsumura M, Uemura M, Kojima H, Okamoto S, Tsujii T. Stepped care medical treatment for cirrhotic ascites: analysis of factors influencing the response to treatment. J Gastroenterol Hepatol. 1995;10:30–35. doi: 10.1111/j.1440-1746.1995.tb01043.x. [DOI] [PubMed] [Google Scholar]
- 27.Fukui H, Uemura M, Tsujii T. Pathopjysiology and treatment of cirrhotic ascites. In: Yamanaka M, Toda G, Tanaka T, et al., editors. Liver cirrhosis update. Amsterdam: Elsevier; 1998. pp. 63–76. [Google Scholar]
- 28.Salerno F, Guevara M, Bernardi M, Moreau R, Wong F, Angeli P, Garcia-Tsao G, Lee SS. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int. 2010;30:937–947. doi: 10.1111/j.1478-3231.2010.02272.x. [DOI] [PubMed] [Google Scholar]
- 29.Gatta A, Caregaro L, Angeli P, Merkel C, Menon F, Rondana M, Ruol A. Impaired renal water excretion in liver cirrhosis. The role of reduced distal delivery of sodium. Scand J Gastroenterol. 1988;23:523–528. doi: 10.3109/00365528809093905. [DOI] [PubMed] [Google Scholar]
- 30.Krag A, Møller S, Pedersen EB, Henriksen JH, Holstein-Rathlou NH, Bendtsen F. Impaired free water excretion in child C cirrhosis and ascites: relations to distal tubular function and the vasopressin system. Liver Int. 2010;30:1364–1370. doi: 10.1111/j.1478-3231.2010.02319.x. [DOI] [PubMed] [Google Scholar]
- 31.Caregaro L, Lauro S, Angeli P, Merkel C, Gatta A. Renal water and sodium handling in compensated liver cirrhosis: mechanism of the impaired natriuresis after saline loading. Eur J Clin Invest. 1985;15:360–364. doi: 10.1111/j.1365-2362.1985.tb00285.x. [DOI] [PubMed] [Google Scholar]
- 32.Madsen M, Pedersen EB, Danielsen H, Jensen LS, Sørensen SS. Impaired renal water excretion in early hepatic cirrhosis. Lack of relationship between renal water excretion and plasma levels of arginine vasopressin, angiotensin II, and aldosterone after water loading. Scand J Gastroenterol. 1986;21:749–755. doi: 10.3109/00365528609011112. [DOI] [PubMed] [Google Scholar]
- 33.John S, Thuluvath PJ. Hyponatremia in cirrhosis: pathophysiology and management. World J Gastroenterol. 2015;21:3197–3205. doi: 10.3748/wjg.v21.i11.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pérez-Ayuso RM, Arroyo V, Camps J, Rimola A, Gaya J, Costa J, Rivera F, Rodés J. Evidence that renal prostaglandins are involved in renal water metabolism in cirrhosis. Kidney Int. 1984;26:72–80. doi: 10.1038/ki.1984.136. [DOI] [PubMed] [Google Scholar]
- 35.Castellano G, Solis-Herruzo JA, Morillas JD, Larrodera L, Coca C, Gonzalez-Gamarra A, Muñoz-Yagüe T. Antidiuretic hormone and renal function after water loading in patients with cirrhosis of the liver. Scand J Gastroenterol. 1991;26:49–57. doi: 10.3109/00365529108996483. [DOI] [PubMed] [Google Scholar]
- 36.Jespersen B, Pedersen EB, Madsen M, Christensen P, Eiskjaer H, Leyssac PP, Sørensen SS. Disturbed relationship between urinary prostaglandin E2 excretion, plasma arginine vasopressin and renal water excretion after oral water loading in early hepatic cirrhosis. Eur J Clin Invest. 1988;18:202–206. doi: 10.1111/j.1365-2362.1988.tb02414.x. [DOI] [PubMed] [Google Scholar]
- 37.Nicholls KM, Shapiro MD, Groves BS, Schrier RW. Factors determining renal response to water immersion in non-excretor cirrhotic patients. Kidney Int. 1986;30:417–421. doi: 10.1038/ki.1986.200. [DOI] [PubMed] [Google Scholar]
- 38.Bichet DG, Groves BM, Schrier RW. Mechanisms of improvement of water and sodium excretion by immersion in decompensated cirrhotic patients. Kidney Int. 1983;24:788–794. doi: 10.1038/ki.1983.229. [DOI] [PubMed] [Google Scholar]
- 39.Epstein M, Weitzman RE, Preston S, DeNunzio AG. Relationship between plasma arginine vasopressin and renal water handling in decompensated cirrhosis. Miner Electrolyte Metab. 1984;10:155–165. [PubMed] [Google Scholar]
- 40.Uemura M, Matsumoto M, Tsujii T, Fukui H, Miyamoto Y, Kojima H, Kikuchi E, Fukui K, Fujimoto M, Mitoro A, et al. Effects of “body compression” on parameters related to ascites formation: therapeutic trial in cirrhotic patients. J Gastroenterol. 1999;34:75–82. doi: 10.1007/s005350050219. [DOI] [PubMed] [Google Scholar]
- 41.Aditya S, Rattan A. Vaptans: A new option in the management of hyponatremia. Int J Appl Basic Med Res. 2012;2:77–83. doi: 10.4103/2229-516X.106347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schrier RW. Water and sodium retention in edematous disorders: role of vasopressin and aldosterone. Am J Med. 2006;119:S47–S53. doi: 10.1016/j.amjmed.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 43.Palmer BF. The role of v2 receptor antagonists in the treatment of hyponatremia. Electrolyte Blood Press. 2013;11:1–8. doi: 10.5049/EBP.2013.11.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gianotti RJ, Cardenas A. Hyponatraemia and cirrhosis. Gastroenterol Rep (Oxf) 2014;2:21–26. doi: 10.1093/gastro/got037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lolait SJ, O’Carroll AM, Mahan LC, Felder CC, Button DC, Young WS, Mezey E, Brownstein MJ. Extrapituitary expression of the rat V1b vasopressin receptor gene. Proc Natl Acad Sci USA. 1995;92:6783–6787. doi: 10.1073/pnas.92.15.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pedersen RS, Bentzen H, Bech JN, Nyvad O, Pedersen EB. Urinary aquaporin-2 in healthy humans and patients with liver cirrhosis and chronic heart failure during baseline conditions and after acute water load. Kidney Int. 2003;63:1417–1425. doi: 10.1046/j.1523-1755.2003.00858.x. [DOI] [PubMed] [Google Scholar]
- 47.Ishikawa SE, Schrier RW. Pathophysiological roles of arginine vasopressin and aquaporin-2 in impaired water excretion. Clin Endocrinol (Oxf) 2003;58:1–17. doi: 10.1046/j.1365-2265.2003.01647.x. [DOI] [PubMed] [Google Scholar]
- 48.Asahina Y, Izumi N, Enomoto N, Sasaki S, Fushimi K, Marumo F, Sato C. Increased gene expression of water channel in cirrhotic rat kidneys. Hepatology. 1995;21:169–173. [PubMed] [Google Scholar]
- 49.Jonassen TE, Christensen S, Kwon TH, Langhoff S, Salling N, Nielsen S. Renal water handling in rats with decompensated liver cirrhosis. Am J Physiol Renal Physiol. 2000;279:F1101–F1109. doi: 10.1152/ajprenal.2000.279.6.F1101. [DOI] [PubMed] [Google Scholar]
- 50.Ivarsen P, Frøkiaer J, Aagaard NK, Hansen EF, Bendtsen F, Nielsen S, Vilstrup H. Increased urinary excretion of aquaporin 2 in patients with liver cirrhosis. Gut. 2003;52:1194–1199. doi: 10.1136/gut.52.8.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chung SH, Jun DW, Kim KT, Chae JD, Park EK, Son BK, Kim SH, Jo YJ, Park YS. Aquaporin-2 urinary excretion in cirrhosis: relationship to vasopressin and nitric oxide. Dig Dis Sci. 2010;55:1135–1141. doi: 10.1007/s10620-009-0829-x. [DOI] [PubMed] [Google Scholar]
- 52.Esteva-Font C, Baccaro ME, Fernández-Llama P, Sans L, Guevara M, Ars E, Jiménez W, Arroyo V, Ballarín JA, Ginès P. Aquaporin-1 and aquaporin-2 urinary excretion in cirrhosis: Relationship with ascites and hepatorenal syndrome. Hepatology. 2006;44:1555–1563. doi: 10.1002/hep.21414. [DOI] [PubMed] [Google Scholar]
- 53.Gaglio P, Marfo K, Chiodo J. Hyponatremia in cirrhosis and end-stage liver disease: treatment with the vasopressin V2-receptor antagonist tolvaptan. Dig Dis Sci. 2012;57:2774–2785. doi: 10.1007/s10620-012-2276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guyader D, Patat A, Ellis-Grosse EJ, Orczyk GP. Pharmacodynamic effects of a nonpeptide antidiuretic hormone V2 antagonist in cirrhotic patients with ascites. Hepatology. 2002;36:1197–1205. doi: 10.1053/jhep.2002.36375. [DOI] [PubMed] [Google Scholar]
- 55.Gerbes AL, Gülberg V, Ginès P, Decaux G, Gross P, Gandjini H, Djian J. Therapy of hyponatremia in cirrhosis with a vasopressin receptor antagonist: a randomized double-blind multicenter trial. Gastroenterology. 2003;124:933–939. doi: 10.1053/gast.2003.50143. [DOI] [PubMed] [Google Scholar]
- 56.Wong F, Blei AT, Blendis LM, Thuluvath PJ. A vasopressin receptor antagonist (VPA-985) improves serum sodium concentration in patients with hyponatremia: a multicenter, randomized, placebo-controlled trial. Hepatology. 2003;37:182–191. doi: 10.1053/jhep.2003.50021. [DOI] [PubMed] [Google Scholar]
- 57.Abraham WT, Decaux G, Josiassen RC, Yagil Y, Kopyt N, Thacker HP, Mannelli M, Bichet DG, Orlandi C. Oral lixivaptan effectively increases serum sodium concentrations in outpatients with euvolemic hyponatremia. Kidney Int. 2012;82:1215–1222. doi: 10.1038/ki.2012.274. [DOI] [PubMed] [Google Scholar]
- 58.Thuluvath PJ, Maheshwari A, Wong F, Yoo HW, Schrier RW, Parikh C, Steare S, Korula J. Oral V2 receptor antagonist (RWJ-351647) in patients with cirrhosis and ascites: a randomized, double-blind, placebo-controlled, single ascending dose study. Aliment Pharmacol Ther. 2006;24:973–982. doi: 10.1111/j.1365-2036.2006.03088.x. [DOI] [PubMed] [Google Scholar]
- 59.Ginès P, Wong F, Watson H, Milutinovic S, del Arbol LR, Olteanu D. Effects of satavaptan, a selective vasopressin V(2) receptor antagonist, on ascites and serum sodium in cirrhosis with hyponatremia: a randomized trial. Hepatology. 2008;48:204–213. doi: 10.1002/hep.22293. [DOI] [PubMed] [Google Scholar]
- 60.Ginès P, Wong F, Watson H, Terg R, Bruha R, Zarski JP, Dudley F. Clinical trial: short-term effects of combination of satavaptan, a selective vasopressin V2 receptor antagonist, and diuretics on ascites in patients with cirrhosis without hyponatraemia--a randomized, double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2010;31:834–845. doi: 10.1111/j.1365-2036.2010.04236.x. [DOI] [PubMed] [Google Scholar]
- 61.Wong F, Gines P, Watson H, Horsmans Y, Angeli P, Gow P, Minini P, Bernardi M. Effects of a selective vasopressin V2 receptor antagonist, satavaptan, on ascites recurrence after paracentesis in patients with cirrhosis. J Hepatol. 2010;53:283–290. doi: 10.1016/j.jhep.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 62.Wong F, Watson H, Gerbes A, Vilstrup H, Badalamenti S, Bernardi M, Ginès P. Satavaptan for the management of ascites in cirrhosis: efficacy and safety across the spectrum of ascites severity. Gut. 2012;61:108–116. doi: 10.1136/gutjnl-2011-300157. [DOI] [PubMed] [Google Scholar]
- 63.Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355:2099–2112. doi: 10.1056/NEJMoa065181. [DOI] [PubMed] [Google Scholar]
- 64.Cárdenas A, Ginès P, Marotta P, Czerwiec F, Oyuang J, Guevara M, Afdhal NH. Tolvaptan, an oral vasopressin antagonist, in the treatment of hyponatremia in cirrhosis. J Hepatol. 2012;56:571–578. doi: 10.1016/j.jhep.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 65.Berl T, Quittnat-Pelletier F, Verbalis JG, Schrier RW, Bichet DG, Ouyang J, Czerwiec FS. Oral tolvaptan is safe and effective in chronic hyponatremia. J Am Soc Nephrol. 2010;21:705–712. doi: 10.1681/ASN.2009080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Okita K, Sakaida I, Okada M, Kaneko A, Chayama K, Kato M, Sata M, Yoshihara H, Ono N, Murawaki Y. A multicenter, open-label, dose-ranging study to exploratively evaluate the efficacy, safety, and dose-response of tolvaptan in patients with decompensated liver cirrhosis. J Gastroenterol. 2010;45:979–987. doi: 10.1007/s00535-010-0240-6. [DOI] [PubMed] [Google Scholar]
- 67.Okita K, Kawazoe S, Hasebe C, Kajimura K, Kaneko A, Okada M, Sakaida I. Dose-finding trial of tolvaptan in liver cirrhosis patients with hepatic edema: A randomized, double-blind, placebo-controlled trial. Hepatol Res. 2014;44:83–91. doi: 10.1111/hepr.12099. [DOI] [PubMed] [Google Scholar]
- 68.Sakaida I, Kawazoe S, Kajimura K, Saito T, Okuse C, Takaguchi K, Okada M, Okita K. Tolvaptan for improvement of hepatic edema: A phase 3, multicenter, randomized, double-blind, placebo-controlled trial. Hepatol Res. 2014;44:73–82. doi: 10.1111/hepr.12098. [DOI] [PubMed] [Google Scholar]
- 69.Sakaida I, Nakajima K, Okita K, Hori M, Izumi T, Sakurai M, Shibasaki Y, Tachikawa S, Tsubouchi H, Oka H, et al. Can serum albumin level affect the pharmacological action of tolvaptan in patients with liver cirrhosis? A post hoc analysis of previous clinical trials in Japan. J Gastroenterol. 2015;50:1047–1053. doi: 10.1007/s00535-015-1052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sakaida I, Yamashita S, Kobayashi T, Komatsu M, Sakai T, Komorizono Y, Okada M, Okita K. Efficacy and safety of a 14-day administration of tolvaptan in the treatment of patients with ascites in hepatic oedema. J Int Med Res. 2013;41:835–847. doi: 10.1177/0300060513480089. [DOI] [PubMed] [Google Scholar]
- 71.Sakaida I, Yanase M, Kobayashi Y, Yasutake T, Okada M, Okita K. The pharmacokinetics and pharmacodynamics of tolvaptan in patients with liver cirrhosis with insufficient response to conventional diuretics: a multicentre, double-blind, parallel-group, phase III study. J Int Med Res. 2012;40:2381–2393. doi: 10.1177/030006051204000637. [DOI] [PubMed] [Google Scholar]
- 72.O’Leary JG, Davis GL. Conivaptan increases serum sodium in hyponatremic patients with end-stage liver disease. Liver Transpl. 2009;15:1325–1329. doi: 10.1002/lt.21836. [DOI] [PubMed] [Google Scholar]
- 73.Ali F, Guglin M, Vaitkevicius P, Ghali JK. Therapeutic potential of vasopressin receptor antagonists. Drugs. 2007;67:847–858. doi: 10.2165/00003495-200767060-00002. [DOI] [PubMed] [Google Scholar]
- 74.Wong BP, Wong NL. Renal hypersensitivity to vasopressin in congestive heart failure. Cardiology. 1998;90:20–27. doi: 10.1159/000006811. [DOI] [PubMed] [Google Scholar]
- 75.Decaux G, Hanson B, Cauchie P, Bosson D, Unger J. Relationship between aldosterone and sodium, potassium, and uric acid clearance in cirrhosis with and without ascites. Nephron. 1986;44:226–229. doi: 10.1159/000183991. [DOI] [PubMed] [Google Scholar]
- 76.Guevara M, Baccaro ME, Torre A, Gómez-Ansón B, Ríos J, Torres F, Rami L, Monté-Rubio GC, Martín-Llahí M, Arroyo V, et al. Hyponatremia is a risk factor of hepatic encephalopathy in patients with cirrhosis: a prospective study with time-dependent analysis. Am J Gastroenterol. 2009;104:1382–1389. doi: 10.1038/ajg.2009.293. [DOI] [PubMed] [Google Scholar]
- 77.Häussinger D, Schliess F. Pathogenetic mechanisms of hepatic encephalopathy. Gut. 2008;57:1156–1165. doi: 10.1136/gut.2007.122176. [DOI] [PubMed] [Google Scholar]
- 78.Ahluwalia V, Heuman DM, Feldman G, Wade JB, Thacker LR, Gavis E, Gilles H, Unser A, White MB, Bajaj JS. Correction of hyponatraemia improves cognition, quality of life, and brain oedema in cirrhosis. J Hepatol. 2015;62:75–82. doi: 10.1016/j.jhep.2014.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dahl E, Gluud LL, Kimer N, Krag A. Meta-analysis: the safety and efficacy of vaptans (tolvaptan, satavaptan and lixivaptan) in cirrhosis with ascites or hyponatraemia. Aliment Pharmacol Ther. 2012;36:619–626. doi: 10.1111/apt.12025. [DOI] [PubMed] [Google Scholar]
- 80.Boyer TD. Tolvaptan and hyponatremia in a patient with cirrhosis. Hepatology. 2010;51:699–702. doi: 10.1002/hep.23522. [DOI] [PubMed] [Google Scholar]
- 81.Kwo PY. Management of hyponatremia in clinical hepatology practice. Curr Gastroenterol Rep. 2014;16:382. doi: 10.1007/s11894-014-0382-4. [DOI] [PubMed] [Google Scholar]
- 82.Zhang X, Wang SZ, Zheng JF, Zhao WM, Li P, Fan CL, Li B, Dong PL, Li L, Ding HG. Clinical efficacy of tolvaptan for treatment of refractory ascites in liver cirrhosis patients. World J Gastroenterol. 2014;20:11400–11405. doi: 10.3748/wjg.v20.i32.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Habib S, Boyer TD. Vasopressin V2-receptor antagonists in patients with cirrhosis, ascites and hyponatremia. Therap Adv Gastroenterol. 2012;5:189–197. doi: 10.1177/1756283X12437357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brown A, Goodman Z. Hepatitis B-associated fibrosis and fibrosis/cirrhosis regression with nucleoside and nucleotide analogs. Expert Rev Gastroenterol Hepatol. 2012;6:187–198. doi: 10.1586/egh.12.4. [DOI] [PubMed] [Google Scholar]
- 85.Fukui H. Gut-liver axis in liver cirrhosis: How to manage leaky gut and endotoxemia. World J Hepatol. 2015;7:425–442. doi: 10.4254/wjh.v7.i3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Uchihara M, Izumi N, Sato C, Marumo F. Clinical significance of elevated plasma endothelin concentration in patients with cirrhosis. Hepatology. 1992;16:95–99. doi: 10.1002/hep.1840160117. [DOI] [PubMed] [Google Scholar]
- 87.Asbert M, Ginès A, Ginès P, Jiménez W, Clària J, Saló J, Arroyo V, Rivera F, Rodés J. Circulating levels of endothelin in cirrhosis. Gastroenterology. 1993;104:1485–1491. doi: 10.1016/0016-5085(93)90360-o. [DOI] [PubMed] [Google Scholar]
- 88.Tsai YT, Lin HC, Yang MC, Lee FY, Hou MC, Chen LS, Lee SD. Plasma endothelin levels in patients with cirrhosis and their relationships to the severity of cirrhosis and renal function. J Hepatol. 1995;23:681–688. doi: 10.1016/0168-8278(95)80034-4. [DOI] [PubMed] [Google Scholar]
- 89.Møller S, Emmeluth C, Henriksen JH. Elevated circulating plasma endothelin-1 concentrations in cirrhosis. J Hepatol. 1993;19:285–290. doi: 10.1016/s0168-8278(05)80584-7. [DOI] [PubMed] [Google Scholar]


