Abstract
Neuroendocrine differentiation in sporadic colorectal cancer has been recognized since decades, but its clinical impact is still controversially discussed. Detailed parameter analyses hint at the possibility that probably not neuroendocrine differentiation itself, but its association with poor grade of tumor differentiation, lymph node metastases, distant metastases and other unfavorable features contribute to worse clinical outcome. However, other studies deny a relationship between neuroendocrine differentiation and prognosis of colorectal cancer. This review elucidates, whether new insights into the origin of neuroendocrine differentiation in the intestinal epithelium, its regulation by mTOR pathway components and its possible link to the intestinal stem cell compartment could determine a role of neuroendocrine cells as prognostic marker and putative therapeutic target in sporadic colorectal cancer.
Keywords: Neuroendocrine differentiation, Colorectal cancer, mTOR pathway, Neuroendocrine tumorigenesis, Targeted therapy
Core tip: Neuroendocrine differentiation in sporadic colorectal cancer has been recognized since decades. In contrast to the clinico-pathologically well-defined pure neuroendocrine tumors and mixed adenoneuroendocrine carcinomas of the colon and rectum, the clinical impact of focal neuroendocrine differentiation in colorectal carcinomas is still controversially discussed. Further insights into the regulation of neuroendocrine differentiation by mTOR pathway components and recent knowledge about a link of enteroendocrine cells to the intestinal stem cell compartment hint at a role of neuroendocrine cells as prognostic marker and putative therapeutic target in sporadic colorectal cancer.
INTRODUCTION
Colorectal cancer (CRC) is the third most commonly diagnosed cancer and the fourth leading cause of cancer-related death worldwide[1]. More than 50% of patients with CRC experience recurrence or metastases despite of curative operations[2]. Cytotoxic drugs applied as monotherapy or combined with monoclonal antibodies targeting proangiogenic vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) have been shown to prolong survival[3,4]. However, a proportion of patients gain little or even no benefit from these therapeutic regimens[5]. These facts underline the need to increase knowledge about special phenotypes, somatic genetic alterations and signaling pathways, which can be translated into prognostic markers or new molecularly defined targets for therapy of CRC.
The recent approval of new drugs for the treatment of advanced pancreatic neuroendocrine tumors brought neuroendocrine differentiation of tumor tissue, also beyond the pancreaticobiliary tract, into the focus of oncologists[6-8]. Whereas pure neuroendocrine tumors (NET) of the gastrointestinal tract have been established as well defined entities, the prognostic and therapeutic relevance of neuroendocrine differentiation in sporadic colorectal cancer has been less extensively evaluated. Insights into the histogenesis, epidemiology and pathogenetic links to known cancer pathways are necessary to elucidate the sufficiency of neuroendocrine cells as prognostic marker or new target for the therapy of CRC.
ORIGIN OF ENTEROENDOCRINE CELLS
Enteroendocrine cells comprise approximately 1% of epithelial cells in the gastrointestinal system and represent the largest population of hormone-producing cells in the body[9].
Detailed investigation revealed that enteroendocrine precursor cells differentiate immediately from self-renewing Lgr5+ intestinal stem cells[10]. This lineage differentiation depends on a regulatory cascade involving Notch signaling[10], the hairy enhancer of split (HES) transcription repressor and proendocrine basic helix-loop-helix (bHLH) transcription factors[11]. Expression of proendocrine bHLH factors, which is positively influenced by inactivation of Notch signaling[11], enables cells to differentiate toward divergent subsets of mature hormone-producing endocrine cells[12,13]. Several key transcription factors are involved in the regulation of enteroendocrine cell differentiation[14], which takes place within the crypts. Completely differentiated enteroendocrine cells are mainly determined to migrate upward along the villus as mature hormone-producing cells[12,13]. However, a small population of enteroendocrine cells migrates downwards to the bottom of the crypt or stays localized at the crypt base[15], where they reside in a Wnt signaling active zone and express both stem and postmitotic mature endocrine cell markers[16]. In both, intestinal and enteroendorine cell populations, expression of stem cell markers and continuous exposure to Wnt signaling could be hallmarks of cells, which are susceptible to neoplastic transformation[16,17].
However, data from a mouse model indicate that probably not these terminally differentiated enteroendocrine cells, but their early precursors respond to abnormal Wnt signaling by developing serotonin-expressing adenomas of the small intestine[18]. The concept that exocrine and endocrine components of CRC have the same cellular origin is supported by studies on mixed adenoneuroendocrine carcinomas (MANEC) and neuroendocrine carcinomas with minor associated exocrine components, which could demonstrate that both components share somatic mutations in several genes as APC, TP53, DCC, KRAS, BRAF, ATM, CTNNB1, ERBB4, JAK3, KDR, RB1, BCL9, FOXP1[19-22] and display identical LOH pattern on different chromosome loci as 5q, 17p, 18q[23]. Evidence of an additional mutation (SMARCA4)[22] or LOH involving 6q, 11p, 18q, APC marker and chromosome 3[23] in the endocrine tumor cell population could indicate that this component corresponds to a higher grade transformation of the tumor[20].
According to these data from mouse and human models, higher grade tumor transformation via development of neuroendocrine compartments can occur in every stage of intestinal or colorectal carcinogenesis and, furthermore, it could be even therapy-related. Probably both, pre- and postoperative as well as cytotoxic and radiation therapy could induce trans-differentiation in carcinomas with completely developed phenotype as indicated by the finding of increased neuroendocrine cells in a subset of distant metastatic compared to primary colorectal carcinomas[24] and in neoadjuvant treated compared to untreated rectal carcinomas[25]. This trans-differentiation from non-neuroendocrine to neuroendocrine tumor cells has already been proven for prostate cancer in an androgen-deprived environment[26,27] and is essentially associated with the phosphatidylinositol 3-kinase-Akt-mammalian target of rapamycin (PI3K-Akt-mTOR) pathway[28]. Studies on both experimental and human sporadic neuroendocrine tumors (NETs) and on familial syndromes, in which NETs arise, point to the involvement of mTOR pathway components in neuroendocrine tumorigenesis in general[29]. Proven or putative activators of the mTOR pathway are mutations in upstream regulators of mTOR (PTEN and TSC2) and overexpression of a microRNA (miR-21) that targets PTEN and reduces its expression[29-31] as displayed in Figure 1 (modified according to Cingarlini et al[29] and McCubrey et al[32]). The association between neuroendocrine trans-differentiation and mTOR pathway could be important for new targeted therapy regimens as discussed later in this review.
Figure 1.

The PI3K/PTEN/Akt/mTOR-cascade (Modified according to Cingarlini et al[29] and McCubrey et al[32]). Phosphatidylinositol-3-kinase (PI3K) is a heterodimeric protein with an p85-kDA regulatory subunit and a p110α-kDa catalytic subunit (PIK3CA). PI3K phosphorylates membrane phospholipids, thereby forming the second messenger lipids phosphatidylinositol 3,4-biphosphate (PIP2) and phosphatidylinositol 3,4,5-triphosphate (PIP3). Pleckstrin-homology (PH) domain of kinase Akt binds to PIP3, thereby promoting activation of Akt via phosphorylation by phosphotidylinositide-dependent kinase 1 (PDK1, not displayed in the Figure). Akt inhibits tuberous sclerosis 2 (TSC2 or tuberin) function through direct phosphorylation. TSC2 phosphorylation by Akt represses activity of the TSC1/TSC2 complex, allowing the small GTPase Rheb to activate the protein kinase function of mTOR (mammalian target of rapamycin). mTOR forms the catalytic core of the mTORC1 complex, which comprises additionally Raptor (Regulatory associated protein of mTOR) adaptor protein, DEPTOR (DEP domain containing mTOR-interacting protein), mLST8 (member of the Lethal-with-Sec-Thirteen gene family), FKBP38 (FK506 binding protein 38), and PRAS40 (proline-rich Akt substrate 40 kDa protein). Activated mTOR phosphorylates p70S6K, which induces a negative feedback loop uncoupling IRS-1 (insulin receptor substrate-1) from PI3K, thus preventing further signal transduction through this pathway. Negative regulation of the PI3K pathway is primarily accomplished though the PTEN tumor suppressor protein, which dephosphorylates phosphoinositide substrates as PIP3. Expression of PTEN is regulated by microRNA miR-21.
NEUROENDOCRINE DIFFERENTIATION IN COLORECTAL TUMORS: DIAGNOSIS, CLASSIFICATION AND EPIDEMIOLOGY
Neuroendocrine cells in non-neoplastic and neoplastic tissue of the gastrointestinal tract and nerve elements express a panel of identical antigens, which are used as neuroendocrine markers[33]. The markers synaptophysin, chromogranin A, B and C, HISL-19, neuron-specific enolase (NSE), the proprotein convertases PC2 and PC3, the lymphoreticular epitope Leu-7, and the neural cell adhesion molecule (or CD56) are sufficient to reveal neuroendocrine differentiation[33,34] independent of hormone production[35]. An example for synaptophysin expression in a colorectal adenocarcinoma is displayed in Figure 2. In addition, syntaxin1, VAMP2, SNAP25, alpha/beta-SNAP[36] and L-dopa decarboxylase (DDC)[37] have been used as neuroendocrine markers. In the pre-immunohistochemistry era, the Churukian-Schenk argyrophil stain[38] and the Grimelius stain[39] were applied to demonstrate neuroendocrine cells, which are argyrophilic. The currently known 15 neuroendocrine cell types of the gastrointestinal tract and pancreas produce different hormones, but all of them express the general neuroendocrine marker synaptophysin[40].
Figure 2.
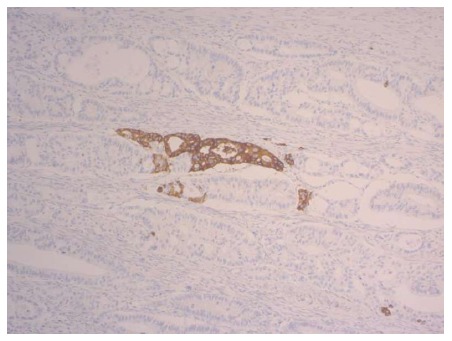
Primary colorectal cancer with focal synaptophysin expression (magnification × 200).
In accordance with the consensus guidelines of the European Neuroendocrine Tumor Society (ENETS)[41,42], the current WHO classification for gastrointestinal neuroendocrine tumors[43] applies a grading system based on mitotic activity and the percentage of Ki-67 labeled proliferating cells: Grade 1, grade 2 and grade 3 (= neuroendocrine carcinoma) are defined by mitotic counts of < 2/10 high power fields (HPF), 2-20/10 HPF and > 20/10 HPF, respectively, and/or by Ki-67 indices of ≤ 2%, 3%-20% and > 20%, respectively. A fourth group, mixed adenoneuroendocrine carcinoma (MANEC) is morphologically recognizable as both gland-forming epithelial and neuroendocrine phenotype, with each component representing at least 30% of the lesion[43]. An additional category considering neuroendocrine differentiation less than 30%, but above the level reported for normal colorectal epithelium (> 1 cell/mm2[44,45], > 2%[36]) similar to that proposed previously by Jansson et al[46] has not been defined by the current WHO classification[43]. However, a detailed study on colorectal tumors with mixed glandular-neuroendocrine differentiation[47] revealed that also neuroendocrine tumor components comprising less than the currently used 30% cut off could have negative impact on the clinical course and patient outcome, which sets the occasional finding of isolated neuroendocrine cells in colorectal cancer into a new focus.
In sporadic colorectal cancer, neuroendocrine cells have been identified in 8%-77.5% of cases[24,25,36,38,39,45,48-63], largely depending on the method used to assess the neuroendocrine cell population[24]. Neuroendocrine differentiation occurs also in hereditary non polyposis colorectal cancer (HNPCC, 51.4%)[64].
HISTOPATHOLOGICAL AND CLINICAL FEATURES OF SPORADIC COLORECTAL CANCER WITH NEUROENDOCRINE DIFFERENTIATION
Chromogranin A and synaptophysin are the most frequently used markers to study the link between neuroendocrine differentiation and clinicopathological characteristics. These markers are expressed in divergent patterns: Co-expression of chromogranin A and synaptophysin[63,65] occurs as well as predominance of one marker concomitant with absence of the other marker[65,66].
Studies focusing on the relationship between occasional neuroendocrine differentiation (i.e., < 30%) and clinicopathological parameters in sporadic colorectal cancer are summarized in Table 1[24,25,39-71]. Detailed parameter analyses hint at the possibility that not neuroendocrine differentiation itself, but its association with poor grade of tumor differentiation, lymph node metastases, distant metastases and other unfavorable features contribute to the worse prognosis of sporadic CRC with neuroendocrine differentiation, which has been reported by several authors (Table 1). However, other authors deny a relationship between neuroendocrine differentiation and the prognosis of CRC (Table 1).
Table 1.
Relationship between neuroendocrine differentiation and clinico-pathological parameters
| Clinical parameter | Link to neuroendocrine differentiation | Ref. |
| Age | No | [39, 55, 63] |
| Gender | No | [39, 55, 63] |
| Preoperative conditions | Association with lower CEA levels | [63] |
| Tumor markers | ||
| DNA ploidy | No | [50] |
| TP53 expression | No | [50, 67] |
| Similar abnormal expression as in conventional adenocarcinomas | [25] | |
| BCL-2 expression | Yes | [68] |
| Ki-67 labeling index | Very low (< 5%) | [25] |
| No | [24] | |
| Tumor localization | No | [24, 25, 39, 45, 48-50, 53, 55, 63, 66] |
| Yes | [38, 59] | |
| Tumor morphology | ||
| Polypoid vs ulceration | No | [55, 63] |
| Tumor differentiation | No | [24, 39, 45, 48-50, 52, 53, 63, 66] |
| Yes | [38, 56, 57, 60, 69] | |
| Tumor size | No | [55, 63] |
| Tumor stage | No | [24, 39, 45, 48-50, 53, 55, 66] |
| Yes | [56] | |
| Lymphatic and venous invasion | No | [55, 63] |
| Perineural invasion | No | [63] |
| Lymph node metastases | No | [55] |
| Yes | [57, 58] | |
| Distant metastases | Yes | [47, 51] |
| Clinical stage | No | [53, 55] |
| Yes | [36] | |
| Therapy response | Associated with better response to radiochemotherapy | [25] |
| Prognosis | No | [38, 44, 50, 53, 55, 62, 63] |
| Better prognosis | [60] | |
| Shorter survival from time of metastasis | [24] | |
| More aggressive behavior | [48] | |
| Poor prognosis | [36, 39, 45, 46, 54, 56, 57, 59, 61 (stage II), 66 (stage III and IV), 70, 71] |
The assumption of a causal relationship between neuroendocrine differentiation and tumor differentiation is growing stronger after introduction of a new histologic grading system based on the number of poorly differentiated cell clusters (PDC) in CRC[72,73]. In a study of 20 consecutive CRCs with high grade PDCs (≥ 10 clusters, grade III CRCs), the PDCs, but not the glandular part expressed synaptophysin[74]. This could be the morphologic correlate for the previously discussed “trans-differentiation”, which initiates the development of a more aggressive tumor[20].
The presence of neuroendocrine cells in the proliferative compartments of gastrointestinal adenocarcinomas is well-documented[38,63]. Moreover, according to recent knowledge obtained from an adult Drosophila midgut model, enteroendocrine cells could function as local regulators of intestinal stem cell proliferation through modulation of the mesenchymal stem cell niche[75]. These findings could hint at the importance of neuroendocrine cells for both, maintenance and progression of tumors, thus contributing to the development of CRC with high survival potential and aggressiveness.
Further evidence for an indirect impact of neuroendocrine cells on the clinical outcome of CRC is given by the previously published link between chromogranin A/antioxidant enzyme co-expressing CRC cells and unfavorable prognosis, probably due to activated antioxidant defense and higher metabolic activity of the tumors[76]. In addition, high expression of MTOR or its downstream targets p-RPS6KB1, p-RPS6 and p-EIF4EBP1 is associated with adverse clinical outcomes in neuroendocrine tumors[77], but this link has not been investigated for neuroendocrine foci within sporadic CRC.
NEUROENDOCRINE DIFFERENTIATION AND THERAPY OF SPORADIC COLORECTAL CANCER
Neuroendocrine differentiation has been recognized as result of therapy and is getting into the focus of oncologist as target for new treatment approaches.
Shia et al[25] demonstrated an increased frequency and density of cells with an endocrine phenotype in rectal adenocarcinomas treated with neoadjuvant radiochemotherapy and found that the extent of endocrine cells appeared proportional to the degree of treatment response. This therapy-related endocrine differentiation of tumor cells could be induced by cytotoxic insult[25].
The approval of new drugs for the treatment of advanced pancreatic neuroendocrine tumors[6-8] harbored the possibility to extend the therapeutic spectrum also for neuroendocrine differentiated tumors beyond the pancreaticobiliary tract. Everolimus plus octreotide long-acting repeatable (LAR) showed significant benefits and improved outcomes for patients with advanced colorectal neuroendocrine tumors[78]. Phase I trials including CRC patients demonstrated a positive effect on stable disease for one of these drugs, everolimus, when it was combined with cetuximab[79] or with 5-fluorouracil/leucovorin (5-FU/LV) or with mFOLFOX6 (5-FU/LV + oxaliplatin)[80]. Everolimus inhibits the PI3K/PTEN/Akt pathway by connecting to the FK-506 binding protein 12 to block mTOR (mammalian target of rapamycin) activation[79]. Considering a possible importance of the PI3K-Akt-mTOR pathway for neuroendocrine differentiation[28], neuroendocrine cells within sporadic CRC could be the putative target for mTOR-inhibitor therapy (for example everolimus). The published pharmacodynamics trials[79,80] did not consider special CRC phenotypes. However, according to new insights into genotype-phenotype correlations, pretreatment histomorphological characterization of CRC could possibly help to increase efficacy of mTOR-inhibitor therapy.
CONCLUSION
A possible role of neuroendocrine differentiation as prognostic marker and therapeutic target in sporadic colorectal cancer should be further elucidated by large cohort studies.
Footnotes
Conflict-of-interest statement: The authors have no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 30, 2015
First decision: June 23, 2015
Article in press: September 15, 2015
P- Reviewer: Tonelli F S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Kelly C, Cassidy J. Chemotherapy in metastatic colorectal cancer. Surg Oncol. 2007;16:65–70. doi: 10.1016/j.suronc.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Köhne CH, Lenz HJ. Chemotherapy with targeted agents for the treatment of metastatic colorectal cancer. Oncologist. 2009;14:478–488. doi: 10.1634/theoncologist.2008-0202. [DOI] [PubMed] [Google Scholar]
- 4.Okines A, Cunningham D. Current perspective: bevacizumab in colorectal cancer--a time for reappraisal? Eur J Cancer. 2009;45:2452–2461. doi: 10.1016/j.ejca.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 5.Kim JC, Kim SY, Cho DH, Ha YJ, Choi EY, Kim CW, Roh SA, Kim TW, Ju H, Kim YS. Novel chemosensitive single-nucleotide polymorphism markers to targeted regimens in metastatic colorectal cancer. Clin Cancer Res. 2011;17:1200–1209. doi: 10.1158/1078-0432.CCR-10-1907. [DOI] [PubMed] [Google Scholar]
- 6.Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 7.Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kulke MH. Are neuroendocrine tumors going mainstream? J Clin Oncol. 2013;31:404–405. doi: 10.1200/JCO.2012.47.3884. [DOI] [PubMed] [Google Scholar]
- 9.Rehfeld JF. A centenary of gastrointestinal endocrinology. Horm Metab Res. 2004;36:735–741. doi: 10.1055/s-2004-826154. [DOI] [PubMed] [Google Scholar]
- 10.Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- 11.Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- 12.Aiken KD, Kisslinger JA, Roth KA. Immunohistochemical studies indicate multiple enteroendocrine cell differentiation pathways in the mouse proximal small intestine. Dev Dyn. 1994;201:63–70. doi: 10.1002/aja.1002010107. [DOI] [PubMed] [Google Scholar]
- 13.Roth KA, Gordon JI. Spatial differentiation of the intestinal epithelium: analysis of enteroendocrine cells containing immunoreactive serotonin, secretin, and substance P in normal and transgenic mice. Proc Natl Acad Sci USA. 1990;87:6408–6412. doi: 10.1073/pnas.87.16.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schonhoff SE, Giel-Moloney M, Leiter AB. Minireview: Development and differentiation of gut endocrine cells. Endocrinology. 2004;145:2639–2644. doi: 10.1210/en.2004-0051. [DOI] [PubMed] [Google Scholar]
- 15.Bjerknes M, Cheng H. The stem-cell zone of the small intestinal epithelium. III. Evidence from columnar, enteroendocrine, and mucous cells in the adult mouse. Am J Anat. 1981;160:77–91. doi: 10.1002/aja.1001600107. [DOI] [PubMed] [Google Scholar]
- 16.Sei Y, Lu X, Liou A, Zhao X, Wank SA. A stem cell marker-expressing subset of enteroendocrine cells resides at the crypt base in the small intestine. Am J Physiol Gastrointest Liver Physiol. 2011;300:G345–G356. doi: 10.1152/ajpgi.00278.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW, Gilbertson RJ. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457:603–607. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Giel-Moloney M, Rindi G, Leiter AB. Enteroendocrine precursors differentiate independently of Wnt and form serotonin expressing adenomas in response to active beta-catenin. Proc Natl Acad Sci USA. 2007;104:11328–11333. doi: 10.1073/pnas.0702665104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vortmeyer AO, Lubensky IA, Merino MJ, Wang CY, Pham T, Furth EE, Zhuang Z. Concordance of genetic alterations in poorly differentiated colorectal neuroendocrine carcinomas and associated adenocarcinomas. J Natl Cancer Inst. 1997;89:1448–1453. doi: 10.1093/jnci/89.19.1448. [DOI] [PubMed] [Google Scholar]
- 20.Karkouche R, Bachet JB, Sandrini J, Mitry E, Penna C, Côté JF, Blons H, Penault-Llorca F, Rougier P, Saint André JP, et al. Colorectal neuroendocrine carcinomas and adenocarcinomas share oncogenic pathways. A clinico-pathologic study of 12 cases. Eur J Gastroenterol Hepatol. 2012;24:1430–1437. doi: 10.1097/MEG.0b013e3283583c87. [DOI] [PubMed] [Google Scholar]
- 21.Scardoni M, Vittoria E, Volante M, Rusev B, Bersani S, Mafficini A, Gottardi M, Giandomenico V, Malleo G, Butturini G, et al. Mixed adenoneuroendocrine carcinomas of the gastrointestinal tract: targeted next-generation sequencing suggests a monoclonal origin of the two components. Neuroendocrinology. 2014;100:310–316. doi: 10.1159/000369071. [DOI] [PubMed] [Google Scholar]
- 22.Vanacker L, Smeets D, Hoorens A, Teugels E, Algaba R, Dehou MF, De Becker A, Lambrechts D, De Greve J. Mixed adenoneuroendocrine carcinoma of the colon: molecular pathogenesis and treatment. Anticancer Res. 2014;34:5517–5521. [PubMed] [Google Scholar]
- 23.Furlan D, Cerutti R, Genasetti A, Pelosi G, Uccella S, La Rosa S, Capella C. Microallelotyping defines the monoclonal or the polyclonal origin of mixed and collision endocrine-exocrine tumors of the gut. Lab Invest. 2003;83:963–971. doi: 10.1097/01.lab.0000079006.91414.be. [DOI] [PubMed] [Google Scholar]
- 24.Volante M, Marci V, Andrejevic-Blant S, Tavaglione V, Sculli MC, Tampellini M, Papotti M. Increased neuroendocrine cells in resected metastases compared to primary colorectal adenocarcinomas. Virchows Arch. 2010;457:521–527. doi: 10.1007/s00428-010-0967-8. [DOI] [PubMed] [Google Scholar]
- 25.Shia J, Tickoo SK, Guillem JG, Qin J, Nissan A, Hoos A, Stojadinovic A, Ruo L, Wong WD, Paty PB, et al. Increased endocrine cells in treated rectal adenocarcinomas: a possible reflection of endocrine differentiation in tumor cells induced by chemotherapy and radiotherapy. Am J Surg Pathol. 2002;26:863–872. doi: 10.1097/00000478-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Burchardt T, Burchardt M, Chen MW, Cao Y, de la Taille A, Shabsigh A, Hayek O, Dorai T, Buttyan R. Transdifferentiation of prostate cancer cells to a neuroendocrine cell phenotype in vitro and in vivo. J Urol. 1999;162:1800–1805. [PubMed] [Google Scholar]
- 27.Cox ME, Deeble PD, Lakhani S, Parsons SJ. Acquisition of neuroendocrine characteristics by prostate tumor cells is reversible: implications for prostate cancer progression. Cancer Res. 1999;59:3821–3830. [PubMed] [Google Scholar]
- 28.Wu C, Huang J. Phosphatidylinositol 3-kinase-AKT-mammalian target of rapamycin pathway is essential for neuroendocrine differentiation of prostate cancer. J Biol Chem. 2007;282:3571–3583. doi: 10.1074/jbc.M608487200. [DOI] [PubMed] [Google Scholar]
- 29.Cingarlini S, Bonomi M, Corbo V, Scarpa A, Tortora G. Profiling mTOR pathway in neuroendocrine tumors. Target Oncol. 2012;7:183–188. doi: 10.1007/s11523-012-0226-9. [DOI] [PubMed] [Google Scholar]
- 30.Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, Schulick RD, Tang LH, Wolfgang CL, Choti MA, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199–1203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roldo C, Missiaglia E, Hagan JP, Falconi M, Capelli P, Bersani S, Calin GA, Volinia S, Liu CG, Scarpa A, et al. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J Clin Oncol. 2006;24:4677–4684. doi: 10.1200/JCO.2005.05.5194. [DOI] [PubMed] [Google Scholar]
- 32.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Montalto G, Cervello M, Nicoletti F, Fagone P, Malaponte G, Mazzarino MC, et al. Mutations and deregulation of Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascades which alter therapy response. Oncotarget. 2012;3:954–987. doi: 10.18632/oncotarget.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klöppel G, Rindi G, Anlauf M, Perren A, Komminoth P. Site-specific biology and pathology of gastroenteropancreatic neuroendocrine tumors. Virchows Arch. 2007;451 Suppl 1:S9–27. doi: 10.1007/s00428-007-0461-0. [DOI] [PubMed] [Google Scholar]
- 34.Lloyd RV, Mervak T, Schmidt K, Warner TF, Wilson BS. Immunohistochemical detection of chromogranin and neuron-specific enolase in pancreatic endocrine neoplasms. Am J Surg Pathol. 1984;8:607–614. doi: 10.1097/00000478-198408000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Lloyd RV. Practical markers used in the diagnosis of neuroendocrine tumors. Endocr Pathol. 2003;14:293–301. doi: 10.1385/ep:14:4:293. [DOI] [PubMed] [Google Scholar]
- 36.Grabowski P, Schönfelder J, Ahnert-Hilger G, Foss HD, Heine B, Schindler I, Stein H, Berger G, Zeitz M, Scherübl H. Expression of neuroendocrine markers: a signature of human undifferentiated carcinoma of the colon and rectum. Virchows Arch. 2002;441:256–263. doi: 10.1007/s00428-002-0650-9. [DOI] [PubMed] [Google Scholar]
- 37.Gazdar AF, Helman LJ, Israel MA, Russell EK, Linnoila RI, Mulshine JL, Schuller HM, Park JG. Expression of neuroendocrine cell markers L-dopa decarboxylase, chromogranin A, and dense core granules in human tumors of endocrine and nonendocrine origin. Cancer Res. 1988;48:4078–4082. [PubMed] [Google Scholar]
- 38.Smith DM, Haggitt RC. The prevalence and prognostic significance of argyrophil cells in colorectal carcinomas. Am J Surg Pathol. 1984;8:123–128. doi: 10.1097/00000478-198402000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Pagani A, Papotti M, Abbona GC, Bussolati G. Chromogranin gene expressions in colorectal adenocarcinomas. Mod Pathol. 1995;8:626–632. [PubMed] [Google Scholar]
- 40.Solcia E, Capella C, Fiocca R, Sessa F, La Rosa S, Rindi G. Disorders of the endocrine system. In: Ming SC, Goldman H, editors. Pathology of the gastrointestinal tract. Philadelphia: Williams and Wilkins; 1998. pp. 295–322. [Google Scholar]
- 41.Rindi G, Klöppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, Erikssson B, Falchetti A, Falconi M, Komminoth P, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449:395–401. doi: 10.1007/s00428-006-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rindi G, Klöppel G, Couvelard A, Komminoth P, Körner M, Lopes JM, McNicol AM, Nilsson O, Perren A, Scarpa A, et al. TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2007;451:757–762. doi: 10.1007/s00428-007-0452-1. [DOI] [PubMed] [Google Scholar]
- 43.Bosman FT, Carneiro F, Hruban RH, Theise ND, editors . World Health Organization Classification of Tumors. WHO classification of Tumors of the Digestive System. 4th ed. Lyon: IARC Press; 2010. pp. 13–14. [Google Scholar]
- 44.Lloyd RV, Schroeder G, Bauman MD, Krook JE, Jin L, Goldberg RM, Farr GH. Prevalence and Prognostic Significance of Neuroendocrine Differentiation in Colorectal Carcinomas. Endocr Pathol. 1998;9:35–42. doi: 10.1007/BF02739950. [DOI] [PubMed] [Google Scholar]
- 45.Hamada Y, Oishi A, Shoji T, Takada H, Yamamura M, Hioki K, Yamamoto M. Endocrine cells and prognosis in patients with colorectal carcinoma. Cancer. 1992;69:2641–2646. doi: 10.1002/1097-0142(19920601)69:11<2641::aid-cncr2820691104>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 46.Jansson D, Gould VE, Gooch GT, Rittenhouse HG, Shin SS, Manderino GL, Tomita JT, Staren ED. Immunohistochemical analysis of colon carcinomas applying exocrine and neuroendocrine markers. APMIS. 1988;96:1129–1139. doi: 10.1111/j.1699-0463.1988.tb00991.x. [DOI] [PubMed] [Google Scholar]
- 47.Li Y, Yau A, Schaeffer D, Magliocco A, Gui X, Urbanski S, Waghray R, Owen D, Gao ZH. Colorectal glandular-neuroendocrine mixed tumor: pathologic spectrum and clinical implications. Am J Surg Pathol. 2011;35:413–425. doi: 10.1097/PAS.0b013e3182093657. [DOI] [PubMed] [Google Scholar]
- 48.Arends JW, Wiggers T, Verstijnen K, Bosman FT. The occurrence and clinicopathological significance of serotonin immunoreactive cells in large bowel carcinoma. J Pathol. 1986;149:97–102. doi: 10.1002/path.1711490204. [DOI] [PubMed] [Google Scholar]
- 49.Lapertosa G, Baracchini P, Delucchi F. Prevalence and prognostic significance of endocrine cells in colorectal adenocarcinomas. Pathologica. 1994;86:170–173. [PubMed] [Google Scholar]
- 50.Ferrero S, Buffa R, Pruneri G, Siccardi AG, Pelagi M, Lee AK, Coggi G, Bosari S. The prevalence and clinical significance of chromogranin A and secretogranin II immunoreactivity in colorectal adenocarcinomas. Virchows Arch. 1995;426:587–592. doi: 10.1007/BF00192113. [DOI] [PubMed] [Google Scholar]
- 51.Shinji S, Naito Z, Ishiwata T, Tanaka N, Furukawa K, Suzuki H, Seya T, Kan H, Tsuruta H, Matsumoto S, et al. Neuroendocrine cell differentiation of poorly differentiated colorectal adenocarcinoma correlates with liver metastasis. Int J Oncol. 2006;29:357–364. [PubMed] [Google Scholar]
- 52.Park JG, Choe GY, Helman LJ, Gazdar AF, Yang HK, Kim JP, Park SH, Kim YI. Chromogranin-A expression in gastric and colon cancer tissues. Int J Cancer. 1992;51:189–194. doi: 10.1002/ijc.2910510205. [DOI] [PubMed] [Google Scholar]
- 53.Secco GB, Campora E, Fardelli R, Lapertosa G, De Lucchi F, Gianquinto D, Bonfante P. Chromogranin-A expression in neoplastic neuroendocrine cells and prognosis in colorectal cancer. Tumori. 1996;82:390–393. doi: 10.1177/030089169608200419. [DOI] [PubMed] [Google Scholar]
- 54.de Bruïne AP, Wiggers T, Beek C, Volovics A, von Meyenfeldt M, Arends JW, Bosman FT. Endocrine cells in colorectal adenocarcinomas: incidence, hormone profile and prognostic relevance. Int J Cancer. 1993;54:765–771. doi: 10.1002/ijc.2910540510. [DOI] [PubMed] [Google Scholar]
- 55.Mori M, Mimori K, Kamakura T, Adachi Y, Ikeda Y, Sugimachi K. Chromogranin positive cells in colorectal carcinoma and transitional mucosa. J Clin Pathol. 1995;48:754–758. doi: 10.1136/jcp.48.8.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Atasoy P, Ensari A, Demirci S, Kurşun N. Neuroendocrine differentiation in colorectal carcinomas: assessing its prognostic significance. Tumori. 2003;89:49–53. doi: 10.1177/030089160308900111. [DOI] [PubMed] [Google Scholar]
- 57.Yin J, Liang Y, Wang H. [Significance of endocrine cells and their hormones in colorectal cancer] Zhonghua Zhong Liu Za Zhi. 1997;19:192–195. [PubMed] [Google Scholar]
- 58.Indinnimeo M, Cicchini C, Memeo L, Stazi A, Provenza C, Ricci F, Mingazzini PL. Correlation between chromogranin-A expression and pathological variables in human colon carcinoma. Anticancer Res. 2002;22:395–398. [PubMed] [Google Scholar]
- 59.Syversen U, Halvorsen T, Mårvik R, Waldum HL. Neuroendocrine differentiation in colorectal carcinomas. Eur J Gastroenterol Hepatol. 1995;7:667–674. [PubMed] [Google Scholar]
- 60.Yao GY, Zhou JL, Lai MD, Chen XQ, Chen PH. Neuroendocrine markers in adenocarcinomas: an investigation of 356 cases. World J Gastroenterol. 2003;9:858–861. doi: 10.3748/wjg.v9.i4.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y, Xu J, Jiao Y, Hu Y, Yi C, Li Q, Tong Z, Wang X, Hu L, Xiao Q, et al. Neuroendocrine differentiation is a prognostic factor for stage II poorly differentiated colorectal cancer. Biomed Res Int. 2014;2014:789575. doi: 10.1155/2014/789575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Foley EF, Gaffey MJ, Frierson HF. The frequency and clinical significance of neuroendocrine cells within stage III adenocarcinomas of the colon. Arch Pathol Lab Med. 1998;122:912–914. [PubMed] [Google Scholar]
- 63.Cho YB, Yang SS, Lee WY, Song SY, Kim SH, Shin HJ, Yun SH, Chun HK. The clinical significance of neuroendocrine differentiation in T3-T4 node-negative colorectal cancer. Int J Surg Pathol. 2010;18:201–206. doi: 10.1177/1066896909332112. [DOI] [PubMed] [Google Scholar]
- 64.Sun MH. Neuroendocrine differentiation in sporadic CRC and hereditary nonpolyosis colorectal cancer. Dis Markers. 2004;20:283–288. doi: 10.1155/2004/379053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kleist B, Kempa M, Novy M, Oberkanins C, Xu L, Li G, Loland C, Poetsch M. Comparison of neuroendocrine differentiation and KRAS/NRAS/BRAF/PIK3CA/TP53 mutation status in primary and metastatic colorectal cancer. Int J Clin Exp Pathol. 2014;7:5927–5939. [PMC free article] [PubMed] [Google Scholar]
- 66.Grabowski P, Schindler I, Anagnostopoulos I, Foss HD, Riecken EO, Mansmann U, Stein H, Berger G, Buhr HJ, Scherübl H. Neuroendocrine differentiation is a relevant prognostic factor in stage III-IV colorectal cancer. Eur J Gastroenterol Hepatol. 2001;13:405–411. doi: 10.1097/00042737-200104000-00018. [DOI] [PubMed] [Google Scholar]
- 67.Grabowski P, Sturm I, Schelwies K, Maaser K, Buhr HJ, Dörken B, Zeitz M, Daniel PT, Scherübl H. Analysis of neuroendocrine differentiation and the p53/BAX pathway in UICC stage III colorectal carcinoma identifies patients with good prognosis. Int J Colorectal Dis. 2006;21:221–230. doi: 10.1007/s00384-005-0779-5. [DOI] [PubMed] [Google Scholar]
- 68.Atasoy P, Bozdoğan O, Oztürk S, Ensari A. Bcl2 expression and its correlation with neuroendocrine differentiation in colon carcinomas. Tumori. 2004;90:233–238. doi: 10.1177/030089160409000213. [DOI] [PubMed] [Google Scholar]
- 69.Seretis E, Gavrill A, Agnantis N, Golematis V, Voloudakis-Baltatzis IE. Comparative study of serotonin and bombesin in adenocarcinomas and neuroendocrine tumors of the colon. Ultrastruct Pathol. 2001;25:445–454. doi: 10.1080/019131201753343485. [DOI] [PubMed] [Google Scholar]
- 70.Staren ED, Gould VE, Jansson DS, Hyser M, Gooch GT, Economou SG. Neuroendocrine differentiation in “poorly differentiated” colon carcinomas. Am Surg. 1990;56:412–419. [PubMed] [Google Scholar]
- 71.Zeng YJ, Lai W, Liu L, Wu H, Luo XX, Wang J, Chu ZH. Prognostic significance of neuroendocrine differentiation in colorectal adenocarcinoma after radical operation: a meta-analysis. J Gastrointest Surg. 2014;18:968–976. doi: 10.1007/s11605-014-2480-x. [DOI] [PubMed] [Google Scholar]
- 72.Ueno H, Kajiwara Y, Shimazaki H, Shinto E, Hashiguchi Y, Nakanishi K, Maekawa K, Katsurada Y, Nakamura T, Mochizuki H, et al. New criteria for histologic grading of colorectal cancer. Am J Surg Pathol. 2012;36:193–201. doi: 10.1097/PAS.0b013e318235edee. [DOI] [PubMed] [Google Scholar]
- 73.Barresi V, Reggiani Bonetti L, Branca G, Di Gregorio C, Ponz de Leon M, Tuccari G. Colorectal carcinoma grading by quantifying poorly differentiated cell clusters is more reproducible and provides more robust prognostic information than conventional grading. Virchows Arch. 2012;461:621–628. doi: 10.1007/s00428-012-1326-8. [DOI] [PubMed] [Google Scholar]
- 74.Gurzu S, Serester O, Jung I. Possible neuroendocrine phenotype of poorly differentiated cell clusters in colorectal carcinoma, as a prognostic parameter. Am J Surg Pathol. 2014;38:143–144. doi: 10.1097/PAS.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 75.Scopelliti A, Cordero JB, Diao F, Strathdee K, White BH, Sansom OJ, Vidal M. Local control of intestinal stem cell homeostasis by enteroendocrine cells in the adult Drosophila midgut. Curr Biol. 2014;24:1199–1211. doi: 10.1016/j.cub.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gulubova M, Vlaykova T. Expression of the xenobiotic- and reactive oxygen species-detoxifying enzymes, GST-pi, Cu/Zn-SOD, and Mn-SOD in the endocrine cells of colorectal cancer. Int J Colorectal Dis. 2010;25:1397–1405. doi: 10.1007/s00384-010-1041-3. [DOI] [PubMed] [Google Scholar]
- 77.Qian ZR, Ter-Minassian M, Chan JA, Imamura Y, Hooshmand SM, Kuchiba A, Morikawa T, Brais LK, Daskalova A, Heafield R, et al. Prognostic significance of MTOR pathway component expression in neuroendocrine tumors. J Clin Oncol. 2013;31:3418–3425. doi: 10.1200/JCO.2012.46.6946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Castellano D, Bajetta E, Panneerselvam A, Saletan S, Kocha W, O’Dorisio T, Anthony LB, Hobday T. Everolimus plus octreotide long-acting repeatable in patients with colorectal neuroendocrine tumors: a subgroup analysis of the phase III RADIANT-2 study. Oncologist. 2013;18:46–53. doi: 10.1634/theoncologist.2012-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ciunci CA, Perini RF, Avadhani AN, Kang HC, Sun W, Redlinger M, Harlacker K, Flaherty KT, Giantonio BJ, Rosen MA, et al. Phase 1 and pharmacodynamic trial of everolimus in combination with cetuximab in patients with advanced cancer. Cancer. 2014;120:77–85. doi: 10.1002/cncr.28294. [DOI] [PubMed] [Google Scholar]
- 80.McRee AJ, Davies JM, Sanoff HG, Goldberg RM, Bernard S, Dees EC, Keller K, Ivanova A, O’Neil BH. A phase I trial of everolimus in combination with 5-FU/LV, mFOLFOX6 and mFOLFOX6 plus panitumumab in patients with refractory solid tumors. Cancer Chemother Pharmacol. 2014;74:117–123. doi: 10.1007/s00280-014-2474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


