Abstract
The revised Atlanta classification of acute pancreatitis was adopted by international consensus, and is based on actual local and systemic determinants of disease severity. The local determinant is pancreatic necrosis (sterile or infected), and the systemic determinant is organ failure. Local complications of pancreatitis can include acute peri-pancreatic fluid collection, acute necrotic collection, pseudocyst formation, and walled-off necrosis. Interventional endoscopic ultrasound (EUS) has been increasing utilized in managing these local complications. After performing a PubMed search, the authors manually applied pre-defined inclusion criteria or a filter to identify publications relevant to EUS and pancreatic collections (PFCs). The authors then reviewed the utility, efficacy, and risks associated with using therapeutic EUS and involved EUS devices in treating PFCs. Due to the development and regulatory approval of improved and novel endoscopic devices specifically designed for transmural drainage of fluid and necrotic debris (access and patency devices), the authors predict continuing evolution in the management of PFCs. We believe that EUS will become an indispensable part of procedures used to diagnose PFCs and perform image-guided interventions. After draining a PFC, the amount of tissue necrosis is the most important predictor of a successful outcome. Hence, it seems logical to classify these collections based on their percentage of necrotic component or debris present when viewed by imaging methods or EUS. Finally, the authors propose an algorithm for managing fluid collections based on their size, location, associated symptoms, internal echogenic patterns, and content.
Keywords: Endoscopic ultrasound, Drainage, Pancreatic fluid collection, Pseudocyst, Patency device, Abscess, Walled of necrosis, Pancreas
Core tip: The revised Atlanta classification of acute pancreatitis was approved by international consensus, and is based on actual local and systemic determinants of disease severity. Local complications of pancreatitis can include acute peri-pancreatic fluid collection, acute necrotic collection, pseudocyst formation, and walled-off necrosis. Interventional endoscopic ultrasound (EUS) has been increasingly utilized in managing pancreatitis. This review describes the utility, efficacy, and risks associated with using therapeutic EUS and involved EUS devices to manage acute pancreatitis. The authors propose an algorithm for use in managing pancreatic fluid collections based on their size, location, associated symptoms, internal echogenic patterns, and content.
INTRODUCTION
In 1992, the Atlanta classification of acute pancreatitis was adopted by international consensus. While the Atlanta classification attempted to standardize reporting and communication among health care professionals, some of the terminology used was confusing and failed to objectively describe complications associated with pancreatitis. This made it difficult to provide proper treatment. A revised Atlanta classification was released in 2012 after years of web-based consultation among a global panel of experts and international pancreatic associations[1-3]. The new classification system is based on actual local and systemic determinants of severity, rather than descriptions of events correlated with severity[1,2]. The local determinant is pancreatic necrosis (sterile or infected) and the systemic determinant is organ failure (transient or persistent). Acute pancreatitis is now classified into two phases (early and late), and its severity is classified as mild, moderate or severe. Mild acute pancreatitis is not accompanied by organ failure, local or systemic complications, and usually resolves in the first week. Moderately severe acute pancreatitis is accompanied by transient organ failure, as well as local complications or exacerbation of a co-morbid disease. Severe acute pancreatitis presents with persistent organ failure (> 48 h duration) (Table 1).
Table 1.
Revised Atlanta classification (2012) of pancreatic/peripancreatic fluid collections
| Type of pancreatic or peripancreatic fluid collection | Etiology | Capsule | Specific features |
| Acute peripancreatic fluid collection, APFC | ≤ 4 wk after onset of acute interstitial-edematous pancreatitis | - | Homogeneous, liquid, infection +/-, no features of a pseudocyst, usually resolves spontaneously |
| Pancreatic pseudocyst, PPC | > 4 wk after onset of acute interstitial-edematous pancreatitis | + | Round/oval, Liquid, no non-liquid contents, persistent |
| Acute necrotic collection, ANC | Acute necrotizing pancreatitis | - | Heterogeneous, liquid and necrotic contents, usually resolves spontaneously |
| Walled-off pancreatic necrosis, WOPN | > 4 wk after onset of necrotizing pancreatitis | + | Heterogeneous, liquid and necrotic contents, infection +/- |
ANC: Acute necrotic collection.
Local complications of pancreatitis can include acute peri-pancreatic fluid accumulation, acute necrotic collection (ANC, sterile or infected), pseudocyst formation, and development of walled-off necrosis (WON) (sterile or infected). WON is characterized by a distinct rim that forms around areas of tissue necrosis and adjacent pancreatic parenchyma. Interventional endoscopic ultrasound (EUS) has been increasing utilized to help manage these local complications[4,5]. To gather information for this review, the authors searched English language medical literature and reviewed articles which described the utility, efficacy, and risks associated with using therapeutic EUS and its involved devices in these clinical settings, which we grouped together as pancreatic fluid collections (PFCs). The authors propose an algorithm for use in managing pancreatic fluid collections based on their size, location, associated symptoms, internal echogenic pattern, and structure.
METHODS AND REVIEW STRATEGY
On November 15, 2014, the authors performed a PubMed search using the abbreviation EUS in combination with phrases or words related to pancreatic fluid collection; such as pseudocyst, fluid collection, abscess, and WON. Next, pre-defined inclusion criteria or filters were manually applied to the PubMed search results (Figure 1). The inclusion criteria were: (1) original report; (2) case number > 6; and (3) English language publication only. The authors then manually reviewed the publications and their listed references, i.e., cross-reference search. Finally, each published paper was jointly reviewed by two authors of this review article, and relevant important information was extracted.
Figure 1.
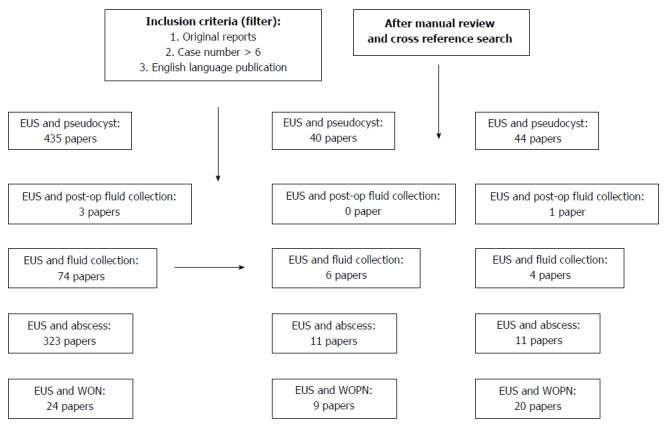
On November 15, 2014, the authors performed a PubMed search using following key word sets: endoscopic ultrasound in combination with terminologies related to pancreatic fluid collections such as pseudocyst, fluid collection, abscess, and walled off necrosis. Each published paper was simultaneously reviewed by two authors who extracted important information related to this review. EUS: Endoscopic ultrasound; WON: Walled off necrosis.
INDICATIONS AND TIMING FOR TRANSMURAL DRAINAGE
The decision to drain a pancreatic fluid collection depends on several factors, including the patient’s clinical condition and symptoms, the change in amount of accumulated fluid over time, the time from the onset of symptoms, and the presence of infection or other complications. Asymptomatic pancreatic and/or extra-pancreatic fluid collections do not warrant intervention regardless of their size, location, and/or extension. Instead, drainage is considered only when a fluid collection causes clinical symptoms or displays signs of infection[6]. Infection is most common in fluid surrounding necrotic tissue, and is suggested by the presence of air pockets inside the accumulation and visible on a computerized tomography (CT) scan. If a clinical scenario strongly suggests an infected fluid collection, it can be verified by performing and examining the contents of a fine needle aspiration. Patients with sterile accumulations, luminal or biliary obstruction resulting from external compression, persistent abdominal pain requiring narcotics, or an undiagnosed sepsis syndrome should receive drainage[7]. PFCs can be drained using surgical, percutaneous or endoscopic methods.
Proper timing is critical for successful endoscopic drainage in cases of necrotizing pancreatitis. Interventions made within the first several weeks of necrotizing pancreatitis generally lead to poor outcomes. The guiding principle for timing of debridement is to delay any intervention until the collection has become encapsulated and liquefied as much as possible. Encapsulation does not usually occur until at least 4 wk after the initial injury.
Endoscopic methods for draining collected fluid have shown efficacies comparable to those achieved using surgical methods. Furthermore, endoscopic treatments usually result in shorter hospital stays, better patient physical and mental health, and lower treatment costs compared to surgery[8]. Percutaneous drainage requires the patient to have an external drain implanted for an extended period of time. This may lead to development of pancreatico-cutaneous fistulas; especially in patients with ductal disruption. In contrast to percutaneous drainage, an endoscopic approach allows placement of multiple drainage modalities through a single puncture site.
EUS permits a physician to visualize the entire abdominal cavity, assess the maturity of the wall, measure the distance between the collection and the luminal wall, and identify intervening vessels (collaterals) along the puncture site (Figures 2 and 3). Furthermore, the rates of technical success achieved when using EUS-guided drainage have been higher than those achieved using conventional transmural endoscopic drainage techniques performed without EUS guidance[9]. EUS-guided drainage is the preferred modality in cases where there is no visible luminal bulge, portal hypertension and collateral formation are suspected, or when treating patients with coagulopathy[4,6,7].
Figure 2.
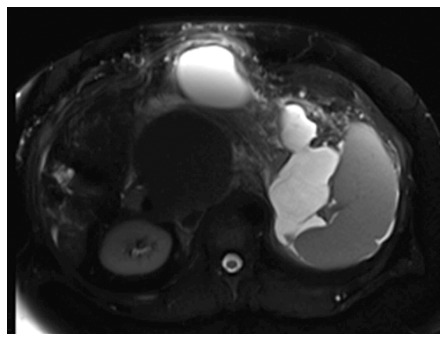
Selected magnetic resonance imaging frame showing a large peri-pancreatic pseudocyst extending from the pancreatic tail to the anterior abdominal wall in a patient with pancreatitis and splenic vein thrombosis.
Figure 3.
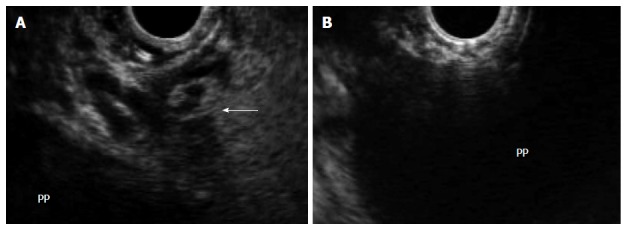
In the same patient, endoscopic ultrasound permits visualization of the pancreatic pseudocyst, assessment of wall maturity, determination of distance between the collection and the luminal wall, identification of intervening vessels or collaterals (arrow) (A), and selection of the optimal puncture site (B). PP: Pancreatic pseudocyst.
SHOULD ERCP WITH TRANS-PAPILLARY DRAINAGE BE PERFORMED ON THESE PATIENTS?
Endoscopic drainage of PFCs may be performed either during endoscopic retrograde cholangiopancreatography (ERCP) with drainage through the main ampulla of Vater or via a transmural route - either the duodenum or stomach. Currently, no comparative or randomized studies have been reported from which solid data can be extracted regarding the preferred method for drainage. Only case series in which inconsistent methods and guidelines were used based on expert opinions have been published[10,11]. Based on the available published information, transpapillary drainage is preferred to EUS-guided transmural drainage as a first-step procedure for treating small fluid collections which communicate with the main pancreatic duct in the head or body of the pancreas (Figure 4). Moreover, most published cases which describe the use of EUS-guided drainage, fail to mention whether the patients had undergone ERCP prior to EUS-guided drainage. In one series of 116 patients, 15 patients received transpapillary drainage, 60 received transmural drainage, and 41 received both types of drainage. In that series, successful drainage was achieved in 88% of the patients[12]. However, there was no difference in the rates of success achieved using the different methods. Hence, little evidence exists to support a recommendation that pancreatic fluid collections should preferably be drained via the pancreatic papilla and pancreatic duct.
Figure 4.
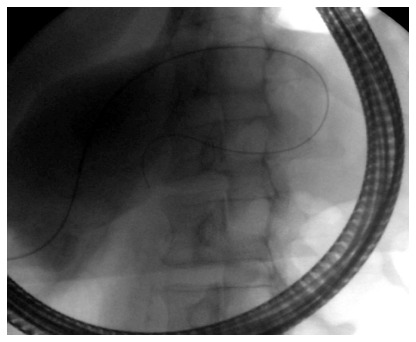
Fluoroscopic image showing transpapillary drainage of a pancreatic pseudocyst that is shared with the main pancreatic duct.
DOES LOCATION OF THE FLUID COLLECTION MATTER?
Transmural drainage has been attempted as a method for treating pancreatic pseudocysts (Figure 5A) and WON (Figure 5B) of suitable size, and located in the head, body or tail of the pancreas. However, this method requires that the distance between the lumen wall and cyst is < 1 cm. Due to their location in the lesser sac or extension to the pararenal space, PFCs in the pancreatic tail do not cause luminal compression, and can be accessed only by EUS. Varadarajulu et al[13] noted that the location of a pseudocyst is not predictive of treatment success. However, two cases of perforation have been reported when transgastric drainage was attempted for pseudocysts located in the uncinate process of the pancreas. This complication did not occur when uncinate pseudocysts were drained via the duodenum. Following transmural stenting, a low hanging pseudocyst in the uncinate region becomes decompressed, and may disconnect from the stomach wall, leading to perforation[14].
Figure 5.
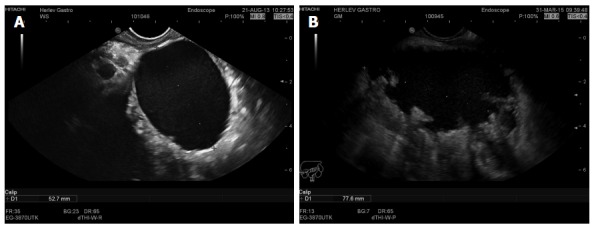
Endoscopic ultrasound image of a 5 cm chronic pseudocyst with a thin wall (A) or a 7.8 cm irregular pseudocyst with walled off necrosis (B).
DOES THE AMOUNT OF INTERNAL DEBRIS MATTER?
In the context of draining PFCs, technical success refers to achieving access to a PFC and the placement of transmural stents, whereas clinical success means resolution of the collection. Very high clinical success rates (90%-100%) have been achieved when draining pseudocysts[9,12,15]. However, when treating cases of walled off pancreatic necrosis, the clinical success rates are generally poor. In a recent study of 211 patients with symptomatic PFCs, the reported success rate for treating sterile and infective pseudocysts was 93.5%, but only 63.2% when treating a WON[13]. Baron et al[16] reported a 92% success rate when performing pseudocyst drainage in patients without necrosis, compared to 72% in patients with necrosis. Although that study utilized non-EUS-guided endoscopic drainage, it illustrates the principle that outcomes achieved when performing endoscopic drainage of pseudocysts are superior to those achieved when draining collections with infected necrosis. In another study, drainage of a necrosis was clinically successful in only 25% of cases, but technically successful in 50% of cases[12]. If an aggressive endoscopic approach using endoscopic necrosectomy is adopted, success rates up to 81% can be achieved when treating a WON[17]; however, adjunctive surgical and percutaneous drainage may be required. Varadarajulu et al[18] suggested multiple transluminal gateway treatment (MTGT) for a WON, by which they attained a successful response in 92% of patients. In those cases, two or three transmural tracts were created between the necrotic cavity and gastrointestinal lumen by using EUS guidance. While one tract was used to flush normal saline solution via a nasocystic catheter, multiple stents were deployed in the tracts to facilitate drainage of the necrotic contents.
As the amount of necrotic component in a collection increases, the success rate of draining the collection progressively decreases, unless aggressive endoscopic necrosectomy or concomitant percutaneous drainage is also used. The need for surgical intervention is also more common in these groups of patients. The revised Atlanta classification describes a WON as a well encapsulated fluid collection which occurs 4 wk in the setting of necrotic pancreatitis. However, this may not always be true, as previous necrotic collections can liquefy over time. A study conducted in India had patients undergo follow-up EUS examinations at 6 wk, 3 mo, and 6 mo after the onset of acute necrotic pancreatitis, and found that not all fluid collections following acute necrotic pancreatitis had a solid necrotic component. During the time period studied, the collections tended to decrease in size and their solid content tended to liquefy, with almost half of the PFCs being completely liquid at 6 mo[19].
Another study conducted by the same group examined 43 patients with a symptomatic WON treated by endoscopic drainage[20]. The WONs had a mean size of 9.95 ± 2.75 cm, and were found to contain < 10%, 10%-40%, and > 40% solid debris in 6, 33, and 4 patients, respectively. Patients with < 10% necrotic debris required only a single session of endoscopic drainage, whereas patients with 10%-40% solid debris required two or more sessions. Patients with > 40% solid debris required either direct endoscopic debridement or surgical necrosectomy. The extent of necrosis was significantly correlated with the type of treatment received by the patient (r = 0.703, P < 0.001).
CHOICE OF TRANSMURAL ACCESS DEVICES
The widespread use of EUS-guided PFC drainage has been limited by a lack of dedicated accessories. This factor necessitates using multiple steps to place a transluminal stent. The fluid collection is first visualized using a linear echoendoscope, and Doppler technology is used to ensure that no blood vessels lie in the line of puncture. The PFC is then visualized and punctured using a 19-gauge FNA needle, a cystotome or needle wire. After puncturing, a 0.035 guide wire is inserted into the PFC. When multiple stents need to be placed, some physicians prefer using a double guide wire approach in which two guide wires are simultaneously inserted after the first puncture[21]. A novel lumen-apposing self-expandable metal stent (AXIOSTM system, Xlumina; Mountain View, CA, United States) has recently been developed that can be deployed in a single step (Figures 6 and 7). The stent has a dumbbell-shaped configuration that foreshortens on deployment, and thereby minimizes the possibility of leakage or perforation[22].
Figure 6.
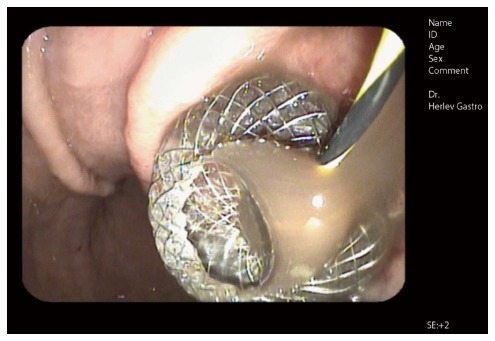
Endoscopic image of a self-expandable metal stent immediately after endoscopic ultrasound guided drainage (AXIOSTM system, Xlumina, Mountain View, CA, United States). Note the fluid floating through the stent opening. A guidewire extending through the stent lumen is still visible.
Figure 7.
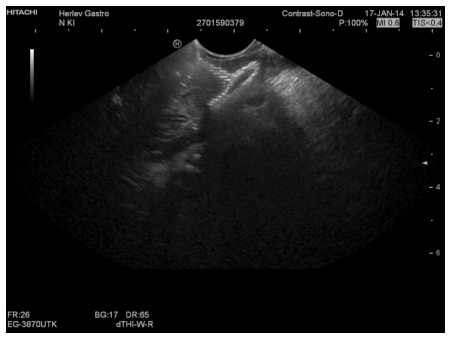
Corresponding endoscopic ultrasound image of a collapsed pancreatic pseudocyst immediately after drainage. Note reflexions from the stent mesh inside the collapsed cyst.
CYSTOENTEROTOMY PATENCY DEVICES
A variety of stents have been used to maintain patency of the fistulous tract between the gut lumen and the PFC. Single plastic stents (straight or double pigtail), multiple plastic stents, nasocystic drainage catheters, enteral metal stents, and biliary metal stents have all been tried. Some studies have also used combined modalities such as plastic stents in combination with nasocystic drainage catheters or double pigtail stents axially placed through a metal stent. While the available literature includes studies which used a variety of stents in combination, there is no clear evidence to suggest that metal stents are better than plastic stents, or that one type of plastic stent is better than another.
Pseudocysts
Lopes et al[23] used single plastic stents for draining pseudocysts. While drainage was successful in 93% of patients, 25% of patients experienced a recurrence. Those investigators also noted that complications occurred more frequently when using straight stents as compared to double pigtail stents; however, the difference was not statistically significant. Straight stents do not have anchorage, and thus can migrate more easily than double pigtail stents. Additionally, straight stents have been reported to cause bleeding and perforation.
Antillon et al[24] performed a single center prospective cohort study which examined the efficacy of single-step EUS-guided transmural drainage of pseudocysts. During the index procedure, complete resolution of the pseudocyst was achieved in 27 (82%) of 33 patients. Four additional patients (12%) had partial resolution (50% reduction in cyst size) accompanied by symptom resolution. Recurrence was observed in only one patient during a median follow-up period of 46 wk.
In a retrospective study, 87 consecutive patients with pancreatic pseudocysts were managed by EUS-guided drainage. Sixty-three patients with solid debris were drained via nasocystic drains placed alongside stents, while 24 patients with solid debris were drained via transmural stents. The short-term success rate among patients with viscous solid debris-laden fluid and whose pseudocysts were drained by both stents and nasocystic tubes was 3-fold greater than that among patients who were drained by stents alone (OR = 3.6; 95%CI: 1.2-10.7; P = 0.03). Long-term follow-up results showed a non-significant trend suggesting that pseudocysts were better resolved when debris was drained using nasocystic drains placed alongside stents compared to using stents alone (79% vs 58% respectively, OR = 2.7; P = 0.059). Moreover, when draining debris-laden cysts, the rate of stent occlusion was higher when using stents alone rather than nasocystic drains placed alongside of stents (33% vs 13%, P = 0.03)[25].
Seewald et al[21] used a single step, simultaneous double-wire technique in conjunction with a prototype device to drain symptomatic cysts in eight patients. After puncturing a cyst, two 0.035-inch guide wires were simultaneously inserted into the cyst cavity. Next, transmural stenting was performed with an 8.5F double pigtail stent, and a 7 French nasocystic catheter was inserted into the cavity. The cavity was irrigated with a total of 1500 mL of saline solution daily (7-21 d duration) administered through the nasocystic catheter to prevent accumulation of pus and debris. Follow-up CT scans showed that all patients experienced complete resolution of their pseudocysts, and no recurrence was found during follow-up periods ranging 6 to 16 mo.
Due et al[26] described 10 patients who underwent pancreatic pseudocyst drainage; among whom, 7 patients received a 10 mm × 20 mm covered double-flanged metal stent. Three of the 7 patients who received the metal stent developed sepsis due to stent blockage, and one patient experienced persistent leakage. Two of the patients with stent blockage and the one patient with a leak ultimately required surgical intervention. Fabbri et al[27] reported the drainage of 20 patients with infected fluid collections using covered self-expanding metal stents (SEMS; 4 cm or 6 cm long, 10 mm diameter). The procedure was technically successful in all of the patients, and the treatment success rate was 90%. One month after insertion, the stents were removed, and the removal procedure was successful in all except one patient. Additionally, one stent had migrated and one patient required surgery. Several attempts have been made to provide better anchorage to SEMS by deploying plastic stents through them, and thereby reduce the likelihood of migration. Talreja et al[28] described a series of 16 patients with PFCs who underwent endoscopic drainage. SEMS (10 mm × 60 mm) were inserted, and double pigtail plastic stents were deployed through or alongside the metal stents to provide better anchorage. That study showed a 95% treatment response rate, and stent migration occurred in only one patient. Comparable results were reported by Penn et al[29], who used the same technique to drain pseudocysts.
A study from California[30] evaluated the safety and efficacy of EUS-guided drainage of PFCs using a one-step access device (NAVIXTM, Xlumina), followed by placement of a fully covered SEMS. Eighteen patients with a PFC showing indeterminate adherence were enrolled. After 7-10 d, the fully covered SEMSs were removed and replaced with double-pigtail stents. When indicated, tract dilation and endoscopy-guided cyst debridement were performed. Fully covered SEMS placement was technically successful in all 18 patients, and there were no complications. Cyst resolution was achieved in 78% of the patients, and the median procedure time was 37.5 min. Berzosa et al[31] reported 100% technical and clinical success rates when using SEMSs, and found no instance of stent migration. The majority of SEMSs used in that study were tubular stents designed for transluminal drainage, including bile duct drainage. When used for transmural drainage, these SEMSs have some limitations, including a high risk of stent migration and a possibility of causing tissue injury and bleeding. As a result of those limitations, a novel large-diameter SEMS with bilateral flanges (the AXIOS stent) has been specifically designed for transmural drainage. It consists of a barbell-shaped, flexible, fully covered, self-expanding nitinol stent housed within a catheter-based delivery system. The new SEMS is available in two sizes (10 mm × 10 mm and 15 mm × 10 mm), and its 10 mm saddle length is designed to appose the stomach or duodenum to the PFC wall. In 2012, Itoi et al[32] first described the use of AXIOS stents in a series of 15 patients with symptomatic pseudocysts who underwent drainage. All stents were successfully deployed without complications; the pseudocysts resolved after a single drainage procedure, and the median time to removal was 35 d. Although one stent migrated into the stomach, the remaining 14 stents were found to be patent at the time of removal. Moreover, there was no pseudocyst recurrence during a median follow-up period of 11-mo. In 2013, a Spanish study[33] reported the use of AXIOS stents for pseudocyst drainage. In that study, the technical success rate was 88% (8/9 patients), as the stent delivery system failed in one case. However, no stent migration was reported and all stents were easily removed. Moreover, all patients experienced a complete resolution of their cyst.
Walled-off necrosis
A WON often leads to the severe clinical deterioration of a patient, and requires treatment with open debridement or endoscopic necrosectomy. Infection is a common complication which occurs during endoscopic drainage of pancreatic fluid collections, and is more common in patients with a WON than patients with a pseudocyst. This increased incidence of infection in cases of WON is presumably due to stent occlusion by solid debris and subsequent bacterial colonization. Hence, most physicians favor placing multiple stents, and especially in cases of WON. When performing MTGT, two or three transmural tracts are created between the necrotic cavity and gastrointestinal lumen under EUS guidance. While one tract is used to flush normal saline solution via a nasocystic catheter, multiple stents are inserted into the other tracts to facilitate drainage of necrotic contents. Varadarajulu et al[18] compared MTGT with conventional EUS-guided drainage in 60 patients with a symptomatic WON. Twelve of the patients were managed by MTGT and 48 by conventional drainage. Treatment was successful in 11 of 12 (91.7%) patients managed by MTGT vs 25 of 48 (52.1%) patients managed by conventional drainage (P = 0.01). Although 1 patient in the MTGT cohort required endoscopic necrosectomy, 17 patients who received conventional drainage required surgery, 3 underwent endoscopic necrosectomy, and 3 died of multiple-organ failure. A multicenter prospective study[22] from the United States evaluated the outcome of AXIOS stent placement in 33 patients with symptomatic pancreatic pseudocysts and WONs. The devices were successfully placed under endoscopic ultrasound guidance in 30 patients (91%), while the remaining 3 patients each received two double-pigtail stents. One subject could not be evaluated due to a pseudoaneurysm. Among the 29 patients who received an AXIOS stent, 27 patients (93%) showed resolution of their PFC, and stent migration was noted in only one patient. In a large multicenter trial involving 15 European centers, 61 patients with either a pseudocyst (n = 46, 75%) or WON (n = 15, 25%) were drained using AXIOS stents. In that study, stent placement was judged to be technically successful in 60 (98%) of the 61 patients. Clinical success, defined as resolution of clinical symptoms combined with a decrease in the PFC size to ≤ 2 cm on imaging, was achieved in 93% of patients with a pancreatic pseudocyst and 81% of patients with a WON. Treatment failure occurred in nine patients (16%), including four patients who required surgical intervention. Stent removal was performed in 82% of patients after a median time of 32 d, and the removal procedure was rated as “easy” by all but one patient. Endoscopic stent removal was not performed in a total of 10 patients due to stent migration (n = 3), stent dislodgement during necrosectomy (n = 3), stent removal during surgery (n = 2), or patient refusal (n = 2).
SINGLE OR MULTIPLE STENTS?
No randomized control trial has compared the benefits of using a single plastic stent vs multiple plastic stents vs a metal stent for treating a pseudocyst. However, retrospective studies have shown that insertion of even a single stent provides high rates of clinical resolution. This is probably because stent occlusion has not occurred due to the absence of solid necrotic debris. A recent meta-analysis of 14 studies involving 698 patients found no difference in the rates of treatment success between patients managed with multiple plastic stents vs metal stents (89%, 95%CI: 87-91 vs 87% 95%CI: 76 to 91; P = 0.22, respectively). Furthermore, the two cohorts showed no difference in their respective rates of adverse events or pseudocyst recurrence[34]. However, these results may not apply in cases of WON, because stent occlusion and consequent treatment failure are likely occurrences, and the chances of achieving clinical resolution and treatment success depend on providing adequate drainage. This notion is reinforced by the successful use of MTGT to treat cases of WON as proposed by Varadarajulu et al[18], who placed multiple stents at different sites in conjunction with a nasocystic drain to achieve resolution of a WON in > 90% of cases.
DEFINITION OF CLINICAL AND RADIOLOGICAL SUCCESS
Technical success is defined as the ability to access and drain a pancreatic pseudocyst by placement of a stent. Treatment success involves both clinical and radiologic improvement. Clinical success refers to the resolution of symptoms that prompted an intervention. Radiologic success refers to a decrease in the size of a cyst or its resolution. In a randomized clinical trial comparing endoscopic and surgical drainage of pseudocysts, treatment success for endoscopy was defined as complete resolution or a decrease in the size of a fluid collection to 2 cm or less as seen on a CT scan, in combination with the resolution of clinical symptoms as determined at an 8-wk outpatient follow-up evaluation[8]. Follow-up is usually done using upper endoscopies and imaging techniques (either a CT scan or abdominal ultrasound).
EFFICACY OF EUS-GUIDED DRAINAGE OF PANCREATIC FLUID COLLECTIONS
Very high clinical success rates (90%-100%) have been achieved by draining pseudocysts[9,12,23]; however, less data is available concerning the clinical success rates achieved when draining an abscess. While high treatment success rates ranging from 80%[35] to > 90%[12] have been reported, the rates for clinical resolution of a WON are generally poor. In a recent study of 211 patients with symptomatic PFCs, the rate of success in treating sterile and infected pseudocysts was 93.5%, compared to only 63.2% when treating WONs[36]. However, the success rate for treating WONs can be improved to 81%[17] if an aggressive endoscopic approach using endoscopic necrosectomy is adopted; although adjunctive surgical and percutaneous drainage may be needed. Varadarajulu et al[18] suggested multiple transluminal gateway treatment for a WON, and achieved a clinically positive response in 92% of patients when using this method. In a retrospective review of 31 patients who received EUS-guided drainage of fluid collections after pancreatic resection, EUS-guided drainage was performed with a technical success rate of 100%, and clinical success was achieved in 29 of the 31 patients (93%)[37].
COMPLICATIONS AND RISKS OF EUS-GUIDED TRANSMURAL DRAINAGE
When using EUS-guided transmural drainage, the rates of complication range from 1%-18%[12,24,36,38-40], and complications most frequently manifest as bleeding, perforation, secondary infection or stent migration. A retrospective study conducted by Varadarajulu et al[13] reported a significantly higher complication frequency in cases of pancreatic necrosis (15%) when compared to cases involving a pseudocyst or abscess (5%). Secondary infection is caused by contamination of an incompletely drained WON or pseudocyst resulting from premature stent occlusion or its uneven collapse, and occurs in about 10% of cases. The perforation risk increases when a pseudocyst wall is poorly defined or is located > 1 cm from the intestinal lumen. Very few cases of procedure-related mortality have been reported, and the ones that have were mainly related to bleeding[12,41,42]. Because surgery is required in 5%-11% of cases, most complications are conservatively managed by an interventional radiologist or endoscopy[43]. Complications such as pneumothorax, air embolism, and intra-abdominal abscess have been seldom reported in the literature.
WHEN TO CONSIDER SURGERY
Walled-off pancreatic collections can be surgically managed with either an open surgical procedure or a laparoscopic approach. Most surgical literature has described the use of open surgical drainage procedures. Open surgical drainage can be accomplished via cystgastrostomy, cystenterostomy (direct drainage or via a Roux limb) or resection. However, drainage can also be accomplished using laparoscopic techniques. Laparoscopic cystgastrostomy can be performed via an anterior transgastric approach or a posterior approach through the lesser sac. The latter approach requires only a single gastrotomy in continuity with the walled-off pancreatic fluid collection. During the last 10 years, endoscopic drainage has come to the forefront and demonstrated efficacy comparable to that of surgical drainage; additionally, it is less expensive to perform and requires a shorter hospital stay.
Surgical drainage is a multidisciplinary decision and should only be considered for patients who have experienced previous endoscopic failures, disease recurrence following a successful endoscopic drainage, and patients who do not satisfy the criteria for endoscopic or percutaneous drainage. In 2011, Seewald et al[44] published a paper describing the long term results of patients who underwent endoscopic drainage of PFCs. Their retrospective analysis of 80 patients with symptomatic PFCs showed that fluid collections were clinically resolved by endoscopic methods in 67 (83.8%) patients, and surgery was required for 13 patients (perforation: four patients; endoscopically inaccessible areas: two patients; inadequate drainage: seven patients). Five patients required surgery within 6 mo after their first treatment due to recurrent fluid collection. Moreover, during a mean followup period of 31 mo, an additional four patients required surgery due to recurrent collections as a consequence of underlying pancreatic duct abnormalities that could not be treated endoscopically. The long-term success rate of endoscopic treatment was 72.5% (58/80 patients), and 28% of patients required surgery.
The traditional approach to treating necrotizing pancreatitis accompanied by a secondary infection of necrotic tissue is open necrosectomy to completely remove the infected necrotic tissue. However, this invasive approach is associated with high rates of complications and death (11%-39%), as well as a risk of long-term pancreatic insufficiency. The Dutch pancreatitis study group showed that an incremental approach consisting of percutaneous drainage followed, if necessary, by minimally invasive retroperitoneal necrosectomy is a better strategy than open necrosectomy for treating patients with necrotizing pancreatitis and a secondary infection[45]. Additionally, new-onset multiple-organ failure occurred less often in patients treated using the incremental approach than in those treated via open necrosectomy (12% vs 40%, P = 0.002).
FUTURE DIRECTIONS
The revised Atlanta classification of acute pancreatitis better defines local complications which can be associated with pancreatitis: acute PFC, acute necrotic collection, pseudocyst formation, and WON. In recent decades, interventional EUS has been increasingly utilized in the management of these local complications. With development of improved and novel endoscopic devices dedicated to transmural drainage of fluid and necrotic debris (access and patency devices), we believe that EUS will become an indispensable part of procedures used to diagnose PFCs and image guided interventions.
The evidence provided in this review suggests that the amount of necrosis is the most important predictor of a successful outcome following drainage of a PFC. Hence, it seems logical to classify these collections based on their percentage of necrotic component or debris as indicated by radiological imaging or EUS. Thus we propose using a classification system in which fluid collections can be categorized into 3 groups: those with a solid component < 20%, those with a solid component between 20%-50%, and those with solid a component > 50%. As patient clinical outcomes are directly related to the type of fluid collection being treated, it is important to accurately distinguish a PFC before initiating intervention. In this review, the authors proposed using a management algorithm based on the amount of internal debris present in a PFC (Figure 8). For PFCs with < 20% internal debris, transmural drainage with 1-2 double pigtail plastic stents or a lumen apposing metal stent would probably be sufficient. For PFCs with > 50% internal debris, endoscopic necrosectomy with placement multiple plastic stents or a lumen apposing metal is required. If the pancreatic duct is connected to the cyst, trans-papillary drainage via ERCP can be performed on any of these patients at the discretion of an endoscopist.
Figure 8.
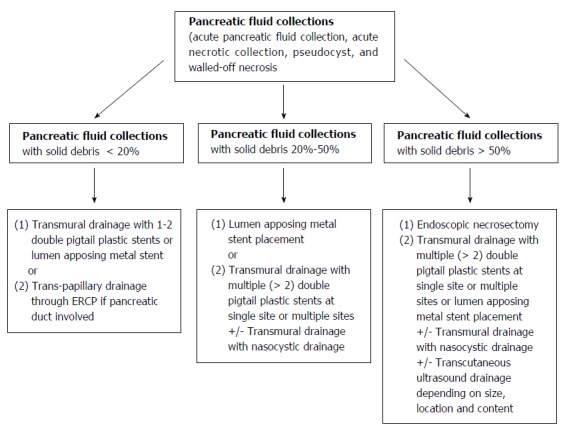
Authors' proposed endoscopic ultrasound-guided management algorithm based on the amount of internal debris inside a pancreatic fluid collection.
Footnotes
Conflict-of-interest statement: Vilmann AS, Menarchy J, Srinivasan I and Tang SJ have no potential conflicts of interest. Vilmann P reported a conflict as a consultant for MediGlobe, GmbH, Grassau, Germany.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 6, 2015
First decision: June 2, 2015
Article in press: September 2, 2015
P- Reviewer: Tomizawa M, Wilcox CM S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Wang CH
References
- 1.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 2.Dellinger EP, Forsmark CE, Layer P, Lévy P, Maraví-Poma E, Petrov MS, Shimosegawa T, Siriwardena AK, Uomo G, Whitcomb DC, et al. Determinant-based classification of acute pancreatitis severity: an international multidisciplinary consultation. Ann Surg. 2012;256:875–880. doi: 10.1097/SLA.0b013e318256f778. [DOI] [PubMed] [Google Scholar]
- 3.Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology. 2012;262:751–764. doi: 10.1148/radiol.11110947. [DOI] [PubMed] [Google Scholar]
- 4.Kaul V, Adler DG, Conway JD, Farraye FA, Kantsevoy SV, Kethu SR, Kwon RS, Mamula P, Pedrosa MC, Rodriguez SA, et al. Interventional EUS. Gastrointest Endosc. 2010;72:1–4. doi: 10.1016/j.gie.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez-Sánchez MV, Jenssen C, Faiss S, Napoléon B. Interventional endoscopic ultrasonography: an overview of safety and complications. Surg Endosc. 2014;28:712–734. doi: 10.1007/s00464-013-3260-5. [DOI] [PubMed] [Google Scholar]
- 6.Gardner TB. Endoscopic management of necrotizing pancreatitis. Gastrointest Endosc. 2012;76:1214–1223. doi: 10.1016/j.gie.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 7.Gardner TB, Chahal P, Papachristou GI, Vege SS, Petersen BT, Gostout CJ, Topazian MD, Takahashi N, Sarr MG, Baron TH. A comparison of direct endoscopic necrosectomy with transmural endoscopic drainage for the treatment of walled-off pancreatic necrosis. Gastrointest Endosc. 2009;69:1085–1094. doi: 10.1016/j.gie.2008.06.061. [DOI] [PubMed] [Google Scholar]
- 8.Varadarajulu S, Bang JY, Sutton BS, Trevino JM, Christein JD, Wilcox CM. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology. 2013;145:583–90.e1. doi: 10.1053/j.gastro.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 9.Varadarajulu S, Christein JD, Tamhane A, Drelichman ER, Wilcox CM. Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts (with videos) Gastrointest Endosc. 2008;68:1102–1111. doi: 10.1016/j.gie.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 10.Dumonceau JM, Delhaye M, Tringali A, Dominguez-Munoz JE, Poley JW, Arvanitaki M, Costamagna G, Costea F, Devière J, Eisendrath P, et al. Endoscopic treatment of chronic pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2012;44:784–800. doi: 10.1055/s-0032-1309840. [DOI] [PubMed] [Google Scholar]
- 11.Tenner S, Baillie J, DeWitt J, Vege SS. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400–115; 1416. doi: 10.1038/ajg.2013.218. [DOI] [PubMed] [Google Scholar]
- 12.Hookey LC, Debroux S, Delhaye M, Arvanitakis M, Le Moine O, Devière J. Endoscopic drainage of pancreatic-fluid collections in 116 patients: a comparison of etiologies, drainage techniques, and outcomes. Gastrointest Endosc. 2006;63:635–643. doi: 10.1016/j.gie.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 13.Varadarajulu S, Bang JY, Phadnis MA, Christein JD, Wilcox CM. Endoscopic transmural drainage of peripancreatic fluid collections: outcomes and predictors of treatment success in 211 consecutive patients. J Gastrointest Surg. 2011;15:2080–2088. doi: 10.1007/s11605-011-1621-8. [DOI] [PubMed] [Google Scholar]
- 14.Varadarajulu S, Christein JD, Wilcox CM. Frequency of complications during EUS-guided drainage of pancreatic fluid collections in 148 consecutive patients. J Gastroenterol Hepatol. 2011;26:1504–1508. doi: 10.1111/j.1440-1746.2011.06771.x. [DOI] [PubMed] [Google Scholar]
- 15.Varadarajulu S, Lopes TL, Wilcox CM, Drelichman ER, Kilgore ML, Christein JD. EUS versus surgical cyst-gastrostomy for management of pancreatic pseudocysts. Gastrointest Endosc. 2008;68:649–655. doi: 10.1016/j.gie.2008.02.057. [DOI] [PubMed] [Google Scholar]
- 16.Baron TH, Harewood GC, Morgan DE, Yates MR. Outcome differences after endoscopic drainage of pancreatic necrosis, acute pancreatic pseudocysts, and chronic pancreatic pseudocysts. Gastrointest Endosc. 2002;56:7–17. doi: 10.1067/mge.2002.125106. [DOI] [PubMed] [Google Scholar]
- 17.Seifert H, Biermer M, Schmitt W, Jürgensen C, Will U, Gerlach R, Kreitmair C, Meining A, Wehrmann T, Rösch T. Transluminal endoscopic necrosectomy after acute pancreatitis: a multicentre study with long-term follow-up (the GEPARD Study) Gut. 2009;58:1260–1266. doi: 10.1136/gut.2008.163733. [DOI] [PubMed] [Google Scholar]
- 18.Varadarajulu S, Phadnis MA, Christein JD, Wilcox CM. Multiple transluminal gateway technique for EUS-guided drainage of symptomatic walled-off pancreatic necrosis. Gastrointest Endosc. 2011;74:74–80. doi: 10.1016/j.gie.2011.03.1122. [DOI] [PubMed] [Google Scholar]
- 19.Rana SS, Bhasin DK, Reddy YR, Sharma V, Rao C, Sharma RK, Gupta R. Morphological features of fluid collections on endoscopic ultrasound in acute necrotizing pancreatitis: do they change over time? Ann Gastroenterol. 2014;27:258–261. [PMC free article] [PubMed] [Google Scholar]
- 20.Rana SS, Bhasin DK, Sharma RK, Kathiresan J, Gupta R. Do the morphological features of walled off pancreatic necrosis on endoscopic ultrasound determine the outcome of endoscopic transmural drainage? Endosc Ultrasound. 2014;3:118–122. doi: 10.4103/2303-9027.131039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seewald S, Thonke F, Ang TL, Omar S, Seitz U, Groth S, Zhong Y, Yekebas E, Izbicki J, Soehendra N. One-step, simultaneous double-wire technique facilitates pancreatic pseudocyst and abscess drainage (with videos) Gastrointest Endosc. 2006;64:805–808. doi: 10.1016/j.gie.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 22.Walter D, Will U, Sanchez-Yague A, Brenke D, Hampe J, Wollny H, López-Jamar JM, Jechart G, Vilmann P, Gornals JB, et al. A novel lumen-apposing metal stent for endoscopic ultrasound-guided drainage of pancreatic fluid collections: a prospective cohort study. Endoscopy. 2015;47:63–67. doi: 10.1055/s-0034-1378113. [DOI] [PubMed] [Google Scholar]
- 23.Lopes CV, Pesenti C, Bories E, Caillol F, Giovannini M. Endoscopic ultrasound-guided endoscopic transmural drainage of pancreatic pseudocysts. Arq Gastroenterol. 2008;45:17–21. doi: 10.1590/s0004-28032008000100004. [DOI] [PubMed] [Google Scholar]
- 24.Antillon MR, Shah RJ, Stiegmann G, Chen YK. Single-step EUS-guided transmural drainage of simple and complicated pancreatic pseudocysts. Gastrointest Endosc. 2006;63:797–803. doi: 10.1016/j.gie.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 25.Siddiqui AA, Dewitt JM, Strongin A, Singh H, Jordan S, Loren DE, Kowalski T, Eloubeidi MA. Outcomes of EUS-guided drainage of debris-containing pancreatic pseudocysts by using combined endoprosthesis and a nasocystic drain. Gastrointest Endosc. 2013;78:589–595. doi: 10.1016/j.gie.2013.03.1337. [DOI] [PubMed] [Google Scholar]
- 26.Due SL, Wilson TG, Chung A, Chen JWC. Endoscopic cyst-gastrostomy for pancreatic pseudocysts: refining the indications. ANZ J Surg. 2014:Epub ahead of print. doi: 10.1111/ans.12648. [DOI] [PubMed] [Google Scholar]
- 27.Fabbri C, Luigiano C, Cennamo V, Polifemo AM, Barresi L, Jovine E, Traina M, D’Imperio N, Tarantino I. Endoscopic ultrasound-guided transmural drainage of infected pancreatic fluid collections with placement of covered self-expanding metal stents: a case series. Endoscopy. 2012;44:429–433. doi: 10.1055/s-0031-1291624. [DOI] [PubMed] [Google Scholar]
- 28.Talreja JP, Shami VM, Ku J, Morris TD, Ellen K, Kahaleh M. Transenteric drainage of pancreatic-fluid collections with fully covered self-expanding metallic stents (with video) Gastrointest Endosc. 2008;68:1199–1203. doi: 10.1016/j.gie.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 29.Penn DE, Draganov PV, Wagh MS, Forsmark CE, Gupte AR, Chauhan SS. Prospective evaluation of the use of fully covered self-expanding metal stents for EUS-guided transmural drainage of pancreatic pseudocysts. Gastrointest Endosc. 2012;76:679–684. doi: 10.1016/j.gie.2012.04.457. [DOI] [PubMed] [Google Scholar]
- 30.Weilert F, Binmoeller KF, Shah JN, Bhat YM, Kane S. Endoscopic ultrasound-guided drainage of pancreatic fluid collections with indeterminate adherence using temporary covered metal stents. Endoscopy. 2012;44:780–783. doi: 10.1055/s-0032-1309839. [DOI] [PubMed] [Google Scholar]
- 31.Berzosa M, Maheshwari S, Patel KK, Shaib YH. Single-step endoscopic ultrasonography-guided drainage of peripancreatic fluid collections with a single self-expandable metal stent and standard linear echoendoscope. Endoscopy. 2012;44:543–547. doi: 10.1055/s-0031-1291710. [DOI] [PubMed] [Google Scholar]
- 32.Itoi T, Binmoeller KF, Shah J, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, et al. Clinical evaluation of a novel lumen-apposing metal stent for endosonography-guided pancreatic pseudocyst and gallbladder drainage (with videos) Gastrointest Endosc. 2012;75:870–876. doi: 10.1016/j.gie.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 33.Gornals JB, De la Serna-Higuera C, Sánchez-Yague A, Loras C, Sánchez-Cantos AM, Pérez-Miranda M. Endosonography-guided drainage of pancreatic fluid collections with a novel lumen-apposing stent. Surg Endosc. 2013;27:1428–1434. doi: 10.1007/s00464-012-2591-y. [DOI] [PubMed] [Google Scholar]
- 34.Navaneethan U, Njei B, Sanaka MR. 734 Endoscopic Transmural Drainage of Pancreatic Pseudocysts: Multiple Plastic Stents Versus Metal Stents- a Systematic Review and Meta-Analysis. Gastrointest Endosc. 2014;79:AB167–8. [Google Scholar]
- 35.Seewald S, Groth S, Omar S, Imazu H, Seitz U, de Weerth A, Soetikno R, Zhong Y, Sriram PV, Ponnudurai R, et al. Aggressive endoscopic therapy for pancreatic necrosis and pancreatic abscess: a new safe and effective treatment algorithm (videos) Gastrointest Endosc. 2005;62:92–100. doi: 10.1016/s0016-5107(05)00541-9. [DOI] [PubMed] [Google Scholar]
- 36.Varadarajulu S, Tamhane A, Blakely J. Graded dilation technique for EUS-guided drainage of peripancreatic fluid collections: an assessment of outcomes and complications and technical proficiency (with video) Gastrointest Endosc. 2008;68:656–666. doi: 10.1016/j.gie.2008.03.1091. [DOI] [PubMed] [Google Scholar]
- 37.Tilara A, Gerdes H, Allen P, Jarnagin W, Kingham P, Fong Y, DeMatteo R, D’Angelica M, Schattner M. Endoscopic ultrasound-guided transmural drainage of postoperative pancreatic collections. J Am Coll Surg. 2014;218:33–40. doi: 10.1016/j.jamcollsurg.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Lopes CV, Pesenti C, Bories E, Caillol F, Giovannini M. Endoscopic-ultrasound-guided endoscopic transmural drainage of pancreatic pseudocysts and abscesses. Scand J Gastroenterol. 2007;42:524–529. doi: 10.1080/00365520601065093. [DOI] [PubMed] [Google Scholar]
- 39.Kahaleh M, Shami VM, Conaway MR, Tokar J, Rockoff T, De La Rue SA, de Lange E, Bassignani M, Gay S, Adams RB, et al. Endoscopic ultrasound drainage of pancreatic pseudocyst: a prospective comparison with conventional endoscopic drainage. Endoscopy. 2006;38:355–359. doi: 10.1055/s-2006-925249. [DOI] [PubMed] [Google Scholar]
- 40.Barthet M, Lamblin G, Gasmi M, Vitton V, Desjeux A, Grimaud JC. Clinical usefulness of a treatment algorithm for pancreatic pseudocysts. Gastrointest Endosc. 2008;67:245–252. doi: 10.1016/j.gie.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 41.Trevino JM, Tamhane A, Varadarajulu S. Successful stenting in ductal disruption favorably impacts treatment outcomes in patients undergoing transmural drainage of peripancreatic fluid collections. J Gastroenterol Hepatol. 2010;25:526–531. doi: 10.1111/j.1440-1746.2009.06109.x. [DOI] [PubMed] [Google Scholar]
- 42.Ardengh JC, Coelho DE, Coelho JF, de Lima LF, dos Santos JS, Módena JL. Single-step EUS-guided endoscopic treatment for sterile pancreatic collections: a single-center experience. Dig Dis. 2008;26:370–376. doi: 10.1159/000177024. [DOI] [PubMed] [Google Scholar]
- 43.Sadik R, Kalaitzakis E, Thune A, Hansen J, Jönson C. EUS-guided drainage is more successful in pancreatic pseudocysts compared with abscesses. World J Gastroenterol. 2011;17:499–505. doi: 10.3748/wjg.v17.i4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seewald S, Ang TL, Richter H, Teng KY, Zhong Y, Groth S, Omar S, Soehendra N. Long-term results after endoscopic drainage and necrosectomy of symptomatic pancreatic fluid collections. Dig Endosc. 2012;24:36–41. doi: 10.1111/j.1443-1661.2011.01162.x. [DOI] [PubMed] [Google Scholar]
- 45.van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, van Goor H, Schaapherder AF, van Eijck CH, Bollen TL, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362:1491–1502. doi: 10.1056/NEJMoa0908821. [DOI] [PubMed] [Google Scholar]


