Abstract
AIM: To assist in the selection of suitable nomograms for obtaining desired predictions in daily clinical practice.
METHODS: We conducted electronic searches for journal articles on colorectal cancer (CRC)-associated nomograms using the search terms colon/rectal/colorectal/nomogram. Of 174 articles initially found, we retrieved 28 studies in which a nomogram for CRC was developed.
RESULTS: We discuss the currently available CRC-associated nomograms, including those that predict the oncological prognosis, the short-term outcome of treatments, such as surgery or neoadjuvant chemoradiotherapy, and the future development of CRC. Developing nomograms always presents a dilemma. On the one hand, the desire to cover as wide a patient range as possible tends to produce nomograms that are too complex and yet have C-indexes that are not sufficiently high. Conversely, confining the target patients might impair the clinical applicability of constructed nomograms.
CONCLUSION: The information provided in this review should be of use in selecting a nomogram suitable for obtaining desired predictions in daily clinical practice.
Keywords: Colon, Rectum, Nomograms, Prognosis, Cancer
Core tip: In this review, we discuss currently available colorectal cancer (CRC)-associated nomograms, including those that predict the oncological prognosis, the short-term outcome of treatments, such as surgery or neoadjuvant chemoradiotherapy, and the future development of CRC. This review aims to assist in the selection of suitable nomograms for obtaining desired predictions in daily clinical practice.
INTRODUCTION
Colorectal cancer (CRC) is one of the most common malignancies in Asia as well as in most Western countries[1]. A number of studies have suggested scoring or stratifying the risk associated with CRC, as represented by the American Joint Committee on Cancer TNM classifications[2-4]. A nomogram is a graphic calculating scale designed to provide the likelihood of the occurrence of a specific event. In clinical practice, a nomogram is typically used to predict the probability of a particular outcome as related to a disease. The clinical use of nomograms extends as far back as 1928 when nomograms were first used by Lawrence Henderson[5]. In recent years, a number of nomograms concerning the treatment of cancers, including prostate cancers[6], gastric cancers[7], and CRCs, have been reported because of their user friendly interface and strong statistical ability to predict individualized outcome.
In this systematic review, we discuss the currently available CRC-related nomograms, including those that predict the prognosis, the short-term outcome of treatments, such as surgery or neoadjuvant chemoradiotherapy (CRT), and CRC prevalence.
MATERIALS AND METHODS
Evidence acquisition
We used PubMed to perform electronic searches for publications on CRC-associated nomograms. Our search included all English language entries from inception until February 2015 and incorporated the following keywords: nomogram/colon/rectal/colorectal in all fields. Only human studies were eligible for inclusion; case reports, editorials, letters, commentaries, and nomograms that were not published in print were excluded. Studies that only validated previously published nomograms without describing the development of new nomograms were also excluded. The initial search resulted in 174 publications. After title and abstract screening, 41 studies remained, and 28 were finally selected for the present review after full text screening (Figure 1).
Figure 1.
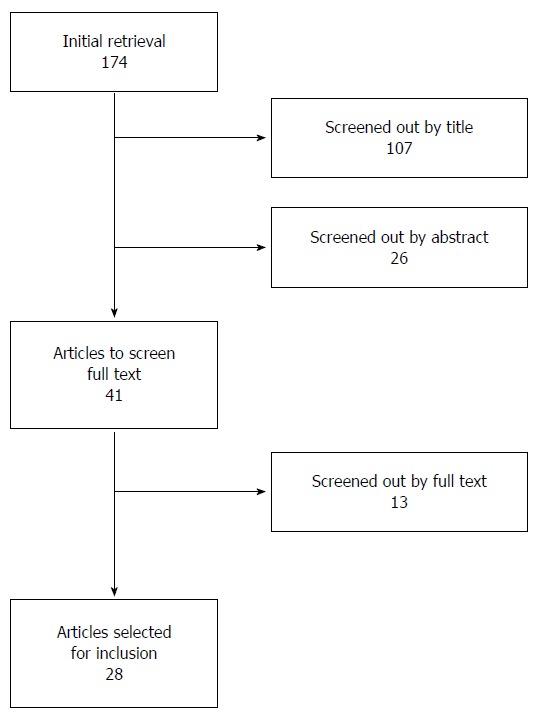
Flow chart of the study selection process.
RESULTS
Assessment of the predictive quality of nomograms
Before applying published nomograms to clinical practice, understanding the reliability of the predictions as well as the limitations of each nomogram is essential. First, the targeted patient characteristics and predicted outcomes should be noted. The targeted cancer location and the TNM stage varies among nomograms, and inputting data into a nomogram that was not developed pursuant to a particular patient’s disease type might result in misreporting the probabilities. In Tables 1, 2, 3 and 4[8-42], we tabulated nomograms according to patient backgrounds for which the nomograms were developed as well as the intended outcomes with the aim of assisting clinical doctors in selecting the appropriate nomogram for their particular needs.
Table 1.
Nomograms predicting stage I-III colorectal cancer oncological prognosis
| Ref. | Year | Cancer location | Targeted patients | Predicted outcome | Number of patients | C-index | Validation | Calibration | Variables | Comments |
| Weiser et al[8] | 2008 | Colon | Stage I-III | RFS | 1320 | 0.77 | Absent | Present | Age, CEA, No. of positive and negative nodes, pT, adjuvant chemotherapy, cancer location, differentiation, lymphovascular invasion, perineural invasion | |
| Segelman et al[9] | 2014 | Colorectal | Stage I-III | Peritoneal carcinomatosis | 8044 | 0.78-0.80 | Absent | Present | Age, cancer location, pT, pN, radicality, type of surgery, preoperative radiotherapy, nodes examined, adjuvant chemotherapy | Only web-calculator was available |
| Ying et al[10] | 2014 | Colorectal | Stage I-III | RFS, OS, CSS | 205 | 0.80-0.81 | Absent | Absent | Chemotherapy, tumor size, cell differentiation, TNM stage, neutrophil-to-lymphocyte ratio | |
| Zhang et al[11] | 2013 | Colon | Stage II | RFS | 735 | 0.65-0.82 | Present | Present | Expression of microRNA, pT, internal obstruction or perforation, nodes examined, tumor grade | |
| Goossens-Beumer et al[12] | 2015 | Colorectal | Stage II/III | RFS | 93 | 0.80 | Present | Present | Expression of microRNA, TNM stage, age, gender | |
| Peng et al[13] | 2014 | Rectal | Stage II/III | OS, distant metastasis | 883 | 0.68-0.76 | Present | Absent | Gender, age, CEA, cancer location, pT, pN, ratio of metastatic lymph nodes, adjuvant chemotherapy, adjuvant chemoradiotherapy | |
| Valentini et al[14] | 2011 | Rectal | Clinical stage II/III patients undergoing adjuvant radiotherapy or chemora | OS, local recurrence, distant metastasis | 2795 | 0.68-0.73 | Present | Present | pT, cT, pN, age, concomitant and adjuvant chemotherapy, surgical procedure, gender, dose of radiotherapy | |
| van Gijn et al[15] | 2015 | Rectal | Stage I-III | OS, local recurrence, distant metastasis | 2881 | 0.75-0.79 | Absent | Absent | Age, pT, pN, PA-stage, distance, residual cancer, surgical type, gender, radiotherapy, complications |
RFS: Recurrence-free survival; OS: Overall survival; CSS: Cancer-specific survival; C-index: Concordance index; CEA: Carcinoembryonic antigen.
Table 2.
Nomograms predicting stage IV colorectal cancer oncological prognosis
| Ref. | Year | Targeted cancer | Treatment | Predicted outcome | Number of patients | C-index | Validation | Calibration | Variables |
| Beppu et al[18] | 2012 | Liver metastasis | Hepatic resection | DFS | 727 | Not assessed | Validated by Okuno et al[26] | Absent | Metachronous or synchronous, pN, No. of tumors, largest tumor diameter, extrahepatic metastasis, CA19-9 |
| Kanemitsu et al[19] | 2008 | Liver metastasis | Hepatic resection | OS, CSS | 578 | 0.66-0.68 | Validated by Takakura et al[27] | Present | Histology, No. of lymph node metastases, No. of tumors, extrahepatic metastasis, metastasis of hilar lymph nodes, surgical margin, CEA |
| Kattan et al[20] | 2008 | Liver metastasis | Hepatic resection | CSS | 1477 | 0.61 | Validated by Takakura et al[27], Reddy et al[28], and Nathan et al[29] | Present | Gender, age, primary site, disease-free interval, CEA, No. of tumors, largest tumor diameter, bilateral resection, > 1 lobe, pN |
| Kanemitsu et al[21] | 2004 | Lung metastasis | Thoracotomy | OS | 313 | 0.66-0.72 | Validated by Kanemitsu et al[30] | Present | Histology, No. of tumors, hilar/mediastinal lymph nodes, extrathoracic metastasis, CEA |
| Elias et al[22] | 2014 | Liver and/or Peritoneal metastasis | Optimal surgery plus chemotherapy | OS | 287 | 0.61 | Absent | Present | No. of lymph node metastases, peritoneal carcinomatosis index, planified procedure |
| Kawai et al[23] | 2015 | Metastatic CRC | Curative resection | DFS, OS | 1133 | 0.60-0.64 | Present | Present | Postoperative CEA, pT, pN, No. of metastatic organs, peritoneal dissemination |
| Manceau et al[24] | 2014 | Metastatic CRC, KRAS-wild-type, refractory to chemotherapy | Anti-EGFR antibodies | Risk of progression | 132 | > 0.7 | Present | Absent | MicroRNA expression and BRAF mutations |
| Massacesi et al[25] | 2000 | Locally advanced or metastatic CRC | Chemotherapy | OS | 1057 | Not assessed | Absent | Absent | Response to chemotherapy, No. of metastatic sites, CEA, performance status |
DFS: Disease-free survival; OS: Overall survival; CSS: Cancer-specific survival; CRC: Colorectal cancer; C-index: Concordance index; CEA: Carcinoembryonic antigen; KRAS: Kirsten rat sarcoma viral oncogene homolog; EGFR: Epidermal growth factor receptor; BRAF: B-Raf proto-oncogene, serine/threonine kinase.
Table 3.
Nomograms predicting short-term outcomes of surgery for colorectal cancer
| Ref. | Year | Cancer location | Targeted patients | Predicted outcome | Number of patients | C-index | Validation | Calibration | Variables | Comments |
| Kiran et al[31] | 2013 | Colorectal | All colorectal surgeries | 30-d mortality | 30900 | 0.89 | Present | Present | Age, ASA, albumin, functional dependency, renal failure, emergency surgery, disseminated cancer | |
| Hedrick et al[32] | 2013 | Colorectal | All colorectal surgeries | Superficial SSI, deep incisional SSI, and combination thereof | 18403 | 0.64-0.65 | Absent | Present | Diabetes, smoking, disseminated cancer, BMI, open or laparoscopic surgery | |
| de Campos-Lobato et al[33] | 2009 | Small bowel/colorectal | All colorectal surgeries | Organ space SSI | 12373 | 0.65 | Present | Present | Surgical site, smoking, ASA, wound class, diabetes, steroid use, prior surgery, radiotherapy, open or laparoscopic surgery, age, BMI, creatinine, albumin, gender, transfusion, operative time | |
| Frasson et al[34] | 2014 | Colon | All colorectal surgeries | Anastomotic leakage | 3193 | 0.62-0.63 | Absent | Absent | Oral anticoagulants, intraoperative complications, BMI, total protein, gender, No. of beds | Decision-tree diagram was also presented |
| Yao et al[35] | 2014 | Rectal | Laparoscopic anterior resection with intracorporeal rectal transection and double-stapling technique anastomosis | Anastomotic leakage | 476 | 0.84 | Internal validation | Absent | Cancer location, operative time, preservation of the left colic artery | |
| Russell et al[36] | 2013 | Rectal/rectosigmoid | Stage I-III | Rate of margin positivity | 85190 | 0.75 | Absent | Present | Age, gender, ethnicity, cancer location, TNM stage, tumor size, tumor grade, insurance status, histology |
SSI: Surgical site infection; ASA: American Society of Anesthesiologists; BMI: Body mass index; C-index: Concordance index.
Table 4.
Other nomograms relevant to colorectal cancer
| Ref. | Year | Targeted patients | Treatment | Predicted outcome | Number of patients | C-index | Validation | Calibration | Variables | Comments |
| Jwa et al[37] | 2014 | Non-metastatic rectal cancer | CRT + surgery | ypN status | 891 | 0.77-0.81 | Present | Present | ypT, cN, histology, lymphovascular invasion, perineural invasion, age | |
| van Stiphout et al[38] | 2011 | Rectal cancer | CRT + surgery | Pathologic complete response | 953 | Not assessed | Present | Present | tumor length, RI, SUV | Pre- and post-CRT PET-CTs were used to predict response |
| van Stiphout et al[39] | 2014 | Rectal cancer | CRT + surgery | Pathologic complete response | 190 | 0.70-0.78 | Present | Absent | Maximal diameter at day 15, RI, cN | Pre- and intra-CRT PET-CTs were used to predict response |
| Omata et al[40] | 2011 | Asymptomatic individuals | Colorectal neoplasms | 1085 | Not assessed | Absent | Absent | Quantitative fecal immunochemical test, gender, age, BMI | ||
| Kawai et al[41] | 2014 | Colorectal cancer | Surgery | Postoperative development of metachronous colorectal neoplasms | 309 | 0.71 | Present | Present | Gender, age, No. of synchronous adenomas and colorectal cancers | |
| Wells et al[42] | 2014 | Age > 45 | Colorectal cancer development | 180630 | 0.68 | Absent | Present | Age, ethnicity, smoking, alcoholic drinks, BMI, education, aspirin, estrogen, family history of CRC, NSAIDs, multivitamins, red meat intake, diabetes, physical activity |
CRT: Chemoradiotherapy; PET-CT: Positron emission tomography-computed tomography; RI: Response index; SUV: Standardized uptake value; CRC: Colorectal cancer; BMI: Body mass index; C-index: Concordance index; NSAID: Non-steroidal anti-inflammatory drug.
Second, the concordance index (C-index) is important. The C-index represents the ability of a model to reliably predict whether individuals more likely to experience the intended result and is equivalent to the area under the receiver-operator characteristic curve if there are no censored cases. A value of 0.5 indicates no predictive discrimination, whereas a value of 1.0 indicates perfect separation of patients with different outcomes. C-indexes of most nomograms ranged from 0.7 to 0.8, and those below 0.7 were regarded to have a relatively low prediction ability. Third, whether the validation of the nomogram was disclosed or not is also essential. Because the outcome of a treatment varies substantially between institutions, results from a single institution tend to be biased. If a reported C-index that used patient data from an external institution was comparable to the C-index of the derivation data set, the nomogram was regarded as generally applicable across institutions. Finally, a calibration plot should be provided. The C-index only provides the overall stratifying ability of a nomogram, whereas a calibration plot represents the actual correlation between the nomogram-predicted probability and the observed incidence.
Nomograms predicting stage I-III CRC oncological prognosis
In terms of nomograms that predict long-term prognosis after CRC surgery, no nomogram that predicts prognosis for all stages has been developed because the prognosis for stages I-III differs substantially from that of stage IV and variables associated with prognosis also differ markedly. As shown in Table 1, our search retrieved 8 nomograms predicting the prognosis of stage I-III CRC patients[8-15]. Two nomograms were for colon cancer, three were for colorectal cancer, and the remaining three were for rectal cancer. Most of these nomograms were published within the past few years.
In 2008, Weiser et al[8] developed a nomogram predicting recurrence after surgery using general clinicopathological variables. Although the C-index of this nomogram was sufficiently high, the overall survival (OS) was not included in the outcome, and external validation was not performed. Recently, two nomograms for CRC, which were available in municipal hospitals, were published. One nomogram, developed by Segelman et al[9] was unique because it specialized in predicting peritoneal carcinomatosis recurrence. The other nomogram, developed by Ying et al[10], succeeded in achieving a high (greater than 0.8) C-index by adding preoperative neutrophil-to-lymphocyte ratio (NLR) to the conventional clinicopathological variables as an additional predictor. In several precedent studies, high NLR has been reported to correlate with a poorer prognosis in CRC[16,17], and this group established the clinical applicability of NLR by incorporating it into nomograms that calculated the probabilities of recurrence free survival (RFS), OS, and cancer-specific survival (CSS). Because the number of patients included was relatively small and no validation was performed, future studies validating the nomograms developed by Ying et al[10] with larger amounts of external patient data would reinforce their results. MicroRNA classifiers were incorporated in the remaining two nomograms. One such nomogram developed by Zhang et al[11] demonstrated that six microRNAs (miR-21-5p, miR-20a-5p, miR-103a-3p, miR-106b-5p, miR-143-5p, and miR215) independently predict prognosis, and one nomogram developed by Goossens-Beumer et al[12] focused on two microRNAs (miR-25-3p and miR-339-5p). Although these studies demonstrated the importance of microRNAs in CRC prognosis, currently, it may be difficult to apply these nomograms at municipal hospitals.
Three nomograms for rectal cancer prognosis have been reported to date. Most notably, the nomograms by Valentini et al[14] were developed using data from five major European clinical trials. Because OS, local recurrence, and distant metastasis were all included in the predicted outcome and because both validation and calibration were presented, these nomograms should have high clinical applicability. However, their usage is limited to patients who underwent radiotherapy or chemoradiotherapy (CRT).
Therefore, of the nomograms predicting stage I-III CRC prognosis, the nomograms developed by Weiser and Valentini for colon and rectal cancer, respectively, appear to be the most promising for clinical practice because, in these nomograms, the number of patients enrolled was large, no variables that are unavailable in municipal hospitals were incorporated, and the developed nomograms were well calibrated.
Nomograms predicting Stage IV colorectal cancer oncological prognosis
Nomograms predicting the prognosis of metastatic CRC are presented in Table 2[18-25]. Because stage IV CRC includes a wide variety of clinical settings, the C-indexes were relatively low with most being below 0.70. In contrast, most C-indexes of the nomograms for stage I-III CRC were above 0.75, as shown in Table 1. In terms of patients who underwent complete resection of metastases, three nomograms predicting the prognosis after resection of liver metastasis with curative intent have been established[18-20]; the widespread applicability of these nomograms was demonstrated by external validation studies[26-29]. These nomograms include both synchronous and metachronous liver metastasis, and two of these nomograms incorporated the interval between primary CRC surgery and hepatic resection as a variable because the prognosis of metachronous liver metastasis was better than that of synchronous lesions. Kanemitsu et al[21] and Kattan et al[20] demonstrated carcinoembryonic antigen (CEA) to be a strong prognosis-predictive marker, whereas Beppu focused on CA19-9. Kanemitsu et al[21] also constructed a nomogram predicting OS after thoracotomy for lung metastasis from CRC[21], which they subsequently validated in a separate study[30]. Elias et al[22] reported a nomogram specifically for those with liver and/or peritoneal metastasis and for those that underwent surgery including hyperthermic intraperitoneal chemotherapy (HIPEC) with no macroscopically residual cancer[22]. The nomogram was unique in that it was based on the outcome of 156 HIPEC patients. Recently, we built nomograms predicting DFS and OS after curative resection of stage IV CRC, namely, the complete resection of both primary CRC and synchronous distant metastasis[23]. We focused on the CEA concentration shortly after surgery because high postoperative CEA may be indicative of residual cancer cells and, consequently, of recurrence. The nomograms should have an advantage over previous nomograms because they may apply to all stage IV cases regardless of the metastatic organ, although their C-indexes were no greater than 0.7, which is similar to other stage IV nomograms.
The remaining two nomograms predicted the outcome of chemotherapy for those who were unable to undergo complete surgical resection. One nomogram demonstrated the significance of hsa-miR-31-3p expression as a risk factor for cancer progression in patients who were refractory to chemotherapy and were treated with anti-EGFR therapy[24], and the other nomogram demonstrated the 2-year survival of locally advanced or metastatic CRC patients[25]. Because the latter was developed using patient data gathered between 1990 and 1998, the predicted survival may currently be improved due to the subsequent development of diverse chemotherapeutic agents.
Nomograms predicting short-term outcomes of surgery for colorectal cancer
There have been six published nomograms predicting short-term operative outcomes, namely, mortality[31], surgical site infection (SSI)[32,33], anastomotic leakage[34,35], and the rate of margin positivity[36] (Table 3). Because of the low incidences of these outcomes, most of the nomograms were constructed using large national databases such as the American College of Surgeons’ National Surgical Quality Improvement Program; consequently, the numbers of enrolled patients were greater than 10000 in five of these nomograms, which was an order of magnitude greater than the number of patients in the majority of the studies predicting long-term oncological prognosis.
The 30-d mortality risk, which was the most serious postoperative complication, was predicted by Kiran et al[31]’s nomogram. This nomogram achieved a C-index of 0.89 by focusing particularly on age. There have also been three nomograms for calculating the incidence of SSI or anastomotic leakage in general colorectal surgery[32-34]. Because the occurrence of these complications was largely affected by the surgical procedures, it may be difficult to accurately anticipate the complications in advance using statistical models. Therefore, the C-indexes of these nomograms were only 0.65 at most. Recently, Yao et al[35] reported another nomogram predicting anastomotic leakage. Although its C-index was high (0.84), this exclusive nomogram only covered patients who underwent laparoscopic anterior resection with intracorporeal rectal transection and anastomosis using the double-stapling technique. In addition to postoperative complications, the rate of margin positivity in rectal cancer surgery was also predicted. Because the circumferential resection margin is a major determinant of local recurrence, predicting the rate preoperatively should be of considerable clinical benefit. However, a nomogram developed by Russell et al[36] incorporated factors that could not be confirmed preoperatively, such as tumor stage and size, and its actual clinical applicability was therefore limited.
Other nomograms relevant to CRC
Among the remaining six nomograms related to CRC, three concerned the prediction of the response to preoperative CRT in rectal cancer[37-39]. This was quite important in deciding the post-CRT treatment because accurate prediction of lymph node metastasis after CRT might enable the reduction of the surgical resection to local excision of the tumor instead of performing total mesorectal excision. Similarly, perfect prediction of the pathological complete response (pCR) might make it possible to omit even the surgery itself. One nomogram reported by Jwa et al[37] predicted the lymph node metastasis status of rectal cancer after CRT. Because this nomogram used the ypT stage, lymphovascular invasion, and perineural invasion as variables, it could not determine a suitable surgical procedure in advance. Alternatively, to clinically utilize the nomogram, local excision and pathological examination must first be performed, and if the risk of nodal metastasis calculated by the final pathological findings is acceptably low, omission of further surgical treatment accompanied by lymph node dissection could be one of the therapeutic options. van Stiphout et al[38] reported two nomograms predicting pCR by using positron emission tomography (PET)-computer tomography (CT) as the predictor[38,39]. In their first study, PET-CT was performed before and after CRT, and they incorporated the response ratio calculated by the standardized uptake values of these two PET-CTs into their nomogram[38]. Alternatively, they performed PET-CT before and two weeks after the start of CRT in their latter nomogram and demonstrated that the response ratio between the two PET-CT scans (i.e., early response to CRT) is also a promising predictive factor available in the nomogram[39]. In the future, the accumulation of these data may enable the identification of patients who can either avoid unnecessary overtreatment or who should receive additional chemotherapy or radiotherapy.
Finally, we describe three nomograms that attempt to detect or predict newly developed CRCs[40-42]. Omata et al[40] demonstrated the diagnostic performance of the quantitative fecal immunochemical test (QTFIT) for colorectal neoplasms in asymptomatic individuals, and the addition of sex, age, and body mass index to the nomograms could amplify the accuracy of QTFIT as a screening test. Recently, we developed a nomogram that could predict the development of metachronous colorectal neoplasms after surgical resection of primary CRC[41] because patients who previously had CRC are at a high risk for developing second primary adenoma or CRC. Wells et al[42] also provided a nomogram calculating the 10-year risk of CRC development. The latter two nomograms were of clinical utility in identifying those patients who should receive intensive colonoscopy screening.
DISCUSSION
In the field of prostate cancer, a number of nomograms predicting a wide variety of outcomes, such as cancer prognosis[43], diagnosis[44], and screening[45], have been developed and well validated. In contrast, nomograms for CRC fall behind nomograms for prostate cancer, with the targeted patients and performed validation studies being limited. Therefore, further developments and validations of novel nomograms for CRC are needed. Developing nomograms always presents a dilemma. On the one hand, the desire to cover as wide a patient range as possible tends to produce nomograms that are too complex and yet have C-indexes that are not sufficiently high. Conversely, confining the target patients might impair the clinical applicability of constructed nomograms. The information provided in this review should be of use in selecting a nomogram suitable for obtaining desired predictions in daily clinical practice.
COMMENTS
Background
A nomogram is a graphic calculating scale designed to provide the likelihood of the occurrence of a specific event. In clinical practice, a nomogram is typically used to predict the probability of a particular outcome as related to a disease.
Research frontiers
In recent years, a number of nomograms concerning the treatment of cancers, including prostate cancers, gastric cancers, and colorectal cancers (CRCs), have been reported because of their user friendly interface and strong statistical ability to predict individualized outcome.
Applications
In this systematic review, the authors discuss the currently available CRC-related nomograms, including those that predict the prognosis, the short-term outcome of treatments, such as surgery or neoadjuvant chemoradiotherapy, and CRC prevalence. The information provided in this review should be of use in selecting a nomogram suitable for obtaining desired predictions in daily clinical practice.
Peer-review
It is an interesting paper with a good review of a frequently disperse information, well-written review and may have a potential significance for clinical practice of CRC.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 28, 2015
First decision: May 18, 2015
Article in press: September 30, 2015
P- Reviewer: Aparicio J, Cui GL S- Editor: Yu J L- Editor: A E- Editor: Liu XM
References
- 1.Hyodo I, Suzuki H, Takahashi K, Saito Y, Tanaka S, Chiu HM, Kim NK, Li J, Lim R, Villalon A, et al. Present status and perspectives of colorectal cancer in Asia: Colorectal Cancer Working Group report in 30th Asia-Pacific Cancer Conference. Jpn J Clin Oncol. 2010;40 Suppl 1:i38–i43. doi: 10.1093/jjco/hyq125. [DOI] [PubMed] [Google Scholar]
- 2.Edge S, Byrd D, Compton C, Fritz A, Greene F, A . T. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. [Google Scholar]
- 3.Ishizuka M, Nagata H, Takagi K, Horie T, Kubota K. Inflammation-based prognostic score is a novel predictor of postoperative outcome in patients with colorectal cancer. Ann Surg. 2007;246:1047–1051. doi: 10.1097/SLA.0b013e3181454171. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi H, Kotake K, Sugihara K. Prognostic scoring system for stage IV colorectal cancer: is the AJCC sub-classification of stage IV colorectal cancer appropriate? Int J Clin Oncol. 2013;18:696–703. doi: 10.1007/s10147-012-0433-5. [DOI] [PubMed] [Google Scholar]
- 5.Henderson JL. Blood: A study in general physiology. London: Yale University Press; 1928. [Google Scholar]
- 6.Eastham JA, May R, Robertson JL, Sartor O, Kattan MW. Development of a nomogram that predicts the probability of a positive prostate biopsy in men with an abnormal digital rectal examination and a prostate-specific antigen between 0 and 4 ng/mL. Urology. 1999;54:709–713. doi: 10.1016/s0090-4295(99)00213-7. [DOI] [PubMed] [Google Scholar]
- 7.Kattan MW, Karpeh MS, Mazumdar M, Brennan MF. Postoperative nomogram for disease-specific survival after an R0 resection for gastric carcinoma. J Clin Oncol. 2003;21:3647–3650. doi: 10.1200/JCO.2003.01.240. [DOI] [PubMed] [Google Scholar]
- 8.Weiser MR, Landmann RG, Kattan MW, Gonen M, Shia J, Chou J, Paty PB, Guillem JG, Temple LK, Schrag D, et al. Individualized prediction of colon cancer recurrence using a nomogram. J Clin Oncol. 2008;26:380–385. doi: 10.1200/JCO.2007.14.1291. [DOI] [PubMed] [Google Scholar]
- 9.Segelman J, Akre O, Gustafsson UO, Bottai M, Martling A. Individualized prediction of risk of metachronous peritoneal carcinomatosis from colorectal cancer. Colorectal Dis. 2014;16:359–367. doi: 10.1111/codi.12552. [DOI] [PubMed] [Google Scholar]
- 10.Ying HQ, Deng QW, He BS, Pan YQ, Wang F, Sun HL, Chen J, Liu X, Wang SK. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol. 2014;31:305. doi: 10.1007/s12032-014-0305-0. [DOI] [PubMed] [Google Scholar]
- 11.Zhang JX, Song W, Chen ZH, Wei JH, Liao YJ, Lei J, Hu M, Chen GZ, Liao B, Lu J, et al. Prognostic and predictive value of a microRNA signature in stage II colon cancer: a microRNA expression analysis. Lancet Oncol. 2013;14:1295–1306. doi: 10.1016/S1470-2045(13)70491-1. [DOI] [PubMed] [Google Scholar]
- 12.Goossens-Beumer IJ, Derr RS, Buermans HP, Goeman JJ, Böhringer S, Morreau H, Nitsche U, Janssen KP, van de Velde CJ, Kuppen PJ. MicroRNA classifier and nomogram for metastasis prediction in colon cancer. Cancer Epidemiol Biomarkers Prev. 2015;24:187–197. doi: 10.1158/1055-9965.EPI-14-0544-T. [DOI] [PubMed] [Google Scholar]
- 13.Peng J, Ding Y, Tu S, Shi D, Sun L, Li X, Wu H, Cai S. Prognostic nomograms for predicting survival and distant metastases in locally advanced rectal cancers. PLoS One. 2014;9:e106344. doi: 10.1371/journal.pone.0106344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valentini V, van Stiphout RG, Lammering G, Gambacorta MA, Barba MC, Bebenek M, Bonnetain F, Bosset JF, Bujko K, Cionini L, et al. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J Clin Oncol. 2011;29:3163–3172. doi: 10.1200/JCO.2010.33.1595. [DOI] [PubMed] [Google Scholar]
- 15.van Gijn W, van Stiphout RG, van de Velde CJ, Valentini V, Lammering G, Gambacorta MA, Påhlman L, Bujko K, Lambin P. Nomograms to predict survival and the risk for developing local or distant recurrence in patients with rectal cancer treated with optional short-term radiotherapy. Ann Oncol. 2015;26:928–935. doi: 10.1093/annonc/mdv023. [DOI] [PubMed] [Google Scholar]
- 16.Chua W, Charles KA, Baracos VE, Clarke SJ. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer. 2011;104:1288–1295. doi: 10.1038/bjc.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malietzis G, Giacometti M, Askari A, Nachiappan S, Kennedy RH, Faiz OD, Aziz O, Jenkins JT. A preoperative neutrophil to lymphocyte ratio of 3 predicts disease-free survival after curative elective colorectal cancer surgery. Ann Surg. 2014;260:287–292. doi: 10.1097/SLA.0000000000000216. [DOI] [PubMed] [Google Scholar]
- 18.Beppu T, Sakamoto Y, Hasegawa K, Honda G, Tanaka K, Kotera Y, Nitta H, Yoshidome H, Hatano E, Ueno M, et al. A nomogram predicting disease-free survival in patients with colorectal liver metastases treated with hepatic resection: multicenter data collection as a Project Study for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2012;19:72–84. doi: 10.1007/s00534-011-0460-z. [DOI] [PubMed] [Google Scholar]
- 19.Kanemitsu Y, Kato T. Prognostic models for predicting death after hepatectomy in individuals with hepatic metastases from colorectal cancer. World J Surg. 2008;32:1097–1107. doi: 10.1007/s00268-007-9348-0. [DOI] [PubMed] [Google Scholar]
- 20.Kattan MW, Gönen M, Jarnagin WR, DeMatteo R, D’Angelica M, Weiser M, Blumgart LH, Fong Y. A nomogram for predicting disease-specific survival after hepatic resection for metastatic colorectal cancer. Ann Surg. 2008;247:282–287. doi: 10.1097/SLA.0b013e31815ed67b. [DOI] [PubMed] [Google Scholar]
- 21.Kanemitsu Y, Kato T, Hirai T, Yasui K. Preoperative probability model for predicting overall survival after resection of pulmonary metastases from colorectal cancer. Br J Surg. 2004;91:112–120. doi: 10.1002/bjs.4370. [DOI] [PubMed] [Google Scholar]
- 22.Elias D, Faron M, Goéré D, Dumont F, Honoré C, Boige V, Malka D, Ducreux M. A simple tumor load-based nomogram for surgery in patients with colorectal liver and peritoneal metastases. Ann Surg Oncol. 2014;21:2052–2058. doi: 10.1245/s10434-014-3506-z. [DOI] [PubMed] [Google Scholar]
- 23.Kawai K, Ishihara S, Yamaguchi H, Sunami E, Kitayama J, Miyata H, Sugihara K, Watanabe T. Nomograms for predicting the prognosis of stage IV colorectal cancer after curative resection: a multicenter retrospective study. Eur J Surg Oncol. 2015;41:457–465. doi: 10.1016/j.ejso.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 24.Manceau G, Imbeaud S, Thiébaut R, Liébaert F, Fontaine K, Rousseau F, Génin B, Le Corre D, Didelot A, Vincent M, et al. Hsa-miR-31-3p expression is linked to progression-free survival in patients with KRAS wild-type metastatic colorectal cancer treated with anti-EGFR therapy. Clin Cancer Res. 2014;20:3338–3347. doi: 10.1158/1078-0432.CCR-13-2750. [DOI] [PubMed] [Google Scholar]
- 25.Massacesi C, Norman A, Price T, Hill M, Ross P, Cunningham D. A clinical nomogram for predicting long-term survival in advanced colorectal cancer. Eur J Cancer. 2000;36:2044–2052. doi: 10.1016/s0959-8049(00)00286-0. [DOI] [PubMed] [Google Scholar]
- 26.Okuno M, Hatano E, Seo S, Taura K, Yasuchika K, Nakajima A, Yazawa T, Furuyama H, Kawamoto H, Yagi S, et al. Indication for neoadjuvant chemotherapy in patients with colorectal liver metastases based on a nomogram that predicts disease-free survival. J Hepatobiliary Pancreat Sci. 2014;21:881–888. doi: 10.1002/jhbp.149. [DOI] [PubMed] [Google Scholar]
- 27.Takakura Y, Okajima M, Kanemitsu Y, Kuroda S, Egi H, Hinoi T, Tashiro H, Ohdan H. External validation of two nomograms for predicting patient survival after hepatic resection for metastatic colorectal cancer. World J Surg. 2011;35:2275–2282. doi: 10.1007/s00268-011-1194-4. [DOI] [PubMed] [Google Scholar]
- 28.Reddy SK, Kattan MW, Yu C, Ceppa EP, de la Fuente SG, Fong Y, Clary BM, White RR. Evaluation of peri-operative chemotherapy using a prognostic nomogram for survival after resection of colorectal liver metastases. HPB (Oxford) 2009;11:592–599. doi: 10.1111/j.1477-2574.2009.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nathan H, de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Gigot JF, Schulick RD, Choti MA, Aldrighetti L, et al. Conditional survival after surgical resection of colorectal liver metastasis: an international multi-institutional analysis of 949 patients. J Am Coll Surg. 2010;210:755–764, 764-766. doi: 10.1016/j.jamcollsurg.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 30.Kanemitsu Y, Kato T, Komori K, Fukui T, Mitsudomi T. Validation of a nomogram for predicting overall survival after resection of pulmonary metastases from colorectal cancer at a single center. World J Surg. 2010;34:2973–2978. doi: 10.1007/s00268-010-0745-4. [DOI] [PubMed] [Google Scholar]
- 31.Kiran RP, Attaluri V, Hammel J, Church J. A novel nomogram accurately quantifies the risk of mortality in elderly patients undergoing colorectal surgery. Ann Surg. 2013;257:905–908. doi: 10.1097/SLA.0b013e318269d337. [DOI] [PubMed] [Google Scholar]
- 32.Hedrick TL, Sawyer RG, Friel CM, Stukenborg GJ. A method for estimating the risk of surgical site infection in patients with abdominal colorectal procedures. Dis Colon Rectum. 2013;56:627–637. doi: 10.1097/DCR.0b013e318279a93e. [DOI] [PubMed] [Google Scholar]
- 33.de Campos-Lobato LF, Wells B, Wick E, Pronty K, Kiran R, Remzi F, Vogel JD. Predicting organ space surgical site infection with a nomogram. J Gastrointest Surg. 2009;13:1986–1992. doi: 10.1007/s11605-009-0968-6. [DOI] [PubMed] [Google Scholar]
- 34.Frasson M, Flor-Lorente B, Rodríguez JL, Granero-Castro P, Hervás D, Alvarez Rico MA, Brao MJ, Sánchez González JM, Garcia-Granero E. Risk Factors for Anastomotic Leak After Colon Resection for Cancer: Multivariate Analysis and Nomogram From a Multicentric, Prospective, National Study With 3193 Patients. Ann Surg. 2015;262:321–330. doi: 10.1097/SLA.0000000000000973. [DOI] [PubMed] [Google Scholar]
- 35.Yao HH, Shao F, Huang Q, Wu Y, Qiang Zhu Z, Liang W. Nomogram to predict anastomotic leakage after laparoscopic anterior resection with intracorporeal rectal transection and double-stapling technique anastomosis for rectal cancer. Hepatogastroenterology. 2014;61:1257–1261. [PubMed] [Google Scholar]
- 36.Russell MC, You YN, Hu CY, Cormier JN, Feig BW, Skibber JM, Rodriguez-Bigas MA, Nelson H, Chang GJ. A novel risk-adjusted nomogram for rectal cancer surgery outcomes. JAMA Surg. 2013;148:769–777. doi: 10.1001/jamasurg.2013.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jwa E, Kim JH, Han S, Park JH, Lim SB, Kim JC, Hong YS, Kim TW, Yu CS. Nomogram to predict ypN status after chemoradiation in patients with locally advanced rectal cancer. Br J Cancer. 2014;111:249–254. doi: 10.1038/bjc.2014.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Stiphout RG, Lammering G, Buijsen J, Janssen MH, Gambacorta MA, Slagmolen P, Lambrecht M, Rubello D, Gava M, Giordano A, et al. Development and external validation of a predictive model for pathological complete response of rectal cancer patients including sequential PET-CT imaging. Radiother Oncol. 2011;98:126–133. doi: 10.1016/j.radonc.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 39.van Stiphout RG, Valentini V, Buijsen J, Lammering G, Meldolesi E, van Soest J, Leccisotti L, Giordano A, Gambacorta MA, Dekker A, et al. Nomogram predicting response after chemoradiotherapy in rectal cancer using sequential PETCT imaging: a multicentric prospective study with external validation. Radiother Oncol. 2014;113:215–222. doi: 10.1016/j.radonc.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Omata F, Shintani A, Isozaki M, Masuda K, Fujita Y, Fukui T. Diagnostic performance of quantitative fecal immunochemical test and multivariate prediction model for colorectal neoplasms in asymptomatic individuals. Eur J Gastroenterol Hepatol. 2011;23:1036–1041. doi: 10.1097/MEG.0b013e32834a2882. [DOI] [PubMed] [Google Scholar]
- 41.Kawai K, Ishihara S, Yamaguchi H, Sunami E, Kitayama J, Miyata H, Watanabe T. Nomogram prediction of metachronous colorectal neoplasms in patients with colorectal cancer. Ann Surg. 2015;261:926–932. doi: 10.1097/SLA.0000000000000881. [DOI] [PubMed] [Google Scholar]
- 42.Wells BJ, Kattan MW, Cooper GS, Jackson L, Koroukian S. Colorectal cancer predicted risk online (CRC-PRO) calculator using data from the multi-ethnic cohort study. J Am Board Fam Med. 2014;27:42–55. doi: 10.3122/jabfm.2014.01.130040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyoshi Y, Noguchi K, Yanagisawa M, Taguri M, Morita S, Ikeda I, Fujinami K, Miura T, Kobayashi K, Uemura H. Nomogram for overall survival of Japanese patients with bone-metastatic prostate cancer. BMC Cancer. 2015;15:338. doi: 10.1186/s12885-015-1330-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hansen J, Auprich M, Ahyai SA, de la Taille A, van Poppel H, Marberger M, Stenzl A, Mulders PF, Huland H, Fisch M, et al. Initial prostate biopsy: development and internal validation of a biopsy-specific nomogram based on the prostate cancer antigen 3 assay. Eur Urol. 2013;63:201–209. doi: 10.1016/j.eururo.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 45.Nam RK, Toi A, Klotz LH, Trachtenberg J, Jewett MA, Appu S, Loblaw DA, Sugar L, Narod SA, Kattan MW. Assessing individual risk for prostate cancer. J Clin Oncol. 2007;25:3582–3588. doi: 10.1200/JCO.2007.10.6450. [DOI] [PubMed] [Google Scholar]


