Abstract
Respiratory melioidosis is a disease presentation of the biodefense pathogen, Burkholderia pseudomallei, which is frequently associated with a lethal septicemic spread of the bacteria. We have recently developed an improved respiratory melioidosis model to study the pathogenesis of Burkholderia pseudomallei in the lung (intubation-mediated intratracheal [IMIT] inoculation), which more closely models descriptions of human melioidosis, including prominent septicemic spread from the lung and reduced involvement of the upper respiratory tract. We previously demonstrated that the Type 3 Secretion System cluster 3 (T3SS3) is a critical virulence determinant for B. pseudomallei when delivered directly into the lung. We decided to comprehensively identify all virulence determinants required for respiratory melioidosis using the Tn-seq phenotypic screen, as well as to investigate which virulence determinants are required for dissemination to the liver and spleen. While previous studies have used Tn-seq to identify essential genes for in vitro cultured B. pseudomallei, this represents the first study to use Tn-seq to identify genes required for in vivo fitness. Consistent with our previous findings, we identified T3SS3 as the largest genetic cluster required for fitness in the lung. Furthermore, we identified capsular polysaccharide and Type 6 Secretion System cluster 5 (T6SS5) as the two additional major genetic clusters facilitating respiratory melioidosis. Importantly, Tn-seq did not identify additional, novel large genetic systems supporting respiratory melioidosis, although these studies identified additional small gene clusters that may also play crucial roles in lung fitness. Interestingly, other previously identified virulence determinants do not appear to be required for lung fitness, such as lipopolysaccharide. The role of T3SS3, capsule, and T6SS5 in lung fitness was validated by competition studies, but only T3SS3 was found to be important for respiratory melioidosis when delivered as a single strain challenge, suggesting that competition studies may provide a higher resolution analysis of fitness factors in the lung. The use of Tn-seq phenotypic screening also provided key insights into the selective pressure encountered in the liver.
Keywords: respiratory melioidosis, intubation-mediated intratracheal (IMIT) inoculation, mouse infection models, Tn-seq, type 3 secretion, type 6 secretion, capsular polysaccharide
Introduction
Burkholderia pseudomallei, the etiological agent of melioidosis, is a motile Gram-negative bacterium classified as a Tier 1 Select Agent. While endemic to Southeast Asia and northern Australia (Cheng and Currie, 2005), an increasing number of melioidosis cases have been reported in Latin America (Inglis et al., 2006), the Caribbean (Wiersinga et al., 2006), Africa (Schweizer et al., 2014) and other tropical regions worldwide (Cheng and Currie, 2005). The bacterium is an environmental saprophyte generally found in the soil and water reservoirs in indigenous areas (Galyov et al., 2010) where it opportunistically infects an array of plant (Kaestli et al., 2012), animal, and human hosts (Cheng and Currie, 2005). The most common routes of infection in humans include percutaneous inoculation, ingestion, or inhalation of the bacterium from the environment (Brett and Woods, 2000; Wiersinga et al., 2006; Lazar Adler et al., 2009). Melioidosis manifestations may include asymptomatic seroconversion, febrile illness, pneumonia, hepatic and splenic abscesses, chronic melioidosis and sepsis syndrome (Holden et al., 2004; Wiersinga et al., 2006; Cheng et al., 2013). Risk factors associated with melioidosis include diabetes mellitus, alcoholism, renal failure, and chronic pulmonary disease (Wiersinga et al., 2006).
Respiratory melioidosis is a disease presentation of high relevance to both naturally-occurring and biodefense-related studies; indeed, B. pseudomallei is lung tropic even in cases of percutaneous inoculation (Currie et al., 2000). Both our lab and others have previously shown that the nasal cavity represents the predominant site of B. pseudomallei colonization in intranasally-inoculated mice (Owen et al., 2009; Warawa et al., 2011), whereas direct lung instillation of bacteria can abrogate nasal cavity colonization and the associated central nervous system involvement (Revelli et al., 2012; Gutierrez et al., 2015). We have further characterized that lung-specific instillation of B. pseudomallei results in a shift of moribund endpoint from a predominant nasal cavity infection in the intranasal model to a greater bacterial proliferation in the lung and disseminated spread (Gutierrez et al., 2015), more closely resembling descriptions of human melioidosis. Furthermore, the intubation-mediated intratracheal (IMIT) inoculation method has facilitated the discovery of a spread-deficiency phenotype for a capsular polysaccharide mutant from the lung (Gutierrez et al., 2015), which had not been discovered in mice succumbing to nasal cavity colonization (Warawa et al., 2011), and yet meets the predicted role for capsule in mediating protection from complement during dissemination (Reckseidler-Zenteno et al., 2005). Thus, the IMIT lung-specific melioidosis model has begun to provide unique insights into the roles of B. pseudomallei virulence determinants that have not been identified in other respiratory melioidosis models. We therefore sought to identify the roles for additional B. pseudomallei virulence determinants using the IMIT model of lung-specific disease.
Tn-seq is a powerful new tool used to identify a full repertoire or genes required for an organism's viability in a selective environment by combining saturation mutagenesis and Next Generation Sequencing (Van Opijnen et al., 2009). Recent Tn-seq studies identified essential genes required to support in vitro growth for the Burkholderia pseudomallei K96243 strain (Moule et al., 2014) and the closely related Burkholderia thailandensis E264 strain (Baugh et al., 2013; Gallagher et al., 2013). These studies identified potential antimicrobial drug targets in key constituents of metabolic pathways, cell structure and genes required for nucleotide and amino acid synthesis, and further estimated that ~8% of the Burkholderia genome represent essential genes (Baugh et al., 2013; Moule et al., 2014). Importantly, Tn-seq has not previously been used to identify B. pseudomallei genes required to support growth in the selective pressure of mammalian host tissues. In the present study, we investigated the potential of Tn-seq to identify virulence determinants required by B. pseudomallei to colonize mammalian lungs in a mouse model of respiratory melioidosis as well as to disseminate to the liver and spleen. These studies take advantage of our IMIT respiratory melioidosis model to non-invasively target delivery of a Tn-seq library directly into the lungs of mice to specifically identify genes supporting pulmonary disease in the absence of potential interplay between upper and lower respiratory tract infections.
Materials and methods
Bacterial strains and culture
Burkholderia pseudomallei and Escherichia coli strains (Table 1) were routinely cultured in Lennox Broth (LB) at 37°C. For inoculum preparation, B. pseudomallei strains were subcultured 1:25 in dialyzed and chelated Trypticase Soy Broth (TSBDC) supplemented with 50 μM monosodium glutamate from overnight LB cultures and grown for 3 h at 37° C. Antibiotics were used at the following concentrations when appropriate: kanamycin (Km), 25 μg/mL; polymyxin B (Pm), 50 μg/mL; streptomycin (Sm), 100 μg/mL; gentamicin (Gm), 20 μg/mL.
Table 1.
Strains and plasmids used in this study.
| Strain | Description | References |
|---|---|---|
| S17−1 | E. coli conjugation strain, λpir | Simon et al., 1983 |
| S17−1/pKAS46−araPtolClux | Bioluminescence allelic exchange construct | Warawa et al., 2011 |
| S17−1/pKAS46−ΔtssC−5 | T6SS5 mutant allelic exchange construct | This study |
| DD503 | B. pseudomallei 1026b derivative, PmR SmR KmS GmS, lacking AmrAB-OprA efflux pump | Moore et al., 1999 |
| DD503 ΔtssC−5 | T6SS5 mutant | This study |
| DD503 ΔsctUBp3 | T3SS3 mutant | Warawa and Woods, 2005 |
| JW270 | DD503 Δwcb capsule mutant | Warawa et al., 2009 |
| JW280 | DD503::PtolC-luxCDABE | Warawa et al., 2011 |
| JW280 ΔtssC−5 | Bioluminescent T6SS5 mutant | This study |
| MGBP001 | DD503::PtolC-luxCDABE-Gm | This study |
| MGBP001 ΔtssC-5 | Bioluminescent, GmR T6SS5 mutant | This study |
| MGBP001 ΔsctUBp3 | Bioluminescent, GmR T3SS3 mutant | This study |
| MGBP001 Δwcb | Bioluminescent, GmR capsule mutant | This study |
| Plasmid | ||
| pBTK30 | Vector containing Himar1 C9 transposase | Goodman et al., 2004 |
| pSAM-Bt | Tn-seq vector for use in Bacteroides thetaiotaomicron | Goodman et al., 2009 |
| pSAM-DYH | pSAM_Bt with the transposase from pBTK30 inserted as a BamHI fragment to replace the native transposase and promoter | Skurnik et al., 2013a |
| pEXKm5 | Gene replacement vector for Burkholderia species | López et al., 2009 |
| pSAM-DKm | Tn-seq vector | This study |
| pKAS46 | Allelic exchange vector providing KmR and SmS in DD503 genetic background | Skorupski and Taylor, 1996 |
| pGSV4 | Promoterless bioreporter vector harboring luxCDABE operon | Gutierrez et al., 2015 |
| pCR®-Blunt II-TOPO | Topoisomerase-conjugated cloning vector | Invitrogen |
| pUC19 | Cloning vector | New England Biolabs |
Tn-seq library preparation
Plasmids used in this study are identified in Table 1. To construct the transposon delivery vector pSAM-DKm, the mariner-family transposon, Himar1 C9 transposase and its upstream regulatory region were PCR amplified from pBTK30 and cloned as a BamHI restriction fragment into pSAM-Bt to yield pSAM-DYH. The kanamycin resistance cassette from pEXKm5 was PCR amplified and inserted into the MfeI and XbaI restriction sites in place of the erythromycin resistance cassette and the resulting plasmid, pSAM-DKm was verified by PCR. To generate the B. pseudomallei Tn-seq library, the E. coli S17-1 strain transformed with pSAM-DKm was conjugated with the luminescent B. pseudomallei parent strain JW280 for 2 h at 37°C. Transposon mutants were selected for on PmKm LB agar plates and grown at 37°C for 24 h. A library of 2 × 104 mutants were pooled, grown in PmKm LB broth with shaking at 37°C and cryopreserved.
Ethics and biosafety statement
All animal studies were conducted under Biosafety Level 3 conditions using 8–10 week old female albino C57BL/6J mice (B6 (Cg)-Tyrc−2J/J, Jackson Laboratories) bred at the University of Louisville (Protocol Number 11113). These studies were approved by the University of Louisville Institutional Animal Care and Use Committee (Protocol numbers 10073 and 13053) in agreement with NIH guidelines and the “Guide for the Care and Use of Laboratory Animals” (NRC). University of Louisville is approved for use of the Tier 1 select agent B. pseudomallei with continuous registration from the Centers for Disease Control since 2010.
Tn-seq screen
A B. pseudomallei Tn-seq library TSBDC culture was washed into phosphate buffered saline (PBS) and OD600 readings were used to estimate bacterial concentrations in order to prepare an inoculum of 104.74 CFU/50 μL. A group of three mice were inoculated with 50 μL of bacterial suspension of Tn-seq library by intubation mediated intratracheal (IMIT) instillation, as described (Lawrenz et al., 2014). Disease progression was monitored twice daily by optical diagnostic imaging of bioluminescent B. pseudomallei until study completion, as described (Gutierrez et al., 2015). Animals were euthanized at moribund endpoints (66 h post-infection) and necropsied to collect lungs, liver and spleen. Tissues were homogenized and bacteria from these tissues were cultured in LB broth overnight at 37°C with shaking. Bioluminescence imaging data was processed as described (Lawrenz et al., 2014).
Chromosomal DNA was purified from triplicate biological samples, as described (Beji et al., 1987), and pooled for each tissue. Genomic DNA was digested with the MmeI restriction enzyme overnight at 37°C, treated for 20 min at 80°C to inactivate the enzyme, purified using the QIAquick, PCR purification kit (Qiagen) according to the manufacturer's instructions, and concentrated with a speed vacuum to a final volume of 30 μL. Restricted fragments were separated by electrophoresis in a 1% agarose gel and fragments corresponding to 1.2–1.5 Kb were isolated using the QIAquick Gel Extraction Kit (Qiagen). Fragments were ligated to barcoded double stranded adaptors using T4 DNA ligase in 50 μL reactions overnight at 16°C, treated at 65°C for 10 min to inactivate the ligase, and purified using the QIAquick, PCR purification kit. Transposon-genome junctions were amplified by PCR using the LIB_PCR_5 (5′-CAAGC AGAAGACGGCATACGAAGACCGGGGACTTATCATCCAACC TGT-3′) and LIB_PCR_3 (5′-AATGA TACGGCGACCACCGAACACTCTTTCCCTACACGACGCTCTTCCGA TCT-3′) primers (Goodman et al., 2009) using HiFi DNA Polymerase (KAPA Biosystems). Confirmation of a 125 base pair product was conducted using 1% agarose gel electrophoresis, and libraries were sequeced using Illumina 50-bp single end sequencing at the Harvard Medical School Biopolymer Facility. Data was analyzed by sorting based on barcodes using a custom script in Java Eclipse, then using CLC Genomics Workbench v. 7.2 for subsequent bioinformatics. Reads were trimmed to remove adapter and transposon sequences and aligned to the B. pseudomallei 1026b genome using the default settings in the RNA-seq function in CLC. Reads that mapped to multiple locations were discarded. The resultant reads were counted and normalized to the reads per kilobase of transcript per million reads mapped (RPKM) and compared across sample sites. Significance was assessed using the On Proportions function of CLC using the Kals's test option and the Bonferroni multiple testing correction was applied to the comparisons. Initially, fold change measurements of less than −3 (reduced in the mouse samples compared to the input pool) with a Bonferroni-adjusted p-value of less than 0.05 were considered significant.
Early dissemination studies
Early dissemination studies were conducted using the B. pseudomallei Tn-seq library strain to infect groups of three animals by IMIT as described above, euthanizing groups at 6, 12, and 24 h post-infection. Lung, liver and spleen from individual animals were collected at necropsy, homogenized and serial diluted for bacterial enumeration as described (Lawrenz et al., 2014).
Type 6 secretion system mutagenesis
To generate a Type 6 Secretion System cluster 5 (T6SS5) mutant in B. pseudomallei, an internal in-frame 1437 bp region of the tssC-5 gene was deleted. Briefly, flanking fragments in the 5′ and 3′ region of the tssC-5 gene were amplified with the following primer sets: 5-tssC-F, GAAGAATTCGCGTAGAACAGCAGCAGCAGCAGCCCCGC; 5-tssC-R, GTCAAGCTTCGGGGATTGCAGGTGTTCGCCTTCCATGGTC; 3-tssC-F, GTCAAGCTTTCGCTCGTCGGCAAGCTCGAAAAGCGCTAGG; 3-tssC-R, GGCGGTACCTTGCGCGAAGCCGCCCGCGAG. The amplified 5′ tssC-5 fragment was cloned into pSK as an EcoRI/HindIII fragment to yield pSK-5′tssC. The amplified 3′ tssC-5 fragment was cloned into pSK-5′tssC as a HindIII/KpnI fragment to yield pSK-ΔtssC-5. The assembled fragment was cloned into pKAS46 as an EcoRI/KpnI fragment to yield pKAS46-ΔtssC-5 and conjugated with the parent B. pseudomallei strain DD503. Finally, the bioluminescent strain JW280 ΔtssC-5 was constructed by conjugation of DD503 ΔtssC-5 with S17-1/pKAS46-araPtolClux, as described previously (Warawa et al., 2011).
Virulence assessment of a T6SS5 mutant in respiratory melioidosis
Animal studies were conducted to compare the virulence of JW280 and the JW280 ΔtssC-5 mutant by IMIT infection, as described above. Groups of five animals were euthanized at moribund end point defined as loss of righting reflex. Thus, we define LD50 in our studies as onset of moribund symptoms rather than to use death as an endpoint. Mice were necropsied and lung, liver and spleen were isolated and imaged for bioluminescence in 24-well black plates. The equations used to perform correlations between bioluminescent signal and colony forming units (CFU) have been previously defined (Gutierrez et al., 2015).
Competition studies
A gentamicin resistance cassette was amplified with flanking NotI restriction sites from the pGSV4 vector (Gutierrez et al., 2015) with the primers Gm cass NotI(+) (GCGGCCGCAGATTTAAATTAATTAAGAGCTAGAATTGACATAAGCCTG) and Gm cass NotI(−) (GAAGCGGCCGCGGCGTTGTGACAATTTACCGAACAACTC). The Gm cassette was cloned into the pCR®-Blunt II-TOPO cloning vector (Invitrogen) and was then cloned into the pUC19-araPtolClux vector as a NotI/NotI fragment to yield pUC19-araPtolClux-Gm. The araPtolClux-Gm fragment was cloned into pKAS46 to generate the pKAS46-araPtolClux-Gm and was transformed into S17-1 cells to generate the S17-1/pKAS46-araPtolClux-Gm strain. This strain was conjugated with the B. pseudomallei parent strain DD503, capsule polysaccharide mutant (JW270), Type 3 Secretion System translocation-deficient mutant (DD503 ΔsctUBp3) and Type 6 Secretion System mutant (DD503 ΔtssC-5) to generate MGBP001, MGBP001 Δwcb, MGBP001 ΔsctUBp3, and MGBP001 ΔtssC-5, respectively, by allelic exchange as previously described (Warawa and Woods, 2005).
Competition studies were conducted by challenging animals by IMIT with ~104.0 CFU of a 1:1 mixture of non-luminescent B. pseudomallei DD503 plus one of the four luminescent, gentamicin-resistant MGBP001 strains. The study was completed at 67 h post-infection at which point lung, liver and spleen were isolated and homogenized. Homogenates were plated in replicate on both LB and LB-Gm plates for bacterial enumeration and competitive index calculations.
Statistical analysis
Two-Way ANOVA followed by the Bonferroni post-test, Student's T-test, Log-rank survival analysis (Mantel-Cox test and Gehan-Breslow-Wilcoxon test), One-Way ANOVA followed by Tukey's Multiple Comparison test were conducted using GraphPad Prism.
Results
Lung-specific mouse infection with the Tn-seq insertion library
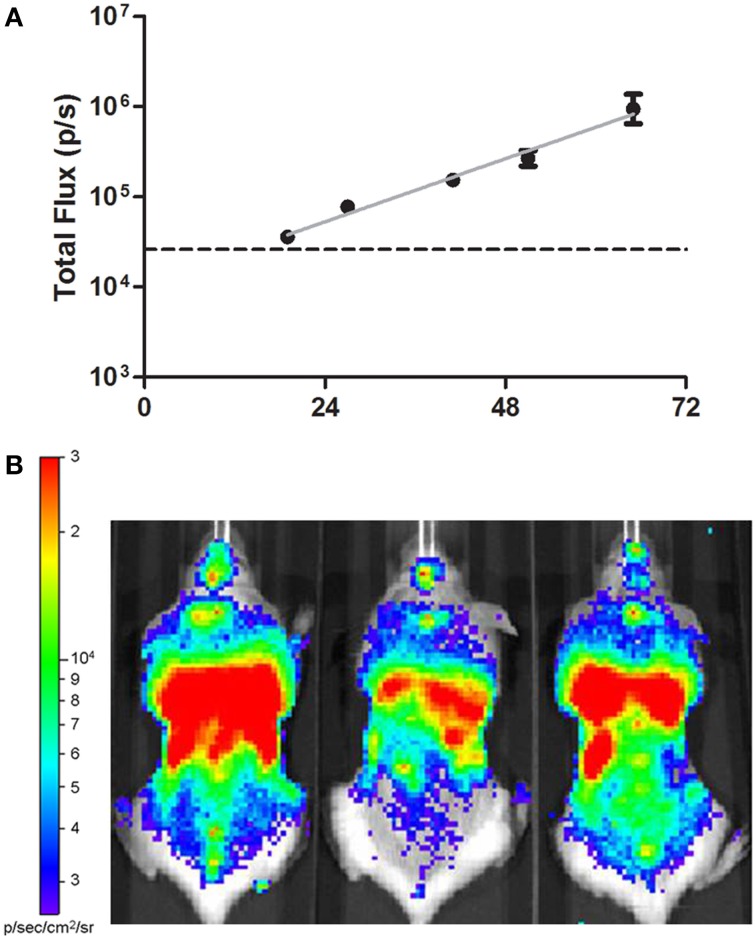
We sought to identify virulence determinants required by B. pseudomallei to cause disease specifically in the lung, as well as for subsequent disseminated spread to liver and spleen. Accordingly, we generated a transposon insertion library composed of 20,000 insertion mutants in the genome of the B. pseudomallei luminescent strain JW280, a derivative of the 1026b clinical strain. To select against transposon-inactivated genes required for lung colonization and systemic spread in vivo, we challenged pools of C57BL/6J albino female mice with 104.74 CFU of a transposon insertion library. This inoculum was within 10 median lethal doses (LD50) of the JW280 strain (Gutierrez et al., 2015), and achieved ~4x coverage of the genome and 4x mutant strain representation in the challenge inoculum. We monitored the growth rate of the mutant library by bioluminescence starting at 19 h post-infection and observed that in the lungs, the library pool exhibited logarithmic growth at a rate consistent with our previously observed JW280 parent strain growth rate (Gutierrez et al., 2015; Figure 1A). By 66 h post-infection, all animals had reached moribund stage, characterized by acute respiratory infection and systemic spread (Figure 1B) and at this point all animals were euthanized and lungs, liver and spleen were isolated. These data indicate that in vivo disease progression in C57BL/6J mice infected with the transposon insertion library followed an acute course of infection comparable to that of the parent B. pseudomallei strain (Gutierrez et al., 2015).
Figure 1.
In vivo disease progression of Burkholderia pseudomallei Tn-seq transposon library. (A) In vivo growth of the B. pseudomallei transposon library in the thoracic cavity of three C57BL/6J female albino mice infected by IMIT was monitored twice daily with an in vivo imaging system using luminescence as a read out for bacterial replication. Total luminescence was enumerated using a region of interest (ROI) centered on the thoracic cavity. The dashed line indicates technical 95% limit of detection. (B) Whole body imaging of moribund C57BL/6J mice infected with B. pseudomallei transposon library at 66 h post-infection. The logarithmic scale bar is provided for the standardized data presentation of 2.5 × 103–3 × 104 p/s/cm2/sr.
Transposon insertion sequencing and mapping
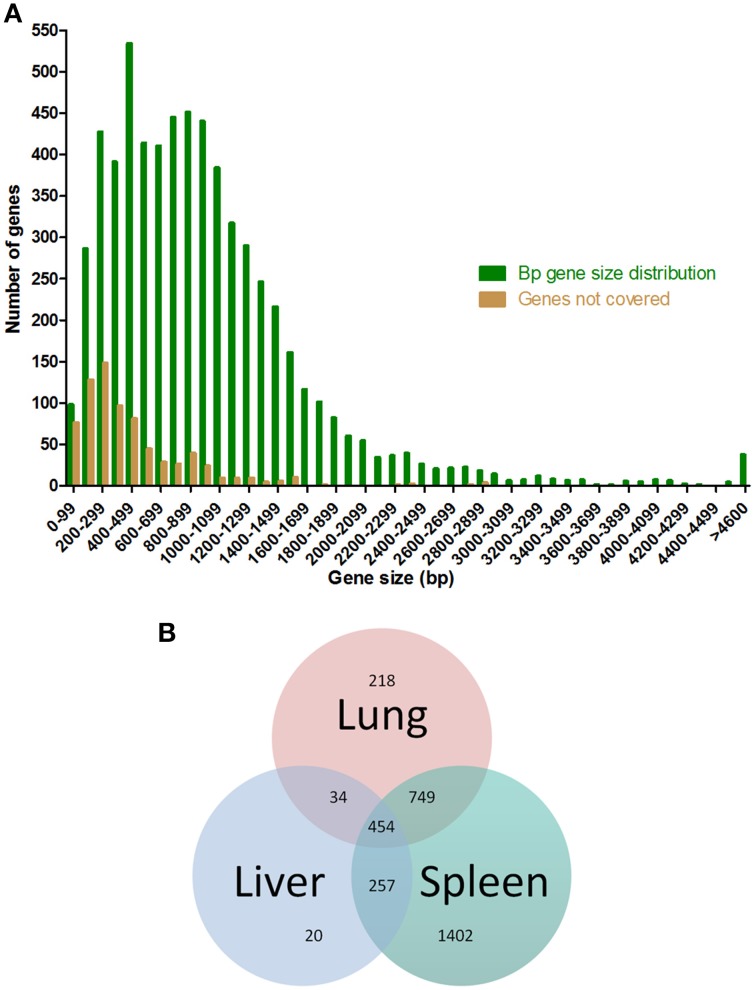
We performed sequencing of the input transposon insertion library to determine transposon insertion density and assess coverage of the B. pseudomallei JW280 genome. Sequencing of the input mutant library mapped 1.1268 million reads to the B. pseudomallei 1026b genome and revealed that 88% percent of genes contained a transposon insertion. Approximately 8% of the B. pseudomallei genome is estimated to represent essential genes required for in vitro growth (Moule et al., 2014); thus only 92% of B. pseudomallei genes can be mutanted and our Tn-seq library approached full saturation. As expected, larger genes received better coverage efficiency, corresponding to the random nature of Marineer transposon insertion events favoring higher frequency insertion into larger genes (Figure 2A).
Figure 2.
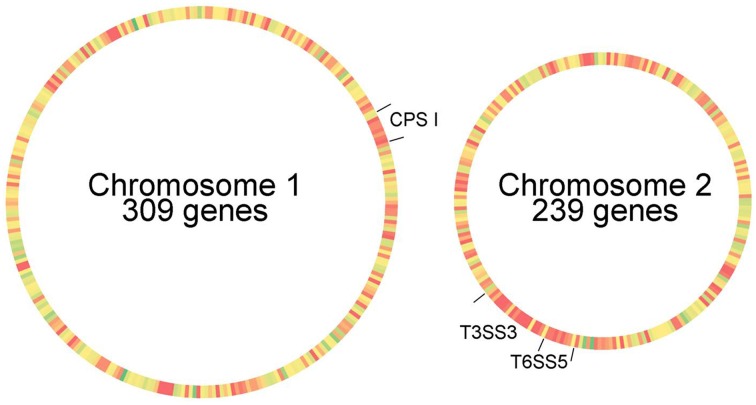
Gene size distribution of the Burkholderia pseudomallei genome and transposon insertion coverage. (A) Histogram of the B. pseudomallei genome (green bars) and percentage of genes lacking transposon insertions (brown bars), both binned at 100 bp gene size intervals. (B) Tn-seq data sets were analyzed for B. pseudomallei Tn-seq mutants lost by selective pressures in the lung (1455); liver (765); spleen (2862) at a 3-fold reduction in relative abundance relative to the input pool. Venn diagram illustrating mutants lost in the three tissues, with 454 genes being selected against in all three tissues.
Tn-seq analyses typically investigate changes in library diversity between two conditions using a 2-fold cutoff criteria for very large libraries investigated in in vitro studies (Skurnik et al., 2013a,b; Wiles et al., 2013). However, we had limited the size of our Tn-seq library to achieve a biologically relevant infection and therefore we increased the stringency of our initial data analysis to a 3-fold cutoff to account for an increase in variation noise with a smaller library studied in vivo. Sequencing of the output libraries showed that at a 3-fold loss relative to the input library, transposon mutants in the lungs sustained selective pressure leading to clearance of 1455 transposon-inactivated genes required for lung colonization relative to the input pool (Figure 2B and Supplemental Table 1). We hypothesized that lung-specific delivery of the B. pseudomallei mutant library would sustain selective pressure in the lung, which would lead to an initial loss of library diversity and subsequent additional diversity loss following spread to the liver and spleen. As expected, the transposon library was further reduced in the spleen (2862 total genes lost relative to input pool), suggestive of loss of library diversity both in the lung and due to loss of genes required for disseminated spread. Surprisingly, we observed a greater retention of diversity of the transposon library in the liver relative to the lung (765 mutants lost in the liver compared to 1455 in the lung), suggesting that a representative library pool reached this site prior to effects of the selective pressure exhibited in the lung (Figure 2B). Further, the retention of library diversity in the liver relative to the lung suggests that the liver does not exhibit as strong a selective environment as the lung, and that the early spread to the liver is at high titer so as to avoid potential bottlenecking effects of spread from one tissue to another. Taken together, these data suggest that B. pseudomallei spread from the lung to the liver is a prominent dissemination path which occurs as an early event, resulting in a reduced selective pressure in the liver.
Burkholderia pseudomallei early hepatic dissemination
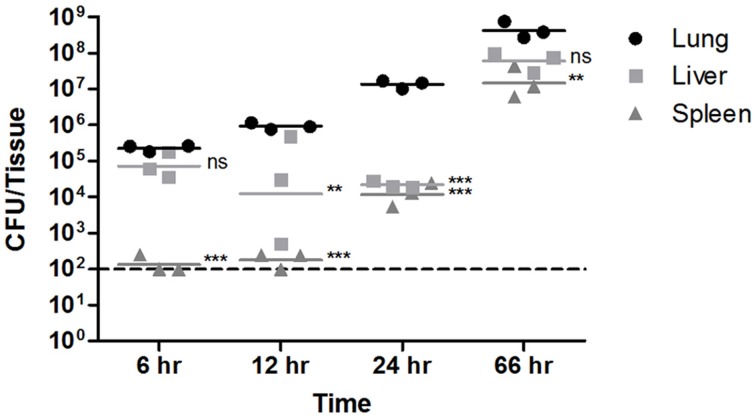
To characterize the kinetics of disseminated spread from the lung to other tissues, a time course analysis was conducted. Groups of three C57BL/6J female albino mice were infected with 104.62 CFU of the Tn-seq library, and then lungs, liver and spleen were isolated at 6, 12, and 24 h post-infection and bacterial titers were enumerated from each organ. By 6 h post-infection, bacteria were cultured from the lungs with 5.5-fold higher titers than the original instilled amount and grew exponentially throughout the course of infection (Figure 3), consistent with growth patterns observed by optical diagnostic imaging (Figure 1A). At 6 h post-infection, 104.87 CFU were also present in the liver, which were similar titers to those found in the lungs (Figure 3), confirming the indications of the Tn-seq data that hepatic spread is early and at high titer. Colonization of the spleen by 12 h post-infection was at or below the limit of detection (100 CFU), where pronounced splenic colonization only occurred 24 h post-infection (Figure 3), suggesting that the enhanced loss of Tn-seq library diversity in the spleen relative to the lung is associated with later spread from the lung, subsequent to the effects of selective pressure in the lung. Thus, B. pseudomallei colonizes host lungs very early during infection and persists at this site through the onset of acute respiratory disease. Similarly, the bacterium can spread from the lungs at high titer to colonize the host liver very early following infection but bacterial titers at this site remain constant until late in infection. This finding corroborates a unique observation made from the Tn-seq data set, shedding new light on B. pseudomallei dissemination.
Figure 3.
Burkholderia pseudomallei Tn-seq transposon library tissue burdens at key sites of infection. Groups of three C57BL/6 female albino mice infected with the B. pseudomallei Tn-seq transposon library were euthanized at 6, 12, and 24 h post-infection. Bacterial titers in the lungs, liver and spleen were enumerated by plate counting and compared to bacterial titers of moribund mice at 66 h post-infection from the initial Tn-seq transposon library challenge. The limit of detection of bacterial enumeration from host tissue is indicated by a dashed line. Tissue counts below the limit of detection were set to the limit of detection for statistical analysis. Statistical significance was calculated by Two-Way ANOVA followed by Bonferroni post-test (**P < 0.01;***P < 0.001; ns, not significant).
Identification of virulence determinants required for B. pseudomallei colonization of host lungs
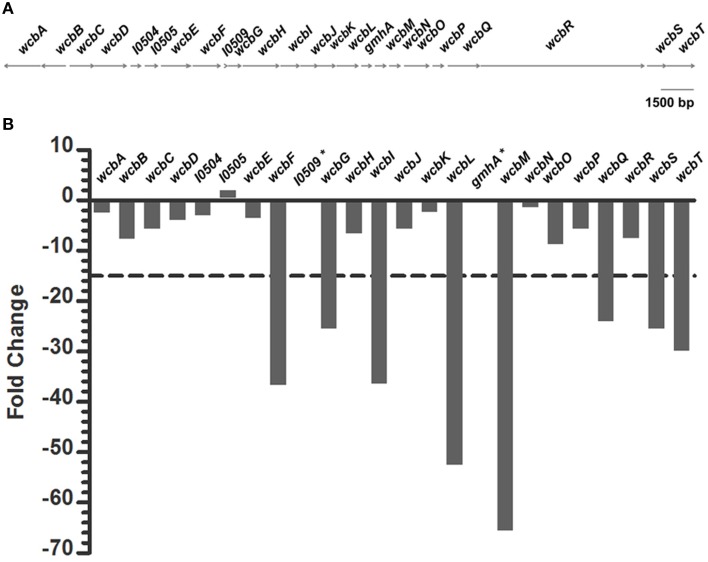
We initially focused our analysis of the Tn-seq data set on identifying virulence determinants required by B. pseudomallei to colonize the lungs of mammalian hosts, of which 1455 genes were required to colonize the murine lung at a 3-fold relative abundance cutoff ratio relative to the input pool (Figure 2B). As a refined stringency to focus our identification of key virulence determinants, we made use of a targeted analysis of the capsular polysaccharide biosynthetic cluster (CPS or CPS I, Reckseidler-Zenteno et al., 2009). We have previously demonstrated that a capsule mutant was attenuated 6.8-fold relative to the isogenic parent strain in the IMIT model, but that this level of attenuation was not statistically significant (Gutierrez et al., 2015). We therefore performed a detailed analysis of the Tn-seq lung results for CPS biosynthetic cluster as a benchmark genetic system which exhibits a marginal level of attenuation by LD50 analysis. We found that while the majority of CPS genes were reduced in the lung relative to the input pool, pronounced variation in degree of response was observed across the genetic cluster (Figure 4). This variation was suggestive of bottlenecking effects which are possible under the parameters of the current study, designed for a biologically relevant infection while achieving saturating library coverage. While variation was observed across the CPS locus, the locus itself exhibited an average 14.92-fold reduction in mutant prevalence for the lung vs. input pool. Thus, to further analyze the Tn-seq lung data set, we set a more stringent fold-change cut off of 15-fold, reflecting the magnitude of the average fold gene reduction of the CPS locus. Further, we decided to identify clusters of genes which meet this 15-fold cutoff to mitigate the impact which bottlenecking might have on dataset variation at the individual gene level (Figure 4).
Figure 4.
Analysis of variation within capsular polysaccharide operon genes required for lung colonization by Tn-seq. (A) Scale representation of the capsular polysaccharide genetic locus. (B) Variation in fold-change of capsule genes by Tn-seq analysis of the lung data set relative to the input pool. The average fold reduction across all capsule genes was 14.92-fold (dashed horizontal line). *Denotes genes not covered in the input library.
A total of 548 genes underwent selective pressure in the lung at a 15-fold cutoff relative to the input pool. These genes were heat mapped for nearest neighbor relationships (Figure 5) to identify genetic clusters with consecutive hits of no more than a 4 gene distance from the previous hit. The three genetic loci which provided the largest assemblage of genes meeting these criteria included: (i) CPS (8 genes), (ii) Type 6 Secretion System cluster 5 (T6SS5; 8 genes), and (iii) Type 3 Secretion System cluster 3 (T3SS3; 7+5 genes) (Table 2). There are six distinct T6SS clusters in the B. pseudomallei genome, and the T6SS5 is identified as cluster 5 by the NCBI database (Shalom et al., 2007), and this same cluster is identified as cluster 1 in Burkholderia mallei (Schell et al., 2007). Numerous additional small genetic clusters may provide important contributions to the fitness of B. pseudomallei in the lung; however, these data indicate that the three prominent genetic systems contributing to B. pseudomallei lung pathogenesis are CPS, T3SS3, and T6SS5. Given that we have previously characterized the contribution of CPS and T3SS3 to respiratory melioidosis using the IMIT model (Gutierrez et al., 2015), we decided to address what impact the T6SS5 system has on the virulence of B. pseudomallei.
Figure 5.
Proximity heat map of genes required for respiratory melioidosis. The Tn-seq lung data set was filtered for genes required by B. pseudomallei at a 15-fold reduction cut off relative to the input pool (548 genes). Genes were sorted for their arrangement on the two circular chromosomes of the B. pseudomallei genome, with similar distribution density found on the larger chromosome 1 (309 genes) and smaller chromosome 2 (239 genes). The heat map provides graphical cluster analysis of gene hits located in close proximity (red = 1 gene distance) or distal hits (green >70 gene neighbor distance). The three largest genetic clusters of hits separated by no more than 4 genes per hit are indicated: capsular polysaccharide (CPS I), Type 3 Secretion System cluster 3 (T3SS3), and Type 6 Secretion System cluster 5 (T6SS5).
Table 2.
Identification of prominent genetic loci required for pulmonary disease.
| Feature ID | Fold change | Distance to previous hit | Feature ID | Fold change | Distance to previous hit |
|---|---|---|---|---|---|
| T3SS3 | T6SS5 | ||||
| bprA | −70.3781 | BP1026B_II1587 | −83.2298 | ||
| bipC | −35.0191 | 1 | hcp−5 | −367.19 | 4 |
| bipB | −15.6668 | 1 | tssF−5 | −22.3609 | 3 |
| bicA | −15.2996 | 1 | clpV−5 | −23.6294 | 1 |
| bsaZ | −56.991 | 1 | tagB−5 | −21.4194 | 3 |
| spaP | −15.6396 | 3 | tagC−5 | −22.6434 | 1 |
| bsaV | −26.665 | 1 | tssK−5 | −31.7466 | 2 |
| bsaO | −82.8959 | 7 | tssL−5 | −111.177 | 2 |
| BP1026B_II1643 | −34.0416 | 2 | Capsule | ||
| BP1026B_II1644 | −59.6684 | 1 | wcbF | −36.6525 | |
| BP1026B_II1645 | −40.3909 | 1 | wcbG | −25.4066 | 2 |
| BP1026B_II1646 | −33.6591 | 1 | wcbI | −36.413 | 3 |
| wcbL | −52.5586 | 3 | |||
| wcbM | −65.6182 | 2 | |||
| wcbQ | −24.1121 | 4 | |||
| wcbS | −25.4738 | 2 | |||
| wcbT | −29.8059 | 1 |
Only a single gene met the 15-fold cutoff criterion in the liver (thioredoxin: -16.2-fold liver:input), suggesting that the liver does not offer a strong selective pressure against B. pseudomallei relative to that of the lung. Conversely, 2255 genes met this more stringent 15-fold cutoff criterion in the spleen, thus this criterion did not effectively reduce the number of hits in the spleen from the 2862 hits at a 3-fold cutoff criterion. Due to the late spread to the spleen and the large number of gene hits, we interpret that the colonization of the spleen in this current model system is a bottle-necked event that does not facilitate a biologically relevant data interpretation. This study therefore focused on validating the results from the lung dataset, specifically the identification of capsule, T3SS3 and T6SS5 as the largest genetic loci contributing to respiratory melioidosis.
T6SS5 mutant is not attenuated in the IMIT model
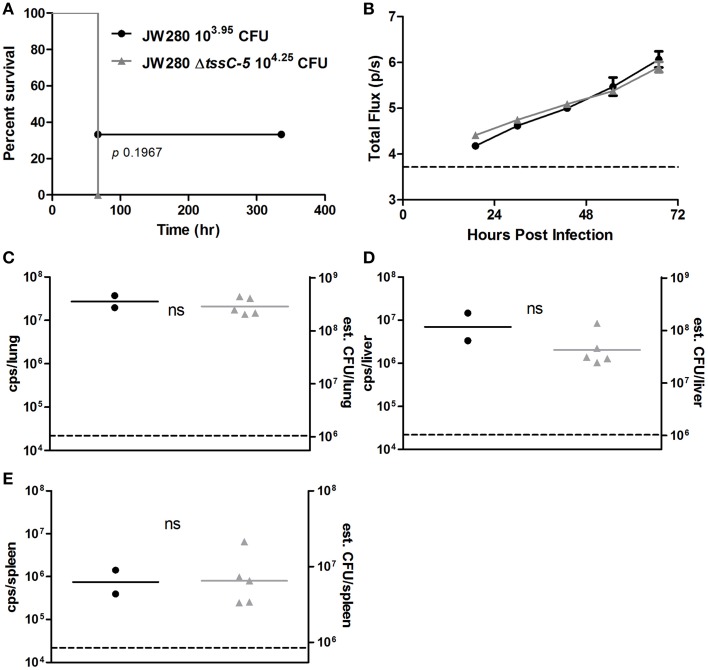
We previously calculated the LD50 of a B. pseudomallei luminescent strain JW280 to be 103.87 CFU, a T3SS3 translocation-deficient mutant, JW280 ΔsctUBp3 to be 106.19 CFU and a CPS operon mutant to be 104.57 (Gutierrez et al., 2015). While CPS, T3SS3, and T6SS5 all contributed to B. pseudomallei pulmonary fitness by the Tn-seq screen, single strain challenge with a CPS mutant did not reveal an attenuation by LD50, whereas a T3SS3 mutant was significantly attenuated >200-fold (Gutierrez et al., 2015). We therefore challenged C57BL/6J albino female mice with 103.95 CFU of the JW280 luminescent strain and 104.25 CFU of the JW280 ΔtssC-5 luminescent T6SS5 mutant strain to investigate the role of T6SS5 in respiratory melioidosis. Infection with the parent JW280 strain close to its LD50 resulted in 67% mortality (Figure 6A), whereas infection with the luminescent T6SS5 mutant was 100% lethal with the 104.25 CFU dose, which is 0.52-log above the parent LD50. Thus, the LD50 of the tssC-5 mutant is < 104.25 CFU, which was not significantly attenuated by Log-rank survival analysis relative to the parent control (Figure 6A). Therefore, like the CPS mutant, the T6SS5 mutant is not attenuated in single strain respiratory model challenge. Optical diagnostic imaging was used to monitor bacterial proliferation in the lung of lethally infected mice, and by 19 h post-infection, both the parent and the T6SS5 mutant were detectable by bioluminescent imaging, however the bioluminescent signal intensity increased at similar rates in the thoracic cavity (Figure 6B). While the T6SS5 mutant appeared to be slightly less fit than parent strain in proliferation rate, this difference was not significant. We characterized the bacterial tissue burden in moribund mice and found no significant difference between the T6SS5 and parent bacterial titers in the lungs, liver and spleen (Figures 6C–E). Thus, although our Tn-seq analysis revealed that the T6SS5 cluster is required for the full fitness of B. pseudomallei in the lung, single strain pulmonary challenge studies did not support this role for T6SS5.
Figure 6.
In vivo characterization of a T6SS mutant. (A) Survival curve of C57BL/6 female albino mice by IMIT infected with either JW280 or JW280 ΔtssC-5. (B) In vivo growth of B. pseudomallei strains JW280 and JW280 ΔtssC-5 infecting C57BL/6 mice, where bioluminescence was monitored twice per day and thoracic cavity total flux (p/s) was collected from region of interest measurements. The technical 95% limit of detection is indicated by a horizontal dashed line. Bacterial titers in the lungs (C), liver (D), and spleen (E) of C57BL/6 mice infected with either JW280 or JW280 ΔtssC-5 were enumerated by bioluminescence measurements of ex vivo tissues (counts per second [cps]/tissue), as described (Lawrenz et al., 2014). Statistical significance was calculated using the Student's T-test (ns: not significant).
A B. pseudomallei T6SS5 mutant is attenuated in competition studies
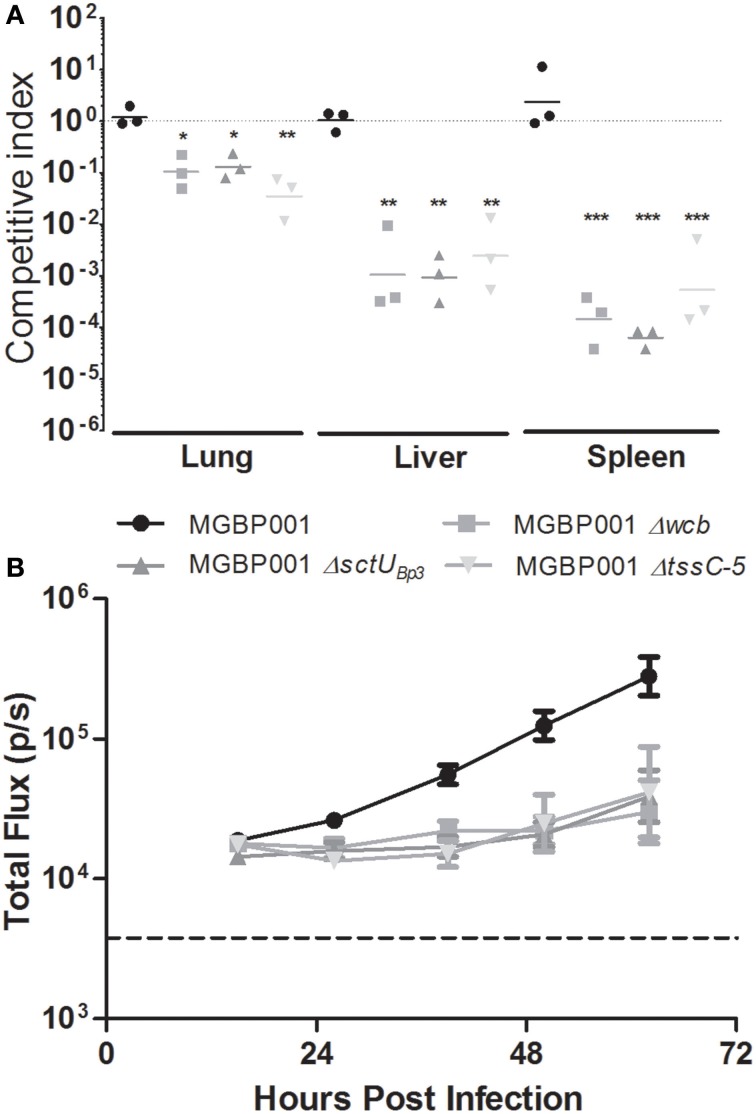
Given that Tn-seq is a genome-wide competition study, and initial validation of our Tn-seq results using traditional LD50 estimations did not identify attenuation for the tssC-5 mutant, we decided to investigate whether this T6SS5 mutant is attenuated by direct competition studies. We generated a novel gentamicin-marked PtolCluxCDABE bioluminescence reporter strain, MGBP001, which we also generated for the CPS, T3SS3, and T6SS5 mutants, and used these in competition studies against gentamicin-sensitive, non-luminescent, DD503. Accordingly, four groups of three C57BL/6J albino female mice were inoculated with ~104.0 B. pseudomallei at a 1:1 ratio of DD503 combined with either: (i) MGBP001, (ii) MGBP001 Δwcb (CPS−), (iii) MGBP001 ΔsctUBp3 (T3SS3-), or (iv) MGBP001 ΔtssC-5 (T6SS5-). Infections persisted for 67 h (late stage disease) before lungs, liver and spleen were collected and homogenates differentially plated on agar to identify proportions of gentamicin resistant and sensitive strains. The MGBP001 parent strain exhibited a competition index which was not significantly different from a ratio of 1.0 (Figure 7A), indicating that DD503 and MGBP001 shared the same virulence potential in all tissues, suggesting that neither the luminescence operon nor gentamicin resistance impact the virulence of the MGBP001 B. pseudomallei parent strain. Interestingly, all three mutant strains exhibited significantly reduced competition indices in the lungs, liver and spleen of mice relative to the non-luminescent DD503 competition partner (Figure 7A). Furthermore, optical diagnostic imaging of the thoracic cavity demonstrated that the parent MGBP001 strain proliferated within the lung (Figure 7B), consistent with the pulmonary colonization observed by the JW280 strain (Gutierrez et al., 2015). Imaging was able to successfully monitor the decreased fitness of the three mutants in the lung during the course of infection (Figure 7B), with significant decreases in thoracic cavity bioluminescence for all but the capsule mutant by 39 h, and for all strains by 50 h. Thus, optical diagnostic imaging successfully provides an early indication of fitness defects in vivo during competition studies. Therefore, CPS, T3SS3, and T6SS5 are all critical virulence determinants to support the fitness of B. pseudomallei in the lung, but importantly, attenuation of these virulence systems by single strain challenge studies has only been able to identify a role for T3SS3. We propose that competition analyses, including Tn-seq, provide a higher resolution assay by which to assess the role of B. pseudomallei virulence determinants in the lung, and that our current Tn-seq screen successfully identified CPS, T3SS3, and T6SS5 as the largest genetic loci required to facilitate respiratory melioidosis.
Figure 7.
Burkholderia pseudomallei major virulence determinants are attenuated in competition assays. (A) Competitive index of luminescent, Gm-marked parent strain (MGBP001), capsular polysaccharide deletion mutant (MGBP001 Δwcb) and T3SS (MGBP001 ΔsctUBp3) and T6SS5 (MGBP001 ΔtssC-5) mutants compared to Gm-sensitive, non-luminescent strain, DD503. Competitive index was calculated as the output ratio of mutant to parent divided by the input ratio of mutant to parent, having differentially plated lung, liver and spleen homogenates on agar plates with or without gentamicin selection. The horizontal dashed line indicates a competitive index of 1. Statistical significance was calculated using One-Way ANOVA followed by Tukey's Multiple Comparison Test (*P < 0.01; **P < 0.001; ***P < 0.0001).(B) In vivo growth of MGBP001, MGBP001 Δwcb, MGBP001 ΔsctUBp3, MGBP001 ΔtssC-5 in the lungs was monitored twice daily using luminescence as a read out for bacterial replication, and total flux (p/s) measurements collected from regions of interest centered on the thoracic cavity. The horizontal dashed line indicates the 95% technical limit of detection.
Discussion
In this study, we performed Tn-seq analysis to identify virulence determinants required to cause respiratory disease in a mouse model of lung-specific melioidosis. The primary design criterion of our screen was achieving the balance of saturating transposon mutagenesis while also limiting the challenge dose to allow for a biologically relevant disease progression. Previous transposon-directed insertion site sequencing and Tn-seq libraries have been generated in the Burkholderia pseudomallei strain K96243 (Moule et al., 2014) and its close relative Burkholderia thailandensis (Baugh et al., 2013; Gallagher et al., 2013) to identify essential genes required for survival of these pathogens in vitro. Importantly, these in vitro screens allowed for much larger library sizes to identify essential genes which were not amenable to transposon mutagenesis, which for B. pseudomallei was estimated to be 8.0% of the genome (Moule et al., 2014). In spite of limiting library size in our study to allow for infection studies, we achieved coverage of 88% of the 6141 estimated genes, where the theoretical maximum coverage is 92% based on estimates of number for essential genes. While our Tn-seq library was not designed to identify essential genes, we performed a ortholog search of the B. pseudomallei K96243 TraDIS-identified essential genes (Moule et al., 2014), and of the 483 orthologs in the 1026b genome, 53 genes were similarly identified with no reads in our Tn-seq library. An additional 202 K96243 essential orthologs were low read hits in our Tn-seq data set at one standard deviation below the log transformed mean read density. Thus, our Tn-seq data set confirms ~53% of the essential genes in the K96243 TraDIS essential gene list as confirmed essential genes in 1026b orthologs. The remaining half of the predicted essential genes were successfully read in our Tn-seq dataset, and we propose that these are not essential genes in 1026b, including such systems as Type 3 Secretion System cluster 1 (T3SS1) and three of the Type 6 Secretion Systems (T6SS2, T6SS4, and T6SS5), notably including T6SS5 which was a subject of investigation in these studies. Thus, consistent with the wider body of literature, some of the genes previously reported as essential may not be so, notably including the report that amrA and amrB are essential genes (Moule et al., 2014); while we do not read these genes in our Tn-seq dataset due to the fact that they have been deleted from DD503-linage strains, these are clearly not essential genes due to the availability of strains lacking these genes (Moore et al., 1999). These findings may suggest that the essential gene repertoire of B. pseudomallei is smaller than previously reported, and may reflect contributions from differences in strain (K96243 vs. 1026b lineage), transposon (Tn5 vs. Marineer) and other potential culturing techniques.
An unexpected result of our Tn-seq dataset was the revelation that B. pseudomallei is capable of reaching the liver very early in the course of infection, which was subsequently validated by a time course infection study. These findings were facilitated by using IMIT instillation which has been reported to efficiently deliver < 98% of an instilled dose directly into the lung (Lawrenz et al., 2014). Within 6 h of delivery directly into the lung by IMIT instillation, B. pseudomallei not only replicates in the lung, but also spreads to the liver at numbers similar to that cultured from the lung. Interestingly, few genes were critical for B. pseudomallei to maintain a presence in the liver, relative to the lung, suggesting that the lung does not persistently seed the liver over the course of infection, nor is the liver a source of a strong selective pressure to B. pseudomallei. Furthermore, B. pseudomallei does not appear to replicate in the liver until late in the infection, suggesting that the systemic fitness of the host may impact the replication potential of B. pseudomallei in the liver, again given that Tn-seq results do not support the possibility that the late infection increase in hepatic burden results from spread from the lung. It is noteworthy that in other respiratory models, the liver becomes colonized at later time points; for instance, aerosol delivery of B. pseudomallei did not result in detection of bacteria in the liver until 3 days post-infection (Tan et al., 2008). Thus, the observation of rapid dissemination from the lung to the liver may be a unique feature of the IMIT model, as is the prominent moribund septicemia which is not observed in other respiratory melioidosis models. Future work will be required to understand the role of the liver as a replicative niche for B. pseudomallei during respiratory melioidosis.
We developed a high specificity filter to prioritize follow-up analysis of the Tn-seq results of the lung. The selection criteria was benchmarked to the capsule polysaccharide genetic locus which we previously demonstrated was not a critical virulence determinant in the IMIT model of respiratory melioidosis, yet was attenuated 6.8-fold relative to parent (Gutierrez et al., 2015). Thus, our criteria was chosen to capture all virulence systems, including those which may not be significantly required by LD50 analysis. Our screen identified 548 genes, 8.7% of the genome's predicted ORFs, to be required by the bacterium for mammalian lung colonization, and of these, 32% (175 ORFs) accounted for hypothetical proteins, suggesting that additional novel virulence systems may participate in mediating fitness within the mammalian lung. Using cluster analysis to further prioritize the Tn-seq data set, we identified the three dominant genetic loci contributing to respiratory melioidosis as CPS, T3SS3, and T6SS5. Interestingly, these systems have been previously identified as virulence systems in systemic animal models (Reckseidler et al., 2001; Warawa and Woods, 2005; Burtnick et al., 2011); however, other virulence determinants described as important in systemic disease models did not meet our selection criteria, including the lipopolysaccharide (LPS) biosynthetic locus which had just two genes meeting our 15-fold selection criterion. Thus, virulence determinants required to support systemic infection may not be the same as those required in the lung. Our Tn-seq analysis was notably biased to the identification of large genetic systems which contribute to respiratory melioidosis, and it is therefore likely that additional smaller genetic loci have also been identified by this Tn-seq dataset, which will be the subject of future investigation.
Most B. pseudomallei virulence factors have been characterized primarily in systemic infection models, including intravenous, subcutaneous, and intraperitoneal inoculation. Indeed, the three major virulence systems targeted from our Tn-seq screen have been well studied as major contributors to disease in hamster and mouse systemic intraperitoneal infection models. The capsular polysaccharide has been characterized by several groups as providing one of the most significant contributions to the systemic disease potential of B. pseudomallei, with capsule mutants being attenuated ~5 log in these infection models (Atkins et al., 2002; Reckseidler-Zenteno et al., 2005). The significant role for capsule in B. pseudomallei systemic disease is consistent with a demonstrated role of this virulence factor in resisting complement opsonization of the bacteria (Reckseidler-Zenteno et al., 2005), and therefore likely a critical virulence factor for traffic between tissues, suggestive of B. pseudomallei spread as an extracellular pathogen. Interestingly, the capsule mutant does not appear to have a major role in the respiratory system of mice by single strain challenge, with attenuation levels of < 2 log by intranasal delivery and < 1 log by IMIT instillation (Warawa et al., 2009; Gutierrez et al., 2015). The T3SS3 system is known to importantly allow for escape of B. pseudomallei from phagosomes to facilitate rapid replication in the cytoplasm of phagocytes (Stevens et al., 2002), and the ubiquitous requirement for T3SS3 in both systemic (Warawa and Woods, 2005) and respiratory disease models (Gutierrez et al., 2015) suggests that the intracellular lifestyle of B. pseudomallei is critical in all host tissues. Indeed, T3SS3 is the only virulence system examined thus far in the IMIT instillation method which exhibits significant attenuation by single strain challenge, suggesting that this virulence system is one of the most critical systems for both respiratory and systemic disease. The primary function of the T6SS5 system is not well described, though there is evidence that T6SS5 supports the intracellular lifestyle of B. pseudomallei and cell-to cell spread (Pilatz et al., 2006; Burtnick et al., 2011). T6SS5 system mutants are attenuated >3 log in a systemic hamster intraperitoneal model (Burtnick et al., 2011) and are attenuated ~2–3 log in intranasal murine models (Pilatz et al., 2006; Hopf et al., 2014). In this current study, the role for T6SS5 in respiratory melioidosis in single strain challenges using IMIT delivery does not appear to suggest a prominent role given that we were unable to demonstrate significant attenuation in single strain challenge studies, which is in contrast to what has been previously reported for the intranasal models. We have previously demonstrated that intranasal inoculation of B. pseudomallei in mice results in a moribund disease state primarily associated with the nasal cavity rather than the systemic disease state observed following IMIT inoculation (Gutierrez et al., 2015). Thus, T6SS5 may be an important factor for nasal cavity colonization, but does not play the same critical role in the lung as seen for the T3SS3 system. In spite of the inability of capsule and T6SS5 mutants to demonstrate T3SS3-like significant attenuation by single strain challenges, they are clearly virulence determiants when assessed in competition studies.
A technical caveat of these studies relates to the use of a cloning strain of B. pseudomallei to perform the Tn-seq screen. The Tn-seq library was developed using the JW280 bioluminescent strain, which was derived from the aminoglycoside-sensitive strain DD503, which is itself derived from the 1026b clinical isolate. We have previously demonstrated that the introduction of the luxCDABE bioluminescent operon into the B. pseudomallei genome did not impact the virulence of the bacteria (Warawa et al., 2011), which was validated in this study by the finding that introduction of both luxCDABE and the gentamicin resistance marker (MGBP001) did not impact the competition index relative to the parent strain, DD503. It is important to note that the deletion of the AmrAB-OprA efflux pump in the DD503 strain may have an impact on virulence of B. pseudomallei and thus impact which genes are required for lung fitness in that specific strain background.
A key finding of our studies was the ability of competition studies to provide a higher resolution identification of virulence factors in the fitness of B. pseudomallei in the lung than single strain challenges/LD50 analyses. Similar findings have been made for other disease model systems, where competition index provides a higher resolution methodology for defining the contribution of virulence genes to a pathogen's fitness in animal model systems (Auerbuch et al., 2001; Logsdon and Mecsas, 2003; Yang et al., 2012). We had previously reported that a CPS mutant was not significantly attenuated by LD50 in the IMIT model (Gutierrez et al., 2015), but presently that CPS was significantly attenuated by competition study in the IMIT model. Similarly, we were unable to validate a significant attenuation for a T6SS5 mutant by single strain challenge, but identified a significant attenuation by competition index. The Tn-seq screen itself is a genome wide competition assay, thus our competition studies were able to successfully recapitulate the phenotypes we identified from the Tn-seq screen results, whereas single strain challenges did not have the resolution to defined roles in respiratory melioidosis to either capsular polysaccharide or T6SS5. These observations indicate that competition studies have a higher resolution to ascribe roles in virulence to both CPS and T6SS5 in respiratory melioidosis, and thus far, T3SS3 is the sole virulence determinant which exhibits attenuation by LD50 in the IMIT respiratory melioidosis model (Gutierrez et al., 2015). As an added feature of our competition assay, we have included a unique optical imaging approach which is further capable of detecting significant fitness defects in competition studies as early as 39 h post-infection.
An additional aspect of these studies worth highlighting is the finding that significant phenotypes were able to be resolved using small group sizes of animals. The three Rs of responsible animal research include: (1) replacement, (2) reduction, and (3) refinement; it is notable that reduction in group size was achievable through the methods we employed. By using groups of three mice inoculated by IMIT inoculation and infection monitoring by bioluminescence, we are successful in very targeted, efficient delivery of reagents directly into the lung (Lawrenz et al., 2014), which reduces variation in study metrics. This was particularly apparent in the demonstrated ability to synchronize infections and achieve excellent bacterial burden analysis throughout our studies. Most host-pathogen interactions are successfully studied by triplicate analysis, and we have demonstrated that n = 3 studies can be successfully carried out using whole animal host-pathogen interaction studies as well. This is consistent also with the previous use of small groups of animals (3–4 animals) to perform pooled in vivo Tn-seq analysis, and such study design has successfully resolved the detection of virulence systems for other bacterial pathogens (Skurnik et al., 2013a,b; Wang et al., 2014).
In summary, we provide the first phenotypic screen for B. pseudomallei virulence factor function in the mammalian lung, and have identified the largest critical genetic systems as CPS, T3SS3, and T6SS5. While previously identified as virulence systems, this study represents the first comparative study to validate their importance to respiratory melioidosis. These virulence systems therefore represent excellent targets for the future development of therapeutics against this Tier 1 Select Agent pathogen.
Author contributions
All authors contributed to experimental design, conducting studies, and composing the manuscript (MG, DY, JW).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by internal funding at the University of Louisville, including a Departmental Bridge Grant to JW.
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fcimb.2015.00078
References
- Atkins T., Prior R., Mack K., Russell P., Nelson M., Prior J., et al. (2002). Characterisation of an acapsular mutant of Burkholderia pseudomallei identified by signature tagged mutagenesis. J. Med. Microbiol. 51, 539–547. 10.1099/0022-1317-51-7-539 [DOI] [PubMed] [Google Scholar]
- Auerbuch V., Lenz L. L., Portnoy D. A. (2001). Development of a competitive index assay to evaluate the virulence of Listeria monocytogenes actA mutants during primary and secondary infection of mice. Infect. Immun. 69, 5953–5957. 10.1128/IAI.69.9.5953-5957.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh L., Gallagher L. A., Patrapuvich R., Clifton M. C., Gardberg A. S., Edwards T. E., et al. (2013). Combining functional and structural genomics to sample the essential Burkholderia structome. PLoS ONE 8:e53851. 10.1371/journal.pone.0053851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beji A., Izard D., Gavini F., Leclerc H., Leseine-Delstanche M., Krembel J. (1987). A rapid chemical procedure for isolation and purification of chromosomal DNA from gram-negative bacilli. Anal. Biochem. 162, 18–23. 10.1016/0003-2697(87)90005-4 [DOI] [PubMed] [Google Scholar]
- Brett P. J., Woods D. E. (2000). Pathogenesis of and immunity to melioidosis. Acta Trop. 74, 201–210. 10.1016/S0001-706X(99)00071-6 [DOI] [PubMed] [Google Scholar]
- Burtnick M. N., Brett P. J., Harding S. V., Ngugi S. A., Ribot W. J., Chantratita N., et al. (2011). The cluster 1 type VI secretion system is a major virulence determinant in Burkholderia pseudomallei. Infect. Immun. 79, 1512–1525. 10.1128/IAI.01218-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A. C., Currie B. J. (2005). Melioidosis: epidemiology, pathophysiology, and management. Clin. Microbiol. Rev. 18, 383–416. 10.1128/CMR.18.2.383-416.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A. C., Currie B. J., Dance D. A., Funnell S. G., Limmathurotsakul D., Simpson A. J., et al. (2013). Clinical definitions of melioidosis. Am. J. Trop. Med. Hyg. 88, 411–413. 10.4269/ajtmh.12-0555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie B. J., Fisher D. A., Howard D. M., Burrow J. N., Selvanayagam S., Snelling P. L., et al. (2000). The epidemiology of melioidosis in Australia and Papua New Guinea. Acta Trop. 74, 121–127. 10.1016/S0001-706X(99)00060-1 [DOI] [PubMed] [Google Scholar]
- Gallagher L. A., Ramage E., Patrapuvich R., Weiss E., Brittnacher M., Manoil C. (2013). Sequence-defined transposon mutant library of Burkholderia thailandensis. MBio 4, e00604–00613. 10.1128/mBio.00604-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galyov E. E., Brett P. J., DeShazer D. (2010). Molecular insights into Burkholderia pseudomallei and Burkholderia mallei pathogenesis. Annu. Rev. Microbiol. 64, 495–517. 10.1146/annurev.micro.112408.134030 [DOI] [PubMed] [Google Scholar]
- Goodman A. L., Kulasekara B., Rietsch A., Boyd D., Smith R. S., Lory S. (2004). A signaling network reciprocally regulates genes associated with acute infection and chronic persistence in Pseudomonas aeruginosa. Dev. Cell 7, 745–754. 10.1016/j.devcel.2004.08.020 [DOI] [PubMed] [Google Scholar]
- Goodman A. L., McNulty N. P., Zhao Y., Leip D., Mitra R. D., Lozupone C. A., et al. (2009). Identifying genetic determinants needed to establish a human gut symbiont in its habitat. Cell Host Microbe 6, 279–289. 10.1016/j.chom.2009.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez M. G., Pfeffer T. L., Warawa J. M. (2015). Type 3 Secretion system cluster 3 is a critical virulence determinant for lung-specific melioidosis. PLoS Negl. Trop. Dis. 9:e3441. 10.1371/journal.pntd.0003441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden M. T., Titball R. W., Peacock S. J., Cerdeño-Tárraga A. M., Atkins T., Crossman L. C., et al. (2004). Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei. Proc. Natl. Acad. Sci. U.S.A. 101, 14240–14245. 10.1073/pnas.0403302101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf V., Göhler A., Eske-Pogodda K., Bast A., Steinmetz I., Breitbach K. (2014). BPSS1504, a cluster 1 type VI secretion gene, is involved in intracellular survival and virulence of Burkholderia pseudomallei. Infect. Immun. 82, 2006–2015. 10.1128/IAI.01544-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis T. J., Rolim D. B., Sousa Ade Q. (2006). Melioidosis in the Americas. Am. J. Trop. Med. Hyg. 75, 947–954. [PubMed] [Google Scholar]
- Kaestli M., Schmid M., Mayo M., Rothballer M., Harrington G., Richardson L., et al. (2012). Out of the ground: aerial and exotic habitats of the melioidosis bacterium Burkholderia pseudomallei in grasses in Australia. Environ. Microbiol. 14, 2058–2070. 10.1111/j.1462-2920.2011.02671.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrenz M. B., Fodah R. A., Gutierrez M. G., Warawa J. (2014). Intubation-mediated intratracheal (IMIT) instillation: a noninvasive, lung-specific delivery system. J. Vis. Exp. 17:e52261. 10.3791/52261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar Adler N. R., Govan B., Cullinane M., Harper M., Adler B., Boyce J. D. (2009). The molecular and cellular basis of pathogenesis in melioidosis: how does Burkholderia pseudomallei cause disease? FEMS Microbiol. Rev. 33, 1079–1099. 10.1111/j.1574-6976.2009.00189.x [DOI] [PubMed] [Google Scholar]
- Logsdon L. K., Mecsas J. (2003). Requirement of the Yersinia pseudotuberculosis effectors YopH and YopE in colonization and persistence in intestinal and lymph tissues. Infect. Immun. 71, 4595–4607. 10.1128/IAI.71.8.4595-4607.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López C. M., Rholl D. A., Trunck L. A., Schweizer H. P. (2009). Versatile dual-technology system for markerless allele replacement in Burkholderia pseudomallei. Appl. Environ. Microbiol. 75, 6496–6503. 10.1128/AEM.01669-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. A., DeShazer D., Reckseidler S., Weissman A., Woods D. E. (1999). Efflux-mediated aminoglycoside and macrolide resistance in Burkholderia pseudomallei. Antimicrob. Agents Chemother. 43, 465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moule M. G., Hemsley C. M., Seet Q., Guerra-Assunção J. A., Lim J., Sarkar-Tyson M., et al. (2014). Genome-wide saturation mutagenesis of Burkholderia pseudomallei K96243 predicts essential genes and novel targets for antimicrobial development. MBio 5:e00926-13. 10.1128/mBio.00926-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen S. J., Batzloff M., Chehrehasa F., Meedeniya A., Casart Y., Logue C. A., et al. (2009). Nasal-associated lymphoid tissue and olfactory epithelium as portals of entry for Burkholderia pseudomallei in murine melioidosis. J. Infect. Dis. 199, 1761–1770. 10.1086/599210 [DOI] [PubMed] [Google Scholar]
- Pilatz S., Breitbach K., Hein N., Fehlhaber B., Schulze J., Brenneke B., et al. (2006). Identification of Burkholderia pseudomallei genes required for the intracellular life cycle and in vivo virulence. Infect. Immun. 74, 3576–3586. 10.1128/IAI.01262-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reckseidler S. L., DeShazer D., Sokol P. A., Woods D. E. (2001). Detection of bacterial virulence genes by subtractive hybridization: identification of capsular polysaccharide of Burkholderia pseudomallei as a major virulence determinant. Infect. Immun. 69, 34–44. 10.1128/IAI.69.1.34-44.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reckseidler-Zenteno S. L., DeVinney R., Woods D. E. (2005). The capsular polysaccharide of Burkholderia pseudomallei contributes to survival in serum by reducing complement factor C3b deposition. Infect. Immun. 73, 1106–1115. 10.1128/IAI.73.2.1106-1115.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reckseidler-Zenteno S. L., Moore R., Woods D. E. (2009). Genetics and function of the capsules of Burkholderia pseudomallei and their potential as therapeutic targets. Mini Rev. Med. Chem. 9, 265–271. 10.2174/138955709787316047 [DOI] [PubMed] [Google Scholar]
- Revelli D. A., Boylan J. A., Gherardini F. C. (2012). A non-invasive intratracheal inoculation method for the study of pulmonary melioidosis. Front. Cell. Infect. Microbiol. 2:164. 10.3389/fcimb.2012.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell M. A., Ulrich R. L., Ribot W. J., Brueggemann E. E., Hines H. B., Chen D., et al. (2007). Type VI secretion is a major virulence determinant in Burkholderia mallei. Mol. Microbiol. 64, 1466–1485. 10.1111/j.1365-2958.2007.05734.x [DOI] [PubMed] [Google Scholar]
- Schweizer H. P., Limmathurotsakul D., Peacock S. J. (2014). New insights from the 7th World Melioidosis Congress 2013. Emerging Infect. Dis. 20. 10.3201/eid2007.131737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalom G., Shaw J. G., Thomas M. S. (2007). In vivo expression technology identifies a type VI secretion system locus in Burkholderia pseudomallei that is induced upon invasion of macrophages. Microbiology 153, 2689–2699. 10.1099/mic.0.2007/006585-0 [DOI] [PubMed] [Google Scholar]
- Simon R., Priefer U., Pühler A. (1983). A broad range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology 1, 784–791. 10.1038/nbt1183-784 [DOI] [Google Scholar]
- Skorupski K., Taylor R. K. (1996). Positive selection vectors for allelic exchange. Gene 169, 47–52. 10.1016/0378-1119(95)00793-8 [DOI] [PubMed] [Google Scholar]
- Skurnik D., Roux D., Aschard H., Cattoir V., Yoder-Himes D., Lory S., et al. (2013a). A comprehensive analysis of in vitro and in vivo genetic fitness of Pseudomonas aeruginosa using high-throughput sequencing of transposon libraries. PLoS Pathog. 9:e1003582. 10.1371/journal.ppat.1003582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurnik D., Roux D., Cattoir V., Danilchanka O., Lu X., Yoder-Himes D. R., et al. (2013b). Enhanced in vivo fitness of carbapenem-resistant oprD mutants of Pseudomonas aeruginosa revealed through high-throughput sequencing. Proc. Natl. Acad. Sci. U.S.A. 110, 20747–20752. 10.1073/pnas.1221552110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M. P., Wood M. W., Taylor L. A., Monaghan P., Hawes P., Jones P. W., et al. (2002). An Inv/Mxi-Spa-like type III protein secretion system in Burkholderia pseudomallei modulates intracellular behaviour of the pathogen. Mol. Microbiol. 46, 649–659. 10.1046/j.1365-2958.2002.03190.x [DOI] [PubMed] [Google Scholar]
- Tan G. Y., Liu Y., Sivalingam S. P., Sim S. H., Wang D., Paucod J. C., et al. (2008). Burkholderia pseudomallei aerosol infection results in differential inflammatory responses in BALB/c and C57Bl/6 mice. J. Med. Microbiol. 57, 508–515. 10.1099/jmm.0.47596-0 [DOI] [PubMed] [Google Scholar]
- Van Opijnen T., Bodi K. L., Camilli A. (2009). Tn-seq: high-throughput parallel sequencing for fitness and genetic interaction studies in microorganisms. Nat. Methods 6, 767–772. 10.1038/nmeth.1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Ozer E. A., Mandel M. J., Hauser A. R. (2014). Genome-wide identification of Acinetobacter baumannii genes necessary for persistence in the lung. MBio 5:e01163-14. 10.1128/mBio.01163-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warawa J., Woods D. E. (2005). Type III secretion system cluster 3 is required for maximal virulence of Burkholderia pseudomallei in a hamster infection model. FEMS Microbiol. Lett. 242, 101–108. 10.1016/j.femsle.2004.10.045 [DOI] [PubMed] [Google Scholar]
- Warawa J. M., Long D., Rosenke R., Gardner D., Gherardini F. C. (2009). Role for the Burkholderia pseudomallei capsular polysaccharide encoded by the wcb operon in acute disseminated melioidosis. Infect. Immun. 77, 5252–5261. 10.1128/IAI.00824-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warawa J. M., Long D., Rosenke R., Gardner D., Gherardini F. C. (2011). Bioluminescent diagnostic imaging to characterize altered respiratory tract colonization by the Burkholderia pseudomallei capsule mutant. Front. Microbiol. 2:133. 10.3389/fmicb.2011.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersinga W. J., Van der Poll T., White N. J., Day N. P., Peacock S. J. (2006). Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat. Rev. Microbiol. 4, 272–282. 10.1038/nrmicro1385 [DOI] [PubMed] [Google Scholar]
- Wiles T. J., Norton J. P., Russell C. W., Dalley B. K., Fischer K. F., Mulvey M. A. (2013). Combining quantitative genetic footprinting and trait enrichment analysis to identify fitness determinants of a bacterial pathogen. PLoS Genet. 9:e1003716. 10.1371/journal.pgen.1003716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Lv Y., Xiao J., Wu H., Zheng H., Liu Q., et al. (2012). Edwardsiella comparative phylogenomics reveal the new intra/inter-species taxonomic relationships, virulence evolution and niche adaptation mechanisms. PLoS ONE 7:e36987. 10.1371/journal.pone.0036987 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.