Abstract
Glucose-regulated protein 78 (GRP78) is a member of the heat-shock protein 70 family. We evaluated the expression of GRP78 using tissue microarray-based immunohistochemistry in tumor tissues and adjacent nontumor tissues from 180 pancreatic ductal adenocarcinoma (PDAC) patients. The associations between the expression levels of GRP78, clinicopathological factors, and overall survival were evaluated. The results showed that the expression of GRP78 was significantly higher in PDAC cells than in normal pancreatic duct cells within adjacent nontumor tissues (p < 0.05). The increased expression of GRP78 in the tumor tissues was significantly correlated with a higher T-stage (p < 0.05) and a shorter overall survival (OS, p < 0.05). In an in vitro study, the regulation of GRP78 in the PDAC cell lines affected the proliferation, migration, and invasion of PDAC cells through the regulation of CyclinD1, cyclin-dependent kinase (CDK) 4, CDK6, phospho-signal transducer, activator of transcription 3 (p-STAT3), janus kinase 2 (JAK2), ras homolog gene family member A (RhoA), Rho-associated kinase 1 (ROCK1), and sterile alpha motif domain containing protein 4 (Smad4). The present data suggest that GRP78 plays a crucial role in the proliferation, migration, and invasion of pancreatic cancer cells and may be a suitable prognostic marker in PDAC.
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive types of malignant tumors, and approximately 46,420 new cases will be diagnosed and approximately 39,590 patients will die of PDAC in the United States in 20141. Although surgery offers the only opportunity for a cure, the prognosis of patients after resection is still disappointing in many cases2. The average 5-year survival rate of PDAC patients remains at only approximately 6%3; therefore, the identification of new and specific factors that can provide a possible prognosis of PDAC is of great importance. Except for conventional clinicopathological factors, such as lymph node status, perineural invasion (PNI), pathological grade, and stage, many molecules, such as mucin-1 (MUC1), mesothelin (MSLN), and mucin-2 (MUC2), were studied as prognostic biomarkers that are involved in tumor initiation and development. However, more research is needed to accumulate additional data on prognostic biomarkers4.
Glucose-regulated protein 78 (GRP78), also known as immunoglobulin heavy chain binding protein, is a member of the heat-shock protein 70 family and is localized in the endoplasmic reticulum (ER). GRP78 has been implicated in the folding and transport of proteins, and plays an essential role as a regulator in ER homeostasis, the activation of the ER stress sensors, and the unfolded protein response (UPR)5,6. Many investigations have indicated that GRP78 plays a critical role in the proliferation, invasion, and metastasis of human cancer cells7,8,9,10,11,12,13,14,15. Some studies indicated a positive correlation between GRP78 and tumor malignancy (epithelial ovarian tumors7, diffuse large B cell lymphoma8, renal cell carcinoma9, endometrial carcinoma10, gastric carcinoma11, and prostate cancer12), while some other studies came to an opposite conclusion (colorectal carcinoma13, oral squamous cell carcinoma14, and esophageal adenocarcinoma15). Therefore, its effect on the outcome of cancer might be tissue-type specific and involved in different signaling pathways. The correlation between GRP78 expression and the clinicopathological characteristics of PDAC and its role in pancreatic cancer have not been reported.
In the present study, the correlations between GRP78 expression and the clinicopathological characteristics and overall survival of patients with PDAC were investigated using tissue microarray (TMA)-based immunohistochemistry. In addition, the effect of GRP78 expression on the proliferation, migration, and invasion of three PDAC cell lines were evaluated in vitro. The changes in the associated proteins were detected by western blotting.
Results
Expression of GRP78 and its clinicopathological relevance in human PDAC
We first examined the expression of GRP78 using tissue microarray-based immunohistochemistry on the tumor tissues and adjacent nontumor tissues from 180 patients with PDAC. GRP78-positive staining was observed in the cytoplasm of both the PDAC cells and in the pancreatic duct cells in the adjacent nontumor tissues. Compared to the normal pancreatic duct cells in the adjacent nontumor tissues, the level of GRP78 staining was significantly higher in the PDAC cells (p < 0.001) (Fig. 1). As shown in Table 1, the expression of GRP78 in the tumor tissue was significantly associated with the tumor (T) stage (p = 0.026), indicating that GRP78 may play a role in the progression of PDAC. No significant association was detected between GRP78 expression and the other clinicopathological characteristics.
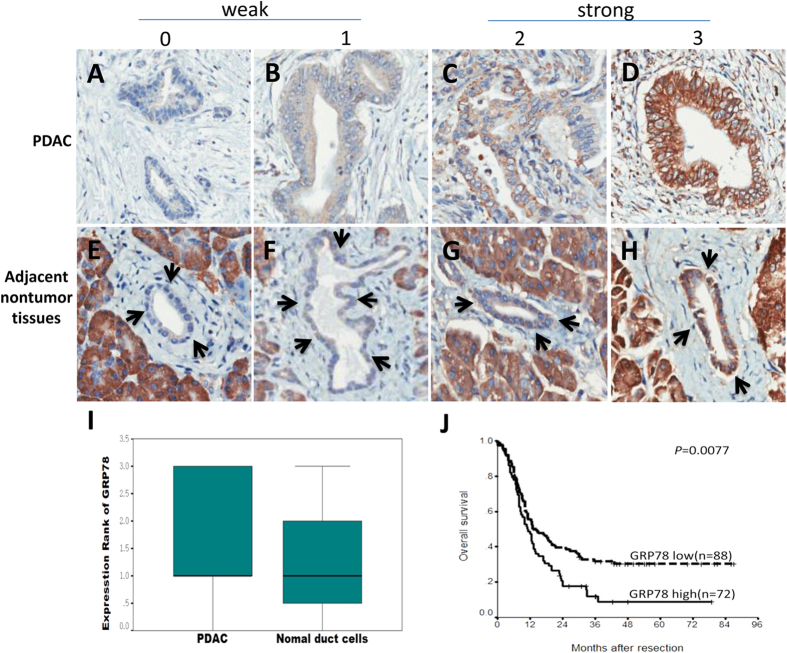
Figure 1. Immunohistochemical staining of GRP78 and the Kaplan-Meier survival analysis.
(A–D) Different staining intensities of GRP78 in the PDAC tumor tissues. (E-H) Different staining intensities of GRP78 in the adjacent nontumor tissues. The cellular staining was classified using a scale of 0-3 as follows: 0 = negative (A, E), 1 = weakly positive (B,F), 2 = moderately positive (C,G) and 3 = strongly positive (D,H) (original magnification 200×). (I) Comparison of the GRP78 staining score between the PDAC and nontumor pancreatic duct cells (Mann–Whitney U-test, p = 0.009). (J) Survival analysis of the PDAC patients after surgical resection based on the GRP78 expression in the tumor tissues (log-rank test, p = 0.0077).
Table 1. Association between the GRP78 expression in the tumor tissues and the clinicopathologic variables of PDAC.
| Variable | GRP78 expression | p value* | |
|---|---|---|---|
| No. of cases | Low high | ||
| Age (years) | 0.361 | ||
| ≥65 | 62 | 37 25 | |
| <65 | 118 | 62 56 | |
| Gender | 0.566 | ||
| Male | 113 | 64 49 | |
| Female | 67 | 35 32 | |
| Tumor location | 0.876 | ||
| Head | 101 | 54 47 | |
| Body/tail | 67 | 35 32 | |
| Tumor size | 0.079 | ||
| >4 cm | 76 | 35 41 | |
| ≤4 cm | 94 | 56 38 | |
| Histological grade | 0.406 | ||
| G1–2 | 120 | 62 58 | |
| G3 | 37 | 22 15 | |
| pT stage | 0.026 | ||
| T1–2 | 96 | 58 38 | |
| T3–4 | 74 | 32 42 | |
| pN stage | 0.487 | ||
| N0 | 95 | 52 43 | |
| N1 | 71 | 35 36 | |
| Perineural invasion | 0.111 | ||
| Positive | 84 | 40 44 | |
| Negative | 87 | 52 35 |
Note: The boldface type indicates a significant difference. PDAC, pancreatic ductal adenocarcinoma; G1, well differentiated; G2, moderately differentiated; G3, poorly differentiated; T, tumor; N, lymph node. * Chi-square test.
Effect of GRP78 expression on prognosis
We next examined the effect of GRP78 expression on prognosis using the Kaplan–Meier method and log-rank test; the univariate analysis showed that the poorer overall survival of patients was significantly associated with high GRP78 expression in the tumor tissues (p = 0.0077) (Fig. 1J), sex (p = 0.0075), histologic grade (p = 0.015), T stage (p = 0.0139), and peripheral nerve infiltration (PNI) (p = 0.0455) (Table 2). The Cox regression analysis showed that GRP78 expression as well as PNI, sex, tumor tissues, histologic grade, and T-stage are independent prognostic markers (p < 0.05) (Table 2). Additionally, the GRP78 expression in the tumor tissues was also an independent prognostic marker (p < 0.05) in many subgroups (female, G1-2, PNI-negative) of PDAC patients (Table 3; Fig. 2).
Table 2. Univariate and multivariate Cox regression analyses of the association of the prognosis with the clinicopathological parameters and GRP78 expression in PDAC.
| Variable | No. of cases | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| Median ± SE | 95% CI | p value# | RR | 95% CI | p value* | ||
| Age (years) | 0.9272 | ||||||
| ≥65 | 53 | 11 ± 2 | 7–15 | ||||
| <65 | 107 | 13 ± 1 | 10–15 | ||||
| Gender | 0.0075 | 0.041 | |||||
| Male | 104 | 11 ± 1 | 9–13 | 1.617 | 1.017–2.187 | ||
| Female | 56 | 14 ± 5 | 5–24 | 1 | |||
| Tumor location | 0.4050 | ||||||
| 95 | 13 ± 1 | 10–16 | |||||
| 60 | 11 ± 1 | 8–14 | |||||
| Tumor size | 0.9543 | ||||||
| >4 cm | 94 | 13 ± 1 | 10–16 | ||||
| ≤4 cm | 69 | 11 ± 1 | 8–14 | ||||
| Histological grade | 0.0150 | 0.013 | |||||
| G1–2 | 108 | 14 ± 2 | 9–19 | 1 | |||
| G3 | 36 | 9 ± 1 | 7–11 | 1.696 | 1.116–2.576 | ||
| pT stage | 0.0139 | 0.036 | |||||
| T1–2 | 94 | 13 ± 4 | 6–20 | 1 | |||
| T3–4 | 64 | 11 ± 2 | 8–14 | 1.508 | 1.026–2.514 | ||
| pN stage | 0.0778 | ||||||
| N0 | 91 | 15 ± 3 | 10–20 | ||||
| N1 | 62 | 10 ± 2 | 7–13 | ||||
| Perineural invasion | 0.0455 | 0.348 | |||||
| Positive | 77 | 11 ± 1 | 9–13 | 1.204 | 0.817–1.776 | ||
| Negative | 81 | 15 ± 3 | 9–21 | 1 | |||
| GRP78 in tumor | 0.0077 | 0.041 | |||||
| High | 72 | 11 ± 1 | 8–14 | 1.491 | 1.017–2.187 | ||
| Low | 88 | 13 ± 4 | 6–20 | 1 | |||
Note: The boldface type indicates a significant difference. PDAC, pancreatic ductal adenocarcinoma; SE, standard error; RR, relative risk; CI, confidence interval; G1, well differentiated; G2, moderately differentiated; G3, poorly differentiated; T, tumor; N, lymph node. #Log-rank test; *Cox regression test.
Table 3. Multivariate Cox regression analyses of the prognostic relevance of GRP78 expression in PDAC subgroups in which the poorer overall survival of patients was significantly associated with high GRP78 expression (identified by the log-rank test).
| Subgroups | RR | 95% CI | p value * |
|---|---|---|---|
| Females | 2.222 | 1.031–4.790 | 0.042 |
| G1–2 | 1.808 | 1.136–2.876 | 0.012 |
| Size > 4 cm | 1.668 | 0.824–3.378 | 0.155 |
| Head of pancreas | 1.639 | 0.996–2.697 | 0.052 |
| PNI negative | 2.101 | 1.143–3.863 | 0.017 |
| N1 | 1.763 | 0.955–3.254 | 0.070 |
Note: The boldface type indicates a significant difference. PDAC, pancreatic ductal adenocarcinoma; RR, relative risk; CI, confidence interval; G1, well differentiated; G2, moderately differentiated; PNI, perineural invasion; N, lymph node. *Cox regression test.
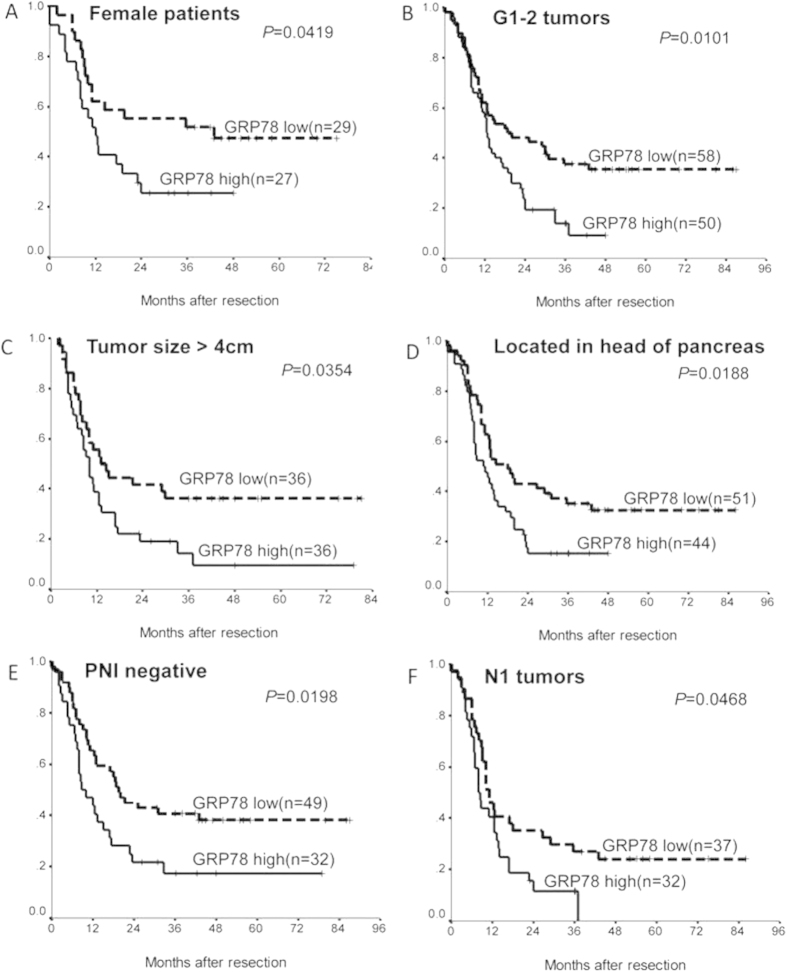
Figure 2. Kaplan-Meier survival analysis of the PDAC patients after surgical resection based on the GRP78 expression in the tumor tissues in many subgroups.
(A) female patients (p = 0.0419); (B) G1-2 tumors (p = 0.0101); (C) patients with tumor size > 4 cm (p = 0.0354); (D) patients with carcinoma of the head of the pancreas (p = 0.0188); (E) PNI-negative tumors (p = 0.0198); (F) N1 tumors (p = 0.0468). G1, well differentiated; G2, moderately differentiated; PNI, perineural invasion; N, lymph node.
Expression of GRP78 in six PDAC cell lines
To investigate the effects of GRP78 on the behaviors of PDAC cells, we performed in vitro experiments to determine the proliferation and invasion of the cell lines. The expression of GRP78 in six PDAC cell lines, including AsPC-1, BxPC-3, Capan-1, MIA PaCa-2, PCT-3, and SU.86.86 cells, was evaluated by western blotting. As shown in Fig. 3, higher GRP78 expression was observed in the BxPC-3 and SU.86.86 cells. Additionally, relatively low GRP78 expression was observed in the Capan-1 cell line. Therefore, these three cell lines were chosen for additional in vitro experiments.
Figure 3. Expression of GRP78 in six PDAC cell lines.

Cropped blots/gels were used in the figure and the gels had been run under the same experimental conditions; the full-length blots/gels are presented in Supplementary Figure S3B.
GRP78 promoted cell proliferation and regulated the cell cycle in PDAC cell lines
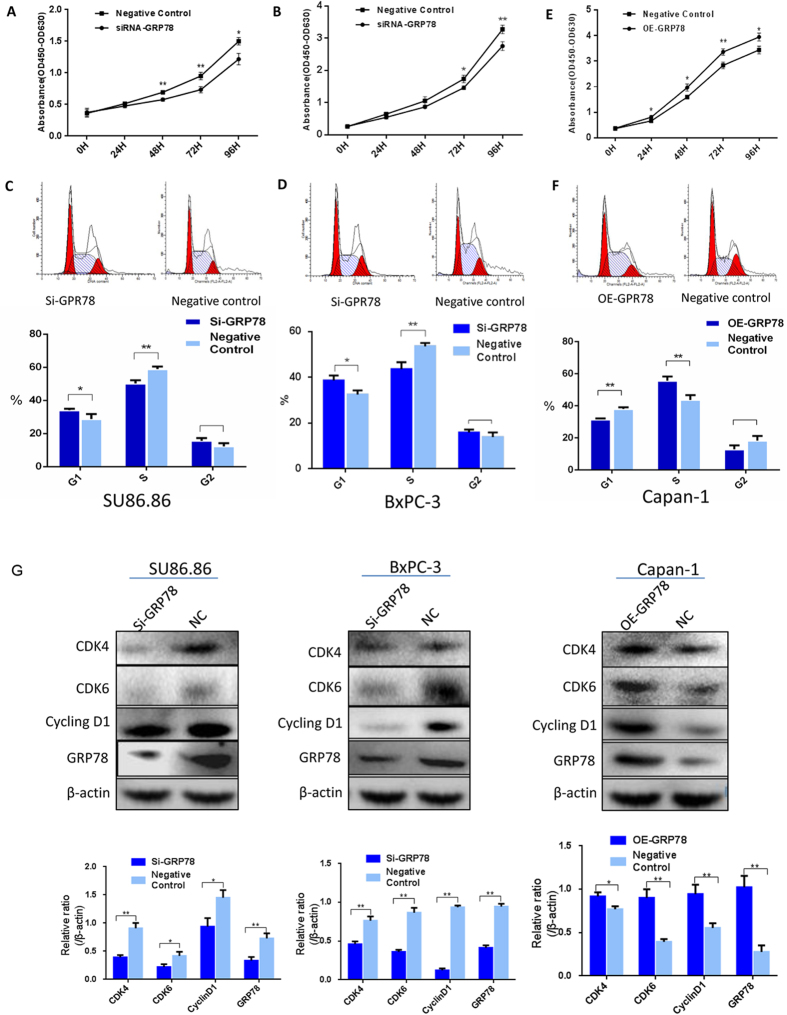
GRP78 was silenced using transient transfection of siRNAs in the BxPC-3 and SU.86.86 cell lines, which highly expressed GRP78. The expression of GRP78 was successfully suppressed at 48 hours after transfection and verified by western blotting (Fig. 4). The CCK8 assay showed that the proliferation of the two GRP78 knockdown cell lines was dramatically reduced compared to the negative control (Fig. 4A,B).
Figure 4. Impact of GRP78 on the proliferation and cell cycle of the PDAC cell lines.
(A,B) The CCK8 assay showed that the proliferation capacity of the SU86.86 and BXPC-3 cells was decreased after GRP78 knockdown. (C,D) The percentage of SU.86.86 and BXPC-3 cells in G0/G1 phase was significantly increased after GRP78 knockdown. (E) The CCK8 assay showed that the proliferation capacity of the Capan-1 cells was increased after forced overexpression of GRP78. (F) After the forced overexpression of GRP78, the percentage of Capan-1 cells in G0/G1 phase was significantly decreased, while the percentage of cells in S phase was significantly increased. (G) Western blots for CyclinD1, CDK4, and CDK6 in the SU86.86, BXPC-3 and Capan-1 cells after knockdown or overexpression of GRP78 or the negative control. si-GRP78, siRNA-GRP78; OE-GRP78, overexpression of GRP78; NC, negative control. Cropped blots/gels were used in the figure and the gels had been run under the same experimental conditions; the full-length blots/gels are presented in Supplementary Figure S4.
We also evaluated the effect of GRP78 on the cell cycle. Compared to the control group, the downregulation of GRP78 in PDAC cells by transfecting siRNAs significantly decreased the percentage of cells in S phase and increased the percentage of cells in G0/G1 phase (Fig. 4C,D).
Additionally, the Capan-1 cell line was transfected with plasmids to overexpress GRP78 to confirm the effect of GRP78 on cell proliferation. Cell proliferation was significantly increased in the GRP78-overexpressing Capan-1 cell line compared to the negative control (Fig. 4E). Furthermore, the overexpression of GRP78 increased the percentage of cells in S phase in the cell cycle analysis (Fig. 4F).
Western blotting showed that the expression of cell cycle proteins, including CyclinD1, CDK4, and CDK6, was simultaneously suppressed in the GRP78 knockdown groups, while the expression levels of these proteins was upregulated in the GRP78-overexpressing group (Fig. 4G).
Impact of GRP78 on the invasion and migration of the PDAC cell lines
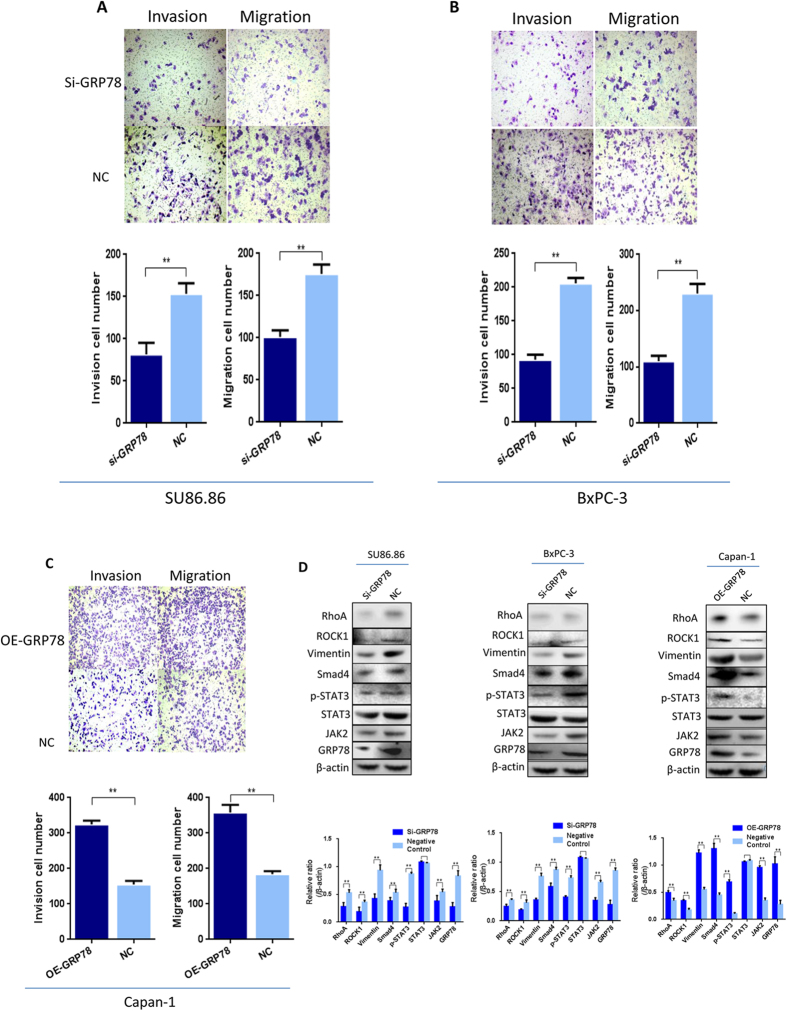
After silencing GRP78, the invasion and migration of the BXPC-3 and SU.86.86 cells were significantly reduced compared to those of the negative control by the transwell array (Fig. 5A,B). However, after GRP78 overexpression, the invasion and migration of Capan-1 cells were significantly increased compared to the control group (Fig. 5C).
Figure 5. Impact of GRP78 on the invasion and migration of the PDAC cell lines.
(A) The invasion and migration of the SU.86.86 cells were decreased after GRP78 knockdown compared to the NC group (*p < 0.01). (B) The invasion and migration of the BXPC-3 cells were decreased after GRP78 knockdown compared to the NC group. (C) The invasion and migration of the Capan-1 cells were increased after the forced overexpression of GRP78 compared to the NC group (*p < 0.01, original magnification, 100×). (D) The levels of RhoA, ROCK1, JAK2, vimentin, Smad4, and p-STAT3 were decreased by GRP78 knockdown and increased by GRP78 overexpression in the SU86.86, BXPC-3 and Capan-1 cell lines (*p < 0.05). siRNA, siRNA-GRP78; OE-GRP78, overexpression of GRP78; NC, negative control. Cropped blots/gels were used in the figure and the gels had been run under the same experimental conditions; the full-length blots/gels are presented in Supplementary Figure S5.
To examine the mechanisms by which GRP78 regulates cell invasion and migration, we tested the expression of p-STAT3, JAK2, vimentin, RhoA, ROCK1 and Smad4. In the GRP78 knockdown group, the expression of these proteins was downregulated. In contrast, in the GRP78-overexpressing group, the levels of these proteins were significantly increased (Fig. 5D).
Discussion
Due to the extremely aggressive progression of PDAC, it is important to select prognostic markers that help clinicians to select therapy options that will avoid unnecessary toxicity. GRP78 is involved in cancer development and progression, with tumor-specific characteristics7,8,9,10,11,12,13,14,15. Although it remains unclear how GRP78 participates in tumor proliferation, invasion, and metastasis, a number of studies have reported correlations between GRP78 expression and the clinicopathological characteristics of various human cancers, and the possible mechanisms were explored. Some researchers assume that because of the rapid and unrestrained proliferation of cancer cells, the protein load might exceed the ER capacity, causing the accumulation of misfolded and unfolded proteins in the ER. This causes ER stress and triggers the UPR to restore ER homeostasis by activating a cascade of signaling molecules to induce the ER molecular chaperones and enzymes that enhance the protein folding capacity, and to initiate a process to export and degrade the misfolded and unfolded proteins16. As the most well-studied molecular chaperone in the UPR, GRP78 plays an important role in maintaining ER homeostasis and contributes to cancer cell survival and progression. The prognostic significance of GRP78 in human cancers was controversial, according to previous reports11,13. Increased overall 5-year survival was associated with high GRP78 expression in colorectal cancer13, whereas the opposite conclusion was found in gastric cancer11 and hepatocellular carcinoma17.
A recent study has shown increased GRP78 expression in human PDAC tissue samples18. Similar to our results, they also found high expression of GRP78 in normal pancreatic acinar cells18, which might be because the acinar cells around the PDAC always suffered from chronic inflammation19, and GRP78 has long been found to be highly expressed in inflammation20. However, the correlations between GRP78 expression and the clinicopathological characteristics or prognosis of PDAC have not been investigated. In the present study, we tested GRP78 expression in PDAC by immunohistochemistry. Because neoadjuvant chemotherapy might induce ER stress, we excluded those samples. The data showed that compared to the normal pancreatic duct cells, GRP78 was expressed at significantly higher levels in the cytoplasm of PDAC cells, indicating that the cancer cells were often under ER stress and the UPR was triggered to restore ER homeostasis. Moreover, increased GRP78 expression was significantly correlated with a higher tumor (T) stage, suggesting that GRP78 played a crucial role in cancer progression and may serve as a prognostic marker for PDAC patients after surgery. Further analysis revealed that elevated GRP78 expression is associated with a poor prognosis in PDAC patients after surgery, in addition to some conventional clinicopathologic factors, including sex, PNI, histological grade, and T stage. Multivariate analysis proved that GRP78 expression was an independent prognostic factor of overall survival in PDAC. The data from a univariate analysis in some subgroups also supported this conclusion.
We performed in vitro experiments to verify our findings. Our findings indicated that GRP78 promoted the proliferation of PDAC cells via elevated expression of CyclinD1, CDK4, and CDK6, which was in accord with a previous report on a renal cell carcinoma cell line21. Unrestrained proliferative capacity is a hallmark of cancer22. CyclinD1, CDK4, and CDK6 are key proteins that control the G1 checkpoint in cell proliferation23,24. Our tests on the cell cycle after regulating GRP78 expression reached the same conclusion, suggesting that GRP78 might affect the proliferation of PDAC cells by changing the cell cycle. Overexpression of CyclinD1 and CDK4/CDK6 was also observed in many human malignancies, including gall bladder cancer, endometrial carcinoma, and ovarian serous carcinomas25,26,27,28. Moreover, the apoptosis assay was also performed by FCS, but no significant difference was observed, likely because the spontaneous apoptosis of PDAC cell lines occurred at a relatively low level (supplementary material).
The present in vitro study also revealed that GRP78 may promote PDAC migration and invasion through a few pathways. The expression of vimentin, a widely used maker for the epithelial-mesenchymal transition (EMT), a common characteristic of cancer cells that contributes to tumor invasion and metastasis29, was decreased in accord with the knockdown of GRP78. Vimentin has been reported to be an important prognostic factor for PDAC and to be associated with portal vein invasion and lymph node metastasis29. The expression of Smad4 was decreased after the knockdown of GRP78. Smad4 is known as the common mediator of transforming growth factor-β (TGF-β) superfamily signaling, which plays a central role in the EMT. Although Smad4 is generally considered to be a tumor suppressor gene, it is indispensable in the regulation of the TGF-β-inducible EMT. Ya’an, K indicated that the migration and invasion of human pancreatic ductal epithelial cells was diminished by the decreased N-cadherin expression caused by Smad4 knockdown30. We found that the expression of RhoA and ROCK1 were synchronously downregulated after GRP78 knockdown. RhoA is a small GTPase protein that is believed to regulate cell motility. Because of its significant role in regulating cell shape, polarity, and locomotion, RhoA and ROCK1 have been well studied in many cancers and proven to promote cancer cell migration and invasion to other tissues31. Tavares, AL indicated that TGF-β-mediated RhoA expression is necessary for the EMT32. We assume that after knockdown of GRP78, the EMT of the PDAC cells was decreased, causing the decreased invasion and migration capacity, which needs to be investigated in further studies. STAT3 activation has been found to enhance the expression of the vimentin gene33, and a close relationship between STAT3 signaling and RhoA has also been found34. A study of breast cancer has revealed that the EMT promotes cancer progression via a fibronectin-dependent STAT3 signaling pathway35. In our study, the expression of JAK2 and p-STAT3 was downregulated after GRP78 knockdown of GRP78, and upregulated after GRP78 overexpression in PDAC cell lines. The JAK2/STAT3 pathway had been proven to play a crucial role in mediating cancer cell migration and invasion in many human cancers through different mechanisms36,37,38. Guo, K and Li, H found that STAT3 promoted perineural invasion, and STAT3 knockdown could reduce PDAC cell invasion and matrix metalloproteinase-7 expression39,40.
In summary, the present study showed that the level of GRP78 was elevated in PDAC cells and was associated with a higher T stage and a poor prognosis. In the in vitro study, we explored the various mechanisms by which GRP78 might contribute to the proliferation, migration, and invasion of PDAC cell lines. In addition, several reports implicated GRP78 as a potential target in cancer therapy, and corresponding inhibitors have been studied41,42,43,44. Our study indicated that GRP78 played an important role in PDAC growth and metastasis, and that it could be a practical prognostic marker and potential target in PDAC therapy.
Materials and Methods
Patient information and follow-up
The PDAC tumor tissues and corresponding adjacent nontumor tissues were collected from 180 patients (113 males and 67 females) using the following inclusion criteria: (1) the PDAC samples were histologically proven to be ductal adenocarcinoma by hematoxylin–eosin staining; (2) both the paired tumor and nontumor tissues were obtained; and (3) the patients were not undergoing neoadjuvant chemotherapy. The diagnosis and staging were based on the Staging Manual of the American Joint Committee on Cancer, 7th edition. The adjacent nontumor tissue was excised from 3 cm around the cancerous tissue. The exclusion criteria included patients with other organic diseases or the inability to provide informed consent. Of the 180 patients, follow-up data was obtained for 160 cases (approximately 0–87 months; median: 12.55 months), as shown in Tables 1 and 2. Overall survival was defined as the survival time after surgery. This study was approved by the Ethics Committee of Peking Union Medical College Hospital and adhered to the tenets of the Declaration of Helsinki. Written informed consents for the storage of the tissue samples and publication of this study were obtained before surgery after a complete verbal explanation.
Tissue microarray construction and immunohistochemistry
The TMA was constructed with a manual tissue microarray (Beecher Instruments, Sun Prairie, WI, USA) using formalin-fixed paraffin-embedded blocks. Two cores of the tumor and adjacent nontumor tissue for each case were sampled from representative areas using a 1.5-mm punch.
Immunohistochemistry was performed to detect GRP78 expression. A rabbit GRP78 monoclonal antibody (ab108613, Abcam Biotech Company, Cambridge, UK) and an EnVision+ two-step staining kit (Dako, Glostrup, Denmark) were used for staining. Briefly, the 4-μm-thick formalin-fixed, paraffin-embedded tumor sections were mounted, deparaffinized in xylene, and rehydrated in a graded alcohol series. The slides were washed with phosphate-buffered saline (PBS), immersed in a 0.01 mol/L citrate buffer solution (pH 6.0), and heated in a microwave for 10 min for antigen retrieval. Peroxidase was quenched using 3% hydrogen peroxide for 10 min. After washing again with PBS, the slides were incubated at 4 °C overnight with the primary antibody (1:50). After washing with PBS four times, the horseradish peroxidase-conjugated secondary antibody was added for 30 min and diaminobenzidine was used as a chromogen. Finally, the slides were counterstained with hematoxylin (Sigma-Aldrich Co., LLC, Munich, Germany) to visualize the nuclei. Preimmune rabbit serum was used at the same dilution as the negative control.
Staining evaluation
A blinded staining evaluation was performed by two experienced pathologists who did not have access to the clinicopathological or follow-up data. Based on a previous study13, the frequencies of GRP78 staining were almost at the same level in the tissues; the staining intensity was used to evaluate the expression of GRP78. The proportion of stained cells was first classified using a scale of 0–3 as follows: 0 = negative, 1 = weakly positive, 2 = moderately positive and 3 = strongly positive. Finally, GRP78 expression was simply summarized as low (intensity 0 or 1) or high (intensity 2 or 3) as shown in Fig. 1.
Cell lines and culture conditions
The human PDAC cell lines AsPC-1, BxPC-3, Capan-1, MIA PaCa-2, PCT-3, and SU.86.86 were donated by the University of Heidelberg. The cells were cultured in HyClone RPMI-1640 medium (GE Healthcare Life Sciences, Logan, Utah, USA) supplemented with 10% HyClone fetal bovine serum (FBS, GE Healthcare Life Sciences, Logan, Utah, USA) in a humidified incubator with 5% CO2 at 37 °C.
Small interfering RNA and transfection
The small interfering RNA for GRP78 (5′-CUAUGAAGCCCGUCCAGAAtt-3′) was purchased from Thermo Fisher Scientific Corporation (Waltham, MA, USA), and a nonsense sequence that is unrelated to siGRP78, 5′-UUCUCCGAACGUGUCACGUtt-3′, was used as a negative control. Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) was used for transfection according to the manufacturer’s instructions. The SU.86.86 and BxPC-3 cells were plated in 6-well plates at a concentration of 5 × 105 cells/well in 2 mL of medium supplemented with 10% FBS. After 18 h, when the confluence of cells reached approximately 80%, the cells were used for transient transfection. A mixture containing the 80 pM of the siGRP78 duplexes and the Lipofectamine 2000 reagent was added to each well for transfection. The cells were collected after 48 h.
Plasmids and transfection
The recombinant vectors encoding human GRP78 were constructed by PCR-based amplification and were then subcloned into the pcDNA3.1 expression vector (Thermo Fisher Scientific, Waltham, MA, USA). The vectors were transfected into the cells using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturers’ protocol.
Western blot analysis
To obtain the whole-cell lysates, the transfected cells were digested with a 0.25% trypsin solution and lysed with RIPA buffer (Thermo Fisher Scientific, Waltham, MA, USA) containing a protease and phosphatase inhibitor cocktail (Sigma-Aldrich Co., LLC, St. Louis, MO, USA). The protein concentrations were measured with a BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA). The total proteins (50 μg) were first separated on 10% SDS-PAGE and then electrotransferred to a polyvinylidene difluoride membrane (PVDF, Millipore Corporation, Billerica, MA, USA). The PVDF membranes were blocked with 5% nonfat dry milk in TBS/Tween 0.5% (TBS-T) for 1 h, and then incubated overnight with primary antibodies at 4 °C. After washing with TBS-T three times for 5 min, the membranes were incubated in horseradish peroxidase–conjugated mouse or rabbit secondary antibodies (1:3,000; Golden Bridge Biological Technology, Beijing, China) in 5% (w/v) nonfat dry milk in TBST for 1 h at room temperature. The protein bands were detected using an enhanced chemiluminescence system (Merck & Co., Inc. Whitehouse Station, NJ, USA) and the intensities were quantified using Quantity One software. Equal protein sample loading was guaranteed by incubating the membranes with a β-actin antibody as the internal control standard. The anti-human-GRP78 primary antibody was used at a dilution of 1:1000 as described above. The anti-human-CyclinD1, anti-human-CDK4, anti-human-CDK6, anti-human-p-STAT3, anti-human-STAT3, anti-human-Smad4, anti-human-JAK2, anti-human-vimentin, anti-human-RhoA, and anti-human-ROCK1 primary antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA) and used at a dilution of 1:500.
Cell proliferation analyses
Cell proliferation was detected using the CCK-8 kit (Dojindo Laboratories, Kumamoto, Japan). Briefly, the cells were seeded in flat-bottomed 96-well plates at 1000 cells/well with 100 μL medium, 10 μL of the CCK-8 solution was added to each well, and the plates were incubated for 3 h at 37 °C. The absorbance was measured at 450 nm using the SpectraMax 190 microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA) at time points from days 1 to 4. A wavelength of 630 nm was used as a reference.
Cell cycle assays
The cell cycle analysis was performed by fluorescence-activated cell sorting. The PDAC cells were harvested at 24 h after transfection. After washing with cold phosphate-buffered saline (PBS), the cells were resuspended in ice-cold PBS, and fixed in 70% ice-cold ethanol overnight at 4 °C. The cells were stained with a solution containing 0.2 mg/ml RNase A, 0.1% Triton X-100 and 20 μg/ml propidium iodide and analyzed using the Accuri C6 flow cytometer (Becton Dickinson, New Jersey USA).
Migration and invasion assays
The cell migration and invasion assays were performed using a double-chamber assay with a pore size of 8 μm (Corning Life Sciences, New York, NY, USA). The membranes for the invasion assay were coated with diluted Engelbreth-Holm-Swarm murine sarcoma extracellular matrix proteins (1:8, E1270, Sigma-Aldrich Co., LLC, St. Louis, MO, USA) and then incubated at 37 °C for 4 h. According to the manufacturer’s protocol, the cells (4 × 104 cells per well) were added to the upper chambers containing serum-free media, and 600 μL of medium containing 10% FBS was added to the lower chamber. The cells for migration assays were carefully removed from the upper side of the membrane with a swab after incubating for 12 hours, and the cells for the invasion assays were removed after incubating for 24 hours. The migration and invasion activity was assessed by counting the number of cells on the lower side of the filter. The membranes were fixed with methanol and then stained with crystal violet. The number of cells in five randomly selected fields was counted under a light microscope at a magnification of 200×. All in vitro experiments were independently repeated in triplicate under identical conditions.
Statistical analyses
A McNemar’s test and the Mann–Whitney U-test were used to compare GRP78 staining between the tumor tissues and the adjacent nontumor tissues. A chi-square test was used to detect the correlations of the categorical variables. The Kaplan–Meier method and log-rank test were used to evaluate the survival analysis, and the Cox regression model was used to analyze the prognostic factors. The Student’s t-test was used to determine the significance of the differences in the in vitro cell invasion, migration, cell cycle and apoptosis assays. SPSS (Statistical Package for the Social Sciences version 11.05; SPSS Inc., Chicago, IL, USA) was used for the statistical analyses. P < 0.05 was defined as being statistically significant.
Additional Information
How to cite this article: Niu, Z. et al. Elevated GRP78 expression is associated with poor prognosis in patients with pancreatic cancer. Sci. Rep. 5, 16067; doi: 10.1038/srep16067 (2015).
Supplementary Material
Acknowledgments
This work is supported by the grants from the National High Technology Research and Development Program (No. 2012AA02A212) and the National Natural Science Foundation of China (81272573).
Footnotes
Author Contributions Y.P.Z. and Q.L. designed the study; Z.Y.N and M.Y.W. contributed to the experiments, discussed the results, analyzed the data and wrote the manuscript. L.Z. contributed to the statistical analysis. LT.Y. contributed to the supplementary experiments and the statistical analysis.
References
- Siegel R., Ma J., Zou Z. & Jemal A. Cancer statistics, 2014. CA Cancer J Clin 64, 9–29 (2014). [DOI] [PubMed] [Google Scholar]
- Witkowski E. R., Smith J. K. & Tseng J. F. Outcomes following resection of pancreatic cancer. J Surg Oncol 107, 97–103 (2013). [DOI] [PubMed] [Google Scholar]
- Vincent A., Herman J., Schulick R., Hruban R. H. & Goggins M. Pancreatic cancer. Lancet 378, 607–620 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J. M., Yeo C. J. & Brody J. R. Diagnostic, prognostic, and predictive biomarkers in pancreatic cancer. J Surg Oncol 107, 15–22 (2013). [DOI] [PubMed] [Google Scholar]
- Lee A. S. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res 67, 3496–3499 (2007). [DOI] [PubMed] [Google Scholar]
- Fu Y. & Lee A. S. Glucose regulated proteins in cancer progression, drug resistance and immunotherapy. Cancer Biol Ther 5, 741–744 (2006). [DOI] [PubMed] [Google Scholar]
- Huang L. W., Lin C. Y., Lee C. C., Liu T. Z. & Jeng C. J. Overexpression of GRP78 is associated with malignant transformation in epithelial ovarian tumors. Appl Immunohistochem Mol Morphol 20, 381–3855 (2012). [DOI] [PubMed] [Google Scholar]
- Mozos A. et al. The expression of the endoplasmic reticulum stress sensor BiP/GRP78 predicts response to chemotherapy and determines the efficacy of proteasome inhibitors in diffuse large b-cell lymphoma. Am J Pathol 179, 2601–2610 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda K. et al. Glucose-regulated protein 78 positivity as a predictor of poor survival in patients with renal cell carcinoma. Urol Int 87, 450–456 (2011). [DOI] [PubMed] [Google Scholar]
- Teng Y., Ai Z., Wang Y., Wang J. & Luo L. Proteomic identification of PKM2 and HSPA5 as potential biomarkers for predicting high-risk endometrial carcinoma. J Obstet Gynaecol Res 39, 317–325 (2013). [DOI] [PubMed] [Google Scholar]
- Zhang J. et al. Association of elevated GRP78 expression with increased lymph node metastasis and poor prognosis in patients with gastric cancer. Clin Exp Metastasis 23, 401–410 (2006). [DOI] [PubMed] [Google Scholar]
- Wu C. T. et al. Glucose-regulated protein 78 mediates hormone-independent prostate cancer progression and metastasis through maspin and COX-2 expression. Tumour Biol 35, 195–204 (2014). [DOI] [PubMed] [Google Scholar]
- Thornton M. et al. The unfolded protein response regulator GRP78 is a novel predictive biomarker in coloretcal cancer. Int J Cancer 133, 1408–1418 (2013). [DOI] [PubMed] [Google Scholar]
- Huang T. T. et al. Decreased GRP78 protein expression is a potential prognostic marker of oral squamous cell carcinoma in Taiwan. J Formos Med Assoc 109, 326–337 (2010). [DOI] [PubMed] [Google Scholar]
- Langer R., Feith M., Siewert J. R., Wester H. J. & Hoefler H. Expression and clinical significance of glucose regulated proteins GRP78 (BiP) and GRP94 (GP96) in human adenocarcinomas of the esophagus. BMC Cancer 8, 70 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo B. & Lee A. S. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene 32, 805–18 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su R. et al. Grp78 promotes the invasion of hepatocellular carcinoma. BMC Cancer 10, 20 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujumdar N. et al. Triptolide activates unfolded protein response leading to chronic ER stress in pancreatic cancer cells. Am J Physiol Gastrointest Liver Physiol 306, G1011–20 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondi S., Lowenfels A. B., Morselli-Labate A. M., Maisonneuve P. & Pezzilli R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol 24, 349–358 (2010). [DOI] [PubMed] [Google Scholar]
- Shkoda A. et al. Interleukin-10 blocked endoplasmic reticulum stress in intestinal epithelial cells: impact on chronic inflammation. Gastroenterology 132, 190–207 (2007). [DOI] [PubMed] [Google Scholar]
- Lin J. A. et al. Silencing glucose-regulated protein 78 induced renal cell carcinoma cell line G1 cell-cycle arrest and resistance to conventional chemotherapy. Urol Oncol 32, 29.e1–11 (2014). [DOI] [PubMed] [Google Scholar]
- Hanahan D. & Weinberg R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- Harbour J. W., Luo R. X., Dei S. A., Postigo A. A. & Dean D. C. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell 98, 859–869 (1999). [DOI] [PubMed] [Google Scholar]
- Lundberg A. S. & Weinberg R. A. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol 18, 753–761 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava V., Patel B., Kumar M., Shukla M. & Pandey M. Cyclin D1, retinoblastoma and p16 protein expression in carcinoma of the gallbladder. Asian Pac J Cancer Prev 14, 2711–2715 (2013). [DOI] [PubMed] [Google Scholar]
- Seiler R., Thalmann G. N., Rotzer D., Perren A. & Fleischmann A. CCND1/CyclinD1 status in metastasizing bladder cancer: a prognosticator and predictor of chemotherapeutic response. Mod Pathol 27, 87–95 (2014). [DOI] [PubMed] [Google Scholar]
- Liang S. et al. CyclinD1, a prominent prognostic marker for endometrial diseases. Diagn Pathol 8, 138 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. et al. Overexpression of beta-catenin and cyclinD1 predicts a poor prognosis in ovarian serous carcinomas. Int J Clin Exp Pathol 7, 264–271 (2014). [PMC free article] [PubMed] [Google Scholar]
- Yamada S. et al. Epithelial-to-mesenchymal transition predicts prognosis of pancreatic cancer. Surgery 154, 946–954 (2013). [DOI] [PubMed] [Google Scholar]
- Kang Y. et al. SMAD4 regulates cell motility through transcription of N-cadherin in human pancreatic ductal epithelium. PLoS One 9, e107948 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor K. & Chen M. Dynamic functions of RhoA in tumor cell migration and invasion. Small GTPases 4, 141–147 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares A. L., Mercado-Pimentel M. E., Runyan R. B. & Kitten G. T. TGF beta-mediated RhoA expression is necessary for epithelial-mesenchymal transition in the embryonic chick heart. Dev Dyn 235, 1589–98 (2006). [DOI] [PubMed] [Google Scholar]
- Wu Y., Diab I., Zhang X., Izmailova E. S. & Zehner Z. E. Stat3 enhances vimentin gene expression by binding to the antisilencer element and interacting with the repressor protein, ZBP-89. Oncogene 23, 168–178 (2004). [DOI] [PubMed] [Google Scholar]
- Raptis L., Arulanandam R., Geletu M. & Turkson J. The R(h)oads to Stat3: Stat3 activation by the Rho GTPases. Exp Cell Res 317, 1787–1795 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balanis N. et al. Epithelial to mesenchymal transition promotes breast cancer progression via a fibronectin-dependent STAT3 signaling pathway. J Biol Chem 288, 17954–67 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd L. M. et al. Inhibition of the JAK2/STAT3 pathway reduces gastric cancer growth in vitro and in vivo. PLoS One 9, e95993 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stechishin O. D. et al. On-target JAK2/STAT3 inhibition slows disease progression in orthotopic xenografts of human glioblastoma brain tumor stem cells. Neuro Oncol 15, 198–207 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senft C. et al. Inhibition of the JAK-2/STAT3 signaling pathway impedes the migratory and invasive potential of human glioblastoma cells. J Neurooncol 101, 393–403 (2011). [DOI] [PubMed] [Google Scholar]
- Guo K. et al. Interaction of the sympathetic nerve with pancreatic cancer cells promotes perineural invasion through the activation of STAT3 signaling. Mol Cancer Ther 12, 264–273 (2013). [DOI] [PubMed] [Google Scholar]
- Li H. et al. STAT3 knockdown reduces pancreatic cancer cell invasiveness and matrix metalloproteinase-7 expression in nude mice. PLoS One 6, e25941 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. et al. Blockade of GRP78 sensitizes breast cancer cells to microtubules-interfering agents that induce the unfolded protein response. J Cell Mol Med 13, 3888–3897 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov S. I., Greten F. R. & Karin M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S. et al. Chemical genomics identifies the unfolded protein response as a target for seletcive cancer cell killing during glucose deprivation. Cancer Res 69, 4225–4234 (2009). [DOI] [PubMed] [Google Scholar]
- Backer J. M. et al. Chaperone-targeting cytotoxin and endoplasmic reticulum stress-inducing drug synergize to kill cancer cells. Neoplasia 11, 1165–1173 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.