Abstract
H2S is produced mainly by two enzymes:cystathionine-β-synthase (CBS) and cystathionine-γ-lyase (CSE), using L-cysteine (L-Cys) as the substrate. In this study, we investigated the role of H2S in gastric accommodation using CBS+/− mice, immunohistochemistry, immunoblot, methylene blue assay, intragastric pressure (IGP) recording and electrical field stimulation (EFS). Mouse gastric fundus expressed H2S-generating enzymes (CBS and CSE) and generated detectable amounts of H2S. The H2S donor, NaHS or L-Cys, caused a relaxation in either gastric fundus or body. The gastric compliance was significantly increased in the presence of L-Cys (1 mM). On the contrary, AOAA, an inhibitor for CBS, largely inhibited gastric compliance. Consistently, CBS+/− mice shows a lower gastric compliance. However, PAG, a CSE inhibitor, had no effect on gastric compliances. L-Cys enhances the non-adrenergic, non-cholinergic (NANC) relaxation of fundus strips, but AOAA reduces the magnitude of relaxations to EFS. Notably, the expression level of CBS but not CSE protein was elevated after feeding. Consistently, the production of H2S was also increased after feeding in mice gastric fundus. In addition, AOAA largely reduced food intake and body weight in mice. Furthermore, a metabolic aberration of H2S was found in patients with functional dyspepsia (FD). In conclusion, endogenous H2S, a novel gasotransmitter, involves in gastric accommodation.
The stomach has variety of functions including reservoir functions. Disorders of the reservoir functions result in symptoms of early satiety and anorexia, which are the major symptoms of patients with functional dyspepsia (FD). Gastric accommodation consists of two types of relaxation: the receptive relaxation and the adaptive relaxation. These physiological responses are important to accommodate the intake of food and liquid. Adaptive relaxation is a reflex in which the fundus of the stomach dilates in response to small increases in intragastric pressure when food enters the stomach. Receptive relaxation is a reflex in which the gastric fundus dilates when food passes down the pharynx and the esophagus.
Some gastrointestinal hormones and chemical mediators such as gastrin, histamine1, serotonin, vasoactive intestinal peptide (VIP)2 and nitric oxide (NO)3,4,5 have been shown to mediate these two types of relaxations.
In the gastrointestinal tract, NO is an important non-adrenergic, non-cholinergic (NANC) inhibitory neurotransmitter which is released in response to nerve stimulation and relaxes smooth muscles6,7. Animal studies have consistently shown that basal tone is decreased by vagal stimulation and that this effect is blocked by the NO inhibitor8,9,10,11. Besides NO and CO, hydrogen sulfide (H2S) is the third gasotransmitter. H2S is produced mainly by two enzymes:cystathionine–β–synthase (CBS) and cystathionine-γ–lyase (CSE), using L-cysteine (L-Cys) as the substrate12,13,14. CBS and CSE are expressed in the enteric nervous system (ENS)15. In the gastrointestinal tract, sodium hydrogen sulfide (NaHS), a source of H2S, can reduce spontaneous or acetylcholine (ACh)-induced contraction of ileal smooth muscles16,17. H2S also causes concentration-dependent relaxation of pre-contracted smooth muscles in the mouse gastric fundus and distal colon18,19. Muscle contractions of the mouse colon and jejunum were also inhibited by application of NaHS20. H2S is similar with the two kinds of endogenous gas signal molecules of CO and NO, they are very important bio-regulating substances, and share some common characteristics. We hypothesize that beside NO, H2S is another gasotransmitter which involves in the mechanical accommodation of the stomach. In the present study, we therefore examined the role of H2S in receptive and adaptive relaxation of the mouse stomach.
Materials and Methods
Animals
Male BLAB/c mice weighing 35–45 g, kept in individual cages with raised mesh bottoms, were deprived of food but allowed free access to tap water for 18 hr before the experiments. Animals were sacrificed by cervical dislocation and the stomach was quickly removed and placed into aerated (5% CO2 and 95% O2) Krebs solution. Wild-type (WT) and CBS+/− mice on C57BL/6J background were obtained from the Jackson Laboratory (BarHarbor, ME). All experimental procedures were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals of Shandong University, and the present study was approved by the Experimental Animal Research Committee of Shandong University China (number ECAESDUSM 2012029).
Western blots
Gastric biopsy specimens were obtained from 8 patients with FD fulfilling the Rome III criteria and 7 healthy volunteers. Biopsy samples were taken for western blot detection. Informed consent was obtained from each patient and approval granted from the Medical Ethics Committees of Shandong University (number MECSDUMS 2013023). Tissue was homogenized in ice-cold lysis buffer. The ice-cold lysis buffer contained: 50 mM Tris (pH 7.4), 150 mM NaCl, 1%TritonX-100, 1% sodium deoxycholate, 0.1% SDS, 1 mM NaF, 1 mM Na3VO4, 1 mM EDTA and 0.5 μg/ml leupeptin. After centrifugation, the supernatant was boiled for 10 min. Ten to thirty mg of denatured proteins were separated on 10% SDS polyacrylamide gels and then transferred to a PVDF membrane. Membranes were blocked for one hour using 5% non-fat dry milk in Tris-buffered saline with 0.05% Tween-20, then washed in Tween-Tris-buffered saline (0.1% Tween 20, 50 mM Tris and 150 mM NaCl), followed by overnight incubation at 4 °C with a rabbit polyclonal CBS antibody (Santa Cruz Biotechnology, Santa Cruz, CA, 1:1000 dilution) or a rabbit polyclonal CSE antibody (Abcam, Cambridge, UK, 1:1000 dilution). Membranes were washed in Tween-Tris-buffered saline and incubated with an anti-horseradish-peroxidase conjugated secondary antibody (ZSGB biology, Beijing, China, 1:20000) for one hour. The membranes were washed again and exposed to ECL. The blot films were scanned, and the band densities were calculated using the Quantity One analysis software (Bio-Rad). The values of blot densities were normalized to the levels of respective β-actin blots.
Immunofluorescence
Paraffin sections were roasted 90 min at 65 °C, dewaxed in xylene twice for 10 min and then rehydrated in 100, 100, 95, 95, 90 and 80% of ethanol and running tap water for 5 min each, in order. Tissue sections underwent antigen retrieval in a solution consisting of 0.01 M citrate and 0.01 M sodium citrate before they were blocked in PBS containing 10% goat serum for 1 h at room temperature. The sections were incubated with a polyclonal CBS antibody (Santa Cruz Biotechnology, Santa Cruz, CA, 1:100 dilution) or polyclonal CSE antibody (Abcam, Cambridge, UK, 1:100 dilution) overnight at 4 °C. After being washed in PBS, the sections were incubated for 1 h with Alexa Fluor 568 goat anti-rabbit IgG (HtL) (1: 600; Invitrogen Carlsbad, CA, USA) at room temperature. The sections were washed again and incubated in DAPI (1:1,000) for 10 min at room temperature. In negative controls, the sections were incubated with PBS instead of the primary antibody. We repeated the immunohistochemistry of each protein in eight tissue slices of four samples. The fluorescence intensity for a specific protein stain was set below the threshold for the negative control.
The release of H2S in fundus
Tissues were homogenized in 50 mM ice-cold potassium phosphate buffer pH 6.8. The reaction mixture contained (mM): 10% (w/v) tissue homogenate (0.5 ml), 100 mM potassium phosphate buffer (pH = 7.4, 0.5 ml), 20 mM pyridoxal 5′-phosphate (0.1 ml) and 10 mM L-Cys (0.1 ml). The reaction was performed in a 25-ml flask containing the reaction mixture. Before being sealed, the flask was flushed with N2. The reaction was initiated by transferring the flasks from ice to a 37 °C shaking water bath. After incubation at 37 °C for 90 min, trichloroacetic acid (50%, 0.5 ml) was added to the reaction mixture to stop the reaction and incubated at 37 °C for an additional 60 min. The contents were then transferred to test tubes, each containing 3.5 ml of ultra-pure water. Subsequently, 0.5 ml of 20 mM N,N-dimethyl-p-phenylenediamine sulphate in 7.2 M HCl was added, immediately followed by the addition of 0.4 ml 30 mM FeCl3. After 20 min of incubation at room temperature, the optical absorbance of the resulting solutions was measured at 670 nm. A standard curve was generated with known concentrations of NaHS. The H2S concentration was calculated against the calibration curve of the standard H2S solutions.
Muscle tension experiment
The fundic portion of the stomach was dissected free. One full wall thickness fundus strips (2 × 10 mm) were prepared by cutting in the direction of the longitudinal muscle layer. After a silk thread (USP 4/0) was attached to both ends of the strips, they were mounted in 7 ml organ baths. One end of each strip was fixed, while the other was connected to a force displacement transducer for continuous recording of isometric tension. After an equilibration period of 30 min with flushing every 10 min at a load of 1 g (±0.2 g), the length-tension relationship was determined. Strips were subsequently incubated with NaHS (10−3 mol/L) and determine the tension.
Recordings of intragastric pressure in vivo (IGP)
Mice were anesthetized in urethane (25%, 1.5 g/kg, ip). A homemade balloon (maximum volume 1.5 ml) attached to pressure sensor was inserted into the bottom of the stomach from incision on anterior wall of duodenal bulb. The volume of balloon was increased stepwise from 0.1 to 0.3, 0.5 ml by injection water through T-branch pipe. The pressure inside balloon increased sharply and then slowly lower to reach a platform due to the relaxation of the bottom of the stomach. IGP was recorded and viewed in real time using customized PowerLab Chart 5 v5.1 software (AD Instruments). IGPs were set at 0 mmHg and recorded in response to stepwise isovolumetric distensions. Gastric adaptive relaxation compliance expressed as the rate of decline of IGPs to each volume stimuli (0–20 s) and plateau pressure expressed as plateau values minus basal values were evaluated using the same software. Responses with or without pretreatment with L-Cys, AOAA, PAG, SAM and NaHS were evaluated.
The NANC relaxation of fundus strips induced by electrical field stimulation (EFS)
The fundic portion of the stomach was dissected free. One full wall thickness fundus strips (2 × 10 mm) were prepared by cutting in the direction of the longitudinal muscle layer. Muscle strips were mounted in 7 ml double-jacketed organ baths containing Krebs solution, gassed with 95% O2–5% CO2 mixture. Prewarmed water (37 °C) was circulated through the outer jacket of the tissue bath via a constant-temperature circulator pump. One end of each strip was fixed, while the other was connected to a force displacement transducer for continuous recording of isometric tension. EFS was applied via two platinum electrodes (6 mm apart). All experiments were performed at optimal load. Therefore, after an equilibration period of 30 min with flushing every 10 min at a load of 1.5 g (±0.2 g), the length-tension relationship was determined. To investigate the effects of H2S on NANC relaxant responses, isoproterenol (1 μM), atropine (2 μM) were added to the bath medium, to rule out the adrenergic and the cholinergic influences, respectively. Each fundus strip was allowed to equilibrate for at least 30 min before 5-HT (0.5 μM) was added to produce a sustained increase. After a further 10-min equilibration period, the responses to electrical field stimulation (EFS; 80 V, 0.5 ms, 4–8–16 Hz for 15 s with a 2 min interval) were obtained in the presence or absence of L-Cys (1 mM) or AOAA (1 mM).
Effects of H2S signal pathway on food intake
Mice were randomly divided into six groups. Abdominal cavity injection was performed every 48 hours with normal saline (10 ml/kg), L-Cys (50 mg/kg), AOAA (50 mg/kg), PAG (100 mg/kg), SAM (50 mg/kg) and NaHS (5 mg/kg), respectively, from 0 day to 16 day. In this period, average food intake, water intake and body weight of each group were measured every 24 hours.
Solutions and drugs
Krebs solution was a buffer solution containing (mmol/L): NaCl 120.6, KCl 5.9, CaCl2 2.5, NaH2 PO4 1.2, MgCl2 1.2, NaHCO3 15.4 and glucose 11.5. PH was 7.4. For immunohistochemical experiments, phosphate buffered saline (PBS) was used containing (mmol/L):NaCl 135, KCl 2.7, KH2PO4 1.5, and K2HPO4 8, pH was 7.4. For western blot experiments, Tris-HCL buffered saline (TBS) was used containing (mmol/L):Tris 50, NaCl 150. pH was adjusted to 7.4 with HCl.
L-Cys, NaHS, AOAA, SAM and PAG were from Sigma. Pyridoxal 5-phosphate and dimethyl aniline hydrochloride (DMPD) were from Aladdin (Shanghai, China). Zinc acetate, FeC l3 and trichloroacetic acid were from Damao chemical reagent company (Tianjin, China). If not indicated specially, the drugs were from chemical reagent co., LTD of national medicine bloc (Shanghai, China).
Statistical analysis
The data are presented as means ± standard error of the mean (SEM), n is the number of tissues examined. The Student’s t-test was used for comparison between the two sets of data, and ANOVA analysis was used for group comparison. A P < 0.05 was considered statistically significant.
Results
Western Blot and immunoflurence studies
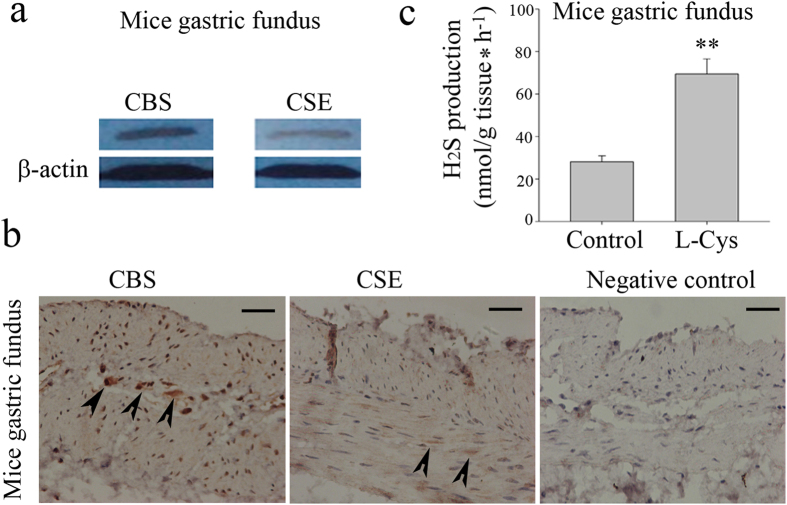
CBS and CSE were expressed in mice fundus as demonstrated by Western blot studies (Fig. 1a). The immunohistochemistry study shows that CBS was expressed on the soma of the myenteric neurons of gastric fundus muscle myenteric plexus (Fig. 1b). In addition, bundles of muscular tissue showed a clear immunoreactivity for CSE (Fig. 1b).
Figure 1. Mice gastric fundus expressed CBS and CSE and generated detectable amounts of H2S.
CBS and CSE were expressed in mice fundus (a). CBS was expressed on the soma of the myenteric neurons of gastric fundus muscle myenteric plexus. Bundles of muscular tissue showed a clear immunoreactivity for CSE (b). The gastric fundus of mouse generated detectable amounts of H2S. The biosynthesis of H2S was increased by 3- fold over basal values after incubation of tissue homogenates with L-Cys, the CBS/CSE substrate (c). Scale bar: 50 μm. n = 8; **P < 0.01.
H2S Production in gastric fundus
The gastric fundus of mouse generated detectable amounts of H2S (Fig. 1c). The biosynthesis of H2S was increased by 3- fold over basal values after incubation of tissue homogenates with L-Cys, the CBS/CSE substrate (Fig. 1c). Therefore, gastric fundus is capable of synthesizing H2S from L-Cys.
Effect of H2S donor, NaHS or L-Cys in gastric fundus strips
The gastric fundus or body strips from mice showed spontaneous contraction (Fig. 2). Exogenous H2S donor, NaHS (1 mM) caused a relaxation in either gastric body or fundus (Fig. 2a). L-Cys (1 mM), a substrate of CBS/CSE, also induced an inhibition of contractile activity in mice gastric fundus (Fig. 2b).
Figure 2. Effect of H2S donor, NaHS or L-Cys in gastric fundus Strips.
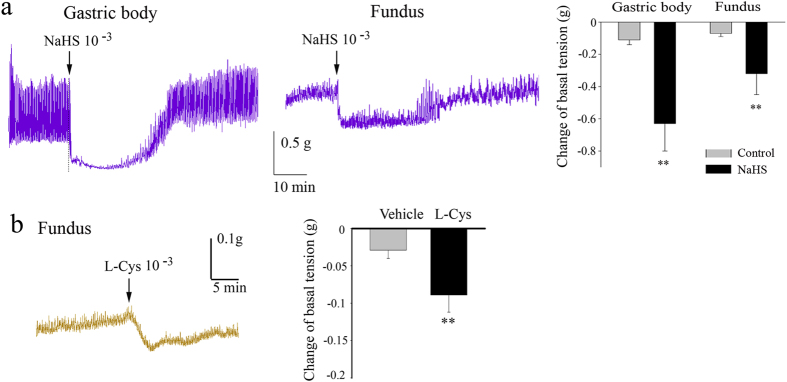
Representative recordings of the effects of NaHS or L-Cys on the contraction of gastric body and fundus muscle strips of mouse. The H2S donor, NaHS (a) or L-Cys (b) caused a relaxation in either gastric body or fundus (b). n = 8–10; **P < 0.01.
Effect of H2S signal pathway on IGP in vivo
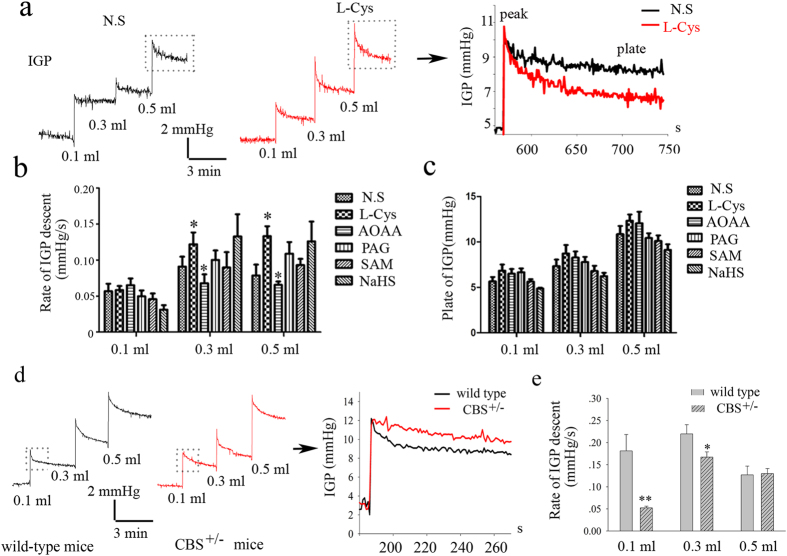
We hypothesized that CBS expressed in inhibitory motor neurons of the gastric myenteric plexus may detect changes in IGP and enhance gastric compliance.
To test this hypothesis, we measured changes in IGP of mouse stomach responding to volume stimuli in vivo in the presence of L-Cys, a substrate of CBS and CSE, or AOAA, an inhibitor for CBS. The results showed that the descent rate of IGP (reflecting gastric compliance) was significantly increased in the presence of L-Cys (1 mM) (Fig. 3a,b). On the contrary, AOAA largely inhibited gastric compliance. However, plateau IGPs were not affected by pretreatment with either L-Cys or AOAA. Notably, exogenous H2S donor NaHS, CSE inhibitor PAG or CBS activator SAM had no effect on gastric compliances (Fig. 3c). To confirm the roles of endogenous H2S, the CBS knocked out mice was used. The present result showed that the gastric compliance was lower in CBS+/− mice than that of littermate wild-type mice (Fig. 3d,e).
Figure 3. Effect of H2S signal pathway on IGP in vivo.
Representative recordings of the effects of L-Cys on IGP (a).The rate of IGP decrease was significantly larger upon pretreatment with the L-Cys (1 mM) than that of control group, which was reduced by AOAA, an inhibitor for CBS (b). Plateau IGPs were not affected by pretreatment with either L-Cys or AOAA (c). Notably, either exogenous H2S donor NaHS or CSE inhibitor PAG had no effect on IGP. Furthermore, the descent rate of IGP was lower in CBS+/− mice than that of littermate wild-type mice (d,e). Rate of IGP descent: the rate of pressure decrease within 20 s. Plate of IGP: difference between plateau pressure and basal pressure. n = 4–6; *P < 0.05; **P < 0.01.
Responses to NANC nerve stimulation
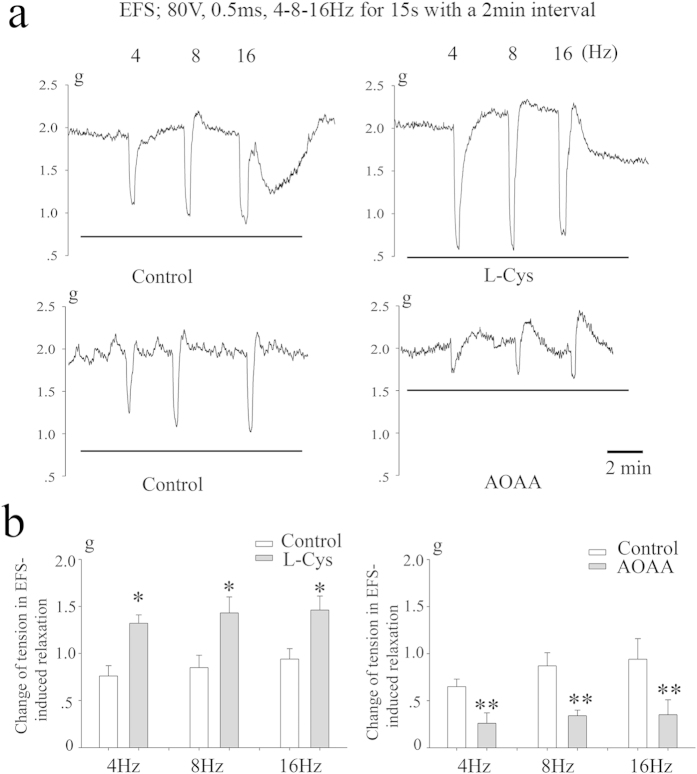
EFS (4–16 Hz, 80V, 0.5 ms, 15-s train) produced rapid, frequency-dependent relaxations (Fig. 4). Relaxant responses of gastric fundus muscle strips to EFS were significantly enhanced following exposure to L-Cys. However, following incubation with AOAA, the magnitude of relaxations to EFS was greatly reduced (Fig. 4).
Figure 4. Responses to NANC nerve stimulation.
Representative traces of the effects of L-Cys or AOAA on EFS-induced relaxation in fundus muscle strips of mouse (a). EFS (4–16 Hz, 80 V, 0.5 ms, 15-s train, 2 min intervals) produced rapid, frequency-dependent relaxations. Relaxant responses of gastric fundus to EFS were significantly enhanced following exposure to L-Cys. However, following incubation with AOAA, the magnitude of relaxations to EFS was greatly reduced (b). n = 11; *P < 0.05; **P < 0.01 vs control group.
Effect of feeding on the expression of the H2S-producing enzyme in mice fundus
The expression of CBS protein was elevated at 5 min and peak at 10 min after feeding, and then be back at 20 min after feeding. However, another H2S-generating enzyme CSE is not changed after feeding. The production of H2S was also increased after feeding in mice gastric fundus, but not gastric body (Fig. 5).
Figure 5. Effect of feeding on the expression of CBS and CSE in mice fundus.
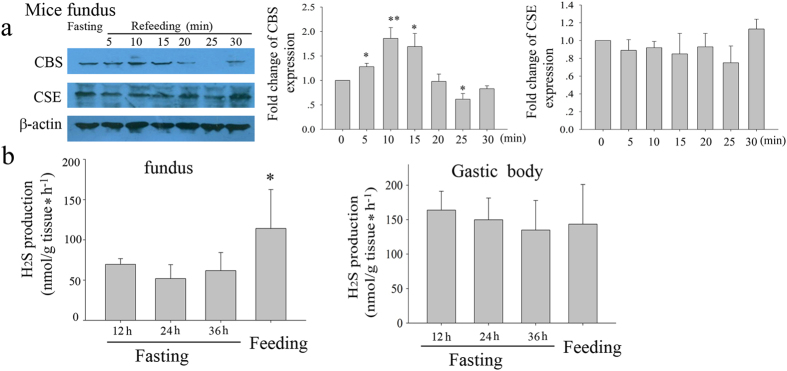
The expression of CBS was elevated at 5 min after feeding, and then be back to normal before at 20 min after feeding. However, another H2S-generating enzyme CSE is not changed after feeding (a). The production of H2S was also increased after feeding in mice gastric fundus, but not gastric body (b). n = 5; *P < 0.05; **P < 0.01 vs 0 (a) or 12 h of fasting (b).
Effects of H2S signal pathway on food intake and body weight
Because H2S involves in regulation of IGP, it is possible that H2S signal may affect the food intake and body weight. To test this hypothesis, we measured changes in food intake and body weight of mouse after injecting ip L-Cys, AOAA, PAG, SAM and NaHS, respectively. Food intake and body weight were significantly reduced after injecting AOAA (n = 8). However, either L-Cys or NaHS, both H2S donors, had no effect on food intake and body weight in mouse. CSE inhibitor PAG or CBS activator SAM also did not affect food intake and body weight, which is consistent with the results of gastric compliances assay (Fig. 6).
Figure 6. Effects of H2S signal pathway on food intake and body weight.
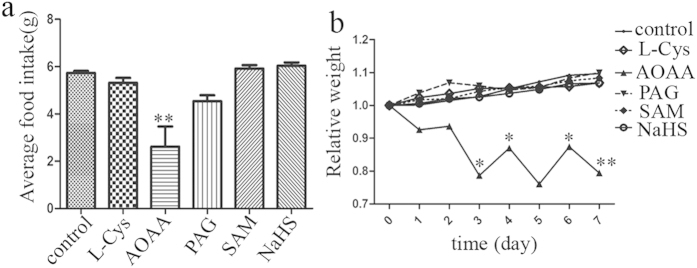
Food intake and body weight were largely reduced after injecting AOAA. n = 8; *P < 0.05; **P < 0.01 vs control group (a) or the corresponding time point of control group (b).
Dysregulation of H2S production in FD patients
The expression of CBS protein is downregulated in gastric biopsy sample taken from patients with FD compared with healthy volunteers. However, the expression of CSE, another H2S generating enzyme, is not changed (Fig. 7a). By the enzymatic H2S production assays, we further confirmed the downregulation of H2S production in FD patients (Fig. 7b).
Figure 7. An aberration in H2S signal pathway in FD patients.
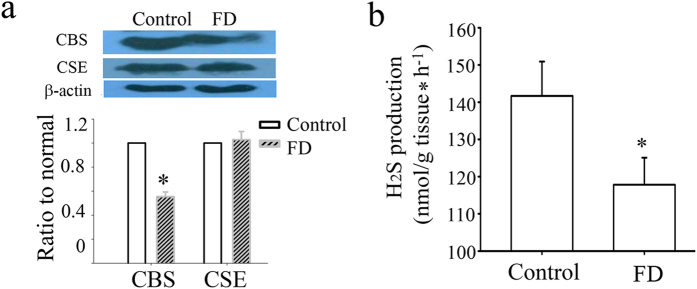
The expression of CBS is downregulated in gastric biopsy sample taken from patients with FD compared with healthy volunteers. However, the expression of CSE, another H2S generating enzyme, is not changed (a). H2S production also was decreased in FD patients (b). n = 7–8; *P < 0.05 vs control group.
Discussion
H2S has been considered as the third biological gasotransmitter along with NO and CO. Two H2S generating enzymes-CBS and CSE have been identified in mammalian systems. CBS and CSE have been documented to be expressed in certain neurons of the mouse21, rat22, guinea-pig and human enteric nervous systems15. In our present study, we demonstrated that both H2S generating enzymes also exist in gastric fundus from mouse or human. Moreover, the gastric fundus is capable of synthesizing H2S indicated by enzyme activity assay. Growing studies demonstrated that exogenous H2S relaxes the gastrointestinal smooth muscle16,17. H2S has an inhibitory role on spontaneous and agonist-mediated rhythmic contractile activity. In isolated ICC of the mouse small intestine, H2S inhibits pacemaker activity in ICC23 and interacts with nitric oxide in regulating functional pacemaker activity24. An endogenous H2S contributes to resting membrane potential and spontaneous contractions in the rat colon25. However, there are studies that have demonstrated that NaHS at low concentrations increased basal tension in the gastric antrum in vitro26,27 and enhances the gastric emptying in vivo28. Whether H2S is excitatory or inhibitory on gastrointestinal smooth muscle is dependent upon the concentration, regions and species.
In our study, we found that NaHS (1 mM) largely decrease the smooth muscle contraction in the gastric fundus from mouse. Notably, L-Cys, an endogenous H2S donor, also exert an inhibitory effect on the spontaneous contraction of gastric fundus smooth muscle in mice. These results suggest that L-Cys/H2S regulates the spontaneous contraction of gastric fundus smooth muscle. Recent studies have begun to reveal that H2S interacts with NO29. H2S induces phosphorylation of eNOS and also prevents its degradation30,31,32. CSE knockout mice exhibits dysfunctional eNOS and diminished NO levels, which can be restored by acute H2S therapy30,31. Similarly, CBS (−/+) mice exhibits impaired vascular functions33, which is caused by decreased eNOS activity and bioavailability of NO34,35. H2S selectively restored chronic ischemic tissue function and viability by enhancing NO production involving both endothelial NO synthase and sulfide-dependent nitrite reduction mechanisms36. However, some studies indicate that H2S downregulates the expression of NOS and inhibits the production of NO37,38,39. In our study, L-NAME, a NOS inhibitor, did not influence the L-Cys-evoked relaxation of mice gastric fundus smooth muscle, suggesting that this effect does not depend on NO signaling pathways (data not shown).
The receptive relaxation in response to gastric distention provides an appropriate gastric reservoir for food and enables the stomach to increase the intraluminal volume without rise in the intragastric pressure. In the present study, we observed that the adaptive relaxation induced by gastric distention was enhanced by L-Cys, while inhibited by AOAA in vivo. The experiment using CBS knocked out mice further confirmed CBS-derived H2S involves the receptive relaxation in response to gastric distention. We further demonstrated in this study that the NANC relaxation of fundus strips induced by EFS was significantly enhanced by L-Cys. In contrast, AOAA attenuated the EFS-induced relaxation of fundus strips, suggesting that endogenous H2S may also be involved in the basal receptive relaxation. In addition, the evidences that the expression of CBS and the production of H2S in mouse gastric fundus was significantly elevated after feeding further confirm the involvement of endogenous H2S in the receptive relaxation.
In FD patients, gastric accommodation is impaired40,41,42. Although impaired gastric accommodation is considered an important pathophysiological mechanism in the development of FD, surprisingly little is known about the aetiology of impaired gastric accommodation. In our study, we found that the H2S production was abnormal in FD patients, suggesting that dysregulation of H2S production may contribute to FD.
In conclusion, we demonstrate for the first time to our knowledge that a functional H2S signal system exists in gastric fundus, and the endogenous H2S regulates the gastric accommodation of mouse. The present study suggest the modification of CBS-derived H2S pathway is a useful alternative strategy for the treatment of FD-related gastrointestinal disorders.
Significance of this study
What is already known on this subject?
Hydrogen sulfide (H2S) is the third gasotransmitter besides NO and CO.
Two kind of H2S-generating enzymes CBS and CSE are expressed in the enteric nervous system (ENS).
H2S regulates gastrointestinal motility.
What are the new findings?
Here we reveal that beside NO, H2S, another gasotransmitter, involves in gastric accommodation.
H2S is a regulator of gastric accommodation.
CBS-derived H2S involves in receptive and adaptive relaxation of the mouse stomach.
A metabolic aberration of H2S was found in patients with functional dyspepsia (FD).
How might it impact on clinical practice in the foreseeable future?
Detection of plasma H2S concentration may serve as a biomarker in cancer in patients patients with FD. Importantly, this work reveals a potential novel way to treat FD.
Additional Information
How to cite this article: Xiao, A. et al. H2S, a novel gasotransmitter, involves in gastric accommodation. Sci. Rep. 5, 16086; doi: 10.1038/srep16086 (2015).
Acknowledgments
We thank Dr. Fang Yi (director of department of Pharmacology, Shandong University, China) for providing us with the CBS+/− mice used in the present study. This work was funded by grants from the National Natural Science Foundation of China (NSFC 31171108). Natural Science Foundation of Shandong Province (ZR2013HQ045) and Jinan Young Star Plan of Science and Technology (20100324).
Footnotes
Author Contributions A.X., H.W., X.L., J.Z., D.H. and T.X. carried out all the experiments. H.W. supplied human tissues. C.L. and J.G. revised whole manuscript. J.L. conceived the experiments. J.L. is the principal investigator of the laboratory in which the research was performed, wrote the manuscript. All of the authors read and approved the final manuscript.
References
- Takasugi S. et al. Neural and humoral factors influence gastric receptive relaxation in dogs. Jpn. J. Surg. 12, 208–213 (1982). [DOI] [PubMed] [Google Scholar]
- Fahrenkrug J. et al. Nervous release of vasoactive intestinal polypeptide in the gastrointestinal tract of cats: possible physiological implications. J. Physiol. 284, 291–305 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley M. N. & Mackie C. R. Gastric adaptive relaxation and symptoms after vagotomy. Br. J. Surg. 78, 24–27 (1991). [DOI] [PubMed] [Google Scholar]
- Desai K. M., Sessa W. C. & Vane J. R. Involvement of nitric oxide in the reflex relaxation of the stomach to accommodate food or fluid. Nature 351, 477–479, 10.1038/351477a0 (1991). [DOI] [PubMed] [Google Scholar]
- Desai K. M., Zembowicz A., Sessa W. C. & Vane J. R. Nitroxergic nerves mediate vagally induced relaxation in the isolated stomach of the guinea pig. Proc. Natl Acad. Sci. USA 88, 11490–11494 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre R. A., Baert E. & Barbier A. J. Influence of NG-nitro-L-arginine on non-adrenergic non-cholinergic relaxation in the guinea-pig gastric fundus. Br. J.Pharmacol. 106, 173–179 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre R. A., Smits G. J. & Timmermans J. P. Study of NO and VIP as non-adrenergic non-cholinergic neurotransmitters in the pig gastric fundus. Br. J.Pharmacol. 116, 2017–2026 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulemans A. L., Eelen J. G. & Schuurkes J. A. NO mediates gastric relaxation after brief vagal stimulation in anesthetized dogs. Am. J. Physiol. 269, G255–261 (1995). [DOI] [PubMed] [Google Scholar]
- Paterson C. A., Anvari M., Tougas G. & Huizinga J. D. Nitrergic and cholinergic vagal pathways involved in the regulation of canine proximal gastric tone: an in vivo study. Neurogastroenterol. Motil. 12, 301–306 (2000). [DOI] [PubMed] [Google Scholar]
- Kuiken S. D., Vergeer M., Heisterkamp S. H., Tytgat G. N. & Boeckxstaens G. E. Role of nitric oxide in gastric motor and sensory functions in healthy subjects. Gut 51, 212–218 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack J., Demedts I., Meulemans A., Schuurkes J. & Janssens J. Role of nitric oxide in the gastric accommodation reflex and in meal induced satiety in humans. Gut 51, 219–224 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowicka E. & Beltowski J. Hydrogen sulfide (H2S) - the third gas of interest for pharmacologists. Pharmacol. Rep. 59, 4–24 (2007). [PubMed] [Google Scholar]
- Kimura H. Hydrogen sulfide: its production, release and functions. Amino acids 41, 113–121, 10.1007/s00726-010-0510-x (2011). [DOI] [PubMed] [Google Scholar]
- Wang R. Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 16, 1792–1798, 10.1096/fj.02-0211hyp (2002). [DOI] [PubMed] [Google Scholar]
- Schicho R. et al. Hydrogen sulfide is a novel prosecretory neuromodulator in the Guinea-pig and human colon. Gastroenterology 131, 1542–1552, 10.1053/j.gastro.2006.08.035 (2006). [DOI] [PubMed] [Google Scholar]
- Hosoki R., Matsuki N. & Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem. Biophys. Res. Commun. 237, 527–531, 10.1006/bbrc.1997.6878 (1997). [DOI] [PubMed] [Google Scholar]
- Teague B., Asiedu S. & Moore P. K. The smooth muscle relaxant effect of hydrogen sulphide in vitro: evidence for a physiological role to control intestinal contractility. Br. J.Pharmacol. 137, 139–145, 10.1038/sj.bjp.0704858 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaese I. & Lefebvre R. A. Myosin light chain phosphatase activation is involved in the hydrogen sulfide-induced relaxation in mouse gastric fundus. Eur. J. Pharmacol. 606, 180–186, 10.1016/j.ejphar.2009.01.011 (2009). [DOI] [PubMed] [Google Scholar]
- Dhaese I., Van Colen I. & Lefebvre R. A. Mechanisms of action of hydrogen sulfide in relaxation of mouse distal colonic smooth muscle. Eur. J. Pharmacol. 628, 179–186, 10.1016/j.ejphar.2009.11.024 (2010). [DOI] [PubMed] [Google Scholar]
- Gallego D. et al. The gaseous mediator, hydrogen sulphide, inhibits in vitro motor patterns in the human, rat and mouse colon and jejunum. Neurogastroenterol. Motil. 20, 1306–1316, 10.1111/j.1365-2982.2008.01201.x (2008). [DOI] [PubMed] [Google Scholar]
- Linden D. R. et al. Production of the gaseous signal molecule hydrogen sulfide in mouse tissues. J. Neurochem. 106, 1577–1585, 10.1111/j.1471-4159.2008.05502.x (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W. et al. H2 S modulates duodenal motility in male rats via activating TRPV1 and K (ATP) channels. Br. J.Pharmacol. 171, 1534–1550, 10.1111/bph.12562 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parajuli S. P. et al. The inhibitory effects of hydrogen sulfide on pacemaker activity of interstitial cells of cajal from mouse small intestine. Korean. J. Physiol. Pharmacol. 14, 83–89, 10.4196/kjpp.2010.14.2.83 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon P. J. et al. Interplay of hydrogen sulfide and nitric oxide on the pacemaker activity of interstitial cells of cajal from mouse small intestine. Chonnam medical journal 47, 72–79, 10.4068/cmj.2011.47.2.72 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil V., Gallego D. & Jimenez M. Effects of inhibitors of hydrogen sulphide synthesis on rat colonic motility. Br. J.Pharmacol. 164, 485–498, 10.1111/j.1476-5381.2011.01431.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X. et al. Different regulatory effects of hydrogen sulfide and nitric oxide on gastric motility in mice. Eur. J. Pharmacol. 720, 276–285, 10.1016/j.ejphar.2013.10.017 (2013). [DOI] [PubMed] [Google Scholar]
- Han Y. F. et al. Evidence that endogenous hydrogen sulfide exerts an excitatory effect on gastric motility in mice. Eur. J. Pharmacol. 673, 85–95, 10.1016/j.ejphar.2011.10.018 (2011). [DOI] [PubMed] [Google Scholar]
- Medeiros J. V. et al. Role of KATP channels and TRPV1 receptors in hydrogen sulfide-enhanced gastric emptying of liquid in awake mice. Eur. J. Pharmacol. 693, 57–63, 10.1016/j.ejphar.2012.07.004 (2012). [DOI] [PubMed] [Google Scholar]
- Kolluru G. K., Shen X. & Kevil C. G. A tale of two gases: NO and H2S, foes or friends for life? Redox biology 1, 313–318, 10.1016/j.redox.2013.05.001 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. L. et al. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc. Natl Acad. Sci. USA 111, 3182–3187, 10.1073/pnas.1321871111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K. et al. H(2)S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation 127, 1116–1127, 10.1161/CIRCULATIONAHA.112.000855 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y. P., Liu C. T., Sheen L. Y., Chen H. W. & Lii C. K. Diallyl disulfide and diallyl trisulfide protect endothelial nitric oxide synthase against damage by oxidized low-density lipoprotein. Mol. Nutr. Food. Res. 54 Suppl 1, S42–52, 10.1002/mnfr.200900278 (2010). [DOI] [PubMed] [Google Scholar]
- Eberhardt R. T. et al. Endothelial dysfunction in a murine model of mild hyperhomocyst (e)inemia. J. Clin. Invest. 106, 483–491, 10.1172/JCI8342 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upchurch G. R. Jr. et al. Homocyst (e)ine decreases bioavailable nitric oxide by a mechanism involving glutathione peroxidase. J. Biol. Chem. 272, 17012–17017 (1997). [DOI] [PubMed] [Google Scholar]
- Zhang X. et al. Effects of homocysteine on endothelial nitric oxide production. Am. J. Physiol. Renal. Physiol. 279, F671–678 (2000). [DOI] [PubMed] [Google Scholar]
- Bir S. C. et al. Hydrogen sulfide stimulates ischemic vascular remodeling through nitric oxide synthase and nitrite reduction activity regulating hypoxia-inducible factor-1alpha and vascular endothelial growth factor-dependent angiogenesis. J. Am. Heart Assoc. 1, e004093, 10.1161/JAHA.112.004093 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng B. et al. Hydrogen sulfide downregulates the aortic L-arginine/nitric oxide pathway in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R1608–1618, 10.1152/ajpregu.00207.2006 (2007). [DOI] [PubMed] [Google Scholar]
- Kubo S. et al. Hydrogen sulfide inhibits activity of three isoforms of recombinant nitric oxide synthase. Toxicology 241, 92–97, 10.1016/j.tox.2007.08.087 (2007). [DOI] [PubMed] [Google Scholar]
- Oh G. S. et al. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic. Biol. Med. 41, 106–119, 10.1016/j.freeradbiomed.2006.03.021 (2006). [DOI] [PubMed] [Google Scholar]
- Gilja O. H., Hausken T., Wilhelmsen I. & Berstad A. Impaired accommodation of proximal stomach to a meal in functional dyspepsia. Dig. Dis. Sci. 41, 689–696 (1996). [DOI] [PubMed] [Google Scholar]
- Troncon L. E., Bennett R. J., Ahluwalia N. K. & Thompson D. G. Abnormal intragastric distribution of food during gastric emptying in functional dyspepsia patients. Gut 35, 327–332 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldarella M. P., Azpiroz F. & Malagelada J. R. Antro-fundic dysfunctions in functional dyspepsia. Gastroenterology 124, 1220–1229 (2003). [DOI] [PubMed] [Google Scholar]