Abstract
Cholangiocarcinoma (CCA) is a common biliary malignancy. Despite continuing advances, novel indicators are urgently needed to identify patients with a poor prognosis. Several microRNAs (miRNAs) have been reported to be dysregulated in CCA tissues. The purpose of the current study was to explore the potential use of certain miRNAs as serum indicators. A total of 157 individuals, including103 CCA patients, were recruited into this study. We first used qRT-PCR to evaluate 5 CCA-related miRNAs in the serum of 95 individuals to identify significantly deregulated miRNAs. A logistic regression was used to analyse the potential variables influencing lymph node metastasis. Cox proportional hazards regression models were applied to determine the association between possible prognostic variables and overall survival (OS). We observed that decreased serum miR-106a confers a higher likelihood of lymph node metastasis [hazard ratio (HR) 18.3, 95% confidence interval (CI) 5.9–56.4, p < 0.01]. Additionally, lower circulating miR-106a levels (HR 5.1; 95% CI 2.2–11.8; p < 0.01) and non-radical surgery (HR 4.2; 95% CI 2.3–7.7; p < 0.01) were independent predictors for poor prognosis. Together, reduced expression of serum miR-106a is a powerful prognostic indicator for CCA patients. The dismal outcome of these CCA patients might correlate with a higher risk of lymph node metastasis.
Cholangiocarcinoma (CCA) is a malignant tumour originating from the bile duct epithelium, and it frequently metastasizes to the lymph node. The morbidity associated with CCA has risen in recent years, but the pathogenesis mechanism and its predisposing factors remain unclear1. The only potentially curative treatment for CCA is radical resection2. Because of its lack of early symptoms, when clinical symptoms appear, most patients have reached an advanced stage, and radical resection is not a viable option3,4. Unfortunately, to date there are no definite sensitive and specific indicators for the early diagnosis of CCA5. The prognosis of CCA patients is dismal, usually measured by months, with death generally resulting from tumour metastasis6. In a retrospective analysis performed in our department, 40.4% of CCA patients developed lymph node metastasis, which was an independent prognostic predictor7. However, little is known regarding the exact molecular mechanisms underlying lymph node metastasis. In the clinical setting, the serum level of carbohydrate antigen 19-9 (CA19-9) is a marker that is frequently used for diagnosis and prognosis prediction in CCA patients. Unfortunately, CA19-9 levels are neither very sensitive nor particularly specific. Therefore, there is an urgent need to identify new indicators that will facilitate the identification of patients with a poor prognosis and permit adjuvant therapy for patients with a high risk of metastasis. In addition, with numerous chemotherapeutic drugs aimed to treat CCA, dynamic molecular indicators in the blood would be ideal to isolate CCA cohorts and monitor the potential benefits and side effects of different treatments.
MicroRNA (miRNA) is a type of endogenous coding small molecular RNA that widely exists in eukaryotes8. The discovery that miRNA expression is frequently dysregulated in malignant tumours underpins their pivotal role both from a basic science perspective and for its clinical usefulness9. Various studies have shown that miRNAs play critical roles in the development of human cancers10,11,12,13,14,15,16. In CCA tissues, several studies have identified some dysregulated miRNAs13,14,17. Some studies have indicated that miRNAs are also involved in lymph node metastasis18,19. Profiles of dysregulated miRNA isolated from plasma and serum have been generated and suggest that these miRNAs have diagnostic potential for human disease20,21. Serum circulating miRNAs are promising indicators for CCA for which the best chance of successful treatment is timely diagnosis and management; however, to date, few studies have specifically addressed the significance of circulating miRNAs in CCA patients. In the current study, we first performed a pooled analysis on the clinical validity of certain CCA-related miRNAs in 95 individuals to identify the specific miRNA as a dynamic indicator. The CCA cohort was extended to 103 cases for further clinicopathological and prognostic investigation.
Results
Patient characteristics
A total of 157 individuals including 103 CCAs, 34 benign bile-duct diseases (BBDDs) controls and 20 healthy controls were recruited into this study (Table 1). There were no significant differences in age (Student’s t-test) or gender (Pearson χ2 test) between cases and controls. In the CCA cohort, 60 patients (58.3%) acquired R0 resection. Overall survival (OS) was 74.8% at 1 year and 26.2% at 3 years. OS and recurrence rates for the R0 resections patients were 80.0% and 41.7% at 1 year, and 40.0% and 51.7% at 3 years, respectively. There were 52 (50.5%) patients confirmed dead and 15 (14.6%) patients confirmed with tumour recurrence at last follow-up. The mean age of patients was 58 years (range, 33 to 83). The median follow-up period (22.6 ± 27.1 months) was 26.9 months (range, 1 to 71 months). In addition, the CCA group and the other two control groups showed significant differences of T-Bil, CA-199, AST and ALT (p < 0.01). In CCA patients, 45 cases (43.7%) demonstrated lymph node metastasis.
Table 1. Summary of clinical parameters of the enrolled individuals.
| CCA (n = 103) | BBDD (n = 34) | Healthy Control (n = 20) | |
|---|---|---|---|
| Male n (%) | 55 (53.3) | 22 (64.7) | 12 (60.0) |
| Age (median, range) | 58 (33, 83) | 45 (20, 78) | 45 (19, 83) |
| Laboratory values (median, range) | |||
| Tbil (μmol/L) | 183 (9, 493) | 78 (33, 135)* | 11 (9, 15)* |
| AST (U/L) | 94 (69, 212) | 31 (27, 45)* | 19 (16, 23)* |
| ALT (U/L) | 101 (35, 368) | 22 (13, 37)* | 17 (14, 26)* |
| CA19-9 (U/ml) | 205 (1, 1000) | 42 (12, 56)* | 23 (6, 31)* |
*mean p < 0.01 compared with CCA group.
CCA: cholangiocarcinoma, BBDD: benign bile-duct disease, Tbil: total bilirubin, AST: aspartate transaminase, ALT: alanine aminotransferase, CA19-9: carbohydrate antigen 19-9.
Indicator Selection and Validation in Serum Samples
The goal of the present study was to explore the potential use of certain serum miRNAs as prognostic factors for CCA. First, a panel of 5 CCA-associated miRNAs was chosen on the basis of their reported relevance to CCA. Their expression levels were examined by RT-qPCR and quantitative PCR in 41 CCAs, 34 BBDDs and 20 healthy controls. The serum level of miR-106a was downregulated in CCA patients (1.27 ± 0.65) compare with BBDD patients (2.15 ± 1.80, p < 0.01) or healthy controls (3.27 ± 1.85, p < 0.01) using miR-16 as normalization control. Moreover, the serum level of miR-21 was higher in CCA patients (3.12 ± 3.80) than in BBDD controls (1.92 ± 2.72, p = 0.13) although the difference did not reach statistically significance. However, circulating miR-21 was significantly upregulated in CCA patients compared with healthy controls (1.29 ± 0.97, p = 0.04). The differences of serum levels of miR-224 and miR-224-2 were not significant among the three groups (p > 0.05). With regard to miR-370 the detection rates were <50% in all serum samples analysed by RT-qPCR; subsequently, the above three miRNAs (miR-224, miR-224-2, and miR-370) were excluded from further analytical studies. Of the two dysregulated miRNAs, the difference in the expression levels of miR-21 between CCA and BBDD patients did not achieve significance. On the basis of above results, we focused on miR-106a for its diagnostic and prognostic value. The CCA cohort was extended to 103 cases for further clinicopathological and prognostic analysis. The serum level of miR-106a was confirmed to be significantly downregulated in CCA patients (1.10 ± 0.77, p < 0.01). The results are shown in Fig. 1.
Figure 1. Expression analysis of miR-106a and miR-21 in the serum of patients with CCA, BBDD and healthy controls.
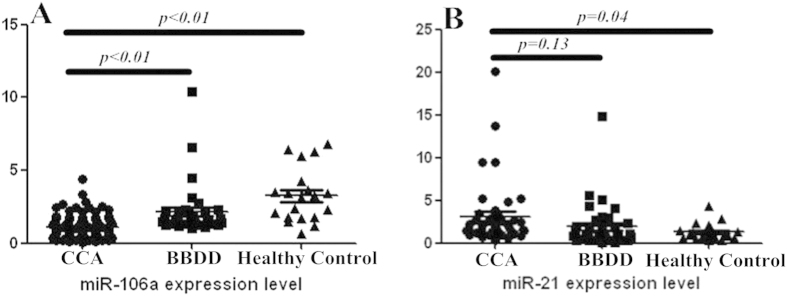
(A) Serum miR-106a levels of CCA patients were significantly downregulated compared with those of BBDD patients and healthy controls; (B) MiR-21 levels in serum from patients with CCA were significantly elevated compared with healthy controls; however, the difference did not demonstrate significance compared with BBDD patients.
The Diagnostic Value of miR-106a for CCA patients
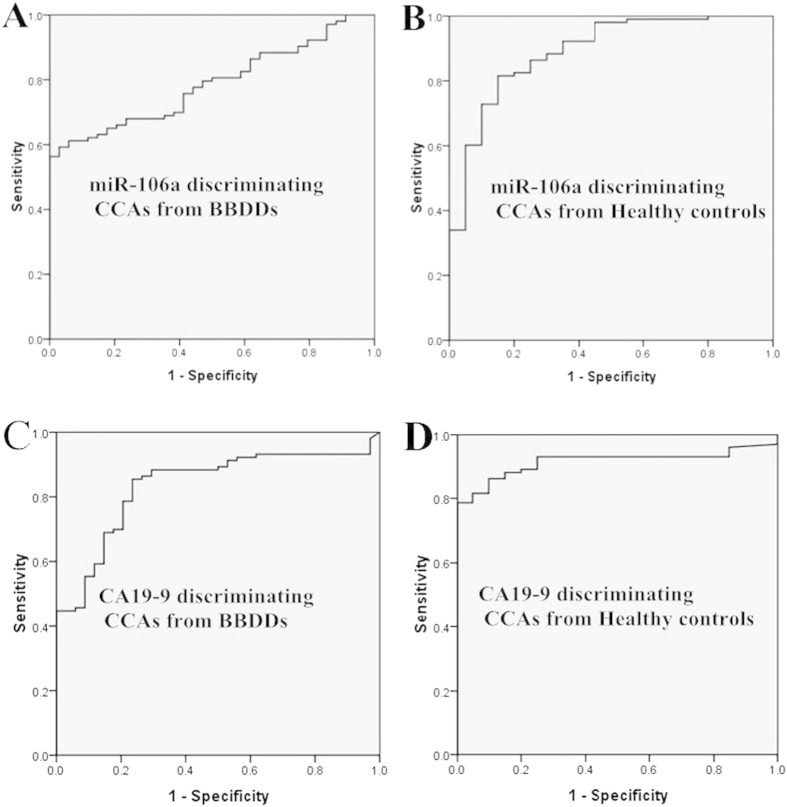
ROC curve analyses were performed to evaluate the potential of serum miR-106a to distinguish CCA from BBDD patients and/or healthy controls. The AUC of serum miR-106a for discriminating CCA patients from BBDD controls was 0.79 (95% CI: 0.71–0.86; Fig. 2A). At the cut-off value of 1.00, the sensitivity and specificity were 56.3% and 100%, respectively, and the positive and negative likelihood ratios were 1.68 and 0.04, respectively. The AUC of serum miR-106a for discriminating CCA patients from healthy controls was 0.89 (95% CI 0.81–0.97; Fig. 2B). At the cut-off value of 1.68, the sensitivity and specificity for this marker were 81.6% and 85.0%, respectively, and the positive and negative likelihood ratios were 1.76 and 0.09, respectively. As a control variable, the AUC of serum CA19-9 for discriminating CCA patients from BBDD controls was 0.84 (95% CI 0.76–0.91; Fig. 2C), and at the cut-off value of 34.2, the sensitivity and specificity for this marker were 85.4% and 86.5%, respectively. The AUC of serum CA19-9 for discriminating CCA patients from healthy controls was 0.92 (95% CI 0.87–0.97; Fig. 2D), and at the cut-off value of 57.1, the sensitivity and specificity for this marker were 78.6% and 100.0%, respectively. Based on the above results, we conclude that the diagnostic value of miR-106a is moderate and superior to serum CA19-9.
Figure 2. ROC curve analysis of serum miR-106a and CA19-9 for the diagnosis of CCA form BBDD or healthy controls.
(A) AUC of serum miR-106a for discriminating CCA patients from BBDD patients; (B) AUC of serum miR-106a for discriminating CCA patients from healthy controls; (C) AUC of serum CA19-9 for discriminating CCA patients from BBDD patients; (D) AUC of serum CA19-9 for discriminating CCA patients from healthy controls.
Serum miR-106a level and clinicopathological factors
CCA has the biological property of metastasis to regional lymph nodes in its early stage. It is well known that the expression levels of certain miRNAs are associated with clinicopathological variables in several cancers. As shown in Fig. 3A, serum miR-106a expression levels in CCA patients with lymph node metastasis were significantly decreased compared with those without metastasis (0.62 ± 0.40 vs.1.48 ± 0.78, respectively, p < 0.01), indicating that lower miR-106a levels might contribute to the lymph node metastasis of CCA. In contrast, no significant difference of serum CA19-9 levels was observed between these two groups (340.2 ± 352.8 vs. 326.9 ± 338.2, respectively, p = 0.82; Fig. 3B). Therefore, we examined the association between the expression level of circulating miR-106a and clinicopathological characteristics in 103 CCA patients. We defined the miR-106a level as ‘high expression’ when it was higher than a cut-off value of 1. The results are shown in Table 2. The circulating miR-106a level was significantly associated with lymph node metastasis (p < 0.01). To further investigate whether circulating miR-106a can serve as a predictor of lymph node metastasis, we performed multivariate logistic regression analysis, including serum miR-106a, CA19-9 level, tumour differentiation, neural invasion, p53, and MUC1 expression. Circulating miR-106a was identified as the only independent predictor of lymph node metastasis [hazard ratio (HR) 18.3, 95% confidence interval (CI) 5.9–56.4, p < 0.01].
Figure 3. Expression analysis of miR-106a and CA19-9 in the serum of patients with CCA subdivided by metastasis to lymph node.
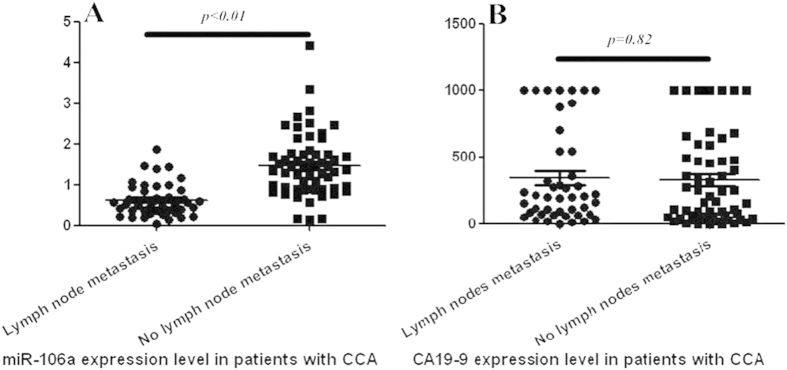
(A) miR-106a (B) CA19-9.
Table 2. The correlation of circulating miR-106a with clinicopathological factors in CCA patients.
| Low expression | High expression | p value | |
|---|---|---|---|
| Age (y) | |||
| ≲65 | 40 | 29 | 0.44 |
| >65 | 17 | 17 | |
| Gender | |||
| Male | 33 | 22 | 0.31 |
| Female | 24 | 24 | |
| Serum CA19-9 level (U/ml) | |||
| ≤37 | 9 | 8 | 0.83 |
| >37 | 48 | 38 | |
| Radical resection | |||
| Yes | 30 | 30 | 0.20 |
| No | 27 | 16 | |
| Well differentiation | |||
| Yes | 2 | 4 | 0.26* |
| No | 55 | 42 | |
| Lymph node metastasis | |||
| Yes | 39 | 6 | <0.01 |
| No | 18 | 40 | |
| Nerve invasion | |||
| Yes | 39 | 26 | 0.21 |
| No | 18 | 20 | |
| p53 | |||
| Positive | 22 | 15 | 0.53 |
| Negative | 35 | 31 | |
| MUC1 | |||
| Positive | 24 | 18 | 0.76 |
| Negative | 33 | 28 | |
*means result of Fisher exact test.
Down-regulation of miR-106a in serum samples was associated with poor prognosis in CCA patients
As the serum expression of miR-106a was significantly reduced in CCA patients, we explored the association between miR-106 serum levels with survival time. Initially, the median miR-106a serum level was utilized to divide the CCA patients into high and low groups by the cut-off value of 1.00 (miR-106a high, n = 46; miR-106a low, n = 57). The mean OS time for the entire CCA cohort was 32.8 ± 3.1 months. The miR-106a low expression group exhibited a shorter OS (p < 0.01, Fig. 4A). A Kaplan-Meier analysis also indicated that radical resection (p < 0.01, Fig. 4B) and no lymph node metastasis (p < 0.01) were associated with a longer OS. In contrast, age, gender, serum CA19-9 level, tumour diameter, tumour differentiation, p53, MUC1, and nerve invasion did not manifest a significant impact on OS. The detailed results are shown in Table 3. The mean OS time was 11.4 ± 1.2 months for patients with serum miR-106a level <1.00 compared with 45.0 ± 3.8 months for patients with serum miR-106a level >1.00. In addition, patients who received a radical resection had a mean OS time of 43.7 ± 4.1 months, while patients who did not had a mean OS time of 17.4 ± 3.7 months. Patients with lymph node metastasis had a mean OS time of 17.7 ± 3.8 months compared with 40.5 ± 3.8 months for the patients without.
Figure 4. Kaplan-Meier survival curves of patients with CCA subdivided by serum miR-106a levels or radical resection.
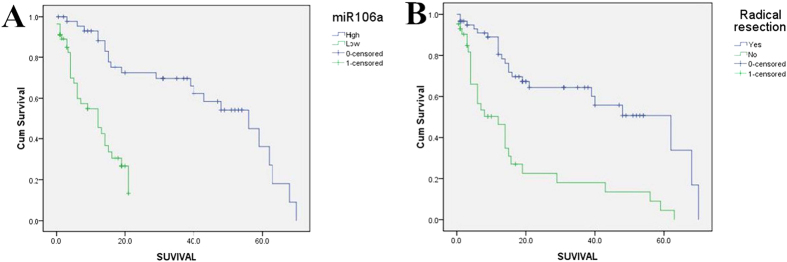
(A) miR-106a (B) Radical resection.
Table 3. Prognostic factors for survival by univariate analysis.
| Factors | Patients (n) | Mean survival | Standard error | 95% Confidence Interval (CI) | p value |
|---|---|---|---|---|---|
| Age (years) | |||||
| <65 | 67 | 39.4 | 3.4 | 26.4–42.1 | 0.51 |
| ≧65 | 36 | 24.6 | 5.1 | 20.9–39.9 | |
| Gender | |||||
| Male | 55 | 32.0 | 3.6 | 24.8–39.1 | 0.64 |
| Female | 48 | 36.3 | 6.2 | 24.1–48.4 | |
| Serum CA19-9 level (U/L) | |||||
| ≤37 | 17 | 40.2 | 7.5 | 25.4–55.0 | 0.39 |
| >37 | 86 | 31.2 | 3.3 | 24.7–37.8 | |
| Serum miR-106a level | |||||
| ≤1 | 57 | 11.4 | 1.2 | 9.1–13.7 | <0.01 |
| >1 | 46 | 45.0 | 3.8 | 37.5–52.5 | |
| Radical resection | |||||
| Yes | 60 | 43.7 | 4.1 | 35.7–51.9 | <0.01 |
| No | 43 | 17.4 | 3.7 | 10.2–24.6 | |
| Neural invasion | |||||
| Yes | 65 | 31.0 | 4.8 | 21.7–40.3 | 0.57 |
| No | 38 | 33.6 | 4.0 | 25.7–41.4 | |
| Tumor diameter (cm) | |||||
| <3 | 48 | 34.2 | 4.2 | 26.0–42.6 | 0.31 |
| ≧3 | 55 | 32.0 | 4.5 | 23.2–40.7 | |
| Lymph node metastasis | |||||
| Yes | 45 | 17.7 | 3.8 | 10.2–25.2 | <0.01 |
| No | 58 | 40.5 | 3.8 | 33.1–47.9 | |
| Well differentiation | |||||
| Yes | 6 | 29.2 | 8.8 | 12.1–46.4 | 0.93 |
| No | 97 | 32.8 | 3.2 | 26.5–39.0 | |
| p53 | |||||
| Positive | 37 | 34.7 | 6.3 | 22.4–47.0 | 0.77 |
| Negative | 66 | 32.7 | 3.6 | 25.7–39.7 | |
| MUC1 | |||||
| Positive | 42 | 26.9 | 4.2 | 18.6–35.2 | 0.20 |
| Negative | 61 | 36.9 | 4.2 | 28.6–45.2 | |
Factors that were demonstrated to be significant in the univariate analysis entered a Cox hazard model to confirm the independent impact on OS. Based on multivariate analysis, low serum miR-106a level was identified as an independent prognostic factor for OS (HR 5.1; 95% CI 2.2-11.8; p < 0.01), which was the strongest factor among indices (Table 4). Radical resection also demonstrated independent influence on OS time (HR 4.2; 95% CI 2.3-7.7; p < 0.01). However, lymph node metastasis did not maintain a significant influence on OS time in multivariate analysis (HR1.4; 95% CI 0.7-2.8; p = 0.38). This influence was not independent in this series likely because of the colinearity between lymph node metastasis and circulating miR-106a levels.
Table 4. Prognostic factors for survival by Cox proportional hazards model.
| Independent factors | Hazard Ratio | 95% CI | p value |
|---|---|---|---|
| low serum miR-106a level | 5.1 | 2.2–11.8 | <0.01 |
| radical resection | 4.2 | 2.3–7.7 | <0.01 |
| Factors evaluated: | |||
| serum miR-106a level | |||
| radical resection | |||
| lmph node metastasis |
Discussion
The initial purpose of our work was to identify a set of miRNAs differentially expressed in healthy, BBDD, and CCA patients, that may aid in diagnosis and prognosis evaluation. Beginning with a pool of miRNAs, miR-106a manifested a moderate diagnostic value for CCA although the sensitivity and specificity were inferior to CA19-9. Our results supported that lower serum miR-106a levels were associated with higher risk of metastasis to lymph node. Additionally, we identified that circulating miR-106a was a prognostic predictor for OS, and a higher serum miR-106a level demonstrated a 33.6-months survival benefit in the current cohort. The overall mean survival time for the entire series was 32.8 ± 3.1 months, consistent with results reported in previous studies7,22,23,24. Based on these results, we believe that higher serum miR-106a level is strongly associated with a significantly better survival. This advantage might be attributed to less opportunity to metastasis to lymph nodes. From a clinical perspective, our study showed that the preoperative serum miR-106a level was an independent variable for predicting lymph node metastasis and prognosis evaluation in CCA patients. Moreover, this study confirmed the independent prognostic power of radical resection consistent with results reported previously7,22,24. In contrast, serum CA19-9 level, tumour differentiation, p53 protein expression, MUC1 protein expression, and nerve invasion demonstrated little prognostic value in the current cohort.
A number of studies have identified the stability of miRNAs in serum; therefore serum circulating miRNAs may become non-invasive and specific molecular diagnostic or prognostic markers for human diseases20,25. Circulating miRNAs have been postulated as novel biomarkers or indicators for various cancers26,27,28,29,30. In CCA patients, various miRNAs have been detected in tissues. MiR-106a and miR-21 have been indicated to be upregulated in CCA tissues14. After measuring plasma levels by qRT-PCR, Tomoya and colleagues suggested that miR-21 was upregulated in CCA patients and suggested that it could be used as a diagnostic biomarker for CCA31. In the current series, we confirmed that miR-21 was elevated in CCA patient serum compared with healthy controls, but the expression difference between CCA patients and BBDDs did not achieve significance. However, the expression levels of circulating miR-106a demonstrated a significant difference not only between CCAs and healthy controls but also between CCAs and BBDDs.
MiR-106a is a member of the miR-106a-363 cluster, located on chromosome X in humans32. MiR-106a plays an important role in the tumorigenesis of several human malignancies33,34,35,36,37. Chen et al.14 determined that miR-106a was increased by 110-fold in CCA tissues. MiR-106a was also found to be overexpressed in gastric cancer38, colorectal cancer39,40, and pancreatic cancer37. However, miR-106a was found to be down-regulated in glioma and play tumor suppressor role41. Only recently higher miR-106a tissue levels have been described to be associated with glioma invasion by targeting metalloproteinases-234.
Lymph node metastasis is a dependent prognostic factor as confirmed in the current series. However, this influence was not independent in this series likely because of the colinearity between lymph node metastasis and circulating miR-106a level. Interestingly, we found that the circulating miR-106a level significantly correlated with lymph node metastasis. Patients with a lower serum miR-106a level conferred more opportunity to lymph node metastasis (HR 18.3, 95% CI 5.9-56.4, p < 0.01). In addition, a low circulating miR-106a level was confirmed to be an independent poor prognostic predictor (HR 5.1; 95% CI 2.2-11.8; p < 0.01). Several reports have confirmed the prognostic value of miR-106a in astrocytoma42, glioblastoma43, and gastric carcinoma44. The downregulation of circulating miR-106a in the current patient group is not consistent with findings drawn from the CCA tissues. This inconsistency may be explained by the non-secreting nature of miR-106a and likely the effect of the tumour microenvironment. The source of circulating miRNAs has been investigated by several studies but is still a source of debate. Elhelw and colleagues argued that serum miRNA levels not only were a result of tumours, but also maybe be a result of the immune response45. This discrepancy has been demonstrated by several reports, such as miR-195 in breast cancer46,47 and miR-12248,49,50 and miR181a51,52 in hepatocellular carcinoma. Notably, miR-106a has recently been implicated in chemotherapy resistance. Circulating miR-106a was indicated to be upregulated in non-responders after oxaliplatin-based chemotherapy in metastatic colorectal cancer patients and as a biomarker to predict outcome53. In ovarian cancer, Huh and colleagues published an article and suggested upregulated miR-106a was associated with paclitaxel resistance54. However, the mechanisms of these results have not been addressed, and this characterization is fundamentally necessary to acquire a deeper understanding of cancer progression.
Taken together, these findings demonstrate that serum miR-106a level is downregulated in CCA patients and associated with metastasis to lymph node and prognosis. Higher circulating miR-106a level confers a survival benefit. A lower miR-106a level confers a higher risk for lymph node metastasis in CCA patients. The diagnostic value of miR-106a for CCA patients is moderate. Collectively, these results indicate that miR-106a presents a clinically promising indicator that can facilitate lymph node metastasis risk assessment and prognosis evaluation in CCA patients. These findings require large-scale prospective validation.
Methods
The methods were performed in accordance with the approved guidelines.
Study Design and Patients
This study was approved by ethics boards of Eastern Hepatobiliary Surgery Hospital, and informed consent was obtained from all subjects recruited. No attempt was made to define a target statistical power. From February 2010 through January 2014, we prospectively enrolled a cohort of individuals, including CCA patients (n = 103) who underwent resection with a curative intent, 34 BBDD patients (20 primary bile duct stone and 14 congenital biliary duct cyst patients) and 20 healthy controls. The distribution of gender and age was not significantly different between patients and healthy controls. All CCA patients were required to have histologically confirmed adenocarcinoma. According to the AJCC 7th TNM stage system, there were 76 peri-hilar CCAs and 27 extrahepatic CCAs in this cohort. Tumour stage was determined according to the AJCC 7th TNM stage. In current study, the peripheral-blood samples (fasting) were drawn from all patients preoperatively. None of the patients had received prior treatment, in particular, chemotherapy or radiotherapy for CCA patients, and none suffered from any tumours or any relevant critical illnesses. CA19-9 levels in serum samples were measured by standard enzyme immunoassay as a routine clinical test. After resection, tissue samples were examined histopathologically by at least two pathologists. Routine analyses were performed on all CCA specimens (pathological grade, lymph node metastasis, nerve invasion, vascular invasion, and immunostaining for p53, MUC1, CK19 and CA19-9). Complete clinicopathological data were collected for each patient. The data regarding the subjects were obtained from medical records, pathology reports and personal interviews with the subjects. The data collected included age, gender, total bilirubin level (T-Bil), alanine aminotransferase (ALT) level, aspartate transaminase (AST) level, CA19-9 level, and lymph node metastasis status according to previous surgical operative notes. As a control, serum samples were drawn from 20 healthy subjects confirmed through comprehensive medical examination in Changzheng Hospital (Shanghai, China). The comparative baseline clinical characteristics of CCA, BBDD patients, and healthy controls are described in Table 1.
Selection of circulating miRNAs as candidate markers
According to previous reports11,13,14, a group of most CCA-associated miRNAs, including miR-21, miR-106a, miR-224, miR-224-2, and miR-370, was selected to evaluate their potential as circulating indicators for CCA. These miRNAs were previously found to be differentially expressed in CCA tissues and normal bile duct mucosa. Although multiple miRNAs with dysregulated expression in CCA have been discovered, we focused on these miRNAs because they have been reported as the most dramatically dysregulated. Using miR-16 as normalization control, the potential miRNAs markers chosen were verified on serum samples through reverse transcription (RT) and quantitative PCR. The diagnostic efficacy and correlation with lymph node metastasis and survival of CCA patients were analysed. Serum preparation, RNA extraction, reverse transcription (RT) and quantitative PCR (qPCR) procedures have been previously described55. RT and qPCR kits allowed for accurate miRNA analysis (Applied Biosystems) and were used to evaluate the expression of the chosen miRNAs.
CCA patient follow-up
CCA patients were followed up every 3 months. All CCA patients were prospectively monitored by serum carcinoembryonic antigen (CEA), CA19-9, abdomen ultrasonography with an interval of 1 month during the first year postpoperatively. A computed tomography scan of the abdomen was performed every 3 months. If recurrence was suspected, a computed tomography scan or magnetic resonance imaging was performed immediately. Most causes of death were recurrence and metastasis. Patients with confirmed recurrence received further treatment, which was mainly based oral tegafur chemotherapy and external radiotherapy. Otherwise, symptomatic treatment was provided. Follow-up was terminated on May 6, 2015. OS was defined as the interval elapsing between the date of surgery and date of death or censoring at the most recent follow-up.
Statistical Analyses
Statistical analyses were performed using SPSS Statistical Software version 17.0 (SPSS, Inc.). Because of the magnitude and range of relative miRNAs expression levels observed, the results were log transformed for the analysis. There was no evidence against normality for the log transformed data as confirmed using the Kolmogorov-Smirnov test. Descriptive statistics for quantitative variables are given as the mean ± standard deviation. The difference in quantitative variable was tested using Student’s t-test. The Pearson Chi-square test or Fisher’s exact test was used to compare qualitative variable. Receiver operating characteristics (ROC) curves were constructed and the area under the curve (AUC) was calculated to evaluate the sensitivity and specificity for predicting CCAs and BBDDs or healthy controls based on the expression level of dysregulated miRNAs. Logistic regression was used to analyse the potential variables influencing lymph node metastasis. Survival analyses were executed following the Kaplan-Meier method, and comparisons were made using the log rank test. Beginning with a pool of significant predictors identified in the univariate analyses, variables were evaluated in multivariable Cox proportional hazards models, including only variables with a p value < 0.05. Two-sided p values < 0.05 were considered significant.
Additional Information
How to cite this article: Cheng, Q. et al. Circulating miR-106a is a Novel Prognostic and Lymph Node Metastasis Indicator for Cholangiocarcinoma. Sci. Rep. 5, 16103; doi: 10.1038/srep16103 (2015).
Acknowledgments
We sincerely thank Prof. Chunfang Gao for the invaluable support for current study. This study was supported by the Shanghai Natural Science Foundation (13ZR1410200), the Scientific research project of Shanghai science and Technology Committee (14411967700), and the National Natural Science Fund (81472280).
Footnotes
Author Contributions Q.C., F.F. and Y.Z. collected and analysed the data. Q.C. and L.Z. wrote the main manuscript. L.Z. performed experiments and followed up patients with CCA. C.L., X.L., B.Y. and X.J. performed surgery. X.J. designed this study. All authors reviewed the manuscript.
References
- Razumilava N. & Gores G.J. Cholangiocarcinoma. Lancet 383, 2168–2179, 10.1016/S0140-6736(13)61903-0 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel T. Cholangiocarcinoma. Nat Clin Pract Gastroenterol Hepatol 3, 33–42, 10.1038/ncpgasthep0389 (2006). [DOI] [PubMed] [Google Scholar]
- Malhi H. & Gores G.J. Cholangiocarcinoma: modern advances in understanding a deadly old disease. J Hepatol 45, 856–867, 10.1016/j.jhep.2006.09.001 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarnagin W.R. & Shoup M. Surgical management of cholangiocarcinoma. Semin Liver Dis 24, 189–199, 10.1055/s-2004-828895 (2004). [DOI] [PubMed] [Google Scholar]
- Blechacz B. & Gores G.J. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology 48, 308–321, 10.1002/hep.22310 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley D.R., Weaver A.L. & Nagorney D.M. “Natural history” of unresected cholangiocarcinoma: patient outcome after noncurative intervention. Mayo Clin Proc 70, 425–429 (1995). [DOI] [PubMed] [Google Scholar]
- Cheng Q.B. et al. Resection with total caudate lobectomy confers survival benefit in hilar cholangiocarcinoma of Bismuth type III and IV. Eur J Surg Oncol 38, 1197–1203, 10.1016/j.ejso.2012.08.009 (2012). [DOI] [PubMed] [Google Scholar]
- Cummins J.M. & Velculescu V.E. Implications of micro-RNA profiling for cancer diagnosis. Oncogene 25, 6220–6227, 10.1038/sj.onc.1209914 (2006). [DOI] [PubMed] [Google Scholar]
- van Kouwenhove M., Kedde M. & Agami R. MicroRNA regulation by RNA-binding proteins and its implications for cancer. Nat Rev Cancer 11, 644–656, 10.1038/nrc3107 (2011). [DOI] [PubMed] [Google Scholar]
- Murakami Y. et al. Comprehensive analysis of microRNA expression patterns in hepatocellular carcinoma and non-tumorous tissues. Oncogene 25, 2537–2545, 10.1038/sj.onc.1209283 (2006). [DOI] [PubMed] [Google Scholar]
- Meng F. et al. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology 130, 2113–2129, 10.1053/j.gastro.2006.02.057 (2006). [DOI] [PubMed] [Google Scholar]
- Iorio M.V. et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res 65, 7065–7070, 10.1158/0008-5472.CAN-05-1783 (2005). [DOI] [PubMed] [Google Scholar]
- Selaru F.M. et al. MicroRNA-21 is overexpressed in human cholangiocarcinoma and regulates programmed cell death 4 and tissue inhibitor of metalloproteinase 3. Hepatology 49, 1595–1601, 10.1002/hep.22838 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. et al. The role of microRNA expression pattern in human intrahepatic cholangiocarcinoma. J Hepatol 50, 358–369, 10.1016/j.jhep.2008.09.015 (2009). [DOI] [PubMed] [Google Scholar]
- Yanaihara N. et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell 9, 189–198, 10.1016/j.ccr.2006.01.025 (2006). [DOI] [PubMed] [Google Scholar]
- Wang C. et al. A panel of five serum miRNAs as a potential diagnostic tool for early-stage renal cell carcinoma. Sci Rep 5, 7610, 10.1038/srep07610 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q., Yi B., Wang A. & Jiang X. Exploring and exploiting the fundamental role of microRNAs in tumor pathogenesis. Onco Targets Ther 6, 1675–1684, 10.2147/OTT.S52730 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucci P. et al. Loss of p63 and its microRNA-205 target results in enhanced cell migration and metastasis in prostate cancer. Proc Natl Acad Sci USA 109, 15312–15317, 10.1073/pnas.1110977109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. et al. MiR-196a binding-site SNP regulates RAP1A expression contributing to esophageal squamous cell carcinoma risk and metastasis. Carcinogenesis 33, 2147–2154, 10.1093/carcin/bgs259 (2012). [DOI] [PubMed] [Google Scholar]
- Mitchell P.S. et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 105, 10513–10518, 10.1073/pnas.0804549105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skog J. et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10, 1470–1476, 10.1038/ncb1800 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q. et al. Distal bile duct carcinoma: prognostic factors after curative surgery. A series of 112 cases. Ann Surg Oncol 14, 1212–1219, 10.1245/s10434-006-9260-0 (2007). [DOI] [PubMed] [Google Scholar]
- Seyama Y. et al. Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann Surg 238, 73–83, 10.1097/01.SLA.0000074960.55004.72 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q. et al. Predictive factors for prognosis of hilar cholangiocarcinoma: postresection radiotherapy improves survival. Eur J Surg Oncol 33, 202–207, 10.1016/j.ejso.2006.09.033 (2007). [DOI] [PubMed] [Google Scholar]
- Jackson D.B. Serum-based microRNAs: are we blinded by potential? Proc Natl Acad Sci USA 106, E5, 10.1073/pnas.0809999106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho A.S. et al. Circulating miR-210 as a Novel Hypoxia Marker in Pancreatic Cancer. Transl Oncol 3, 109–113, 10.1593/tlo.09256 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng E.K. et al. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut 58, 1375–1381, 10.1136/gut.2008.167817 (2009). [DOI] [PubMed] [Google Scholar]
- Taylor D.D. & Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol 110, 13–21, 10.1016/j.ygyno.2008.04.033 (2008). [DOI] [PubMed] [Google Scholar]
- Huang Z. et al. Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int J Cancer 127, 118–126, 10.1002/ijc.25007 (2010). [DOI] [PubMed] [Google Scholar]
- Resnick K.E. et al. The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol Oncol 112, 55–59, 10.1016/j.ygyno.2008.08.036 (2009). [DOI] [PubMed] [Google Scholar]
- Kishimoto T. et al. Plasma miR-21 is a novel diagnostic biomarker for biliary tract cancer. Cancer Sci 104, 1626–1631, 10.1111/cas.12300 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura A. et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell 132, 875–886, 10.1016/j.cell.2008.02.019 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volinia S. et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA 103, 2257–2261, 10.1073/pnas.0510565103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. et al. Oncogenic miR-20a and miR-106a enhance the invasiveness of human glioma stem cells by directly targeting TIMP-2. Oncogene 34, 1407–1419, 10.1038/onc.2014.75 (2014). [DOI] [PubMed] [Google Scholar]
- Landais S., Landry S., Legault P. & Rassart E. Oncogenic potential of the miR-106-363 cluster and its implication in human T-cell leukemia. Cancer Res 67, 5699–5707, 10.1158/0008-5472.CAN-06-4478 (2007). [DOI] [PubMed] [Google Scholar]
- Yuan R. et al. Upregulated expression of miR-106a by DNA hypomethylation plays an oncogenic role in hepatocellular carcinoma. Tumour Biol 36, 3093–3100, 10.1007/s13277-014-2945-2 (2014). [DOI] [PubMed] [Google Scholar]
- Li P. et al. Upregulated miR-106a plays an oncogenic role in pancreatic cancer. Febs Lett 588, 705–712, 10.1016/j.febslet.2014.01.007 (2014). [DOI] [PubMed] [Google Scholar]
- Cui L. et al. Gastric juice MicroRNAs as potential biomarkers for the screening of gastric cancer. Cancer-Am Cancer Soc 119, 1618–1626, 10.1002/cncr.27903 (2013). [DOI] [PubMed] [Google Scholar]
- Catela I.T., Aralica G., Cacev T., Loncar B. & Kapitanovic S. miR-106a overexpression and pRB downregulation in sporadic colorectal cancer. Exp Mol Pathol 94, 148–154, 10.1016/j.yexmp.2012.11.002 (2013). [DOI] [PubMed] [Google Scholar]
- Bovell L.C. et al. The prognostic value of microRNAs varies with patient race/ethnicity and stage of colorectal cancer. Clin Cancer Res 19, 3955–3965, 10.1158/1078-0432.CCR-12-3302 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G. et al. MiR-106a inhibits glioma cell growth by targeting E2F1 independent of p53 status. J Mol Med (Berl) 89, 1037–1050, 10.1007/s00109-011-0775-x (2011). [DOI] [PubMed] [Google Scholar]
- Zhi F. et al. Identification of 9 serum microRNAs as potential noninvasive biomarkers of human astrocytoma. Neuro Oncol 17, 383–91, 10.1093/neuonc/nou169 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S. et al. MiR-106a is an independent prognostic marker in patients with glioblastoma. Neuro Oncol 15, 707–717, 10.1093/neuonc/not001 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.H., Hong S.W., Kim A., Choi S.H. & Yoon S.O. Prognostic implications for high expression of oncogenic microRNAs in advanced gastric carcinoma. J Surg Oncol 107, 505–510, 10.1002/jso.23271 (2013). [DOI] [PubMed] [Google Scholar]
- Elhelw D.S. et al. Predictive prognostic role of with discrepancy in the liver and serum of genotype 4 hepatitis C virus patients. Biomed Rep 2, 843–848, 10.3892/br.2014.343 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D. et al. Analysis of MiR-195 and MiR-497 expression, regulation and role in breast cancer. Clin Cancer Res 17, 1722–1730, 10.1158/1078-0432.CCR-10-1800 (2011). [DOI] [PubMed] [Google Scholar]
- Heneghan H.M. et al. Circulating microRNAs as novel minimally invasive biomarkers for breast cancer. Ann Surg 251, 499–505, 10.1097/SLA.0b013e3181cc939f (2010). [DOI] [PubMed] [Google Scholar]
- Xu J. et al. Circulating microRNAs, miR-21, miR-122, and miR-223, in patients with hepatocellular carcinoma or chronic hepatitis. Mol Carcinog 50, 136–142, 10.1002/mc.20712 (2011). [DOI] [PubMed] [Google Scholar]
- Gramantieri L. et al. Cyclin G1 is a target of miR-122a, a microRNA frequently down-regulated in human hepatocellular carcinoma. Cancer Res 67, 6092–6099, 10.1158/0008-5472.CAN-06-4607 (2007). [DOI] [PubMed] [Google Scholar]
- Kutay H. et al. Downregulation of miR-122 in the rodent and human hepatocellular carcinomas. J Cell Biochem 99, 671–678, 10.1002/jcb.20982 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhan M.X. et al. Expression of serum microRNAs (miR-222, miR-181, miR-216) in human hepatocellular carcinoma and its clinical significance. Zhonghua Yi Xue Za Zhi 93, 1830–1832 (2013). [PubMed] [Google Scholar]
- Ji J. et al. Identification of microRNA-181 by genome-wide screening as a critical player in EpCAM-positive hepatic cancer stem cells. Hepatology 50, 472–480, 10.1002/hep.22989 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjersem J.B. et al. Plasma microRNAs predicting clinical outcome in metastatic colorectal cancer patients receiving first-line oxaliplatin-based treatment. Mol Oncol 8, 59–67, 10.1016/j.molonc.2013.09.001 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh J.H. et al. Dysregulation of miR-106a and miR-591 confers paclitaxel resistance to ovarian cancer. Br J Cancer 109, 452–461, 10.1038/bjc.2013.305 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi P. et al. Serum microRNAs as biomarkers for hepatocellular carcinoma in Chinese patients with chronic hepatitis B virus infection. Plos One 6, e28486, 10.1371/journal.pone.0028486 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]