Abstract
Background
There are suggestions that patients with attention-deficit/hyperactivity disorder (ADHD) show impairment in executive attention control and emotion regulation. This study investigated emotion regulation as a function of the recruitment of executive attention in patients with ADHD.
Methods
Thirty-five healthy children/adolescents (mean age = 13.91) and twenty-six children/adolescents with ADHD (mean age = 14.53) participated in this fMRI study. They completed the affective Stroop paradigm viewing positive, neutral and negative images under varying cognitive loads. A 3-way ANOVA (diagnosis-by-condition-by-emotion) was conducted on the BOLD response data. Following this, 2 3-way ANOVAs (diagnosis-by-condition-by-emotion) were applied to context-dependent psychophysiological interaction (gPPI) analyses generated from a dorsomedial frontal cortex and an amygdala seed (identified from the BOLD response ANOVA main effects of condition and emotion respectively).
Results
A diagnosis-by-condition interaction within dorsomedial frontal cortex revealed reduced recruitment of dorsomedial frontal cortex as a function of increased task demands in the children/adolescents with ADHD relative to healthy children/adolescents. The level of reduction in recruitment of dorsomedial frontal cortex was significantly correlated with symptom severity (total and hyperactivity) measured by Conner's Parent Report Scale in the children/adolescents with ADHD. In addition, analysis of gPPI data from a dorsomedial frontal cortex seed revealed significant diagnosis-by-condition interactions within lateral frontal cortex; connectivity between dorsomedial frontal cortex and lateral frontal cortex was reduced in the patients with ADHD relative to comparison youth during congruent and incongruent task trials relative to view trials. There were no interactions of group, or main effect of group, within the amygdala in the BOLD response ANOVA (though children/adolescents with ADHD showed increased responses to positive images within temporal cortical regions during task trials; identified by the diagnosis-by-condition-by-emotion interaction). However, analysis of gPPI data from an amygdala seed revealed decreased connectivity between amygdala and lentiform nucleus in the presence of emotional stimuli in children/adolescents with ADHD (diagnosis-by-emotion interaction).
Conclusion
The current study demonstrated disrupted recruitment of regions implicated in executive function and impaired connectivity within those regions in children/adolescents with ADHD. There were also indications of heightened representation of emotional stimuli in patients with ADHD. However, as the findings were specific for positive stimuli, the suggestion of a general failure in emotion regulation in ADHD was not supported.
Keywords: Attention-deficit/hyperactivity disorder, Affective Stroop, Executive attention, Emotion regulation, fMRI
Highlights
-
•
ADHD showed decreased dorsomedial frontal cortex activity with increased cognitive demand.
-
•
Decreased dorsomedial frontal cortex activity was correlated with symptom severity of ADHD.
-
•
Connectivity of dorsomedial frontal cortex–lateral frontal cortex was compromised in ADHD.
-
•
ADHD showed increased activities in emotional responding areas to positive emotional stimuli.
1. Introduction
Attention deficit hyperactivity disorder (ADHD) involves a persistent pattern of inattention and/or hyperactivity–impulsivity that is associated with impairment in at least two domains of functioning, such as at school and in the home (American Psychiatric Association, 2013). Considerable work in patients with ADHD has focused on the cognitive domains of attention, working memory and executive function (De La Fuente et al., 2013, Hart et al., 2013, Rapport et al., 2013, Schulz et al., 2000, Sonuga-Barke et al., 2008). However, recently there has also been a growing interest in potential emotional dysfunction in ADHD (Herrmann et al., 2010, Maier et al., 2013, Posner et al., 2011, Posner et al., 2013, Shaw et al., 2014). There have been suggestions that ADHD is marked by emotion dysregulation and/or poor emotion regulation (Barkley and Fischer, 2010, Reimherr et al., 2005, Shaw et al., 2014, Sobanski et al., 2010). Recent data indicates that patients with ADHD may have increased difficulty in regulating positive emotions relative to healthy individuals (Musser et al., 2013). Moreover, there are suggestions that difficulties in top-down executive functioning and increased bottom-up emotional reactivity were associated in ADHD, and this contributes to more significant symptom severity of aggression, difficulty in behavioral inhibition, and internalizing symptoms (Graziano et al., 2013). Consistent with those suggestions, patients with ADHD show heightened amygdala responses during aversive conditioning (Maier et al., 2013) and when rating fear in neutral facial expressions (Brotman et al., 2010). However, amygdala responses to fearful expressions have not been consistently reported as increased in patients with ADHD (Marsh et al., 2008, Passarotti et al., 2010b, Posner et al., 2011).
Emotion regulation is a broad term that subsumes at least two main sets of control processes (Gyurak et al., 2011, Ochsner and Gross, 2005, Phillips et al., 2003). The first type of emotion regulation involves ventral prefrontal system that represents emotional value and/or a form of emotional conflict adaptation and can be indexed through emotional Stroop paradigms (Etkin et al., 2010). Sonuga-Barke and colleagues have argued that this form of emotion regulatory circuitry is disrupted in at least some patients with ADHD (Posner et al., 2013, Sonuga-Barke, 2002, Sonuga-Barke et al., 2008). In addition to this, recent work using emotional Stroop task variants has reported atypical responses to emotional distractors in patients with ADHD. For example, two groups reported that patients with ADHD showed reduced activity within right orbital/ventromedial and inferior frontal cortices when identifying the hue of negative relative to neutral valenced words (Passarotti et al., 2010a) or the number of negative or neutral words presented on the screen (Posner et al., 2011). Patients with ADHD showed greater responses than healthy controls within vmPFC (Posner et al., 2011) and subgenual ACC (Passarotti et al., 2010a) when the contrast was positive vs. neutral.
The second type of emotion regulation involves prefrontal (both dorsomedial and lateral frontal) regions. Executive attention allows the priming of relevant representations at the expense of irrelevant ones, thereby resolving representational competition (Desimone and Duncan, 1995). Such control processes can be recruited explicitly within cognitive reappraisal paradigms, where subjects willfully attempt to alter stimulus representations by priming non-emotional features (see Kalisch for reviews; Kalisch, 2009, Ochsner et al., 2002, Ochsner and Gross, 2005). It is argued that these processes are recruited implicitly through emotion distraction paradigms (Blair et al., 2007, Erthal et al., 2005, Mitchell et al., 2006, Pessoa et al., 2005, Pessoa et al., 2002). On the basis of a recent influential neurocognitive model of ADHD (the dual pathway model; Sonuga-Barke, 2002, Sonuga-Barke et al., 2008), one might expect both types of emotion regulation to be dysfunctional in ADHD.
In the current study, we implemented one such emotion distraction task, the affective Stroop task (AST) (Blair et al., 2007). In the AST, participants are required to determine the number of digits presented on the screen. These numbers are temporally bracketed by emotional or neutral distracters. A body of studies with the AST and its variants has demonstrated that task performance is associated with increased activity within regions implicated in executive attention and decreased emotional responding (Blair et al., 2007, Mitchell et al., 2007, Mitchell et al., 2008). Importantly, the recruitment of the executive attention system by task demands (“count the numbers”) is not thought to directly inhibit the amygdala. Rather, executive attention will prime task relevant representations (representations of the number stimuli) within temporal cortex. This in turn will suppress representation of emotional distractors following representational competition (Desimone and Duncan, 1995). The reduced representation of the emotional distracters results in reduced amygdala response to these distracters.
Given the previous findings of executive attention dysfunction and emotion dys-regulation in ADHD (Arnsten and Rubia, 2012, Banich et al., 2009, Bush et al., 1999, Christakou et al., 2013, Posner et al., 2011, Rubia et al., 2009, Schneider et al., 2010), and previous findings from the studies using various versions of AST on ADHD population (Passarotti et al., 2010a, Posner et al., 2011), we hypothesized that this process of representation-priming is impaired in ADHD. As such, we predicted that children and adolescents with ADHD would show: (i) reduced recruitment of, and functional connectivity between, executive attention regions (dorsomedial and lateral frontal cortices); (ii) increased recruitment of regions implicated in emotional responding (amygdala) relative to healthy comparison youth during task conditions, due to disruption in priming of representation of task-relevant stimuli by executive attention areas.
2. Methods and materials
2.1. Participants
Sixty-seven children and adolescents participated: 36 healthy and 31 with ADHD. Children and adolescents were recruited from the community through newspaper ads, fliers, and referrals from area mental health practitioners. All children and adolescents and parents completed the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS) (Kaufman et al., 1997). The diagnoses of ADHD were made by expert child and adolescent psychiatrists on the basis of the K-SADS (including the ADHD module) done by a doctoral-level clinical psychologist. Healthy children/adolescents were determined by having no current or past psychiatric diagnoses including ADHD by K-SADS. Exclusion criteria for both healthy children/adolescents and children/adolescents with ADHD were autism spectrum disorders, Tourette syndrome, lifetime history of psychosis, depression, bipolar disorder, generalized, social or separation anxiety disorder, posttraumatic stress disorder (PTSD), neurologic disorder including seizure or epilepsy, history of major head trauma including skull fracture, substance dependence, major medical illness, and IQ of < 80. The parents of 23 children/adolescents with ADHD as well as 31 healthy children/adolescents completed the Conner's Parent Rating Scale for ADHD, version 2 (Conners et al., 1998). IQ was assessed with the Wechsler Abbreviated Scale of Intelligence (2-subtest form) (Wechsler, 1999).
Six participants (1 healthy, 5 with ADHD) were excluded due to performance artifacts during the functional MRI scanning procedure (for example, too much movement; i.e., more than 10% of TRs were discarded due to movement above study limits (1.0 cm)). The five subjects with ADHD who were excluded did not show any significant difference on their behavioral data from the subjects with ADHD who were included [reaction time (RT): F(1,29) = 0.016, p = 0.901; accuracy: F(1,29) = 0.797, p = 0.379]. Five children/adolescents with ADHD who were excluded did not differ on their age, IQ, or Conners Parent Rating for ADHD total score from the children/adolescents with ADHD who were included [t(29) = 1.651, t(29) = 0.275, t(25) = 0.375, & p = 0.110, 0.786, 0.711, respectively]. Thus, data from 35 healthy (17 female, 18 male, average age = 14.53) and 26 ADHD (9 female, 17 male, average age = 13.91, 11 inattentive type, 3 hyperactive-impulsive type, 9 combined type, and 3 ADHD Not otherwise specified) children and adolescents were analyzed. There was no difference in age, IQ, and gender distribution between the healthy and ADHD children/adolescents. Eleven out of 26 children and adolescents with ADHD (42.3%) were currently on stimulant medications; see Table 1. Parents who were on stimulant medications were asked to withhold them at least 24 h prior to scanning.
Table 1.
Characteristics of healthy children/adolescents and children/adolescents with ADHD.
| Healthy children/adolescents (N = 35) | Children/adolescents with ADHD (N = 26) | P value (df) | |
|---|---|---|---|
| Demographics | |||
| Age | 13.91 (2.13) | 14.53 (2.00) | 0.482 (1) |
| IQ | 105.06 (12.67) | 106.42 (13.03) | 0.682 (1) |
| Gender | 18 male, 17 female | 17 male, 9 female | 0.276 (1) |
| Handedness | 9 left, 26 right | 4 left, 22 right | 0.330 (1) |
| ADHD | 0 | 26 | |
| Inattentive | 11 | ||
| H–I | 3 | ||
| Combined | 9 | ||
| NOS | 3 | ||
| ODD | 0 | 1 | |
| SA | 0 | 2 | |
| Conner's Parenting Score | |||
| 2.26 (3.16)a | 31.35 (8.38)b | 0.000 (52) | |
| Range | 0–4 | 17–53 | |
| Medication | |||
| 0 | 11c | ||
ADHD: attention-deficit/hyperactivity disorder; inattentive: predominantly inattentive type; H–I: predominantly hyperactive–impulsive type; combined: combined type; NOS: not otherwise specified type; ODD: comorbidity of oppositional defiant disorder; SA: comorbidity of substance abuse (cannabinoid).
31 subjects.
23 subjects.
8: methylphenidate; 2: amphetamine; 1: risperidone
2.2. Experimental task
We used an adapted version of the affective Stroop task (AST) described previously (Blair et al., 2007); see Fig. 1. On each trial, participants saw a central fixation point (400 milliseconds [ms]), a positive, neutral, or negative image (400 ms), either a numerical array on task trials, or a blank screen on view trials (400 ms), the same image previously displayed (400 ms), and a second blank screen (1300 ms). For task trials, the subjects pressed buttons corresponding to number numerosity; i.e., the number of numbers on the screen. Two to five numbers are presented on the screen during the numerical array. On congruent trials, numerosity matched the actual number values displayed (e.g. three 3 s). On incongruent trials, numerosity did not match the number values displayed (e.g. four 3 s). Participants were asked to respond as quickly as possible, but were free to respond at any time between the initial numerical presentation and the end of the blank screen display (response window: 1700 ms). The participants made no response for view trials (they saw a blank screen temporally bracketed by the emotional stimuli; see Fig. 1).
Fig. 1.
Experiment design. Example trial sequences: (a) positive view trial; (b) positive congruent trial; (c) positive incongruent trial.
The images of emotional stimuli that were presented before and after the numerical arrays (see Fig. 1) consisted of 48 positive, 48 negative, and 48 neutral pictures selected from the International Affective Picture System (Lang et al., 2005). Normative mean image valence and arousal values on a 9-point scale were 3.35 (SD: 0.77) and 5.97 (SD: 1.07) for negative pictures, 7.43 (SD: 0.52) and 4.99 (SD: 1.10) for positive pictures, and 4.87 (SD: 0.28) and 2.66 (SD: 0.54) for neutral pictures.
Subjects completed two runs. Each involved 288 trials (32 in each of 9 categories [3 image type x 3 condition type]) and 96 fixation presentations (each of 2500 ms length to generate a baseline). Trial order was randomized across participants. Participants were asked to withdraw from their current stimulant medications at least 24 h prior to scanning.
2.3. Image acquisition and analysis
Whole-brain blood oxygen level-dependent (BOLD) fMRI data were acquired using a 3-T GE MRI scanner. Following sagittal localization, functional T2*-weighted images were acquired using an echo-planar single-shot gradient echo pulse sequence with a matrix of 64 × 64 mm, repetition time (TR) of 3000 ms, echo time (TE) of 30 ms, field of view (FOV) of 240 mm, and voxels of 3.75 × 3.75 × 4 mm. Images were acquired in 30 continuous 4 mm axial slices per brain volume across two runs. The duration of each run was 8 min 13 s. In the same session, a high-resolution T1-weighed anatomical image was acquired to aid with spatial normalization (three-dimensional Spoiled GRASS; TR = 8.1 ms; TE = 3.2 ms, flip angle 20°; field of view = 240 mm, 128 axial slices, thickness = 1.0 mm; 256 × 256 acquisition matrix).
2.4. Behavioral data analysis
Behavioral data were analyzed via two 2 (diagnosis: healthy children/adolescents, children/adolescents with ADHD) by 2 (task: congruent, incongruent) by 3 (emotion: negative, positive, neutral) between-group repeated measures ANOVAs that were applied to the reaction time (RT) and accuracy data respectively.
2.5. Functional MRI analysis
Data were analyzed within the framework of a random effects general linear model using Analysis of Functional Neuroimages (AFNI). Both individual and group-level analyses were conducted. The first 5 volumes in each scan series, collected before equilibrium magnetization was reached, were discarded. EPI datasets were de-spiked and slice-time corrected. Motion correction was performed by registering all volumes in the EPI dataset to the last volume of the EPI dataset, which was collected shortly before acquisition of the high-resolution anatomical dataset. TRs were censored if the motion was above the motion limit (1 mm). EPI datasets and anatomical were coregistered using a Localized Pearson Correlation cost function.
The EPI datasets for each subject were spatially smoothed (using an isotropic 6 mm Gaussian kernel) to reduce the influence of anatomical variability among the individual maps in generating group maps. Next, the time series data were normalized by dividing the signal intensity of a voxel at each time point by the mean signal intensity of that voxel for each run and multiplying the result by 100. Resultant regression coefficients represented a percent signal change from the mean. The model involved six motion regressors and the following 9 condition regressors (each modeled as a single event from the presentation of the first image): negative congruent, negative incongruent, negative view, neutral congruent, neutral incongruent, neutral view, positive congruent, positive incongruent and positive view. A regressor modeling incorrect responses was also included. All regressors were convolved with a canonical hemodynamic response function (HRF) to account for the slow hemodynamic response (with time point commencing at time of first image onset). There was no significant regressor collinearity.
The participants' anatomical scans were individually registered to the Talairach and Tournoux atlas (Talairach and Tournoux, 1988). The individuals' functional EPI data were then registered to their Talairach anatomical scan within AFNI. Linear regression modeling was performed using the 10 regressors (9 condition plus incorrect responses) described earlier, plus regressors to model a first-order baseline drift function. This produced β coefficients and associated t statistics for each voxel and regressor.
The BOLD data were analyzed via a 2 (diagnosis: healthy children/adolescents, children/adolescents with ADHD) by 3 (condition: congruent, incongruent, view) by 3 (emotion: negative, positive, neutral) between-group repeated measure ANOVA. Statistical maps were created for each main effect and interaction by thresh-holding at a single-voxel p value of p < 0.005. To correct for multiple comparisons, we performed a spatial clustering operation using ClustSim with 10,000 Monte Carlo stimulations taking into account the EPI matrix covering the gray matter. This procedure yielded a minimum cluster size (19 voxels) with a map-wise false-positive probability of p < 0.05, corrected for multiple comparisons.
2.6. Context-dependent psychophysiological interaction (gPPI) analysis
Context-dependent psychophysiological interaction (gPPI) analyses were conducted to examine group differences in functional connectivity following the method described by McLaren and colleagues (McLaren et al., 2012). Our main goal was to examine group differences in functional connectivity within the executive attention network. As such, we took as a seed the strongest activated region showing a main effect to condition from the main ANOVA conducted on the BOLD response data (see Supplement Data Table 1); dorsomedial frontal cortex (coordinates: − 4.5, − 4.5, 47.5). This seed was formed by choosing the entire cluster (119 voxels) at a very conservative p-value (p = 7.0x10-13). This seed can be considered relatively unbiased by group membership as it was identified by the main effect of condition (i.e., significant activity was seen within it in both groups) rather than by the group-by-condition interaction. In addition, we took a right amygdala seed determined by the amygdala region showing a main effect of emotion (peak coordinates: 19.5, − 4.5, − 9.5). This allowed us to examine group differences in functional connectivity within the amygdala (for this area, BOLD responses were greater for emotional (negative and positive) relative to neutral stimuli; t = 3.864 & 3,997, p = 0.000). The average activation from these seed regions was extracted across the time series. Interaction regressors were created by multiplying each of these average time series with nine condition time course vectors (one for each task and emotion condition) which were coded 1 or 0 for condition and emotion present or absent. The average activation for the seeds was entered into a linear regression model along with the nine interaction regressors and 6 motion regressors. A 2 (diagnosis) by 3 (condition) x 3 (emotion) whole-brain repeated measures ANOVA was then applied to the data.
3. Results
3.1. Behavioral data
Two 2 (diagnosis: healthy children/adolescents, children/adolescents with ADHD) by 2 (task: congruent, incongruent) by 3 (emotion: negative, positive, neutral) ANOVAs were applied to the reaction time (RT) and accuracy data respectively; see Table 2. With respect to the RT data, there was a significant main effect of diagnosis [F(1, 59) = 8.09, p = 0.006]; RTs were significantly longer for children/adolescents with ADHD relative to healthy children/adolescents. There was also a significant main effects of task [F(1, 59) = 125.94, p = 0.000] and a trend for emotion [F(2,58) = 2.82, p = 0.064]; RTs were significantly longer for incongruent relative to congruent trials [t(60) = 11.41, p = < 0.001] and were longer for negative trials relative to neutral trials [t(60) = 2.28, p = 0.026; all other emotion contrasts ns]. There were no significant interactions.
Table 2.
Behavioral data (standard deviations in brackets).
| Healthy children/adolescents | Children/adolescents with ADHD | |
|---|---|---|
| RT (milliseconds) | ||
| Negative congruent | 774.24 (35.27) | 942.84 (40.92) |
| Negative incongruent | 865.41 (35.76) | 1006.93 (41.48) |
| Neutral congruent | 764.60 (35.14) | 923.83 (40.77) |
| Neutral incongruent | 860.64 (34.60) | 984.01 (40.22) |
| Positive congruent | 777.72 (35.09) | 931.90 (40.71) |
| Positive incongruent | 854.14 (33.83) | 999.13 (39.25) |
| All congruent | 772.18 (34.65) | 932.81 (40.20)a |
| All incongruent | 860.06 (34.15) | 996.69 (39.62)b |
| All negativec | 819.82 (34.84) | 974.88 (40.43) |
| All neutralc | 815.93 (34.05) | 953.93 (39.82) |
| All positive | 812.61 (34.32) | 965.51 (39.51) |
| Group⁎ | 816.12 (34.11) | 964.78 (39.58)d |
| Accuracy (percent) | ||
| Negative congruent | 71.3 (2.8) | 69.6 (3.2) |
| Negative incongruent | 66.3 (2.9) | 64.8 (3.4) |
| Neutral congruent | 72.3 (2.3) | 70.1 (2.6) |
| Neutral incongruent | 67.8 (2.9) | 69.2 (3.4) |
| Positive congruent | 73.2 (2.8) | 69.5 (3.3) |
| Positive incongruent | 65.5 (2.8) | 66.0 (3.2) |
| All congruent | 72.3 (2.3) | 69.7 (2.7) |
| All incongruent | 66.5 (2.6) | 66.7 (3.0) |
| All negative | 68.8 (2.6) | 67.2 (3.0) |
| All neutral | 70.0 (2.4) | 69.7 (2.7) |
| All positive | 69.4 (2.4) | 67.7 (2.8) |
| Group | 69.4 (2.3) | 68.2 (2.6) |
Groups differed (p < 0.05) in RT for both congruent and incongruent trials (a and b respectively). In addition, participants were slower for negative relative to neutral trials (c). Also there was overall significance group difference in RT between healthy children/adolescents and children/adolescents with ADHD (d).
p < 0.05 (difference between a and b, c and d).
With respect to accuracy data, there was a significant main effect of task [F(1, 59) = 9.03, p = 0.004]; accuracy was significantly lower for incongruent relative to congruent trials [t(60) = 3.18, p = 0.002]. However, no other main effects or interactions were significant.
3.2. Movement data
There were no significant group differences between healthy children/adolescents and children/adolescents with ADHD in movement parameters (average movement across each time point) including delta-roll, delta-pitch, delta-yaw, delta-ds, delta-dp, and delta-dl; [t(58) = 0.697–1.931, p > 0.05].
3.3. MRI data
A whole-brain 2(diagnosis)-by-3(condition)-by-3(emotion) ANOVA was applied to the BOLD data. This revealed regions, corrected for multiple comparisons, showing significant diagnosis-by-condition and diagnosis-by-condition-by-emotion interactions. Regions showing main effects of diagnosis, condition, and emotion, and condition-by-emotion interactions are presented in the Supplemental Information Section 1. No regions showed a diagnosis-by-emotion interaction that survived multiple comparison correction
3.4. Diagnosis-by-condition interaction
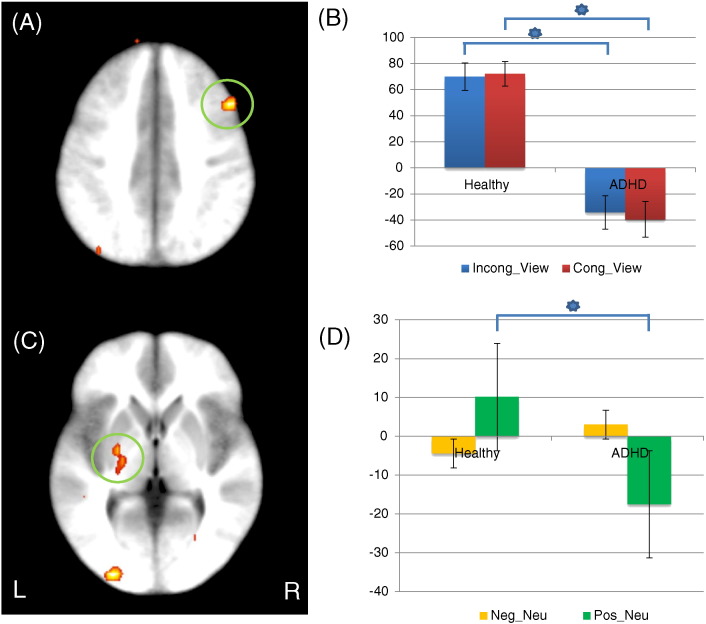
There were diagnosis-by-condition interactions in right dorsomedial frontal gyrus and left cerebellar tonsil; see Table 3. Within both regions, children/adolescents with ADHD showed significantly decreased BOLD responses to incongruent trials relative to both congruent and view trials compared to healthy children/adolescents [t(59) = 3.622/3.121 & 2.62.892, p = 0.001–0.02 respectively], but not for congruent relative to view trials [t(59) = 0.287 & 0.350, p = 0.776 & 0.727, respectively]; see Fig. 2.
Table 3.
Brain regions showing a significant interaction in comparison between healthy children/adolescents and children/adolescents with ADHD. All regions are corrected for multiple comparisons.
| Coordinates of peak activation |
|||||||
|---|---|---|---|---|---|---|---|
| Regiona | Left/Right | BA | x | y | z | F | Voxels |
| Diagnosis-by-condition | |||||||
| Dorsomedial frontal gyrus | Right | 6 | 1.5 | 1.5 | 53.5 | 8.46 | 42 |
| Cerebellar tonsil | Left | − 10.5 | − 52.5 | − 33.5 | 7.33 | 36 | |
| Diagnosis-by-condition-by-emotion | |||||||
| Superior temporal gyrus | Left | 22 | − 49.5 | − 55.5 | 17.5 | 5.65 | 33 |
| Middle temporal gyrus | Left | 22 | − 55.5 | − 37.5 | 5.5 | 6.68 | 102 |
| Middle temporal gyrus | Left | 19 | − 40.5 | − 79.5 | 20.5 | 6.47 | 31 |
| Fusiform gyrus | Left | 37 | − 37.5 | − 58.5 | − 12.5 | 6.78 | 65 |
| Fusiform gyrus | Left | 19 | − 22.5 | − 55.5 | − 6.5 | 5.46 | 51 |
| Lingual gyrus | Right | 18 | 19.5 | − 88.5 | − 9.5 | 5.12 | 35 |
| Cuneus | Left | 18 | − 7.5 | − 91.5 | 11.5 | 5.01 | 26 |
| Culmen | Left | 20 | − 31.5 | − 34.5 | − 18.5 | 6.41 | 25 |
| Culmen | Right | 10.5 | − 52.5 | − 15.5 | 6.18 | 23 | |
According to the Talairach Daemon Atlas (http://www.nitrc.org/projects/tal-daemon).
Fig. 2.
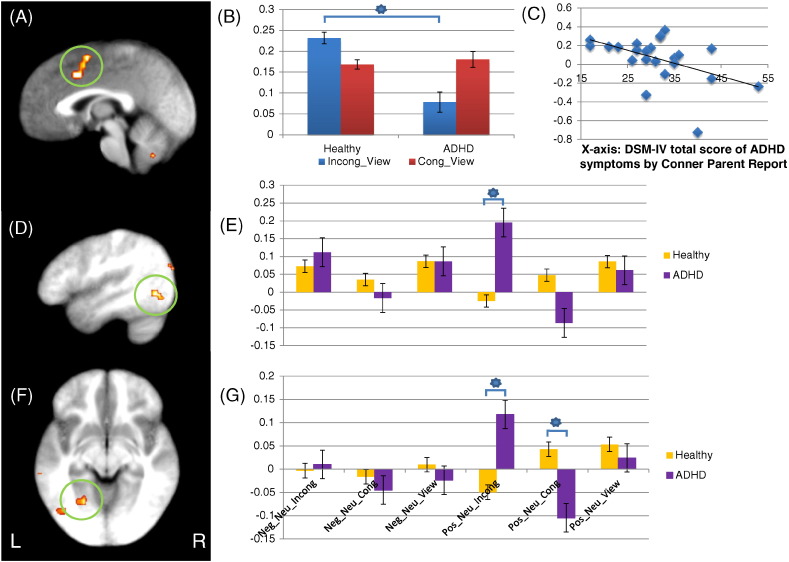
Dorsomedial frontal cortex region showing a significant diagnosis-by-condition interaction and fusiform gyri showing significant diagnosis-by-condition-by-emotion interactions. (A) Right dorsomedial frontal gyrus (coordinates: 1.5, 1.5, 53.5) showing a significant group-by-condition interaction; (B) parameter estimates for right dorsomedial frontal gyrus; (C) negative correlation between symptom severity as measured by Conner parent report scale and BOLD response to incongruent-view trials right dorsomedial frontal gyrus in patients with ADHD; (D) and (F) left fusiform gyri (coordinates: − 37.5, − 58.5, − 12.5 and − 22.5, − 55.5, − 6.5, respectively) showing a significant group-by-condition-by-emotion interaction; (E) and (G) parameter estimates for left fusiform gyri. *: regressor contrasts showing significant group differences. (B), (E), (G) Y axis — parameter estimates. Incong_View: incongruent trials — view trials; Cong_View: congruent trials — view trials; Neg_Neu_Incong: negative incongruent trials — neutral incongruent trials; Neg_Neu_Cong: negative congruent trials — neutral congruent trials; Neg_Neu_View: negative view trials — neutral view trials; Pos_Neu_Incong: positive incongruent trials — neutral incongruent trials; Pos_Neu_Cong: positive congruent trials — neutral congruent trials; Pos_Neu_View: positive view trials — neutral view trials.
3.5. Diagnosis-by-condition-by-emotion interaction
No regions showed a diagnosis-by-emotion interaction that survived multiple comparison correction (k = 22). However, there were regions that showed significant diagnosis-by-condition-by-emotion interactions including left superior temporal gyrus, left middle temporal gyri, left fusiform gyri, right lingual gyrus, left cuneus, and bilateral culmen; see Table 3. Within all these regions, children/adolescents with ADHD showed significantly greater responses to positive stimuli relative to neutral stimuli during incongruent task trials relative to healthy children [t(59) = 2.206–3.866, p = 0.000–0.031]; see Fig. 2.
The significant interactions identified above suggested that group differences were most pronounced during incongruent trials. However, it could be argued that incongruent trials should only be contrasted with congruent trials as only these trials control for motor responses. As such, we conducted a 2(diagnosis)-by-2(task: incongruent and congruent)-by-3(emotion) ANOVA (i.e., the view trials were excluded). This revealed very similar results to the first; i.e., regions, corrected for multiple comparisons, showing significant diagnosis-by-task and diagnosis-by-task-by-emotion interactions (and regions showing main effects of diagnosis, task, and emotion, and task-by-emotion interactions — presented in the Supplemental Information Section 2).
3.6. MRI results: gPPI results
Two 2 (diagnosis)-by-3(condition)-by-3(emotion) ANOVAs were conducted on the gPPI data using the seeds identified from the BOLD response ANOVA via the main effect of condition (left dorsomedial frontal cortex seed) and emotion (right amygdala). We focused on regions showing significant interactions of diagnosis with condition and diagnosis with emotion respectively.
Left dorsomedial frontal cortex seed: regions showing a significant diagnosis-by-condition interaction included right lateral frontal gyrus and left posterior insula; see Table 4. Children/adolescents with ADHD showed significantly reduced connectivity between left dorsomedial frontal gyrus and right lateral frontal gyrus relative to healthy children/adolescents during task (incongruent and congruent) relative to view trials [t = 3.498 & 3.782, p = 0.001 & 0.000, respectively]; see Fig. 3. Children and adolescents with ADHD also showed significantly increased connectivity between left dorsomedial frontal gyrus and left posterior insula relative to healthy children/adolescents during task (incongruent and congruent) relative to view trials [t = 2.715 & 2.330, p = 0.009 & 0.023].
Table 4.
Brain regions showing a significant interaction of connectivity in comparison between healthy children/adolescents and children/adolescents with ADHD. All regions are corrected for multiple comparisons except those marked with an * that were below the ClusterSim cluster size (19 voxels).
| Coordinates of peak activation |
|||||||
|---|---|---|---|---|---|---|---|
| Regiona | Left/right | BA | x | y | z | F | Voxels |
|
(A) Left lateral frontal gyrus seed Diagnosis-by-condition | |||||||
| Lateral frontal gyrus | Right | 9 | 46.5 | 16.5 | 35.5 | 9.50 | 14* |
| Claustrum | Left | − 34.5 | − 22.5 | 2.5 | 12.12 | 36 | |
|
(B) Right amygdala seed Diagnosis-by-emotion | |||||||
| Middle occipital gyrus | Right | 18 | 40.5 | − 79.5 | − 6.5 | 12.53 | 21 |
| Lentiform nucleus | Left | − 22.5 | − 7.5 | − 0.5 | 8.37 | 31 | |
According to the Talairach Daemon Atlas (http://www.nitrc.org/projects/tal-daemon).
Fig. 3.
GPPI connectivity data: (A) lateral frontal gyrus (coordinates: 46.5, 16.5, 35.5) showed a significant diagnosis-by-task interaction in connectivity with right dorsomedial frontal gyrus seed; (B) parameter estimates for lateral frontal gyrus. (C) left lentiform nucleus (coordinates: − 22.5, − 7.5, − 0.5) showing a significant diagnosis-by-emotion interaction in connectivity with right amygdala seed and; (D) parameter estimates for left lentiform nucleus. (B), (D) Y axis: parameter estimates. Incong_View: incongruent trials — view trials; Cong_View: congruent trials — view trials; Neg_Neu: negative trials — neutral trials; Pos_Neu: positive trials — neutral trials.
Right amygdala seed: regions showing significant diagnosis-by-emotion interaction included right middle occipital gyrus and left lentiform nucleus; see Table 4. For both regions, children/adolescents with ADHD showed significantly decreased connectivity with the right amygdala seed compared to healthy children/adolescents in response to positive relative to neutral stimuli [t = 3.275 & 3.581, p = 0.002 & 0.000, respectively], but not in response to negative relative to neutral stimuli [t = 1.072 & 0.599, p > 0.05]; see Fig. 3. Regions showing significant diagnosis-by-condition-by-emotion interaction included bilateral postcentral gyri; see Table 4. For both regions, children and adolescents with ADHD showed significantly decreased connectivity with right amygdala compared to healthy children/adolescents in response to positive relative to neutral stimuli during incongruent trials [t = 2.430 & 2.726, p = 0.018 & 0.008].
3.7. Correlation between BOLD response parameters and symptom severity
Six correlations were conducted to examine the relationship between BOLD responses within right dorsomedial frontal gyrus and left cerebellar tonsil to incongruent trials relative to view trials and severity of the patient's ADHD as measured by the Conner's Parent Scale for ADHD, version 2 (Conners et al., 1998). BOLD response to incongruent trials relative to view trials within dorsomedial frontal cortex correlated with both total Conner Scale score [r = − 0.480, p = 0.002] and level of hyperactivity symptoms [r = − 0.548, p = 0.007] but not with inattention. Also there was no correlation between BOLD response and symptom severity in cerebellar tonsil; see Fig. 2.
3.8. Effect of medication and comorbidity
Participants were asked to withdraw from their current stimulant medications at least 24 h prior to scanning. However, we could not rule out the long term effect of stimulant medications, as well as comorbidity with other psychiatric diagnoses. Thus, we conducted analyses excluding children/adolescents on psychotropic medications (10 stimulant cases and 1 antipsychotic) and comorbidity of substance abuse and oppositional defiant disorder (2 substance abuse cases with a very mild degree of cannabinoid exposure and 1 oppositional defiant disorder). These analyses revealed similar results to the main analysis reported above; see the Supplemental Information Sections 3 and 4.
4. Discussion
The current study investigated the dysfunction of regions implicated in executive attention control and emotional responding in children and adolescents with ADHD. There were four main results: First, compared to healthy controls, children and adolescents with ADHD showed decreased recruitment of right dorsomedial frontal gyrus under high cognitive demand (incongruent trials but not congruent trials relative to view trials). This BOLD signal decrease significantly correlated with symptom severity (total and hyperactivity) measured by Conner's Parent Report Scale in the children/adolescents with ADHD. Second, children and adolescents with ADHD showed significantly decreased connectivity between dorsomedial frontal cortex and lateral frontal gyrus under increased cognitive demand (incongruent and congruent trials relative to view trials) compared to healthy children/adolescents. Third, children/adolescents with ADHD showed increased responses to positive stimuli during incongruent task trials within temporal cortical regions. Fourth, compared to healthy children and adolescents, patients with ADHD showed decreased connectivity between the amygdala and striatum during positive relative to neutral trials.
In line with predictions, patients with ADHD showed reduced recruitment of dorsomedial frontal cortex relative to the comparison group during incongruent task trials. These data support suggestions that the neural circuitry underlying executive attention is disrupted in ADHD (De La Fuente et al., 2013, Rapport et al., 2013, Sonuga-Barke et al., 2008). Moreover, they are consistent with previous studies of ADHD that have reported decreased activity in patients with ADHD within this area (Schneider et al., 2010). Indeed, recent meta-analyses of the literature on ADHD revealed consistent hypo-activation within proximal regions in patients with ADHD relative to comparison individuals (Cortese et al., 2012-peak coordinates: − 0.35, 15.56, 48.61; McCarthy et al., 2013-peak coordinates: − 10, 4, 54; coordinates in current study: 1.5, 1.5, 53.5). Notably, the extent of the BOLD signal decrease in response to incongruent trials in dorsomedial frontal gyrus correlated with both total symptom and hyperactivity scores measured by the Conner's Parent Scale. These data support suggestions that the reduced ability to recruit this region relates to the development of ADHD.
On the basis of the executive attention dysfunction account (De La Fuente et al., 2013, Rapport et al., 2013, Sonuga-Barke et al., 2008), we additionally predicted that the patients with ADHD would show reduced recruitment of lateral frontal cortex. Previous work has reported that patients with ADHD show decreased activity in lateral PFC in the context of executive or sustained attention tasks (Banich et al., 2009, Christakou et al., 2013, Rubia et al., 2009, Schneider et al., 2010). This was not seen in the current study (though task performance shows considerable recruitment of lateral PFC; see Supplemental Information Table 1). However, it is notable that our gPPI analysis indicated reduced connectivity between dorsomedial frontal cortex and right lateral PFC during task trials in the patients with ADHD relative to the healthy youth. Moreover, recent meta-analyses revealed consistent hypo-activation in patients with ADHD within proximal regions relative to comparison individuals (Cortese et al., 2012-coordinates: 42.23, 9.52, 29.36; McCarthy et al., 2013-coordinates: 46, 22, 30; coordinates in current study: 46.5, 16.5, 35.5). These data indicate a relative failure during task performance in the integrated functioning of these regions implicated in executive attention in the patients with ADHD.
On the assumption that patients with ADHD would show deficient recruitment of regions implicated in executive attention and consequent failure of priming of representation of task-relevant stimuli, we predicted that the patients with ADHD would show heightened responding to emotional stimuli during task trials (i.e., reduced emotion regulation). This was partly affirmed by increased BOLD responses to positive (though not negative) stimuli relative to neutral stimuli during task (incongruent) trials in areas implicated in stimulus representation (Desimone and Duncan, 1995); e.g., middle temporal gyri, fusiform gyri and lingual gyrus. Of course, it should be noted that the heightened activity within these regions during incongruent trials was only seen for positive stimuli. This was unpredicted. There have been previous reports of increased responses to positive (but not negative) stimuli in patients with ADHD (Posner et al., 2011). Moreover, patients with ADHD may have increased difficulty relative to comparison individuals regulating positive emotions (Musser et al., 2013). However, our finding of a valence specific increased responsiveness is inconsistent with general problems in emotion regulation. General problems in emotion regulation should result in increased responsiveness to positive and negative stimuli.
There are several previous studies suggesting that ADHD is uniquely associated with disruptions in positive emotion or approach systems (Karalunas et al., 2014, Martel, 2009, Musser et al., 2011, Nigg, 2006). It should also be noted that the patients with ADHD showed decreased connectivity between the amygdala and striatum (lentiform nucleus). This result is also unlikely to be a secondary consequence of executive attention dysfunction, considering this was observed as a diagnosis-by-emotion interaction; i.e., the result reflects heightened integration of response to emotional stimuli generally rather than only under task trials (as would be predicted by a failure of executive attention related emotion regulation). In short, the current results did not support the suggestion of reduced emotion regulation in patients with ADHD at least with respect to emotion regulation via executive attention.
Striatum has been a main focus of interest regarding the pathophysiology of ADHD for some time (Del Campo et al., 2013, Plichta and Scheres, 2014, Valera et al., 2007). Previous studies have reported reduced lentiform nucleus volume in patients with ADHD relative to comparison youth (Nakao et al., 2011), as well as decreased activity in regard to motor response inhibition (Durston et al., 2003, Rubia et al., 1999), or selective sustained attention (Rubia et al., 2009); (see also recent meta-analyses; Cortese et al., 2012, McCarthy et al., 2013). The current data also indicate atypical striatal functioning in patients with ADHD, specifically with respect to its interactions with the amygdala in response to positive emotional stimuli.
Finally, we found both a main effect of emotion within the amygdala (all participants showed greater responses to emotional relative to neutral stimuli) and many regions showed a main effect of emotion for the gPPI connectivity data (there was only a group-by-emotion interaction within lentiform nucleus; see Supplemental Information Section 5). These data also suggest that despite evidence of impaired recruitment and functioning of systems involved in executive attention, emotion regulation in this task was not disrupted.
The results of the current study can be contrasted with those of Passarotti et al. (2010a) and Posner et al. (2011) who used variants of the superficially similar emotional Stroop task. Distracting emotional information in the emotional Stroop task is a component of the task relevant stimulus (the words to be counted are emotional or the word whose hue is to be named is emotional). In contrast, in the affective Stroop task the emotional distracters serve as representational competitors for the target stimulus; they are images that are temporally distinct from the target stimulus. Emotional Stroop tasks have been associated with activity in more rostral and ventral regions of medial frontal cortex (Bush, 2010) and Passarotti et al. (2010a) and Posner et al. (2011) reported dysfunction in patients with ADHD in these more ventromedial regions (decreased activity relative to controls in response to negative distracters and increased in response to positive distracters). In contrast, performance on the affective Stroop task is reliant on more dorsal regions of medial frontal cortex (Blair et al., 2007). It was dysfunction in these regions in the patients with ADHD that was seen here.
There are several potential reasons for the differences in results between the current study and those of Passarotti et al. (2010a) and Posner et al. (2011). First, it is possible that the stimulus properties of the emotional Stroop (where the emotional distracters are also the target stimuli) recruit ventral prefrontal emotion regulation systems while those of the affective Stroop (where the emotional distracters are temporally separated from the target stimuli) rely on more dorsal prefrontal (both dorsomedial and lateral frontal) regions. If the tasks recruit different regions, it is to be expected that they would reveal different results as a function of diagnosis. Second, and perhaps more usefully, it can be noted that a critical contrast for the affective Stroop task concerned the differential recruitment of dorsomedial frontal cortex during incongruent and congruent trials. In contrast, the critical contrast in the emotional Stroop studies has concerned the differential recruitment of regions in the presence of negative (and in an additional contrast positive) relative to neutral distracters (Passarotti et al., 2010a, Posner et al., 2011). Importantly, in the current task incongruent trials involved distracters that were response competitors for the target stimuli; i.e., three 4 s involved the representation of 4 which might be the target stimulus on the next trial. In contrast, the emotional distracters in the emotional Stroop task (or in the affective Stroop task) are not response competitors — they are not associated with responses on the tasks themselves. Considerable fMRI work with the Stroop task attests to dorsomedial frontal cortex in mediating the interference effect induced by distractors (Mayer et al., 2012, Song and Hakoda, 2015, Weissman and Carp, 2013) and, consistent with the current study, the disrupted recruitment of this region in children/adolescents with ADHD when performing classic Stroop tasks (Cortese et al., 2012).
In this regard, the contrasts examined by Passarotti et al. (2010a) and Posner et al. (2011) are more similar to our group-by-emotion and group-by-condition-by-emotion interactions. And here there is some loose similarity between our findings and the previous two studies. Both of these studies report increased responses in vmPFC to positive stimuli and decreased responses within vmPFC to negative stimuli in patients with ADHD relative to comparison youth. Both studies interpreted these findings in terms of deficient emotion regulation. However, this is a reverse inference; no direct evidence was provided that the recruitment seen reflected emotion regulation (and indeed it is unclear why patients with ADHD should show increased/deficient regulation as a function of stimulus valence). Instead, it is plausible that it reflected representation of the value of the emotional distracters. There is considerable evidence that vmPFC is involved in the representation of stimulus value showing increased responses for positive stimuli and decreased responses for negative stimuli (for a recent review, see; Clithero and Rangel, 2014). From this view, the Passarotti et al. (2010a) and Posner et al. (2011) findings would reflect enhanced responses to positive and negative stimuli in the patients with ADHD. In the current study, we also found evidence of enhanced responses to positive stimuli in the patients with ADHD (albeit during task trials and within temportal and fusiform gyri). As such, the findings of these three studies may be somewhat more similar than initially appears.
Seven caveats should be considered with respect to the current data. First, 11 of our 26 participants with ADHD were receiving medication. While participants were instructed to withhold their stimulant medications for at least one day prior to scanning, medication status could have influenced the current data. Mitigating this concern are our results for the treatment naïve patients with ADHD. These individuals showed comparable results to the group of children and adolescents with ADHD as a whole (see the Supplemental Information Section 3). Second, 2 of our participants with ADHD were comorbid for substance abuse disorder, and 1 for oppositional defiant disorder. However, it should be noted that a re-analysis of the data excluding these three participants had a minimal impact of the results (see the Supplemental Information Section 4). In short, the current results do not appear to be a product of comorbid substance abuse disorder. Third, our careful exclusion of patients who were comorbid for both other externalizing and internalizing conditions means that the current results may not generalize to the entire population of individuals diagnosed with ADHD (where such comorbidities are common) (American Psychiatric Association, 2013). However, importantly, these data identify pathology that is specifically associated with ADHD symptomatology and not related to the other diagnoses of behavior disorders (such as Conduct Disorder or Oppositional Defiant Disorder). Fourth, while the diagnostic groups did not significantly differ on gender, it is possible that gender differences had an impact. Mitigating this concern, follow up ANCOVA analyses of the functional data with gender as a covariate revealed no significant interactions of gender with group on BOLD response within any of the regions under consideration. In addition, we conducted a gender (female and male)-by-condition-by-emotion ANOVA on the BOLD response data. This revealed regions showing a main effect of gender and significant gender-by-emotion interactions (see Supplemental Information Section 6). However, none of the regions identified overlapped with those identified as relevant to ADHD in this paper. As such, gender does not to appear to have a significant impact on the results reported here. Fifth, it is possible that the significant group differences in RT might relate to the group differences in BOLD response within dorsomedial frontal cortex. To address this issue, we conducted an additional diagnosis-by-condition-by-emotion ANCOVA using the participant's average reaction time as a covariate (see Supplemental Information Section 7). This ANCOVA revealed a very similar region of dorosomedial frontal gyrus (coordinates: 1.5, 1.5, 53.5, 46 voxels) showing a group-by-condition interaction. In short, it is unlikely that the obtained result within dorsomedial frontal cortex can be attributed to reactive time differences between the groups. Sixth, the valence ratings of the images were based on established norms (Lang et al., 2005) rather than the participant's self-reports. There might be group differences in level of experienced emotion. Of course, this might reflect the patient's pathology; i.e., as such controlling for self-reported levels of valence would control for level of pathology. Seventh, it is important to note that while the current data indicate that patients with ADHD as a group do not show impairment in the form of emotion regulation studied here, this does not mean that all patients with ADHD do not show this impairment. Recent work has argued that patients with ADHD can be subtyped into three different forms according to their emotional responsiveness (Karalunas et al., 2014). It is possible that, for example, the irritable subtype identified in the Karalunas et al. (2014) study might show executive attention based emotion regulation impairment.
In conclusion, the current results support suggestions of disrupted recruitment of regions implicated in executive attention (dorsomedial and lateral frontal cortices) in children and adolescents with ADHD, particularly in the presence of more difficult task demands (incongruent trials). Moreover, they support suggestions of exaggerated emotional responses in patients with ADHD at least to positive stimuli. Future study is warranted to further elucidate the interaction between executive function and emotion dysregulation in ADHD.
Financial disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
Acknowledgments
This work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health under grant number 1-ZIA-MH002860-08 to Dr. Blair. Ethics approval for this study was granted by the NIH Combined Neuroscience Institutional Review Board under protocol number 05-M-0105.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2015.10.005.
Appendix A. Supplementary data
Supplementary material.
References
- Arnsten A.F., Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51(4):356–367. doi: 10.1016/j.jaac.2012.01.008. (doi: S0890-8567(12)00043-3 [pii]) [DOI] [PubMed] [Google Scholar]
- Association, A. P. American Psychiatric Association; Washington D.C.: 2013. Diagnostic and Statistical Manual 5. [Google Scholar]
- Banich M.T., Burgess G.C., Depue B.E., Ruzic L., Bidwell L.C., Hitt-Laustsen S., …, Willcutt E.G. The neural basis of sustained and transient attentional control in young adults with ADHD. Neuropsychologia. 2009;47(14):3095–3104. doi: 10.1016/j.neuropsychologia.2009.07.005. (doi: S0028-3932(09)00298-X [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley R.A., Fischer M. The unique contribution of emotional impulsiveness to impairment in major life activities in hyperactive children as adults. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49(5):503–513. doi: 10.1097/00004583-201005000-00011. (doi: 00004583-201005000-00011 [pii]) [DOI] [PubMed] [Google Scholar]
- Blair K.S., Smith B.W., Mitchell D.G., Morton J., Vythilingam M., Pessoa L., …, Blair R.J. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35(1):430–440. doi: 10.1016/j.neuroimage.2006.11.048. (doi: S1053-8119(06)01117-7 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman M.A., Rich B.A., Guyer A.E., Lunsford J.R., Horsey S.E., Reising M.M., …, Leibenluft E. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. Am. J. Psychiatry. 2010;167(1):61–69. doi: 10.1176/appi.ajp.2009.09010043. (doi: appi.ajp.2009.09010043 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G. Attention-deficit/hyperactivity disorder and attention networks. Neuropsychopharmacology. 2010;35(1):278–300. doi: 10.1038/npp.2009.120. (doi: npp2009120 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G., Frazier J.A., Rauch S.L., Seidman L.J., Whalen P.J., Jenike M.A., …, Biederman J. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop. Biol. Psychiatry. 1999;45(12):1542–1552. doi: 10.1016/s0006-3223(99)00083-9. (doi: S0006322399000839 [pii]) [DOI] [PubMed] [Google Scholar]
- Christakou A., Murphy C.M., Chantiluke K., Cubillo A.I., Smith A.B., Giampietro V., …, Rubia K. Disorder-specific functional abnormalities during sustained attention in youth with attention deficit hyperactivity disorder (ADHD) and with autism. Mol. Psychiatry. 2013;18(2):236–244. doi: 10.1038/mp.2011.185. (doi: mp2011185 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clithero J.A., Rangel A. Informatic parcellation of the network involved in the computation of subjective value. Soc. Cogn. Affect. Neurosci. 2014;9(9):1289–1302. doi: 10.1093/scan/nst106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners C.K., Sitarenios G., Parker J.D., Epstein J.N. The revised Conners' Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J. Abnorm. Child Psychol. 1998;26(4):257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- Cortese S., Kelly C., Chabernaud C., Proal E., Di Martino A., Milham M.P., Castellanos F.X. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am. J. Psychiatry. 2012;169(10):1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Fuente A., Xia S., Branch C., Li X. A review of attention-deficit/hyperactivity disorder from the perspective of brain networks. Front. Hum. Neurosci. 2013;7:192. doi: 10.3389/fnhum.2013.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Campo N., Fryer T.D., Hong Y.T., Smith R., Brichard L., Acosta-Cabronero J., …, Muller U. A positron emission tomography study of nigro-striatal dopaminergic mechanisms underlying attention: implications for ADHD and its treatment. Brain. 2013;136(Pt 11):3252–3270. doi: 10.1093/brain/awt263. (doi: awt263 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R., Duncan J. Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Durston S., Tottenham N.T., Thomas K.M., Davidson M.C., Eigsti I.M., Yang Y., …, Casey B.J. Differential patterns of striatal activation in young children with and without ADHD. Biol. Psychiatry. 2003;53(10):871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- Erthal F.S., de Oliveira L., Mocaiber I., Pereira M.G., Machado-Pinheiro W., Volchan E., Pessoa L. Load-dependent modulation of affective picture processing. Cogn. Affect. Behav. Neurosci. 2005;5(4):388–395. doi: 10.3758/cabn.5.4.388. [DOI] [PubMed] [Google Scholar]
- Etkin A., Prater K.E., Hoeft F., Menon V., Schatzberg A.F. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am. J. Psychiatry. 2010;167(5):545–554. doi: 10.1176/appi.ajp.2009.09070931. (doi: appi.ajp.2009.09070931 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano P.A., McNamara J.P., Geffken G.R., Reid A.M. Differentiating co-occurring behavior problems in children with ADHD: patterns of emotional reactivity and executive functioning. J. Atten. Disord. 2013;17(3):249–260. doi: 10.1177/1087054711428741. [DOI] [PubMed] [Google Scholar]
- Gyurak A., Gross J.J., Etkin A. Explicit and implicit emotion regulation: a dual-process framework. Cogn. Emot. 2011;25(3):400–412. doi: 10.1080/02699931.2010.544160. (doi: 933887834 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H., Radua J., Nakao T., Mataix-Cols D., Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70(2):185–198. doi: 10.1001/jamapsychiatry.2013.277. (doi: 1485446 [pii]) [DOI] [PubMed] [Google Scholar]
- Herrmann M.J., Biehl S.C., Jacob C., Deckert J. Neurobiological and psychophysiological correlates of emotional dysregulation in ADHD patients. Atten. Defic. Hyperact. Disord. 2010;2(4):233–239. doi: 10.1007/s12402-010-0047-6. [DOI] [PubMed] [Google Scholar]
- Kalisch R. The functional neuroanatomy of reappraisal: time matters. Neurosci. Biobehav. Rev. 2009;33(8):1215–1226. doi: 10.1016/j.neubiorev.2009.06.003. (doi: S0149-7634(09)00083-9 [pii]) [DOI] [PubMed] [Google Scholar]
- Karalunas S.L., Fair D., Musser E.D., Aykes K., Iyer S.P., Nigg J.T. Subtyping attention-deficit/hyperactivity disorder using temperament dimensions: toward biologically based nosologic criteria. JAMA Psychiatry. 2014;71(9):1015–1024. doi: 10.1001/jamapsychiatry.2014.763. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P., …, Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Lang P.J., B. M., Cuthbert B.N. University of Florida; Gainesville, FL: 2005. International Affective Picture System (IAPS): Affetive Ratings of Pictures and Instruction Manual. [Google Scholar]
- Maier S.J., Szalkowski A., Kamphausen S., Feige B., Perlov E., Kalisch R., …, van Elst L.T. Altered cingulate and amygdala response towards threat and safe cues in attention deficit hyperactivity disorder. Psychol. Med. 2013:1–14. doi: 10.1017/S0033291713000469. (doi: S0033291713000469 [pii]) [DOI] [PubMed] [Google Scholar]
- Marsh A.A., Finger E.C., Mitchell D.G., Reid M.E., Sims C., Kosson D.S., …, Blair R.J. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am. J. Psychiatry. 2008;165(6):712–720. doi: 10.1176/appi.ajp.2007.07071145. (doi: appi.ajp.2007.07071145 [pii]) [DOI] [PubMed] [Google Scholar]
- Martel M.M. Research review: a new perspective on attention-deficit/hyperactivity disorder: emotion dysregulation and trait models. J. Child Psychol. Psychiatry. 2009;50(9):1042–1051. doi: 10.1111/j.1469-7610.2009.02105.x. [DOI] [PubMed] [Google Scholar]
- Mayer A.R., Teshiba T.M., Franco A.R., Ling J., Shane M.S., Stephen J.M., Jung R.E. Modeling conflict and error in the medial frontal cortex. Hum. Brain Mapp. 2012;33(12):2843–2855. doi: 10.1002/hbm.21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy H., Skokauskas N., Frodl T. Identifying a consistent pattern of neural function in attention deficit hyperactivity disorder: a meta-analysis. Psychol. Med. 2013:1–12. doi: 10.1017/S0033291713001037. (doi: S0033291713001037 [pii]) [DOI] [PubMed] [Google Scholar]
- McLaren D.G., Ries M.L., Xu G., Johnson S.C. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61(4):1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. (doi: S1053-8119(12)00349-7 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D.G., Richell R.A., Leonard A., Blair R.J. Emotion at the expense of cognition: psychopathic individuals outperform controls on an operant response task. J. Abnorm. Psychol. 2006;115(3):559–566. doi: 10.1037/0021-843X.115.3.559. (doi: 2006-09167-017 [pii]) [DOI] [PubMed] [Google Scholar]
- Mitchell D.G., Nakic M., Fridberg D., Kamel N., Pine D.S., Blair R.J. The impact of processing load on emotion. Neuroimage. 2007;34(3):1299–1309. doi: 10.1016/j.neuroimage.2006.10.012. (doi: S1053-8119(06)01038-X [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D.G., Luo Q., Mondillo K., Vythilingam M., Finger E.C., Blair R.J. The interference of operant task performance by emotional distracters: an antagonistic relationship between the amygdala and frontoparietal cortices. Neuroimage. 2008;40(2):859–868. doi: 10.1016/j.neuroimage.2007.08.002. (doi: S1053-8119(07)00701-X [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser E.D., Backs R.W., Schmitt C.F., Ablow J.C., Measelle J.R., Nigg J.T. Emotion regulation via the autonomic nervous system in children with attention-deficit/hyperactivity disorder (ADHD) J. Abnorm. Child Psychol. 2011;39(6):841–852. doi: 10.1007/s10802-011-9499-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser E.D., Galloway-Long H.S., Frick P.J., Nigg J.T. Emotion regulation and heterogeneity in attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52(2):163–171. doi: 10.1016/j.jaac.2012.11.009. (e162. doi: S0890-8567(12)00875-1 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao T., Radua J., Rubia K., Mataix-Cols D. Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am. J. Psychiatry. 2011;168(11):1154–1163. doi: 10.1176/appi.ajp.2011.11020281. [DOI] [PubMed] [Google Scholar]
- Nigg J.T. Temperament and developmental psychopathology. J. Child Psychol. Psychiatry. 2006;47(3–4):395–422. doi: 10.1111/j.1469-7610.2006.01612.x. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. The cognitive control of emotion. Trends Cogn. Sci. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. (doi: S1364-6613(05)00090-2 [pii]) [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Bunge S.A., Gross J.J., Gabrieli J.D. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J. Cogn. Neurosci. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Passarotti A.M., Sweeney J.A., Pavuluri M.N. Differential engagement of cognitive and affective neural systems in pediatric bipolar disorder and attention deficit hyperactivity disorder. J. Int. Neuropsychol. Soc. 2010;16(1):106–117. doi: 10.1017/S1355617709991019. (doi: S1355617709991019 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti A.M., Sweeney J.A., Pavuluri M.N. Emotion processing influences working memory circuits in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49(10):1064–1080. doi: 10.1016/j.jaac.2010.07.009. (doi: S0890-8567(10)00563-0 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L., McKenna M., Gutierrez E., Ungerleider L.G. Neural processing of emotional faces requires attention. Proc. Natl. Acad. Sci. U. S. A. 2002;99(17):11458–11463. doi: 10.1073/pnas.172403899. (172403899 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L., Padmala S., Morland T. Fate of unattended fearful faces in the amygdala is determined by both attentional resources and cognitive modulation. Neuroimage. 2005;28(1):249–255. doi: 10.1016/j.neuroimage.2005.05.048. (doi: S1053-8119(05)00363-0 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol. Psychiatry. 2003;54(5):515–528. doi: 10.1016/s0006-3223(03)00171-9. (doi: S0006322303001719 [pii]) [DOI] [PubMed] [Google Scholar]
- Plichta M.M., Scheres A. Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta-analytic review of the fMRI literature. Neurosci. Biobehav. Rev. 2014;38:125–134. doi: 10.1016/j.neubiorev.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J., Maia T.V., Fair D., Peterson B.S., Sonuga-Barke E.J., Nagel B.J. The attenuation of dysfunctional emotional processing with stimulant medication: an fMRI study of adolescents with ADHD. Psychiatry Res. 2011;193(3):151–160. doi: 10.1016/j.pscychresns.2011.02.005. (doi: S0925-4927(11)00071-0 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J., Rauh V., Gruber A., Gat I., Wang Z., Peterson B.S. Dissociable attentional and affective circuits in medication-naive children with attention-deficit/hyperactivity disorder. Psychiatry Res. 2013;213(1):24–30. doi: 10.1016/j.pscychresns.2013.01.004. (doi: S0925-4927(13)00022-X [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapport M.D., Orban S.A., Kofler M.J., Friedman L.M. Do programs designed to train working memory, other executive functions, and attention benefit children with ADHD? A meta-analytic review of cognitive, academic, and behavioral outcomes. Clin. Psychol. Rev. 2013 doi: 10.1016/j.cpr.2013.08.005. (doi: S0272-7358(13)00121-9 [pii]) [DOI] [PubMed] [Google Scholar]
- Reimherr F.W., Marchant B.K., Strong R.E., Hedges D.W., Adler L., Spencer T.J., …, Soni P. Emotional dysregulation in adult ADHD and response to atomoxetine. Biol. Psychiatry. 2005;58(2):125–131. doi: 10.1016/j.biopsych.2005.04.040. (doi: S0006-3223(05)00501-9 [pii]) [DOI] [PubMed] [Google Scholar]
- Rubia K., Overmeyer S., Taylor E., Brammer M., Williams S.C., Simmons A., Bullmore E.T. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. Am. J. Psychiatry. 1999;156(6):891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- Rubia K., Halari R., Cubillo A., Mohammad A.M., Brammer M., Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naive children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57(7–8):640–652. doi: 10.1016/j.neuropharm.2009.08.013. (doi: S0028-3908(09)00279-2 [pii]) [DOI] [PubMed] [Google Scholar]
- Schneider M.F., Krick C.M., Retz W., Hengesch G., Retz-Junginger P., Reith W., Rosler M. Impairment of fronto-striatal and parietal cerebral networks correlates with attention deficit hyperactivity disorder (ADHD) psychopathology in adults — a functional magnetic resonance imaging (fMRI) study. Psychiatry Res. 2010;183(1):75–84. doi: 10.1016/j.pscychresns.2010.04.005. (doi: S0925-4927(10)00118-6 [pii]) [DOI] [PubMed] [Google Scholar]
- Schulz K.P., Himelstein J., Halperin J.M., Newcorn J.H. Neurobiological models of attention-deficit/hyperactivity disorder: a brief review of the empirical evidence. CNS Spectr. 2000;5(6):34–44. doi: 10.1017/s1092852900007057. [DOI] [PubMed] [Google Scholar]
- Shaw P., Stringaris A., Nigg J., Leibenluft E. Emotion dysregulation in attention deficit hyperactivity disorder. Am. J. Psychiatry. 2014 doi: 10.1176/appi.ajp.2013.13070966. (doi: 1827806 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobanski E., Banaschewski T., Asherson P., Buitelaar J., Chen W., Franke B., …, Faraone S.V. Emotional lability in children and adolescents with attention deficit/hyperactivity disorder (ADHD): clinical correlates and familial prevalence. J. Child Psychol. Psychiatry. 2010;51(8):915–923. doi: 10.1111/j.1469-7610.2010.02217.x. (doi: JCPP2217 [pii]) [DOI] [PubMed] [Google Scholar]
- Song Y., Hakoda Y. An fMRI study of the functional mechanisms of Stroop/reverse-Stroop effects. Behav. Brain Res. 2015;290:187–196. doi: 10.1016/j.bbr.2015.04.047. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E.J. Psychological heterogeneity in AD/HD—a dual pathway model of behaviour and cognition. Behav. Brain Res. 2002;130(1–2):29–36. doi: 10.1016/s0166-4328(01)00432-6. (doi: S0166432801004326 [pii]) [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E.J., Sergeant J.A., Nigg J., Willcutt E. Executive dysfunction and delay aversion in attention deficit hyperactivity disorder: nosologic and diagnostic implications. Child Adolesc. Psychiatr. Clin. N. Am. 2008;17(2):367–384. doi: 10.1016/j.chc.2007.11.008. (ix. doi: S1056-4993(07)00122-8 [pii]) [DOI] [PubMed] [Google Scholar]
- Talairach, Tournoux . Thieme; Stuttgart: 1988. Co-Planar Stereotaxic Atlas of the Human Brain. [Google Scholar]
- Valera E.M., Faraone S.V., Murray K.E., Seidman L.J. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2007;61(12):1361–1369. doi: 10.1016/j.biopsych.2006.06.011. (doi: S0006-3223(06)00803-1 [pii]) [DOI] [PubMed] [Google Scholar]
- Wechsler D. Psychological Corporation; San Antonio, TX: 1999. Wechsler Abbreviated Scale of Intelligence. [Google Scholar]
- Weissman D.H., Carp J. The congruency effect in the posterior medial frontal cortex is more consistent with time on task than with response conflict. PLoS One. 2013;8(4):e62405. doi: 10.1371/journal.pone.0062405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.