Abstract
Background
Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis (NASH) are serious conditions and are being diagnosed at an increased rate. The etiology of these hepatic disorders is not clear but involves insulin resistance and oxidative stress. Remogliflozin etabonate (Remo) is an inhibitor of the sodium glucose-dependent renal transporter 2 (SGLT2), and improves insulin sensitivity in type 2 diabetics. In the current study, we examined the effects of Remo in a diet-induced obese mouse model of NAFLD.
Methods
After 11-weeks on High-Fat-Diet 32 (HFD32), C57BL/6J mice were obese and displayed characteristics consistent with NAFLD. Cohorts of obese animals were continued on HFD32 for an additional 4-week treatment period with or without Remo.
Results
Treatment with Remo for 4 weeks markedly lowered both plasma alanine aminotransferase (76%) and aspartate aminotransferase (48%), and reduced both liver weight and hepatic triglyceride content by 42% and 40%, respectively. Remo also reduced hepatic mRNA content for tumor necrosis factor (TNF)-α (69%), and monocyte chemoattractant protein (MCP)-1 (69%). The diet-induced increase in thiobarbituric acid-reactive substances, a marker of oxidative stress, was reduced following treatment with Remo, as measured in both liver homogenates (22%) and serum (37%). Finally, the oxygen radical absorbance capacity (ORAC) in three different SGLT2 inhibitors was determined: remogliflozin, canagliflozin and dapagliflozin. Only remogliflozin had any significant ORAC activity.
Conclusions
Remo significantly improved markers associated with NAFLD in this animal model, and may be an effective compound for the treatment of NASH and NAFLD due to its insulin-sensitizing and antioxidant properties.
Keywords: hepatic steatosis, NAFLD, NASH, obesity, SGLT2
Abbreviations: AAPH, 2,2′-azobis-2-methyl-propanimidamide dihydrochloride; ALT, Alanine aminotransferase; AST, aspartate aminotransferase; DIO, Diet-induced obesity; ER, Endoplasmic reticulum; FFA, Free fatty acids; FXR, Farnesoid X receptor; HFD32, High fat diet 32; MCP-1, Monocyte chemoattractant protein-1; NAFLD, Nonalcoholic fatty liver disease; NASH, Nonalcoholic steatohepatitis; ORAC, Oxygen radical absorbance capacity; Remo, Remogliflozin etabonate; ROS, Reactive oxygen species; SGLT2, sodium glucose-dependent renal transporter 2; TBARS, Thiobarbituric acid-reactive substances; TG, Triglyceride; TNF-α, Tumor necrosis factor alpha; Trolox, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid
Non-alcoholic fatty liver disease (NAFLD) is a chronic hepatic disorder that affects 25% of the population; including a significant portion of pediatric cases.1 NAFLD can lead to non-alcoholic steatohepatitis (NASH), a more severe condition that includes damage such as fibrosis. Major risk factors for NAFLD include those for metabolic syndrome, especially obesity and insulin resistance.1 While there are clinical trials investigating the use of anti-diabetic therapies,2 antioxidants are also being pursued,3 as well as farnesoid X receptor (FXR) agonists, such as the bile acid analog obeticholic acid.4
Although the exact mechanisms underlying progression of NAFLD are unclear, it likely involves an early lipotoxic response due to hepatic steatosis. The intrahepatic accumulation of free fatty acids (FFA) induces hepatic insulin resistance and both oxidative and endoplasmic reticulum (ER) stress, culminating in decreased hepatic function, apoptosis and fibrosis.5 Elevated concentrations of intracellular FFA are accompanied by numerous alterations in the expression of genes involved in lipid metabolism, leading to increased de novo lipid synthesis and a concomitant decrease in secretion of FFA.6, 7, 8, 9, 10, 11, 12, 13, 14 Chronic exposure to fatty acids also induces ER stress, promoting elevated expression of the ER chaperone Grp78, increased phosphorylation of both eIF2α and PERK, activation of caspase-3, as well as induction of other pro-apoptotic genes and stress-activated transcription factors.15, 16, 17, 18, 19 Oxidative stress is also activated by FFA and is associated with a decline in glutathione, reduced superoxide dismutase activity, elevated lipid peroxidation and increased generation of reactive oxygen species (ROS).13 Thus, it is likely that insulin resistance and cellular stress, resulting from hepatic steatosis, act collectively to promote NAFLD, a pro-fibrotic state and progression to NASH.
Inhibition of the renal specific sodium/glucose transporter 2 (SGLT2) is a contemporary therapeutic approach for the treatment of type 2 diabetes.20 The mechanism of action for this class of compounds is to inhibit the reabsorption of urinary glucose, resulting in excretion of glucose and lowering of plasma glucose. Remogliflozin etabonate (Remo) is a novel inhibitor of SGLT2. The efficacy of Remo in the treatment of type 2 diabetes has been established in several phase II clinical trials.21, 22, 23, 24, 25, 26 Remo and other compounds in this class are effective in improving glucose homeostasis, insulin sensitivity, and beta cell function, as well as reducing body weight.27, 28, 29 In this study, we have examined the potential benefits of Remo on hepatic steatosis and associated parameters of NAFLD in a diet-induced obese (DIO) mouse model.
Methods
Drugs
Remogliflozin etabonate (4-[(4-isopropoxyphenyl)-methyl]-1-isopropyl-5-methyl-1H-pyrazol-3-yl 6-O-ethoxycarbonyl-β-d-glucopyranoside) was synthesized by Kissei Pharmaceutical Co. Ltd. (Matumoto, Japan). Bezafibrate was obtained from Chugai Pharmaceutical Co. Ltd. (Tokyo, Japan).
Vertebrate Animals
All animal experiments were conducted in accordance with the guidelines for animal care and welfare issued by Kissei Pharmaceutical Co. Ltd., which have been approved by The Japanese Pharmacological Society. C57BL/6J mice (4-weeks old) were housed individually with free access to water. The mice were fed a diet of normal chow or High-Fat-Diet 32 (HFD32; Clea, Japan) for 11-weeks. HFD32 contains 25% protein, 29% carbohydrate and 32% fat (with saturated, monounsaturated and polyunsaturated fatty acids at 7, 22 and 4 g/100 g chow, respectively). HFD32 has a caloric value of 507 kcal/100 g. The mice (15 weeks of age) were then randomly divided into 6–7 mice/group. These groups were maintained for 4-weeks on the following diets: group 1 (n = 7) was maintained continuously on a normal diet (Normal); group 2 (n = 7) was given HFD32 (Control group); group 3 (n = 7) received HFD32 supplemented with 0.01% (wt/wt) Remo; group 5 (n = 7), HFD32 with 0.03% Remo; and group 7 (n = 6), HFD32 with 0.2% bezafibrate. The animals receiving Remo ate slightly more than the untreated Control group. Thus, based on the average daily food consumption in the Control group, group 4 and group 6 (n = 7 each) were pair-fed (PF), receiving an equal amount of HFD32 chow as Control group, but containing either 0.01% Remo (group 4) or 0.03% Remo (group 6). Average daily drug intake was determined by multiplying drug concentration in chow by food consumption. Control and Control-PF groups are mice maintained on HFD32, but not treated with Remo.
Glucose, Alanine aminotransferase (ALT) and Aspartate Aminotransferase (AST) Levels
Blood samples were collected from a tail vein into a heparinized hematocrit tube. Plasma ALT, AST and glucose levels were measured by using commercially available kits (Transaminase C-II test, Glucose C-II test; Wako Pure Chemical Industries, Osaka, Japan).
Liver Weights and Triglyceride (TG) Content
At the end of the treatment period liver tissue was removed, weighed and stored at −80 °C. A sample of liver tissue was homogenized and subjected to lipid extraction according to the method of Folch et al.30 Hepatic TG content was measured by using a commercially available kit (TG E-test, Wako Pure Chemical Industries) and normalized for sample weight.
Thiobarbituric Acid Reactive Substances (TBARS) Assay
Serum samples and liver homogenates were used to determine levels of serum and hepatic TBARS, measured by using an OXI-TEK TBARS Assay kit (ALEXIS JAPAN, Tokyo, Japan). The content of hepatic TBARS was normalized for protein concentration.
Liver Histopathology and Fatty Droplet Area
Livers were fixed in 10% buffered formalin, embedded in paraffin, and sectioned at 3–4 μm. For the evaluation of hepatic steatosis, sections were stained with Oil red O. The average area of intracellular fatty droplets was determined as follows: livers were fixed in 10% buffered formalin. Frozen sections of the liver, which were stained with Oil Red O, were prepared for the evaluation of hepatic steatosis. The average area (μm2) of the fat droplets within hepatocytes in 5 fields (each approximately 93000 μm2) for each liver was measured with the aid of an image analyzer (IPAP-WIN, Sumika TechnosCo., Osaka, Japan).
Determination of mRNA Levels
Total RNA was isolated and purified from liver by means of an RNeasy Plus Mini Kit (QIAGEN) with DNase I treatment. The mRNA levels corresponding to various target genes were determined by real-time quantitative RT-PCR using 7500 Fast Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The primers for mouse TNF-α and MCP-1 were as previously described.31, 32
Oxygen Radical Absorbance Capacity (ORAC) Assay
An ORAC antioxidant assay kit (Zen-Bio, NC) was used to determine the antioxidant capacity of dapagliflozin, canagliflozin, remogliflozin etabonate and remogliflozin. The assay measures the ability of the test compounds to block the peroxyl-radical formation during the breakdown of AAPH (2,2′-azobis-2-methyl-propanimidamide dihydrochloride). Compounds are tested in a dose response to generate an activity curve. Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) is used as a positive control and to generate a standard curve. The area under the curve (AUC) of normalized decay curves was determined for each concentration of control and sample. Trolox net AUC was used as a standard curve to calculate the Trolox equivalence of the test samples. Values are μmoles Trolox activity/g of test compounds.
Data Analysis
Data are presented as means ± S.E.M. Calculations were performed using Microsoft Excel 2010 and statistical analysis were performed using SAS Systems, version 8.2 (SAS Institute, Cary, NC). In this analysis, data were analyzed among three or more groups as follows: When equality of variances was indicated by a Bartlett's test, statistical analysis was performed using a one-way analysis of variance followed by a Dunnett's multiple comparison test. When equality of variances was not indicated by a Bartlett's test, statistical analysis was performed using a Kruskal–Wallis test followed by a Dunnett's multiple comparison test. Comparisons between two groups were made as follows: when equality of variances was indicated by an F-test, statistical analysis was performed using a Student's t test. When equality of variances was not indicated by an F-test, statistical analysis was performed using a Welch's t test.
Results
Diet-induced Obesity Model and Drug Treatment
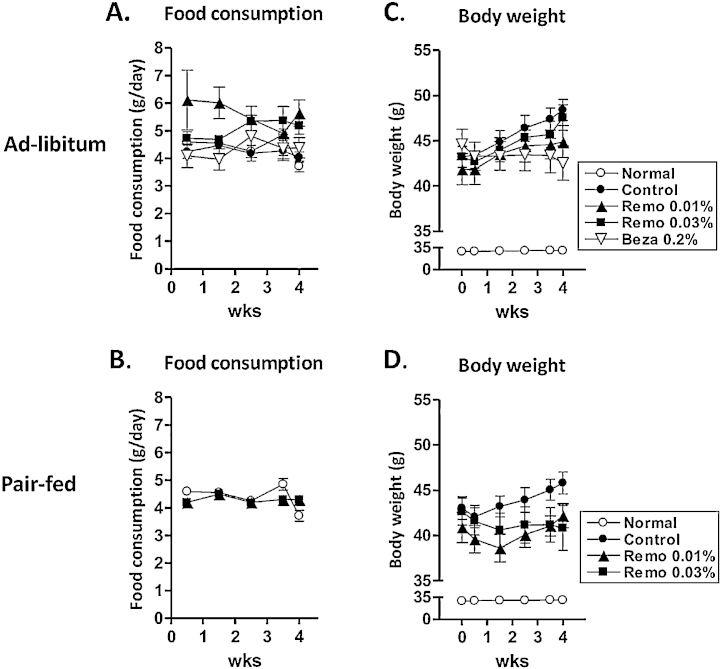
Mice treated with Remo consumed slightly more than untreated animals (Figure 1A). Thus, both ad-libitum and pair-fed (PF) groups (Figure 1B) were established. Daily drug intake was determined by multiplying drug concentration in chow by food consumption. Total drug consumed in animals fed Remo at 0.01% and 0.03% ad-libitum was 13.2 ± 2.2 mg/kg/day and 33.9 ± 2.0 mg/kg/day, respectively. Consumption of Remo in Pair-fed animals fed 0.01% and 0.03% Remo was 10.8 ± 0.4 mg/kg/day and 32.0 ± 1.6 mg/kg/day, respectively. Animals fed bezafibrate 0.2% ad-libitum consumed 198.7 ± 16.1 mg/kg/day.
Figure 1.
Remo inhibited weight gain compared to untreated DIO mice. C57BL/6J mice were given a diet of normal chow or HFD32 for 11-weeks. After the initial feeding period the mice were divided into Ad-libitum (panels A and C) or Pair-Fed (panels B and D) groups. These groups were continued for an additional 4-weeks on a normal diet (Normal), HFD32 (Control group), or HFD32 supplemented with 0.01% Remo, 0.03% Remo or 0.2% bezafibrate (Beza).
At the end of the initial 11-week feeding period, compared to mice receiving normal chow, mice fed HFD32 became obese (body weight; 28.9 ± 0.5 vs 42.7 ± 0.5 g, P < 0.001). The Control and Control-PF groups (untreated and maintained on HFD32) continued to gain weight during the 4-week treatment period (Figure 1C–D). In contrast, pair-fed mice treated with Remo displayed and initial weight loss during the first 10 days of treatment, and continued to maintain the reduced weight during the treatment period. The untreated HFD32-fed mice also displayed elevated non-fasting plasma glucose (169.8 ± 5.9 vs 210.9 ± 4.3 mg/dL, P < 0.001). In addition, and described in more detail below, untreated HFD32-fed mice had elevated liver enzymes (ALT and AST), increased oxidative stress (TBARS) in both liver and serum, as well as elevated expression of TNF-α and MCP-1. Furthermore, untreated HFD32-fed mice displayed significant steatosis and elevated liver weight. Thus, at the end of the initial feeding period, animals fed HFD32 displayed characteristics consistent with NAFLD. Furthermore, the Control and Control-PF groups continued to display the NAFLD phenotype throughout the treatment period.
Plasma Alanine Aminotransferase/Aspartate Aminotransferase
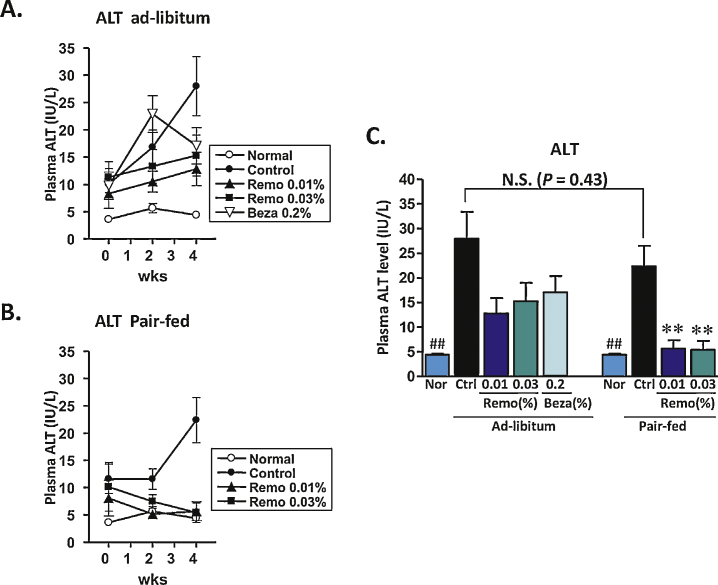
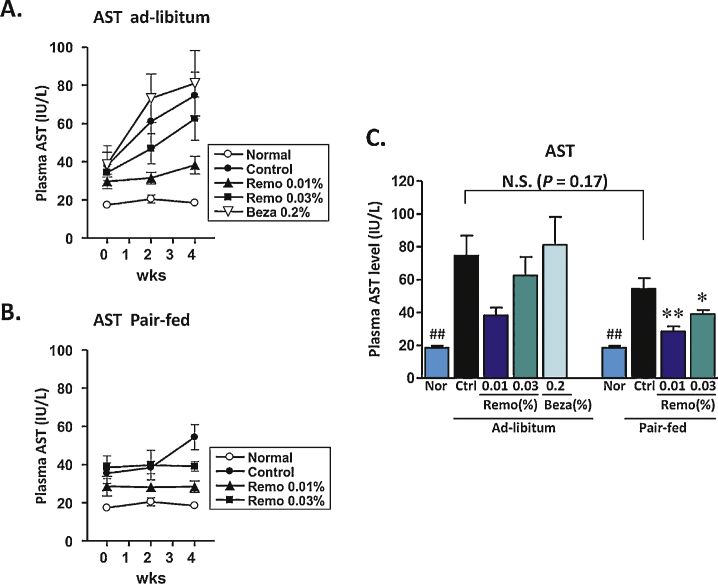
Levels of ALT/AST are routinely followed as markers of NAFLD and NASH, as well as general markers for liver function and potential toxicity. Prior to the beginning of the 4-week treatment period, compared to normal lean mice, the HFD32 fed obese animals had significantly elevated serum levels of ALT and AST. In the obese Control and Control-PF groups, these levels continued to rise during the 4-week period (Figure 2, Figure 3). In the pair-fed animals, Remo significantly reduced the level of ALT by 2-weeks, which was maintained throughout the study (Figure 2B). At the end of the 4-week treatment period serum ALT levels were significantly lower in both Remo-treated ad-libitum and pair-fed groups (Figure 2C). Similarly, at the end of the 4-week treatment period, AST levels in Remo-treated mice were significantly reduced (Figure 3C).
Figure 2.
Treatment with Remo reduced plasma ALT in pair-fed mice. Levels of ALT in the plasma were determined at the beginning of the treatment period and at the end of weeks 2 and 4 in Ad-libitum (panel A) and Pair-fed (panel B) groups. Data from the end of the treatment period are compiled in panel C (**P-value ≤0.001 vs Control Pair-Fed; ## P-value ≤0.005 vs Control or Control Pair-Fed). N.S., not significant; Nor, Normal; Ctrl, Control; Beza, bezafibrate.
Figure 3.
Treatment with Remo reduced plasma AST in pair-fed mice. Levels of AST in the plasma were determined at the beginning of the treatment period and at the end of weeks 2 and 4 in Ad-libitum (panel A) and Pair-fed (panel B) groups. Data from the end of the treatment period are compiled in panel C (* P-value ≤0.05; ** P-value ≤0.001 vs Control Pair-Fed; ## P-value ≤0.005 vs Control or Control Pair-Fed). N.S., not significant; Nor, Normal; Ctrl, Control; Beza, bezafibrate.
Liver Weight
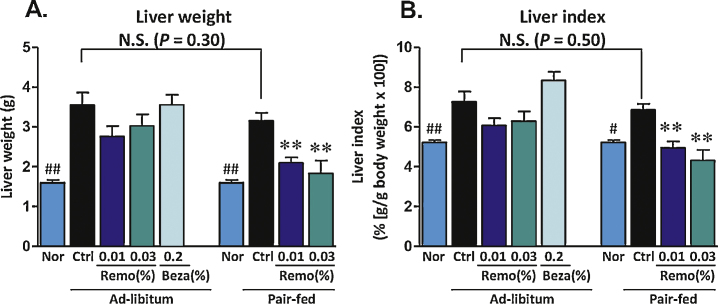
At the end of the 4-week treatment period, liver weights were determined. In the obese Control and Control-PF mice, liver weights were elevated ≥2-fold compared to animals fed a normal chow diet. In contrast, in the pair-fed animals, Remo reduced total liver weight and liver weight indices to levels similar to those observed for normal lean mice (Figure 4).
Figure 4.
Liver weights were reduced in DIO mice treated with Remo. Mice were sacrificed and livers excised and weighed (panel A). A Liver Index was calculated (g liver weight/g body weight × 100) and is shown in panel B. # P-value ≤0.03 vs Control Pair-Fed; ## P-value ≤0.01 vs Control or Control Pair-Fed; ** P-value ≤0.01 compared to Control Pair-Fed. N.S., not significant; Nor, Normal; Ctrl, Control; Beza, bezafibrate.
Hepatic Steatosis
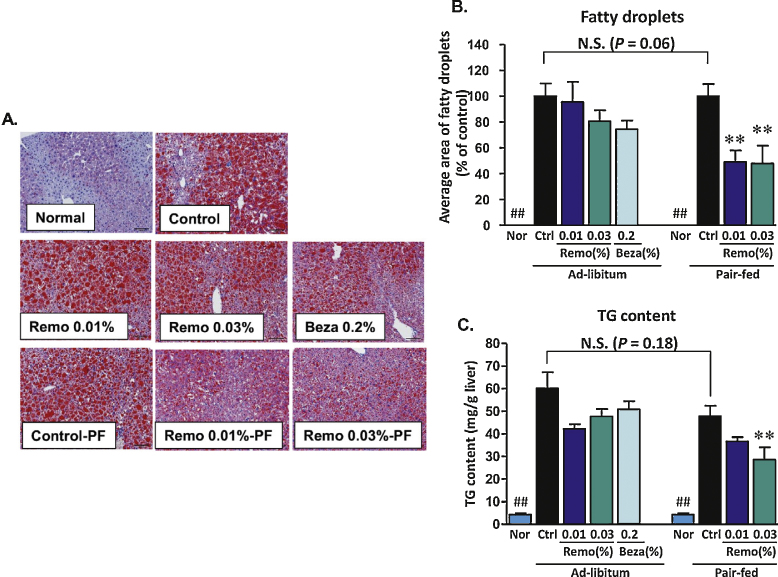
Assessment of hepatic lipid accumulation was determined by calculation of the average area of fatty droplets in liver sections stained with Oil Red O, and by direct measurement of TG levels in liver homogenates. Representative images of Oil Red O stained liver sections obtained for each treatment group are shown in Figure 5A, and indicate a dramatic increase in intracellular lipid accumulation in samples obtained from animals fed a high fat diet. Pair-fed animals treated with Remo showed reduced Oil Red O staining. Consistent with these observations, the average area of fatty droplets was significantly decreased in Remo treated pair-fed animals (Figure 5B). Finally, in the untreated obese animals, there was an approximate 10-fold increase in hepatic lipid content. Pair-fed mice treated with Remo at 0.01% and 0.03% displayed approximately 20% and 40% reduction in total hepatic lipid content, respectively (Figure 5C).
Figure 5.
Remo reduced hepatic triglyceride content and intracellular fatty droplet size. Sections of formalin-fixed livers were stained with Oil red O (panel A). These images were used to calculate the average area of intracellular fatty droplets shown in panel B (## P-value ≤0.0001 compared to Control or Control Pair-Fed; ** P-value ≤0.007 compared to Control Pair-Fed). Total hepatic TG content (panel C) was determined using and values were normalized for sample weight (## P-value ≤0.002 compared to Control or Control Pair-Fed; ** P-value ≤0.009 compared to Control Pair-Fed). N.S., not significant; Nor, Normal; Ctrl, Control; Beza, bezafibrate; PF, Pair-Fed.
Effect of Remogliflozin Etabonate on mRNA Levels of Tumor Necrosis Factor Alpha and Monocyte Chemoattractant Protein-1
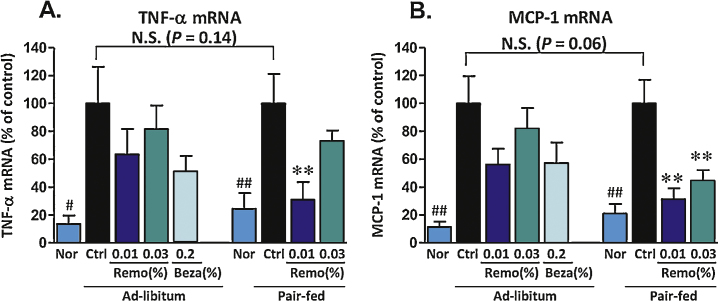
Both TNF-α and MCP-1 are involved in inflammatory processes and are linked to development of insulin resistance.33, 34 Compared to lean animals, untreated obese animals displayed an approximate 5-fold increase in the levels of mRNA for both TNF-α and MCP-1 (Figure 6). Pair-fed animals treated with Remo displayed a maximum 69% reduction in the obesity-induced levels of both TNF-α and MCP-1.
Figure 6.
Effect of Remo on DIO-associated increase in expression of TNF-α and MCP-1. Total RNA purified from liver samples was used to determine levels of expression for TNF-α (panel A; # P-value ≤0.02 compared to Control; ## P-value ≤0.008 compared to Control Pair-Fed; ** P-value ≤0.008) and MCP-1 (panel B; ## P-value ≤0.004; ** P-value ≤0.006). Data are presented as % Control of Ad-libitum or Pair-Fed as indicated. N.S., not significant; Nor, Normal; Ctrl, Control; Beza, bezafibrate.
Thiobarbituric Acid-reactive Substances as a Measure of Oxidative Stress
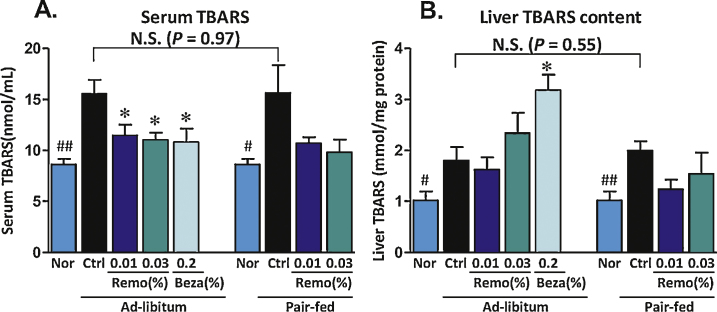
An imbalance in the cellular production of reactive oxygen species (ROS) and the antioxidant capacity of the cell can result in oxidative stress. By-products of lipid peroxidation are associated with oxidative stress and can be quantified using the thiobarbituric acid reactive substances (TBARS) assay. Assessment of TBARS showed reduced levels of oxidative stress markers in both serum (37% decrease) and liver homogenates (22% decrease) isolated from Remo-treated pair-fed mice (Figure 7).
Figure 7.
Remo reduces the obesity-induced increase in the oxidative stress marker TBARS. Thiobarbituric acid reactive substances (TBARS) were determined at the end of the treatment period in serum samples and liver homogenates.
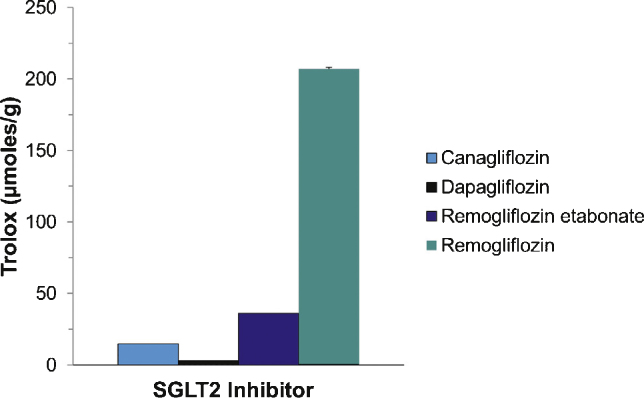
In Vitro Antioxidant Activity of Remogliflozin Etabonate
The antioxidant activity of individual compounds can be determined by measuring the oxygen radical absorbance capacity (ORAC assay). Comparison of the test compound with a known standard (Trolox) provides and index of antioxidant capacity.35 Remogliflozin is the active component of the pro-drug remogliflozin etabonate. The pro-drug is rapidly cleaved in the gut to produce remogliflozin. Using the ORAC assay, we determined the antioxidant capacity of equal concentrations of remogliflozin etabonate, remogliflozin, canagliflozin and dapagliflozin. As shown in Figure 8, remogliflozin displays a robust antioxidant activity compared to the pro-drug and other SGLT2 inhibitors, canagliflozin and dapagliflozin.
Figure 8.
Remogliflozin possesses significant antioxidant capacity. Antioxidant capacity of Remo, remogliflozin, canagliflozin and dapagliflozin was determined in vitro using an oxygen radical absorbance capacity (ORAC) assay.
Discussion
Remo is in an advanced stage of clinical development. Similar to other SGLT2 inhibitors (e.g. canagliflozin and dapagliflozin) in type 2 diabetic subjects, Remo has been shown to reduce postprandial glucose excursions, improve HbA1c, increase insulin sensitivity and improve pancreatic beta cell function.22, 24, 25, 26, 36 We previously reported that the SGLT2 inhibitors, sergliflozin and Remo, exhibited anti-hyperglycemic and insulin sensitizing properties in several rodent models of diabetes including streptozoticin-induced diabetic rats, Zucker fatty rats, KK-Ay mice and GK rats.21, 37, 38, 39 In addition, in these earlier studies, long-term treatment with sergliflozin etabonate improved glucose metabolism and insulin resistance, accompanied with marked reduction in hepatic fatty droplets and hepatic TG content.38 More recently, the SGLT2 inhibitor ipragliflozin, which was approved in Japan, was shown to improve hepatic steatosis and necroinflammation accompanied with significant reduction in hepatic oxidative stress markers and inflammatory makers in rodent models.40, 41 While the exact mechanism remains unclear, taken together the above studies suggest that these SGLT2 inhibitors have the potential to improve fatty liver disease.
While no single animal model can exactly recapitulate development of NAFLD and NASH in humans, the DIO mouse provides a relevant model to follow several early events in nutritionally-induced fatty liver, as well as examine potential therapeutics. In this study, we focused on examining the potential benefits of Remo in C57BL/6J mice fed the high fat diet, HFD32. These mice became obese, displayed elevated plasma glucose, had increased liver weight and developed significant hepatic steatosis that was accompanied by a marked increase in circulating levels of liver enzymes and elevated oxidative stress. After the initial feeding period to establish the disease phenotype, the treatment period was initiated and it was noted that animals receiving Remo ate slightly more than the untreated HFD32 control group; suggesting the loss of calories in the urine caused compensation for calorie intake. Thus, based on the average daily food consumption in the untreated DIO Control group, two additional Remo groups were pair-fed, receiving an equal amount of HFD32 chow as Control group, but containing either 0.01% Remo or 0.03% Remo. HFD32-fed mice that did not receive Remo continued to gain weight and displayed further elevated levels of ALT and AST. In contrast, HFD32 pair-fed mice treated with Remo did not gain additional weight, and had significantly decreased serum levels of ALT and AST. Treatment with Remo also reduced liver weight in parallel with a significant reduction in hepatic steatosis, as indicated by a reduction in both total hepatic TG content and average area of fatty droplets observed within hepatocytes. Thus, Remo was able to reduce the early pathophysiologic parameters associated with development of fatty liver disease. Furthermore, TNF-α has been proposed as a link between obesity and insulin resistance. Moreover, MCP-1 has contributed to macrophage infiltration into adipose and liver tissue, and has also been associated with development of insulin resistance and fibrosis.33, 34 The ability of Remo to inhibit the DIO-induced levels of mRNA for TNF-α and MCP-1 is consistent with improved insulin action and glucose metabolism observed in earlier studies,21, 37, 38, 39 and is suggestive of a decreased inflammatory response.
Remo (remogliflozin etabonate) is rapidly converted in the gut to the active entity remogliflozin.21 Remogliflozin is an O-linked glycoside, where as other SGLT2 inhibitors are C-linked glycosides.21, 27 Interestingly, the chemical structure of remogliflozin suggests that it may possess intrinsic antioxidant properties. Indeed, compared to canagliflozin and dapagliflozin, remogliflozin displays significant antioxidant capacity. Thus, in addition to its insulin sensitizing properties, these data suggest that remogliflozin may act as an effective antioxidant. This is relevant since antioxidant therapeutics, such as vitamin E, are being investigated for the treatment of fatty liver disease.3 Furthermore, we observed decreased TBARS in Remo-treated animals. It is unclear whether this observation is a result of Remo's ability to solely increase insulin sensitivity, act as an antioxidant or both.
In summary, in this DIO mouse model, Remo improved the pathophysiologic characteristics associated with NAFLD. With respect to improvement in hepatic steatosis, these findings are consistent with previous studies examining Remo in genetic models of insulin resistance in rodents; specifically Zucker fatty rats, KK-Ay mice and GK rats.21, 37, 38, 39 Furthermore, since we have observed significant antioxidant activity with Remo in vitro, it is possible that Remo could directly affect hepatic function in a manner entirely independent of its ability to inhibit SGLT2. Additional studies in both animal models and cell-based assays are needed to further examine this hypothesis. Although several potential therapeutics are in clinical trials, no drugs have been approved for the specific treatment of NAFLD or NASH. Experimental treatments under evaluation in patients with NASH include compounds that target oxidative stress such as the antioxidants vitamin E, selenium, and betaine3; and compounds that target insulin resistance such as metformin and thiazolidinediones.2 More recently, the FXR agonist obeticholic acid appears to show some efficacy in patients with NASH.4, 42 In an attempt to block the effects of MCP-1, antagonists of the cell surface receptors, CCR2/CCR5, are also being explored.43 Taken together, the data from this study and others suggest that Remo can target multiple pathways involved in the development of fatty liver disease and may be an effective therapeutic for the treatment of NAFLD/NASH. Clinical trials in patients with histologically proven NASH could validate this hypothesis.
Disclosure statement
The authors of this manuscript include employees from BHV Pharma (BC), Kissei Pharmaceuticals (SK, KK, MI, TN) and Islet Sciences (WW). These institutions have been involved in the pre-clinical and/or clinical development of remogliflozin etabonate and have a financial interest in its commercialization. BB and SW have nothing to disclose.
Conflicts of interest
All authors have none to declare.
References
- 1.Sanyal A.J. NASH: a global health problem. Hepatol Res. 2011;41:670–674. doi: 10.1111/j.1872-034X.2011.00824.x. [DOI] [PubMed] [Google Scholar]
- 2.Trappoliere M., Tuccillo C., Federico A. The treatment of NAFLD. Eur Rev Med Pharmacol Sci. 2005;9:299–304. [PubMed] [Google Scholar]
- 3.Medina J., Fernandez-Salazar L.I., Garcia-Buey L., Moreno-Otero R. Approach to the pathogenesis and treatment of nonalcoholic steatohepatitis. Diabetes Care. 2004;27:2057–2066. doi: 10.2337/diacare.27.8.2057. [DOI] [PubMed] [Google Scholar]
- 4.Mudaliar S., Henry R.R., Sanyal A.J. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–582. doi: 10.1053/j.gastro.2013.05.042. e571. [DOI] [PubMed] [Google Scholar]
- 5.Brookheart R.T., Michel C.I., Schaffer J.E. As a matter of fat. Cell Metab. 2009;10:9–12. doi: 10.1016/j.cmet.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng X.Q., Chen L.L., Li N.X. The expression of SIRT1 in nonalcoholic fatty liver disease induced by high-fat diet in rats. Liver Int. 2007;27:708–715. doi: 10.1111/j.1478-3231.2007.01497.x. [DOI] [PubMed] [Google Scholar]
- 7.Hou X., Xu S., Maitland-Toolan K.A. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohjima M., Higuchi N., Kato M. SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. Int J Mol Med. 2008;21:507–511. [PubMed] [Google Scholar]
- 9.Straub B.K., Stoeffel P., Heid H., Zimbelmann R., Schirmacher P. Differential pattern of lipid droplet-associated proteins and de novo perilipin expression in hepatocyte steatogenesis. Hepatology. 2008;47:1936–1946. doi: 10.1002/hep.22268. [DOI] [PubMed] [Google Scholar]
- 10.Bell M., Wang H., Chen H. Consequences of lipid droplet coat protein downregulation in liver cells: abnormal lipid droplet metabolism and induction of insulin resistance. Diabetes. 2008;57:2037–2045. doi: 10.2337/db07-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed M.H., Byrne C.D. Modulation of sterol regulatory element binding proteins (SREBPs) as potential treatments for non-alcoholic fatty liver disease (NAFLD) Drug Discov Today. 2007;12:740–747. doi: 10.1016/j.drudis.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Nagai Y., Yonemitsu S., Erion D.M. The role of peroxisome proliferator-activated receptor gamma coactivator-1 beta in the pathogenesis of fructose-induced insulin resistance. Cell Metab. 2009;9:252–264. doi: 10.1016/j.cmet.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Videla L.A., Rodrigo R., Araya J., Poniachik J. Insulin resistance and oxidative stress interdependency in non-alcoholic fatty liver disease. Trends Mol Med. 2006;12:555–558. doi: 10.1016/j.molmed.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Darimont C., Avanti O., Zbinden I. Liver X receptor preferentially activates de novo lipogenesis in human preadipocytes. Biochimie. 2006;88:309–318. doi: 10.1016/j.biochi.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Borradaile N.M., Han X., Harp J.D., Gale S.E., Ory D.S., Schaffer J.E. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47:2726–2737. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Hotamisligil G.S. Role of endoplasmic reticulum stress and c-Jun NH2-terminal kinase pathways in inflammation and origin of obesity and diabetes. Diabetes. 2005;54(suppl 2):S73–S78. doi: 10.2337/diabetes.54.suppl_2.s73. [DOI] [PubMed] [Google Scholar]
- 17.Hotamisligil G.S. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int J Obes (Lond) 2008;32(suppl 7):S52–S54. doi: 10.1038/ijo.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozcan U., Cao Q., Yilmaz E. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 19.Brzozowska M.M., Ostapowicz G., Weltman M.D. An association between non-alcoholic fatty liver disease and polycystic ovarian syndrome. J Gastroenterol Hepatol. 2009;24:243–247. doi: 10.1111/j.1440-1746.2008.05740.x. [DOI] [PubMed] [Google Scholar]
- 20.Chao E.C., Henry R.R. SGLT2 inhibition – a novel strategy for diabetes treatment. Nat Rev Drug Discov. 2010;9:551–559. doi: 10.1038/nrd3180. [DOI] [PubMed] [Google Scholar]
- 21.Fujimori Y., Katsuno K., Nakashima I., Ishikawa-Takemura Y., Fujikura H., Isaji M. Remogliflozin etabonate, in a novel category of selective low-affinity sodium glucose cotransporter (SGLT2) inhibitors, exhibits antidiabetic efficacy in rodent models. J Pharmacol Exp Ther. 2008;327:268–276. doi: 10.1124/jpet.108.140210. [DOI] [PubMed] [Google Scholar]
- 22.Dobbins R.L., O'Connor-Semmes R., Kapur A. Remogliflozin etabonate, a selective inhibitor of the sodium-dependent transporter 2 reduces serum glucose in type 2 diabetes mellitus patients. Diabetes Obes Metab. 2012;14:15–22. doi: 10.1111/j.1463-1326.2011.01462.x. [DOI] [PubMed] [Google Scholar]
- 23.Mudaliar S., Armstrong D.A., Mavian A.A. Remogliflozin etabonate, a selective inhibitor of the sodium-glucose transporter 2, improves serum glucose profiles in type 1 diabetes. Diabetes Care. 2012;35:2198–2200. doi: 10.2337/dc12-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapur A., O'Connor-Semmes R., Hussey E.K. First human dose-escalation study with remogliflozin etabonate, a selective inhibitor of the sodium-glucose transporter 2 (SGLT2), in healthy subjects and in subjects with type 2 diabetes mellitus. BMC Pharmacol Toxicol. 2013;14:26. doi: 10.1186/2050-6511-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sykes A.P., Gail L.K., Dobbins R. Randomized efficacy and safety trial of once daily remogliflozin etabonate for the treatment of type 2 diabetes diabetes. Obes Metabolism. 2015;17:98–101. doi: 10.1111/dom.12393. [DOI] [PubMed] [Google Scholar]
- 26.Sykes A.P., O'Connor Semmes R.L., Dobbins R. Randomized trial demonstrating efficacy and safety of twice daily remogliflozin etabonate for the treatment of type 2 diabetes. Diabetes Obes Metab. 2015;17:94–97. doi: 10.1111/dom.12391. [DOI] [PubMed] [Google Scholar]
- 27.Isaji M. SGLT2 inhibitors: molecular design and potential differences in effect. Kidney Int Suppl. 2011:S14–S19. doi: 10.1038/ki.2010.511. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh R.K., Ghosh S.M., Chawla S., Jasdanwala S.A. SGLT2 inhibitors: a new emerging therapeutic class in the treatment of type 2 diabetes mellitus. J Clin Pharmacol. 2012;52:457–463. doi: 10.1177/0091270011400604. [DOI] [PubMed] [Google Scholar]
- 29.Isaji M. Sodium-glucose cotransporter inhibitors for diabetes. Curr Opin Investig Drugs. 2007;8:285–292. [PubMed] [Google Scholar]
- 30.Folch J., Lees M., Sloane Stanley G.H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 31.Nagasawa T., Inada Y., Nakano S. Effects of bezafibrate, PPAR pan-agonist, and GW501516, PPARdelta agonist, on development of steatohepatitis in mice fed a methionine- and choline-deficient diet. Eur J Pharmacol. 2006;536:182–191. doi: 10.1016/j.ejphar.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 32.Trogan E., Choudhury R.P., Dansky H.M., Rong J.X., Breslow J.L., Fisher E.A. Laser capture microdissection analysis of gene expression in macrophages from atherosclerotic lesions of apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A. 2002;99:2234–2239. doi: 10.1073/pnas.042683999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dandona P., Aljada A., Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 34.Kanda H., Tateya S., Tamori Y. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang D., Ou B., Prior R.L. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 36.Hussey E.K., Kapur A., O'Connor-Semmes R. Safety, pharmacokinetics and pharmacodynamics of remogliflozin etabonate, a novel SGLT2 inhibitor, and metformin when co-administered in subjects with type 2 diabetes mellitus. BMC Pharmacol Toxicol. 2013;14:25. doi: 10.1186/2050-6511-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujimori Y., Katsuno K., Ojima K. Sergliflozin etabonate, a selective SGLT2 inhibitor, improves glycemic control in streptozotocin-induced diabetic rats and Zucker fatty rats. Eur J Pharmacol. 2009;609:148–154. doi: 10.1016/j.ejphar.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Katsuno K., Fujimori Y., Ishikawa-Takemura Y., Isaji M. Long-term treatment with sergliflozin etabonate improves disturbed glucose metabolism in KK-A(y) mice. Eur J Pharmacol. 2009;618:98–104. doi: 10.1016/j.ejphar.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Katsuno K., Fujimori Y., Takemura Y. Sergliflozin, a novel selective inhibitor of low-affinity sodium glucose cotransporter (SGLT2), validates the critical role of SGLT2 in renal glucose reabsorption and modulates plasma glucose level. J Pharmacol Exp Ther. 2007;320:323–330. doi: 10.1124/jpet.106.110296. [DOI] [PubMed] [Google Scholar]
- 40.Tahara A., Kurosaki E., Yokono M. Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. Eur J Pharmacol. 2013;715:246–255. doi: 10.1016/j.ejphar.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Tahara A., Kurosaki E., Yokono M. Effects of sodium-glucose cotransporter 2 selective inhibitor ipragliflozin on hyperglycaemia, oxidative stress, inflammation and liver injury in streptozotocin-induced type 1 diabetic rats. J Pharm Pharmacol. 2014;66:975–987. doi: 10.1111/jphp.12223. [DOI] [PubMed] [Google Scholar]
- 42.Lindor K.D. Farnesoid X receptor agonists for primary biliary cirrhosis. Curr Opin Gastroenterol. 2011;27:285–288. doi: 10.1097/MOG.0b013e32834452c8. [DOI] [PubMed] [Google Scholar]
- 43.Miura K., Yang L., van Rooijen N., Ohnishi H., Seki E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1310–G1321. doi: 10.1152/ajpgi.00365.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]