Abstract
Per-cutaneously inserted catheter drainage is an accepted treatment modality for a large amoebic liver abscess. Complications that can arise are; secondary infection, bleeding into the abscess cavity, inadvertent catheter misplacement into the IVC and rupture of abscess with spillage into the peritoneal cavity. We report a case of a large amoebic liver abscess that presented with complications related to per-cutaneously inserted catheter drainage.
Keywords: Amebic liver abscess, Amebiasis, Catheter drainage
Introduction
Liver abscess is a collection of purulent material and necro-inflammatory tissue in the liver parenchyma. Although, a wide variety of organisms can cause liver abscess, yet, in developing countries, Entamoeba histolytica, is the commonest cause. Infection with E. histolytica occurs world-wide and liver abscess is the most common extra-intestinal complication in 3%–9% of patients affected with amebiasis.1 Treatment of amebic liver abscess is mainly by anti-amebic drugs and percutaneous drainage of the abscess cavity.1, 2, 3 We report a case of amebic liver abscess that presented with complications related to percutaneously inserted catheter drainage.
Case report
A 28-year old man, presented with fever, pain in the right upper quadrant of the abdomen of a month's duration and jaundice for 4 days. He had been evaluated at another hospital and diagnosed as right lobe liver abscess on sonography of the abdomen. A percutaneous pigtail catheter was inserted in to the liver abscess and 100 ml of purulent material drained. On day 4, he developed bilateral deep venous thrombosis for which anticoagulation with parenteral Enoxaparin and oral warfarin was initiated. On day 5, development of malena with high grade fever prompted a review sonogram that showed hemorrhage in the liver abscess cavity, intraperitoneal hemorrhage, IVC thrombosis and a minimal right side pleural effusion. He was referred to our institute for further management.
On examination, he was febrile, pulse—110 per minute, blood pressure—130/84 mmHg, respiratory rate—32 per minute and he had jaundice. Examination of the chest and cardiovascular systems was normal. Although, the abdomen was distended with ascites and hepatomegaly (16 cms), yet, bowel sounds were normal. Investigations disclosed anemia (Hb-8.8 g/dL), leucocytosis (28,900/mm3, polymorphs-80%) and prothrombin time index 36%. The renal function tests showed pre-renal azotemia (Urea-166 mg/dL, creatinine-2.3 mg/dL), hyperbilirubinemia-23.9 mg/dL (conjugated bilirubin-18.0 mg/dL), serum ALT-207 U/L, AST-468 U/L and serum alkaline phosphatase −747 U/L. The amoebic serology was positive at titers of 1:1600. A repeat abdominal sonogram disclosed hepatomegaly, a 16 × 6 cm liver abscess in the right lobe of liver, ascitis and a right sided pleural effusion. A CECT examination of the abdomen showed free fluid, a large right lobe liver abscess 14 × 6 cm with peri-hepatic collection, hemorrhage and air in the abscess cavity (Figure 1, Figure 2, Figure 3). Filling defects were also seen in the IVC, common iliac vein and external iliac vein suggesting thrombosis. A CT-abdominal angiogram did not show any pseudo-aneurysm of the mesenteric arteries or the coeliac axis but contrast extravasation from branches of the right hepatic artery into the abscess cavity. The right hepatic artery (aberrantly arising from the superior mesenteric artery), was catheterized and embolization was done using PVA (300–500 μg). Check angiogram after embolization did not show any contrast extravasation from the branches of the right hepatic artery (Figure 4, Figure 5).
Figure 1.
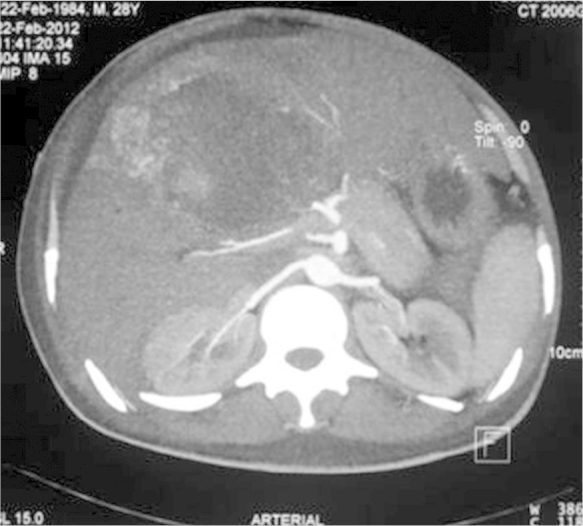
CECT abdomen (arterial phase) showing a large right lobe liver abscess with perihepatic collection and contrast extravasation into liver abscess cavity from a right hepatic artery branch.
Figure 2.
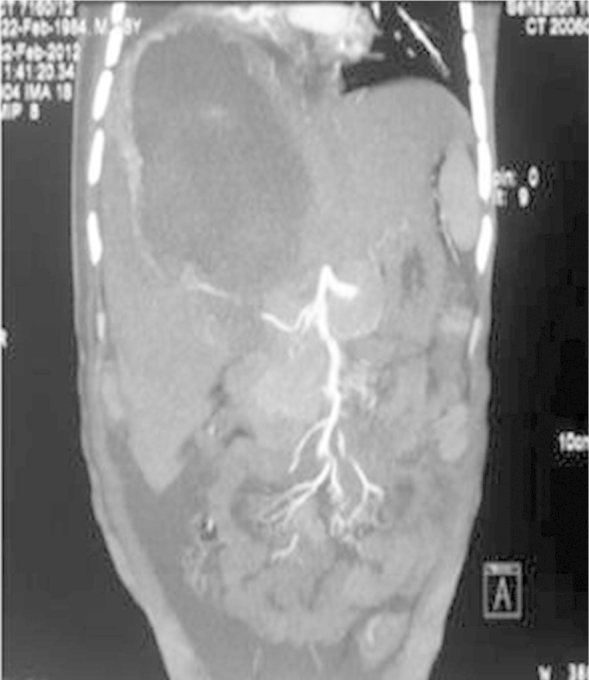
Reconstruction CECT abdomen (arterial phase) image showing a large right lobe liver abscess with contrast extravasation into liver abscess cavity from a right hepatic artery branch.
Figure 3.
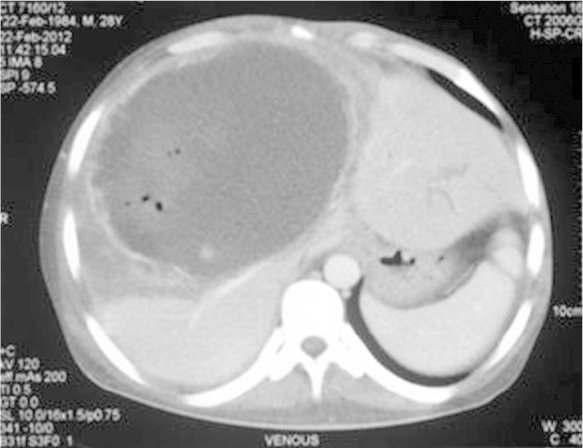
CECT Abdomen (venous phase) showing a large right lobe liver abscess with presence of air and diffuse contrast extravasation from the walls of the abscess cavity.
Figure 4.
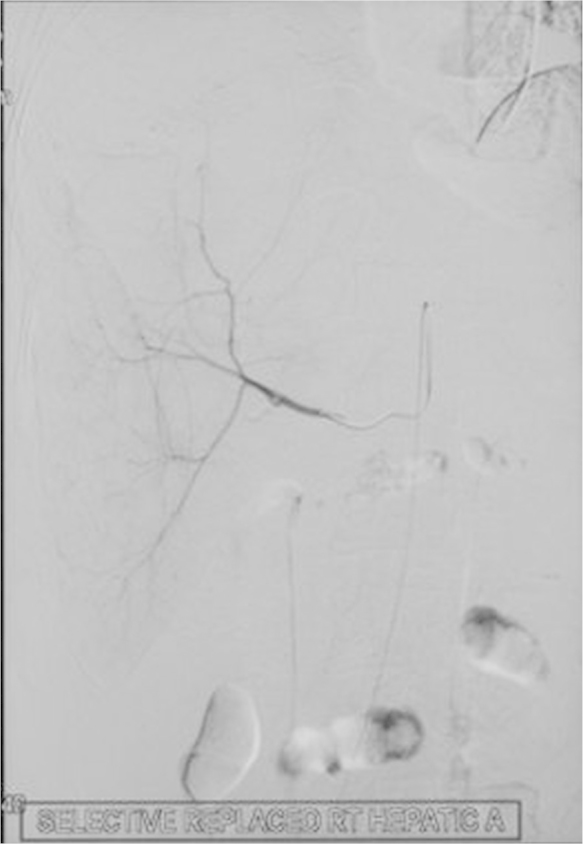
CT Angiogram that shows an aberrant origin of right hepatic artery from the superior mesenteric artery.
Figure 5.
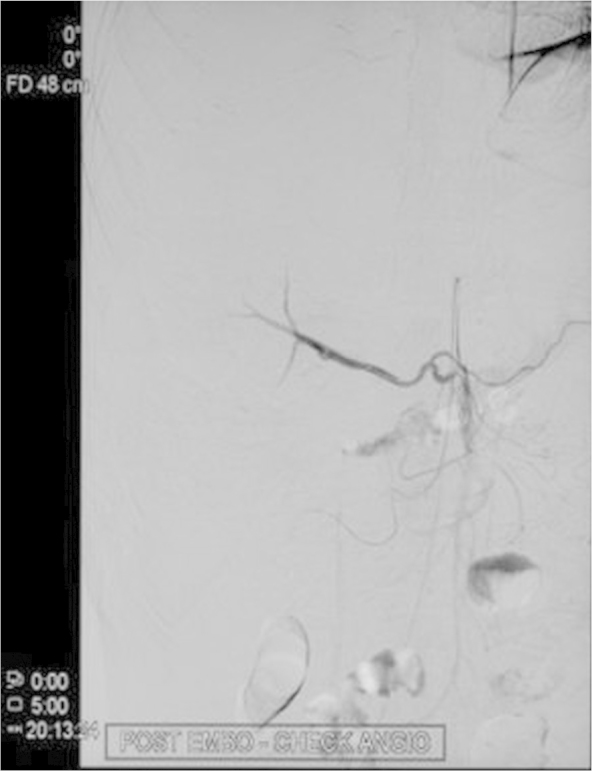
Post embolization angiography (right image) CT Angiogram does not show any contrast extravasation from the right hepatic vein branches.
The patient's coagulopathy was treated with withdrawal of warfarin and parenteral vitamin K. Antibiotic therapy with piperacillin-tazobactam and metronidazole was started. On day 3, under sonographic guidance, three pigtail catheters were per-cutaneously inserted into the abdomen; the first catheter was inserted into the right lobe abscess and the other two in the peritoneal cavity on both sides to drain the peritoneal fluid. On day 9, an increase in the right sided pleural effusion prompted the insertion of a chest tube and 500 ml of hemorrhagic fluid was drained. Over the next few days, he had 4–5 episodes of malena, a drop in hematocrit and coagulopathy that necessitated treatment with 3 units' blood transfusion over 3 days and 4 units of fresh frozen plasma daily for 3 days. A review Doppler ultrasound of the lower limb veins on day 12 revealed normal flow in the lower limb veins. All four catheters drained 100–250 ml hemorrhagic fluid daily till the 14th day after admission and were successively removed. The patient's clinical condition improved and he was discharged 3 weeks later.
Discussion
For abdominal abscesses, percutaneous catheter drainage is an effective method for treatment and is preferentially indicated when sectional imaging demonstrates a single accessible lesion.1, 2, 3 In adults, imaging-guided percutaneous aspiration and insertion of a pigtail catheter in liver abscess is a well-accepted treatment modality.2, 3, 4 Complications that can arise are; secondary infection, bleeding into the abscess cavity, inadvertent catheter misplacement into the IVC and rupture of abscess with spillage into the peritoneal cavity.
In our case, hemorrhage into the liver abscess cavity occurred after insertion of a pigtail catheter with the start of anticoagulation therapy for DVT. DVT occurs in 5–10% of patients admitted to the medical wards world-wide. The 9th ACCP consensus guidelines have classified hospitalized medical patients at a moderate risk for development of DVT.5 In our patient, DVT developed as a result of two factors; immobilization and severe sepsis due to the large amoebic liver abscess. The second time insertions of a percutaneous pigtail catheter into the liver abscess resulted in hemobilia and malena. Furthermore, abdominal distension was caused by hemoperitoneum due to spillage of the hemorrhagic contents from the ruptured liver abscess into the peritoneal cavity. In our patient, jaundice indicated the presence of a biliovascular fistula at the time of initial catheter drainage.6 Biliovascular fistula in such cases is usually due to a communication in between the intrahepatic radicles of the biliary tree and branches of the hepatic or portal veins. The resultant bilhemia, due to admixture of bile and necrotic debris of the liver abscess flows towards the low pressure damaged circuit of hepatic vein branches causing a rapid rise of the serum bilirubin as occurred in our case.
A CT angiogram of the liver showed a diffuse arterial hemorrhage from branches of the damaged right hepatic artery (Figure 1, Figure 2) and venous ooze due to coagulopathy (Figure 3) The arterial hemorrhage was treated with blood transfusions and embolization of the right hepatic artery and the venous ooze by treating the coagulopathy with vitamin-K and fresh frozen plasma.
In conclusion, bleeding into the liver abscess cavity with hemoperitoneum can be a fatal complication of catheter insertion into liver abscess cavity. Hemostatic resuscitation with blood transfusions, vitamin K and fresh frozen plasma followed by per-cutaneous pigtail catheter drainage of the liver abscess cavity and peritoneum can be life-saving.
Conflicts of interest
The authors have none to declare.
References
- 1.Stanley S.L., Jr. Amoebiasis: seminar. Lancet. 2003;361:1025–1034. doi: 10.1016/S0140-6736(03)12830-9. [DOI] [PubMed] [Google Scholar]
- 2.Giorgio A., Tarantino L., Mariniello N. Pyogenic liver abscesses: 13 years of experience in percutaneous needle aspiration with US guidance. Radiology. 1995;195:122–124. doi: 10.1148/radiology.195.1.7892451. [DOI] [PubMed] [Google Scholar]
- 3.Sharma N., Sharma A., Varma S., Lal A., Singh V. Amoebic liver abscess in the medical emergency of a North Indian hospital. BMC Res Notes. 2010;3:21. doi: 10.1186/1756-0500-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma B.C., Garg V., Reddy R. Endoscopic management of liver abscess with biliary communication. Dig Dis Sci. 2012;57:524–527. doi: 10.1007/s10620-011-1872-y. [DOI] [PubMed] [Google Scholar]
- 5.Guyatt G.H., Akln E.A., Crowther M., Gutterman D.A., Schuünemann H.J. Antithrombotic therapy and Prevention of thrombosis, 9th ed: American College of chest Physicians Evidence-Based clinical Practice guidelines. Chest. 2012;141:7S–47S. doi: 10.1378/chest.1412S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh V., Bhalla A., Sharma N., Mahi S.K., Lal A., Singh P. Pathophysiology of jaundice in amoebic liver abscess. Am J Trop Med Hyg. 2008;78:556–559. [PubMed] [Google Scholar]


