Supplemental Digital Content is available in the text.
Keywords: human adipose-derived mesenchymal stem cells, radio-sterilized human amnion, radio-sterilized pig skin, wound healing, wound dressing, cellular response, burn treatment
Abstract
Human adipose-derived mesenchymal stem cells (hADMSCs) are believed to be potential key factors for starting the regenerative process after tissue injury. However, an efficient method of delivering these regenerative cells to an external wound site is still lacking. Human amnion and pig skin have long been used as skin wound dressings for the treatment of burns and other skin lesions. Herein, we present the generation of two constructs using these two biomaterials as effective scaffolds for the culture of hADMSCs. It was found that hADMSCs seeded onto radiosterilized human amnion and pig skin are viable and proliferate. These cells are able to migrate over these scaffolds as demonstrated by using time-lapse microscopy. In addition, the scaffolds induce hADMSCs to secrete interleukin-10, an important negative regulator of inflammation, and interleukin-1β, a proinflammatory protein. The interplay between these two proteins has been proven to be vital for a balanced restoration of all necessary tissues. Thus, radiosterilized human amnion and pig skin are likely suitable scaffolds for delivery of hADMSCs transplants that could promote tissue regeneration in skin injuries like patients with burn injuries.
In 2013, the American Burn Association reported that in the United States, 400,000 people were hospitalized because of lesions related to burns or because of different kinds of accidents that could lead to burns by various other factors. Even though there are many skin substitutes in the market,1,2 patients with burn injuries in underdeveloped countries often do not have access to them because of their high cost. In the past few decades, amniotic membrane and pig skin have been used for the treatment of partial-thickness burns because of their favorable properties: avoiding loss of water, diminishing pain sensation, and decreasing the probability of acquiring an infection.3–5 During a second-degree skin burn, the epidermis and part of the dermis are completely lost, whereas in a third-degree burn, even the adipose tissue and muscle might be affected or lost. The use of a simple dressing for the treatment of these types of burn injuries does not aid in the restoration of damaged tissue. Hence, amnion and porcine skin have been used as skin substitutes in burns6,7 because they have different extracellular matrix components and could support the growth of fibroblasts, keratinocytes, or both. In recent years, the use of mesenchymal stem cells (MSCs) has revealed that these cells are helpful in wound healing because of their capacity to differentiate into the vascular lineage, fibroblasts, keratinocytes, and adipose tissue, altogether important components of the skin. MSCs are also capable of secreting interleukin (IL)-10, an important chemokine for immunoregulation. Several studies have shown that this chemokine can mark the difference between wound healing and regeneration.8–10 The treatment of patients with deep skin injuries such as third-grade burns requires the use of cells and a suitable scaffold for delivery of these cells to the site of wound or lesion. A good scaffold should allow cell adhesion and cell migration and, most importantly, must not be harmful to the cells that it supports. Because radiosterilized human amnion (RHA) and radiosterilized pig skin (RPS) are composed of extracellular matrix components such as type I collagen, type IV collagen, laminin, and fibronectin, they have been believed to be an excellent substrate for derived mesenchymal stem cell (hADMSC) culture.11–13 For this reason, we analyzed cytocompatibility of hADMSCs cultured onto RHA and RPS evaluating viability, cell adhesion, proliferation, and migration. Our results show that both materials are suitable scaffolds promoting viability, adhesion, proliferation, and migration of hADMSC.
Materials and Methods
Human Adipose-Derived Mesenchymal Stem Cells Isolation and Culture
The consent and experimental protocols in this study were reviewed and approved by the ethics committee of the Instituto Nacional de Rehabilitación (México, D.F.). Subcutaneous adipose tissue was obtained from four aesthetic surgeries undergoing elective liposuction. Surgical procedures were performed using a liposuction needle with an internal diameter of 4 mm. Lipoaspirate samples were digested for 45 min at 37°C with shaking at 200 rpm in Dulbecco’s Modified Eagle's Medium (DMEM) medium (GIBCO, Grand Island, NY) containing 0.1% type I collagenase (Worthington Biochemical, Lakewood, NJ). Cells were passed through a 70 µm strainer and centrifuged at 1,200 rpm for 5 min. Cells were seeded at 50,000 cells/cm2. After 24 h, medium was changed, and the adherent hAMSCs were grown to confluence as passage zero cells. Cells were maintained in DMEM medium supplemented with 10% fetal bovine serum (FBS; GIBCO) and 1% penicillin/streptomycin (GIBCO).
Flow Cytometry
To verify the presence of MSC markers, first passage (P1) MSCs were analyzed using a FACSCalibur flow cytometer (FACS; Becton Dickinson, San Jose, CA). We analyzed two positive and two negative markers for the mesenchymal stem cells. The mesenchymal stem cell markers were CD73 (ecto-5′-nucleotidase) and CD90 (Thy-1). The hematopoietic markers were CD34 and CD45 (leukocyte common antigen). At 80% of confluence, cells at P1 were harvested from one 75 cm2 flask, counted, and resuspended to a concentration of 2 × 105 cells per antibody test in incubation buffer (phosphate-buffered saline [PBS]: 0.5% uncomplemented FBS). Fifty microliter aliquots of cells were transferred to flow cytometry tubes and incubated for 45 min at 4°C with CD34-phycoerythrin (Becton Dickinson), CD45-fluorescein isothiocyanate (FITC) (Becton Dickinson), CD73-APC (BD Pharmingen, San Jose, CA), or CD90-FITC (BD Pharmingen) monoclonal antibodies. Negative control staining was performed using a FITC-conjugated mouse IgG1 isotype, a PE-conjugated mouse IgG1 isotype, and an allophycocyanin-conjugated mouse IgG1 isotype antibody (all from BD Biosciences, San Jose, CA). Subsequently, cells were washed with PBS and diluted in 500 µl of PBS. Finally, data were acquired by FACSCalibur (Becton Dickinson) equipped with a laser BLUE 488 nm. Data analysis was performed with the software Cell Quest Pro (Becton Dickinson Immunocytometry Systems).
Cell Differentiation
The hADMSCs at third passage from three independent samples were treated to induce chondrogenic, osteogenic, and adipogenic differentiation. Adipogenic differentiation was induced by culturing hADMSCs for 1 week in adipogenic medium (DMEM containing 10% FBS, 1 µM dexamethasone, 0.5 mM isobutylmethylxanthine, 10 µg/ml insulin, and 60 µM indomethacin) and assessed using an oil red O staining. Osteogenic differentiation was induced by culturing MSCs for 1 week in osteogenic medium (DMEM containing 10% FBS, 100 nM dexamethasone, 10 mM glycerophosphate, 10 ng/ml BMP7, and 50 µM ascorbic acid) and inspected for Runx2 immunofluorescence. Chondrogenic differentiation was induced using chondrogenic medium for 1 week (DMEM containing 1% FBS, 50 µg/ml ascorbic acid, 10 ng/ml transforming growth factor-β1, and 6.25 µg/ml insulin) and confirmed using alcian blue staining and type II collagen immunofluorescence.
Human Amnion and Pig Skin Processing
Both types of scaffolds were kindly supplied by the Banco de Tejidos Radioesterilizados (BTR; Estado de México, México), which is affiliated to the Instituto Nacional de Investigaciones Nucleares (ININ; Estado de México, México). The BTR has a sanitary license for tissue processing since July 7, 1999; the Quality Management System of the BTR is certified by ISO 9001:2008 since August 1, 2003.14 The BTR has been processing human amnions and pig skin since 2001. These tissues have been successfully used in patients with skin loss from several hospitals. In general, tissue processing is as follows: amnions from healthy mothers were collected in authorized hospitals. To procure the pig skin, animals are selected at the authorized slaughterhouse following strict selection criteria. Once the tissues are in the BTR, they are washed, dried, cut, packed, and labeled. The packed tissues are then subjected to terminal sterilization by using the ININ industrial 60Co gamma irradiator at a minimum dose of 25 kGy. Radiosterilization is a process of sterilization using ionizing radiation such as X-rays, gamma rays, or high-energy electrons. Radiation tissue processing is done following good radiation practices. After that, the radiosterilized tissues undergo a sterility test as a final product and are released for clinical application. In the case of the amnios, it is necessary to wait for the negative second serology test results before the release of the sterile scaffolds.15
Histological Analysis
Both RHA and RPS were fixed in 4% paraformaldehyde (Sigma, St Louis, MO) for 10 min, washed three times with PBS, and sections of 10 µm thick were stained with hematoxylin–eosin. The hADMSCs that were differentiated into the chondrocytic lineage were stained using alcian blue, and those differentiated into osteocytes were stained using Von Kossa staining.
Generation of Constructs by Seeding hADMSC onto Radiosterilized Human Amnion and Radiosterilized Pig Skin
Mesenchymal cells isolated from adipose tissue at P1 were cultured onto two different types of scaffolds, RHA and RPS. Under sterile conditions, fragments of approximately 0.5 cm diameter of RHA and RPS were cut and placed onto a 24 well culture plate. Mesenchymal cells previously counted and pelleted were seeded onto each scaffold at a concentration of 30,000 cells/cm2 and cultured in DMEM medium complemented with 10% FBS and 1% antibiotics. The constructs were kept for a week at 37°C and 5% CO2.
Viability Assay
To confirm that RHA and RPS are feasible scaffolds for MSC isolated from adipose tissue, viability of hADMSCs cultured onto RHA or RPS was determined after 48 h using the LIVE/DEAD viability/cytotoxicity for mammalian cells Molecular Probes kit (Thermo Fisher Scientific, Rockford, IL). Following technical specifications established by the manufacturer, 1 μM calcein AM and 2 μM ethidium homodimer-1 (EthD-1) were diluted in Hank’s medium. This solution was used to incubate constructs for 45 min at 37°C. Finally, constructs were washed with PBS and analyzed using a confocal microscope LSM 780 and ZEN 2010 Carl Zeiss software (Zeiss, Jena-Turingia, Germany).
hADMSC Proliferation
After 48 h, hADMSCs cultured onto RHA or RPS were washed and fixed in 4% paraformaldehyde/0.1M PBS buffer (pH = 7.4). Cells were immunolabeled with primary antibody against human Ki67 (1:100, BioLegend, San Diego, CA) and incubated at 4°C overnight. The constructs were washed and incubated with Alexa Fluor 488 (1:500; Rockford, IL) secondary antibody Thermo Fisher Scientific for 2 h. Nuclei were stained using 1 mg/ml of 4′, 6-diamidine-2-phenylidole-dihydrochloride for 10 min. Images were obtained with an AxioVision Observer A.1 microscope (Zeiss).
Cell Migration
For examination of cell migration on the surface of RHA and RPS, cells were stained with DiI and seeded during 48 h onto the scaffolds; cells were photographed using “time series” function in an Axio observer microscope provided with a CO2 incubation chamber during 6 h. Migration distance was calculated using AxioVision software.
ELISA
Conditioned medium from hADMSCs seeded onto RHA or RPS scaffolds was collected after the third day of culture. Enzyme-linked immunosorbent assays (ELISAs) to determine the presence of IL-10 and IL-1β were performed according kit supplier’s protocols (PeproTech, Inc., Rocky Hill, NJ, for IL-10 and Beckon Dickinson for IL-1β).
Confocal Images
For confocal microscope examination, fluorescently labeled cells were visualized and photographed using a laser scanning confocal microscope (LSM780, Carl Zeiss).
Statistical Analysis
All the experiments were repeated at least three times with hADMSCc from three different donors. The materials were tested in parallel with the same batch of cells. The values are expressed as the mean ± standard error. The Student’s t-test was used to determine statistically significant differences between experimental and control samples. Analysis of variance followed by Tukey’s multiple comparison test were used for more than two populations. A p value less than or equal to 0.05 was considered significant.
Results
Phenotype Determination and Differentiation of hADMSC
Multiple studies have demonstrated the existence of a small population of mesenchymal stem cells in adipose tissue. The hADMSCs were obtained from lipoaspirates of patients undergoing cosmetic surgery under signed informed consent. We found that after passing adipose tissue through a size 4 needle, we obtained approximately five times the total number of cells than when we disaggregated the tissue using just a scalpel as it has been reported in previous study.16 Once the tissue was minced (in cases where adipose tissue was collected in big pieces), it was incubated with type I collagenase for 1 h at 37°C and 200 rpm shaking. One characteristic of MSCs is their ability to adhere to the culture dish surface; so we counted the number of cells and seeded them at 50,000 cells/cm2 during 24 h. Cells that did not attach to the culture dish were washed with PBS. Attached cells reached confluency about 1 week later. At the onset of the cultures, we observed cells with varying morphology; a portion of them had a spiculated shape like fibroblasts and others had a rounded morphology and formed colonies (Figure 1). During the culture, cells gradually acquired a fibroblast phenotype. As reported in other studies, MSCs express CD90 and CD73 but show negative expression of CD34 and CD45. We detected the expression of CD90 (84.8%), CD73 (99.9%), CD45 (0.15%), and CD34 (1.6%) in our cultures (Figure 1). These data along with the aforementioned cell morphology phenotypes suggest that we obtained a population of hADMSCs; this was further supported by other investigations that have proven the isolation of MSCs in adipose tissue.17 A third characteristic of MSCs is their multipotent capacity of differentiation and thus, as described in the Materials and Methods, we differentiated MSCs into chondrocytes, osteocytes, and adipocytes. To analyze the presence of chondrocytes after the differentiation process, we performed an immunofluorescence for type II collagen and alcian blue staining; for osteocytes, we analyzed the presence of Runx2 and performed Von Kossa staining. Finally, for adipocytes detection, we performed Oil red staining. Our data suggest that we have a population of hADMSCs in our cultures, and this population is capable of differentiating into other cell types (Figure 2).
Figure 1.
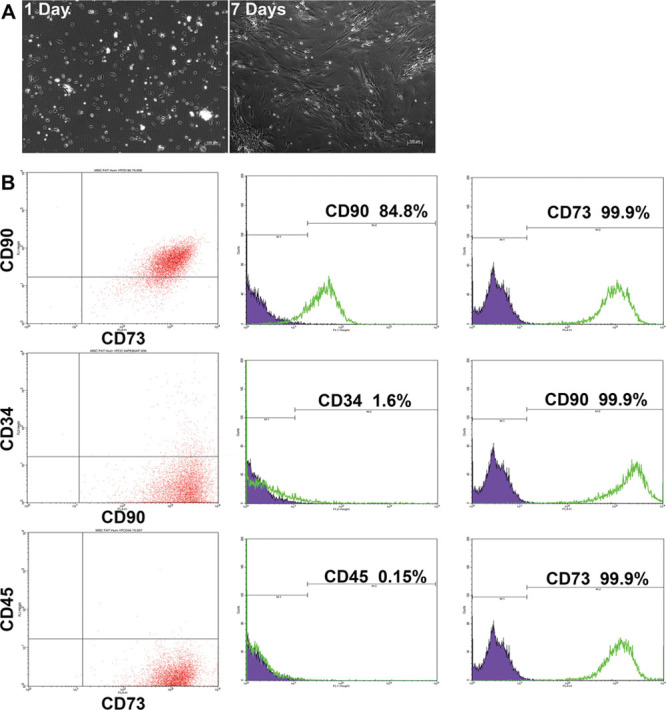
Immunophenotype of human adipose mesenchymal stem cells. A: Primary cultures on day 1 and 7 of adipose tissue cell isolation. B: Cell cytometry for mesenchymal stem cell markers: CD90 and CD73 co-expression (top), CD34 and CD90 expression (middle), and CD45 with CD73 (bottom).
Figure 2.
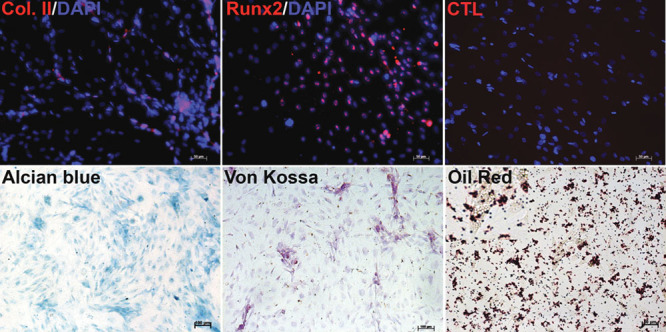
Human adipose-derived mesenchymal stem cells (hADMSCs) differentiation. hADMSCs were differentiated to chondrogenic lineage, and it was evaluated by type II collagen immunofluorescence (red) and alcian blue staining. For osteogenic differentiation, we analyzed Runx2 by immunofluorescences (red) and VonKossa staining. To observe adipogenic lineage, we performed Oil red staining. Nuclei were stained with 6-diamidine-2-phenylidole-dihydrochloride (blue).
hADMSCs Show High Viability in Constructs Made by RHA or RPS
Once the cells were isolated and characterized, we performed histological analysis of the RHA and RPS. These biomaterials have been used for a long time for treating burn patients without any reported problems caused by these wound dressings. Although both materials are pretreated with hypochlorite, they still contain a remaining population of cells from the epithelium in the case of the RHA or the complete epidermis (and a few cells from the dermis) in the case of the RPS (Figure 3). There were generated two constructs by seeding hADMSCs over RHA or RPS. We performed a viability test to confirm that mesenchymal cells isolated from adipose tissue remained viable and can be supported by the scaffolds. This test allows simultaneous determination of live and dead cells using two probes that measure recognized parameters of cell viability—intracellular esterase activity and plasma membrane integrity, using the calcein AM and EthD-1 as principal components. We compared both scaffolds by counting the number of living cells using the software Image J; the 10X and 20X fields and different focal planes were analyzed to count the cells in the whole area of the scaffold. Viability of mesenchymal cells seeded onto RHA was 92.7% and onto RPS was 90.5%. Despite the viability on both scaffolds being very similar, we observed that the number of cells supported by the RPS was larger than that of the RHA. This evidence suggests that this pair of wound dressings could work as good scaffolds and possess a potential application for future autologous or heterologous cell transplants in patients with burn injuries or for other skin problems such as ulcers.
Figure 3.
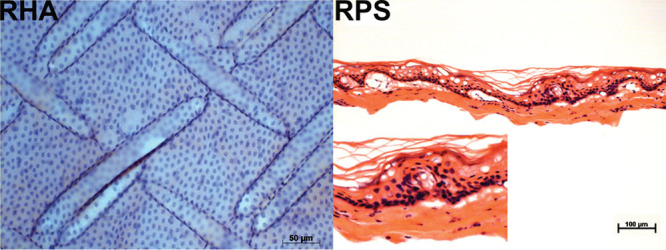
Human amnion and pig skin preserve cellular components after chlorine and radiosterilization treatment. Left, we observe the presence of nuclei in radiosterilized human amnion (RHA), and these nuclei should be the part of amnion epithelium. Right, we see the remnant nuclei of dermis and epidermis of radiosterilized pig skin (RPS).
Radiosterilized Human Amnion and Radiosterilized Pig Skin Promote Cell Proliferation in the Constructs
Previous investigations have described that human mesenchymal stem cells are multipotent, have the capacity to differentiate into various cell types, and possess an extensive self-renewal capacity in vitro.18 The nuclear protein Ki67 plays an important role during the replication phase of the cell cycle and has been a useful tool to evaluate cell proliferation.19 To analyze the self-renewal capacity of hADMSCs, a proliferation assay was performed to determine whether these cells were able to proliferate after 4 day culture onto radiosterilized human amniotic membrane or RPS. As determined, hADMSCs cultured onto RHA showed an increased proliferation (49.5%) when compared with those cultured onto RPS (27.7%). Cells clearly displayed a fluorescent signal inside the nuclei where Ki67 was actively participating in cell division (Figure 4). The increase in cell proliferation is a good parameter because we want to use these materials as carriers of autologous cells to promote new tissue formation in wounds.
Figure 4.
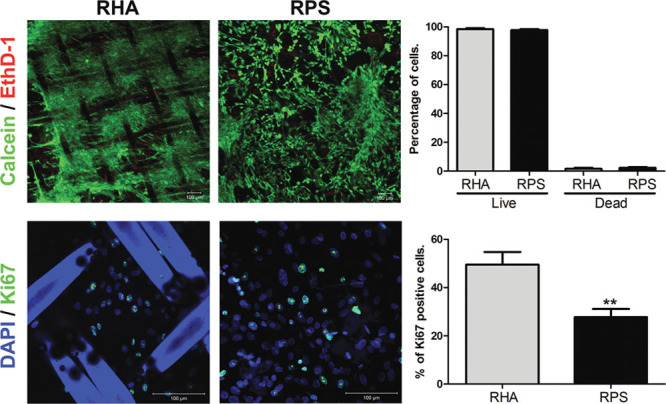
hADMSCs show high cell viability and proliferation when they are seeded onto RHA and RPS. Top images show viability assay where the live cells are stained with calcein (green) and dead cells are positive for ethidium homodimer-1 (red), and we did not find significant differences between RHA and RPS in this test. Bottom images show the presence of proliferation marker Ki67 (green) of hADMSCs seeded onto RHA or RPS. Cells growing on RHA have a greater proliferative capacity in comparison with RPS. Nuclei were stained with DAPI (blue). Student’s t-test, **p < 0.001. DAPI, 4′, 6-diamidine-2-phenylidole-dihydrochloride; hADMSCs, human adipose-derived mesenchymal stem cells; RHA, radio-sterilized human amnion; RPS, radio-sterilized pig skin.
Radiosterilized Human Amnion and Radiosterilized Pig Skin Allow Migration of hADMSCs
One of the integral characteristics of a good scaffold is that it should support cell migration, which is important for tissue organization. To analyze whether these biomaterials were good candidates with regard to cell migration, we captured time-lapse images using an Axio Observer microscope equipped with a CO2 incubation chamber. Cells were stained with DiI before they were cultured onto each material to identify them by fluorescence, (see Supplemental Digital Content 1, 2, and 3, http://links.lww.com/ASAIO/A70, http://links.lww.com/ASAIO/A71, http://links.lww.com/ASAIO/A72) then we measured how far the cells could displace within 6 h. The migration distance was quantified using the program AxioVision. The hADMSCs were capable of migrating over the surface of both biomaterials. We did not find significant differences between cell migration over these two scaffolds (Figure 4).
hADMSCs Secrete Interleukin-10 and Interleukin-1β When Seeded onto Radiosterilized Human Amnion and Radiosterilized Pig Skin
The regulation of inflammation is a very important process during wound healing. Different studies have shown that when inflammation is diminished, the healing is more akin to a regeneration event; and when inflammation is exacerbated, the healing can give rise to hypertrophic scars. However, it is still reported that without inflammation, the healing does not carry on.20,21 The MSCs typically secrete IL-10, which is a cytokine that negatively regulates inflammation22; conversely, IL-1β is one of the most important positive regulators of inflammation that determines whether wound healing will be exacerbated. We analyzed by ELISA whether our cells secrete these two important cytokines after they were seeded onto the scaffolds. We did not find significant differences in the concentration of IL-10 secreted by hADMSCs when they were seeded over RHA or RPS. Interestingly hADMSC secrete more IL-1β when they are grown on RPS rather than RHA (Figure 5). This result shows that RPS promotes IL-1β secretion, and this is possibly related to its xenogeneic origin.
Figure 5.
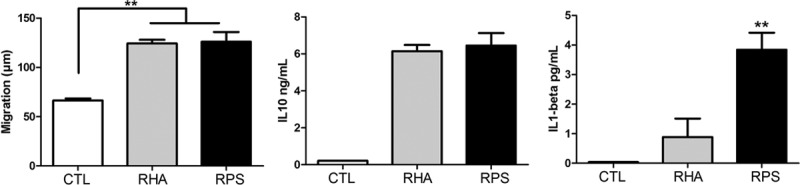
RHA and RPS scaffolds promote cell migration and cytokine release. Left graph shows differences in cell migration. RHA and RPS did not show a difference in migration distance; however, both induced more cell migration compared with cells grown over culture dish. Analysis of variance, Tukey test, **p < 0.001. Center graph reveals IL-10 release by hADMSCs in both biomaterials tested by enzyme-linked immunosorbent assay. There were no significant differences between groups. Right graph represents IL-1β concentrations in conditioned medium, and we found that RPS induced more secretion of this cytokine than RHA. ANOVA, Tukey test, **p < 0.001. CTL, Control; hADMSCs, human adipose-derived mesenchymal stem cells; IL, interleukin; RHA, radiosterilized human amnion; RPS, radiosterilized pig skin.
Discussion
During recent years, there has been increased interest in mesenchymal stem cell research from the field of clinical medicine. The advances in knowledge of MSC biology have generated an important evolution in our perception of their nature and in their clinical application. The advantages for the use of MSC are as follows: we could treat patients with their own cells; in patients with burn injuries, adipose tissue is more probable to remain than epidermis and dermis; MSC could differentiate into fibroblasts that are the principal cellular component in the dermis; they could differentiate to vascular lineages and promote the growth of new tissue; and finally their capacity to release IL-10 promote immunoregulation of the wounded area.23–26 Adipose tissue is a feasible source of hADMSCs because aesthetic surgeries are performed in high quantity and adipose tissue is considered biological waste. This kind of tissue is not only a good source to obtain a population of mesenchymal stem cells (with the appropriate legal documents and the consent of the patient); but also, adipose tissue is a great supply for autologous cell transplants as we propose here using RHA and RPS. Herein, we describe that when we isolate cells from adipose tissue derived from lipoaspirates by passing it through a size 4 needle, the quantity of cells was higher than when the tissue was disaggregated using only a scalpel. This result may occur because the needle helps to obtain tinier fragments that, in effect, increase the total surface area, making it easier for the collagenase to digest the extracellular matrix and release the cells. In our research, these cells can be cultured onto the scaffolds previously used as skin coverages, showing a good anchoring to the scaffolds and they also have high viability. In our cultures, cells from P1 formed small colonies and others were extended; however, after determinated passages, most of them tended to look like fibroblasts, which is a characteristic of MSCs. The presence of hADMSCs was corroborated using two important MSC surface markers: CD90 and CD73; we also observed a low percentage of CD34- and CD45-positive cells in flow cytometry assays. The capacity of our isolated cells to differentiate into chondrocytes, osteocytes, and adipocytes supports the idea that in the mixed cell population, we have a fraction of hADMSCs, and additional evidence shows that these cells are capable of releasing IL-10, which is also a property of MSC. We wanted to analyze the histology of the biomaterials before seeding the cells and we found that, as in many protocols, our procedure of dehydrating and radiosterilizing the human amnion and pig skin did not release the structural components of the cells. Some studies have shown that when biomaterials still contain cells, it is difficult for the new cells to adhere onto the scaffold, but even when there were cell remnants in both materials, hADMSCs were able to attach to them.27 Viability assays proved that hADMSCs could attach to both surfaces even in the presence of cellular remnants, and they also demonstrated that these remnants do not affect viability of the cells. By definition, MSCs are undifferentiated cells with the potential for long-term self-renewal, mainly influenced by growth factors28; however, it also has been described that matrix interactions are crucial for cell differentiation and proliferation.29 In our study, we report that hADMSCs isolated from liposuction aspirates possess the capacity to attach to the surface of the flask within 24 h. Our results demonstrate that interactions established between RHA and hADMSCs favored proliferation 4 days postisolation and suggest that the self-renewal capacity is maintained in these cells. Similar results have shown that porcine dermal matrix promotes the growth and differentiation of human keratinocytes,30 while Huang et al.31 used the amniotic membrane to promote the rapid expansion of epidermal keratinocytes. In our case, both biomaterials induced cell proliferation and cell migration, excellent features to function as cell carriers into skin wound sites; we may also be able to increase the bioactivity of these biomaterials by removing the remaining cellular components. Another study using a rat model has revealed that mesenchymal stem cells seeded onto porcine dermis were able to induce neovascularization.32 We know little about the mechanism by which both biomaterials induce the biological activity of hADMSCs seeded onto them; however, we believe it is because of the extracellular matrix structural proteins present in the original tissue. The list of proteins in the acellular dermis matrix includes type II, IV, and VII collagen; laminin; fibronectin; cytokines; vascular endothelial growth factor that can induce angiogenic activity; and tumor growth factor-β, a potent mitogen that can induce collagen production.33 The amniotic membrane includes proteins such as NGF, HGF, KGF, bFGF, TGF-β1, and EGF, and these proteins create a microenvironment niche for the stem cells similar to the native tissue.34 Many of these proteins can interact with cell membrane receptors and induce growth, proliferation, migration, adhesion, etc. Finally, cells were able to produce IL-10, which is important for diminishing inflammation, but surprisingly, RPS also induced the release of IL-1β that could be detrimental when found in increased amounts. This effect could be related to the presence of pig proteins that are contained in the cell remnants. Although it could be interpreted as a bad indicator, it is important to remember that there is also evidence showing the need to have inflammation to start a regenerative process.21 Another important note is that these biomaterials have been used in patients with burn injuries for a long time and are considered good apposite for healing wounds. For these reasons, our laboratory has started to analyze the effects of these skin substitutes using animal models. It will be important to further analyze how these substitutes and stem cells help to heal wounds caused by burns.
Conclusion
This work shows that these economical and accessible materials could represent a good option to carry out autologous cell transplants using hADMSCs, a tissue more likely to remain during burn incidents. These long-standing biomaterials could be promising scaffolds to be used with new treatments involving human adipose-derived mesenchymal stem cells.
Supplementary Material
Footnotes
Disclosure: The authors have no conflicts of interest to report.
This work was supported by SSA/IMSS/ISSSTE-CONACYT 161687 and 201836 and International Atomic Energy Agency (IAEA) through the IAEA research contract no. 18278/R0.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML and PDF versions of this article on the journal’s Web site (www.asaiojournal.com)
References
- 1.Yildirimer L, Thanh NT, Seifalian AM. Skin regeneration scaffolds: A multimodal bottom-up approach. Trends Biotechnol. 2012;30:638–648. doi: 10.1016/j.tibtech.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Greaves NS, Iqbal SA, Baguneid M, Bayat A. The role of skin substitutes in the management of chronic cutaneous wounds. Wound Repair Regen. 2013;21:194–210. doi: 10.1111/wrr.12029. [DOI] [PubMed] [Google Scholar]
- 3.Rao TV, Chandrasekharam V. Use of dry human and bovine amnion as a biological dressing. Arch Surg. 1981;116:891–896. doi: 10.1001/archsurg.1981.01380190029007. [DOI] [PubMed] [Google Scholar]
- 4.Colocho G, Graham WP, III, Greene AE, Matheson DW, Lynch D. Human amniotic membrane as a physiologic wound dressing. Arch Surg. 1974;109:370–373. doi: 10.1001/archsurg.1974.01360030022006. [DOI] [PubMed] [Google Scholar]
- 5.Song IC, Bromberg BE, Mohn MP, Koehnlein E. Heterografts as biological dressings for large skin wounds. Surgery. 1966;59:576–583. [PubMed] [Google Scholar]
- 6.Feng X, Shen R, Tan J, et al. The study of inhibiting systematic inflammatory response syndrome by applying xenogenic (porcine) acellular dermal matrix on second-degree burns. Burns. 2007;33:477–479. doi: 10.1016/j.burns.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Jiong C, Jiake C, Chunmao H, et al. Clinical application and long-term follow-up study of porcine acellular dermal matrix combined with autoskin grafting. J Burn Care Res. 2010;31:280–285. doi: 10.1097/BCR.0b013e3181d0f42d. [DOI] [PubMed] [Google Scholar]
- 8.Liechty KW, Kim HB, Adzick NS, Crombleholme TM. Fetal wound repair results in scar formation in interleukin-10-deficient mice in a syngeneic murine model of scarless fetal wound repair. J Pediatr Surg. 2000;35:866–872. doi: 10.1053/jpsu.2000.6868. discussion 872. [DOI] [PubMed] [Google Scholar]
- 9.Chavez-Munoz C, Nguyen KT, Xu W, Hong SJ, Mustoe TA, Galiano RD. Transdifferentiation of adipose-derived stem cells into keratinocyte-like cells: Engineering a stratified epidermis. PLoS One. 2013;8:e80587. doi: 10.1371/journal.pone.0080587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer LJ, McIlhenny S, Tulenko T, et al. Endothelial differentiation of adipose-derived stem cells: Effects of endothelial cell growth supplement and shear force. J Surg Res. 2009;152:157–166. doi: 10.1016/j.jss.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toda A, Okabe M, Yoshida T, Nikaido T. The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J Pharmacol Sci. 2007;105:215–228. doi: 10.1254/jphs.cr0070034. [DOI] [PubMed] [Google Scholar]
- 12.Debeer S, Le Luduec JB, Kaiserlian D, et al. Comparative histology and immunohistochemistry of porcine versus human skin. Eur J Dermatol. 2013;23:456–466. doi: 10.1684/ejd.2013.2060. [DOI] [PubMed] [Google Scholar]
- 13.Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008;15:88–99. doi: 10.22203/ecm.v015a07. [DOI] [PubMed] [Google Scholar]
- 14.Martínez-Pardo ME, Mariano-Magaña D. The tissue bank at the Instituto Nacional de Investigaciones Nucleares: ISO 9001:2000 certification of its quality management system. Cell Tissue Bank. 2007;8:221–231. doi: 10.1007/s10561-006-9031-y. [DOI] [PubMed] [Google Scholar]
- 15.Ley-Chávez E, Martínez-Pardo ME, Roman R, Oliveros-Lozano Fde J, Canchola-Martínez E. Application of biological dressings from radiosterilized amnios with cobalt 60 and serologic studies on the handling of burns in pediatric patients. Ann Transplant. 2003;8:46–49. [PubMed] [Google Scholar]
- 16.Vermette M, Trottier V, Ménard V, Saint-Pierre L, Roy A, Fradette J. Production of a new tissue-engineered adipose substitute from human adipose-derived stromal cells. Biomaterials. 2007;28:2850–2860. doi: 10.1016/j.biomaterials.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 17.Strem BM, Hicok KC, Zhu M, et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54:132–141. doi: 10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- 18.Sarugaser R, Hanoun L, Keating A, Stanford WL, Davies JE. Human mesenchymal stem cells self-renew and differentiate according to a deterministic hierarchy. PLoS One. 2009;4:e6498. doi: 10.1371/journal.pone.0006498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlüter C, Duchrow M, Wohlenberg C, et al. The cell proliferation-associated antigen of antibody Ki-67: A very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle-maintaining proteins. J Cell Biol. 1993;123:513–522. doi: 10.1083/jcb.123.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christian LM, Graham JE, Padgett DA, Glaser R, Kiecolt-Glaser JK. Stress and wound healing. Neuroimmunomodulation. 2006;13:337–346. doi: 10.1159/000104862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: Molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 22.Liu WH, Liu JJ, Wu J, et al. Novel mechanism of inhibition of dendritic cells maturation by mesenchymal stem cells via interleukin-10 and the JAK1/STAT3 signaling pathway. PLoS One. 2013;8:e55487. doi: 10.1371/journal.pone.0055487. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Falanga V, Iwamoto S, Chartier M, et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13:1299–1312. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- 24.Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003;139:510–516. doi: 10.1001/archderm.139.4.510. [DOI] [PubMed] [Google Scholar]
- 25.Dabiri G, Heiner D, Falanga V. The emerging use of bone marrow-derived mesenchymal stem cells in the treatment of human chronic wounds. Expert Opin Emerg Drugs. 2013;18:405–419. doi: 10.1517/14728214.2013.833184. [DOI] [PubMed] [Google Scholar]
- 26.Fibbe WE, Nauta AJ, Roelofs H. Modulation of immune responses by mesenchymal stem cells. Ann N Y Acad Sci. 2007;1106:272–278. doi: 10.1196/annals.1392.025. [DOI] [PubMed] [Google Scholar]
- 27.Tauzin H, Rolin G, Viennet C, Saas P, Humbert P, Muret P. A skin substitute based on human amniotic membrane. Cell Tissue Bank. 2014;15:257–265. doi: 10.1007/s10561-014-9427-z. [DOI] [PubMed] [Google Scholar]
- 28.Danišovič L, Varga I, Polák S. Growth factors and chondrogenic differentiation of mesenchymal stem cells. Tissue Cell. 2012;44:69–73. doi: 10.1016/j.tice.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 30.Zajicek R, Mandys V, Mestak O, Sevcik J, Königova R, Matouskova E. Human keratinocyte growth and differentiation on acellular porcine dermal matrix in relation to wound healing potential. ScientificWorldJournal. 2012;2012:727352. doi: 10.1100/2012/727352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang G, Ji S, Luo P, et al. Accelerated expansion of epidermal keratinocyte and improved dermal reconstruction achieved by engineered amniotic membrane. Cell Transplant. 2013;22:1831–1844. doi: 10.3727/096368912X657945. [DOI] [PubMed] [Google Scholar]
- 32.Mestak O, Matouskova E, Spurkova Z, et al. Mesenchymal stem cells seeded on cross-linked and noncross-linked acellular porcine dermal scaffolds for long-term full-thickness hernia repair in a small animal model. Artif Organs. 2014;38:572–579. doi: 10.1111/aor.12224. [DOI] [PubMed] [Google Scholar]
- 33.Hoganson DM, O’Doherty EM, Owens GE, et al. The retention of extracellular matrix proteins and angiogenic and mitogenic cytokines in a decellularized porcine dermis. Biomaterials. 2010;31:6730–6737. doi: 10.1016/j.biomaterials.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 34.Ji SZ, Xiao SC, Luo PF, et al. An epidermal stem cells niche microenvironment created by engineered human amniotic membrane. Biomaterials. 2011;32:7801–7811. doi: 10.1016/j.biomaterials.2011.06.076. [DOI] [PubMed] [Google Scholar]


