Inflammatory monocytes recruited into chronically inflamed intestines become immunosuppressive, inhibit T cell activation and polarization toward Th1 phenotype, but promote generation of regulatory T cells.
Keywords: colitis, Ly6Chigh, suppression, nitric oxide, interferon-γ, polarization, Th1, Th17, regulatory T cell
Abstract
Chronic colitis is accompanied by extensive myelopoiesis and accumulation of CD11b+Gr-1+ cells in spleens and secondary lymphoid tissues. Although cells with similar phenotype have been described in cancer, chronic infection, or autoimmunity, where they were associated with suppression of T cell responses, little is known regarding how these cells affect CD4 T cell responses in the context of chronic intestinal inflammation. Therefore, we undertook this study to characterize the interplay between colitis-induced myeloid cells and CD4 T cell. Within the CD11b+Gr-1+ population, only monocytes (Ly6GnegLy6Chigh) but not other myeloid cell subsets suppressed proliferation and production of cytokines by CD4 T cells. Suppression was mediated by cell-contact, NO and partially by IFN-γ and PGs. Interestingly, Ly6Chigh MDCs, isolated from colitic colons, showed up-regulation of iNOS and arginase-1 and were more potent suppressors than those isolated from spleen. On a single-cell level, MDCs inhibited Th1 responses but enhanced generation of foxp3+ T cells. MDCs, cocultured with activated/Teffs, isolated from inflamed colons under hypoxic (1% O2) conditions typical for the inflamed intestine, suppressed proliferation but not their production of proinflammatory cytokines and chemokines. Taken together, expansion of monocytes and MDCs and activation of their suppressive properties may represent a homeostatic mechanism aimed at restraining excessive T cell activation during chronic inflammatory settings. The contribution of immunosuppressive monocytes/MDCs to chronic colitis and their role in shaping T cell responses in vivo require further investigation.
Introduction
IBDs are chronic, relapsing diseases of the gastrointestinal tract of unknown etiology [1, 2]. IBD is thought to results from a dysregulated immune response to commensal enteric antigens, where T cells have emerged as central players in the disease pathogenesis [1, 3]. Besides T cells, myeloid cells (e.g., monocytes, neutrophils, etc.) are also found in significant numbers infiltrating inflamed regions of the intestine. However, the role that these cells play in disease pathogenesis is poorly defined.
Recruitment of myeloid cells from blood into tissues is accompanied by their differentiation, which changes their phenotype and function. Recent work determined that tissue macrophages and DCs are constantly replenished by their unique circulating precursors. Monocytes differentiate into tissue macrophages, whereas pre-DCs give rise to several subsets of intestinal DCs [4–7]. Under steady-state, mononuclear intestinal phagocytes display a tolerogenic phenotype and respond poorly to stimulation by bacterial antigens [8, 9]. Work by Rivollier et al. [10] showed that under noninflammatory conditions, circulating monocytes (CD11b+Ly6Chigh cells) differentiated into intestinal CX3CR1high macrophages/DCs with immunoregulatory properties. Complementary studies by Zigmond et al. [11], Tamoutounour et al. [12], and Bain et al. [13] found that in normal, noninflamed intestines, monocytes became TLR-hyporesponsive, IL-10-producing tolerogenic macrophages.
We [14] and others [15] showed that development of colitis in mice is associated with a dramatic myelopoiesis and expansion of CD11b+Gr-1+ myeloid cells in BM, spleen, MLNs, and colons. Cotransfer of colitis-induced splenic CD11b+Gr-1+ cells attenuated inflammation in autoimmune CD8-mediated chronic colitis [16] and DSS-induced acute colitis [17]. Treatment of IL-10-deficient mice with resveratrol attenuated development of intestinal inflammation [18] and correlated with expansion of myeloid cells with suppressive properties. Depletion of myeloid cells using anti-Gr-1 antibody exacerbated intestinal inflammation in mice, which led to higher mortality [19–21], suggesting that myeloid cells play a protective role. These latter results, however, must be interpreted with caution, as the anti-Gr-1 antibody (clone RB6-8C5) used in these studies also affects Ly6C-bearing cells, including monocytes, neutrophils, and T cells [22]; therefore, such depletion studies cannot be interpreted conclusively. Although these studies suggest that myeloid cells during chronic colitis may be protective, which specific population is endowed with suppressive properties remains unclear.
Accumulating evidence suggests that the infiltrating myeloid cells in IBD play a dual role in inflammation. Delayed apoptosis in neutrophils, following their exposure to inflammatory cytokines, which inhibits their apoptosis, acquisition of antigen-presenting capacity by these cells [14], and transepithelial migration of neutrophils into the crypts, disrupting epithelial barrier [23, 24], suggests that neutrophils contribute to chronic inflammation. On the other hand, infiltrating neutrophils create an hypoxic environment, which activates hypoxia-inducible factor 1-mediated pathways and contributes to intestinal healing, indicating that neutrophils may also serve a protective role in IBD [25, 26]. Monocytes recruited to the inflamed colons differentiated into proinflammatory macrophages/DCs [10, 12, 13] by differentiating into iNOS-positive macrophages that secreted inflammatory cytokines in response to TLR stimulation [10, 12, 13]. Although these studies suggested that monocytes may contribute to chronic inflammation, this conclusion was made largely based on in vitro studies or cytokine gene expression data. On the other hand, monocytes have been shown to attenuated neutrophil activation via the PG-dependent mechanism [27]. Moreover, monocytes recruited to the injured tissue contribute to repair and healing [28, 29]. Therefore, the role of monocytes, neutrophils, and other myeloid cells in IBD is complex, poorly understood, and requires further investigations.
In our current work, with the use of a model of chronic colitis, we examined more closely the cross-talk between different subsets of CD11b+Gr-1+ myeloid cells and T cells. We found that colitis-induced monocytes acquired regulatory properties and suppressed T cell responses in vitro. We also found that monocytes and MDCs play a contributing role in shaping T cell polarization. Collectively, our results suggest that expansion of monocytes that are capable of controlling T cell responses may represent an important homeostatic mechanism aimed at restraining excessive T cell activation during chronic colitis.
MATERIALS AND METHODS
Animals
WT mice, RAG-1−/− (B6.129S7-Rag1tm1Mom/J) mice, and OVA-specific B6.Cg-Tg(TcraTcrb)425Cbn/J (OT2) mice, all 8–12 weeks of age on a C57Bl/6 background, were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). TNFΔARE mice have been described [30]. Sixteen-week-old male and female TNFΔARE/WT and age- and sex-matched TNFWT/WT or WT littermate control mice were used for analysis and cell isolation. Animals were maintained on 12-h/12-h light/dark cycles in standard animal cages with filter tops under specific pathogen-free conditions in our animal care facility at LSUHSC-S and were given standard laboratory rodent chow and water ad libitum. All experimental procedures involving the use of animals were reviewed and approved by the Institutional Animal Care and Use Committee of LSUHSC-S and Case Western Reserve University and performed according to the criteria outlined by the U.S. National Institutes of Health.
Antibodies and reagents
Antibodies were purchased from BD PharMingen (San Diego, CA, USA), eBioscience (San Diego, CA, USA), or AbD Serotec (Raleigh, NC, USA). Negative selection kits for isolation of mouse CD4+ T cells were purchased from Stemcell Technologies (Vancouver, BC, Canada). For proliferation assays, cells were cultured in RPMI-1640 medium (Gibco, Life Technologies, Grand Island, NY, USA), supplemented with 10% FBS, β-ME, glutamine, and antibiotic/antimycotic.
Induction of intestinal inflammation
Chronic colitis was induced by the transfer of sorted WT CD4+CD45RBhigh T cells into RAG-1−/− mice using our previously published method [31]. Clinical evidence of disease (e.g., body weight loss and loose stool/diarrhea) was followed and recorded weekly from the time of the injection.
Acute colitis was induced by supplementing drinking water with 3% DSS continuously for 7 days. The following parameters were recorded daily: weight loss, stool form, occult blood, and liquid consumption. On Day 7, mice were killed for cell isolation and flow cytometry analysis.
Histopathology
A piece of proximal and distal colon was fixed in 10% phosphate-buffered formalin and processed for H&E staining. Blinded histopathological scoring of each sample was performed by an experienced pathologist using our criteria published previously [32].
Tissue lymphocyte analyses
Lymphocytes were obtained from the spleen, MLNs, and cLP and analyzed by flow cytometry, as described previously [32]. Cells from BM were obtained from femurs and tibias and passed through a 70-μ cell strainer. For analysis, ∼106 cells were surface-stained with the indicated antibodies and then fixed for 20 min on ice in 2% ultrapure formaldehyde (Polysciences, Warrington, PA, USA) and analyzed on FACSCalibur or BD LSR II (BD Biosciences, San Jose, CA, USA). All data were analyzed using FlowJo software (Version 7.2.5 for PC; Tree Star, Ashland, OR, USA).
Leukocyte quantification in the blood
Reconstituted RAG-1−/− mice were bled once/week after CD45RBhigh transfer for 8 weeks. Approximately 75 μl blood was collected from tail vein in heparinized microhematocrit capillary tubes (Thermo Fisher Scientific, Pittsburgh, PA, USA). Red blood cells were lysed using hypotonic buffer, washed and stained with anti-CD11b-FITC (clone M1/70), anti-Gr-1-PE (RB6-8C5), anti-CD4-PerCP (RM4.5), and anti-Ly6C-APC (AL-21). CountBright absolute counting beads (Invitrogen, Life Technologies, Grand Island, NY, USA) were added before acquisition on FACSCalibur (BD Biosciences), according to the manufacturer's instructions. The total cell number/ml blood was calculated as follows, according to the manufacturer's instructions: (number of total events×number of beads added)/(number of bead events×blood volume). Cell number for specific population was calculated as (total number of cells/μL blood) × (frequency of total cells, as determined by flow cytometry analysis).
Myeloid cell isolation and sorting
Mice with macroscopic colitis scores of at least 2 (on a 0–4 scale) [31], corresponding to moderate/severe colitis, were used as donors. Cells from spleens and cLP were isolated, as described above, and pooled from at least three mice. Cells were stained with anti-CD11b, Ly6C, Ly6G (1A8), and where indicated, Dectin-1 (2A11) or Ly-6B.2 (7/4), followed by FACS using the FACSAria cell sorter (BD Biosciences) into CD11b+Ly6ChighLy6G−, CD11b+Ly6Cneg/lowLy6G−, and CD11b+Ly6CintLy6G+ populations.
Proliferation assays
For an antigen-specific proliferation assay, 100,000 total OT2 splenocytes were cocultured in triplicate with sorted myeloid cells in the presence of 10 μg/ml OVA peptide (323–339; AnaSpec, San Jose, CA, USA). For some experiments, CD4 OT2 T cells were flow-purified from spleens of OT2 mice by sorting for (CD8, CD11c, B220, CD11b)neg CD4+ cells. For antigen-nonspecific proliferation assays, 50,000 negatively selected WT CD4+ T cells (>90% purity) were cultured in triplicate with sorted myeloid cells in a 96-well, anti-CD3 antibody, precoated, flat-bottom plates, plus soluble anti-CD28 antibody. The following inhibitors and blocking antibodies were added in the beginning of culturing: L-NIL (0.01–0.5 mM final; Calbiochem, Billerica, MA, USA), L-NMMA (0.1–0.5 mM final; Calbiochem), nor-NOHA (0.5 mM final; Calbiochem), N-(3-aminomethyl)-benzylacetamidine (1400 W, 0.01–0.5 mM final; Calbiochem), catalase (1000 U/mL final; Sigma-Aldrich, St. Louis, MO, USA), anti-IFN-γ (clone XMG1.2, 10 μg/ml final; eBioscience), anti-IL-10 (clone JES5-16E3, 10 μg/ml final; eBioscience), NAC (0.5 mM; Sigma-Aldrich), 1.15 mM L-arginine (Sigma-Aldrich), indomethacin (10μM; Cayman Chemicals, Ann Arbor, MI, USA), Zn (II) PPIX (1μM; Calbiochem), IDO inhibitor (CAY10581, 100 μM; Cayman Chemicals), and ODQ (10μM; Cayman Chemicals). Cells were pulsed with 1 μCi 3H-thymidine (PerkinElmer, Waltham, MA, USA) for the last 8 h of a 72-h culture and harvested onto fiberglass filters. Radioactivity was measured using the liquid scintillation counter Wallac 1409 (PerkinElmer). For the transwell experiments, cells were separated using a 0.4-μm pore-size Millicell, 96-well, cell-culture device (Millipore, Billerica, MA, USA).
RNA isolation and RT-PCR
FACS-purified spleens and cLP CD11b+Ly6G−Ly6Chigh cells were processed for RNA isolation using the RNeasy Mini kit (Qiagen, Valencia, CA, USA), according to the manufacturer's instructions, and treated with DNase I. RNA concentrations and purity were assessed using the NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific). Approximately 50 ng was reversely transcribed with qScript cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD, USA), according to the manufacturer's instructions. PCR reactions were carried out in a total volume of 25 μl using 2720 Thermo Cycler (Applied Biosystems, Life Technologies, Grand Island, NY, USA) with primers specific for β-actin, iNOS, and arginase-1. Primer sequences for β-actin and iNOS were obtained from Sato et al. [33] and for arginase-1, from Kitowska et al. [34]. Amplicons of expected length were resolved on a 1.5% agarose gel.
Cytokine and NO measurements in culture SNs
For cytokine analysis, SNs from replicate wells were collected after 24 h or 48 h, pooled where indicated, and frozen at −80°C. Cytokine concentrations were measured using Mouse Cytokine/Chemokine Immunoassay multiplex kits (Millipore), according to the manufacturer's instructions. Samples were run on a Bio-Plex Luminex instrument (Bio-Rad Laboratories, Hercules, CA, USA), and data were analyzed using Milliplex Analyzer 3.1 xPonent software (Millipore).
For NO determination, SNs were collected from wells containing T cells and Ly6Chigh cells after 64 h of coculture. Samples were mixed with sample storage buffer [1×PBS containing potassium ferricyanide (800 mM), N-ethylmaleimide (17.6 mM), and 6% IGEPAL CA-630] at a ratio of 5:1 and stored at −80°C. Before the analysis, samples were thawed on ice, and 10 μl of the sample was analyzed using Sievers 280i NO analyzer (GE Analytical Instruments, Boulder, CO, USA), according to the manufacturer's instructions. The instrument was calibrated with sodium nitrite standard solution before use.
Cytospin
Sorted cells (104–105) were cytospun onto poly-L-lysine-coated microscope slides, air-dried, and stained with Diff-Quik stain set (Siemens Healthcare Diagnostics, Tarrytown, NY, USA), according to the manufacturer's protocol. The slides were mounted using Permount mounting agent (Thermo Fisher Scientific). Images were taken using a Nikon Eclipse E600 microscope, equipped with a Nikon DS-Fi2 camera.
Statistics
Data are presented as mean ± sem. Statistical significance between more than two groups was evaluated using a one-way ANOVA. Statistical significance between selected groups was evaluated using Dunnett's post hoc test. A probability of P < 0.05 was considered significant.
RESULTS
Development of colitis is accompanied by expansion of myeloid cells in blood, lymphoid, and peripheral tissues of colitic mice
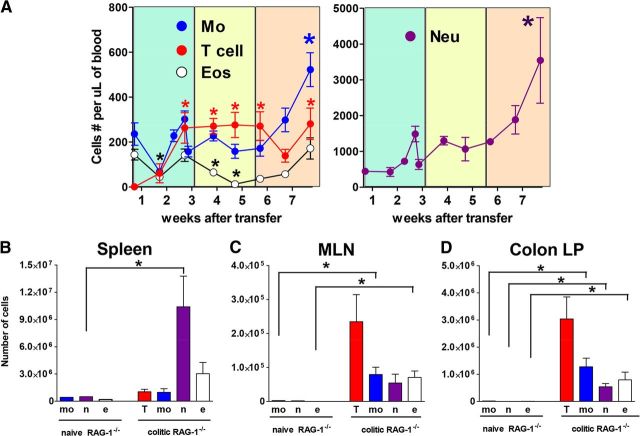
Using multiparameter flow cytometry, cell sorting, and morphological analysis of sorted cells with cytospin and Diff-Quik staining, we were able to distinguish neutrophils (CD11b+Ly6G+Ly6CintDectin-1intLy-6B.2intSSChigh), monocytes (CD11b+Ly6Gneg Ly6ChighDectin-1highLy-6B.2highSSClow), and eosinophils (CD11b+Ly6Glow/negLy6ClowDectin-1lowLy-6B.2lowSSCvery high) within the CD11b+Gr-1+ population in mice with chronic colitis (Supplemental Fig. 1). With the use of these markers, we found that circulating levels of monocytes, neutrophils, and T cells increased as intestinal inflammation progressed with neutrophils and monocytes in colitic mice, increasing approximately eight- and twofold, respectively, by 8 weeks (Fig. 1A). In addition, colitic mice showed dramatic accumulation of myeloid cells in spleens, MLNs, and colons (Fig. 1B–D). Development of colitis was accompanied by increases in plasma levels of myelopoietic cytokines, including G-CSF, IL-1β, IL-6, and IL-17, which corroborated with expansion of granulocytes in colitic mice (Supplemental Fig. 2). Although GM-CSF has been implicated in the development of chronic colitis [15], levels of this cytokine were only modestly increased in colitic mice compared with those that did not develop colitis. Levels of IFN-γ and several chemokines, including those induced by IFN-γ, were also increased, including CXCL10 (IFN-γ-induced protein 10), CCL5 (RANTES), and CXCL9 (monokine induced by IFN-γ). Taken together, development of colitis in mice was accompanied by myelopoiesis and accumulation of myeloid cells in lymphoid and nonlymphoid tissues.
Figure 1. Development of colitis is accompanied by accumulation of circulating and tissue-associated myeloid cells.
(A) Time-course showing appearance of CD4 T cells, neutrophils (Neu; CD11b+Ly6CintLy6G+), monocytes (Mo; CD11b+Ly6ChighLy6G−), and eosinophils (Eos; CD11b+Ly6Clow/negLy6Glow/negSSChigh) in blood following reconstitution with naive T cells. Myeloid cells were identified, as shown in Supplemental Fig. 1; quantification of leukocytes in nonreconstituted and colitic RAG-1−/− mice. Nonpooled individual tissues from mice with colitis were analyzed using flow cytometry, as described in Materials and Methods in spleen (B), MLN (C), and cLP (D). Shown are averaged absolute numbers for five individual mice from a representative experiment. For all graphs, bars show se; significant difference (*P<0.05) in cell levels compared with Day 0. T, T cells; mo, monocytes; n, neutrophils; e, eosinophils.
Immunosuppressive CD11b+Ly6GnegDectin-1+Ly6Chigh cells accumulate in colitic RAG-1−/− mice
Phenotype of CD11b+Ly6GnegLy6Chigh monocytes (hereafter abbreviated as Ly6Chigh) isolated from colitic mice was consistent with that of classical inflammatory monocytes [35], as well as with M-MDSCs, described in tumor-bearing mice [36]. In addition, cells with similar phenotype have been described in autoimmune mouse models [37, 38], where these cells suppressed T cell proliferation and cytokine production. To investigate whether colitis-induced Ly6Chigh cells are immunosuppressive, we purified them from spleen by FACS and cocultured with OVA-specific CD4 T cells in the presence of OVA peptide or with WT CD4 T cells activated using antibodies to CD3/CD28. Only Ly6Chigh cells suppressed T cell proliferation, regardless of the method used for T cell activation (Fig. 2A and B), whereas other myeloid cells were not suppressive (ref. [14], and data not shown). Noteworthy is that suppression was only observed at a 1:1 ratio of T cells:monocytes, whereas when a low number of these cells were added, we observed enhanced proliferation. Also, suppression by Ly6Chigh cells was reversed when an iNOS-specific inhibitor (1400 W) was added or by immunoneutralization of IFN-γ but not when the inhibitor of arginase-1 (nor-NOHA) or excess of L-arginine was added (Fig. 2C).
Figure 2. Suppression of T cell proliferation by spleen Ly6Chigh cells isolated from colitic mice.
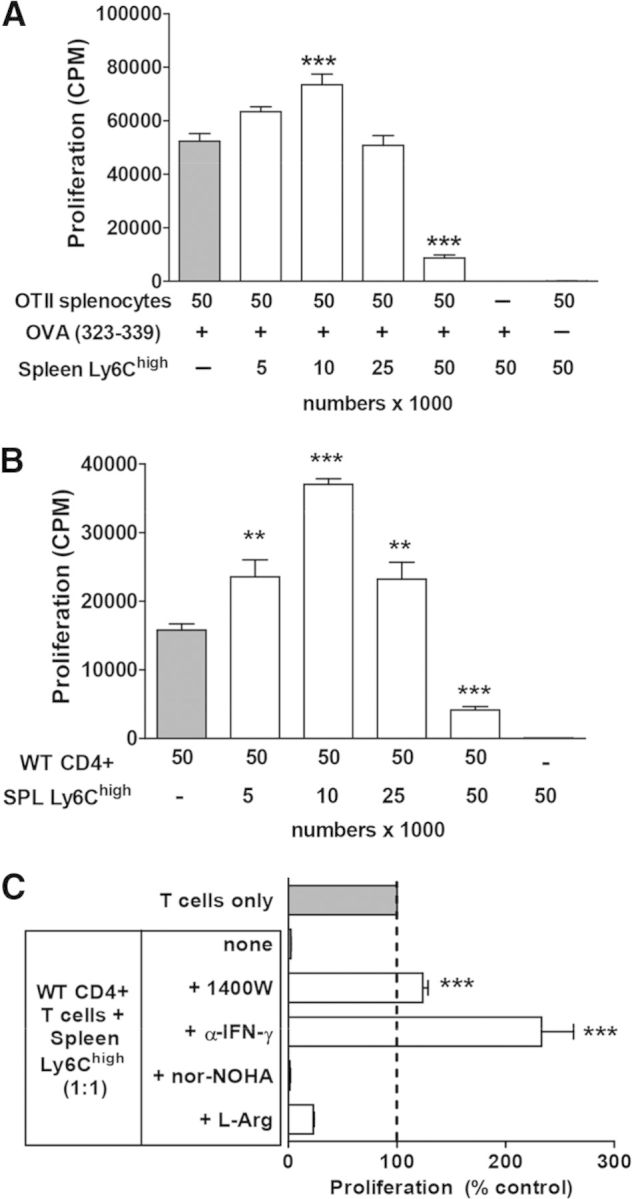
(A) Proliferation data for OT2 (OTII) splenocytes cultured alone (gray bar) or with the indicated number of sorted splenic CD11b+Ly6Chigh cells isolated from colitic mice. Numbers indicate number of cells added × 1000. Ly6Chigh cells were not irradiated before culture. OVA peptide was added at 10 μg/ml final, as indicated. (B) Suppression of WT CD4+ T cell proliferation stimulated with anti-CD3/CD28 antibodies by spleen (SPL) Ly6Chigh cells. Proliferation data are expressed as mean cpm from triplicate wells ± sem. Results from a representative experiment, which was repeated at least three times, are shown. (C) Spleen Ly6Chigh cells suppress proliferation of antibody-stimulated WT T cells in a NO- and IFN-γ-dependent manner. Data are expressed as percentage of T cell proliferation cultured alone (T cells only). Significant differences from T cells only (gray bars) are shown where applicable; **P < 0.01, and ***P < 0.001. L-Arg, L-Arginine.
CD11b+Dectin-1+Ly6GnegLy6Chigh cells were also readily identifiable in the inflamed colons and the MLNs in colitic mice (Supplemental Fig. 1). Addition of cLP Ly6Chigh cells to OT2 splenocytes stimulated with OVA peptide or to WT T cells stimulated with anti-CD3/CD28 antibodies resulted in a dose-dependent suppression of T cell proliferation (Fig. 3A and B). Similar results were obtained with flow-purified CD4 T cells from OT2 mice (Supplemental Fig. 3), suggesting that Ly6Chigh cells suppressed CD4 T cells directly. In agreement with our earlier report [14], neutrophils and Ly6G−Ly6Clow/neg cells did not show immunosuppressive properties but on the contrary, enhanced antigen-specific proliferation of OT2 CD4 T cells (Supplemental Fig. 3).
Figure 3. Mechanisms of T cell suppression by cLP MDCs isolated from colitic mice.
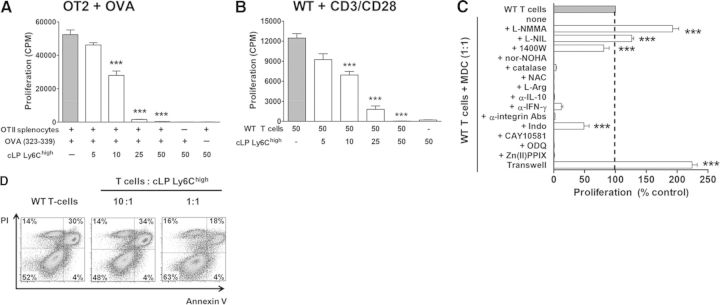
Proliferation of OT2 splenocytes stimulated with OVA peptide (A) or WT CD4+ T cells stimulated with anti-CD3/CD28 antibodies (B). Gray bars show proliferation of T cells cultured alone. Proliferation data are expressed as mean cpm from triplicate wells ± sem. Results from a representative experiment, which was repeated at least three times, are shown; ***P < 0.001. (C) WT T cell proliferation was reversed by inhibition of iNOS (with L-NMMA, L-NIL, 1400 W) and partially reversed by inhibitors of COX-1/2 [indomethacine (Indo)]. Inhibition of arginase-1 (nor-NOHA) and addition of catalase, NAC, L-arginine, or neutralizing antibodies to IFN-γ, CD11a, CD11b, or CD18 did not affect MDC-mediated suppression. IDO-1 (CAY10581), HO-1 [Zn(II)PPIX], and soluble guanylyl cyclase (ODQ) reagents were added in the beginning of the 72-h culture. Data are expressed as percentage of proliferation compared with T cells alone (T cells only). ***Significant differences from T cells cultured with Ly6Chigh cells at 1:1 ratio (none). (D) Viability of WT CD4+ T cells after 72 h of culturing alone or with cLP MDCs was assessed by annexin-V and propidium iodide (PI) analysis. Dot plots were gated on CD4+ T cells.
To dissect the underlying mechanisms of suppression by Ly6Chigh cells, we added pathway-specific inhibitors or blocking antibodies to their cocultures with T cells. In agreement with data obtained with spleen Ly6Chigh cells, suppression by the same subset of cells isolated from the cLP was independent of arginase-1 but required functional iNOS (Fig. 3C). However, in contrast to the results obtained with spleen cells, addition of IFN-γ-neutralizing antibody did not reverse suppression by cLP cells. Moreover, addition of catalase NAC, L-arginine to the cocultures, did not reverse suppression, indicating that suppression does not involve production of ROS or amino acid depletion. T cell proliferation was partly restored by the nonselective inhibitor of COX-1/2, indomethacin, whereas the blocking of HO-1 or IDO had no effect on the reversal of suppression. Interestingly, although production of NO appeared to be critical for the suppression, it was independent of soluble gyanylyl cyclase but required close cellular contact, as separation of T cells and Ly6Chigh cells by semipermeable membrane completely abrogated suppression (Fig. 3C). To determine whether engagement of surface integrins may be important for this, we added different β2 integrin-blocking antibodies, individually or in combination. None of these antibodies, however, abrogated the suppressive activity of Ly6Chigh cells. Lastly, viability of T cells was improved drastically in the presence of myeloid cells (Fig. 3D), indicating that inhibition of proliferation was not a result of induction of T cell apoptosis.
In addition to the T cell transfer model of colitis, we analyzed accumulation of myeloid cells in two other models of colitis: acute model induced by administration of DSS and TNFΔARE model of chronic ileitis [30]. Development of intestinal inflammation in these models was accompanied by massive accumulation of CD11b+Ly6GnegLy6Chigh cells in spleens and inflamed intestines (Supplemental Fig. 4), suggesting that accumulation of cells, phenotypically resembling immunosuppressive Ly6Chigh cells, is not unique to the T cell-induced colitis. Taken together, development of colitis in mice is accompanied by the accumulation of Ly6Chigh myeloid cells that suppressed T cell proliferation in an iNOS-, IFN-γ-, COX-1/2-, and cell contact-dependent manner, without inducing their apoptosis.
Recruitment of Ly6Chigh cells to the inflamed colons triggers their maturation and augments their immunosuppressive properties
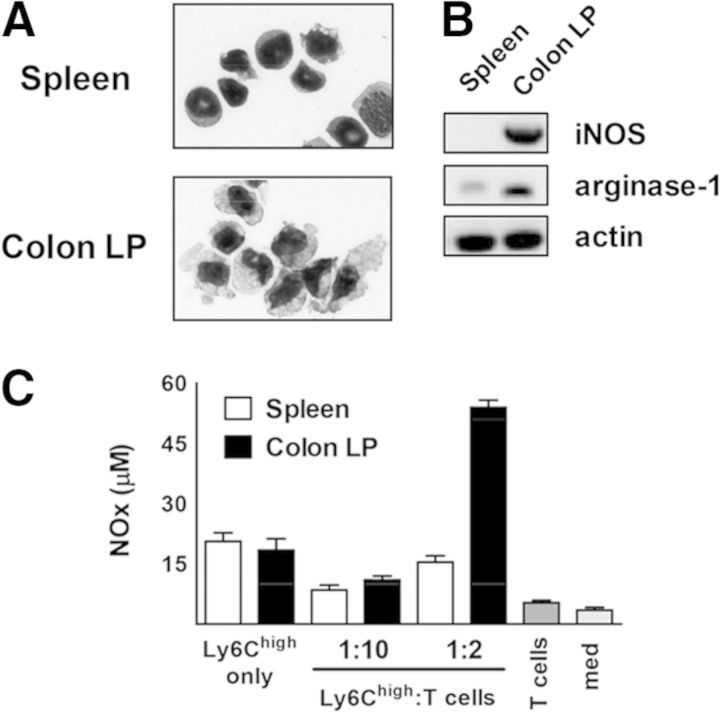
The Ly6Chigh population in spleen consisted of mature monocytes and their precursors (Fig. 4A), whereas the same population in the cLP had only mature cells with a large cytoplasm:nucleus ratio and uneven “ruffled” borders. On a cell-to-cell basis, cLP Ly6Chigh MDCs were more potent suppressors than Ly6Chigh monocytes isolated from spleen. iNOS and arginase-1 are two main enzymes implicated in the suppressive activity by M-MDSC [36]. mRNA levels for iNOS and arginase-1 were substantially higher in MDCs compared with spleen monocytes (Fig. 4B). Moreover, when cocultured with T cells, MDCs produced almost three times more NO (Fig. 4C).
Figure 4. cLP MDCs are more mature than spleen Ly6Chigh cells and produce more NO when cocultured with activated T cells.
(A) Representative Diff-Quik-stained cytospin images of spleen and cLP Ly6Chigh cells were taken at 1000× magnification. (B) Expression of iNOS and arginase-1 in sorted cells was analyzed by semiquantitative RT-PCR. (C) NO (NOx) levels in the SN from cocultures between monocytes/MDCs and T cells were measured, as described in Materials and Methods.
Spleen monocytes expressed M-CSFR (CD115) and IL-4Rα (CD124), whereas cLP cells showed low levels of CD124 and were CD115neg, which was consistent with their mature morphology. However, both populations had minimal or low expression of MHC-II, F4/80, CD11c, or CD80/86 (Fig. 5A, and data not shown). Fixation of cells, but not blocking of MHC-II, abrogated the ability of MDCs to suppress (Fig. 5B). Inhibitory molecules of the B7 family (PD-L1 and PD-L2) play an important role in regulating T cell responses by myeloid cells in vivo and in vitro [39, 40]. Despite prominent expression of both of these molecules on MDCs, addition of blocking antibodies to PD-L1 or PD-L2 did not reverse their ability to suppress T cells (Fig. 5C). Taken together, recruitment of inflammatory monocytes into inflamed colons is accompanied by their maturation and augmentation of their suppressive properties.
Figure 5. Suppression by cLP Ly6Chigh does not require engagement of MHC-II, PD-L1, or PD-L2.
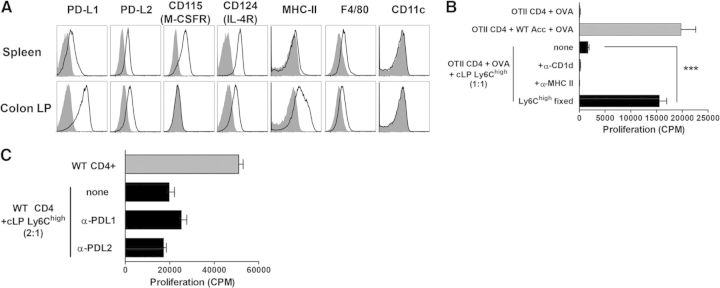
(A) Representative histograms depicting expression of the indicated surface molecules (black lines) were initially gated on CD11b+Ly6ChighSSClowLy6Gneg cells isolated from spleen or cLP. Staining with fluorescently tagged isotype control antibody is depicted by shaded histogram plots. (B) CD4 OT2 T cells were flow-purified from spleens of OT2 mice by sorting for (CD8, CD11c, B220, CD11b)neg CD4+ cells and cocultured together with irradiated accessory splenocytes (WT Acc) or sorted cLP MDCs in the presence of anti-MHC-II-blocking antibody or control antibody (CD1d). Fixation of myeloid cells abrogated their ability to suppress. (C) WT CD4+ T cells were stimulated with anti-CD3/CD28 antibodies and cocultured with MDC in the presence of anti-PD-L1- or anti-PD-L2-blocking antibodies. Data are expressed as mean ± SEM from triplicate wells. Error bars represent SEM. Asterisk indicates significant difference from T cells cocultured with MDCs: ***P < 0.001.
CLP MDCs suppress production of Th1- and Th2-type cytokines and enhance generation of Th17 and foxp3+ T cells
Consistent with the reduction of T cell proliferation, addition of cLP MDC led to a reduction of IFN-γ, GM-CSF, IL-4, and IL-2 (Fig. 6A) in SNs. Suppression of IFN-γ production of T cells by MDC was also confirmed on a single-cell level by intracellular cytokine staining (Fig. 6B and C). At the same time, SN levels of cytokines involved in the polarization of Th17 cells, e.g., IL-17, IL-6, or IL-1β, increased with the addition of MDC. FACS-purified Ly6Chigh cells cultured alone produced significant amounts of IL-1β and IL-6, the two key cytokines involved in Th17 cell polarization, although culturing of T cells with Ly6Chigh cells under standard anti-CD3/CD28-activating conditions did not appreciably enhance the percentage of IL-17+ Th17 cells. When IFN-γ- and IL-4-neutralizing antibodies were added to block differentiation into Th1 and Th2 phenotypes, respectively, the Th17-promoting abilities of MDC became apparent: T cells cocultured with MDC had five- to 10-fold more IL-17+ cells than T cell cultured alone (Fig. 6D and E).
Figure 6. CLP MDCs inhibit Th1 but promote induction of Tregs and Th17 responses.
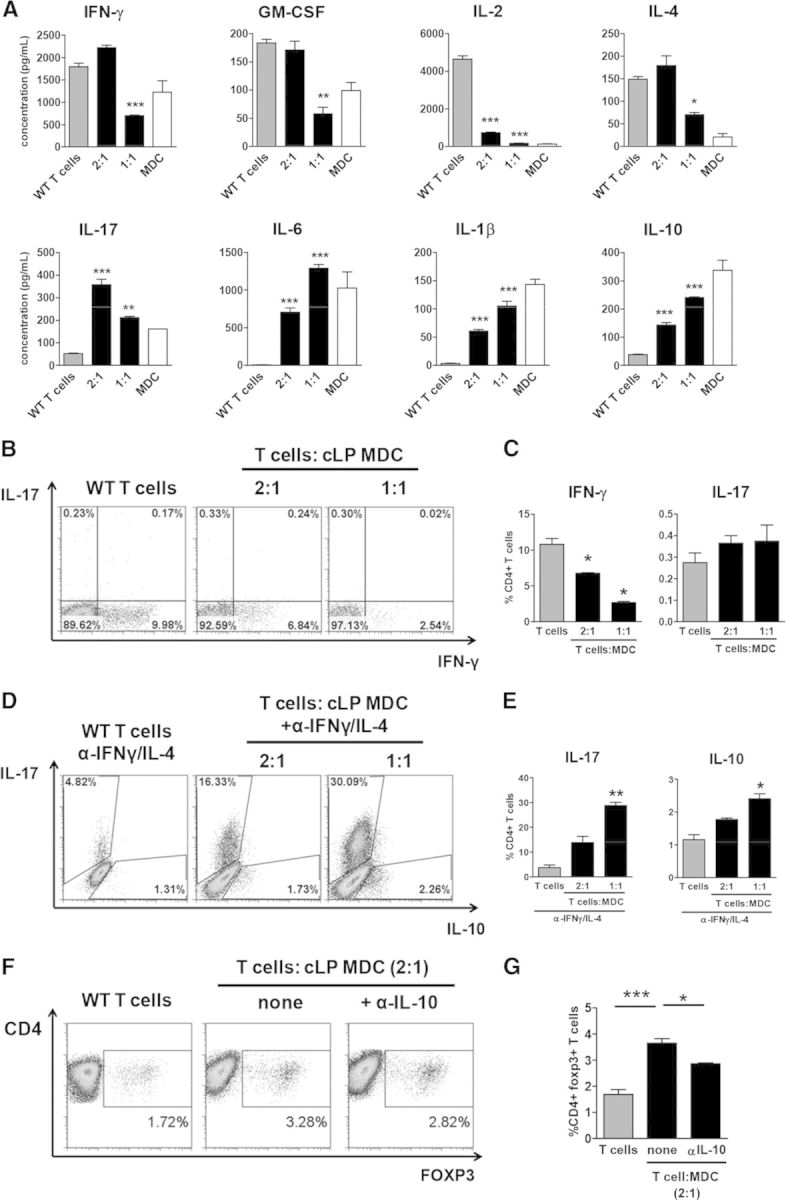
(A) Spleen CD4+ T cells (gray bars) and MDCs (white bars) were cultured alone or cocultured (black bars) at the indicated ratios for 48 h. Cytokine concentrations in the SNs were measured using a multiplex kit. Data are expressed as mean ± sem from triplicate wells. Error bars represent sem. Asterisks indicate significant differences from T cells cultured alone: *P < 0.05, **P < 0.01, and ***P < 0.001. (B and C) T cells cocultured with cLP MDCs produce less IFN-γ. Representative intracellular staining plots, initially gated on viable cells (B), and combined data from triplicate wells from a representative experiment are shown (C). (D and E) Enhanced generation of Th17 cells by MDC when Th1 and Th2 pathways were blocked by neutralizing antibodies to IFN-γ and IL-4, respectively. Representative intracellular staining plots (D) and combined data from triplicate wells from a representative experiment are shown (E). MDCs promote generation of foxp3+ T cells. Representative intracellular staining plots (F) and combined data from duplicate wells from a representative experiment are shown (G). All experiments were repeated at least twice.
In addition, the culturing of T cells with MDC increased levels of foxp3+ T cells (Fig. 6F and G), suggesting that myeloid cells promote inducible Treg differentiation. Steady-state intestinal macrophages have been shown to trigger differentiation of Tregs in an IL-10-dependent manner [41]. Neutralization of IL-10 partially but significantly reduced the percent of foxp3+ T cells. Taken together, colitis-induced MDCs suppress differentiation of naive T cells into Th1 but enhance generation of Th17 and Tregs.
CLP MDCs suppressed proliferation but not cytokine production of CD4 Teffs
The presence of suppressive myeloid cells in colitic mice did not prevent them from developing colitis. We contemplated several possible scenarios to explain this: (1) the number of T cells in the cLP is greater than the numbers of MDCs; (2) the physiological environment of the inflamed colon, such as hypoxia [42, 43], influences the in vivo interaction between myeloid cells and Teffs; or (3) cLP Teffs are “refractory” to suppression by MDCs.
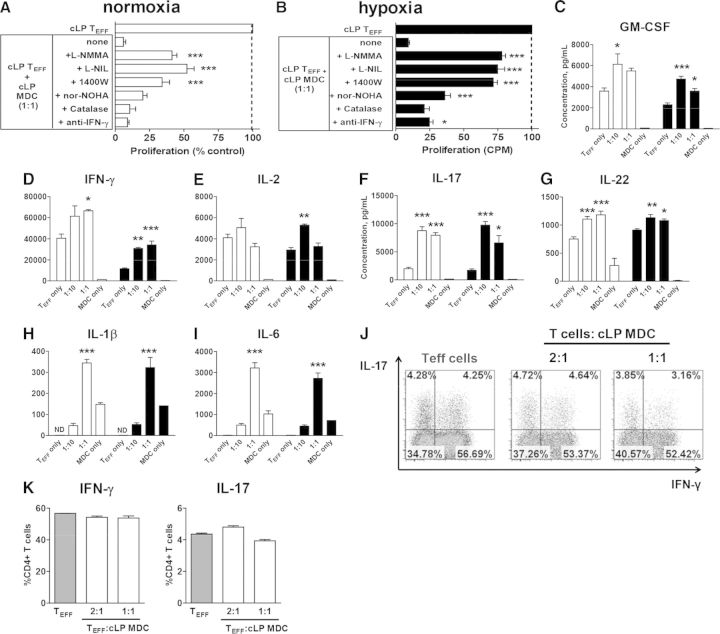
Based on our quantification of myeloid cells and T cells in colitic mice, the ratio of MDC:CD4 T cells in the colon was close to 1:2, respectively (Fig. 1D), suggesting that lack of immunosuppressive myeloid cells is not the likely reason for their inability to prevent disease. As we and others [37, 38] routinely use CD4+ T cells obtained from healthy WT spleens as responder T cells, we next assessed whether Th1/Th17 CD4+ Teffs isolated from inflamed, cLP might be refractory to suppression. When cocultured with MDC, proliferation of Teff was reduced by >90% (Fig. 7A).
Figure 7. MDCs are effective at suppressing proliferation but not cytokine production by CD4+ Teffs isolated from colitic colons.
Suppression of proliferation of Teffs by myeloid cells under ambient (white bars; A) or hypoxic conditions (black bars; 1% O2; B) was mediated by multiple mechanisms. Indicated reagents were added at the start of culture. Data from the lowest effective concentration of L-NMMA (0.1 mM), L-NIL (0.01 mM), 1400 W (0.01 mM), nor-NOHA (0.5 mM), catalase (1000 U/mL), and anti-IFN-γ (10 μg/ml) are shown. Proliferation data from a representative experiment are expressed as percent of T cell proliferation cultured alone. Significant differences from T cells cocultured with MDC at a 1:1 ratio (none) are indicated by asterisks. (C–I) Cytokine levels were measured using a multiplex cytokine kit after 48 h. Mean data from triplicate wells from a representative experiment, which was repeated at least twice with similar results, are shown. Intracellular cytokine production by Teff co-cultured with cLP MDC for 4hrs. Representative intracellular staining plots (J) and combined data from triplicate wells from one of the experiments are shown (K). Statistical significance between Teffs cultured alone or cocultured with myeloid cells was determined by one-way ANOVA with Dunnett's post-test. For all graphs: *P < 0.05, **P < 0.01, and ***P < 0.001.
Emerging literature demonstrates that chronic gut inflammation leads to a significant tissue hypoxia [42], which could affect our in vitro results. However, even hypoxic culture conditions (1% O2) did not influence Teff suppression by Ly6Chigh cells (Fig. 7B). Addition of iNOS inhibitors only partially restored proliferation, whereas addition of the IFN-γ-neutralizing antibody had minimal or no impact on reversing the suppression (Fig. 7A and B), which was similar to our findings with WT T cells (Fig. 3C). Despite reduced proliferation, SN levels of IFN-γ, IL-2, IL-6, IL-17, and GM-CSF were not reduced or were augmented with the addition of MDCs (Fig. 7C–I), which was also confirmed on a single-cell level (Fig. 7J and K). Taken together, our data suggested that monocytes that accumulate in the inflamed colons become immunosuppressive, presumably, in an attempt to restrain Th1 and promote Treg responses, but this is not sufficient to prevent development of colitis effectively.
DISCUSSION
Development of chronic gut inflammation is accompanied by myelopoiesis and accumulation of myeloid cells in intestine and secondary lymphoid tissues [14, 15]. Although studies of intestinal myeloid cell populations during steady-state and inflammatory conditions have traditionally focused on macrophages and DCs, there is an ongoing debate whether neutrophils and monocytes, which constitute a significant portion of immune cell infiltrate in IBDs, play a contributing or a protective role in disease. In the current study, we present evidence supporting the idea that chronic inflammation triggers expansion of myeloid cells with suppressive properties. Our cell-sorting experiments pin-pointed to the inflammatory monocytes/MDCs as the only population capable of suppressing T cell responses in vitro. Specifically, we demonstrated that: (1) monocytes/MDCs that accumulate in colitic mice suppressed proliferation and cytokine production of T cells in a NO-, IFN-γ-, PG-, and cell contact-dependent manner; (2) cLP MDCs inhibited production of cytokines produced by Th1 and Th2 cells but may contribute to Th17 cell differentiation; and (3) MDCs promoted generation of Tregs partly in an IL-10-dependent manner. Taken together, our data suggest that inflammatory monocytes may be the elusive populations of regulatory myeloid cells that play an important role in restraining T cell responses during chronic gut inflammation.
If monocytes are immunosuppressive, why do mice still develop colitis? One of the possibilities may be that robust expansion of monocytes in colitic mice does not occur until very late in disease (Weeks 7–8) when monocytes cannot effectively control T cells in vivo despite the presence of a significant number of these cells in the inflamed tissues. Indeed, based on our in vitro data, MDCs were not as effective at suppressing cytokine production by Teffs as they were at suppressive naive T cells. Finally, as the cellular interactions in vivo are much more complex than can be recapitulated in vitro, additional pathways in mice may still exist that prevent effective suppression of T cells by monocytes/MDCs in vivo. Although recent studies suggested that monocytes recruited to the inflamed colons differentiated into proinflammatory macrophages/DCs [10, 12, 13], this conclusion was made largely based on in vitro and gene-expression data. On the other hand, in vivo depletion of myeloid cells exacerbated colitis and increased associated mortality [19, 20], whereas adoptive transfer of the heterogeneous population of CD11b+Gr-1+ myeloid cells, isolated from colitic mice, had a protective effect on the development of intestinal inflammation [16, 17, 21]. Moreover, a recent report by Grainger et al. [27] found that inflammatory monocytes recruited to the inflammatory sites during acute intestinal inflammation attenuated neutrophil activation via a PG-dependent mechanism, suggesting that regulation by monocytes is not confined to the T cells.
Is accumulation of immunosuppressive monocytes unique to the T cell-induced colitis? We also observed accumulation of CD11b+Ly6Chigh cells in the acute DSS colitis model and in TNFΔARE mice, although we did not test whether these cells were, in fact, suppressive or not. In addition, suppressive Ly6Chigh cells were isolated from the spleens of mice with EAE [37] and autoimmune hepatitis [38] or from the synovial tissues of mice with autoimmune arthritis [44]. Additional suppressive myeloid cells, described as CD11b+Gr-1+, were reported in mice with autoimmune enterocolitis induced by adoptive transfer of self-reactive CD8 T cells [16], in acute DSS-induced colitis [17], and in collagen-induced arthritis [45]. Although these data point to an important role that monocytes and other myeloid cells play in autoimmunity, evidence for their suppressive activity came largely from in vitro studies. Therefore, in vivo studies to dissect the relative importance of myeloid cell subsets are urgently needed to clarify their role in chronic immune-mediated diseases.
A side-by-side comparison of Ly6Chigh cells between spleen and cLP, isolated from colitic mice, revealed notable functional, morphological, and phenotypical differences. The spleen Ly6Chigh subset consisted of mature and immature CD115+ monocytes with a low level of iNOS and arginase-1 expression. Overall, their phenotype was typical of circulating inflammatory monocytes [6]. Suppression of T cells by spleen Ly6Chigh cells was mostly observed at close to a 1:1 ratio of myeloid:T cells and required high purity of the sorted monocyte population. For example, CD11b+Gr-1+ cells isolated from spleens of colitic mice did not always show suppression of T cell proliferation in vitro, which may explain why some studies failed to demonstrate suppression with splenic cells [44]. Moreover, although cLP MDCs displayed some surface markers typical of a monocyte (e.g., high expression of Ly6C and Ly6B.2), their morphology, lack of surface CD115 and L-selectin, and higher mRNA levels of iNOS and arginase-1 suggested that cLP MDCs were more mature than the same population in the spleen [46]. Of note, CD115 expression is sensitive to enzymatic digestions by collagenase (our unpublished observations); thus, it is not clear whether lack of CD115 expression on cLP monocyte-like cells is a result of its down-regulation or enzymatic removal. At the same time, lack of typical DC/macrophage markers indicated that these were not fully differentiated cells. When cocultured with T cells, MDCs produced more NO and were more suppressive on a cell-to-cell basis than the identical population isolated from spleen. Suppression of T cell proliferation by cLP MDCs was also independent of IFN-γ, which was markedly different from spleen monocytes, where neutralization of this cytokine reversed their suppressive abilities. Collectively, these data suggest that peripheral monocytes acquire suppressive abilities after their recruitment into inflamed mucosa or contact with activated T cells, which in turn, stimulated expression of iNOS and led to production of NO [47].
Classically activated M1 macrophages express costimulatory CD80/CD86 molecules and MHC-II, secrete high levels of NO and ROS, and produce Th1-polarizing cytokine IL-12, as well as TNF-α, IL-1β, and IL-6 [48]. Colitis-induced MDCs produced NO; showed low-to-moderate expression of MHC-II; lacked CD80/CD86; secreted IL-1β, IL-6, and TNF-α; but their IL-12 production was minimal. These cells also secreted IL-10, which is usually associated with alternatively activated macrophages [48]. Furthermore, unlike TNF-α/iNOS-producing DCs [49], cLP MDCs produced TNF-α and NO and expressed CD11b but were CD11c-negative. Although it is difficult to “fit” these cells into one of the described categories of macrophages or DCs, they may represent a transitional stage from monocytes to proinflammatory macrophages and/or DCs, as suggested by others [10, 12, 13].
The mechanism of suppression of T cells by NO is thought to result from inhibition of proliferation or induction of T cell apoptosis. At low concentrations (high nanomolar to low micromolar), NO actually promotes T cell proliferation by up-regulating expression of CD25, stimulating production of IL-2, and triggering expression of OX40, which in turn, up-regulates proproliferation factor Survivin in proliferating T cells [50]. However, at higher concentration (high micromolar to low millimolar), NO becomes cytotoxic and triggers T cell apoptosis (Supplemental Fig. 5; refs. [50, 51]). Suppression of T cells by colitis-induced cLP MDCs was largely dependent on NO; however, it also correlated with improved viability of T cells, which supports similar findings in other animal models [52, 53]. Quantification of NO-derived NO2/NO3 in the medium showed ∼50 μM levels. At the same time, Zhu et al. [37] reported that ∼25 μM NO2/NO3 levels in the medium, produced by Ly6Chigh, isolated from mice with EAE, was sufficient to trigger apoptosis in T cells. Differences between our results are not immediately apparent but may be explained by the different animal models and the resulting populations of Ly6Chigh cells. In addition to inducing apoptosis, NO can interfere with IL-2 signaling in T cells by down-regulating STAT5 phosphorylation [54]. We also found that when Teffs were coincubated with MDCs, levels of INF-γ, IL-2, and GM-CSF were augmented. This is in agreement with an earlier study that showed that NO suppresses proliferation but not cytokine production by Th1 cells [55] and suggests that NO may affect naive versus activated/Teffs differently. Taken together, NO emerges as an important molecule produced by monocytes and MDCs and involved in the regulation of T cell functions. It will be imperative for future studies to determine whether NO-mediated regulatory pathways are important in vivo during colitis development in mice.
Based on our unpublished observations, inflammatory milieu of chronically colitic mice is not necessary for monocytes to become suppressive. Monocytes, isolated from the BM of normal mice, suppressed proliferation of T cells by similar mechanisms, as colitis-induced spleen monocytes, and required IFN-γ, functional iNOS, and cell contact (unpublished observations). These results corroborate findings by Slaney et al. [53], with one notable exception: in our hands, suppression by BM monocytes was dependent on IFN-γ, whereas in their experimental set up, IFN-γ was clearly not required for the T cell suppression by circulating monocytes. Ongoing experiments in the laboratory are actively investigating how these discrepancies may be reconciled. One outstanding question that our observations raise is whether monocytes can influence polarization of T cells and pathogen clearance during the course of the normal immune response. Several studies indicated that monocytes play an important role in delivering antigens to the draining lymph nodes, priming T cell responses, or contributing to pathogen clearance [56–59]. Although under noninflammatory conditions, lymph nodes and spleen are devoid of neutrophils and monocytes, these cells may be rapidly recruited there via afferent lymphatics [60, 61] or from blood in a selectin- and chemokine-dependent manner [62, 63]. Splenic monocytes can also expand from an existing precursor pool [64]. Thus, monocytes may play a broader biological role than previously recognized by shaping adaptive immune responses.
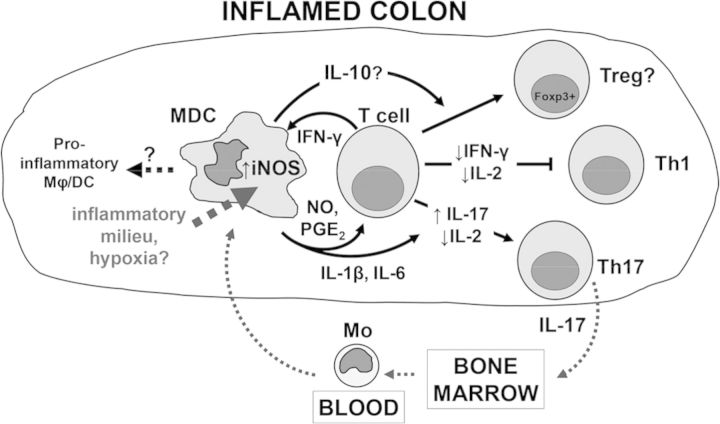
Taken together, our results suggested that monocytes may also play an important, protective role in chronic intestinal inflammation by limiting Teff proliferation and inducing generation of Tregs (Fig. 8). Monocytes recruited to the inflamed intestine become activated by T cell-derived IFN-γ, which stimulates expression of iNOS and leads to production of NO. The antiproliferative effect of NO, in turn, influences polarization of activated T cells away from Th1 cells toward Th17 or an inducible Treg phenotype. The underlying mechanisms may involve reduction in IL-2 levels, which antagonize differentiation of Th17 cells [65], as well as secretion of IL-1β and IL-6 by MDCs. Mechanisms of foxp3 induction in T cells by MDCs are unclear at this time but partially involve IL-10. During active colitis, monocytes are recruited into the inflamed cLP, where proinflammatory cytokines stimulate expression of iNOS. Despite an even greater capacity for NO production and hence, T cell suppression, monocytes/MDCs are unable to control Teffs as a result of insensitivity of these cells to the NO-mediated suppression [55] or other currently unknown mechanisms. Moreover, monocytes are likely themselves influenced by the inflammatory milieu to differentiate into proinflammatory macrophages and DCs, as suggested previously [10, 12, 13].
Figure 8. Proposed control mechanisms of T cell responses by monocytes/MDC during chronic intestinal inflammation.
Monocytes recruited to the inflamed colons differentiate into MDCs and up-regulate expression of iNOS in response to T cell-derived cytokines and the inflammatory milieu of the inflamed colon. MDCs restrain Th1 cell responses via NO, cell contact, and partly, PGE2 production. MDCs also promote generation of foxp3+ Tregs (partly in an IL-10-dependent manner) and Th17 cells. Secretion of IL-17 by Th17 cells stimulates myelopoiesis and release of mature myeloid cells and their precursors from the BM into the blood. It is not clear whether MDCs represent a transient phenotype and eventually differentiate into proinflammatory macrophages (Mϕ)/DC.
Therefore, our study suggests that the expansion of myeloid cells observed in chronic colitis may represent a frustrated attempt by our immune system to limit chronic inflammation and tissue damage. Future studies will determine whether myelopoiesis and expansion of suppressive myeloid cells during the induction phase of disease may be sufficient to attenuate the development of colitis following adoptive transfer of T cells. It will be equally important to evaluate how a suppressive potential of myeloid cells can be harnessed therapeutically to benefit IBD patients.
Supplementary Material
ACKNOWLEDGMENTS
D.V.O. is supported by funds from the Crohn's & Colitis Foundation of America Career Development Award (#2923) and Center of Excellence for Arthritis and Rheumatology, LSUHSC-S.
We are thankful to Dr. Robert Chervenak, Deborah Chervenak, and Shannon Mumphrey for their technical expertise and assistance with flow cytometry. We also thank the laboratory of Dr. Chris Kevil for technical assistance with measurement of NO levels.
SEE CORRESPONDING EDITORIAL ON PAGE 361
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- BM
- bone marrow
- cLP
- colon lamina propria
- COX
- cyclooxygenase
- DSS
- dextran sodium sulfate
- EAE
- experimental autoimmune encephalomyelitis
- foxp3
- forkhead box p3
- HO-1
- heme oxygenase-1
- IBD
- inflammatory bowel diseases
- IDO
- indoleamine-2,3-dioxygenase
- L-NIL
- L-N6-(1-iminoethyl)-L-lysine
- L-NMMA
- NG-monomethyl-L-arginine
- LSUHSC-S
- Louisiana State University Health in Shreveport
- M-MDSC
- monocytic myeloid-derived suppressor cell
- MDC
- monocyte-derived cell
- MLN
- mesenteric lymph nodes
- NAC
- N-acetyl-cystein
- nor-NOHA
- Nω-hydroxy-nor-L-arginine
- ODQ
- 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- OVA
- ovalbumin
- PD-L1/2
- programmed death ligand 1/2
- RAG-1−/−
- RAG-1-deficient
- ROS
- reactive oxygen species
- SN
- supernatant
- SSC
- side-scatter
- Teff
- effector T cell
- Treg
- regulatory T cell
- Zn (II) PPIX
- zinc (II) ptotoporphyrin
AUTHORSHIP
E.K. performed most of the experiments, analyzed data, and wrote the manuscript. D.B. and A.M. carried out the experiments and interpreted results. S.B. contributed intellectually to manuscript preparation. W.G., S.O., and T.P. generated TNFΔARE mice and performed cell isolation and analysis in these mice. D.V.O. formulated hypotheses, designed and interpreted experiments, and wrote the manuscript.
DISCLOSURES
The authors declare no competing financial interests.
REFERENCES
- 1. Abraham C., Cho J. H. (2009) Inflammatory bowel disease. N. Engl. J. Med. 361, 2066–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mayer L. (2010) Evolving paradigms in the pathogenesis of IBD. J. Gastroenterol. 45, 9–16. [DOI] [PubMed] [Google Scholar]
- 3. Sartor R. B. (2006) Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 3, 390–407. [DOI] [PubMed] [Google Scholar]
- 4. Bogunovic M., Ginhoux F., Helft J., Shang L., Hashimoto D., Greter M., Liu K., Jakubzick C., Ingersoll M. A., Leboeuf M., et al. (2009) Origin of the lamina propria dendritic cell network. Immunity 31, 513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Varol C., Vallon-Eberhard A., Elinav E., Aychek T., Shapira Y., Luche H., Fehling H. J., Hardt W. D., Shakhar G., Jung S. (2009) Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity 31, 502–512. [DOI] [PubMed] [Google Scholar]
- 6. Geissmann F., Manz M. G., Jung S., Sieweke M. H., Merad M., Ley K. (2010) Development of monocytes, macrophages, and dendritic cells. Science 327, 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Persson E. K., Scott C. L., Mowat A. M., Agace W. W. (2013) Dendritic cell subsets in the intestinal lamina propria: ontogeny and function. Eur. J. Immunol. 43, 3098–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smythies L. E., Sellers M., Clements R. H., Mosteller-Barnum M., Meng G., Benjamin W. H., Orenstein J. M., Smith P. D. (2005) Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J. Clin. Invest. 115, 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smythies L. E., Shen R., Bimczok D., Novak L., Clements R. H., Eckhoff D. E., Bouchard P., George M. D., Hu W. K., Dandekar S., et al. (2010) Inflammation anergy in human intestinal macrophages is due to Smad-induced IκBα expression and NF-κB inactivation. J. Biol. Chem. 285, 19593–19604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rivollier A., He J., Kole A., Valatas V., Kelsall B. L. (2012) Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J. Exp. Med. 209, 139–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zigmond E., Varol C., Farache J., Elmaliah E., Satpathy A. T., Friedlander G., Mack M., Shpigel N., Boneca I. G., Murphy K. M., et al. (2012) Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity 37, 1076–1090. [DOI] [PubMed] [Google Scholar]
- 12. Tamoutounour S., Henri S., Lelouard H., de Bovis B., de Haar C., van der Woude C. J., Woltman A. M., Reyal Y., Bonnet D., Sichien D., et al. (2012) CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur. J. Immunol. 42, 3150–3166. [DOI] [PubMed] [Google Scholar]
- 13. Bain C. C., Scott C. L., Uronen-Hansson H., Gudjonsson S., Jansson O., Grip O., Guilliams M., Malissen B., Agace W. W., Mowat A. M. (2013) Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6C(hi) monocyte precursors. Mucosal Immunol. 6, 498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ostanin D. V., Kurmaeva E., Furr K., Bao R., Hoffman J., Berney S., Grisham M. B. (2012) Acquisition of antigen-presenting functions by neutrophils isolated from mice with chronic colitis. J. Immunol. 188, 1491–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Griseri T., McKenzie B. S., Schiering C., Powrie F. (2012) Dysregulated hematopoietic stem and progenitor cell activity promotes interleukin-23-driven chronic intestinal inflammation. Immunity 37, 1116–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haile L. A., von Wasielewski R., Gamrekelashvili J., Kruger C., Bachmann O., Westendorf A. M., Buer J., Liblau R., Manns M. P., Korangy F., et al. (2008) Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology 135, 871–881, 881.e1–881.e5. [DOI] [PubMed] [Google Scholar]
- 17. Zhang R., Ito S., Nishio N., Cheng Z., Suzuki H., Isobe K. I. (2011) Dextran sulphate sodium increases splenic Gr1(+)CD11b(+) cells which accelerate recovery from colitis following intravenous transplantation. Clin. Exp. Immunol. 164, 417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singh U. P., Singh N. P., Singh B., Hofseth L. J., Taub D. D., Price R. L., Nagarkatti M., Nagarkatti P. S. (2012) Role of resveratrol-induced CD11b(+) Gr-1(+) myeloid derived suppressor cells (MDSCs) in the reduction of CXCR3(+) T cells and amelioration of chronic colitis in IL-10(−/−) mice. Brain Behav. Immun. 26, 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nemoto Y., Kanai T., Tohda S., Totsuka T., Okamoto R., Tsuchiya K., Nakamura T., Sakamoto N., Fukuda T., Miura O., et al. (2008) Negative feedback regulation of colitogenic CD4+ T cells by increased granulopoiesis. Inflamm. Bowel Dis. 14, 1491–1503. [DOI] [PubMed] [Google Scholar]
- 20. Kuhl A. A., Kakirman H., Janotta M., Dreher S., Cremer P., Pawlowski N. N., Loddenkemper C., Heimesaat M. M., Grollich K., Zeitz M., et al. (2007) Aggravation of different types of experimental colitis by depletion or adhesion blockade of neutrophils. Gastroenterology 133, 1882–1892. [DOI] [PubMed] [Google Scholar]
- 21. Zhang R., Ito S., Nishio N., Cheng Z., Suzuki H., Isobe K. (2011) Up-regulation of Gr1+CD11b+ population in spleen of dextran sulfate sodium administered mice works to repair colitis. Inflamm. Allergy Drug Targets 10, 39–46. [DOI] [PubMed] [Google Scholar]
- 22. Carr K. D., Sieve A. N., Indramohan M., Break T. J., Lee S., Berg R. E. (2011) Specific depletion reveals a novel role for neutrophil-mediated protection in the liver during Listeria monocytogenes infection. Eur. J. Immunol. 41, 2666–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brazil J. C., Louis N. A., Parkos C. A. (2013) The role of polymorphonuclear leukocyte trafficking in the perpetuation of inflammation during inflammatory bowel disease. Inflamm. Bowel Dis. 19, 1556–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fournier B. M., Parkos C. A. (2012) The role of neutrophils during intestinal inflammation. Mucosal Immunol. 5, 354–366. [DOI] [PubMed] [Google Scholar]
- 25. Campbell E. L., Bruyninckx W. J., Kelly C. J., Glover L. E., McNamee E. N., Bowers B. E., Bayless A. J., Scully M., Saeedi B. J., Golden-Mason L., et al. (2014) Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity 40, 66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Colgan S. P., Ehrentraut S. F., Glover L. E., Kominsky D. J., Campbell E. L. (2013) Contributions of neutrophils to resolution of mucosal inflammation. Immunol. Res. 55, 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grainger J. R., Wohlfert E. A., Fuss I. J., Bouladoux N., Askenase M. H., Legrand F., Koo L. Y., Brenchley J. M., Fraser I. D., Belkaid Y. (2013) Inflammatory monocytes regulate pathologic responses to commensals during acute gastrointestinal infection. Nat. Med. 19, 713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arnold L., Henry A., Poron F., Baba-Amer Y., van R. N., Plonquet A., Gherardi R. K., Chazaud B. (2007) Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 204, 1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nahrendorf M., Swirski F. K., Aikawa E., Stangenberg L., Wurdinger T., Figueiredo J. L., Libby P., Weissleder R., Pittet M. J. (2007) The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 204, 3037–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kontoyiannis D., Pasparakis M., Pizarro T. T., Cominelli F., Kollias G. (1999) Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity 10, 387–398. [DOI] [PubMed] [Google Scholar]
- 31. Ostanin D. V., Bao J., Koboziev I., Gray L., Robinson-Jackson S. A., Kosloski-Davidson M., Price V. H., Grisham M. B. (2009) T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G135–G146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ostanin D. V., Pavlick K. P., Bharwani S., D'Souza D., Furr K. L., Brown C. M., Grisham M. B. (2006) T cell-induced inflammation of the small and large intestine in immunodeficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G109–G119. [DOI] [PubMed] [Google Scholar]
- 33. Sato K., Ozaki K., Oh I., Meguro A., Hatanaka K., Nagai T., Muroi K., Ozawa K. (2007) Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood 109, 228–234. [DOI] [PubMed] [Google Scholar]
- 34. Kitowska K., Zakrzewicz D., Konigshoff M., Chrobak I., Grimminger F., Seeger W., Bulau P., Eickelberg O. (2008) Functional role and species-specific contribution of arginases in pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 294, L34–L45. [DOI] [PubMed] [Google Scholar]
- 35. Geissmann F., Jung S., Littman D. R. (2003) Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82. [DOI] [PubMed] [Google Scholar]
- 36. Gabrilovich D. I., Nagaraj S. (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhu B., Bando Y., Xiao S., Yang K., Anderson A. C., Kuchroo V. K., Khoury S. J. (2007) CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J. Immunol. 179, 5228–5237. [DOI] [PubMed] [Google Scholar]
- 38. Cripps J. G., Wang J., Maria A., Blumenthal I., Gorham J. D. (2010) Type 1 T helper cells induce the accumulation of myeloid-derived suppressor cells in the inflamed Tgfb1 knockout mouse liver. Hepatology 52, 1350–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Blank C., Mackensen A. (2007) Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol. Immunother. 56, 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Collins M., Ling V., Carreno B. M. (2005) The B7 family of immune-regulatory ligands. Genome Biol. 6, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Denning T. L., Wang Y. C., Patel S. R., Williams I. R., Pulendran B. (2007) Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat. Immunol. 8, 1086–1094. [DOI] [PubMed] [Google Scholar]
- 42. Colgan S. P., Taylor C. T. (2010) Hypoxia: an alarm signal during intestinal inflammation. Nat. Rev. Gastroenterol. Hepatol. 7, 281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Harris N. R., Carter P. R., Yadav A. S., Watts M. N., Zhang S., Kosloski-Davidson M., Grisham M. B. (2011) Relationship between inflammation and tissue hypoxia in a mouse model of chronic colitis. Inflamm. Bowel Dis. 17, 742–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Egelston C., Kurko J., Besenyei T., Tryniszewska B., Rauch T. A., Glant T. T., Mikecz K. (2012) Suppression of dendritic cell maturation and T cell proliferation by synovial fluid myeloid cells from mice with autoimmune arthritis. Arthritis Rheum. 64, 3179–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fujii W., Ashihara E., Hirai H., Nagahara H., Kajitani N., Fujioka K., Murakami K., Seno T., Yamamoto A., Ishino H., et al. (2013) Myeloid-derived suppressor cells play crucial roles in the regulation of mouse collagen-induced arthritis. J. Immunol. 191, 1073–1081. [DOI] [PubMed] [Google Scholar]
- 46. Gordon S., Taylor P. R. (2005) Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964. [DOI] [PubMed] [Google Scholar]
- 47. Gallina G., Dolcetti L., Serafini P., De Santo C., Marigo I., Colombo M. P., Basso G., Brombacher F., Borrello I., Zanovello P., et al. (2006) Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J. Clin. Invest. 116, 2777–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mosser D. M. (2003) The many faces of macrophage activation. J. Leukoc. Biol. 73, 209–212. [DOI] [PubMed] [Google Scholar]
- 49. Serbina N. V., Salazar-Mather T. P., Biron C. A., Kuziel W. A., Pamer E. G. (2003) TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 19, 59–70. [DOI] [PubMed] [Google Scholar]
- 50. Niedbala W., Cai B., Liu H., Pitman N., Chang L., Liew F. Y. (2007) Nitric oxide induces CD4+CD25+ Foxp3 regulatory T cells from CD4+CD25 T cells via p53, IL-2, and OX40. Proc. Natl. Acad. Sci. USA 104, 15478–15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bogdan C., Rollinghoff M., Diefenbach A. (2000) Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr. Opin. Immunol. 12, 64–76. [DOI] [PubMed] [Google Scholar]
- 52. O'Connor R. A., Li X., Blumerman S., Anderton S. M., Noelle R. J., Dalton D. K. (2012) Adjuvant immunotherapy of experimental autoimmune encephalomyelitis: immature myeloid cells expressing CXCL10 and CXCL16 attract CXCR3+CXCR6+ and myelin-specific T cells to the draining lymph nodes rather than the central nervous system. J. Immunol. 188, 2093–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Slaney C. Y., Toker A., La F. A., Backstrom B. T., Harper J. L. (2011) Naive blood monocytes suppress T-cell function. A possible mechanism for protection from autoimmunity. Immunol. Cell Biol. 89, 7–13. [DOI] [PubMed] [Google Scholar]
- 54. Mazzoni A., Bronte V., Visintin A., Spitzer J. H., Apolloni E., Serafini P., Zanovello P., Segal D. M. (2002) Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J. Immunol. 168, 689–695. [DOI] [PubMed] [Google Scholar]
- 55. Van der Veen R. C., Dietlin T. A., Pen L., Gray J. D. (1999) Nitric oxide inhibits the proliferation of T-helper 1 and 2 lymphocytes without reduction in cytokine secretion. Cell. Immunol. 193, 194–201. [DOI] [PubMed] [Google Scholar]
- 56. Samstein M., Schreiber H. A., Leiner I. M., Susac B., Glickman M. S., Pamer E. G. (2013) Essential yet limited role for CCR2+ inflammatory monocytes during Mycobacterium tuberculosis-specific T cell priming. Elife 2, e01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hohl T. M., Rivera A., Lipuma L., Gallegos A., Shi C., Mack M., Pamer E. G. (2009) Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe 6, 470–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Leon B., Lopez-Bravo M., Ardavin C. (2007) Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity 26, 519–531. [DOI] [PubMed] [Google Scholar]
- 59. Traynor T. R., Herring A. C., Dorf M. E., Kuziel W. A., Toews G. B., Huffnagle G. B. (2002) Differential roles of CC chemokine ligand 2/monocyte chemotactic protein-1 and CCR2 in the development of T1 immunity. J. Immunol. 168, 4659–4666. [DOI] [PubMed] [Google Scholar]
- 60. Abadie V., Badell E., Douillard P., Ensergueix D., Leenen P. J., Tanguy M., Fiette L., Saeland S., Gicquel B., Winter N. (2005) Neutrophils rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes. Blood 106, 1843–1850. [DOI] [PubMed] [Google Scholar]
- 61. Bonneau M., Epardaud M., Payot F., Niborski V., Thoulouze M. I., Bernex F., Charley B., Riffault S., Guilloteau L. A., Schwartz-Cornil I. (2006) Migratory monocytes and granulocytes are major lymphatic carriers of Salmonella from tissue to draining lymph node. J. Leukoc. Biol. 79, 268–276. [DOI] [PubMed] [Google Scholar]
- 62. Leon B., Ardavin C. (2008) Monocyte migration to inflamed skin and lymph nodes is differentially controlled by L-selectin and PSGL-1. Blood 111, 3126–3130. [DOI] [PubMed] [Google Scholar]
- 63. Palframan R. T., Jung S., Cheng G., Weninger W., Luo Y., Dorf M., Littman D. R., Rollins B. J., Zweerink H., Rot A., et al. (2001) Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J. Exp. Med. 194, 1361–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Swirski F. K., Nahrendorf M., Etzrodt M., Wildgruber M., Cortez-Retamozo V., Panizzi P., Figueiredo J. L., Kohler R. H., Chudnovskiy A., Waterman P., et al. (2009) Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Laurence A., Tato C. M., Davidson T. S., Kanno Y., Chen Z., Yao Z., Blank R. B., Meylan F., Siegel R., Hennighausen L., et al. (2007) Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity 26, 371–381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.