New method to safely and effectively diminish the frequency of blood monocytes and tissue-resident macrophages in nonhuman primates.
Keywords: myeloid cells, bisphosphonates
Abstract
Nonhuman primates are critical animal models for the study of human disorders and disease and offer a platform to assess the role of immune cells in pathogenesis via depletion of specific cellular subsets. However, this model is currently hindered by the lack of reagents that safely and specifically ablate myeloid cells of the monocyte/macrophage Lin. Given the central importance of macrophages in homeostasis and host immunity, development of a macrophage-depletion technique in nonhuman primates would open new avenues of research. Here, using LA at i.v. doses as low as 0.1 mg/kg, we show a >50% transient depletion of circulating monocytes and tissue-resident macrophages in RMs by an 11-color flow cytometric analysis. Diminution of monocytes was followed rapidly by emigration of monocytes from the bone marrow, leading to a rebound of monocytes to baseline levels. Importantly, LA was well-tolerated, as no adverse effects or changes in gross organ function were observed during depletion. These results advance the ex vivo study of myeloid cells by flow cytometry and pave the way for in vivo studies of monocyte/macrophage biology in nonhuman primate models of human disease.
Introduction
Circulating monocytes and tissue-resident macrophages are evolutionarily ancient cells involved in a myriad of fundamentally important physiological processes from tissue development and maintenance to metabolic homeostasis, host immunity, and wound-healing [1]. Accordingly, macrophages play a role in nearly every human disease and are appealing targets for drug delivery, novel therapies, and pharmacologic depletion techniques aimed at ameliorating chronic and acute illnesses. Whereas mouse models have proven invaluable for furthering our understanding of macrophage biology, the presence of species-specific differences between mice and humans remains a barrier to the clinical development of macrophage-targeted therapeutics [2, 3].
Given their close phylogenetic proximity to humans, nonhuman primates, such as rhesus and cynomolgus macaques, play a pivotal role in biomedical research by providing physiologically relevant models for the study and development of preclinical therapeutics to treat degenerative, genetic, age-associated, and infectious human diseases [4–7]. Furthermore, experimental in vivo depletion of immune cell subsets in macaques has provided critical insight into the protective and pathogenic role of CD8+ T, CD4+ T, B, and NK cells [8–13]. However, no successful method for depleting macrophages has been described in nonhuman primates. Macrophage depletion by administration of liposomes containing the first-generation bisphosphonate drug clodronate is a well-documented technique in murine models of disease, yet this technique has not been adapted to the nonhuman primate model as a result of a narrow therapeutic window, poor tolerability, and liver toxicity (ref. [14] and Andrew Lackner, personal communication, August 5, 2012).
As conventional LC formulations are ineffective and toxic in macaques, we explored alternate approaches to achieve specific ablation of circulating monocytes and tissue-resident macrophages. We first tested an anti-CD14-depleting antibody but failed to induce in vivo depletion of monocytes. In a more promising approach, we next examined if an optimized liposomal formulation of the second generation, nitrogen-containing bisphosphonate, alendronate, would safely and effectively deplete phagocytic monocytes [15]. We report here that LA consistently depletes blood monocytes in nonhuman primates with no adverse clinical effects or significant changes in serum chemistry. This depletion was also evident in the tissues, where macrophage frequency declined, commensurate with an increase in the frequency of monocytes in the bone marrow. Taken together, these results indicate that LA is a viable method for the depletion of monocyte/macrophage Lin cells in nonhuman primates and as such, will advance our understanding of macrophage biology and facilitate the development of novel macrophage-targeted therapies for the treatment of human disease.
MATERIALS AND METHODS
Animals
All RMs used in this study were housed at the Oregon National Primate Research Center. The Oregon Health & Science University Institutional Animal Care and Use Committee reviewed and approved all study protocols, which were in accordance with the U.S. Department of Health and Human Services' Guide for the Care and Use of Laboratory Animals. Cynomolgus macaques were housed at a separate contract research organization in Israel, according to the animal use and care regulations of that institution.
Liposomal bisphosphonates, BrdU, and anti-CD14 antibody injections
LC was obtained from Nico van Rooijen at VU University Medical Center (Amsterdam, Netherlands) and formulated as described previously [16]. LA was manufactured by BioRest (Yavne, Israel) as described previously [17, 18]. The appropriate mass of LC or LA (determined by animal body weight) was diluted in room temperature, 1× PBS, to a final volume of 20 ml and administered at 4 ml/min by i.v. (saphenous vein) or i.p. injection. BrdU was suspended at 10 mg/ml in HBSS (HyClone Laboratories, Logan, UT, USA). BrdU (60 mg/kg) was injected i.v. (saphenous vein) at a rate of 2–3 ml/min.
The hybridoma-expressing mouse anti-human CD14 antibody, 60BCA, was obtained from American Type Culture Collection (Manassas, VA, USA). Antibody was produced by the U.S. National Institutes of Health Nonhuman Primate Reagent Resource (Beth Israel Deaconess Medical Center, Boston, MA, USA) in bioreactors with serum-free medium, purified by protein affinity chromatography to >95% purity, confirmed to be sterile, and contained <1 endotoxin unit/mg. Antibody was suspended in PBS at 15.5 mg/ml and injected s.c. in a single 50-mg/kg dose. Multiple injection sites were used, with no more than 5 ml antibody/site. Animals were prophylactically treated with Benadryl (5 mg/kg i.m.) before administering antibody and were monitored closely for a period of 45 min after the injection, which is the critical period for development of acute, adverse immunologic reactions to allogeneic protein.
Blood and tissue processing
Whole blood was collected into EDTA-treated tubes (BD Biosciences, San Jose, CA, USA). A 500-μl aliquot was used to assess complete blood counts using an ABX Pentra 60 C+ (Horiba, Irvine, CA, USA). BALs were filtered through 70 μm strainers. Bone marrow was pelleted by centrifugation at 830 g for 4 min and then resuspended and vigorously shaken in 1× PBS containing 2 mM EDTA to disassociate large cell clumps. Cells were washed twice in RPMI 1640 containing 10% FCS (R10; Hyclone Laboratories, Logan, UT, USA). Lymph nodes and spleen were diced with scalpels and then mashed through a 70-μm cell strainer. The strainer was rinsed repeatedly with R10 to obtain a single-cell suspension. Colon and liver were diced into 5 mm [3] pieces, and 25–30 of these pieces were placed in a 50-ml conical containing 25 ml RPMI 1640, supplemented with 3% FCS (R3). DTT was added at a final concentration of 200 μM, and tissues were shaken at 225 rpm for 15 min at room temperature. Tissues were allowed to settle, and the R3 with DTT was aspirated and replaced with R3 containing 5 mM EDTA. Tissues were shaken at 225 rpm for 30 min at 37°C, and the cell-containing supernatant was collected and passed through a cell strainer. R3 containing EDTA was added again, tissues shaken, and cells collected. Tissues were washed twice in 1× HBSS to remove excess EDTA and then were suspended in R3 containing 0.1 mg/ml collagenase (Sigma-Aldrich, St. Louis, MO, USA) and 0.1 mg/ml DNase I (Roche, Indianapolis, IN, USA). Tissues were shaken at 225 rpm for 45 min at 37°C, and the cell-containing supernatant was collected and passed through a 70-μm cell strainer. R3 containing collagenase and DNase I was added again, tissues shaken, and cells collected. Cell fractions collected from the EDTA and collagenase digestion steps were combined (total tissue) and resuspended in 30% isotonic Percoll (GE Healthcare, Buckinghamshire, UK). The cells were then layered over a 60%/40% Percoll gradient and spun at 500 g with the brake off. Mononuclear cells from the lower interface were collected and washed in R10.
Flow cytometric analysis of monocytes/macrophages
We designed and optimized an 11-color flow cytometric staining panel for the analysis of monocytes/macrophages in the blood and tissues of RM (Table 1). Either 100 μl whole blood or 1 × 106 total tissue cells were washed twice in 1× PBS and then surface-stained for 30 min at room temperature. Whole blood and spleen were then incubated in 1 ml FACSLyse for 10 min, spun at 830 g for 4 min, and washed three times in 1× PBS, supplemented with 10% FCS (FACS buffer). Other tissue cells were washed once in 1× PBS and fixed in 2% paraformaldehyde. For intracellular stains, fixed cells were washed twice in FACS buffer containing 1 mg/ml saponin (saponin buffer) and stained for 1 h at room temperature. For intranuclear stains (BrdU and Ki-67), fixed cells were washed twice in a 1:1 mixture of saponin buffer and 2× BD FACSPerm and then washed once in saponin buffer and stained for 1 h at room temperature in the presence of 0.5 mg/ml DNase I. Following staining, samples were washed twice in saponin buffer and then run on an LSR II (Becton Dickinson, Franklin Lakes, NJ, USA). Flow cytometric data were analyzed using FlowJo, version 9.6.4 (TreeStar, Ashland, OR, USA). All positive gates were set by using fluorescence minus one control tubes for the appropriate fluorophore.
Table 1. Monocyte/Macrophage Staining Panel Designed for Flow Cytometry.
| Antibody | Clone | Fluorophore | Manufacturer | Location |
|---|---|---|---|---|
| MAC387 | MAC387 | FITC | Abcam (Cambridge, UK) | Intracellular |
| CD163 | GHI/61 | PE | BioLegend (San Diego, CA, USA) | Surface |
| CD68 | KP1 | PerCP-Cy5.5 | Santa Cruz Biotechnology (Santa Cruz, CA, USA) | Intracellular |
| CD45 | D058-1283 | APC | BD Biosciences | Surface |
| CD3 | SP34-2 | Pacific Blue | BD Biosciences | Surface |
| HLA-DR | G46-6 | Alexa Fluor 700 | BD Biosciences | Surface |
| CD14 | RMO52 | ECD | Beckman Coulter (Brea, CA, USA) | Surface |
| CD8 | B9.11 | PE-Cy5 | Beckman Coulter | Surface |
| CD16 | 3G8 | PE-Cy7 | BD Biosciences | Surface |
| CD20 | 2H7 | APC-H7 | BD Biosciences | Surface |
| ARD | – | LIVE/DEAD Yellow | Invitrogen (Carlsbad, CA, USA) | – |
APC, Allophycocyanin; ECD, energy-coupled dye.
Serum chemistry assessing toxicity of LA
Cell-free serum was collected before and after LA treatment. Liver function was assessed by determination of albumin, alanine transaminase, alkaline phosphatase, and total bilirubin concentrations in the serum using a Cobas C 111 (Roche).
RESULTS AND DISCUSSION
Anti-CD14 antibody does not deplete RMs
Immune cell depletion in nonhuman primates is traditionally achieved by administration of mAb with high affinity for surface receptors demarking specific immune subsets, such as CD4, CD8, CD16, and CD20 [8–13]. Therefore, we first attempted to deplete circulating monocytes with an anti-CD14 mAb (clone 60BCA). We administered 50 mg/kg of this anti-CD14 antibody s.c. into two RMs and monitored the persistence of monocytes in peripheral blood using our 11-color flow cytometric staining panel (Table 1). We defined monocytes as CD14+ and/or CD16+ cells within the CD45+granulocyte−ARD−Lin− population (Fig. 1A). Following antibody administration, we observed a complete lack of CD14 staining in both RMs at 2 d.p.i. (Fig. 1B, upper).
Figure 1. Anti-CD14 antibody administration does not deplete monocytes in RM.
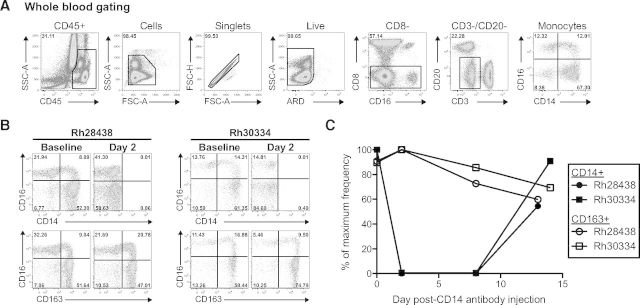
Rh28438 and Rh30334 were injected with 50 mg/kg anti-CD14 antibody (clone 60BCA) s.c. (A) Gating strategy for the identification of monocytes using our 11-color flow cytometric staining panel. Granulocytes were excluded based on their side-scatter (SSC) and CD45dim profile, and Lin-positive cells were excluded based on their expression of CD3, CD8, and CD20. FSC, Forward-scatter; A, area; H, height. (B) Frequency of circulating monocytes following anti-CD14 antibody injection. CD14 staining was abrogated completely by 2 d.p.i., but an analogous marker, CD163, showed increased monocyte frequency. (C) Percent of maximum frequency of CD14+ and CD163+ monocytes through 14 d.p.i.
Although we failed to detect CD14+ monocytes in the blood following injection of the anti-CD14 antibody, it remained unclear whether this was true depletion of the cells or masking of the CD14 antigen. While optimizing our staining panel, we consistently found that nearly all CD14+ RM monocytes also expressed the surface marker CD163, a finding in line with a previous report of the RM model [19]. We therefore compared CD14 with CD163 staining following administration of our anti-CD14 antibody. In contrast to our CD14 staining data, we observed CD163+ monocytes at 2 d.p.i. (Fig. 1B, lower). These stains were repeated over the course of 2 weeks, showing a maintained frequency of CD163+ monocytes in the blood despite our inability to detect any CD14+ monocytes through 8 d.p.i. (Fig. 1C). Taken together, these data demonstrate that our anti-CD14-depleting antibody successfully bound and masked the vast majority of CD14 receptors on the surface of monocytes but failed to induce depletion of these cells.
LC does not deplete monocytes in cynomolgus macaques
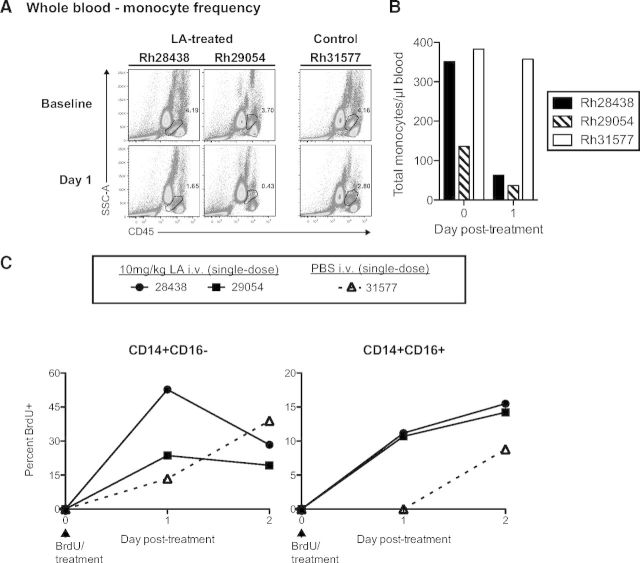
Given the failure of our anti-CD14-depleting antibody, we set out to identify an alternative technique to deplete monocytes and tissue-resident macrophages in nonhuman primates. LC, a first-generation bisphosphonate reagent, has been used to deplete monocytes and macrophages in many animal models, including mice, rats, sheep, gerbils, lambs, and rabbits [20]. We therefore sought to determine if LC could deplete monocytes in nonhuman primates. We injected two cynomolgus macaques with 0.1 mg/kg LC i.v. and monitored the absolute and relative frequencies of CD14+ monocytes over 7 days (Fig. 2A, open symbols). We observed little change in the frequencies of CD14+ monocytes at 1 d.p.i. but instead, detected an increase in CD14+ monocyte frequencies thereafter, peaking at 4 d.p.i. This increase in CD14+ monocyte frequency resolved by 7 d.p.i., at which time, monocyte frequency returned to baseline levels (Fig. 2A, open symbols). Therefore, LC is unsuitable for monocyte depletion in nonhuman primates.
Figure 2. LA depletes monocytes and is well-tolerated in nonhuman primates.
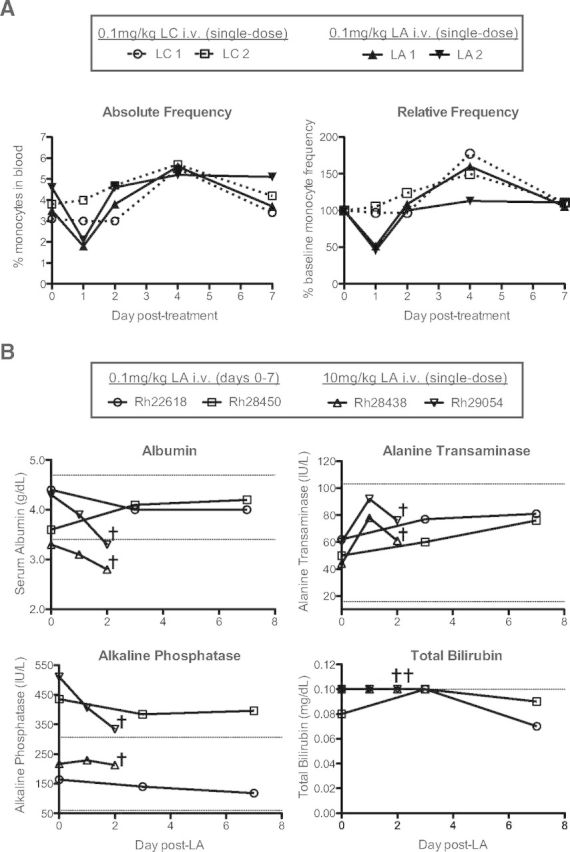
(A) Four cynomolgus macaques were paired into two groups and treated with 0.1 mg/kg i.v. LC (LC 1 and LC 2) or 0.1 mg/kg i.v. LA (LA 1 and LA 2). The absolute and relative frequencies of CD14+ monocytes in whole blood were then assessed through 7 d.p.i. by flow cytometry. (B) Serum chemistry analysis of RM receiving repeated low-dose (0.1 mg/kg; Rh22618 and Rh28450) or single high-dose (10 mg/kg; Rh28438 and Rh29054) i.v. LA administration. Repeated low-dose LA administration revealed no change in liver function, whereas a single high-dose led to perturbation of liver function. Dotted lines represent the expected ranges of albumin, alanine transaminase, alkaline phosphatase, and total bilirubin serum concentrations based on RM population statistics at the Oregon National Primate Research Center. †, Time of euthanasia.
The possibility remained that higher doses of LC would yield depletion of monocytes. However, bisphosphonates, such as clodronate, are toxic at high concentrations, and previous experiments in RM have shown severe, adverse clinical side-effects, such as flu-like symptoms and significant liver toxicity, at doses required for macrophage depletion (Andrew Lackner, personal communication, August 5, 2012). Therefore, LC is neither safe nor effective for the depletion of monocytes and tissue macrophages in nonhuman primates.
LA depletes monocytes and is well-tolerated in nonhuman primates
LC failed to deplete monocytes in cynomolgus macaques when administered i.v. at 0.1 mg/kg, and higher doses of LC have been shown to be toxic in nonhuman primates. Alendronate is a second-generation nitrogenous bisphosphonate that is 50 times more potent than clodronate, thus providing a wider therapeutic window [17, 21, 22]. Additionally, a phase II clinical trial of LA for the treatment of restenosis was completed recently, portending its safe use in nonhuman primates [23]. We therefore tested the ability of LA to deplete monocytes in cynomolgus macaques.
We injected 0.1 mg/kg LA i.v. into two cynomolgus macaques and monitored the absolute and relative frequencies of CD14+ monocytes over 7 days, similar to the LC-treated cynomolgus macaques (Fig. 2A, closed symbols). In contrast to animals receiving LC, the LA-treated animals exhibited a sharp decline in CD14+ monocyte frequencies by 1 d.p.i. (∼50% reduction). This depletion was transient, however, as CD14+ monocyte frequencies returned to baseline levels by 2 d.p.i. (Fig. 2A, closed symbols). Thus, LA is an effective alternative to LC for monocyte depletion in nonhuman primates.
Given the toxicity of LC at high doses, we next wanted to test the safety of LA treatment in nonhuman primates. We therefore monitored the serum concentrations of four key readouts of liver function (albumin, alanine transaminase, alkaline phosphatase, and bilirubin) in four RMs, each receiving one of two different LA-treatment regimens (Fig. 2B). Rh22618 and Rh28450 received a low dose (0.1 mg/kg) of LA i.v., each day for 1 week. We observed no adverse changes in serum chemistry when comparing baseline measurements with post-LA measurements, and all values fell within expected ranges, with the exception of alkaline phosphatase in Rh28450, which was high at baseline (Fig. 2B). Rh28438 and Rh29054 received a single high dose (10 mg/kg) injection of LA i.v., and serum chemistry was followed for 2 days before necropsy. In contrast to the animals receiving daily 0.1 mg/kg LA injections, these animals exhibited decreased serum concentrations of albumin, coincident with elevated concentrations of alanine transaminase, although alanine transaminase concentrations were within expected values (Fig. 2B). These changes in albumin and alanine transaminase serum concentrations are indicative of liver exertion, likely as a result of the depletion of liver Kupffer cells (see below). Despite these findings, we observed no adverse clinical side-effects of LA treatment in these animals nor in any animals receiving LA, regardless of dose or frequency (data not shown). It is important to note that 0.1 mg/kg LA i.v. was sufficient to deplete monocytes, and this dose of LA was safe in RMs, even upon repeated daily injections (Fig. 2A and B). Therefore, in contrast to both LC and anti-CD14 antibody, LA is well-tolerated at effective doses and constitutes a viable, new technique for monocyte depletion in nonhuman primates.
The dose and route of LA treatment can increase monocyte depletion
We discovered that cynomolgus macaques receiving 0.1 mg/kg LA i.v. showed a 50% reduction in monocyte frequency by 1 d.p.i. (Fig. 2A, closed symbols). We next set out to determine whether LA treatment would also deplete monocytes in RMs and whether an alternate dose and route of LA administration could increase the level of monocyte depletion. We injected three RMs (Rh24937, Rh28034, and Rh28099) with 1 mg/kg LA, both i.v. and i.p., and monitored the frequency of monocytes at 1 d.p.i. using our flow cytometry staining panel (Figs. 1A and 3A). Two control RMs (Rh26118 and Rh26144) received PBS injections with the same volumes and routes as the LA-treated animals. We were able to discern the monocyte population from lymphocytes and granulocytes by their distinct CD45 MFI versus side-scatter profile (Fig. 3A, upper). Following LA treatment, we observed large decreases in monocyte frequency at 1 d.p.i., with a particularly pronounced depletion in Rh28034 (baseline: 8.22%; 1 d.p.i.: 1.78%). In contrast, monocyte frequencies in the control animals remained stable at 1 d.p.i. (Fig. 3A, upper).
Figure 3. Alternate routes of LA administration can increase monocyte depletion in RM.
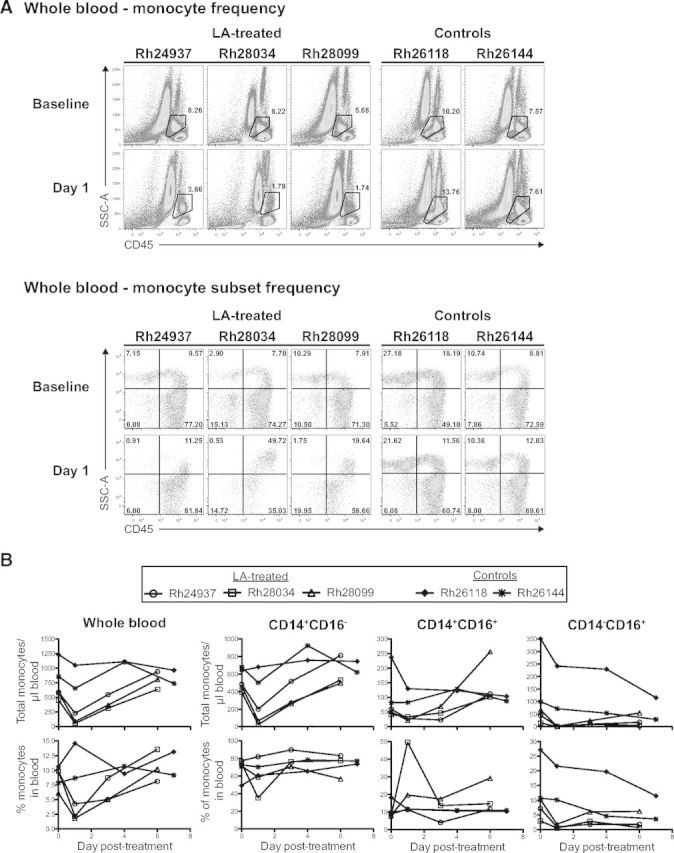
(A, Upper) Frequency of monocytes in whole blood pre- and post-LA treatment, determined by flow cytometric analysis of CD45 MFI versus side-scatter profile. (Lower) Frequency of monocyte subsets in whole blood pre- and post-LA treatment, determined by flow cytometric analysis of CD14+ versus CD16+ staining. (B, Upper) Absolute monocyte counts in whole blood pre- and post-LA treatment. Absolute monocyte counts were calculated by assessing the frequency of CD14+ and/or CD16+ monocytes and comparing this value with the overall white blood cell count (CD45+ cells). (Lower) Frequency of monocytes in whole blood pre- and post-LA treatment assessed by flow cytometry.
We wanted to assess further the monocyte depletion observed in the LA-treated RMs by determining which subsets of monocytes were most affected. Thus, we compared the frequency of three previously described monocyte subsets (classical CD14+CD16−, intermediate CD14+CD16+, and nonclassical CD14−CD16+) within the total monocyte pool, before and after LA treatment (Fig. 3A, lower) [24]. We observed significant animal-to-animal variability when comparing monocyte subset frequencies pre- and post-treatment. Importantly, LA-treated RMs exhibited depletion of nearly all CD14−CD16+ monocytes at 1 d.p.i., whereas the control RMs maintained similar monocyte subset frequencies at 1 d.p.i., supporting the lack of overall monocyte depletion that we observed (Fig. 3A).
Finally, we monitored the absolute counts and frequencies of monocytes through 7 d.p.i. to determine the duration of our depletion (Fig. 3B). In support of our data showing large decreases in monocyte frequency following LA treatment, the absolute counts of monocytes/ml of blood also showed substantial declines at 1 d.p.i. (Fig. 3B, upper left). This was most pronounced in Rh28034 and Rh28099, where absolute monocyte counts decreased 87% and 84%, respectively. Interestingly, absolute monocyte counts also revealed the preservation of CD14+CD16+ monocytes following LA treatment. This was in sharp contrast to the large declines in monocyte counts observed in the CD14+CD16− and CD14−CD16+ populations (Fig. 3B, upper). The monocyte depletion observed in these LA-treated RMs was highly transient, similar to that observed in cynomolgus macaques treated with a single, 0.1-mg/kg dose of LA i.v. (Figs. 2A and 3B, lower). Therefore, LA treatment can safely induce significant, but highly transient, depletion of monocytes in RMs.
Repeated LA injections do not increase the magnitude or sustain the depletion of monocytes
Over the course of our investigation into the effects of LA treatment in nonhuman primates, we implemented numerous regimens of LA administration, the details of which can be found in Supplemental Fig. 1. Importantly, we found that daily, 0.1-mg/kg injections of LA did not continue to reduce monocyte counts or maintain the low monocyte counts achieved following initial LA injection (Supplemental Fig. 1). This finding is consistent with LA use in rats and rabbits, where subsequent administrations within 1 and 4 weeks, respectively, have a diminished effect (unpublished results). Based on our experience, the pharmacodynamics of LA far exceeds its pharmacokinetics in RM, and a “wash-out” period of 4–6 weeks may be necessary between doses (data not shown).
LA treatment reduces the frequency of tissue-resident macrophages and increases the frequency of bone marrow monocytes
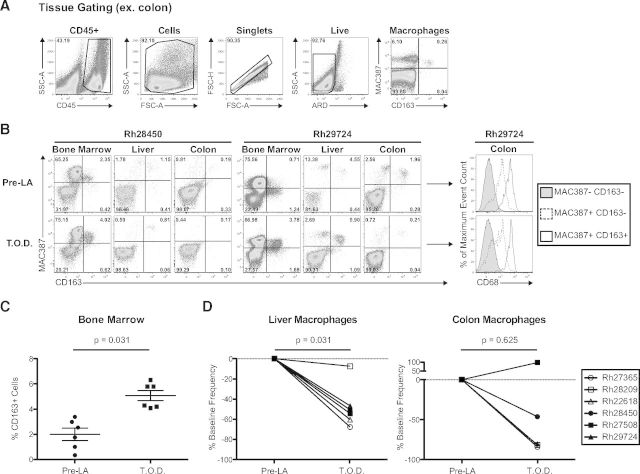
Administration of LA consistently led to monocyte depletion in our nonhuman primate cohorts (Figs. 2A and 3B and Supplemental Fig. 1). We hypothesized that this blood monocyte depletion would be paralleled by a decrease in the frequency of tissue-resident macrophages and bone marrow monocytes. We therefore collected BALs, colon biopsies, liver biopsies, and bone marrow aspirates before and after LA treatment from the six animals outlined in Supplemental Fig. 1 and compared the frequency of tissue-resident macrophages with bone marrow monocytes using our 11-color flow cytometric staining panel (Table 1 and Fig. 4A).
Figure 4. LA consistently depletes tissue-resident macrophages and induces bone marrow monocyte generation.
(A) Gating strategy for the identification of macrophages in the tissues (defined as MAC387+ and/or CD163+) and bone marrow (defined as CD163+). (B) Representative tissue-resident macrophage and bone marrow myeloid precursor staining from Rh28450 and Rh29724. CD68 expression was examined from the colon of Rh29724 before and after LA treatment and found to have similar staining profiles at both time-points. T.O.D.,. (C) Frequency of CD163+ cells in the bone marrow before and after LA treatment showed a significant increase in monocyte precursor production. (D, Left) Tissue-resident macrophage frequency (percent change from baseline), before and after LA treatment, showed a significant decrease in the liver. (Right) Four of five RMs showed decreases in colon macrophages, although this was not significant as a result of a single animal exhibiting an increase in colon macrophage frequency. Significance tests were all Wilcoxon signed-rank. T.O.D., Time of death.
We found no change in the frequency of alveolar macrophages in the BAL following LA treatment (data not shown). Figure 4B shows comparative examples of MAC387 versus CD163 staining before and after LA treatment from bone marrow aspirate, liver, and colon. MAC387 is an antibody that recognizes the calcium-binding myeloid-related protein MRP14, which is expressed in tissue-resident macrophages [25–27]. Our staining panel identified three distinct macrophage populations in these tissues: MAC387+CD163−, MAC387+CD163+, and MAC387−CD163+. In general, MAC387−CD163+ macrophages were found at a much lower frequency than the MAC387+CD163− and MAC387+CD163+ macrophages (Fig. 4B, left). To assess further these populations of tissue macrophages, we examined their expression of CD68, a glycoprotein also expressed in tissue-resident macrophages [26, 28, 29]. We compared CD68 expression between the MAC387+CD163− and MAC387+CD163+ populations from the colon of Rh29724 before and after LA treatment (Fig. 4B, right). MAC387+CD163− and MAC387+CD163+ macrophages expressed CD68, with the latter population showing the highest expression, confirming that our flow cytometric panel was able to identify tissue macrophages (Fig. 4B, right). Thus, we show here, for the first time, the depletion of tissue-resident macrophages following LA treatment of nonhuman primates using three previously described macrophage markers.
We extended this analysis to determine the effectiveness of LA treatment on monocyte depletion in the bone marrow. As blood monocytes express MAC387 and CD163 following emigration from the bone marrow, we looked at the frequency of cells expressing these markers in the bone marrow before and after LA treatment. Our staining of bone marrow aspirates revealed little change in the overall frequency of monocytes when defined as MAC387+ and/or CD163+ (data not shown). However, given that granulocytes in the bone marrow may also express MAC387, we performed a separate analysis defining bone marrow monocytes as CD163+ [25]. This analysis revealed significant increases in bone marrow monocyte frequency following the administration of LA (P=0.031; Fig. 4C). Despite our initial hypothesis that LA treatment would deplete bone marrow monocytes, this finding is not altogether surprising, as SIV-associated depletion of tissue macrophages has been shown to increase myeloid turnover, beginning with monocyte release from the bone marrow [30]. However, we cannot dismiss the possibility that monocyte depletion occurs in the bone marrow before the earliest time-point examined (1 d.p.i.).
In a similar fashion, we assessed tissue macrophage depletion in the liver and colon (Fig. 4D). Macrophage frequencies in the liver were reduced by >45% following LA treatment in five of the six animals studied (P=0.031; Fig. 4D, left). Given that Kupffer cells constitute 80–90% of tissue macrophages in the human body, this result highlights the effectiveness of LA in tissue macrophage depletion [31]. It is important to note that the majority of depleted macrophages in the liver was MAC387+CD163− (Fig. 4B, and data not shown). This same trend was observed in the colon, where overall macrophage frequency was reduced by >45% in four of five animals (Rh22618 excluded from analysis as a result of undetectable macrophage frequency pre-LA; P=0.625). In the colon, however, the depletion was distributed more evenly across the three macrophage populations (Fig. 4B, and data not shown). Surprisingly, one of the animals (Rh27508) showed a large increase in macrophage frequency in the colon. However, this animal also exhibited the expansion described above of CD163+ monocytes in the bone marrow, a >80% reduction in absolute monocyte count and a >50% reduction in macrophage frequency in the liver, providing ample evidence of a direct effect of LA treatment (Fig. 4C and D and Supplemental Fig. 1). We therefore cannot exclude the possibility that the increase in colon macrophage frequency is a result of differential myeloid cell turnover kinetics following LA-associated depletion in this animal.
BrdU reveals monocyte turnover following LA treatment
We consistently detected monocytes following LA treatment but were unable to determine whether they were newly emigrated cells from the bone marrow or simply remainders from the original cellular pool. Our results showing increased frequencies of CD163+ bone marrow monocytes suggested that blood monocyte turnover was exacerbated following LA treatment. However, a more detailed characterization of monocyte turnover would support our depletion results and allow for a better calculation of total blood monocyte depletion. We therefore designed and executed LA treatments in tandem with BrdU administration to ascertain the level of monocyte turnover in our model.
BrdU is a synthetic thymidine analog that is incorporated into the DNA of dividing cells during S-phase and can be detected by intracellular antibody staining [32, 33]. Dividing monocyte precursors in the bone marrow integrate BrdU before being released into the blood as monocytes. Following release from the bone marrow, monocytes and tissue-resident macrophages rarely divide, making BrdU a reliable marker of myeloid cell turnover [30].
To determine the level of monocyte turnover following LA treatment, we injected two RMs (Rh28438 and Rh29054) with 10 mg/kg LA i.v., along with 60 mg/kg BrdU i.v., and monitored the frequency of BrdU+ and BrdU− monocytes through 2 d.p.i. (Fig. 5). Control animal Rh31577 received PBS along with the same 60-mg/kg dose of BrdU. LA treatment in Rh28438 and Rh29054 again led to a profound decrease in the frequency of monocytes by 1 d.p.i. (Fig. 5A, left). Unexpectedly, control animal Rh31577 also exhibited a decline in monocyte frequency, albeit to a smaller extent (Fig. 5A, right).
Figure 5. BrdU administration reveals high monocyte turnover following LA treatment.
Rh28438 and Rh29054 received 10 mg/kg LA i.v., along with 60 mg/kg BrdU i.v. Control animal Rh31577 received PBS along with the same 60-mg/kg dose of BrdU. LA treatment depleted the majority of blood monocytes based on: (A) frequency assessed by CD45 staining versus side-scatter profile and (B) absolute monocyte counts. (C) BrdU staining revealed high levels of monocyte turnover following LA treatment in both the CD14+CD16− (classical) and CD14+CD16+ (intermediate) monocyte populations. Values shown indicate percent of total CD14+CD16− (classical) and CD14+CD16+ (intermediate) populations staining positive for BrdU.
Given the decrease in monocyte frequency observed in Rh31577, we wanted to confirm that this animal was indeed an appropriate control. We therefore assessed absolute monocyte counts before and after LA treatment in all three RMs (Fig. 5B). As expected, we observed large decreases in monocyte counts in the LA-treated animals, whereas monocyte counts in Rh31577 remained unchanged following PBS injection.
Finally, we monitored the emigration of monocytes from the bone morrow (BrdU+), comparing LA-treated animals with the control (Fig. 5C). Interestingly, we found a higher frequency of CD14+CD16−BrdU+ monocytes in LA-treated animals compared with the control animal at 1 d.p.i., with more than one-half of CD14+CD16− monocytes staining positive for BrdU in Rh28438 (Fig. 5C, left). In addition, we found ≧10% of CD14+CD16+ monocytes staining positive for BrdU in LA-treated animals at 1 d.p.i. (Fig. 5C). This was in sharp contrast to control animal Rh31577, which had no CD14+CD16+BrdU+ monocytes at 1 d.p.i. (Fig. 5C). Acute inflammation leads to increased monocyte turnover, as monocytes enter the affected tissues to differentiate into macrophages [34]. In the case of RMs infected with SIV, monocyte turnover is a strong predictor of progression to AIDS [30]. Similarly, high levels of monocyte turnover correlate to the severity of SIV-induced encephalitis in RMs [35]. Remarkably, the levels of monocyte turnover that we observed in LA-treated animals at 1 d.p.i. were similar or greater to those seen in SIV-infected RMs, including rapid progressors with severe encephalitis [30, 35]. Thus, LA treatment increases blood monocyte turnover, a finding in support of our increased bone marrow monocyte generation and blood monocyte depletion data. Additionally, these levels of blood monocyte turnover are comparable with advanced SIV infection, where marked turnover of monocytes is observed.
We show here for the first time that LA is a safe and effective means of monocyte and tissue macrophage depletion in the nonhuman primate model. Importantly, we also demonstrate a selective monocyte subset depletion for the first time, with CD14+CD16− and CD14−CD16+ monocytes exhibiting strong depletion compared with the CD14+CD16+ subset (Fig. 3B, upper). Administration of LA consistently reduced absolute monocyte counts and tissue macrophage frequencies in the liver and colon. This depletion was associated with a significant increase in monocyte generation in the bone marrow, a finding supported by increased monocyte turnover in the blood, as measured by DNA BrdU incorporation.
LA-mediated monocyte depletion was highly transient in all nonhuman primates examined, and this finding is in full support of previous work with rabbits, rats, and humans, where LA depletion of monocytes is also transient. Importantly, transient monocyte depletion has been shown to trigger a profound and sustained anti-inflammatory effect, exemplified by the reduced incidence of restenosis and endometriosis following LA treatment [23, 36, 37]. Consequently, the usefulness of LA administration to nonhuman primates may extend well beyond the time-frame of monocyte depletion.
In contrast to liver and colon, we detected no depletion of alveolar macrophages in the lung following LA treatment. The reason for this difference is unclear but may depend on the biodistribution or macrophage targeting of LA in the lung. The pharmacokinetics and biodistribution of LA have been studied in vivo previously in rabbits and rats, respectively [37]. The half-life of LA in rabbits following a 10-mg/kg LA injection is 35.4 h. However, LA half-life is dependent on dose response, as LA depletes phagocytic cells tasked with clearance of liposomes. Dose response is also key to biodistribution, as the majority of LA is taken up in the liver [15]. Following saturation of the liver, LA can be found at higher concentrations in additional tissues, such as the blood and spleen [15]. Importantly, biodistribution analyses of LA in rats following i.v. or i.p. injections showed minimal penetration of the lung tissue at both high (10 mg/kg) and low (1 mg/kg) doses [37]. Given the large number of macrophages in the lung (∼70% of lung immune cells), it is possible that the mass of LA reaching the lung, following i.v. or i.p. injection, is insufficient to deplete a detectable number of cells [38]. Additionally, our approach of systemic LA administration (i.v. and i.p.) may inhibit the depletion of alveolar macrophages at the mucosal surface of the lung, given that the small amounts of LA leaving the liver will be carried via blood. This LA would encounter and potentially target alternate lung tissue macrophages, such as interstitial macrophages. Indeed, depletion of alveolar macrophages in mice is accomplished by direct intranasal administration of LC, whereas depletion of interstitial lung macrophages is achieved by i.v. injection [39–41].
We observed two major populations of macrophages in the tissues of RMs: MAC387+CD163+ and MAC387+CD163−. However, in humans, MAC387+ macrophages have been characterized as M1-like and CD163+ macrophages as M2-like, with little to no coexpression of these markers on the same cells. Supporting our identification of MAC387+CD163+ tissue macrophages, a recent study in RMs comprehensively characterized the markers expressed by different populations of lung-resident macrophages, showing that both blood monocytes and interstitial macrophages coexpress high levels of MAC387 and CD163 [38]. Functional analysis of this cellular population revealed that MAC387+CD163+ interstitial macrophages respond to classical macrophage activation signals (IFN-γ and LPS). Therefore, MAC387+CD163+ appears to define an M1-like population of macrophages, although anatomical location may affect expression of these markers, as MAC387+CD163+ macrophages are rare in RM brain tissue [26].
The analyses conducted herein were aided by the design of an 11-color flow cytometric staining panel that was universally applied to blood and tissues for the identification of monocytes and macrophages, respectively. The study of macrophage biology in humans and the closely related nonhuman primate have been hampered by the technical limitations of obtaining purified macrophage subsets from tissue directly ex vivo. Indeed, in the absence of such ex vivo sorting techniques for macrophages, studies have relied heavily on macrophage cell lines, such as U937, and in vitro monocyte-derived macrophages, which may not truly recapitulate the complexities of tissue-resident macrophages [42–44]. The evaluation of tissue-resident macrophages by flow cytometry provides the advantage of sorting live cells, a technique not afforded by traditional immunohistochemical and immunofluorescent staining techniques. Given the numerous roles of macrophages in physiological processes, the ability to perform functional assays on macrophages directly ex vivo has implications across multiple fields of study.
Nonhuman primates provide a powerful model of human biology. Here, we advance the nonhuman primate model by showing, for the first time, that monocytes and macrophages can be experimentally depleted by administration of LA. Given the scientific insight gained by depletion of T cells, B cells, and NK cells in nonhuman primates [8–13], we believe that our technique further strengthens the nonhuman primate model and provides a unique avenue for the study of the many physiological processes of blood monocytes and tissue macrophages.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a Creative and Novel Ideas in HIV Research (CNIHR) grant to J.B.S. from the U.S. National Institutes of Health, University of California, San Francisco–Gladstone Institute of Virology and Immunology Center for AIDS Research (P30-AI027763), The American Foundation for AIDS Research (amfAR) with support from the Foundation for AIDS and Immune Research (FAIR) (amfAR grant 108548 to J.B.S.), and P51 OD011092. Anti-CD14 for these studies was obtained from Keith Reimann at the Nonhuman Primate Resource Reagent Program (funded by HHSN272200090037C and OD010976).
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- ARD
- amine reactive dye
- BAL
- bronchoalveolar lavage
- BrdU
- 5′-bromo-2′-deoxyuridine
- d.p.i.
- day(s) postinjection
- LA
- liposomal alendronate
- LC
- liposomal clodronate
- Lin
- lineage
- MFI
- mean fluorescence intensity
- RM
- rhesus macaques
AUTHORSHIP
B.J.B. wrote the manuscript, assisted in project conception, generated all figures, and codesigned and conducted experiments. J.S.R. and K.B.H. conducted experiments and edited the manuscript. M.A.O., S.L.P., and A.W.L. conducted experiments. A.J.E. assisted in project conception and edited the manuscript. Y.R. and G.G. assisted in project conception, provided expert advice, and edited the manuscript. J.B.S. was the principal investigator.
DISCLOSURES
G.G. and Y.R. have a financial interest in BioRest, a company that may have a commercial interest in the results of this research and technology.
REFERENCES
- 1. Pollard J. W. (2009) Trophic macrophages in development and disease. Nat. Rev. Immunol. 9, 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schneemann M., Schoeden G. (2007) Macrophage biology and immunology: man is not a mouse. J. Leukoc. Biol. 81, 579; discussion 580. [DOI] [PubMed] [Google Scholar]
- 3. Murray P. J., Wynn T. A. (2011) Obstacles and opportunities for understanding macrophage polarization. J. Leukoc. Biol. 89, 557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haigwood N. L. (2009) Update on animal models for HIV research. Eur. J. Immunol. 39, 1994–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang S. H., Cheng P. H., Banta H., Piotrowska-Nitsche K., Yang J. J., Cheng E. C., Snyder B., Larkin K., Liu J., Orkin J., Fang Z. H., Smith Y., Bachevalier J., Zola S. M., Li S. H., Li X. J., Chan A. W. (2008) Towards a transgenic model of Huntington's disease in a non-human primate. Nature 453, 921–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sacha J. B., Kim I. J., Chen L., Ullah J. H., Goodwin D. A., Simmons H. A., Schenkman D. I., von Pelchrzim F., Gifford R. J., Nimityongskul F. A., Newman L. P., Wildeboer S., Lappin P. B., Hammond D., Castrovinci P., Piaskowski S. M., Reed J. S., Beheler K. A., Tharmanathan T., Zhang N., Muscat-King S., Rieger M., Fernandes C., Rumpel K., Gardner J. P., Gebhard D. H., Janies J., Shoieb A., Pierce B. G., Trajkovic D., Rakasz E., Rong S., McCluskie M., Christy C., Merson J. R., Jones R. B., Nixon D. F., Ostrowski M. A., Loudon P. T., Pruimboom-Brees I. M., Sheppard N. C. (2012) Vaccination with cancer- and HIV infection-associated endogenous retrotransposable elements is safe and immunogenic. J. Immunol. 189, 1467–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tachibana M., Sparman M., Sritanaudomchai H., Ma H., Clepper L., Woodward J., Li Y., Ramsey C., Kolotushkina O., Mitalipov S. (2009) Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature 461, 367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choi E. I., Reimann K. A., Letvin N. L. (2008) In vivo natural killer cell depletion during primary simian immunodeficiency virus infection in rhesus monkeys. J. Virol. 82, 6758–6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Friedrich T. C., Valentine L. E., Yant L. J., Rakasz E. G., Piaskowski S. M., Furlott J. R., Weisgrau K. L., Burwitz B., May G. E., Leon E. J., Soma T., Napoe G., Capuano S. V., Wilson N. A., Watkins D. I. (2007) Subdominant CD8+ T-cell responses are involved in durable control of AIDS virus replication. J. Virol. 81, 3465–3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ortiz A. M., Klatt N. R., Li B., Yi Y., Tabb B., Hao X. P., Sternberg L., Lawson B., Carnathan P. M., Cramer E. M., Engram J. C., Little D. M., Ryzhova E., Gonzalez-Scarano F., Paiardini M., Ansari A. A., Ratcliffe S., Else J. G., Brenchley J. M., Collman R. G., Estes J. D., Derdeyn C. A., Silvestri G. (2011) Depletion of CD4(+) T cells abrogates post-peak decline of viremia in SIV-infected rhesus macaques. J. Clin. Invest. 121, 4433–4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haberthur K., Engelmann F., Park B., Barron A., Legasse A., Dewane J., Fischer M., Kerns A., Brown M., Messaoudi I. (2011) CD4 T cell immunity is critical for the control of simian varicella virus infection in a nonhuman primate model of VZV infection. PLoS Pathog. 7, e1002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hansen S. G., Powers C. J., Richards R., Ventura A. B., Ford J. C., Siess D., Axthelm M. K., Nelson J. A., Jarvis M. A., Picker L. J., Fruh K. (2010) Evasion of CD8+ T cells is critical for superinfection by cytomegalovirus. Science 328, 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gordon S. N., Cecchinato V., Andresen V., Heraud J. M., Hryniewicz A., Parks R. W., Venzon D., Chung H. K., Karpova T., McNally J., Silvera P., Reimann K. A., Matsui H., Kanehara T., Shinmura Y., Yokote H., Franchini G. (2011) Smallpox vaccine safety is dependent on T cells and not B cells. J. Infect. Dis. 203, 1043–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Rooijen N., Sanders A. (1994) Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods 174, 83–93. [DOI] [PubMed] [Google Scholar]
- 15. Gutman D., Golomb G. (2012) Liposomal alendronate for the treatment of restenosis. J. Control Release 161, 619–627. [DOI] [PubMed] [Google Scholar]
- 16. Van Rooijen N., Hendrikx E. (2010) Liposomes for specific depletion of macrophages from organs and tissues. Methods Mol. Biol. 605, 189–203. [DOI] [PubMed] [Google Scholar]
- 17. Epstein-Barash H., Gutman D., Markovsky E., Mishan-Eisenberg G., Koroukhov N., Szebeni J., Golomb G. (2010) Physicochemical parameters affecting liposomal bisphosphonates bioactivity for restenosis therapy: internalization, cell inhibition, activation of cytokines and complement, and mechanism of cell death. J. Control Release 146, 182–195. [DOI] [PubMed] [Google Scholar]
- 18. Epstein H., Gutman D., Cohen-Sela E., Haber E., Elmalak O., Koroukhov N., Danenberg H. D., Golomb G. (2008) Preparation of alendronate liposomes for enhanced stability and bioactivity: in vitro and in vivo characterization. AAPS J. 10, 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim W. K., Sun Y., Do H., Autissier P., Halpern E. F., Piatak M. J., Lifson J. D., Burdo T. H., McGrath M. S., Williams K. (2010) Monocyte heterogeneity underlying phenotypic changes in monocytes according to SIV disease stage. J. Leukoc. Biol. 87, 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clodronate Liposomes Database. Created by Mohan A., Janan A., Pourpaki M., MirAfzali Z. Encapsula NanoSciences LLC, Brentwood, TN, USA: Last updated September 23, 2011, from http://www.clodrosome.com/database/. [Google Scholar]
- 21. Danenberg H. D., Fishbein I., Epstein H., Waltenberger J., Moerman E., Monkkonen J., Gao J., Gathi I., Reichi R., Golomb G. (2003) Systemic depletion of macrophages by liposomal bisphosphonates reduces neointimal formation following balloon-injury in the rat carotid artery. J. Cardiovasc. Pharmacol. 42, 671–679. [DOI] [PubMed] [Google Scholar]
- 22. Van Beek E. R., Cohen L. H., Leroy I. M., Ebetino F. H., Lowik C. W., Papapoulos S. E. (2003) Differentiating the mechanisms of antiresorptive action of nitrogen containing bisphosphonates. Bone 33, 805–811. [DOI] [PubMed] [Google Scholar]
- 23. Banai S., Finkelstein A., Almagor Y., Assali A., Hasin Y., Rosenschein U., Apruzzese P., Lansky A. J., Kume T., Edelman E. R. (2013) Targeted anti-inflammatory systemic therapy for restenosis: the BioRest Liposomal Alendronate with Stenting Study (BLAST)—a double blind, randomized clinical trial. Am. Heart J. 165, 234.e1–240.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ziegler-Heitbrock L., Ancuta P., Crowe S., Dalod M., Grau V., Hart D. N., Leenen P. J., Liu Y. J., MacPherson G., Randolph G. J., Scherberich J., Schmitz J., Shortman K., Sozzani S., Strobl H., Zembala M., Austyn J. M., Lutz M. B. (2010) Nomenclature of monocytes and dendritic cells in blood. Blood 116, e74–e80. [DOI] [PubMed] [Google Scholar]
- 25. Goebeler M., Roth J., Teigelkamp S., Sorg C. (1994) The monoclonal antibody MAC387 detects an epitope on the calcium-binding protein MRP14. J. Leukoc. Biol. 55, 259–261. [DOI] [PubMed] [Google Scholar]
- 26. Soulas C., Conerly C., Kim W. K., Burdo T. H., Alvarez X., Lackner A. A., Williams K. C. (2011) Recently infiltrating MAC387(+) monocytes/macrophages a third macrophage population involved in SIV and HIV encephalitic lesion formation. Am. J. Pathol. 178, 2121–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Williams D. W., Eugenin E. A., Calderon T. M., Berman J. W. (2012) Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J. Leukoc. Biol. 91, 401–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mansfield K., Lang S. M., Gauduin M. C., Sanford H. B., Lifson J. D., Johnson R. P., Desrosiers R. C. (2008) Vaccine protection by live, attenuated simian immunodeficiency virus in the absence of high-titer antibody responses and high-frequency cellular immune responses measurable in the periphery. J. Virol. 82, 4135–4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rogler G., Hausmann M., Vogl D., Aschenbrenner E., Andus T., Falk W., Andreesen R., Scholmerich J., Gross V. (1998) Isolation and phenotypic characterization of colonic macrophages. Clin. Exp. Immunol. 112, 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hasegawa A., Liu H., Ling B., Borda J. T., Alvarez X., Sugimoto C., Vinet-Oliphant H., Kim W. K., Williams K. C., Ribeiro R. M., Lackner A. A., Veazey R. S., Kuroda M. J. (2009) The level of monocyte turnover predicts disease progression in the macaque model of AIDS. Blood 114, 2917–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bilzer M., Roggel F., Gerbes A. L. (2006) Role of Kupffer cells in host defense and liver disease. Liver Int. 26, 1175–1186. [DOI] [PubMed] [Google Scholar]
- 32. Bischoff R., Holtzer H. (1970) Inhibition of myoblast fusion after one round of DNA synthesis in 5-bromodeoxyuridine. J. Cell Biol. 44, 134–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gunduz N. (1985) The use of FITC-conjugated monoclonal antibodies for determination of S-phase cells with fluorescence microscopy. Cytometry 6, 597–601. [DOI] [PubMed] [Google Scholar]
- 34. Van Furth R., Diesselhoff-den Dulk M. C., Mattie H. (1973) Quantitative study on the production and kinetics of mononuclear phagocytes during an acute inflammatory reaction. J. Exp. Med. 138, 1314–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Burdo T. H., Soulas C., Orzechowski K., Button J., Krishnan A., Sugimoto C., Alvarez X., Kuroda M. J., Williams K. C. (2010) Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog. 6, e1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Danenberg H. D., Golomb G., Groothuis A., Gao J., Epstein H., Swaminathan R. V., Seifert P., Edelman E. R. (2003) Liposomal alendronate inhibits systemic innate immunity and reduces in-stent neointimal hyperplasia in rabbits. Circulation 108, 2798–2804. [DOI] [PubMed] [Google Scholar]
- 37. Haber E., Afergan E., Epstein H., Gutman D., Koroukhov N., Ben-David M., Schachter M., Golomb G. (2010) Route of administration-dependent anti-inflammatory effect of liposomal alendronate. J. Control Release 148, 226–233. [DOI] [PubMed] [Google Scholar]
- 38. Cai Y., Sugimoto C., Arainga M., Alvarez X., Didier E. S., Kuroda M. J. (2014) In vivo characterization of alveolar and interstitial lung macrophages in rhesus macaques: implications for understanding lung disease in humans. J. Immunol. 192, 2821–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harker J. A., Yamaguchi Y., Culley F. J., Tregoning J. S., Openshaw P. J. (2014) Delayed sequelae of neonatal respiratory syncytial virus infection are dependent on cells of the innate immune system. J. Virol. 88, 604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hartwig S. M., Holman K. M., Varga S. M. (2014) Depletion of alveolar macrophages ameliorates virus-induced disease following a pulmonary coronavirus infection. PLoS One 9, e90720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zaynagetdinov R., Sherrill T. P., Kendall P. L., Segal B. H., Weller K. P., Tighe R. M., Blackwell T. S. (2013) Identification of myeloid cell subsets in murine lungs using flow cytometry. Am. J. Respir. Cell Mol. Biol. 49, 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Berger G., Durand S., Fargier G., Nguyen X. N., Cordeil S., Bouaziz S., Muriaux D., Darlix J. L., Cimarelli A. (2011) APOBEC3A is a specific inhibitor of the early phases of HIV-1 infection in myeloid cells. PLoS Pathog. 7, e1002221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sacha J. B., Giraldo-Vela J. P., Buechler M. B., Martins M. A., Maness N. J., Chung C., Wallace L. T., Leon E. J., Friedrich T. C., Wilson N. A., Hiraoka A., Watkins D. I. (2009) Gag- and Nef-specific CD4+ T cells recognize and inhibit SIV replication in infected macrophages early after infection. Proc. Natl. Acad. Sci. USA 106, 9791–9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Song E., Lee S. K., Dykxhoorn D. M., Novina C., Zhang D., Crawford K., Cerny J., Sharp P. A., Lieberman J., Manjunath N., Shankar P. (2003) Sustained small interfering RNA-mediated human immunodeficiency virus type 1 inhibition in primary macrophages. J. Virol. 77, 7174–7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.