Age associated lung inflammation can modify pulmonary macrophage function, which can be reversed with an ibuprofen supplemented diet.
Keywords: M. tuberculosis, inflammaging, age
Abstract
Systemic inflammation that occurs with increasing age (inflammaging) is thought to contribute to the increased susceptibility of the elderly to several disease states. The elderly are at significant risk for developing pulmonary disorders and infectious diseases, but the contribution of inflammation in the pulmonary environment has received little attention. In this study, we demonstrate that the lungs of old mice have elevated levels of proinflammatory cytokines and a resident population of highly activated pulmonary macrophages that are refractory to further activation by IFN-γ. The impact of this inflammatory state on macrophage function was determined in vitro in response to infection with M.tb. Macrophages from the lungs of old mice secreted more proinflammatory cytokines in response to M.tb infection than similar cells from young mice and also demonstrated enhanced M.tb uptake and P-L fusion. Supplementation of mouse chow with the NSAID ibuprofen led to a reversal of lung and macrophage inflammatory signatures. These data indicate that the pulmonary environment becomes inflammatory with increasing age and that this inflammatory environment can be reversed with ibuprofen.
Introduction
Increasing age is associated with an abundance of circulating inflammatory cytokines that are considered to be a significant contributing factor to the susceptibility of the elderly to numerous disease states, including diabetes, heart disease, and chronic pulmonary disorders, such as emphysema, bronchitis, and COPD [1–4]. This systemic increase in basal inflammatory cytokines in old age has been termed inflammaging and is broadly attributed to altered immune cell function, particularly within the myeloid lineage [5]. However, studies of monocyte or macrophage function in vitro have been contradictory with some studies showing that the capacity of myeloid cells to produce inflammatory cytokines can be impaired in old age [6, 7], whereas others have shown that inflammatory cytokine secretion can be enhanced [8, 9]. These disparities have often been attributed to the source of monocytes or derived/resident macrophages, as well as the specific stimulus that has been used [5].
Our own studies have focused on resident tissue-derived pulmonary macrophages and have shown previously that macrophages from the lungs of old mice are capable of secreting abundant TNF-α and IL-12 in response to in vitro or in vivo stimulus with the virulent pulmonary pathogen M.tb [10–12]. M.tb is a significant global threat, accounting for >9 million cases and 1.4 million deaths from tuberculosis each year [13, 14]. M.tb is also responsible for significant morbidity and mortality in the elderly [15], and the aged mouse model has been used extensively to understand innate and adaptive immune function in the context of this infectious disease [16–18]. The accumulated knowledge of immune cell function in vitro and in vivo in response to M.tb in humans and animal models [19] makes this an ideal and globally relevant model system to investigate macrophage function in old age.
Here, we extend our studies on pulmonary macrophage function in old age to characterize further the functional state of macrophages ex vivo and in response to in vitro infection with M.tb. Our data demonstrate that resident pulmonary macrophages from old mice are phenotypically and functionally different than pulmonary macrophages from young mice. Pulmonary macrophages from old mice expressed markers associated with basal activation, had higher levels of M.tb uptake during in vitro infection, and had a greater percentage of phagocytosed M.tb residing in P-L compartments. This activated macrophage phenotype was associated with inflammaging signatures in the lung, as the reversal of the inflammatory state in old mice using an ibuprofen-enriched diet restored the pulmonary macrophage phenotype and function to that observed in young mice.
MATERIALS AND METHODS
Mice
Specific pathogen-free, female C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA, USA) or through rodent resources with the National Institute on Aging (supplied by Charles River Laboratories) at an age of 3 months (young) or 18 months (old). Mice were maintained with sterilized water and chow ad libitum in microisolator cages and acclimatized for at least 1 week before use. For diet modification studies, mice were fed either the Teklad Global 18% protein rodent control diet or Global 18% control diet supplemented with ibuprofen (375 ppm; Harlan Laboratories, Madison, WI, USA) for 2 weeks before experimental manipulation. Mice were killed for experiments by CO2 asphyxiation. All procedures were approved by The Ohio State University Institutional Laboratory Animal Care and Use Committee.
Isolation of lung cells
Lungs of young and old mice were perfused with PBS containing 50 U/ml heparin (Sigma, St. Louis, MO, USA) through the right ventricle of the heart. All lung lobes were extracted and placed in 2 ml-supplemented DMEM containing L-glutamine (Mediatech, Manassas, VA, USA), supplemented with sterile-filtered, 5 ml HEPES buffer (1 M; Sigma), 10 ml MEM nonessential amino acid solution (100×; Sigma), 660 μl 2-ME (50 mM; Sigma), and 45 ml heat-inactivated FBS (Atlas Biologicals, Ft. Collins, CO, USA) (complete DMEM). A single-cell suspension was obtained using enzymatic digestion, as described previously [12]. Briefly, lungs were diced and digested for 30 min at 37°C, 5% CO2, in 4 ml complete DMEM containing collagenase XI from Clostridium histolyticum (0.7 mg/ml; Sigma) and type IV bovine pancreatic DNase (30 μg/ml; Sigma). Complete DMEM (10 ml) was added to stop the enzymatic activity, and lung pieces were gently pressed through a 70-μm cell strainer to achieve a single-cell suspension. Residual erythrocytes were lysed using Gey's lysis buffer (8 mM NH4Cl, 5 mM KHCO3 in water) and suspended in complete DMEM.
Purification of pulmonary macrophages
Lung cells were adhered to tissue-culture grade Petri dishes for 1 h at 37°C, 5% CO2. Petri dishes were washed three times using warm DPBS (Invitrogen, Carlsbad, CA, USA) to remove nonadherent cells. Adherent cells were incubated with 5 ml trypsin-EDTA (Sigma) for 10 min at 37°C, 5% CO2, after which, 10 ml complete DMEM was added to stop the reaction. Adherent cells were harvested by vigorous pipetting and pooled and suspended in 1 ml complete DMEM. Cells were counted using trypan blue (Sigma) and adjusted to working concentrations in complete DMEM. Based on previously published studies from our group [18] and additional analyses here, the pulmonary adherent cell population in old mice expresses more CD11b (Table 1), indicating that in old age, the lung has more cells that resemble infiltrating monocytes. Pulmonary macrophages and dendritic cells both express CD11c [20] and therefore, could not be analyzed independently. Independent of this difference, CD11c- and CD11b-positive cells from the lungs of old mice expressed more MHCII [21], indicative of heightened activation. For simplicity, adherent cells are subsequently referred to as macrophages.
Table 1. Mean Fluorescence Intensity of Cell-Surface Markers.
| Markers | Young control (n=5) | Young ibuprofen (n=5) | Old control (n=5) | Old ibuprofen (n=5) |
|---|---|---|---|---|
| CD11b | 1995 ± 816 | 2893 ± 264 | 3073 ± 169 | 2193 ± 650 |
| CD11c | 1387 ± 237a | 1856 ± 72 | 2218 ± 258 | 2165 ± 283 |
| CD86 | 177 ± 35 | 135 ± 10 | 123 ± 10 | 122 ± 15 |
| MR | 273 ± 140a | 645 ± 172 | 1319 ± 132 | 1506 ± 166 |
| MHCII | 2266 ± 374a | 2203 ± 116 | 4287 ± 366 | 4573 ± 220 |
Pulmonary macrophages from young and old mice on a control or an ibuprofen diet were isolated, fixed, and stained extracellularly with antibodies against CD11b, CD11c, CD86, MR, or MHCII. Mean fluorescence intensity (MFI) of total CD11b and CD11c populations and MFI of CD86, MR, or MHCII on CD11c-positive cells only are expressed. Isotype control antibodies were used to set positive gates. MFI values were normalized by subtracting isotype control values from those obtained using specific antibodies.
Significant differences ( *P<0.05) detected between young control and old control groups. No significant differences were observed between control and ibuprofen treatment in either age group.
Real-time PCR
The right lung lobes of naive, young and old mice were removed and homogenized in 1 ml TRIzol reagent (Invitrogen). RNA was extracted with chloroform, precipitated using isopropanol and 75% ethanol, and reconstituted in DNase/RNase-free water. cDNA was synthesized with random primers using an Omniscript RT Kit (Qiagen, Valencia, CA, USA). Real-time PCR was performed on cDNA from whole lung using TaqMan gene expression assays (Applied Biosystems, Foster City, CA, USA) for IFN-γ, TNF-α, IL-12 p40/p70 (IL-12), IL-10, IL-6, and IL-1β. TaqMan gene expression assays for CIITA, IRGM-1, and IRF-1 were performed on cDNA from macrophages that were purified as described above and RNA-extracted with TRIzol reagent. A Bio-Rad CFX96 Real-Time PCR thermal cycler was used for qPCR (Bio-Rad, Hercules, CA, USA). The ΔΔCT method was used for quantification of the RU of mRNA, using 18S rRNA as an endogenous normalizer and young mice as the calibrator [RU=2−ΔΔCT; ΔCT=CT gene (IFN-γ, TNF-α, etc.)−CT 18S; ΔΔCT=ΔCT sample−ΔCT calibrator (young mouse)].
In vitro bacterial infections
Isolated adherent cells (macrophages) were adjusted to 2× 105 cells/well and placed in wells of a 24-well plate. Confocal studies used acid-treated, flame-sterilized Gold-Seal coverslips (Electron Microscopy Sciences, Hartfield, PA, USA). Cells were allowed to adhere to coverslips or tissue-culture wells overnight at 37°C, 5% CO2. For some experiments, 2 × 105 cells/well were incubated with purified murine rIFN-γ (100 U/ml; Roche, Indianapolis, IN, USA) for 16 h under standard conditions before a 24-h infection. All work involving live M.tb was conducted in BSL3. All infections were completed at a MOI of five, with GFP-M.tb. This strain expresses GFP constitutively through a Mycobacterium bovis bacillus Calmette-Guerin heat shock protein 60 promoter and was generated by Horwitz and colleagues [22]. GFP-M.tb was grown and stored according to our standard protocols [10]. Stock GFP-M.tb was thawed, and clumps were disrupted by four cycles of drawing and ejection through a 26-G needle. The inoculum was subsequently diluted to working concentrations and added to cells in a fixed volume. Cells/M.tb were incubated at 4°C for 10 min and then centrifuged at 200 g, 4°C, for 10 min. The infection was allowed to proceed for 2 h at 37°C, 5% CO2. For confocal studies, cells were washed twice with warm DPBS and then fixed for 10 min with 2% paraformaldehyde. After fixation, coverslips were removed from the BSL3 facility for further processing.
Immunocytochemistry and confocal microscopy
Infected, fixed cells on coverslips were permeabilized with methanol for 3 min at room temperature, washed with DPBS, and blocked overnight at 4°C with blocking buffer PBS [10% heat-inactivated FBS (Atlas Biologicals) and 5 mg/ml BSA (Sigma) in DPBS]. Primary rat anti-mouse IgG2 antibodies against LAMP-1 (Santa Cruz Biotechnology, Dallas, TX, USA) and cathepsin D (R&D Systems, Minneapolis, MN, USA) were used as lysosomal markers. Goat anti-rat IgG antibody conjugated to AlexaFluor 594 (Invitrogen) was used as the secondary antibody. Normal rat IgG2 antibody (R&D Systems) was the isotype control. Coverslips were mounted on slides using ProLong Gold Antifade Reagent containing the nuclear stain 4′,6′-diamidino-2-phenylindole (Invitrogen).
Coverslips were analyzed by laser-scanning confocal microscopy using an Olympus FV1000 Filter Confocal Microscope in the CMIF at The Ohio State University. To measure the percentage of cells containing GFP-M.tb (attached and intracellular), Z-stacks of at least six fields were taken over a depth of 10 μm at a magnification of 400× for all experimental groups so that at least 300 cells were counted/coverslip. Similar methods were used to measure P-L fusion over the same depth and magnification, with at least 100 internalized bacteria counted/coverslip and analyzed for colocalization of phagosomes containing GFP-M.tb (GFP, 488 nm) with LAMP-1 or cathepsin D (lysosomal markers, AlexaFluor 594, 594 nm). Microscopy data were analyzed using the Olympus FluoView Viewer software.
ELISA
Cell culture supernatants were assayed for the presence of IL-12 by ELISA as described previously [18].
Analysis of bacterial growth in macrophages
Macrophages isolated from the lungs were incubated with 5:1 GFP-M.tb for 2 h at 37°C and 5% CO2 before washing with warm complete DMEM to remove nonadherent M.tb. Infected macrophages were then cultured in complete DMEM. Bacterial growth at defined time-points postinfection was assessed using the following protocol. Supernatant from each well was removed and centrifuged down to obtain the pellet of unattached infected cells. Sterile, cold water (300 μl) containing DNase I (500 μm/ml; Sigma) was added to the monolayer. After 10 min with periodic agitation, 560 μl Middlebrook 7H9 culture broth (BD Biosciences, San Jose, CA, USA) containing the pellet of unattached-infected cells was added to the monolayer, followed by lysis with 240 μl 0.25% SDS (Fisher Scientific International, Fair Lawn, NJ, USA) in DPBS. After 10 min with periodic agitation, 300 μl 20% BSA (Sigma) was added to all wells to stop lysis and mixed by pipetting. Contents of each well were transferred to a 1.5-ml tube with a tether cap (Fisher Scientific International) containing two flame-sterilized glass beads. Tubes were pulsed (5×) on a vortex, and serial dilutions were plated on 7H11 agar plates, supplemented with OADC (Remel, Lenexa, KS, USA). Colonies were enumerated after 21 days incubation at 37°C.
Flow cytometry
All antibodies were obtained from BD Biosciences, unless stated otherwise. Isolated lung cells were suspended in deficient RPMI (Mediatech), supplemented with 0.1% sodium azide (Sigma). Surface targets were detected, as described previously [18]. Specific antibodies included rat IgG2a FITC (553929), anti-CD86 FITC (553691), rat IgG2b PE (556925), and anti-MHCII PE (ab24842; Abcam, Cambridge, UK) and hamster IgG1 APC-Cy7 (561206), anti-CD11c APC-Cy7 (561241), rat IgG2a APC (553932), and anti-CD206 APC (141708; BioLegend, San Diego, CA, USA). Samples were read using the FACSCanto II flow cytometer (BD Biosciences) and analyzed with FACSDiva software (BD Biosciences).
Statistical analyses
Data were plotted and analyzed using the GraphPad Prism 5.0 software (GraphPad Software, La Jolla, CA, USA). The unpaired two-tailed Student's t-test was used to determine statistical significance. The Grubbs' test was used to identify outlying data points.
RESULTS
The pulmonary environment in old mice contains increased proinflammatory cytokines and highly activated macrophages
Human aging is signified by an up-regulation of inflammatory responses with increased circulating levels of the proinflammatory cytokines IL-1, IL-6, and TNF-α, resulting in a low-grade, chronic, systemic, proinflammatory state [23]. To determine whether the pulmonary environment has similar proinflammatory characteristics, we determined the expression levels of IFN-γ, TNF-α, IL-12, IL-1β, IL-6, and IL-10 in whole lung homogenates by RT-qPCR. Data were normalized to young mice. The expression of IFN-γ (Fig. 1A), TNF-α (Fig. 1B), and IL-12 mRNA (Fig. 1C) was elevated significantly in the lungs of old mice compared with young mice. Similar trends were also observed for IL-1β and IL-6 mRNA expression (data not shown). Expression of mRNA for the anti-inflammatory cytokine IL-10 did not differ significantly between old and young mice (data not shown). These data demonstrate that the basal properties of the lungs of old mice are inflammatory.
Figure 1. Lungs of old mice demonstrate an enhanced inflammatory state, and macrophages isolated from this environment have elevated IFN-γ-induced activation markers.
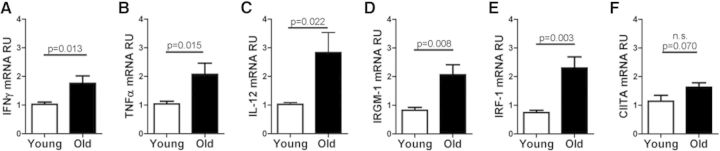
Relative expression of IFN-γ (A), TNF-α (B), IL-12 (C), IRGM-1 (D), IRF-1 (E), and CIITA (F), as determined by RT-qPCR in old (solid bars) and young (open bars) mice. Whole-lung homogenates (A–C) or purified lung macrophages (D–F) were collected into TRIzol reagent, cDNA was synthesized using the Omniscript RT Kit, and real-time PCR was performed using TaqMan gene expression assays. Data were combined from three independent experiments using five individual young or old mice/experiment (A–C) or from two independent experiments using five individual young or old mice/experiment (D–F). Student's t-test was used to determine statistical significance.
IFN-γ and TNF-α are known to activate directly various functions of macrophages [24, 25], and therefore, we anticipated that resident macrophages isolated directly from the lungs of old mice would express markers and function associated with activation. Pulmonary macrophages were isolated from old and young mice, and mRNA expression was determined for genes associated with macrophage activation [26–28] by RT-qPCR. Macrophages isolated from the lungs of old mice expressed significantly more mRNA for IRGM-1 (Fig. 1D) and IRF-1 (Fig. 1E). Although not statistically significant, a similar trend was observed for the expression of CIITA (Fig. 1F). Together, these data demonstrate that mRNA expression for products known to be up-regulated by IFN-γ is increased in resident macrophages isolated from the lungs of old mice.
Pulmonary macrophages isolated from old mice have increased M.tb uptake and P-L fusion
To determine whether the lung-inflammatory state and the preactivated nature of pulmonary macrophages from old mice led to altered function, we determined the capacity of resident pulmonary macrophages from old mice to phagocytose and kill M.tb in vitro. The pathway for M.tb uptake and intracellular trafficking has been elucidated previously in young mice and humans [19], providing a well-characterized baseline for investigating the properties of macrophage function and extending our previous in vivo and in vitro studies of M.tb infection in old mice [10–12, 18, 29].
Macrophages isolated from the lungs of young and old mice were incubated with GFP-M.tb and examined by confocal microscopy. An infected cell was defined as one that had attached or internalized at least one GFP-M.tb bacillus (Fig. 2A). The mean percentage of infected macrophages from old mice was found to be significantly higher than the percentage of infected macrophages from young mice (Fig. 2B). This suggests that macrophages from old mice phagocytose M.tb more robustly than young macrophages.
Figure 2. Pulmonary macrophages from old mice have increased M.tb uptake and P-L fusion but a defect in intracellular control of bacterial growth.
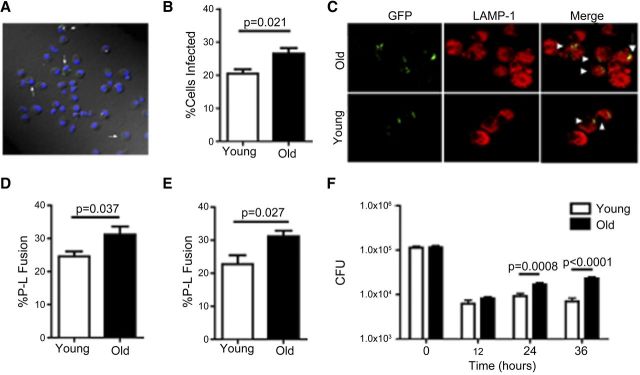
Pulmonary macrophages isolated from old (solid bars) and young (open bars) mice were adhered to coverslips and incubated with 5:1 GFP-M.tb for 2 h and analyzed by confocal microscopy (A–E). White arrows indicate infected pulmonary macrophages (A). Percentage of cells from both groups found to be infected with GFP-M.tb (B). P-L fusion was observed in macrophages from young and old mice using the lysosomal marker LAMP-1 (C and D) and cathepsin D (E). White arrowhead indicates P-L colocalization events (yellow) between GFP-M.tb (green) and LAMP-1 (red). Macrophages isolated from the lungs of young and old mice were incubated with 5:1 GFP-M.tb for 2 h, and M.tb intracellular growth was assessed at the indicated time-points postinfection (F). CFU was normalized between macrophages from young and old mice at time zero. Colonies were enumerated after 21 days of incubation at 37°C. Data were combined from three independent experiments from pools of five mice. Student's t-test was used to determine statistical significance.
Following uptake by macrophages, M.tb normally limits the degree of P-L fusion in macrophages to enhance its survival [19]. To determine the percent P-L fusion for phagocytosed bacteria, we determined colocalization of M.tb with LAMP-1 or cathepsin D, both of which are classically used to identify late endosome and lysosome markers [30–36]. The mean percentage of GFP-M.tb and LAMP-1 colocalization (P-L fusion) seen in macrophages from old mice was significantly higher than that observed for macrophages from young mice (Figs. 2C and D). Determination of percent P-L fusion using cathepsin D (Fig. 2E) showed similar findings to LAMP-1, with significantly higher P-L fusion events in macrophages from old mice compared with macrophages from young mice.
In studies of human monocyte-derived macrophages or macrophages from young mice, increased P-L fusion correlates with enhanced M.tb killing, which can be detected by CFU determination [37]. Two hours after infection with M.tb, macrophages from young and old mice had equivalent M.tb CFU, and both were equally capable of reducing the CFU tenfold within the first 12 h of infection (Fig. 2F). However, as the culture period extended (≥24 h), macrophages from old mice became more permissive to M.tb growth than macrophages from young mice.
IFN-γ boosts P-L fusion and cytokine production in pulmonary macrophages from young but not old mice
Macrophages isolated from the lungs of old mice expressed several molecules that are associated with IFN-γ activation and had enhanced uptake and P-L fusion events in response to M.tb infection. In addition, IFN-γ was found in abundance in the lung homogenate of old mice. We therefore hypothesized that resident pulmonary macrophages from old mice were endogenously exposed to IFN-γ and would be in a preactivated state in vivo and ex vivo.
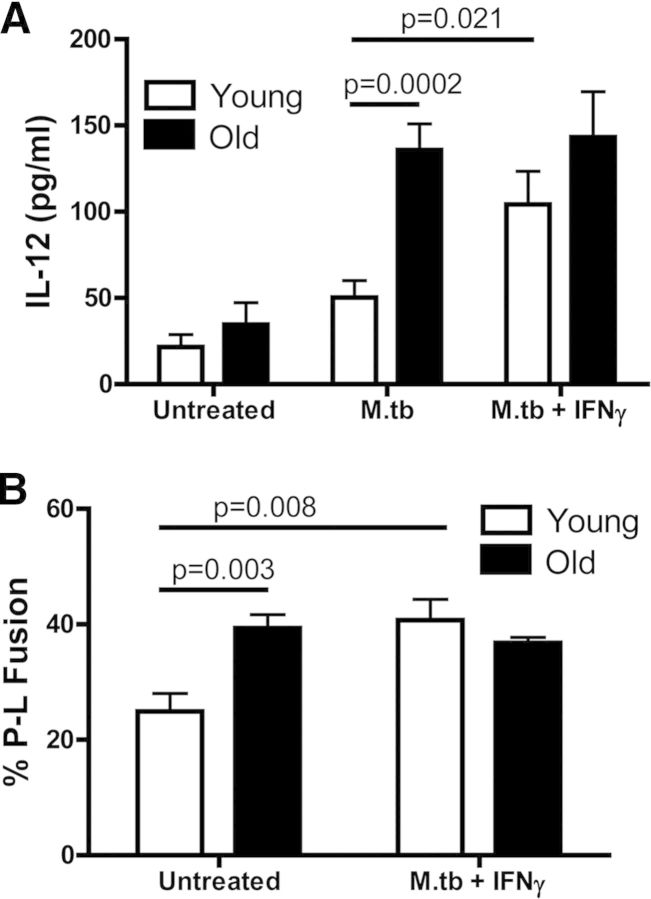
To test this hypothesis, macrophages from the lungs of young and old mice were stimulated with murine rIFN-γ, 16 h before M.tb infection, and IL-12 was determined in culture supernatants, 24 h later. The basal level of IL-12 production from untreated cells was increased slightly in macrophages from old mice, although this difference was not statistically significant (Fig. 3A). When incubated with M.tb, IL-12 production by macrophages isolated from the lungs of young mice increased modestly versus a highly significant increase for macrophages isolated from the lungs of old mice. In line with our hypothesis, IFN-γ activation did not enhance IL-12 production further by macrophages from old mice, whereas IFN-γ activation increased IL-12 production significantly by macrophages from young mice in response to M.tb infection.
Figure 3. IFN-γ fails to boost P-L fusion and cytokine production in response to M.tb infection in pulmonary macrophages from old mice.
Pulmonary macrophages from old (solid bars) and young (open bars) mice were plated onto coverslips and incubated with murine rIFN-γ or left untreated for 16 h. For cytokine production, cells were infected with 5:1 GFP-M.tb for 24 h, and the concentration of IL-12 (A) in the supernatants was analyzed by ELISA. For P-L fusion, cells were infected with 5:1 GFP-M.tb for 2 h, fixed, permeabilized, blocked, and stained for cathepsin D and P-L fusion assessed by confocal microscopy (B). Data were combined from three independent experiments from pools of five mice/experiment. Student's t-test was used to determine statistical significance.
To extend these findings further, we determined whether IFN-γ activation of macrophages could modify intracellular trafficking, resulting in increased P-L fusion. Pulmonary macrophages from young and old mice were incubated with murine rIFN-γ or left untreated for 16 h and subsequently infected with M.tb. P-L fusion was assessed 2 h after M.tb infection. The percent P-L fusion observed in macrophages from old mice was significantly higher than macrophages from young mice (Fig. 3B), as we demonstrated previously (Fig. 2D and E). However, similar to cytokine production, there were no differences in percent P-L fusion between resting old macrophages and those that were preactivated by IFN-γ. In contrast, the percent P-L fusion observed in young macrophages was enhanced significantly by IFN-γ activation. Thus, these data provide evidence that IFN-γ activation cannot enhance further the function of pulmonary macrophages from old mice, potentially as a result of their basal state of IFN-γ-induced activation in the lung.
Ibuprofen can decrease the pulmonary inflammatory environment of old mice and alter macrophage function
As the pulmonary environment of old mice was inflammatory in nature, and increased basal IFN-γ levels appeared to modify resident macrophages sufficiently to alter their function, we hypothesized that the inflammatory state was directly responsible for the hyper-responsiveness of macrophages from old mice. To test this, we used ibuprofen, a NSAID, to modify the basal levels of inflammation in old mice in vivo. Old mice were placed on a diet supplemented with ibuprofen (or control chow) for 2 weeks before pulmonary macrophage isolation.
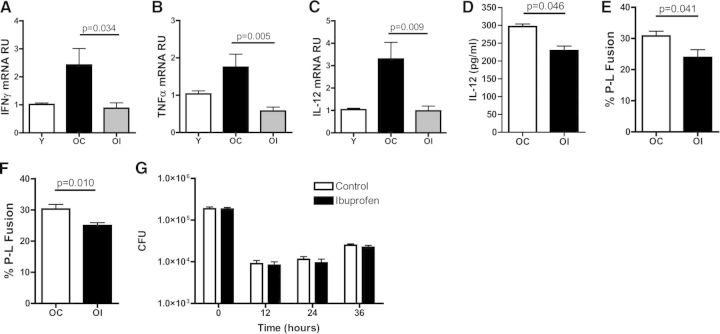
A 2-week diet modification with ibuprofen was sufficient to reduce significantly the levels of IFN-γ (Fig. 4A), TNF-α (Fig. 4B), and IL-12 (Fig. 4C) mRNA in the lungs of old mice to levels similar to young mice. IL-12 cytokine production was similarly reduced after M.tb stimulation (Fig. 4D). No differences in cell-surface marker expression for CD86, MR, or MHCII were observed between control and ibuprofen-fed mice in either age group (Table 1). Furthermore, the ibuprofen diet modification significantly decreased the percentage of P-L fusion events detected in macrophages from old mice infected with M.tb (Fig. 4E and F). However, the ibuprofen-supplemented diet did not translate to any alteration in long-term CFU control in vitro, with a similar CFU reduction at 12 h and subsequent CFU increase by 36 h for macrophage from old mice, as well as from old mice supplemented with ibuprofen (Fig. 4G). No differences were seen in P-L fusion between young mice fed either a control or ibuprofen diet (not shown). Taken together, these data suggest that an ibuprofen-supplemented diet can reduce the inflammatory lung environment of old mice and reverse the P-L fusion phenotype of macrophages from old mice but remains ineffective at boosting the in vitro control of M.tb growth in macrophage cultures.
Figure 4. Ibuprofen decreases the inflammatory environment and restores macrophage function.
Relative expression of IFN-γ (A), TNF-α (B), and IL-12 (C), as determined by RT-qPCR of whole-lung homogenates from naïve, young mice (Y; open bars) and naïve, old mice on either a control (OC; black bars) or an ibuprofen diet (OI; gray bars). The ΔΔCT method was used to analyze data, normalizing to endogenous 18S rRNA. For cytokine production, cells isolated from old mice on a control (OC; open bars) or an ibuprofen (OI; black bars) diet were infected with 5:1 GFP-M.tb for 24 h, and the secretion of IL-12 was analyzed in supernatants by ELISA (D). For P-L fusion, cells were infected with GFP-M.tb at a MOI of 5:1 for 2 h, fixed, permeabilized, blocked, and stained for LAMP-1 (E) or cathepsin D (F) and P-L fusion events evaluated by confocal microscopy. Macrophages, isolated from the lungs of old mice on a control or an ibuprofen-supplemented diet, were incubated with GFP-M.tb at a MOI of 5:1 for 2 h, and plates were washed with warm DPBS. Bacterial growth (CFUs) at defined time-points postinfection was then assessed by plating lysed macrophages on OADC-supplemented 7H11 agar plates (G). Data were combined from three independent experiments using five individual young, old control, or old ibuprofen mice/experiment (A–C) or three (E and F), two (D), or one (G) independent experiment(s) using pools of five mice/experiment. Student's t-test was used to determine statistical significance.
DISCUSSION
Inflammaging, defined as a systemic phenotype of increased basal levels of circulating cytokines in old age [23], has become an established concept that is thought to contribute directly to the increased incidence of numerous diseases of the elderly [1], including infectious diseases [38]. In this study, we have extended our knowledge of systemic inflammaging to include the pulmonary environment, where naïve, old mice had elevated basal levels of mRNA and protein for multiple pro-inflammatory cytokines. This inflammatory state could be restored to levels akin to naïve, young mice with a diet supplemented with the NSAID ibuprofen.
We also determined that resident pulmonary macrophages from old mice were in an activated state within the lung. Macrophages from old mice expressed increased mRNA for IFN-γ-inducible genes that regulate cytokine production and apoptosis (IRF-1) [39], autophagy (IRGM-1) [27], and MHCII expression (CIITA) [40]. Uptake of M.tb was increased by macrophages from the lungs of old mice, and intracellular bacteria were found more frequently in phago-lysosomes. Notably, the increased P-L fusion and cytokine production by M.tb-infected macrophages from old mice could not be enhanced further with IFN-γ activation. Although this basal-activated state may lend itself to an early and enhanced response upon encounter with a pathogen, the elderly are more susceptible to succumbing to infectious diseases [17]. Therefore, it is important to consider whether a robust macrophage-mediated response in the lung may actually be detrimental to the long-term control of an infection. This may occur at the cellular level, where increased macrophage activation predisposes these same cells to apoptosis, [41] or at the systemic level by further increasing the inflammaging state. Alternatively, a robust, inflammatory response could stimulate a subsequent anti-inflammatory response [42] that interferes with the generation of adaptive immunity [43], as we have demonstrated previously for young mice [44].
Interestingly, we observed a significant increase in uptake and P-L fusion events in macrophages from old mice, but this did not translate to an enhanced reduction in CFU after 2 h, relative to macrophages from young mice. Our interpretation of these data as a whole is that the CFU assay was not as sensitive as microscopy for detecting differences in uptake or possibly, that the increase in M.tb uptake (more M.tb in cells) nullified any increased P-L fusion (more killing of M.tb) observed in macrophages from old mice, resulting in an observed, equivalent M.tb CFU in vitro between macrophages from old and young mice after 2 h, followed by equivalent M.tb killing during the first 12 h of infection. However, in an environment with limited M.tb–macrophage interactions, such as during natural infection in vivo, enhanced control of M.tb by macrophages from old mice may be evident. Of additional interest was the demonstration that pulmonary macrophages from old mice were permissive for M.tb growth at later time-points (24 h and 36 h). The reason for this subsequent failure to limit M.tb growth could reflect defects in M.tb-killing mechanisms in old age, such as a failure to reduce phagosomal pH, reduced lysosomal enzyme function, or capacity to restrict phagosomal iron [45], or a preference to activate autophagy in macrophages from old mice, as indicated by increased mRNA for IRGM-1 [27]. Additional studies have been initiated by our group to determine why macrophages from old mice fail to kill or limit the growth of M.tb as efficiently as macrophages from young mice at these later time-points.
One potential mechanism for the altered functional data that we observe is that the differential expression of receptors on macrophages (Table 1) could represent different myeloid lineage subsets in old and young mice. We isolated macrophages by adherence to obtain sufficient cell yield for many of the experiments that were performed, which could include infiltrating monocytes or moderately adherent dendritic cells that may be responsible for the increased binding/P-L fusion that we report. Furthermore, altered receptor expression on cells of a similar lineage (e.g., increased MR on CD11c+ cells) could also account for the modified binding and trafficking that we observed. It is important to note that although we have shown previously that CD11c+ cell numbers are reduced in the lungs of old mice [18], these are predominantly the cell subset that is responsive to M.tb infection [18]. Therefore, the altered function that we observed may, in fact, be greater in a purified CD11c+ population. Regardless of outcome, the makeup of the cell population that we investigated in our studies is likely representative of the heterogeneous lung environment that M.tb encounters in vivo in old age and provides us with important information about how early host-pathogen events occur in the aged lung.
We used ibuprofen-supplemented food as a mechanism to modify successfully the inflammatory state in old mice in vivo. Ibuprofen is a nonspecific COX inhibitor that inhibits COX1 and COX2 isoforms. COX enzymes convert arachidonic acid to PGH2, which, in turn, is converted by specific isomerase and synthase enzymes to several other PGs (PGE2, PGI2, PGD2, PGF2α) that have been shown to be mediators of pain, inflammation, and fever. At this time, the specific mechanism of action for ibuprofen in our model is unclear, but one potential scenario is a direct influence on macrophage function via the action of PGs, which play a central role in the generation of inflammatory responses and conversely, are also involved in resolving inflammation [46]. For example, 15d-PDJ2, a product of PGE2, is a ligand for peroxisome proliferator-activated receptor γ, which is an important regulator of alternatively activated macrophages [47]. However, 15d-PDJ2 is also an activator of the Nrf2-Keap pathway that promotes the oxidative stress pathway [48]. Further investigation into the specific mechanisms of action of ibuprofen in our in vitro model, as well as the study of whether ibuprofen can modify the control of M.tb infection [49] in old mice in vivo, is necessary. In this regard, how inflammation modulates the documented interaction between highly activated pulmonary macrophages and innate CD8 T cells that contribute to an early resistance to M.tb infection in vivo in old mice [10] must also be considered.
As the population continues to age, the overall health of the elderly has become a public concern. Recent evidence suggests that the inflammation that occurs with increasing age contributes to the susceptibility of the elderly to many diseases, including diabetes [2], COPD [3], and emphysema [4] and potentially, to infectious diseases [38]. Interventions that can reduce age-associated inflammation would positively contribute to reducing the incidence of several diseases that are of significance to the elderly. That this can be achieved in vivo with a drug that is relatively low in cost and with limited contraindications is significant.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a Julie Martin Mid-Career Award from the American Federation for Aging Research (to J.T.); The Ohio State University Public Health Preparedness for Infectious Diseases (PHPID) Pilot Award (to J.T., J.B.T., L.S.S., and W.P.L.); and The Ohio State University's College of Medicine Systems and Integrative Biology (SIB) Training Program (to C.H.C.).
We thank the CMIF at The Ohio State University for their services.
Footnotes
- 15d-PDJ2:
- 15-deoxy-delta 12,14-PG D2
- APC
- allophycocyanin
- BSL3
- biosafety level 3
- CIITA
- class II, MHC, transactivator
- CMIF
- Campus Microscopy and Imaging Facility
- COPD
- chronic obstructive pulmonary disease
- COX
- cyclooxygenase
- CT
- cycle threshold
- DPBS
- Dulbecco's PBS
- GFP-M.tb
- GFP expressing M.tb strain Erdman
- IRF-1
- IFN regulatory factor 1
- IRGM-1
- immunity-related GTPase family M member 1
- LAMP-1
- lysosomal-associated membrane protein 1
- M.tb
- Mycobacterium tuberculosis
- MOI
- multiplicity of infection
- MR
- mannose receptor
- NSAID
- nonsteroidal anti-inflammatory drug
- OADC
- oleic albumin dextrose catalase
- P-L
- phago-lysosomal
- PGH2
- PG H2
- qPCR
- quantitative PCR
- RU
- relative unit(s)
AUTHORSHIP
C.H.C. and N.S.G. performed the majority of experiments and contributed to the study design. B.C. optimized experimental protocols and contributed to the study design. W.P.L. and L.S.S. contributed to the study design and data interpretation. J.B.T. established experimental protocols, performed some experiments, and contributed to the study design and data interpretation. J.T. generated the study's conception, optimized experimental protocols, and contributed to the study design and data interpretation. All authors contributed to the manuscript preparation.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1. Vasto S., Candore G., Balistreri C. R., Caruso M., Colonna-Romano G., Grimaldi M. P., Listi F., Nuzzo D., Lio D., Caruso C. (2007) Inflammatory networks in ageing, age-related diseases and longevity. Mech. Ageing Dev. 128, 83–91. [DOI] [PubMed] [Google Scholar]
- 2. De Rekeneire N., Peila R., Ding J., Colbert L. H., Visser M., Shorr R. I., Kritchevsky S. B., Kuller L. H., Strotmeyer E. S., Schwartz A. V., Vellas B., Harris T. B. (2006) Diabetes, hyperglycemia, and inflammation in older individuals: the health, aging and body composition study. Diabetes Care 29, 1902–1908. [DOI] [PubMed] [Google Scholar]
- 3. Provinciali M., Cardelli M., Marchegiani F. (2011) Inflammation, chronic obstructive pulmonary disease and aging. Curr. Opin. Pulm. Med. 17 (Suppl. 1), S3–S10. [DOI] [PubMed] [Google Scholar]
- 4. MacNee W. (2011) Aging, inflammation, and emphysema. Am. J. Respir. Crit. Care Med. 184, 1327–1329. [DOI] [PubMed] [Google Scholar]
- 5. Shaw A. C., Joshi S., Greenwood H., Panda A., Lord J. M. (2010) Aging of the innate immune system. Curr. Opin. Immunol. 22, 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boehmer E. D., Goral J., Faunce D. E., Kovacs E. J. (2004) Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J. Leukoc. Biol. 75, 342–349. [DOI] [PubMed] [Google Scholar]
- 7. Chelvarajan R. L., Collins S. M., Van Willigen J. M., Bondada S. (2005) The unresponsiveness of aged mice to polysaccharide antigens is a result of a defect in macrophage function. J. Leukoc. Biol. 77, 503–512. [DOI] [PubMed] [Google Scholar]
- 8. Gomez C. R., Hirano S., Cutro B. T., Birjandi S., Baila H., Nomellini V., Kovacs E. J. (2007) Advanced age exacerbates the pulmonary inflammatory response after lipopolysaccharide exposure. Crit. Care Med. 35, 246–251. [DOI] [PubMed] [Google Scholar]
- 9. Turnbull I. R., Clark A. T., Stromberg P. E., Dixon D. J., Woolsey C. A., Davis C. G., Hotchkiss R. S., Buchman T. G., Coopersmith C. M. (2009) Effects of aging on the immunopathologic response to sepsis. Crit. Care Med. 37, 1018–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vesosky B., Flaherty D. K., Turner J. (2006) Th1 cytokines facilitate CD8-T-cell-mediated early resistance to infection with Mycobacterium tuberculosis in old mice. Infect. Immun. 74, 3314–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vesosky B., Rottinghaus E. K., Davis C., Turner J. (2009) CD8 T cells in old mice contribute to the innate immune response to Mycobacterium tuberculosis via interleukin-12p70-dependent and antigen-independent production of γ interferon. Infect. Immun. 77, 3355–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rottinghaus E. K., Vesosky B., Turner J. (2009) Interleukin-12 is sufficient to promote antigen-independent interferon-γ production by CD8 T cells in old mice. Immunology 128, e679–e690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Corbett E., Watt C., Walker N., Maher D., Williams B., Raviglione M., Dye C. (2003) The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Int. Med. 163, 1009–1021. [DOI] [PubMed] [Google Scholar]
- 14. Zumla A., Raviglione M., Hafner R., von Reyn C. F. (2013) Tuberculosis. N. Engl. J. Med. 368, 745–755. [DOI] [PubMed] [Google Scholar]
- 15. Vesosky B., Turner J. (2005) The influence of age on immunity to infection with Mycobacterium tuberculosis. Immunol. Rev. 205, 229–243. [DOI] [PubMed] [Google Scholar]
- 16. Orme I. M. (1987) Aging and immunity to tuberculosis: increased susceptibility of old mice reflects a decreased capacity to generate mediator T lymphocytes. J. Immunol. 138, 4414–4418. [PubMed] [Google Scholar]
- 17. Turner J., Frank A. A., Orme I. M. (2002) Old mice express a transient early resistance to pulmonary tuberculosis that is mediated by CD8 T cells. Infect. Immun. 70, 4628–4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rottinghaus E. K., Vesosky B., Turner J. (2010) TLR-2 independent recognition of Mycobacterium tuberculosis by CD11c+ pulmonary cells from old mice. Mech. Ageing Dev. 131, 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schlesinger L., Azad A., Torrelles J., Roberts E., Vergne I., Deretic V. Determinants of phagocytosis, phagosome biogenesis and autophagy for Mycobacterium tuberculosis. Handbook of Tuberculosis: Immunology and Cell Biology (Kaufmann S. H., ed.), Weinheim:Wiley-VCH Verlag GmbH & Co, Weinheim, Germany. [Google Scholar]
- 20. Gonzalez-Juarrero M., Shim T. S., Kipnis A., Junqueira-Kipnis A. P., Orme I. M. (2003) Dynamics of macrophage cell populations during murine pulmonary tuberculosis. J. Immunol. 171, 3128–3135. [DOI] [PubMed] [Google Scholar]
- 21. Wang S-H., Carruthers B., Turner J. (2012) The influence of increasing age on susceptibility of the elderly to tuberculosis. Open Longev. Sci. 6, 73–82. [Google Scholar]
- 22. Tullius M. V., Harth G., Horwitz M. A. (2001) High extracellular levels of Mycobacterium tuberculosis glutamine synthetase and superoxide dismutase in actively growing cultures are due to high expression and extracellular stability rather than to a protein-specific export mechanism. Infect. Immun. 69, 6348–6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Franceschi C., Bonafe M., Valensin S., Olivieri F., De Luca M., Ottaviani E., De Benedictis G. (2000) Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 908, 244–254. [DOI] [PubMed] [Google Scholar]
- 24. Schroder K., Hertzog P. J., Ravasi T., Hume D. A. (2004) Interferon-γ: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 75, 163–189. [DOI] [PubMed] [Google Scholar]
- 25. Eriks I. S., Emerson C. L. (1997) Temporal effect of tumor necrosis factor α on murine macrophages infected with Mycobacterium avium. Infect. Immun. 65, 2100–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ting J. P., Trowsdale J. (2002) Genetic control of MHC class II expression. Cell 109 (Suppl.), S21–S33. [DOI] [PubMed] [Google Scholar]
- 27. Feng C. G., Zheng L., Lenardo M. J., Sher A. (2009) Interferon-inducible immunity-related GTPase Irgm1 regulates IFN γ-dependent host defense, lymphocyte survival and autophagy. Autophagy 5, 232–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kamijo R., Harada H., Matsuyama T., Bosland M., Gerecitano J., Shapiro D., Le J., Koh S. I., Kimura T., Green S. J., et al. (1994) Requirement for transcription factor IRF-1 in NO synthase induction in macrophages. Science 263, 1612–1615. [DOI] [PubMed] [Google Scholar]
- 29. Turner J., Orme I. M. (2004) The expression of early resistance to an infection with Mycobacterium tuberculosis by old mice is dependent on IFN type II (IFN-γ) but not IFN type I. Mech. Ageing Dev. 125, 1–9. [DOI] [PubMed] [Google Scholar]
- 30. Kang B. K., Schlesinger L. S. (1998) Characterization of mannose receptor-dependent phagocytosis mediated by Mycobacterium tuberculosis lipoarabinomannan. Infect. Immun. 66, 2769–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kang P. B., Azad A. K., Torrelles J. B., Kaufman T. M., Beharka A., Tibesar E., DesJardin L. E., Schlesinger L. S. (2005) The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J. Exp. Med. 202, 987–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ferguson J. S., Martin J. L., Azad A. K., McCarthy T. R., Kang P. B., Voelker D. R., Crouch E. C., Schlesinger L. S. (2006) Surfactant protein D increases fusion of Mycobacterium tuberculosis-containing phagosomes with lysosomes in human macrophages. Infect. Immun. 74, 7005–7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clemens D. L., Horwitz M. A. (1995) Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J. Exp. Med. 181, 257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Malik Z. A., Denning G. M., Kusner D. J. (2000) Inhibition of Ca(2+) signaling by Mycobacterium tuberculosis is associated with reduced phagosome-lysosome fusion and increased survival within human macrophages. J. Exp. Med. 191, 287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Via L. E., Fratti R. A., McFalone M., Pagan-Ramos E., Deretic D., Deretic V. (1998) Effects of cytokines on mycobacterial phagosome maturation. J. Cell Sci. 111, 897–905. [DOI] [PubMed] [Google Scholar]
- 36. Malik Z. A., Iyer S. S., Kusner D. J. (2001) Mycobacterium tuberculosis phagosomes exhibit altered calmodulin-dependent signal transduction: contribution to inhibition of phagosome-lysosome fusion and intracellular survival in human macrophages. J. Immunol. 166, 3392–3401. [DOI] [PubMed] [Google Scholar]
- 37. Flynn J. L., Chan J. (2001) Immunology of tuberculosis. Annu. Rev. Immunol. 19, 93–129. [DOI] [PubMed] [Google Scholar]
- 38. Stout-Delgado H. W., Du W., Shirali A. C., Booth C. J., Goldstein D. R. (2009) Aging promotes neutrophil-induced mortality by augmenting IL-17 production during viral infection. Cell Host Microbe 6, 446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harada H., Fujita T., Miyamoto M., Kimura Y., Maruyama M., Furia A., Miyata T., Taniguchi T. (1989) Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell 58, 729–739. [DOI] [PubMed] [Google Scholar]
- 40. Steimle V., Siegrist C. A., Mottet A., Lisowska-Grospierre B., Mach B. (1994) Regulation of MHC class II expression by interferon-γ mediated by the transactivator gene CIITA. Science 265, 106–109. [DOI] [PubMed] [Google Scholar]
- 41. Qian X. S., Zhu Q. L., Yang J., Yin T., Wang S. W. (2005) [Apoptosis of pulmonary alveolar macrophages in aged and adult rats: a comparative study]. Zhonghua Yi Xue Za Zhi 85, 253–256. [PubMed] [Google Scholar]
- 42. Chiu B. C., Stolberg V. R., Freeman C. M., Chensue S. W. (2007) Mononuclear phagocyte-derived interleukin-10 suppresses the innate pulmonary granuloma cytokine response in aged mice. Am. J. Pathol. 171, 829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Opal S. M., DePalo V. A. (2000) Anti-inflammatory cytokines. Chest 117, 1162–1172. [DOI] [PubMed] [Google Scholar]
- 44. Cyktor J. C., Carruthers B., Kominsky R. A., Beamer G. L., Stromberg P., Turner J. (2013) IL-10 inhibits mature fibrotic granuloma formation during Mycobacterium tuberculosis infection. J. Immunol. 190, 2778–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guayerbas N., De La Fuente M. (2003) An impairment of phagocytic function is linked to a shorter life span in two strains of prematurely aging mice. Dev. Comp. Immunol. 27, 339–350. [DOI] [PubMed] [Google Scholar]
- 46. Ricciotti E., FitzGerald G. A. (2011) Prostaglandins and inflammation. Arterioscl. Thromb.Vasc. Biol. 31, 986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rajaram M. V., Brooks M. N., Morris J. D., Torrelles J. B., Azad A. K., Schlesinger L. S. (2010) Mycobacterium tuberculosis activates human macrophage peroxisome proliferator-activated receptor γ linking mannose receptor recognition to regulation of immune responses. J. Immunol. 185, 929–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Itoh K., Mochizuki M., Ishii Y., Ishii T., Shibata T., Kawamoto Y., Kelly V., Sekizawa K., Uchida K., Yamamoto M. (2004) Transcription factor Nrf2 regulates inflammation by mediating the effect of 15-deoxy-delta(12,14)-prostaglandin j(2). Mol. Cell. Biol. 24, 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vilaplana C., Marzo E., Tapia G., Diaz J., Garcia V., Cardona P. J. (2013) Ibuprofen therapy resulted in significantly decreased tissue bacillary loads and increased survival in a new murine experimental model of active tuberculosis. J. Infect. Dis. 208, 199–202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.