Abstract
It is unknown whether associations between blood pressure (BP) and stroke vary between Europeans and South Asians, despite higher stroke rates in the latter. We report findings from a UK cohort study of 1375 European and 1074 South Asian men, not receiving antihypertensive medication, aged 40 to 69 years at baseline (1988–1991). Assessment included BP, blood tests, anthropometry, and questionnaires. Incident stroke was established at 20 years from death certification, hospital and primary care records, and participant report. South Asians had higher systolic BP, diastolic BP, and mean arterial pressure than Europeans, and similar pulse pressure. Associations between systolic BP or diastolic BP and stroke were stronger in South Asians than Europeans, after adjustment for age, smoking status, waist/hip ratio, total/high-density lipoprotein-cholesterol ratio, diabetes mellitus, fasting glucose, physical activity, and heart rate (systolic BP: Europeans [odds ratio, 1.22; 95% confidence interval, 0.98–1.51], South Asians [1.56; 1.24–1.95]; ethnic difference P=0.04; diastolic BP: Europeans [0.90; 0.71–1.13], South Asians [1.68; 1.32–2.15]; P<0.001). Hemodynamic correlates of stroke risk differed by ethnicity: in combined models, mean arterial pressure but not pulse pressure was detrimentally associated with stroke in South Asians, whereas the converse was true for Europeans. The combination of hyperglycemia and hypertension appeared particularly detrimental for South Asians. There are marked ethnic differences in associations between BP parameters and stroke. Undue focus on systolic BP for risk prediction, and current age and treatment thresholds may be inappropriate for individuals of South Asian ancestry.
Keywords: blood pressure, diabetes mellitus, fasting glucose, heart rate, stroke
Stroke is the second leading cause of death globally, with high blood pressure (BP) the strongest risk factor.1-3 South Asians experience a 1.5- to 2-fold higher stroke risk than Europeans.4 Differences in hypertension prevalence between South Asians and Europeans do not explain the greater stroke risk in South Asian groups,5,6 however, associations between BP and its constituents (ie, systolic BP [SBP], diastolic BP [DBP], or, from a hemodynamic perspective, mean arterial pressure [MAP] and pulse pressure [PP]) and stroke risk have not been compared directly in South Asians and Europeans. This is important as studies in European-origin populations indicate that SBP or PP is the major driver of risk7; consequently, it has been proposed that SBP should be the sole target for intervention,8 and many risk estimators only include SBP in their calculations.9,10 Such assumptions need to be tested in non-European populations. Furthermore, diabetes mellitus is much more prevalent in South Asian than European populations5 and increases stroke risk independent of other cardiovascular risk factors,11 but the influence of hyperglycemia on associations between BP and stroke risk in the former group is unexplored.
Using data from a community-based follow-up study, we compared associations between BP constituents and stroke in South Asian and European men, and secondly explored reasons for differences, for example, interethnic variation in glycemia.
Methods
Study Participants and Design
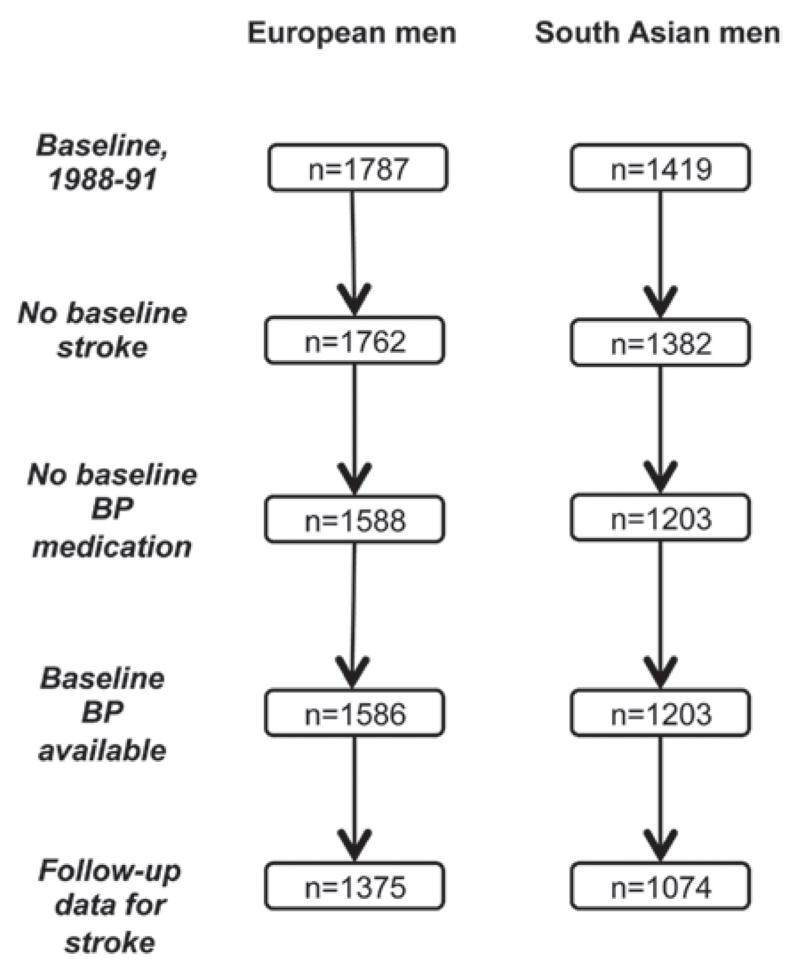
The Southall and Brent Revisited (SABRE) study is a multiethnic cohort study; details are published elsewhere.12 Participants aged 40 to 69 years at baseline (1988–1991, n=4857) were randomly selected from primary care physician lists and workplaces in northwest London. South Asian participants were first-generation migrants originating from the Indian subcontinent. All participants were followed for death, hospitalization, and primary care consultations from baseline to 2011 (outcome data were available for 4196). We report findings from a subset of 1375 European and 1074 South Asian men without stroke at baseline, who were not in receipt of baseline antihypertensive medication (Figure 1).
Figure 1.
Follow-up of the Southall and Brent Revisited (SABRE) cohort 1988 to 2011.
All participants gave written informed consent. Approval for the baseline study was obtained from Ealing, Hounslow and Spelthorne, Parkside and University College London research ethics committees, and at follow-up from St. Mary’s Hospital Local Research Ethics Committee (reference: 07/HO712/109).
Baseline and Follow-Up Measurements
Participants underwent anthropometric measurement and completed a health, lifestyle, and occupation questionnaire. Physical activity comprised the total weekly energy expended (MJ) on sports, walking, cycling, and daily activities. Circulating lipids, fasting and postload glucose, insulin, and glycated haemoglobin (HbA1c) were measured, as described.12 Physician diagnosis or World Health Organization 1999 criteria for fasting and oral glucose tolerance test blood glucose measurements13 defined diabetes mellitus. Homeostasis Model Assessment version 2 for Insulin Resistance (HOMA2-IR) was used to quantify insulin resistance.14 Renal function was quantified by estimated glomerular filtration rate, using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) validated equations.15
Between 2008 and 2011, survivors were invited for examination at St. Mary’s Hospital, London. New cases of diabetes mellitus since baseline were identified from record review, questionnaire, clinic blood results, or death certificate data (International Classification of Diseases-Ninth Revision [ICD-9]: 2500–2509, ICD-10: E100-E149). Atrial fibrillation or flutter (AF) at follow-up was established from record review, questionnaire, and hospital episode statistics (ICD-9: 4273, ICD-10: I48). Incident stroke was defined as the first postbaseline event from the following sources: death certification data (ICD-9: 430–439, ICD-10: I600-I698), hospital episode statistics (ICD codes as above), primary care record review adjudicated by 2 clinicians, as per Anglo-Scandinavian Cardiac Outcomes Trial criteria16 and participant report of physician-diagnosed stroke with duration of symptoms ≥24 hours.
Seated resting brachial BP and heart rate were measured at baseline after a 15-minute rest using a random zero sphygmomanometer (Hawksley, London, United Kingdom) and at follow-up using an Omron 705IT; the mean of 2 measurements from each time point was used in analyses. A correction was applied to follow-up BP measurements (SBP: −1.35 mm Hg, DBP: −1.97 mm Hg) to ensure comparability with those at baseline because the Hawksley random zero sphygmomanometer may underestimate BP when compared with a standard mercury sphygmomanometer.17 Concordance correlation coefficients18 for 30 interobserver repeated measurements of follow-up SBP and DBP were 0.78 and 0.89, respectively. MAP was calculated as DBP+([1/3]×[SBP−DBP]).
Statistical Analysis
Baseline characteristics were compared by follow-up status, incident stroke, and ethnicity. Logistic and linear regression methods determined age-adjusted differences.
Associations between a 1 SD increase in SBP, DBP, MAP, and PP and incident stroke were studied using age-adjusted logistic regression models. We then additionally adjusted these models for potential confounders (smoking, waist/hip ratio, total/high-density lipoprotein-cholesterol ratio, diabetes mellitus, fasting glucose, physical activity, and heart rate). Smoking status and total/high-density lipoprotein-cholesterol ratio were deemed potential confounders a priori,19 whereas the remainder were selected on the basis of associations (P<0.10) with stroke in either ethnic group (Table 1). Interactions between BP measures and ethnicity were sought in all models. In addition, we inspected models with BP measures in combination (SBP+DBP or MAP+PP) by ethnicity. We elected to use logistic regression as the proportional hazards assumption was violated for several models when using Cox regression techniques.
Table 1. Baseline Characteristics of European and South Asian Men in the SABRE Study, by Stroke Status.
| Europeans |
South Asians |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | All | No Stroke | Stroke | P Value* | All | No Stroke | Stroke | P Value* | P Value† |
| n | 1375 | 1271 (92) | 104 (8) | … | 1074 | 972 (90) | 102 (10) | … | … |
| Age, y | 52 (46–58) | 52 (46–58) | 51 (46–56) | <0.001 | 50 (45–55) | 49 (44–55) | 53 (48–59) | <0.001 | <0.001 |
| Ever smoked | 1011 (74) | 928 (73) | 83 (80) | 0.21 | 292 (27) | 260 (27) | 32 (32) | 0.27 | <0.001 |
| Manual occupation | 852 (62) | 778 (61) | 74 (71) | 0.14 | 819 (77) | 736 (76) | 83 (82) | 0.17 | <0.001 |
| Alcohol, units/wk | 12 (3–26) | 12 (3–26) | 13 (1–31) | 0.37 | 3 (0–15) | 3(0–15) | 4 (0–20) | 0.34 | 0.003 |
| Veg/fruit, daily/most days | 931 (68) | 862 (68) | 69 (66) | 0.58 | 711 (67) | 646 (67) | 65 (65) | 0.36 | 0.99 |
| Physical activity, MJ/wk | 11 (7–16) | 12 (7–16) | 11 (7–19) | 0.06 | 10 (6–13) | 10 (6–13) | 9 (5–14) | 0.08 | <0.001 |
| Waist/hip ratio | 0.94±0.07 | 0.94±0.06 | 0.95±0.08 | 0.41 | 0.98±0.06 | 0.98±0.06 | 1.00±0.06 | 0.08 | <0.001 |
| BMI, kg/m2 | 26.1±3.8 | 26.1±3.7 | 25.9±4.1 | 0.51 | 25.7±3.2 | 25.7±3.1 | 25.8±3.7 | 0.71 | 0.02 |
| HDL, mmol/L | 1.3 (1.1–1.5) | 1.3 (1.1–1.5) | 1.3 (1.1–1.5) | 0.73 | 1.2 (1.0–1.4) | 1.2 (1.0–1.4) | 1.2 (1.0–1.4) | 0.98 | <0.001 |
| Triglycerides, mmol/L | 1.4 (1.0–2.1) | 1.4 (1.0–2.1) | 1.6 (1.1–2.2) | 0.20 | 1.7 (1.2–2.5) | 1.7 (1.2–2.5) | 1.8 (1.1–2.6) | 0.57 | <0.001 |
| Total/HDL-cholesterol | 4.7 (3.8–5.9) | 4.7 (3.8–5.9) | 4.8 (3.9–5.6) | 0.93 | 5.0 (4.2–6.1) | 5.0 (4.2–6.1) | 5.1 (4.4–6.1) | 0.23 | <0.001 |
| Diabetes mellitus | 84 (6) | 76 (6) | 8 (8) | 0.92 | 212 (20) | 168 (17) | 44 (43) | <0.001 | <0.001 |
| Fasting glucose, mmol/L | 5.4 (5.1–5.9) | 5.4 (5.1–5.9) | 5.5 (5.1–5.9) | 0.26 | 5.6 (5.2–6.2) | 5.6 (5.1–6.1) | 5.9 (5.3–7.7) | <0.001 | <0.001 |
| Postload glucose, mmol/L | 5.0 (4.1–5.9) | 5.0 (4.1–5.9) | 5.0 (3.9–5.8) | 0.14 | 5.5 (4.6–6.6) | 5.5 (4.6–6.5) | 5.9 (4.8–8.3) | 0.01 | <0.001 |
| HbA1c, % | 5.6 (5.4–5.8) | 5.6 (5.4–5.8) | 5.6 (5.4–5.8) | 0.24 | 5.8 (5.5–6.2) | 5.8 (5.5–6.2) | 6.1 (5.7–6.9) | 0.02 | <0.001 |
| HOMA2-IR | 0.8 (0.6–1.2) | 0.8 (0.6–1.2) | 0.7 (0.5–1.2) | 0.69 | 1.2 (0.8–1.8) | 1.2 (0.8–1.8) | 1.4 (0.9–2.1) | 0.04 | <0.001 |
| eGFR, mL/min | 117±36 | 118±36 | 107±40 | 0.05 | 109±37 | 109±37 | 107±38 | 0.81 | <0.001 |
| Median heart rate, bpm | 64 (57–72) | 63 (57–72) | 65 (56–71) | 0.71 | 68 (61–75) | 67 (61–75) | 71 (63–77) | 0.03 | <0.001 |
| Systolic BP, mm Hg | 122±16 | 122±16 | 127±22 | 0.05 | 124±17 | 123±17 | 134±17 | <0.001 | <0.001 |
| Diastolic BP, mm Hg | 77±11 | 77±11 | 76±12 | 0.40 | 80±10 | 80±10 | 84±11 | <0.001 | <0.001 |
| Mean arterial BP, mm Hg | 92±12 | 92±11 | 93±14 | 0.70 | 95±12 | 94±11 | 101±12 | <0.001 | <0.001 |
| Pulse pressure, mm Hg | 45±12 | 45±11 | 50±17 | 0.001 | 44±13 | 43±12 | 50±13 | 0.003 | 0.90 |
Excludes participants with baseline stroke or receipt of antihypertensive medication. Data are n (%), median (interquartile range), or mean±SD. BMI indicates body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; HOMA2-IR, Homeostasis Model Assessment version 2 for Insulin Resistance; and SABRE, Southall and Brent Revisited study.
Age-adjusted P for difference in participants who experienced stroke vs those who did not.
Age-adjusted P for ethnic difference, regardless of stroke status. HbA1c available for 1082 Europeans and 803 South Asians, and eGFR available for 1015 Europeans and 959 South Asians.
We further explored ethnic differences in associations between BP and stroke by inspecting interaction terms between BP measures and baseline diabetes mellitus, fasting glucose, HbA1c, or HOMA2-IR in models of stroke for each ethnic group. This analysis was extended by graphically displaying stroke incidence by dichotomized SBP or DBP plotted against dichotomized HbA1c or fasting glucose for each ethnic group. Next, we compared associations between age and SBP or DBP in each ethnic group to establish whether age-related trends in BP differed by ethnicity. Subsequently, for participants with BP data at follow-up, we subtracted follow-up BP (with correction applied, see earlier) from baseline BP to elucidate ethnic differences in BP change over time, whether associations between BP change and stroke risk varied by ethnicity, and if this effect was modified by diabetes mellitus. Following this, for individuals in receipt of antihypertensive medication, we examined ethnic differences in SBP, DBP, and BP control (defined as BP≤140/90 mm Hg) by diabetes mellitus status at follow-up. Finally, we contrasted rates of antihypertensive use by ethnicity for people with BP>140/90 mm Hg at baseline and follow-up, to establish ethnic differences in hypertension management.
Sensitivity analyses were conducted by: (1) including individuals in receipt of baseline antihypertensive medication, adding 10/5 mm Hg to BPs of those receiving treatment,20 (2) excluding people with baseline diabetes mellitus, (3) adjusting for diabetes mellitus as a time-varying covariate, (4) adjusting for HbA1c or HOMA2-IR instead of fasting glucose, (5) using the alternative formula for MAP (MAP=DBP+0.4×PP) proposed by Bos,21 (6) adjusting for follow-up use of antithrombotic medication (antiplatelets or anticoagulant agents), (7) adjusting for presence of AF at follow-up (the latter 2 variables were not available at baseline), (8) adjusting for body mass index instead of waist/hip ratio, and (9) additionally adjusting for estimated glomerular filtration rate in cardiovascular disease risk factor–adjusted models (as a sensitivity analysis because of lower data availability). We repeated analyses by age subgroup (<55 years and ≥55 years).
Results
A total of 1375 (87%) European and 1074 (88%) South Asian men had complete data for baseline BP measures and stroke follow-up (Figure 1). There were no consistent differences in baseline characteristics by follow-up status. Over a median of 20-year follow-up, incident stroke was higher in South Asians (n=102, 10%; P=0.02) than Europeans (n=104, 8%). South Asian men were more centrally obese, with more adverse lipid and glycemic profiles and BP measures than European men (Table 1). The exception to this was PP, which was similar by ethnicity.
All measures of BP (SBP, DBP, MAP, and PP) were strongly positively associated with incident stroke in South Asians, even on multivariable adjustment (Table 2). In contrast, with the exception of PP, associations were either weaker (SBP) or absent for Europeans. These ethnic differences in associations between BP and stroke risk were significant as interactions. When both SBP and DBP were included in models, SBP was positively, and DBP negatively related to stroke risk in Europeans, whereas in South Asians, DBP remained positively associated with stroke risk (ethnicity interaction P<0.001), in addition to SBP. In the MAP+PP models, PP but not MAP was associated with stroke in Europeans, whereas the opposite was true for South Asians; ethnicity interactions were present for both parameters.
Table 2. Associations Between Baseline Blood Pressure Measures and Incident Stroke, by Ethnicity.
| Europeans |
South Asians |
|||||
|---|---|---|---|---|---|---|
| BP Measure | Model Factors | OR | 95% CI | OR | 95% CI | P Value* |
| SBP | Age | 1.21 | 1.00–1.47† | 1.63 | 1.34–1.97‡ | 0.04 |
| Age+CVD risk factors§ | 1.22 | 0.98–1.51 | 1.56 | 1.24–1.95‡ | 0.04 | |
| DBP | Age | 0.91 | 0.74–1.13 | 1.64 | 1.33–2.03‡ | <0.001 |
| Age+CVD risk factors§ | 0.90 | 0.71–1.13 | 1.68 | 1.32–2.15‡ | <0.001 | |
| MAP | Age | 1.04 | 0.85–1.28 | 1.69 | 1.38–2.08‡ | 0.001 |
| Age+CVD risk factors§ | 1.04 | 0.82–1.30 | 1.69 | 1.34–2.14‡ | 0.001 | |
| PP | Age | 1.39 | 1.15–1.68∥ | 1.35 | 1.10–1.64∥ | 0.73 |
| Age+CVD risk factors§| | 1.40 | 1.13–1.72∥ | 1.24 | 0.99–1.55 | 0.80 | |
| SBP | Age | 1.58 | 1.22–2.07∥ | 1.38 | 1.04–1.82† | 0.44 |
| DBP | 0.66 | 0.50–0.87∥ | 1.28 | 0.95–1.74 | 0.001 | |
| SBP | Age+CVD risk factors§ | 1.59 | 1.20–2.13∥ | 1.24 | 0.91–1.70 | 0.50 |
| DBP | 0.66 | 0.49–0.89∥ | 1.43 | 1.01–2.01∥ | 0.001 | |
| MAP | Age | 0.88 | 0.71–1.11 | 1.64 | 1.30–2.08‡ | <0.001 |
| PP | 1.46 | 1.18–1.81 ‡ | 1.06 | 0.84–1.34 | 0.04 | |
| MAP | Age+CVD risk factors§ | 0.88 | 0.68–1.12 | 1.72 | 1.31–2.25‡ | <0.001 |
| PP | 1.47 | 1.17–1.85∥ | 0.97 | 0.75–1.26 | 0.04 | |
Excludes participants with baseline stroke or receipt of antihypertensive medication. Data are OR for a 1 SD increase in BP measure unless otherwise stated. CI indicates confidence interval; CVD, cardiovascular disease; DBP, diastolic blood pressure; MAP, mean arterial pressure; OR, odds ratio; PP, pulse pressure; and SBP, systolic blood pressure.
P for ethnicity×BP interaction.
P<0.05.
P<0.001.
CVD risk factors comprise: smoking, waist/hip ratio, total/high-density lipoprotein-cholesterol ratio, diabetes mellitus, fasting glucose, physical activity, and heart rate.
P<0.01.
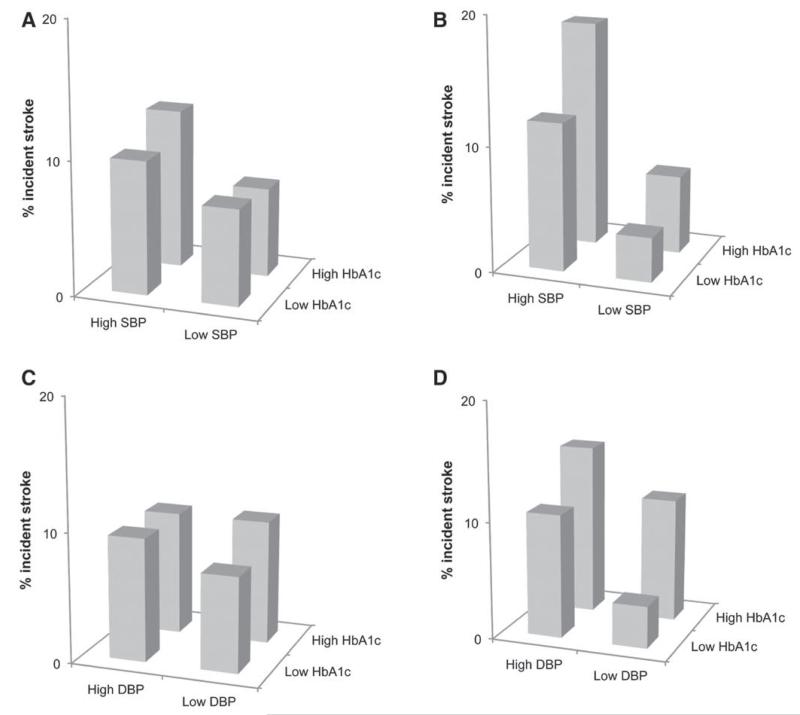
There were no significant interactions for associations between each BP measure and diabetes mellitus, fasting glucose, HbA1c or HOMA2-IR (the latter 3 exposures as continuous variables), and stroke in age-adjusted models. However, inspection of graphical plots of incident stroke by dichotomized SBP or DBP plotted against dichotomized HbA1c indicated that participants in both the highest category for BP measure and HbA1c appeared to have greater risk, compared with those in the lowest categories for each parameter, in South Asians but not Europeans (SBP: Europeans [age-adjusted odds ratio, 0.80; 95% confidence interval, 0.41–1.54], South Asians [5.11; 2.28–11.47]; ethnic difference P=0.001; DBP: Europeans [0.77; 0.39–1.54], South Asians [5.96; 2.25–15.77]; ethnic difference P=0.001; Figure 2). Results were similar for fasting glucose. Relationships between SBP or DBP and age did not vary with ethnicity nor were consistent ethnic differences observed for BP change between baseline and follow-up. Equally, there were no ethnic differences in associations between BP change measures and stroke. Of the original participants, 534 Europeans and 444 South Asians additionally attended the follow-up clinic in 2008–2011. There were no ethnic differences in BP control in these individuals (Table S1 in the online-only Data Supplement) or in the proportions of people with BP>140/90 mm Hg taking antihypertensive medication at baseline or follow-up (baseline: 19% Europeans versus 23% South Asians, P=0.39; follow-up: 66% versus 86%; P=0.25).
Figure 2.
Proportions of incident stroke by blood pressure and glycated haemoglobin (HbA1c) categories in European and South Asian men: for (A) systolic blood pressure (SBP) in Europeans, (B) SBP in South Asians, (C) diastolic BP (DBP) in Europeans, and (D) DBP in South Asians. Excludes participants with baseline stroke or receipt of antihypertensive medication.
Sensitivity analyses showed that inclusion of participants on baseline antihypertensive medication (Europeans: 155/1530 [10%] and South Asians: 154/1228 [13%], P=0.04; Table 3), exclusion of participants with baseline diabetes mellitus (Table S2), adjustment for diabetes mellitus development as a time-varying covariate (Table S3) adjustment for HbA1c or HOMA2-IR instead of fasting glucose, use of an alternative formula for MAP,21 adjustment for antithrombotic medication use at follow-up (Europeans: 395/712 [55%], South Asians: 401/584 [69%]; P<0.001) or AF at follow-up (Europeans: 96/712 [13%] and South Asians 43/584 [7%]; P<0.001; Table 4), substitution of body mass index for waist/hip ratio and additional adjustment for estimated glomerular filtration rate gave similar results to the main analyses. A subgroup analysis contrasting associations by younger versus older age group (<55 versus ≥55 years) demonstrated little evidence for associations between BP measures and stroke risk in younger Europeans, whereas SBP and PP had strong positive associations with stroke in the older group. In contrast, for South Asians, associations between BP measures and stroke did not differ greatly by age group and were similar to those reported in the main analyses (Table S4).
Table 3. Associations Between Blood Pressure Measures and Incident Stroke in Men, Including Participants in Receipt of Baseline Antihypertensive Medication.
| Europeans |
South Asians |
|||||
|---|---|---|---|---|---|---|
| BP Measure | Model Factors | OR | 95% CI | OR | 95% CI | P Value* |
| SBP | Age | 1.32 | 1.11–1.57† | 1.61 | 1.35–1.92‡ | 0.16 |
| Age+CVD risk factors§ | 1.31 | 1.08–1.58† | 1.52 | 1.25–1.86‡ | 0.16 | |
| DBP | Age | 1.01 | 0.85–1.21 | 1.55 | 1.28–1.80‡ | 0.001 |
| Age+CVD risk factors§ | 1.02 | 0.83–1.24 | 1.53 | 1.23–1.89‡ | 0.002 | |
| MAP | Age | 1.15 | 0.97–1.38 | 1.64 | 1.36–1.97‡ | 0.009 |
| Age+CVD risk factors§ | 1.16 | 0.95–1.41 | 1.58 | 1.29–1.95‡ | 0.01 | |
| PP | Age | 1.44 | 1.21–1.70‡ | 1.39 | 1.17–1.66‡ | 0.61 |
| Age+CVD risk factors§ | 1.40 | 1.16–1.67‡ | 1.29 | 1.06–1.57∥ | 0.69 | |
A correction of +10/5 mm Hg was added to the blood pressure of participants in receipt of antihypertensive medication. Data are OR for a 1 SD increase in BP measure unless otherwise stated. CI indicates confidence interval; CVD, cardiovascular disease; DBP, diastolic blood pressure; MAP, mean arterial pressure; OR, odds ratio; PP, pulse pressure; and SBP, systolic blood pressure.
P for ethnicity×BP interaction.
P<0.01.
P<0.001.
CVD risk factors comprise: smoking, waist/hip ratio, total/high-density lipoprotein-cholesterol ratio, diabetes mellitus, fasting glucose, physical activity, and heart rate.
P<0.05.
Table 4. Associations Between Blood Pressure and Incident Stroke, Adjusting for Presence of Antithrombotic Medication (Anticoagulant or Antiplatelet Agents) or Atrial Fibrillation at Follow-Up, by Ethnicity.
| Europeans |
South Asians |
|||||
|---|---|---|---|---|---|---|
| BP Measure | Model Factors | OR | 95% CI | OR | 95% CI | P Value* |
| SBP | Age | 1.02 | 0.76–1.36 | 1.68 | 1.28–2.20† | 0.02 |
| Age+antithrombotic medication at follow-up | 0.90 | 0.66–1.22 | 1.57 | 1.18–2.08‡ | 0.006 | |
| Age+atrial fibrillation at follow-up | 1.00 | 0.75–1.34 | 1.64 | 1.24–2.17 | 0.02 | |
| DBP | Age | 0.82 | 0.62–1.09 | 1.66 | 1.24–2.21‡ | 0.001 |
| Age+antithrombotic medication at follow-up | 0.78 | 0.58–1.04 | 1.54 | 1.14–2.08‡ | 0.001 | |
| Age+atrial fibrillation at follow-up | 0.83 | 0.63–1.10 | 1.58 | 1.18–2.13 | 0.002 | |
| MAP | Age | 0.89 | 0.66–1.19 | 1.70 | 1.29–2.24† | 0.001 |
| Age+antithrombotic medication at follow-up | 0.81 | 0.60–1.09 | 1.59 | 1.20–2.11 ‡ | 0.001 | |
| Age+atrial fibrillation at follow-up | 0.88 | 0.66–1.19 | 1.64 | 1.24–2.18 | 0.002 | |
| PP | Age | 1.20 | 0.92–1.57 | 1.45 | 1.07–1.95§ | 0.36 |
| Age+antithrombotic medication at follow-up | 1.10 | 0.83–1.44 | 1.37 | 1.02–1.85§ | 0.21 | |
| Age+atrial fibrillation at follow-up | 1.17 | 0.90–1.54 | 1.45 | 1.08–1.97 | 0.27 | |
Participants are men not in receipt of baseline antihypertensive medication with follow-up data for medication use and presence of atrial fibrillation; n=712 European and 584 South Asian men. Data are OR for a 1 SD increase in BP measure. CI indicates confidence interval; CVD, cardiovascular disease; DBP, diastolic blood pressure; MAP, mean arterial pressure; OR, odds ratio; PP, pulse pressure; and SBP, systolic blood pressure.
For ethnicity×BP interaction.
P<0.001.
P<0.01.
P<0.05.
Discussion
South Asians had modestly higher SBP, DBP, and MAP than Europeans, although PP was similar. We report the following novel findings: (1) SBP, DBP, and MAP were more adversely associated with stroke in South Asians than Europeans, (2) when MAP+PP were considered together, PP but not MAP was detrimentally associated with stroke in Europeans, whereas the converse was true for South Asians, these findings persisted on adjustment for other cardiovascular risk factors, (3) associations between BP and stroke risk were greater in older (≥55 years) than younger Europeans, but similar by age group in South Asians, (4) the combined effects of high BP and high glycemia appeared to be more deleterious in South Asians than Europeans.
Our findings of higher BP in South Asians than Europeans accord with most5,6 but not all22 previous studies. Also consistent with earlier work,5,6 we showed only marginally elevated SBP and DBP (mean difference, +2 and +3 mm Hg, respectively) in South Asians when compared with Europeans; disparities that do not adequately explain the 1.5-fold higher stroke incidence in the former group.2,4
A novel finding was that SBP, DBP, and MAP were more strongly associated with stroke in South Asians than Europeans. South Asians had more adverse glycemic profiles than Europeans, thus we investigated whether the excess risk of stroke from high BP in South Asians was linked to greater hyperglycemia. Neither excluding people with diabetes mellitus at baseline nor adjusting for mellitus, hyperglycemia, or insulin resistance in the main models altered the observed ethnic difference in the impact of BP measures on stroke. Nevertheless, the combination of high BP and glycemia as dichotomized variables appeared more deleterious for South Asians than Europeans. We have previously shown that South Asians have poorer cerebral autoregulation than Europeans, in part because of greater levels of hyperglycemia.23 This may enhance the South Asian vulnerability to stroke.
Because DBP tends to plateau or fall in Europeans after ≈55 years of age,24 we postulated that the greater effects of DBP on stroke risk in South Asians were because of differences in this age-related DBP decline; this was not the case nor were there ethnic differences in the impact of BP change over 20-year follow-up on stroke risk. In addition, we explored the influence of medication use over follow-up. Our main analyses excluded people in receipt of antihypertensive medication at baseline, but we also showed no ethnic differences in either BP control (BP≤140/90 mm Hg) for participants receiving antihypertensive medication at follow-up or antihypertensive receipt for those with BP>140/90 mm Hg at baseline or follow-up. This suggests ethnic differences in BP management and control in those with hypertension are unlikely to contribute to the excess risk of stroke in South Asians with higher BP, commensurate with findings from UK primary care data.25 In addition, adjustment for antithrombotic use at follow-up, which was greater in South Asians than Europeans, did not alter the ethnic differential in associations between SBP, DBP, or MAP and stroke. Although we did not have data on use of antithrombotic medication at baseline, use of these drugs in primary prevention was not widespread until the late 1990s, hence any confounding effects are likely to be less than for contemporary cohorts. Our follow-up data indicated a greater prevalence of AF in Europeans than South Asians (baseline data were unavailable), in keeping with previous findings,26 however, a sensitivity analysis restricted to follow-up data indicated that AF did not have an important impact on associations between BP and stroke in either ethnic group. Other possible explanations for the ethnic differences in these associations include interethnic variation in diurnal BP. However, a previous study reported no ethnic differences in 24-hour BP, including nocturnal dipping,27 and unpublished data from the SABRE cohort are consistent with this. Alternatively, genetic or epigenetic factors may contribute; we were unable to explore these further.
We contrasted the relative contribution of the hemodynamic components of BP: PP and MAP, to stroke risk by examining models featuring these measures in combination. These indicated that PP was more adversely related to stroke than MAP in Europeans, consistent with the postulated role of arterial stiffness in cardiovascular risk for this ethnic group, demonstrated in the Framingham Heart Study.7 Conversely, MAP was much more adversely related to stroke risk than PP in South Asians. This may imply that increased peripheral resistance might be relatively more important to stroke risk in this group.28 Reasons for these ethnic differences are unknown, but they are consistent with evidence indicating a comparatively greater prevalence of intracranial and small vessel disease in South Asian people with stroke5,26 and increased microvascular disease in South Asian individuals.29 These mechanisms could be further studied by observing ethnic differences in microvascular responses to PP and MAP, for example, using near infrared spectroscopy.30 If stroke pathogenesis differs by ethnicity, this has important implications for risk management strategies, particularly for measures of BP.
This longitudinal study is unique in comparing associations between BP and stroke in Europeans and South Asians, with adequate power to show ethnic differences and excellent follow-up rates. A single BP measurement may not adequately represent lifetime exposure, however, we found no evidence of marked differences in age-related changes in BP by ethnicity and imprecision in measurement of exposures is more likely to obscure differences. Because the main analyses did not involve comparison of baseline and follow-up BPs, base-line/follow-up differences in BP ascertainment method are unlikely to have affected results. Potential confounders may have varied over time, especially use of antihypertensive, lipid-lowering, and antithrombotic medication. We did not have sufficient power to compare stroke subtypes or fatal and nonfatal events, although the majority of strokes in both ethnic groups are ischemic in nature31 and data from the Prospective Studies Collaboration suggests the BP-associated risks for ischemic and hemorrhagic strokes are similar.2 Our study was limited to men and the South Asian population was of Indian origin, mostly Punjabi Sikhs, resident in the United Kingdom, thus caution should be exercised in applying these findings to women and other South Asian subgroups.
Perspectives
To summarize, in this longitudinal comparison of UK ethnic groups, we have shown that SBP, and particularly DBP and MAP, were more strongly associated with stroke risk in South Asians than Europeans, independent of other cardiovascular risk factors. Associations remained strong across the age spectrum in South Asians, unlike Europeans where associations were apparent only in those aged ≥55 years. If substantiated, our findings support the need for trials of BP reduction beyond current thresholds, and in younger individuals, to address the greater stroke risk in South Asian groups. The majority of cardiovascular risk scores guiding primary prevention use SBP alone,9,10 and it has been proposed that DBP may be largely immaterial to cardiovascular disease risk and does not warrant measurement in clinical practice.8 Our findings suggest that DBP is an important therapeutic target for stroke prevention in South Asian men.
Supplementary Material
Novelty and Significance.
What Is New?
No studies compare associations between different blood pressure (BP) measures and stroke risk in South Asian and European groups.
Mid-life systolic BP, diastolic BP, and mean arterial pressure were more strongly related to stroke risk in South Asian than European men. Associations between pulse pressure and stroke were similar.
Pulse pressure contributed most to stroke risk in Europeans, and mean arterial pressure in South Asians.
What Is Relevant?
Risk prediction scores are based on SBP, but DBP/MAP may contribute more to stroke risk in South Asian groups.
More aggressive BP treatment thresholds may be warranted to address the excess stroke risk experienced by South Asians.
The different relative contributions of MAP and pulse pressure suggest ethnic differences in stroke pathogenesis.
Summary
Systolic, diastolic, and mean arterial BP were more strongly associated with stroke risk in South Asian than European men.
Acknowledgments
All authors contributed to study design and interpretation, and approved the final manuscript. S.V. Eastwood had full access to all the data in the study, performed the statistical analyses, wrote the first draft of the manuscript, and has primary responsibility for the final content and decision to submit for publication. We thank all members of the SABRE group for their contributions to study design, management, data collection, and analyses, especially Drs Ajay Gupta and Neil Chapman for their assistance in identifying stroke events.
Sources of Funding
The study was funded at baseline by the UK Medical Research Council, Diabetes UK, and the British Heart Foundation, and at follow-up by the Wellcome Trust and British Heart Foundation. Funders played no role in the study design, conduct, or analysis, or the decision to submit the manuscript for publication. The SABRE study group is entirely independent from the funding bodies.
Footnotes
Disclosures
None.
The online-only Data Supplement is available with this article at http://hyper.ahajournals.org/lookup/suppl/doi:10.1161/HYPERTENSIONAHA.115.05672/-/DC1.
References
- 1.Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global Burden of Diseases, Injuries, and Risk Factors Study 2010 (GBD 2010) and the GBD Stroke Experts Group Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–254. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 3.Lawes CM, Bennett DA, Parag V, Woodward M, Whitlock G, Lam TH, Suh I, Rodgers A, Asia Pacific Cohort Studies Collaboration Blood pressure indices and cardiovascular disease in the Asia Pacific region: a pooled analysis. Hypertension. 2003;42:69–75. doi: 10.1161/01.HYP.0000075083.04415.4B. doi: 10.1161/01.HYP.0000075083.04415.4B. [DOI] [PubMed] [Google Scholar]
- 4.Tillin T, Hughes AD, Mayet J, Whincup P, Sattar N, Forouhi NG, McKeigue PM, Chaturvedi N. The relationship between metabolic risk factors and incident cardiovascular disease in Europeans, South Asians, and African Caribbeans: SABRE (Southall and Brent Revisited) – a prospective population-based study. J Am Coll Cardiol. 2013;61:1777–1786. doi: 10.1016/j.jacc.2012.12.046. doi: 10.1016/j.jacc.2012.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anand SS, Yusuf S, Vuksan V, Devanesen S, Teo KK, Montague PA, Kelemen L, Yi C, Lonn E, Gerstein H, Hegele RA, McQueen M. Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: the Study of Health Assessment and Risk in Ethnic groups (SHARE) Lancet. 2000;356:279–284. doi: 10.1016/s0140-6736(00)02502-2. [DOI] [PubMed] [Google Scholar]
- 6.Chowdhury TA, Lasker SS, Mahfuz R. Ethnic differences in control of cardiovascular risk factors in patients with type 2 diabetes attending an Inner London diabetes clinic. Postgrad Med J. 2006;82:211–215. doi: 10.1136/pgmj.2005.036673. doi: 10.1136/pgmj.2005.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franklin SS, Gustin W, 4th, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 8.Williams B, Lindholm LH, Sever P. Systolic pressure is all that matters. Lancet. 2008;371:2219–2221. doi: 10.1016/S0140-6736(08)60804-1. doi: 10.1016/S0140-6736(08)60804-1. [DOI] [PubMed] [Google Scholar]
- 9.Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, Minhas R, Sheikh A, Brindle P. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ. 2008;336:1475–1482. doi: 10.1136/bmj.39609.449676.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson KM, Wilson PW, Odell PM, Kannell WB. An updated coronary risk profile. A statement for health professionals. Circulation. 1991;83:356–362. doi: 10.1161/01.cir.83.1.356. [DOI] [PubMed] [Google Scholar]
- 11.Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tillin T, Forouhi NG, McKeigue PM, Chaturvedi N, SABRE Study Group Southall And Brent REvisited: Cohort profile of SABRE, a UK population-based comparison of cardiovascular disease and diabetes in people of European, Indian Asian and African Caribbean origins. Int J Epidemiol. 2012;41:33–42. doi: 10.1093/ije/dyq175. doi: 10.1093/ije/dyq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization . Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. WHO; Geneva, Switzerland: [Accessed June 29, 2015]. 1999. p. 6. http://apps.who.int/iris/handle/10665/66040. [Google Scholar]
- 14.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 15.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS, CKD-EPI Investigators Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, McInnes GT, Mehlsen J, Nieminen M, O’Brien E, Ostergren J, ASCOT investigators Rationale, design, methods and baseline demography of participants of the Anglo-Scandinavian Cardiac Outcomes Trial. J Hypertens. 2001;19:1139–1147. doi: 10.1097/00004872-200106000-00020. [DOI] [PubMed] [Google Scholar]
- 17.Mackie A, Whincup P, McKinnon M. Does the Hawksley random zero sphygmomanometer underestimate blood pressure, and by how much? J Hum Hypertens. 1995;9:337–343. [PubMed] [Google Scholar]
- 18.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268. [PubMed] [Google Scholar]
- 19.Kinlay S. Changes in stroke epidemiology, prevention, and treatment. Circulation. 2011;124:e494–e496. doi: 10.1161/CIRCULATIONAHA.111.069633. doi: 10.1161/CIRCULATIONAHA.111.069633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui JS, Hopper JL, Harrap SB. Antihypertensive treatments obscure familial contributions to blood pressure variation. Hypertension. 2003;41:207–210. doi: 10.1161/01.hyp.0000044938.94050.e3. [DOI] [PubMed] [Google Scholar]
- 21.Bos WJ, Verrij E, Vincent HH, Westerhof BE, Parati G, van Montfrans GA. How to assess mean blood pressure properly at the brachial artery level. J Hypertens. 2007;25:751–755. doi: 10.1097/HJH.0b013e32803fb621. doi: 10.1097/HJH.0b013e32803fb621. [DOI] [PubMed] [Google Scholar]
- 22.Bhopal R, Unwin N, White M, Yallop J, Walker L, Alberti KG, Harland J, Patel S, Ahmad N, Turner C, Watson B, Kaur D, Kulkarni A, Laker M, Tavridou A. Heterogeneity of coronary heart disease risk factors in Indian, Pakistani, Bangladeshi, and European origin populations: cross sectional study. BMJ. 1999;319:215–220. doi: 10.1136/bmj.319.7204.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bathula R, Hughes AD, Panerai RB, Potter JF, McG Thom SA, Tillin T, Shore AC, Hale R, Chambers J, Kooner J, Chaturvedi N. South Asians have adverse cerebrovascular haemodynamics, despite equivalent blood pressure, compared with Europeans. This is due to their greater hyperglycaemia. Int J Epidemiol. 2011;40:1490–1498. doi: 10.1093/ije/dyr101. doi: 10.1093/ije/dyr101. [DOI] [PubMed] [Google Scholar]
- 24.Falaschetti E, Chaudhury M, Mindell J, Poulter N. Continued improvement in hypertension management in England: results from the Health Survey for England 2006. Hypertension. 2009;53:480–486. doi: 10.1161/HYPERTENSIONAHA.108.125617. doi: 10.1161/HYPERTENSIONAHA.108.125617. [DOI] [PubMed] [Google Scholar]
- 25.Millett C, Gray J, Saxena S, Netuveli G, Khunti K, Majeed A. Ethnic disparities in diabetes management and pay-for-performance in the UK: the Wandsworth Prospective Diabetes Study. PLoS Med. 2007;4:e191. doi: 10.1371/journal.pmed.0040191. doi: 10.1371/journal.pmed.0040191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunarathne A, Patel JV, Gammon B, Gill PS, Hughes EA, Lip GY. Ischemic stroke in South Asians: a review of the epidemiology, pathophysiology, and ethnicity-related clinical features. Stroke. 2009;40:e415–e423. doi: 10.1161/STROKEAHA.108.535724. doi: 10.1161/STROKEAHA.108.535724. [DOI] [PubMed] [Google Scholar]
- 27.Acharya DU, Heber ME, Doré CJ, Raftery EB. Ambulatory intraarterial blood pressure in essential hypertension. Effects of age, sex, race, and body mass–the Northwick Park Hospital Database Study. Am J Hypertens. 1996;9(10 Pt 1):943–952. doi: 10.1016/0895-7061(96)00177-X. doi: 10.1016/0895-7061(96)00177-X. [DOI] [PubMed] [Google Scholar]
- 28.Pires PW, Dams Ramos CM, Matin N, Dorrance AM. The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol. 2013;304:H1598–H1614. doi: 10.1152/ajpheart.00490.2012. doi: 10.1152/ajpheart.00490.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park C, Bathula R, Shore AC, Tillin T, Strain WD, Chaturvedi N, Hughes AD. Impaired post-ischaemic microvascular hyperaemia in Indian Asians is unexplained by diabetes or other cardiovascular risk factors. Atherosclerosis. 2012;221:503–507. doi: 10.1016/j.atherosclerosis.2011.11.025. doi: 10.1016/j.atherosclerosis.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 30.Reinhard M, Wehrle-Wieland E, Grabiak D, Roth M, Guschlbauer B, Timmer J, Weiller C, Hetzel A. Oscillatory cerebral hemodynamics–the macro- vs. microvascular level. J Neurol Sci. 2006;250:103–109. doi: 10.1016/j.jns.2006.07.011. doi: 10.1016/j.jns.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Wolfe CD, Rudd AG, Howard R, Coshall C, Stewart J, Lawrence E, Hajat C, Hillen T. Incidence and case fatality rates of stroke subtypes in a multiethnic population: the South London Stroke Register. J Neurol Neurosurg Psychiatry. 2002;72:211–216. doi: 10.1136/jnnp.72.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.