Summary
Background
Artemether–lumefantrine is the most widely used artemisinin-based combination therapy for malaria, although treatment failures occur in some regions. We investigated the effect of dosing strategy on efficacy in a pooled analysis from trials done in a wide range of malaria-endemic settings.
Methods
We searched PubMed for clinical trials that enrolled and treated patients with artemether–lumefantrine and were published from 1960 to December, 2012. We merged individual patient data from these trials by use of standardised methods. The primary endpoint was the PCR-adjusted risk of Plasmodium falciparum recrudescence by day 28. Secondary endpoints consisted of the PCR-adjusted risk of P falciparum recurrence by day 42, PCR-unadjusted risk of P falciparum recurrence by day 42, early parasite clearance, and gametocyte carriage. Risk factors for PCR-adjusted recrudescence were identified using Cox’s regression model with frailty shared across the study sites.
Findings
We included 61 studies done between January, 1998, and December, 2012, and included 14 327 patients in our analyses. The PCR-adjusted therapeutic efficacy was 97·6% (95% CI 97·4–97·9) at day 28 and 96·0% (95·6–96·5) at day 42. After controlling for age and parasitaemia, patients prescribed a higher dose of artemether had a lower risk of having parasitaemia on day 1 (adjusted odds ratio [OR] 0·92, 95% CI 0·86–0·99 for every 1 mg/kg increase in daily artemether dose; p=0·024), but not on day 2 (p=0·69) or day 3 (0·087). In Asia, children weighing 10–15 kg who received a total lumefantrine dose less than 60 mg/kg had the lowest PCR-adjusted efficacy (91·7%, 95% CI 86·5–96·9). In Africa, the risk of treatment failure was greatest in malnourished children aged 1–3 years (PCR-adjusted efficacy 94·3%, 95% CI 92·3–96·3). A higher artemether dose was associated with a lower gametocyte presence within 14 days of treatment (adjusted OR 0·92, 95% CI 0·85–0·99; p=0·037 for every 1 mg/kg increase in total artemether dose).
Interpretation
The recommended dose of artemether–lumefantrine provides reliable efficacy in most patients with uncomplicated malaria. However, therapeutic efficacy was lowest in young children from Asia and young underweight children from Africa; a higher dose regimen should be assessed in these groups.
Funding
Bill & Melinda Gates Foundation.
Introduction
Artemisinin-based combination therapies are the first-line treatment for uncomplicated Plasmodium falciparum malaria in most malaria-endemic countries,1 and they have been advocated to counter the threat of antimalarial drug resistance by delaying its emergence and spread.2 As such, artemisinin-based combination therapies are a key component of malaria elimination efforts.3
The combination of artemether and lumefantrine was originally introduced as a four-dose regimen that proved to be efficacious in studies done in China,4 Africa,5 and India;6 however, after detailed pharmacokinetic–pharmacodynamic assessment,7 the regimen was revised to comprise six doses, which was safe and effective even against multidrug-resistant parasites.8–10 In 2011, the six-dose regimen of artemether–lumefantrine (Coartem, Novartis, Basel, Switzerland) was the first artemisinin-based combination therapy to be prequalified by WHO.11 It is now registered in 86 countries and accounts for three-quarters of all artemisinin-based combination therapies used in clinical practice.1 In 2009, artemether–lumefantrine dispersible tablets (Coartem Dispersible) were approved for use in young children,11 with more than 200 million treatments of this formulation dispensed since then.12
Although the six-dose artemether–lumefantrine regimen has high efficacy in most endemic areas, the usefulness of this combination is under threat from the emergence of parasites with reduced susceptibility to the artemisinins.13 Optimum dosing of antimalarial drug regimens is vital for containment of the spread of drug resistance; however, in clinical practice, pragmatic drug distribution results in dosing being based on weight or age banding, with patients at the margins of the bands having either lower or higher weight-adjusted doses than those in the middle of the bands.14 Young children are particularly vulnerable to suboptimum dosing because drugs are often given as tablets or fractions of tablets rather than paediatric formulations or suspensions.15 In this pooled analysis, we investigated the key determinants of the therapeutic efficacy of artemether–lumefantrine, with particular attention to the range of artemether and lumefantrine doses and the effect of these factors on clinical outcome.
Methods
Search strategy and selection criteria
We did a systematic literature review in PubMed to identify all clinical trials published from 1960 to December, 2012, selecting those that enrolled and treated patients with artemether–lumefantrin (appendix). We selected studies for the meta-analysis if patients were treated with the six-dose artemether–lumefantrine regimen and were prospectively assessed for clinical efficacy against P falciparum (either alone or in mixed infections) for a minimum of 28 days. Investigators were contacted by email and asked to share individual patient data and any unpublished study they might have. We included studies in the analysis if information was available on the dose given and on the age and weight of the patient, and if PCR genotyping was done to distinguish between recrudescence and new infections. Individual patient data were uploaded into the Worldwide Antimalarial Resistance Network (WWARN) secure repository, anonymised, and processed using standard methods described in the data management and statistical analysis plan.16
All data included in this analysis were obtained in accordance with ethical approvals from the country of origin. Ethical approval to undertake individual participant data meta-analyses was granted by the Oxford Tropical Research Ethics Committee.
Procedures
We calculated the doses of artemether and lumefantrine administered from the individual number of daily tablets given to each patient. For studies in which the daily tablet count was not available, we did back calculations on the basis of the dosing plan presented in the study protocol, assuming correct adherence. Only patients who completed the six-dose treatment regimen over 3 days were included in the final analysis. Study sites were categorised as low, moderate, or high transmission settings on the basis of the transmission estimates obtained from the Malaria Atlas Project (appendix).17 In children younger than 5 years, we assessed nutritional status using the weight-for-age Z score, with standardised age-specific and sex-specific growth references according to WHO 2006 recommendations.18 Patients were classified as being underweight for age if the weight-for-age Z score was less than −2. Scores outside the range −6 to 6 were treated as outliers.
Outcomes
The primary endpoint was the PCR-adjusted risk of P falciparum recrudescence by day 28. Secondary endpoints consisted of the PCR-adjusted risk of P falciparum recurrence by day 42, PCR-unadjusted risk of P falciparum recurrence by day 42, early parasite clearance, and gametocyte carriage
Statistical analysis
We did all statistical analyses using R (version 2.14.0), on the basis of an a-priori statistical plan.19 We computed the incidence risks for the primary endpoint by survival analysis and compared Kaplan-Meier curves by the log-rank test after stratifying by study site. Definitions of outcome status and censoring are detailed in the WWARN clinical module data management and statistical analysis plan.16 The dose of lumefantrine was regarded primarily as a risk factor for recrudescence because of its long half-life, whereas the dose of artemether was regarded as the primary determinant of the early parasitological response. We assessed risk factors associated with recrudescence with Cox’s proportional hazards model with shared frailty fitted on the combination of study and study sites to account for within-study clustering and any unreported heterogeneity.20 In the multivariable analysis, we forced known confounders (ie, age and parasitaemia) and dose (in mg/kg) into the model irrespective of their statistical significance. We categorised the origin of the studies into three groups: Africa, Asia, and South America. Other covariates significant at the 10% level in univariable analyses were added to the multivariable analyses and their inclusion was on the basis of a likelihood ratio test.21 We calculated the population-attributable risks associated with the risk factors on the basis of the prevalence and adjusted hazard ratio (HR).22 The proportions of patients with patent (microscopy-detected) parasitaemia were computed on days 1, 2, and 3 (parasite positivity rates), and gametocyte carriage was assessed as the proportion of patients with microscopy-detected P falciparum gametocytes (gametocyte positivity rates) on any given day during the follow-up period. Risk factors for parasite positivity rates and gametocyte positivity rates were analysed with mixed-effects logistic regression with sites (combination of study and study site) fitted as a random effect.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
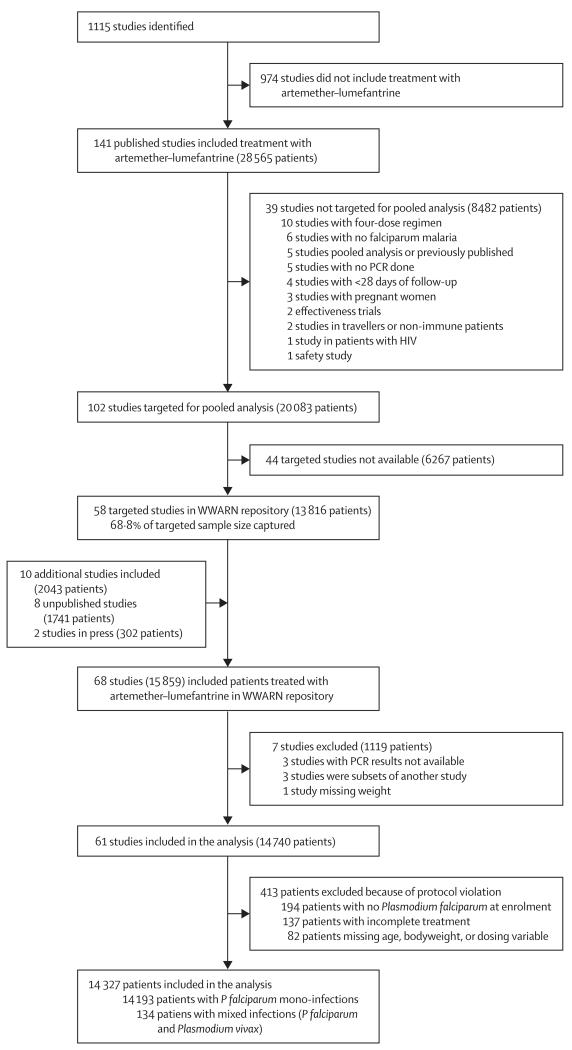
We identified 151 clinical trials of artemether–lumefantrine done between January, 1998, and December, 2012 (141 published, two in press, and eight unpublished), of which 115 (76%) were randomised trials (figure 1; appendix). Individual patient data were available from 58 published studies (n=13 816 [69% of the targeted sample of 20 083]), two studies in press (n=302), and eight unpublished studies (n=1741). Seven studies (n=1119) did not meet the inclusion criteria and an additional 413 patients were excluded for protocol violations. Patients were followed up for 28 days in 34 studies (n=7460), for 42 days in 23 studies (n=6004), for 56 days in one study (n=359), and for 63 days or longer in three studies (n=504). Parasite genotyping was done in all of the studies, with at least three markers used in 34 (56%) studies.
Figure 1. Study flowchart.
WWARN=Worldwide Antimalarial Resistance Network.
Table 1 details the baseline characteristics of patients included in the analysis. The median age of patients was 4 years (range 1 month to 80 years), with 815 (6%) younger than 1 year and 7333 (51%) aged 1 year to younger than 5 years. Patients from Africa were significantly younger than those from either Asia or South America (p<0·0001) and had higher baseline parasitaemia (p=0·03; table 1). All the patients from Asia and South America were from low or moderate transmission settings, whereas only 6415 (54%) of 11 809 patients in Africa were from low or moderate transmission settings.
Table 1. Demographics and baseline characteristics.
| Asia (n=2359) | Africa (n=11 809) | South America (n=159)* |
Overall (n=14 327) | |
|---|---|---|---|---|
| Study period | 1998–2010 | 2002–12 | 2007–08 | 1998–2012 |
| Sex† | ||||
| Female | 929 (39%) | 5538 (47%) | 63 (40%) | 6530 (46%) |
| Male | 1430 (61%) | 5973 (51%) | 96 (60%) | 7499 (52%) |
| Age, years | ||||
| Median (range) | 16·0 (0·5–80·0) | 3·5 (0·0–77·0) | 23·0 (12·0–56·0) | 4·0 (0·0–80·0) |
| <1 | 6 (<1%) | 809 (7%) | 0 | 815 (6%) |
| 1 to <5 | 373 (16%) | 6960 (59%) | 0 | 7333 (51%) |
| 5 to <12 | 503 (21%) | 2477 (21%) | 0 | 2980 (21%) |
| ≥12 | 1477 (63%) | 1563 (13%) | 159 (100%) | 3199 (22%) |
| Weight, kg | ||||
| Median (range) | 41·0 (6·0–88·0) | 13·7 (5·0–102·0) | 64·0 (30·0–110·0) | 15·0 (5·0–110·0) |
| 5 to <10 | 61 (3%) | 2013 (17%) | 0 | 2074 (14%) |
| 10 to <15 | 334 (14%) | 4623 (39%) | 0 | 4957 (35%) |
| 15 to <25 | 404 (17%) | 2992 (25%) | 0 | 3396 (24%) |
| 25 to <35 | 206 (9%) | 753 (6%) | 4 (3%) | 963 (7%) |
| 35 to <70 | 1341 (57%) | 1278 (11%) | 105 (66%) | 2724 (19%) |
| ≥70 | 13 (1%) | 150 (1%) | 50 (31%) | 213 (1%) |
| Treatment supervision‡ | ||||
| Full | 1824 (77%) | 9086 (77%) | 159 (100%) | 11 069 (77%) |
| Partial | 373 (16%) | 1563 (13%) | 0 | 1936 (14%) |
| Unsupervised | 162 (7%) | 1160 (10%) | 0 | 1322 (9%) |
| Treatment coadministration | ||||
| With fatty meal | 656 (28%) | 5635 (48%) | 159 (100%) | 6450 (45%) |
| Without fatty meal | 0 | 1191 (10%) | 0 | 1191 (8%) |
| Advised to consume with fat | 820 (35%) | 1326 (11%) | 0 | 2146 (15%) |
| Not stated | 883 (37%) | 3657 (31%) | 0 | 4540 (32%) |
| Drug tradename | ||||
| Coartem (Novartis) | 2359 (100%) | 11 126 (94%) | 159 (100%) | 13 644 (95%) |
| Coartem dispersible (Novartis) | 0 | 431 (4%) | 0 | 431 (3%) |
| Co-artesiane (Dafra, Turnhout, Belgium) | 0 | 134 (1%) | 0 | 134 (1%) |
| Atrin (LIC Pharmaceuticals, Abidjan, Côte d’Ivoire) | 0 | 118 (1%) | 0 | 118 (1%) |
| Enrolment clinical variables | ||||
| Parasitaemia, parasites per μL | 9559 (13–450 440) | 21 360 (16–420 360) | 4241 (1008–44 744) | 19 921 (13–450 440) |
| Parasitaemia >100 000/μL | 226 (10%) | 1131 (10%) | 0 | 1357 (9%) |
| Mixed infection with Plasmodium vivax | 134 (6%) | 0 | 0 | 134 (1%) |
| Haemoglobin, g/L | 114 (25·8) | 101 (21·2) | NR | 104 (22·9) |
| Anaemic, haemoglobin <100 g/L | 600/2179 (28%) | 4030/8287 (49%) | NR | 4630/10 466 (44%) |
| Gametocytes present | 128/1118 (11%) | 541/7850 (7%) | 10 (6%) | 679/9127 (7%) |
| Fever, temperature >37·5°C | 1199/2195 (55%) | 6973/10 854 (64%) | 103 (65%) | 8275/13 208 (63%) |
| Children underweight for age§ | 173/471 (37%) | 1352/7825 (17%) | NR | 1525/8296 (18%) |
Data are number (%), median (IQR), or mean (SD), unless otherwise specified. Some percentages do not add up to 100 because of rounding. NR=not reported.
Data from one study done in Colombia.
Data were not available for 298 patients from Africa.
Patients with only morning daily doses supervised and evening doses taken at home with no supervision were classified as partly supervised. Patients were classified as unsupervised if all six doses were unobserved or if the first dose was observed at the clinic with remaining five doses unobserved.
Defined using a weight-for-age score <−2 in children <5 years of age. Scores outside the range −6 to 6 were treated as outliers.
Overall, the median total dose of lumefantrine given was 68·6 mg/kg (IQR 57·6–80·0, range 26·2–144·0) and that of artemether was 11·4 mg/kg (IQR 9·6–13·3, range 4·4–24·0). The total dose of both components varied significantly between the different weight categories (p<0·0001; table 2). The proportion of patients who did not receive the therapeutic dose of lumefantrine varied across weight categories and regions (appendix). The lowest median lumefantrine doses were given to children aged 3–5 years (62·6 mg/kg, IQR 55·4–84·7) and to patients weighing more than 70 kg (38·4 mg/kg, IQR 36·0–40·6). Treatment administration was fully supervised in 11 069 (77%) of 14 327 patients, partly supervised in 1936 (14%), and unsupervised in 1322 (9%).
Table 2. Total lumefantrine and artemether doses.
| Number |
Lumefantrine dose, mg/kg
|
Artemether dose, mg/kg
|
|||
|---|---|---|---|---|---|
| Median (IQR) | Range | Median (IQR) | Range | ||
| Overall | 14 327 | 68·6 (57·6–80·0) | 26·2–144·0 | 11·4 (9·6–13·3) | 4·4–24·0 |
| Age category, years | |||||
| <1 | 815 | 90·0 (81·8–102·9) | 40–144 | 15·0 (13·6–17·1) | 6·7–24 |
| 1 to <5 | 7333 | 68·6 (60·0–80·0) | 37·9–144 | 11·4 (10–13·3) | 6·3–24 |
| 5 to <12 | 2980 | 73·8 (65·5–83·1) | 32–120 | 12·3 (10·9–13·8) | 5·3–20 |
| ≥12 | 3199 | 57·6 (49·7–65·5) | 26·2–91·9 | 9·6 (8·3–10·9) | 4·4–15·3 |
| Weight category, kg | |||||
| 5 to <10 | 2074 | 85·7 (80·0–94·7) | 37·9–144·0 | 14·3 (13·3–15·8) | 6·3–24·0 |
| 10 to <15 | 4957 | 60·0 (55·4–67·3) | 42·9–100·7 | 10·0 (9·2–11·2) | 7·1–16·8 |
| 15 to <25 | 3396 | 80·0 (72·0–90·0) | 39·6–105·0 | 13·3 (12·0–15·0) | 6·6–17·5 |
| 25 to <35 | 963 | 74·5 (68·4–83·1) | 42·4–96·9 | 12·4 (11·4–13·8) | 7·1–16·2 |
| 35 to <70 | 2724 | 57·0 (50·5–64·0) | 30·9–82·3 | 9·5 (8·4–10·7) | 5·1–13·7 |
| ≥70 | 213 | 38·4 (36·0–40·6) | 26·2–41·1 | 6·4 (6·0–6·8) | 4·4–6·9 |
| Region | |||||
| Asia | 2359 | 61·3 (55·4–72·0) | 30·9–120·0 | 10·2 (9·2–12·0) | 5·1–20·0 |
| Africa | 11 809 | 71·3 (60·0–80·9) | 28·2–144·0 | 11·9 (10·0–13·5) | 4·7–24·0 |
| South America | 159 | 45·0 (40·6–53·3) | 26·2–82·3 | 7·5 (6·8–8·9) | 4·4–13·7 |
| Drug tradename | |||||
| Artrin | 118 | 74·8 (60–90·0) | 51·4–120·0 | 12·5 (10–15·0) | 8·6–20·0 |
| Co-artesiane | 134 | 71·1 (69·4–74·8) | 60·7–79·2 | 11·8 (11·6–12·5) | 10·1–13·2 |
| Coartem | 14 075 | 68·4 (57·6–80·0) | 26·2–144·0 | 11·4 (9·6–13·3) | 4·4–24·0 |
Information regarding acute vomiting of drugs was available for 14 studies (n=5024), with 221 (4%) of 5024 patients vomiting at least one dose of drug within 1 h of administration during the treatment course; this proportion was greatest (18 [11%] of 171) in infants younger than 1 year (appendix). After adjusting for age, fever at presentation, and baseline parasitaemia, the risk of acute vomiting was associated with increasing dose of lumefantrine, with every 5 mg/kg increase in lumefantrine dose associated with an 8·7% (95% CI 3·4–14·2; p=0·001) increased risk of vomiting. There was no relation between the dose of lumefantrine and either late vomiting or diarrhoea in the first week.
The early therapeutic response was rapid. At 24 h after starting treatment, 5534 (60%) of 9208 patients were parasite positive, falling to 910 (8%) of 12 055 by day 2 and 96 (1%) of 12 829 by day 3; the age-stratified proportion of individuals who were parasite positive on these days are presented in the appendix. In multivariable analysis, baseline parasitaemia was the only independent predictor of parasite positivity on all days (table 3). Patients from Asia were at increased risk of parasite positivity on days 1 and 2, and those from South America were at increased risk on day 2 compared with patients from Africa (table 3). After controlling for baseline parasitaemia, region, and age category, a higher total daily dose of artemether was associated with a lower risk of parasite positivity on day 1 (p=0·024). There was no significant association between artemether dose and parasite positivity on days 2 or 3 (table 3).
Table 3. Risk factors for patent parasitaemia on days 1, 2, and 3.
|
Risk for positivity on day 1
|
Risk for positivity on day 2
|
Risk for positivity on day 3
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Total N (n)* | Adjusted OR (95% CI) | p value | Total N (n)* | Adjusted OR (95%CI) | p value | Total N (n)* | Adjusted OR (95%CI) | p value | |
| Baseline parasitaemia, parasites per μL every ten- times increase |
9208 (5534) | 3·39 (3·09–3·73) | <0·0001 | 12 055 (910) | 2·28 (1·97–2·64) | <0·0001 | 12 829 (96) | 1·85 (1·23–2·78) | 0·003 |
| Artemether daily dose, mg/kg† |
9208 (5534) | 0·92 (0·86–0·99) | 0·024 | 12 055 (910) | 0·96 (0·87–1·07) | 0·69 | 12 829 (96) | 0·76 (0·56–1·04) | 0·087 |
| Age category, years | |||||||||
| ≥12 | 2205 (1155) | Reference | · · | 2541 (206) | Reference | · · | 2860 (14) | Reference | · · |
| <1 | 474 (286) | 1·70 (1·21–2·39) | 0·002 | 734 (57) | 1·43 (0·89–2·3) | 0·14 | 751 (7) | 4·18 (1·16–15·09) | 0·029 |
| 1 to <5 | 4641 (2993) | 1·82 (1·47–2·26) | <0·0001 | 6256 (493) | 1·38 (1–1·89) | 0·050 | 6500 (50) | 2·17 (0·88–5·35) | 0·094 |
| 5 to <12 | 1888 (1100) | 1·38 (1·14–1·67) | 0·001 | 2524 (154) | 1·11 (0·83–1·49) | 0·483 | 2718 (25) | 3·08 (1·33–7·15) | 0·009 |
| Region | |||||||||
| Africa | 7194 (4335) | Reference | · · | 9976 (677) | Reference | · · | 10 766 (64) | Reference | · · |
| Asia | 2014 (1199) | 7·16 (1·49–34·4) | 0·014 | 1923 (183) | 4·40 (1·19–16·27) | 0·026 | 1904 (29) | 4·35 (0·98–19·35) | 0·054 |
| South America | NT | NT | NT | 156 (50) | 40·36 (1·22–1336·69) | 0·038 | 159 (3) | 19·41 (0·52–727·38) | 0·108 |
OR=odds ratio. NT=measurements not taken.
Number of patients (number with positive parasitaemia).
For every mg/kg increase in total daily artemether dose.
2310 (16%) of 14 327 patients had a recurrent parasitaemia detected during follow-up, and in 386 (17%) cases these could be confirmed as recrudescent infections by PCR. In patients who were followed up for 42 days, 139 (70%) of 200 recrudescences occurred before day 28. The overall PCR-adjusted Kaplan-Meier therapeutic efficacy was 97·6% (95% CI 97·4–97·9) at day 28 and 96·0% (95·6–96·5) at day 42. The overall risk of recrudescence was similar between patients from Asia and Africa (table 4). The PCR unadjusted and adjusted risk of recurrence for individual studies are presented in the appendix.
Table 4. Risk factors for PCR-confirmed recrudescence at day 28.
| Total N (n)* |
Univariable analyses
|
Multivariable analyses
†
|
PAR calculations
‡
|
||||
|---|---|---|---|---|---|---|---|
| Crude HR (95% CI) | p value | Adjusted HR (95% CI) | p value | Frequency§ | PAR | ||
| Age, every 1-year increase | 14 139 (304) | 0·96 (0·94–0·99) | 0·001 | · · | · · | · · | · · |
| Bodyweight, every 1 kg increase | 14 139 (304) | 0·98 (0·97–0·99) | 0·001 | · · | · · | · · | · · |
| Lumefantrine dose, every 5 mg/kg increase |
14 139 (304) | 0·98 (0·95–1·02) | 0·380 | 0·98 (0·94–1·02) | 0·380 | 27·46% | 6·30% |
| Enrolment clinical variables | |||||||
| Parasitaemia, parasites per μL every ten-times increase |
14 139 (304) | 1·47 (1·2–1·81) | 0·0002 | 1·41 (1·15–1·74) | 0·0012 | 9·51% | 4·01% |
| Fever, temperature >37·5°C | 13 024 (290) | 1·09 (0·85–1·41) | 0·500 | · · | · · | · · | · · |
| Haemoglobin, every 10 g/L increase | 10 303 (221) | 0·95 (0·88–1·01) | 0·100 | · · | · · | · · | · · |
| Anaemia, haemoglobin <100 g/L | 10 303 (221) | 1·24 (0·93–1·65) | 0·150 | · · | · · | · · | · · |
| Gametocytes present | 9008 (198) | 1·01 (0·62–1·65) | 0·970 | · · | · · | · · | · · |
| Sex | |||||||
| Female | 6448 (143) | Reference | · · | · · | · · | · · | · · |
| Male | 7393 (156) | 0·95 (0·76–1·20) | 0·690 | · · | · · | · · | · · |
| Age category, years | |||||||
| ≥12 | 3160 (34) | Reference | · · | · · | · · | · · | · · |
| <1 | 809 (21) | 1·73 (0·92–3·29) | 0·091 | 1·78 (0·89–3·55) | 0·100 | 5·72% | 4·76% |
| 1 to <5 | 7231 (204) | 2·17 (1·35–3·47) | 0·001 | 2·00 (1·23–3·23) | 0·005 | 51·14% | 37·92% |
| 5 to <12 | 2939 (45) | 1·33 (0·81–2·18) | 0·260 | 1·27 (0·76–2·12) | 0·360 | 20·79% | 7·96% |
| Transmission setting | |||||||
| Low | 3432 (60) | Reference | · · | · · | · · | · · | · · |
| High | 5336 (160) | 1·75 (0·92–3·33) | 0·086 | · · | · · | · · | · · |
| Moderate | 5371 (84) | 1·13 (0·58–2·23) | 0·720 | · · | · · | · · | · · |
| Region | |||||||
| Africa | 11 674 (260) | Reference | · · | · · | · · | · · | · · |
| Asia | 2306 (43) | 0·81 (0·34–1·90) | 0·630 | · · | · · | · · | · · |
| South America | 159 (1) | 0·25 (0·02–4·16) | 0·330 | · · | · · | · · | · · |
| Treatment supervision | |||||||
| Full | 10 929 (232) | Reference | · · | · · | · · | · · | · · |
| Partial | 1909 (51) | 1·40 (0·76–2·59) | 0·280 | · · | · · | · · | · · |
| Unsupervised | 1301 (21) | 1·31 (0·52–3·28) | 0·570 | · · | · · | · · | · · |
| Coadministration with fat | |||||||
| With fatty meal | 6346 (142) | Reference | · · | · · | · · | · · | · · |
| Without fatty meal | 1181 (23) | 0·95 (0·34–2·67) | 0·920 | · · | · · | · · | · · |
| Advised with fatty meal | 2120 (39) | 0·98 (0·40–2·41) | 0·960 | · · | · · | · · | · · |
| Dose calculation method | |||||||
| Per protocol | 9473 (231) | Reference | · · | · · | · · | · · | · · |
| Tablet counts | 4666 (73) | 0·73 (0·43–1·24) | 0·250 | · · | · · | · · | · · |
| Drug tradename | |||||||
| Coartem | 13 891 (296) | Reference | · · | · · | · · | · · | · · |
| Generic artemether–lumefantrine | 248 (8) | 1·12 (0·42–2·99) | 0·820 | · · | · · | · · | · · |
HR=hazard ratio. PAR=population-attributable risk.
Number of patients (number with recrudescence by day 28).
The assumption of proportional hazard held for the model (p=0·47 for global test) and for all the individual covariates in the multivariable model (p>0·05). The variance of random eff ect was 0·95. The likelihood ratio test was not significant for bodyweight (p=0·19), haemoglobin (p=0·29), and transmission (p=0·53) in the presence of mg/kg dose, parasitaemia, and age category; thus, these were dropped from the multivariable analysis.
Overall PAR for model: 51·1%. Cumulative PAR for parasitaemia >100 000 parasites per μL and age 1 to <5 years: 40·4%.
The proportion of patients with the risk factor. Continuous covariates were categorised as follows: baseline parasitaemia at 100 000 parasites per μL and mg/kg lumefantrine dose at 60 mg/kg (lower bound of WHO therapeutic range). The adjusted HR used for estimating the PARs (obtained from the categorised model) were 1·44 for baseline parasitaemia, 1·24 for lumefantrine dose <60 mg/kg, 1·87 for age <1 year, 2·19 for age 1–5 years, and 1·42 for age 5–12 years.
In univariable analyses, three risk factors on presentation were associated with a greater risk of recrudescence by day 28: age (p=0·001), bodyweight (p=0·001), and baseline parasitaemia (p=0·0002; table 4). The risk of recrudescence was similar between patients who were fully supervised and those who were partly or not supervised, and in patients who took the drug with or without a fatty meal (table 4). In multivariable analyses, baseline parasitaemia (p=0·0012) and young age (1–5 years; p=0·005) were the only independent risk factors associated with recrudescence by day 28 (table 4). We further investigated the relation between age and weight for children younger than 5 years. 679 (18%) of 3752 children aged 1–3 years were underweight for their age. Children aged between 1 and 3 years who were underweight for age had an increased risk of recrudescence compared with those who were not (HR 1·56, 95% CI 1·04–2·43; p=0·033; figure 2), but there was no difference between groups for those aged 3–5 years (0·59, 0·31–1·14; p=0·12).
Figure 2. Cumulative risk of PCR-confirmed recrudescence by day 28 in children aged 1–5 years.
Error bars are 95% CIs.
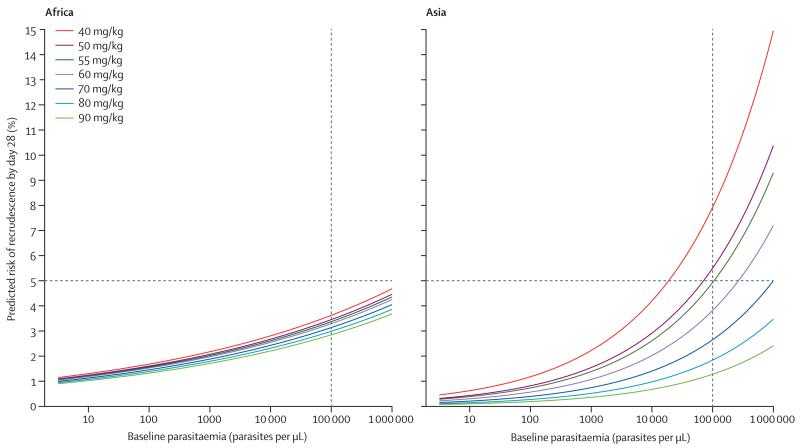
There was a significant interaction between regions and both lumefantrine dose (pinteraction=0·005) and baseline parasitaemia (pinteraction=0·012). Therefore, we generated separate models for Asia and Africa. We could not fit a model for patients from South America because of the small sample size. In Africa, the greatest risk of recrudescence was in underweight for age children between 1 and 3 years, among whom the PCR-adjusted efficacy was 94·3% (95% CI 92·3–96·3) compared with 96·9% (96·2–97·5) in those of similar age who were not underweight (adjusted HR 1·66, 95% CI 1·05–2·63; p=0·028; table 5). For Asia, the corresponding adjusted HR was 1·07 (95% CI 0·17–6·78; p=0·94). The dose of lumefantrine was not associated with the risk of recrudescence in Africa, either overall (adjusted HR for every 5 mg/kg increase in dose 0·98, 95% CI 0·94–1·03; p=0·42) or in the subgroup of children who were underweight for their age (0·95, 0·81–1·10; p=0·47). By contrast, in Asia, the dose of lumefantrine was associated with the risk of recrudescence (adjusted HR 0·77, 95% CI 0·67–0·90; figure 3); efficacy was lowest in children weighing 10–15 kg who received a total lumefantrine dose of less than 60 mg/kg (91·7%, 95% CI 86·5–96·9). Patients who received less than 60 mg/kg (the WHO recommended lower bound) were at greater risk of recrudescence (adjusted HR 2·73, 95% CI 1·40–5·32; p=0·003), accounting for 41% of all treatment failures. Using the same model, we predicted that if patients from Asia with parasitaemia less than 267 000 parasites per μL received a minimum total lumefantrine dose of 60 mg/kg, then adequate cure would be achieved in at least 95% of cases (figure 3).
Table 5. Multivariable models for risk for recrudescence in patients from Africa and Asia by day 28.
|
Africa
*
|
Asia
*
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total N (n)† | Adjusted HR (95% CI) | p value | Frequency | PAR | Total N (n)† | Adjusted HR (95% CI) | p value | Frequency | PAR | |
| Lumefantrine dose <60 mg/kg | 11 547 (255) | 1·20 (0·85–1·68) | 0·30 | 24·1% | 4·5% | 2300 (43) | 2·73 (1·40–5·32) | 0·003 | 41·0% | 41·5% |
| Baseline parasitaemia, >100 000 parasites per μL‡ |
11 547 (255) | 1·44 (0·99–2·1) | 0·054 | 9·6% | 4·1% | 2300 (43) | 1·76 (0·78–3·97) | 0·18 | 9·8% | 6·9% |
| Age category, kg | ||||||||||
| ≥12, reference | 1551 (10) | 1 | · · | · · | · · | 1450 (23) | 1 | · · | · · | · · |
| <1§ | 803 (21) | 2·20 (0·94–5·17) | 0·071 | 7·0% | 7·7% | · · | · · | · · | · · | · · |
| 1 to <3 UWA | 641 (31) | 4·05 (1·78–9·18) | 0·001 | 5·6% | 14·5% | 38 (2) | 3·97 (0·87–18·16) | 0·075 | 1·7% | 4·7% |
| 1 to <3 not UWA | 2986 (84) | 2·52 (1·19–5·34) | 0·016 | 25·9% | 28·2% | 87 (3) | 3·38 (0·92–12·43) | 0·067 | 3·8% | 8·2% |
| 3 to <5 | 3113 (73) | 2·18 (1·04–4·56) | 0·038 | 27·0% | 24·2% | 239 (6) | 1·73 (0·66–4·51) | 0·27 | 10·4% | 7·0% |
| 5 to <12 | 2453 (36) | 1·67 (0·77–3·59) | 0·19 | 21·2% | 12·4% | 486 (9) | 1·50 (0·65–3·46) | 0·34 | 21·1% | 9·6% |
HR=hazard ratio. PAR=population-attributable risk. UWA=underweight for age.
The assumption of proportional hazards was met for each model (p=0·54 for Africa and p=0·18 for Asia by the Schoenfeld’s test). The overall PAR was 66·5% for Africa and 59·9% for Asia.
Number of patients (number with recrudescence by day 28).
Adjusted HRs were 1·31 (95% CI 1·03–1·66) and 2·2 (1·37–3·54) for every increase of ten times.
Only six patients aged <1 year in Asia had no PCR-confirmed failure. HR could not be estimated for this group.
Figure 3. Risk of recrudescence by day 28 in Africa and Asia.
The predicted risk of recrudescence by day 28 for a given enrolment parasitaemia and total lumefantrine dose in Africa and Asia. The risks were estimated using the coefficients for parasitaemia and lumefantrine dose from a Cox’s model containing age, dose, and baseline parasitaemia for Africa. For Asia, the model contained dose and baseline parasitaemia. We assumed zero study effects. The horizontal line represents the 5% treatment failure rate threshold from WHO that should be used to assess if a new drug can be introduced for treatment of uncomplicated malaria. The vertical line is the parasitaemia of 100 000 per μL, a threshold used in the multivariable models for calculating the population-attributable risk estimates.
272 patients had patent gametocytaemia during follow-up. In 198 (73%) of these cases, gametocytes were present on or before day 14 of follow-up. In a multivariable model, after controlling for baseline gametocyte carriage, asexual parasite density, and age category, a higher artemether dose was associated with a lower gametocyte presence within 14 days of treatment (adjusted odds ratio 0·92, 95% CI 0·85–0·99; p=0·037 for every 1 mg/kg increase in total artemether dose).
Discussion
This pooled analysis of individual patient data comprises more than half of all clinical trials that have been published on the six-dose artemether–lumefantrine treatment regimen. Our findings confirm that artemether–lumefantrine treatment is an efficacious antimalarial regimen, resulting in a rapid therapeutic response. In more than 90% of patients, fever was resolved and peripheral parasitaemia was cleared within 48 h. Overall, the therapeutic efficacy of artemether–lumefantrine was 97·6% on day 28 and 96·0% on day 42, with only a slight effect of the total dose on these parameters. Patients who received a lower daily dose of artemether had an increased risk of parasitaemia on day 1 (8% for each 1 mg/kg decrease in daily artemether dose) and an increased risk of gametocyte carriage within 14 days (8% for each 1 mg/kg decrease in total artemether dose). Although high treatment efficacy was achieved in all age and weight categories, there were important regional differences. Patients from Asia had a slower initial therapeutic response compared with patients from Africa and a greater risk of recrudescence. The dose of artemether–lumefantrine was an independent predictor of recrudescence in Asia but not Africa. These regional differences could represent either lower host immunity, in what are mostly low-transmission settings, or reduced parasite susceptibility to lumefantrine and artemether in the P falciparum parasites circulating in the Greater Mekong region.23,24 Our analysis shows that patients receiving less than 60 mg/kg of lumefantrine accounted for almost 42% of treatment failures in Asia, the effect being most noticeable in young children. Ensuring a lumefantrine dose above 60 mg/kg would achieve greater than 95% cure in all patients, provided they presented with a parasitaemia less than 267 000 parasites per μL.
Since there was a significant interaction between regions and both lumefantrine dose and baseline parasitaemia, we generated separate models for Asia and Africa. In Africa, the risk of recrudescence was greatest in young children, especially those who were underweight for their age. The higher efficacy in older children and adults in Africa is probably a result of previous and repeated exposure to malaria, associated with the development of premunition.25,26 By contrast, infections in younger patients with lower host immunity are associated with higher baseline parasitaemia, increasing the risk of treatment failure,27 especially in underweight children.28
The main determinant of artemether–lumefantrine clinical efficacy is the area under the curve of lumefantrine.29 The highly lipophilic lumefantrine is erratically absorbed, its bioavailability being affected by coadministration of food and the acute phase of the infection.29 The area under the curve for lumefantrine varies markedly with age and the nutritional status of the patient, with young children having a more rapid dose-normalised drug clearance compared with adults.30 To accommodate these differences, the dosing schedule of artemether–lumefantrine was modified in the early stages of drug development to ensure a higher mg/kg dose in young children.7 Our study shows the importance of this factor in this vulnerable group. Those who were underweight for their age were at increased risk of treatment failure. This effect could be due to either reduced drug absorption or an increased volume of distribution in malnourished children, both of which result in lower plasma drug concentrations.31
An increase in dose, particularly in children weighing 13–15 kg, might not necessarily result in higher blood concentrations since the absorption of lumefantrine saturates at recommended doses.32 Furthermore, an increase to the next dose band would result in these children receiving over 100 mg/kg of lumefantrine—a level that is associated with an increased risk of acute vomiting. A dispersible formulation of artemether–lumefantrine for administration in young children is now available, but this might not necessarily allow greater precision in achieving therapeutic blood concentrations, and its absorption kinetics and gastrointestinal tolerability at higher than recommended doses have yet to be well characterised. Administration of a 5-day (augmented dose) regimen might circumvent the dose-limited absorption and tolerability issues8 and increase the efficacy in high-risk patients, but effectiveness in clinical practice needs to be assessed.
Our study has several limitations. First, although the clinical data used in the analysis constitute almost 60% of the relevant published work on this treatment regimen, 44 studies (n=6267) were not available, most of which (27 [61%]) were from Africa. However, there was no obvious selection bias between studies that were excluded from the analysis. Second, in only 4718 (33%) of 14 327 patients could drug doses be calculated from the actual number of tablets given; in the remainder of patients the total dose was extrapolated from the number of tablets to have been given per protocol, assuming complete adherence. However, when the method of dose calculation was included in the multivariable analysis the results remained unchanged. Third, ideally the weight-for-age Z scores to define nutritional status of the children should be calibrated to take regional variation into account. We controlled for these differences by stratifying the analysis on the basis of region (Asia and Africa), and the relation between nutritional status and treatment outcome remained. However, we were not able to differentiate between acute and chronic malnutrition, because data on patients’ height were not available.
In summary, the efficacy of artemether–lumefantrine remains excellent in most endemic areas. However, young children in the 10–15 kg weight band received a lower mg/kg total dose of artemether–lumefantrine, and this was associated with reduced efficacy, particularly in patients from Asia who presented with high parasitaemia and in malnourished patients from Africa. Further studies are warranted to optimise treatment strategies in these vulnerable populations. Although we found no evidence of temporal patterns in treatment failure, continued surveillance of artemether–lumefantrine efficacy is crucial to assure that appropriate responses to any decline can be implemented to prolong the clinical efficacy of this antimalarial drug in the long term.
Supplementary Material
Acknowledgments
We thank the patients and all the staff who participated in these clinical trials at all the sites and the WWARN team for technical and administrative support.
WWARN AL Dose Impact Study Group
Australia N M Anstey, R N Price (Global and Tropical Health Division, Menzies School of Health Research and Charles Darwin University, Darwin, NT); T M E Davis (School of Medicine and Pharmacology, University of Western Australia, Crawley, WA); H A Karunajeewa, I Mueller (Infection and Immunity Division, Walter and Eliza Hall Institute, Melbourne, VIC); H A Karunajeewa (Division of Medicine, Western Health, VIC). Belgium U D’Alessandro (Unit of Malariology, Institute of Tropical Medicine, Antwerp). Bénin A Massougbodji (Centre d’Etudes et de Recherche sur le Paludisme Associé à la Grossesse et à l’Enfant, Faculté des Sciences de la Santé, Université d’Abomey-Calavi, Cotonou). Burkina Faso F Nikiema, J-B Ouédraogo, H Tinto, I Zongo (Institut de Recherche en Sciences de la Santé, Direction Régionale de l’Ouest, Bobo-Dioulasso); J-B Ouédraogo, H Tinto (Centre Muraz, Bobo-Dioulasso). Cameroon A Same-Ekobo (Faculté de Médecine et des Sciences Biomédicales, Centre Hospitalo-Universitaire de Yaoundé, Yaoundé). Côte d’Ivoire M Koné, H Menan, W Yavo (Department of Parasitology and Mycology, Faculty of Pharmacy, University of Cocody, Abidjan); A O Touré (Malariology Department, Institut Pasteur de Côte d’Ivoire, Abidjan); W Yavo (Malaria Research and Control Center, National Institute of Public Health, Abidjan). Denmark P-E Kofoed (Department of Paediatrics, Kolding Hospital, Kolding). Ethiopia B H Alemayehu (International Center for AIDS Care and Treatment Programs, Addis Ababa); D Jima (Federal Ministry of Health, Addis Ababa). France E Baudin, E Espié, C Nabasumba, L Pinoges, B Schramm (Epicentre, Paris); M Cot, P Deloron, J-F Faucher (Institut de Recherche pour le Développement, Mother and Child Health in the Tropics Research Unit, Paris); M Cot, P Deloron, J-F Faucher (PRES Sorbonne Paris Cité, Université Paris Descartes, Paris); J-F Faucher (Department of Infectious Diseases, Besançon University Medical Center, Besançon); J-P Guthmann (Département des Maladies Infectieuses, Institut de Veille Sanitaire, Saint Maurice). Gabon B Lell (Centre de Recherches Médicales de Lambaréné, Lambaréné). Germany S Borrmann (Department of Microbiology, Magdeburg University School of Medicine, Magdeburg); B Lell (Institute for Tropical Medicine, University of Tubingen, Tubingen). Ghana G O Adjei (Centre for Tropical Clinical Pharmacology and Therapeutics, University of Ghana Medical School, Accra). Guinea-Bissau P-E Kofoed, J Ursing (Projecto de Saúde de Bandim, Bissau). Indonesia E Tjitra (National Institute of Health Research and Development, Ministry of Health, Jakarta). Kenya S Borrmann, K Marsh, J Peshu (Kenya Medical Research Institute and Wellcome Trust Research Programme, Kilifi); E Juma (Kenya Medical Research Institute, Nairobi); B R Ogutu (Kenya Medical Research Institute and United States Army Medical Research Unit, Kisumu); S A Omar, P Sawa (Human Health Division, International Centre for Insect Physiology and Ecology, Mbita Point); A O Talisuna (WWARN-East Africa Regional Centre, Nairobi); A O Talisuna (University of Oxford, KEMRI, and Wellcome Trust Research Programme, Nairobi). Laos M Khanthavong (Centre of Malariology, Parasitology and Entomology, Vientiane); M Mayxay, P N Newton (Wellcome Trust-Mahosot Hospital-Oxford University Tropical Medicine Research Collaboration, Mahosot Hospital, Vientiane); M Mayxay (Faculty of Medical Science, National University of Laos, Vientiane). Madagascar P Piola (Institut Pasteur de Madagascar, Antananarivo). Mali A A Djimdé, O K Doumbo, B Fofana, I Sagara (Malaria Research and Training Center, Department of Epidemiology of Parasitic Diseases, Faculty of Medicine, Pharmacy and Odonto-Stomatology, University of Bamako, Bamako). Mozambique Q Bassat, R González, C Menéndez (Centro de Investigacao em Saude de Manhica, Manhica). Myanmar (Burma) F Smithuis (Myanmar Oxford Clinical Research Unit, Yangon), F Smithuis (Myanmar and Medical Action Myanmar, Yangon). Netherlands T Bousema (Department of Medical Microbiology, Radboud University Nijmegen Medical Centre, Njimegen); P A Kager, P F Mens (Centre for Infection and Immunity Amsterdam [CINEMA], Division of Infectious Diseases, Tropical Medicine and AIDS, Academic Medical Centre, Amsterdam); P F Mens, H D F H Schallig (Royal Tropical Institute, KIT Biomedical Research, Amsterdam); I Van den Broek (Centre for Infectious Disease Control, National Institute for Public Health and the Environment, Bilthoven); M Van Vugt (Division of Infectious Diseases, Center for Tropical Medicine and Travel Medicine, Academic Medical Center, University of Amsterdam). Niger M L Ibrahim (Centre de Recherche Médicale et Sanitaire, Niamey). Nigeria C O Falade (College of Medicine, University of Ibadan, Ibadan); M Meremikwu (Department of Paediatrics, University of Calabar, Calabar); M Meremikwu (Institute of Tropical Diseases Research and Prevention, Calabar). Portugal J P Gil (Drug Resistance and Pharmacogenetics, Center for Biodiversity, Functional and Integrative Genomics, Faculdade de Ciências, Universidade de Lisboa, Lisbon). Rwanda C Karema (Malaria and Other Parasitic Diseases Division-RBC, Ministry of Health, Kigali). Senegal M S Ba, B Faye, O Faye, O Gaye, J-L Ndiaye, M Pene, D Sow, K Sylla, R C K Tine (Department of Parasitology and Mycology, Faculty of Medicine, University Cheikh Anta Diop, Dakar); L K Penali (WWARN-West Africa Regional Centre, Dakar). South Africa K I Barnes, L J Workman (WWARN, Pharmacology Module, Cape Town); K I Barnes, L J Workman (Division of Clinical Pharmacology, Department of Medicine, University of Cape Town, Cape Town). Spain Q Bassat, R González, C Menéndez, I Mueller (Centre de Recerca en Salut Internacional de Barcelona, Barcelona); A Lima (Médecins Sans Frontières—Operational Centre Barcelona-Athens, Barcelona). Sudan I Adam (Faculty of Medicine, University of Khartoum, Khartoum); N B Gadalla (Department of Epidemiology, Tropical Medicine Research Institute, National Centre for Research, Khartoum); E F M Malik (Federal Ministry of Health, Khartoum). Sweden A Björkman (Infectious Diseases Unit, Department of Medicine, Karolinska University Hospital, Karolinska Institutet, Stockholm); J P Gil (Department of Physiology and Pharmacology, Drug Resistance Unit, Section of Pharmacogenetics, Karolinska Institutet, Stockholm); A Mårtensson, B E Ngasala, J Ursing (Malaria Research, Infectious Disease Unit, Department of Medicine Solna, Karolinska Institutet); A Mårtensson (Department of Public Health Sciences, Division of Global Health, Karolinska Institutet, Stockholm); L Rombo (Malaria Research Laboratory, Unit of Infectious Diseases, Department of Medicine, Karolinska University Hospital, Karolinska Institutet); L Rombo (Department of Infectious Diseases, Eskilstuna); L Rombo (Centre for Clinical Research, Sörmland County Council, Sörmland). Switzerland P Aliu (Novartis Pharma AG, Basel); S Duparc (Medicine for Malaria Venture, Geneva); S Filler (The Global Fund to Fight AIDS, Tuberculosis and Malaria, Geneva); B Genton (Department of Epidemiology and Public Health, Swiss Tropical and Public Health Institute, Basel); B Genton (Division of Infectious Diseases and Department of Ambulatory Care and Community Medicine, University Hospital, Lausanne); E M Hodel (Swiss Tropical and Public Health Institute, Basel); P Olliaro (UNICEF, UNDP, World Bank, and WHO Special Programme for Research and Training in Tropical Diseases, Geneva). Tanzania S Abdulla (Ifakara Health Institute, Dar es Salaam); E Kamugisha (Catholic University of Health and Allied Sciences, Mwanza); B E Ngasala, Z Premji (Department of Parasitology, Muhimbili University of Health and Allied Sciences, Dar es Salaam); S A Shekalaghe (Kilimanjaro Clinical Medical Research Institite, Kilimanjaro Christian Medical Centre, Moshi); S A Shekalaghe (Ifakara Health Institute, Bagamoyo). Thailand E A Ashley, V I Carrara, R McGready, F Nosten (Shoklo Malaria Research Unit, Mahidol-Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Mae Sot); E A Ashley, A M Faiz, S J Lee, N J White (Faculty of Tropical Medicine, Mahidol University, Bangkok); V I Carrara, A M Dondorp (Mahidol Oxford University Research Unit, Mahidol University, Bangkok); J J Smith (WWARN-Asia Regional Centre, Bangkok). The Gambia U D’Alessandro (Medical Research Council Unit, Fajara); J Tarning (Mahidol Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Bangkok). Uganda J Achan, H Bukirwa, A Yeka (Uganda Malaria Surveillance Project, Kampala); E Arinaitwe, S G Staedke (Infectious Diseases Research Collaboration, Kampala); M R Kamya (College of Health Sciences, Makerere University, Kampala); F Kironde (Department of Biochemistry, Makerere University, Kampala); C Nabasumba (Faculty of Medicine, Mbarara University of Science and Technology, Mbarara). UK T Bousema, C J Drakeley, N B Gadalla, M Oguike, C J Sutherland (Immunology and Infection Department, London School of Hygiene and Tropical Medicine, London); F Checchi (Humanitarian Department, Save the Children, London); P Dahal, J A Flegg, P J Guerin, C Moreira, P N Newton, C Nsanzabana, R N Price, C H Sibley, K Stepniewska, J Tarning (Worldwide Antimalarial Resistance Network, Oxford); P Dahal, A M Dondorp, J A Flegg, P J Guerin, S J Lee, K Marsh, R McGready, C Moreira, P N Newton, F Nosten, C Nsanzabana, P Olliaro, R N Price, J Tarning, N J White (Centre for Tropical Medicine and Global Health, Nuffield Department of Clinical Medicine, University of Oxford, Oxford); P W Gething, S I Hay (Spatial Ecology and Epidemiology Group, Department of Zoology, University of Oxford, Oxford); B Greenwood (Department of Diseases Control, London School of Hygiene and Tropical Medicine, London); E M Hodel, S A Ward (Parasitology Department, Liverpool School of Tropical Medicine, Liverpool); S G Staedke (Department of Clinical Research, Faculty of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London); I Van den Broek (Médecins sans Frontières, London); P A Winstanley (Warwick Medical School, University of Warwick, Coventry). USA G Dorsey, B Greenhouse, P J Rosenthal (Department of Medicine, University of California San Francisco, San Francisco, CA); N B Gadalla (National Institute of Allergy and Infectious Diseases, Rockville, MD); J P Gil (The Harpur College of Arts and Sciences, Binghamton University, The State University of New York, Binghamton, NY); A Grivoyannis (Division of Emergency Medicine, University of Washington, Seattle, WA); K Hamed (Novartis, East Hanover, NJ); J Hwang, P S Kachur (US Centers for Disease Control and Prevention, Atlanta, GA); J Hwang (Global Health Group, University of California San Francisco, San Francisco, CA); C H Sibley (Department of Genome Sciences, University of Washington, Seattle, WA). Zambia M Nambozi (Tropical Diseases Research Centre, Ndola, Zambia).
Contributors
SA, JA, IA, GOA, BHA, PA, NMA, EA, EAA, MSB, KIB, QB, EB, AB, SB, TB, HB, VIC, FC, MC, UD’A, TMED, PDe, AAD, AMD, GD, OKD, CJD, SD, EE, AMF, COF, J-FF, BFa, OF, SF, BFo, NBG, OG, BGe, JPGi, RG, BryGr BriGr, AG, PJG, J-PGu, KH, EMH, JH, DJ, EJ, PSK, PAK, EK, MRK, CK, HAK, MKh, FK P-EK, MKo, IML, SJL, BL, AL, EMM, KM, AMar, AMas, MMa, RMG, HM, CMe, PFM, MMe, IM, CNa, MN, J-LN, PNN, BEN, FNi, FNo, MO, BRO, PO, SAO, J-BO, LKP, MP, JP, LP, PP, ZP, RNP, LR, PJR, IS, AS-E, PS, HDFHS, BS, SAS, CHS, FS, DS, SGS, CJS, KSy, AOT, JT, ET, RCKT, HT, OAT, JU, IVDb, MVV, SAW, NJW, PAW, WY, AY, and IZ conceived and designed the experiments. SA, JA, IA, GOA, BHA, PA, NMA, EA, EAA, MSB, KIB, QB, EB, AB, SB, TB, HB, VIC, FC, MC, UD’A, TMED, PDe, AAD, AMD, GD, OKD, CJD, SD, EE, AMF, COF, J-FF, BFa, OF, SF, JAF, BFo, NBG, OG, BGe, PWG, JPGi, RG, BryGr, BriGr, AG, PJG, J-PGu, KH, SIH, EMH, JH, DJ, EJ, PSK, PAK, EK, MRK, CK, HAK, MKh, FK, P-EK, MKo, IML, SJL, BL, AL, EMM, KM, AMar, AMas, MMa, RMG, HM, CMe, PFM, MMe, IM, CNa, MN, J-LN, PNN, BEN, FNi, FNo, MO, BRO, PO, SAO, J-BO, LKP, MP, JP, LP, PP, ZP, RNP, LR, PJR, IS, A-SE, PS, HDFHS, BS, SAS, FS, DS, SGS, CJS, KSy, AOT, JT, ET, RCKT, HT, OAT, JU, IVdB, MVV, SAW, NJW, PAW, WY, AY, and IZ did the experiments. PDa, PJG, CMo, CNs, RNP, and KSt analysed the pooled individual patient data. JAF, PWG, SIH, and LJW contributed reagents, materials, or analysis instruments. PDa, PJG, CNs, and RNP wrote the first draft of the manuscript. All study group members contributed to the writing of the manuscript. SA, JA, IA, GOA, BHA, PA, NMA, EA, EAA, MSB, KIB, QB, EB, AB, SB, TB, HB, VIC, FC, MC, UDA, TMED, PDe, AAD, AMD, GD, OKD, CJD, SD, EE, AMF, COF, J-FF, BFa, OF, SF, JAF, BFo, NBG, OG, BGe, PWG, JPGi, RG, BryGr, BriGr, AG, PJG, J-PGu, KH, SIH, EMH, JH, DJ, EJ, PSK, PAK, EK, MRK, CK, HAK, MKh, FK, P-EK, MKo, IML, SJL, BL, AL, EMM, KM, AMar, AMas, MMa, RMG, HM, CMe, PFM, MMe, IM, CNa, MN, J-LN, PNN, BEN, FNi, FNo, AO, MO, BRO, PO, SAO, JBO, LKP, MP, JP, LP, PP, ZP, RNP, LR, PJR, IS, AS-E, PS, HDFHS, BS, SAS, FS, DS, SGS, CJS, KSy, AOT, JT, ET, RCKT, HT, OAT, JU, IVdB, MVV, SAW, NJW, PAW, WY, AY, and IZ enrolled patients.
Footnotes
Declaration of interests
PA is an employee of Novartis Pharma, Basel, Switzerland. SD is an employee of Medicines for Malaria Venture, Geneva, Switzerland. KH is an employee of Novartis Pharmaceuticals, East Hanover, NJ, USA. KIB, ET, and NJW are members of the WHO Technical Expert Group on Malaria Chemotherapy. QB and UD’A have received speaker fees and travel grants from Novartis. All other members of the study group declare no competing interests.
See Online for appendix
References
- 1.WHO [accessed March 19, 2014];World malaria report 2013. http://www.who.int/malaria/publications/world_malaria_report_2013/en/
- 2.White N. Antimalarial drug resistance and combination chemotherapy. Philos Trans R Soc L B Biol Sci. 1999;354:739–49. doi: 10.1098/rstb.1999.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White NJ. The role of anti-malarial drugs in eliminating malaria. Malar J. 2008;7(suppl 1):S8. doi: 10.1186/1475-2875-7-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiao X, Liu GY, Shan CO, et al. Phase II trial in China of a new, rapidly-acting and effective oral antimalarial, CGP 56697, for the treatment of Plasmodium falciparum malaria. Southeast Asian J Trop Med Public Health. 1997;28:476–81. [PubMed] [Google Scholar]
- 5.Hatz C, Abdulla S, Mull R, et al. Efficacy and safety of CGP 56697 (artemether and benflumetol) compared with chloroquine to treat acute falciparum malaria in Tanzanian children aged 1–5 years. Trop Med Int Health. 1998;3:498–504. doi: 10.1046/j.1365-3156.1998.00250.x. [DOI] [PubMed] [Google Scholar]
- 6.Kshirsagar NA, Gogtay NJ, Moorthy NS, et al. A randomized, double-blind, parallel-group, comparative safety, and efficacy trial of oral co-artemether versus oral chloroquine in the treatment of acute uncomplicated Plasmodium falciparum malaria in adults in India. Am J Trop Med Hyg. 2000;62:402–08. doi: 10.4269/ajtmh.2000.62.402. [DOI] [PubMed] [Google Scholar]
- 7.Ezzet F, van Vugt M, Nosten F, Looareesuwan S, White NJ. Pharmacokinetics and pharmacodynamics of lumefantrine (benflumetol) in acute falciparum malaria. Antimicrob Agents Chemother. 2000;44:697–704. doi: 10.1128/aac.44.3.697-704.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vugt MV, Wilairatana P, Gemperli B, et al. Efficacy of six doses of artemether–lumefantrine (benflumetol) in multidrug-resistant Plasmodium falciparum malaria. Am J Trop Med Hyg. 1999;60:936–42. doi: 10.4269/ajtmh.1999.60.936. [DOI] [PubMed] [Google Scholar]
- 9.Falade C, Makanga M, Premji Z, Ortmann CE, Stockmeyer M, de Palacios PI. Efficacy and safety of artemether–lumefantrine (Coartem) tablets (six-dose regimen) in African infants and children with acute, uncomplicated falciparum malaria. Trans R Soc Trop Med Hyg. 2005;99:459–67. doi: 10.1016/j.trstmh.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Hutagalung R, Paiphun L, Ashley EA, et al. A randomized trial of artemether–lumefantrine versus mefloquine–artesunate for the treatment of uncomplicated multi-drug resistant Plasmodium falciparum on the western border of Thailand. Malar J. 2005;4:46. doi: 10.1186/1475-2875-4-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdulla S, Sagara I, Borrmann S, et al. Efficacy and safety of artemether–lumefantrine dispersible tablets compared with crushed commercial tablets in African infants and children with uncomplicated malaria: a randomised, single-blind, multicentre trial. Lancet. 2008;372:1819–27. doi: 10.1016/S0140-6736(08)61492-0. [DOI] [PubMed] [Google Scholar]
- 12.MMV [accessed Dec 18, 2013];Coartem Dispersible 200 million treatments dispatched. 2013 http://www.mmv.org/achievements-challenges/achievements/coartem-d.
- 13.Song J, Socheat D, Tan B, et al. Randomized trials of artemisinin–piperaquine, dihydroartemisinin–piperaquine phosphate and artemether–lumefantrine for the treatment of multi-drug resistant falciparum malaria in Cambodia–Thailand border area. Malar J. 2011;10:231. doi: 10.1186/1475-2875-10-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarning J, Zongo I, Some FA, et al. Population pharmacokinetics and pharmacodynamics of piperaquine in children with uncomplicated falciparum malaria. Clin Pharmacol Ther. 2012;91:497–505. doi: 10.1038/clpt.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WorldWide Antimalarial Resistance Network (WWARN) DP Study Group The effect of dosing regimens on the antimalarial efficacy of dihydroartemisinin–piperaquine: a pooled analysis of individual patient data. PLoS Med. 2013;10:e1001564. doi: 10.1371/journal.pmed.1001564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WWARN . Clinical module: data management and statistical analysis plan. Version 1.2. Oxford: [accessed March 19, 2014]. 2012. http://www.wwarn.org/sites/default/files/ClinicalDMSAP.pdf. [Google Scholar]
- 17.Gething PW, Patil AP, Smith DL, et al. A new world malaria map: Plasmodium falciparum endemicity in 2010. Malar J. 2011;10:378. doi: 10.1186/1475-2875-10-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO . WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. World Health Organization; Geneva: 2006. [Google Scholar]
- 19.WWARN . Statistical analysis plan AL dose impact study group. Version 1.9. Oxford: [accessed March 19, 2014]. 2012. http://www.wwarn.org/sites/default/files/DHA-PQPDoseImpactStudyGroupSAP.pdf. [Google Scholar]
- 20.Glidden DV, Vittinghoff E. Modelling clustered survival data from multicentre clinical trials. Stat Med. 2004;23:369–88. doi: 10.1002/sim.1599. [DOI] [PubMed] [Google Scholar]
- 21.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–41. [Google Scholar]
- 22.Levin ML. The occurrence of lung cancer in man. Acta Unio Int Contra Cancrum. 1953;9:531–41. [PubMed] [Google Scholar]
- 23.Dondorp AM, Nosten F, Yi P, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–67. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venkatesan M, Gadalla NB, Stepniewska K, et al. Polymorphisms in Plasmodium falciparum chloroquine resistance transporter and multidrug resistance 1 genes: parasite risk factors that affect treatment outcomes for P. falciparum malaria after artemether–lumefantrine and artesunate–amodiaquine. Am J Trop Med Hyg. 2014;91:833–43. doi: 10.4269/ajtmh.14-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price R, Luxemburger C, van Vugt M, et al. Artesunate and mefloquine in the treatment of uncomplicated multidrug-resistant hyperparasitaemic falciparum malaria. Trans R Soc Trop Med Hyg. 1998;92:207–11. doi: 10.1016/s0035-9203(98)90750-7. [DOI] [PubMed] [Google Scholar]
- 26.Nacher M, Carrara VI, Ashley E, et al. Seasonal variation in hyperparasitaemia and gametocyte carriage in patients with Plasmodium falciparum malaria on the Thai-Burmese border. Trans R Soc Trop Med Hyg. 2004;98:322–28. doi: 10.1016/j.trstmh.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 27.White NJ. The assessment of antimalarial drug efficacy. Trends Parasitol. 2002;18:458–64. doi: 10.1016/s1471-4922(02)02373-5. [DOI] [PubMed] [Google Scholar]
- 28.Verret WJ, Arinaitwe E, Wanzira H, et al. Effect of nutritional status on response to treatment with artemisinin-based combination therapy in young Ugandan children with malaria. Antimicrob Agents Chemother. 2011;55:2629–35. doi: 10.1128/AAC.01727-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White NJ, van Vugt M, Ezzet F. Clinical pharmacokinetics and pharmacodynamics and pharmacodynamics of artemether– lumefantrine. Clin Pharmacokinet. 1999;37:105–25. doi: 10.2165/00003088-199937020-00002. [DOI] [PubMed] [Google Scholar]
- 30.Salman S, Page-Sharp M, Griffin S, et al. Population pharmacokinetics of artemether, lumefantrine, and their respective metabolites in Papua New Guinean children with uncomplicated malaria. Antimicrob Agents Chemother. 2011;55:5306–13. doi: 10.1128/AAC.05136-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oshikoya KA, Senbanjo IO. Pathophysiological changes that affect drug disposition in protein-energy malnourished children. Nutr Metab (Lond) 2009;6:50. doi: 10.1186/1743-7075-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashley EA, Stepniewska K, Lindegardh N, et al. Pharmacokinetic study of artemether–lumefantrine given once daily for the treatment of uncomplicated multidrug-resistant falciparum malaria. Trop Med Int Heal. 2007;12:201–08. doi: 10.1111/j.1365-3156.2006.01785.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.