Abstract
Objective
To determine the relationship between maternal vitamin D status in pregnancy and body composition in the offspring.
Design
Prospective mother-offspring cohort study.
Setting
Southampton, UK.
Participants
977 pregnant women whose serum 25-hydroxyvitamin D concentration (25(OH)D) was measured in late pregnancy and their offspring, followed up within 3 weeks of birth, and at 4 and 6 years of age.
Main outcome measures
Offspring lean and fat mass assessed using Dual X-ray Absorptiometry.
Results
Median daily vitamin D intake (from food and supplements) in late pregnancy was 3.7μg/day (IQR 2.7 to 5.7). 22% of the women took vitamin D supplements in late pregnancy, but only 8.5% of the women complied with UK guidance to take 10μg per day. Median maternal serum 25(OH)D in late pregnancy was 62nmol/l (IQR 43-89); 35% of the women studied had values below 50 nmol/l. Lower vitamin D status was associated with lower fat mass in the offspring at birth, but with greater fat mass at 4 and 6 years. It was not associated with lean mass at any of the ages studied. The opposing associations seen between maternal 25(OH)D (SDs) and fat mass (SDs) in the offspring at birth and at 6 years were robust to adjustment for a range of confounding factors, including maternal BMI and weight gain in pregnancy (β (95% CI) 0.08 (0.02, 0.15) and −0.10 (−0.17, − 0.02 respectively). The key independent predictors of higher maternal vitamin D status were season of measurement and taking vitamin D in dietary supplements in late pregnancy.
Conclusion
These data suggest that insufficient maternal vitamin D status in pregnancy could result in programmed differences in offspring fat mass. The findings require replication but add to a growing evidence base for a role of vitamin D in the origins of adiposity.
INTRODUCTION
There is a growing recognition of the diversity of the metabolic roles played by vitamin D, beyond its effects on bone health. Low vitamin D status has been linked to impaired glucose tolerance, insulin resistance and the metabolic syndrome in adults1. It has also been proposed that vitamin D insufficiency may be causally associated with adiposity in both adults and children2-4; consistent with this proposition, vitamin D receptor gene polymorphisms have recently been linked to adiposity phenotypes5.
Vitamin D insufficiency is common in the UK6. A particular concern is that almost a third of young women have low vitamin D status, and are likely to start pregnancy with low maternal stores6. Whilst women are recommended to take an additional 10μg/day of vitamin D in pregnancy, supplementation is not a routine part of antenatal care in the UK, and low status is prevalent7. The long-term effects on children born to mothers who have a low vitamin D status in pregnancy have not been widely studied. Maternal vitamin D insufficiency has been linked to insulin resistance and low muscle mass8, and to poorer bone mineral accrual9 in the offspring; little is known about effects on adiposity10. The primary determinant of vitamin D status is sunshine exposure11. Adult BMI has been shown to differ with season of birth12 and it is possible that higher BMI, indicating greater adiposity, among adults born in winter and spring could be linked to lower maternal vitamin D status in the winter months. Recent findings from a follow-up study of Indian children support this possibility, as low maternal vitamin D status in pregnancy was associated with greater adiposity8. However, in an earlier study of UK children body composition was not related to maternal vitamin D status13.
In the context of current concerns about the high prevalence of vitamin D insufficiency in young women, and increasing rates of childhood obesity in the UK, the metabolic consequences for children born to mothers with low maternal status require further investigation. We therefore examined how maternal vitamin D status in late pregnancy related to the body composition of their children at birth, 4 and 6 years of age in a prospective cohort of 977 mothers who were studied in detail throughout pregnancy.
METHODS
Study sample
The Southampton Women’s Survey (SWS) is a prospective cohort study that has assessed the diet, body composition, physical activity and social circumstances of a large group of non-pregnant women aged 20 to 34 years living in the city of Southampton, UK. Comprehensive details of the study have been published previously14. Women were recruited through General Practices across the city between April 1998 and December 2002. Each woman was invited to take part by letter, followed by a telephone call when an interview date was arranged; 12,583 women agreed to take part, 75% of all women contacted. Trained research nurses visited the women at home and collected information about their health, diet and lifestyles, as well as taking anthropometric measurements. Women who subsequently became pregnant were followed up at 11, 19 and 34 weeks gestation and their offspring were studied in infancy and childhood.
Maternal data
Details of mothers’ parity, educational attainment (defined in 6 groups according to highest academic qualification) and social class were obtained at the pre-pregnancy interview, and height and weight were measured. Amongst women who became pregnant, smoking status in pregnancy was ascertained at the 11 and 34 week interviews. At 34 weeks a research nurse obtained a venous blood sample and an aliquot of maternal serum was frozen at −80°C. Serum 25-hydroxyvitamin D concentrations were analyzed by radioimmunoassay (Diasorin, Stillwater, Minnesota, USA). This assay measures both vitamin D2 and vitamin D3. The assay met the requirements of the UK National Vitamin D External Quality Assurance Scheme (DEQAS), and intraassay and interassay coefficients of variation were less than 10%. At 34 weeks the research nurses weighed the women again; pregnancy weight gain from before to late pregnancy was defined as inadequate, adequate or excessive according to the Institute of Medicine (IOM) 2009 recommendations15, as described previously16. In late pregnancy, diet during the preceding 3 months was assessed using a 100-item validated food frequency questionnaire (FFQ)17 and supplement use was ascertained. Dietary vitamin D intake was calculated by multiplying the frequency of consumption of a portion of each food by its vitamin D content according to the UK food composition tables or manufacturers’ composition data. Vitamin D intake from dietary supplements was calculated from the frequency and dose of individual supplements reported by the participant using composition data obtained from the manufacturer.
Infancy and childhood data
The infants were visited at the ages of 6, 12 months and 24 months, and the dates of the last breastfeed as recorded at these visits were used to define duration of breastfeeding and categorised into 6 groups. Crown-heel length at birth was measured using a neonatometer (CMS Ltd, UK) and childhood height was measured at age 4 and 6 years using a portable stadiometer (Leicester height measurer). Within 3 weeks of birth and at 4 and 6 years of age subsets of children attended for an assessment of body composition by Dual X-ray Absorptiometry (DXA) using a Lunar DPX-L instrument (GE Corp, Madison, WI) in infancy and, in childhood, a Hologic Discovery instrument (Hologic Inc., Bedford, MA, USA), both cross-calibrated. Fat mass and fat-free mass were derived from the whole body scan, using paediatric software. The total X-ray dose for the whole body scans was approximately 10.5 microsieverts (paediatric scan mode), equivalent to around 1-2 days background radiation. At 3 years of age dietary vitamin D intake was ascertained from an 80-item FFQ18 and supplementary vitamin D intake from reported supplement use. Physical activity was assessed as the reported time the child was actively on the move each day.
Sample size
A total of 1981 women became pregnant and delivered a live-born singleton infant before the end of 2003. Six infants died in the neonatal period and 2 had major congenital growth abnormalities, which left 1973 mother-offspring pairs. 121 mothers who delivered before 37 weeks gestation and a further 156 mothers who did not have measures of 25(OH)D were not included in the analysis. Of these, 977 children who had whole body DXA scans at birth, 4 years or 6 years were included in the analysis sample: 574 with DXA data at birth, 565 at 4 years and 447 at 6 years. 116 children had DXA scans at all three time points.
Statistical analysis
All children’s fat mass variables were positively skewed and thus were transformed using Fisher-Yates normal scores19 to a normally distributed variable with a mean of 0 and a standard deviation of 1. Whilst fat-free mass variables were normally distributed, they were similarly Fisher-Yates transformed so that all outcome variables were on the same scale of measurement. Maternal serum 25(OH)D concentrations were not normally distributed and were transformed using Fisher-Yates normal scores, as were other variables that were non-normal (maternal pre-pregnancy BMI, 3 year vitamin D intake and 3 year physical activity). Linear regression models were fitted with body composition variables as the outcomes and maternal serum 25(OH)D concentration as the predictor. Outcomes at birth were adjusted for sex, gestation, age at measurement, age squared and crown-heel length. All outcomes at 4 and 6 years were adjusted for sex, age at measurement and childhood height; by including length or height as covariates the effects of pregnancy weight gain on body composition were independent of child’s stature. Additional adjustments were made for potential confounding factors: maternal educational attainment, smoking in pregnancy, pre-pregnancy BMI, height, parity, social class, IOM weight gain category, breastfeeding duration, vitamin D intake at 3 years, and physical activity at 3 years (the latter two only for models with outcomes at 4 or 6 years). A cubic spline model was fitted to describe the association between vitamin D status and 6 year fat mass in more detail.
A form of Fourier analysis was used to model the association between vitamin D status and date of sample. A variable was derived that described the number of years the sample was taken after the first sample in the study, and multiplied by 2π to give a value θ, in radians. Maternal serum 25(OH)D concentration was regressed on cos θ and sin θ (representing one cycle per annum), cos 2θ and sin 2θ (representing two cycles per annum), and cos 3θ and sin 3θ (representing three cycles per annum). The most parsimonious model was the regression of maternal serum 25(OH)D on cos θ, sin θ, cos 2θ and sin 2θ.
Maternal height, age, parity, educational qualifications, social class, smoking in pregnancy, IOM weight gain category, pre-pregnancy BMI, supplementary vitamin D intake in late pregnancy and vitamin D intake from food in late pregnancy were considered as predictors of late pregnancy vitamin D status. The predicted seasonal component of vitamin D as described by the Fourier analysis model was also considered as a predictor of vitamin D status. Predictors with a P-value of < 0.2 in univariate linear regression models were entered into a multivariate linear regression model and a backwards stepwise procedure was employed to identify significant predictors at the 5% level. Statistical analysis was performed using Stata 11.120.
RESULTS
The characteristics of the mothers studied and their children are given in Table 1. Vitamin D status was measured in late pregnancy at a mean (SD) of 34.6 (0.7) weeks gestation. It was not associated with gestational age at measurement (P = 0.51).
Table 1. Descriptive statistics.
| Characteristic | n | Value |
|---|---|---|
| Mother | ||
| Pre-pregnancy BMI (kg/m2)a | 968 | 24.3 (22.2 to 27.6) |
| Height (m)b | 973 | 1.64 (0.64) |
| Degree qualification or above (%) | 975 | 23.8 |
| Smoked in pregnancy (%) | 977 | 14.6 |
| Age at child’s birth (years)b | 977 | 30.4 (3.8) |
| Primiparous (%) | 977 | 45.9 |
| Total vitamin D intake in late preg. (μg/day)a | 969 | 3.9 (2.7 to 5.7) |
| Use vit. D supplements in late preg. (%) | 969 | 22.2 |
| Use > 10μg vit. D supplements in late preg. (%) | 969 | 8.5 |
| Late pregnancy vitamin D status (nmol/l)a | 977 | 62 (43 to 89) |
| − 50 nmol/l (%) | 35.1 | |
| − 75 nmol/l (%) | 28.3 | |
| > 75 nmol/l (%) | 36.6 | |
| Child | ||
| Male (%) | 977 | 52.3 |
| Birth fat mass (kg)a | 574 | 0.53 (0.40 to 0.68) |
| Birth fat-free mass (kg)b | 574 | 3.01 (0.35) |
| 4 year fat mass (kg)a | 565 | 4.65 (4.07 to 5.55) |
| 4 year fat-free mass (kg)b | 565 | 13.20 (1.59) |
| 6 year fat mass (kg)a | 447 | 5.21 (4.26 to 6.53) |
| 6 year fat-free mass (kg)b | 447 | 18.07 (2.20) |
Median (Interquartile range)
Mean (Standard deviation)
Compared with the 875 participants who were eligible for the analyses but who did not have a measure of maternal vitamin D status or did not have DXA scans at any time point, the 977 participants in the current study were better educated (P < 0.0001), less likely to smoke during pregnancy (P=0.0004), older (P=0.0006), slightly taller (P=0.02), and more likely to be from a white ethnic group (P=0.0002). However, there were no differences in pre-pregnancy BMI between the two groups (P=0.86).
We examined associations between maternal 25(OH)D in late pregnancy and offspring fat mass and fat-free mass at birth, 4 years and 6 years (Table 2). The unadjusted results indicate associations between lower vitamin D status and greater fat mass at both 4 and 6 years. After adjustment for maternal educational attainment, smoking in pregnancy, pre-pregnancy BMI, height, parity, social class, IOM weight gain category, child’s breastfeeding duration, and vitamin D intake and physical activity at 3 years, the association between maternal 25(OH)D and 4 year fat mass became non-significant. After adjustment for confounders there was a positive association between maternal 25(OH)D and fat mass at birth (P=0.02); also the inverse association with 6 year fat mass remained (P=0.01). There was no association between maternal 25(OH)D and fat-free mass at birth, 4 or 6 years, either before or after adjustment for confounders.
Table 2. Late pregnancy vitamin D status (SD) as a predictor of offspring body composition.
| Unadjusted | Adjusted for confoundersb | |||
|---|---|---|---|---|
| Outcomea | β (95% CI) | P-value | β (95% CI) | P-value |
| Birth fat mass (SD) | 0.06 (−0.01, 0.12) | 0.09 | 0.08 (0.02, 0.15) | 0.02 |
| Birth fat-free mass (SD) | 0.02 (−0.03, 0.07) | 0.44 | 0.04 (−0.02, 0.09) | 0.17 |
| 4 year fat mass (SD) | −0.09 (−0.16, −0.02) | 0.02 | −0.01 (−0.08, 0.07) | 0.81 |
| 4 year fat-free mass (SD) | 0.03 (−0.02, 0.08) | 0.21 | 0.03 (−0.02, 0.08) | 0.30 |
| 6 year fat mass (SD) | −0.16 (−0.23, −0.08) | < 0.001 | −0.10 (−0.17, −0.02) | 0.01 |
| 6 year fat-free mass (SD) | 0.01 (−0.04, 0.06) | 0.65 | 0.02 (−0.03, 0.07) | 0.43 |
Birth outcomes adjusted for sex, gestation, age at measurement, age squared and length; childhood outcomes adjusted for sex, age and height
Birth confounders were maternal educational attainment, smoking in pregnancy, pre-pregnancy BMI, height, parity, social class and IOM weight gain category; childhood confounders were maternal educational attainment, smoking in pregnancy, pre-pregnancy BMI, height, parity, social class, IOM weight gain category, breastfeeding duration, vitamin D intake at 3 years, and physical activity at 3 years
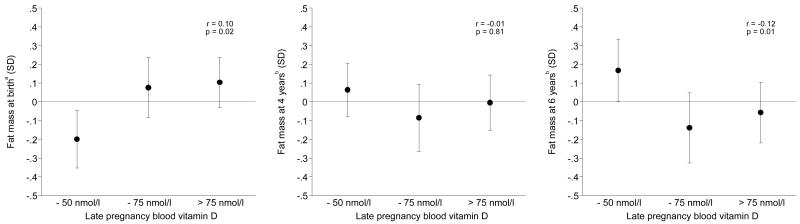
The associations between maternal vitamin D status in late pregnancy and offspring’s fat mass at birth, 4 and 6 years are illustrated in Figure 1. Compared with the offspring of mothers with serum 25(OH)D concentrations of up to 50 nmol/l, fat mass at birth was 8% (95% CI 1, 16) greater amongst those born to mothers whose 25(OH)D was between 50 and 75 nmol/l, and 10% (95% CI 3, 17) greater amongst those whose mothers had a 25(OH)D above 75 nmol/l. Compared with mothers who had a vitamin D status of up to 50 nmol/l, 6 year fat mass was 8% (95% CI 2, 14) lower amongst mothers who had a vitamin D status between 50 and 75 nmol/l, and 6% (95% CI 0, 12) lower amongst mothers who had a vitamin D status above 75 nmol/l.
Figure 1. Mean (95% CI) fat mass at birth, 4 years and 6 years according to maternal vitamin D status in late pregnancy.
aAdjusted for sex, gestation, age at measurement, age squared, length, maternal educational attainment, smoking in pregnancy, pre-pregnancy BMI, height, parity, social class and IOM weight gain categories
bAdjusted for sex, age, height, maternal educational attainment, smoking in pregnancy, pre-pregnancy BMI, height, parity, social class, IOM weight gain categories, breastfeeding duration, vitamin D intake at 3 years, and physical activity at 3 years
It appeared there might be a threshold effect of vitamin D status on 6 year fat mass. A cubic spline model fitted to the data indicated a plateauing of the effect at around 64 nmol/l. Amongst mothers whose serum 25(OH)D concentrations were below 64 nmol/l there was a significant negative association between vitamin D status and 6 year fat mass (β = −0.24 (95% CI −0.41 to −0.07), P = 0.006), whilst amongst mothers with higher 25(OH)D there was no association (β = 0.00 (95% CI −0.19 to 0.18), P = 0.99), after adjustment for confounders. This interaction was statistically significant (P = 0.03).
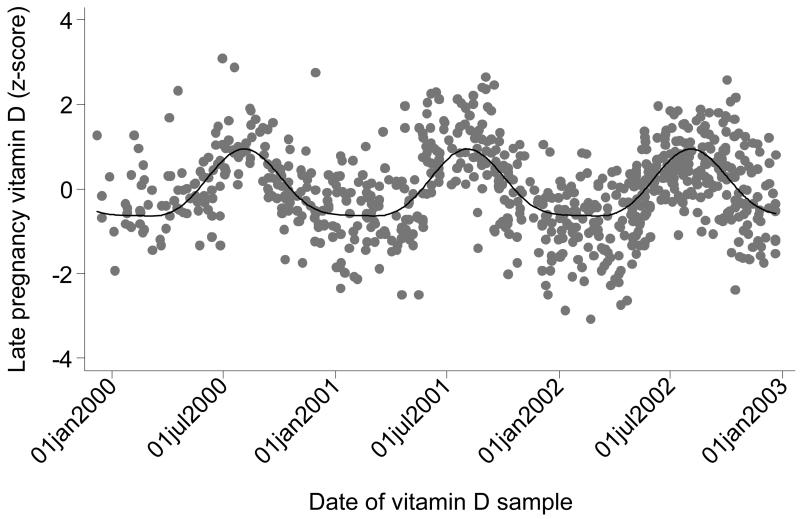
We examined a number of potential influences on vitamin D status in pregnancy. The multivariate linear regression model of predictors of maternal 25(OH)D is presented in Table 3. The most important predictor of higher vitamin D status was season of measurement, as described by the pattern in Figure 2. Amongst women whose samples were taken in July-September, 84% had 25(OH)D greater than 64 nmol/l, whereas amongst women whose samples were taken in December-April, only 23% had values greater than 64 nmol/l. The second most important predictor was taking vitamin D in dietary supplements in late pregnancy. Of the 8.5% of women taking the recommended 10μg of vitamin D, 94% had serum 25(OH)D concentrations greater than 64 nmol/l, whereas amongst the rest of the cohort only 44% had values greater than 64 nmol/l. Higher late pregnancy vitamin D intake from food, and not smoking in pregnancy, were less important predictors of higher maternal vitamin D status. When maternal pre-pregnancy BMI was added to this multivariate model it was not statistically significant (P = 0.11).
Table 3. Predictors of maternal vitamin D status in late pregnancy (SD).
| Predictor | Coefficient (95% CI) | t-statistic | P-value | |
|---|---|---|---|---|
| Seasona | 1.51 (1.37, 1.65) | 21.6 | < 0.001 | 5 |
| Taking vitamin D supplements in late pregnancy (Yes/No) | 0.67 (0.55, 0.79) | 11.3 | < 0.001 | |
| Late pregnancy vitamin D food intake (SDs) | 0.08 (0.04, 0.13) | 3.4 | 0.001 | |
| Smoking in pregnancy (No/Yes) | −0.18 (−0.32, −0.04) | −2.6 | 0.01 |
Season defined as the predicted seasonal component of vitamin D as described by the Fourier analysis model and scaled so that a one unit change describes the difference between the summer maximum and the winter minimum.
Figure 2. Fourier analysis of maternal vitamin D status in late pregnancy.
DISCUSSION
Statement of principal findings
We have demonstrated that maternal vitamin D status measured in late pregnancy is associated with differences in DXA-assessed adiposity in the offspring. However the pattern of association differed at birth and in later childhood; lower vitamin D status was associated with lower fat mass assessed at birth, and with higher fat mass assessed at 6 years. These associations were robust to adjustment for a range of confounding factors, including childhood vitamin D intake and known predictors of adiposity in children. To our knowledge these associations between maternal vitamin D status and adiposity in the offspring at birth and in later childhood, have not been described before. In contrast to the relationships observed with adiposity, we found no association between maternal vitamin D status and fat-free mass in these children.
Comparison with other studies
Although the potential effects of maternal vitamin D deficiency on offspring body composition have been recognised10, there are few published studies with which we can compare our findings directly. We found that lower vitamin D status was associated with lower adiposity at birth (Figure 1); the significance of variations in fat mass at birth measured directly using DXA, as in the current study, are not yet fully understood21. We have previously described limited ‘tracking’ of DXA-assessed fat mass between birth and 4 years in the SWS cohort (r = 0.24), whereas the correspondence between 4 and 6 years is much stronger (r = 0.86)16. This suggests that a lower fat mass at birth is not necessarily associated with lower adiposity in later childhood.
By the age of 4 years, the pattern of association between maternal vitamin D status and fat mass in the SWS children had reversed, such that lower status in late pregnancy was predictive of greater adiposity. At this age the association was not robust to adjustment for the effects of confounding influences. However the same pattern of association between lower vitamin D status in late pregnancy and greater offspring adiposity was evident at 6 years, and this remained after taking account of a wide range of confounders. There are two studies that are relevant to our findings. Firstly, in a recent study of Indian children aged 9.5 years8, percentage fat determined using bioimpedance analysis was greater in offspring of mothers with a lower vitamin D status assessed at 28-32 weeks gestation although this was of borderline significance after adjustment for the effects of maternal covariates. Unlike our study, in the Indian children positive associations were found between maternal vitamin D status and a measure of fat-free mass (arm-muscle area). There are methodological differences between the studies that may be important, as we assessed body composition directly using DXA. However it may also be difficult to compare the findings as so many maternal characteristics differ, including body composition and vitamin D status. For example 67% of Indian women studied had low 25(OH)D (<50 nmol/l), compared with 35% in the present study. A study that allows a more direct comparison with our current data measured 178 children aged 9 years from a previous Southampton birth cohort, whose body composition was also assessed using DXA13. In contrast with the present findings, maternal vitamin D status in late pregnancy was not related to offspring fat mass in the earlier study. It is not clear why these findings differ. Apart from differences in the size of the cohorts there are other differences between the cohorts that may be relevant. Amongst these, the children in the previous study were approaching puberty and the greater use of vitamin D supplements (22% compared with 7%) and higher maternal vitamin D status in late pregnancy (median 25(OH)D 62 vs 50 nmol/l) in the SWS may be important. In our continued follow-up of the SWS children we will be able to reassess the relationship between maternal vitamin D status and body composition in later childhood, but the differences between the findings of the studies are currently not fully explained.
Further investigation of the association between vitamin D status and 6 year fat mass in the SWS children demonstrated a possible threshold effect at a maternal 25(OH)D around 64 nmol/l; the negative association between vitamin D and fat mass was confined to the 48% of women who had lower values; there was no association above this threshold. When we examined the predictors of maternal vitamin D status we found that season of measurement was a key influence, such that women who reached 34 weeks gestation in the summer months had much higher vitamin D concentrations than those measured in the winter (Figure 2). Such seasonal effects on vitamin D status are expected. However, vitamin D status was also increased by use of supplements. In the UK an additional 10μg/day has been recommended since 1991. Although NICE guidance in 200822 endorsed the original recommendation, use of supplements is not routine in the UK. This is borne out in this sample of SWS women; 8.5% of women were taking the recommended amount of vitamin D in late pregnancy. It is important to note that among these women 94% had vitamin D concentrations above 64 nmol/l, irrespective of the season when they were studied.
Strengths and weaknesses
The SWS provides data from a contemporary cohort of women and their offspring from a wide range of sociodemographic backgrounds. Vitamin D status was measured in a large number of participants and outcomes were assessed at three different time points. We used DXA to provide measurements of fat mass and fat-free mass, and by including length or height measures in the models we were able to describe effects independent of infant or childhood stature. Because we have detailed data on this cohort we were able to control for a large number of other potential confounding factors, including important predictors of childhood fat mass such as maternal education, pre-pregnancy BMI, smoking in pregnancy and duration of breastfeeding. For example, whilst we have previously shown that excessive weight gain in pregnancy is associated with offspring adiposity16, we were able to demonstrate that the association between vitamin D status and fat mass at 6 years was independent of this effect (Table 2). A further consideration is that of reverse causality, as increased storage of vitamin D in women who have greater fat stores results in lower 25(OH)D23-25. An apparent association between lower maternal vitamin D status and adiposity in their offspring could therefore be due to the effects of shared diet and lifestyle, rather than causally related to variations in status. Whilst we cannot exclude this possibility, the association between vitamin D status and 6 year fat mass was independent of maternal pre-pregnancy BMI in our study (Table 2); we therefore do not think this would explain the pattern of associations we observed.
Although the use of DXA is well-validated in adults, there are potential problems with neonates and young children due to their low absolute BMC and tendency to move. However, we used specific paediatric software and movement artifact was modest; the few individuals with excessive movement at each time point were excluded from our analyses. The accuracy of DXA for the assessment of body composition in small animals has been demonstrated in piglets26;27.
DXA measurements and vitamin D status were available on a subset of 977 children and their mothers, who, when compared with a similar group without measurements, were better educated, less likely to smoke in pregnancy, older, taller and more likely to be from a white ethnic group. However, unless the associations between vitamin D status and offspring body composition are different in the remainder of the cohort, it is unlikely that selection bias could explain the relationships observed.
Interpretation and implications
We will be able to address some of the implications of the findings of the present study in our continued follow-up of these children. Whilst the findings require extension and replication, the mechanisms that link vitamin D insufficiency to a lower fat mass in the baby at birth but greater adiposity in later childhood also need to be elucidated. The role of vitamin D in adipocyte metabolism is complex4;5 and the mechanisms that link vitamin D status to adiposity are currently unknown4. One interpretation of our data is that there are programmed effects on the fetus arising from maternal vitamin D insufficiency that remain with the individual and that may predispose them to gain excess body fat in later childhood. We speculate that there may be different routes to childhood obesity arising in prenatal life since both insufficient (associated with maternal vitamin D insufficiency or inadequate pregnancy weight gain16), as well as excess (associated with maternal adiposity and excessive pregnancy weight gain16) maternal nutrition have been shown to be associated with greater adiposity in childhood.
Our study shows that low vitamin D status in pregnancy, among a population of healthy pregnant women living in the south of England, is common. These data contribute to a new evidence base that links maternal vitamin D insufficiency to adverse outcomes in the offspring. Whilst further studies are needed, the findings add weight to current concerns about the prevalence of low vitamin D status among women of reproductive age in the UK6. Analyses of an ongoing vitamin D supplementation trial amongst pregnant women will provide a further opportunity to assess the associations between vitamin D insufficient and offspring body composition in an experimental study28.
What is already known on this topic
Low vitamin D status is common in women of reproductive age in the UK.
Low vitamin D status has been linked to higher BMI and to greater adiposity in children and adults, but the consequences of low maternal status in pregnancy for offspring body composition is not known.
What this study adds
In a large general population sample of women, lower vitamin D status in late pregnancy was associated with greater adiposity in their offspring at the age of 6 years.
These data suggest that maternal vitamin D insufficiency may influence fetal development and thereby increase the risk of childhood adiposity in the offspring.
ACKNOWLEDGEMENTS
We thank the general practitioners and midwives in Southampton for their support. We are grateful to the research nurses and other staff of the Southampton Women’s Survey for all their work in recruiting and interviewing the participants, and processing the data and samples. We also thank the women of Southampton and their children who gave their time to take part in the study.
Funding Funding for the components of the Southampton Women’s Survey contributing to this research came from the Medical Research Council, British Heart Foundation, Food Standards Agency and Arthritis Research UK. KG is supported by the National Institute for Health Research through the Southampton NIHR Biomedical Research Unit in Nutrition, Diet & Lifestyle.
Abbreviations
- SWS
Southampton Women’s Survey
- IOM
Institute of Medicine
- DXA
Dual X-ray Absorptiometry
Footnotes
Competing interests All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work beyond that mentioned under Funding; CC has received consultancy and lecture fees from Servier, Eli Lilly, Merck, Amgen, Novartis and Medtronic, KMG has received consultancy fees from Abbott Nutrition, is part of an academic consortium that has received research funding from Abbott Nutrition, Nestec and Danone, has received payment for lectures to Nestlé Nutrition, and has received travel expenses from ILSI Europe, HMI has received payment for consultancy from Bayer Healthcare, SMR has received travel expenses from ILSI Europe; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval The Southampton Women’s Survey was approved by the Southampton and South West Hampshire Local Research Ethics Committee (307/97, 153/99w, 005/03/t and 06/Q1702/104), and all study participants gave written informed consent to be included.
Data sharing No additional data available.
REFERENCES
- (1).Martini L, Wood R. Vitamin D status and the metabolic syndrome. Nutr Rev. 2006;64(11):479–486. doi: 10.1111/j.1753-4887.2006.tb00180.x. [DOI] [PubMed] [Google Scholar]
- (2).Young K, Engelman C, Langefeld C, Hairston K, Haffner S, Bryer-Ash M, et al. Association of plasma vitamin D levels with adiposity in Hispanic and African Americans. J Clin Endocr Metab. 2009;94(9):3306–3313. doi: 10.1210/jc.2009-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Rajakumar K, de las Heras J, Chen TC, Lee S, Holick MF, Arslanian SA. Vitamin D status, adiposity and lipids in Black American and Caucasian children. J Clin Endocr Metab. 2011;96(5):1560–1567. doi: 10.1210/jc.2010-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Gilbert-Diamond D, Baylin A, Mora-Plazas M, Marin C, Arsenault JE, Hughes MD, et al. Vitamin D deficiency and anthropometric indicators of adiposity in school-age children: a prospective study. Am J Clin Nutr. 2010;92:1446–1451. doi: 10.3945/ajcn.2010.29746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Ochs-Balcom HM, Chennamaneni R, Millen AE, Shields PG, Marian C, Trevisan M, et al. Vitamin D receptor gene polymorphisms are associated with adiposity phenotypes. Am J Clin Nutr. 2011;93:5–10. doi: 10.3945/ajcn.2010.29986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Scientific Advisory Committee on Nutrition . Update of Vitamin D. The Stationery Office; Norwich: 2007. [Google Scholar]
- (7).Hyppönen E, Boucher BJ. Avoidance of vitamin D deficiency in pregnancy in the United Kingdom: the case for a unified approach in national policy. Brit J Nutr. 2010;104:309–314. doi: 10.1017/S0007114510002436. [DOI] [PubMed] [Google Scholar]
- (8).Krishnaveni GV, Veena SR, Winder NR, Hill JC, Noonan K, Boucher BJ, et al. Maternal vitamin D status during pregnancy and body composition and cardiovascular risk markers in Indian children: the Mysore Parthenon Study. Am J Clin Nutr. 2011;93:628–635. doi: 10.3945/ajcn.110.003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, et al. Maternal vitamin D status during pregnancy and childhood bone mass at 9 years: a longitudinal study. Lancet. 2006;367:36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- (10).Pasco JA, Wark JD, Carlin JB, Ponsonby A-L, Vuillermin PJ, Morley R. Maternal vitamin D in pregnancy may influence not only offspring bone mass but other aspects of musculoskeletal health and adiposity. Med Hypotheses. 2008;71:266–269. doi: 10.1016/j.mehy.2008.01.033. [DOI] [PubMed] [Google Scholar]
- (11).Mason RS, Sequeira VB, Gordon-Thomson C. Vitamin D: the light side of sunshine. Eur J Clin Nutr. 2011 doi: 10.1038/ejcn.2011.105. doi:10.1038/ejcn.2011.105:1-8. [DOI] [PubMed] [Google Scholar]
- (12).Phillips DIW, Young JB. Birth weight, climate at birth and the risk of obesity in adult life. Int J Obesity. 2000;24:281–287. doi: 10.1038/sj.ijo.0801125. [DOI] [PubMed] [Google Scholar]
- (13).Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, et al. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. 2008;62:68–77. doi: 10.1038/sj.ejcn.1602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Inskip HM, Godfrey KM, Robinson SM, Law CM, Barker DJP, Cooper C, et al. Cohort profile: The Southampton Women’s Survey. Int J Epidemiol. 2006;35(1):42–48. doi: 10.1093/ije/dyi202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Institute of Medicine . Weight gain during pregnancy: reexamining the guidelines. The National Academies Press; Washington, DC: 2009. [PubMed] [Google Scholar]
- (16).Crozier SR, Inskip HM, Godfrey KM, Cooper C, Harvey NC, Cole ZA, et al. Weight gain in pregnancy and childhood body composition: findings from the Southampton Women’s Survey. Am J Clin Nutr. 2010;91:1745–1751. doi: 10.3945/ajcn.2009.29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Robinson S, Godfrey K, Osmond C, Cox V, Barker D. Evaluation of a food frequency questionnaire used to assess nutrient intakes in pregnant women. Eur J Clin Nutr. 1996;50:302–308. [PubMed] [Google Scholar]
- (18).Fisk CM, Crozier SR, Inskip HM, Godfrey KM, Cooper C, Robinson SM, et al. Influences on the quality of young children’s diets: the importance of maternal food choices. Br J Nutr. 2010;105:287–296. doi: 10.1017/S0007114510003302. [DOI] [PubMed] [Google Scholar]
- (19).Armitage P, Berry G. Statistical methods in medical research. Blackwell Science Ltd; Oxford: 2002. [Google Scholar]
- (20).StataCorp . Stata: Release 11. Statistical Software. StataCorp LP; College Station, TX: 2009. [Google Scholar]
- (21).Harvey NC, Poole JR, Javaid MK, Dennison EM, Robinson S, Inskip HM, et al. Parental determinants of neonatal body composition. J Clin Endocr Metab. 2007;92:523–526. doi: 10.1210/jc.2006-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).National Institute of Clinical Excellence . Improving the nutrition of pregnant and breastfeeding mothers and children in low-income households. NICE; London: 2008. [Google Scholar]
- (23).Vimaleswaran KS, Loos RJF. Progress in the genetics of common obesity and type 2 diabetes. Expert Rev Mol Med. 2010;12:e7. doi: 10.1017/S1462399410001389. [DOI] [PubMed] [Google Scholar]
- (24).Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(690):693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- (25).Jorde R, Sneve M, Emaus N, Figenschau Y, Grimnes G. Cross-sectional and longitudinal relation between serum 25-hydroxyvitamin D and body mass index: the Tromsø study. Eur J Nutr. 2010;49:401–407. doi: 10.1007/s00394-010-0098-7. [DOI] [PubMed] [Google Scholar]
- (26).Abrams SA, Schanler RJ, Sheng HP, Evans HJ, Leblanc AD, Garza C. Bone mineral content reflects total body calcium in neonatal miniature piglets. Pediatr Res. 1988;24(6):693–695. doi: 10.1203/00006450-198812000-00008. [DOI] [PubMed] [Google Scholar]
- (27).Brunton JA, Bayley HS, Atkinson SA. Body composition analysis by dual energy X-ray absorptiometry compared to chemical analysis of fat, lean and bone mass in small piglets. Basic Life Sci. 1993;60:157–160. doi: 10.1007/978-1-4899-1268-8_35. [DOI] [PubMed] [Google Scholar]
- (28).Williams EL, Harvey NC, Dennison EM, Edwards CC, Cooper C. Maternal nutrition and bone health in the offspring. Int J Clin Rheumatol. 2009;4(2):133–145. [Google Scholar]